Exploring Sustainable Mineralization Pathways: Multi-Factor Impacts on Microbial-Induced Carbonate Precipitation and Crystals Characteristics
Abstract
1. Introduction
2. Materials and Methods
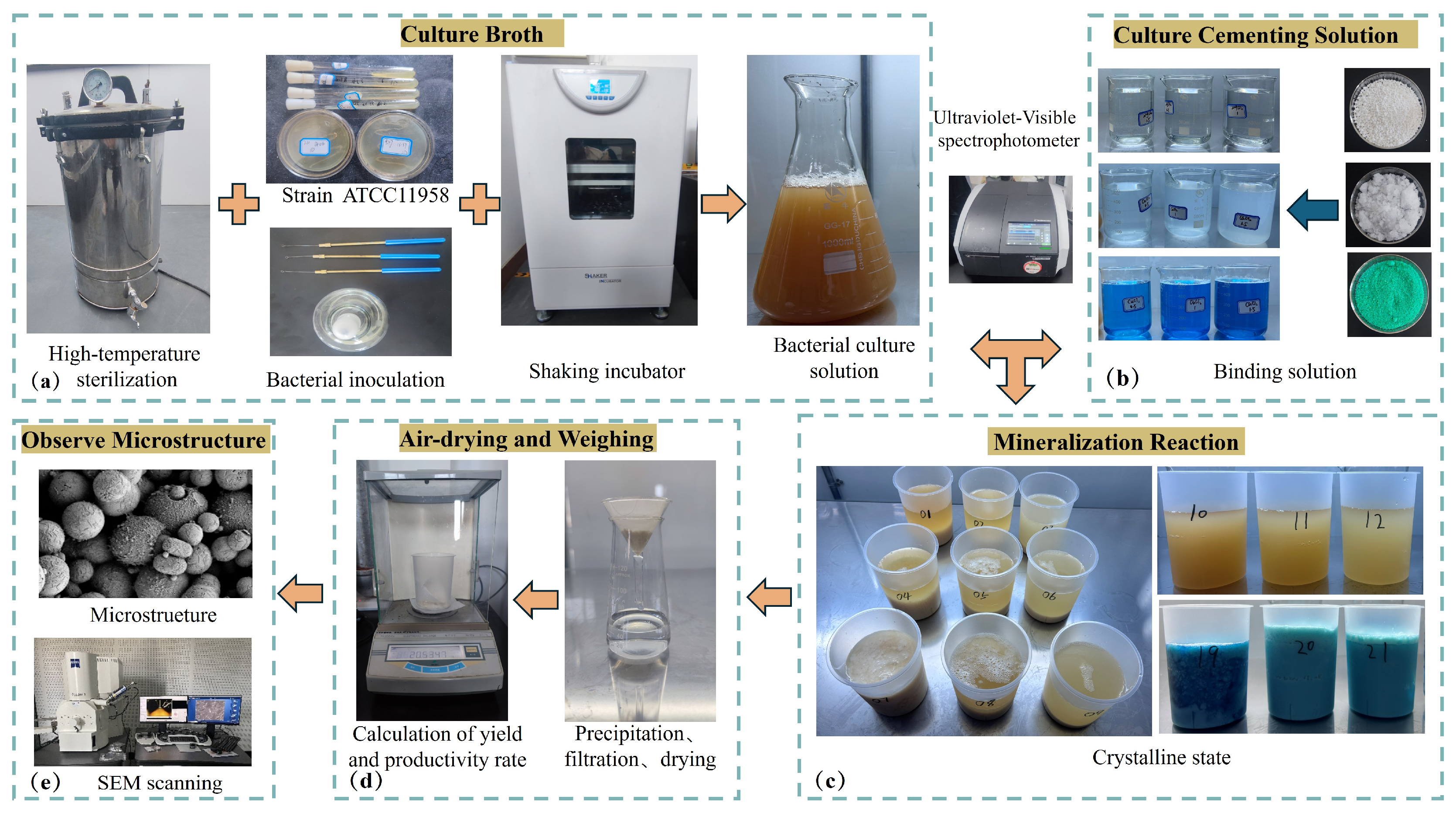
2.1. Preparation of Bacteria
2.2. Crystallization Principle
2.3. Experimental Design
2.4. Microscopic Experimentation
3. Results and Discussion
3.1. Precipitate Yield and Precipitation Rate
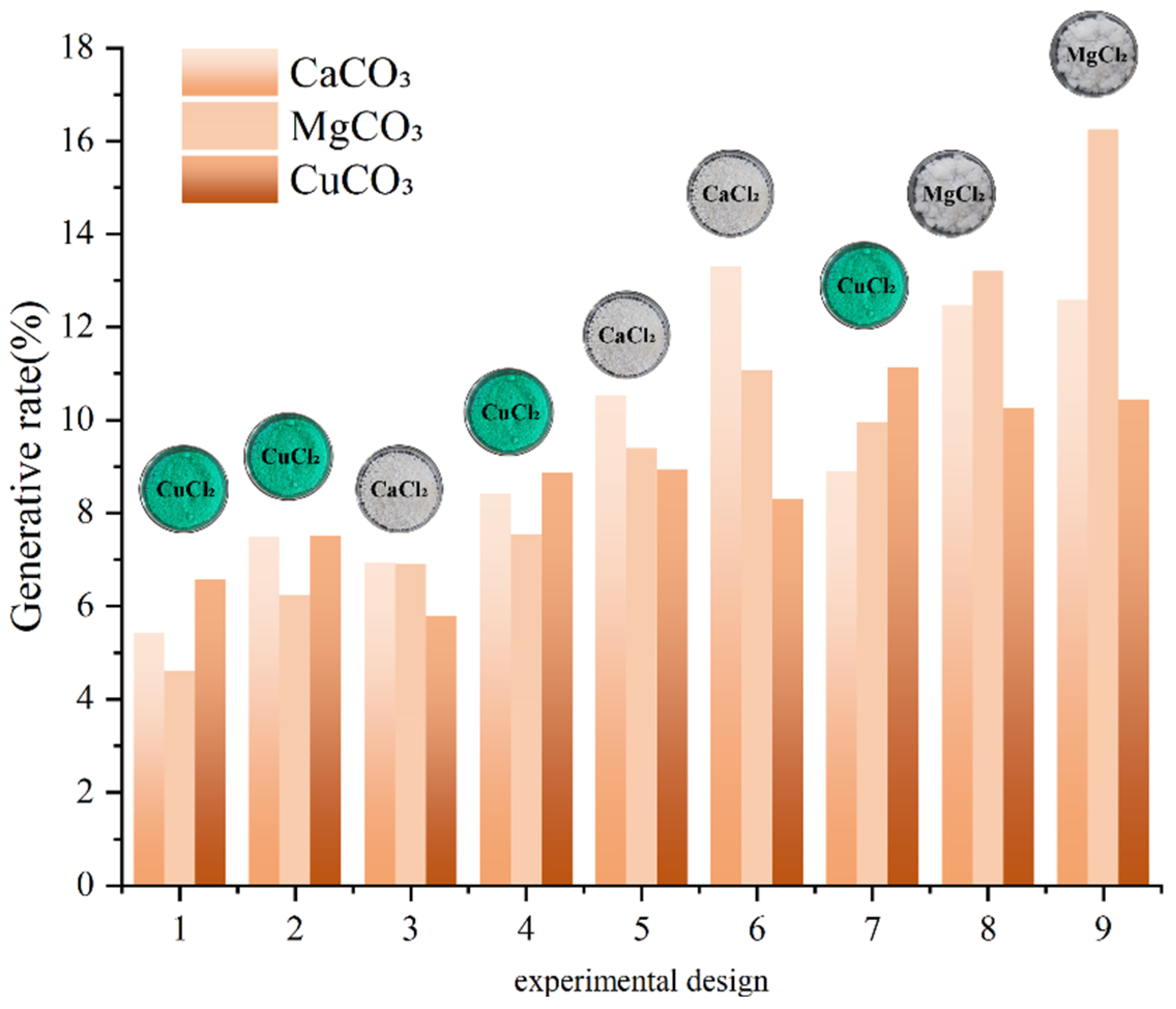
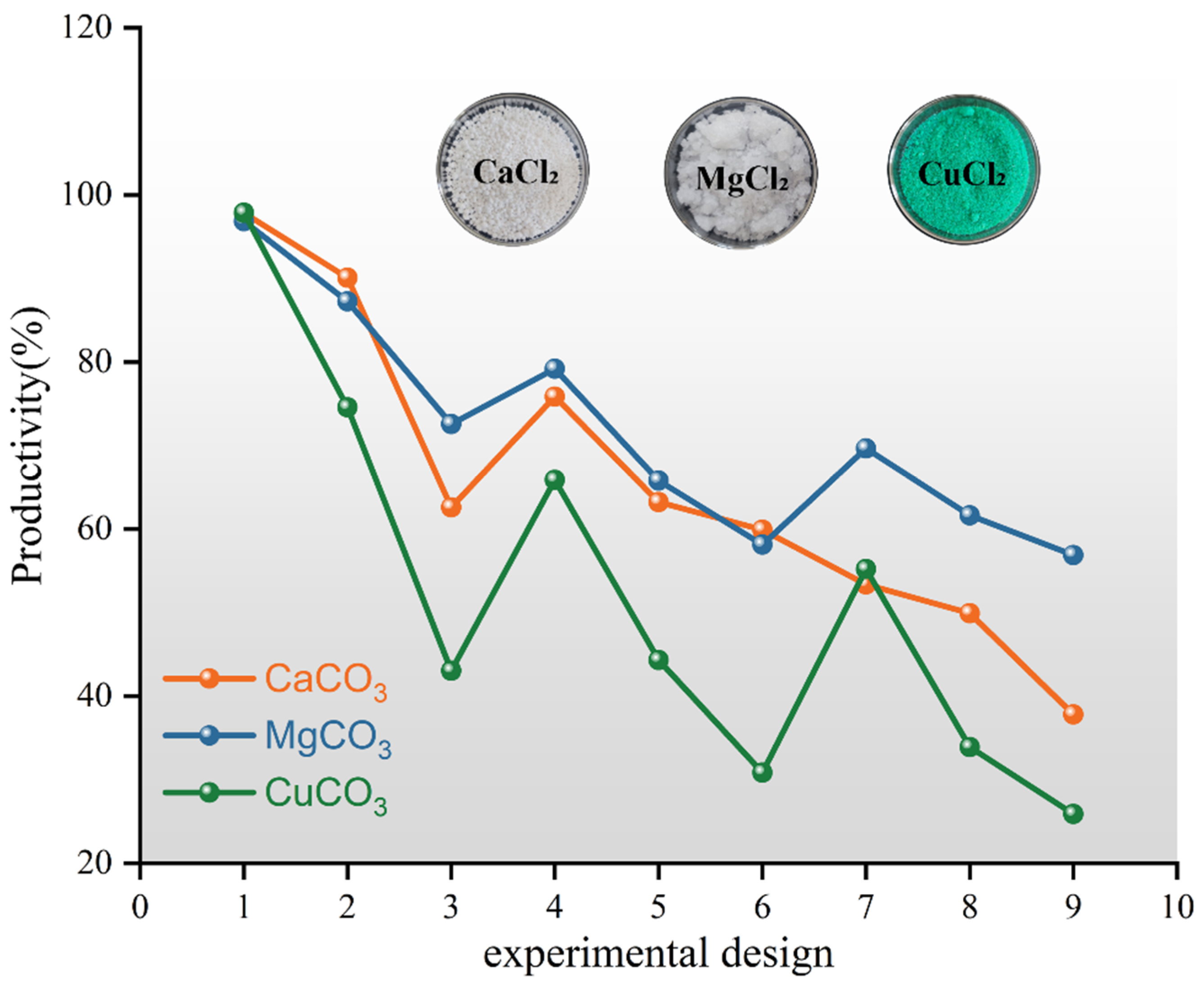
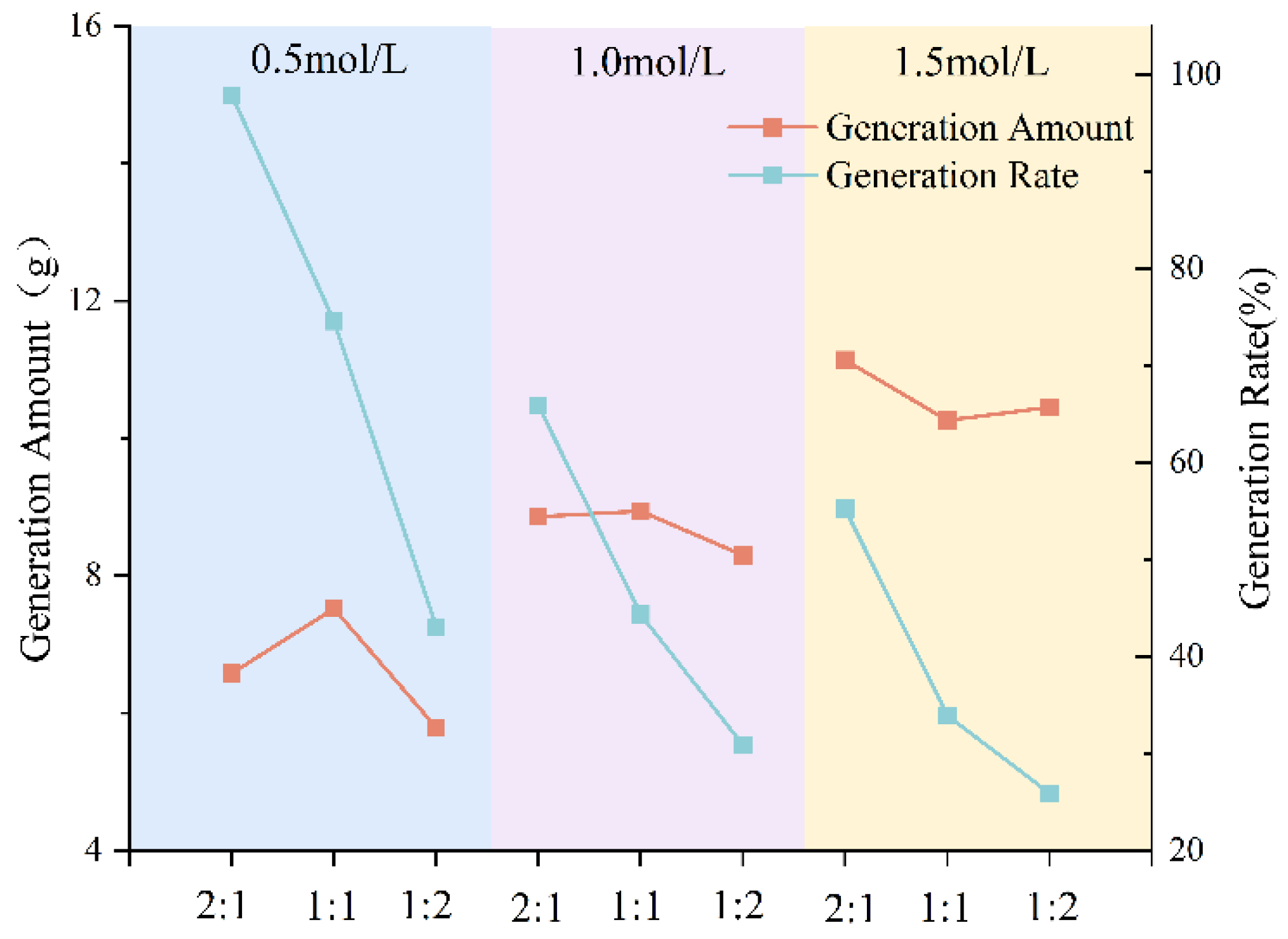
3.1.1. Effect of Metal Source on Precipitation Rate
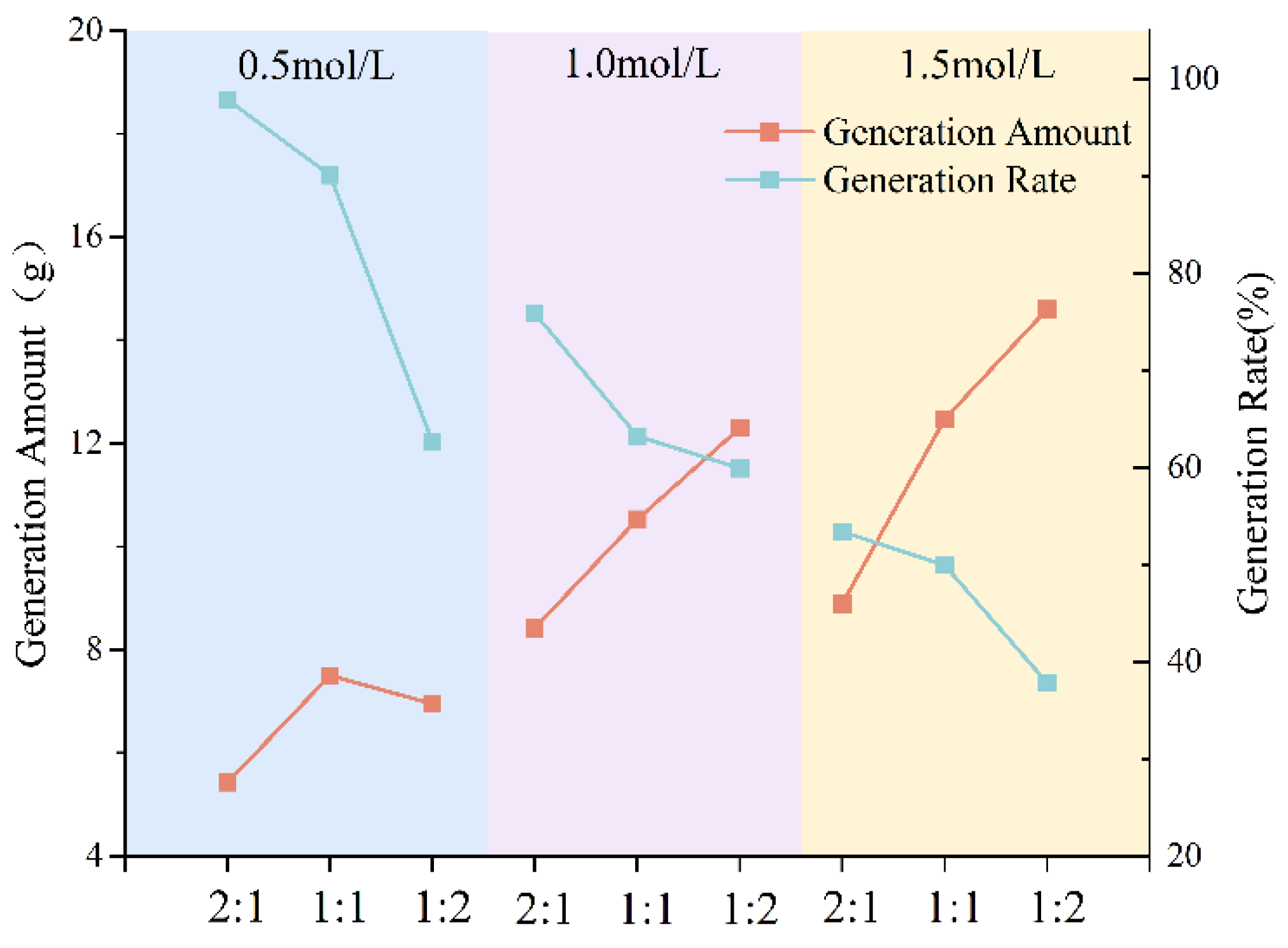
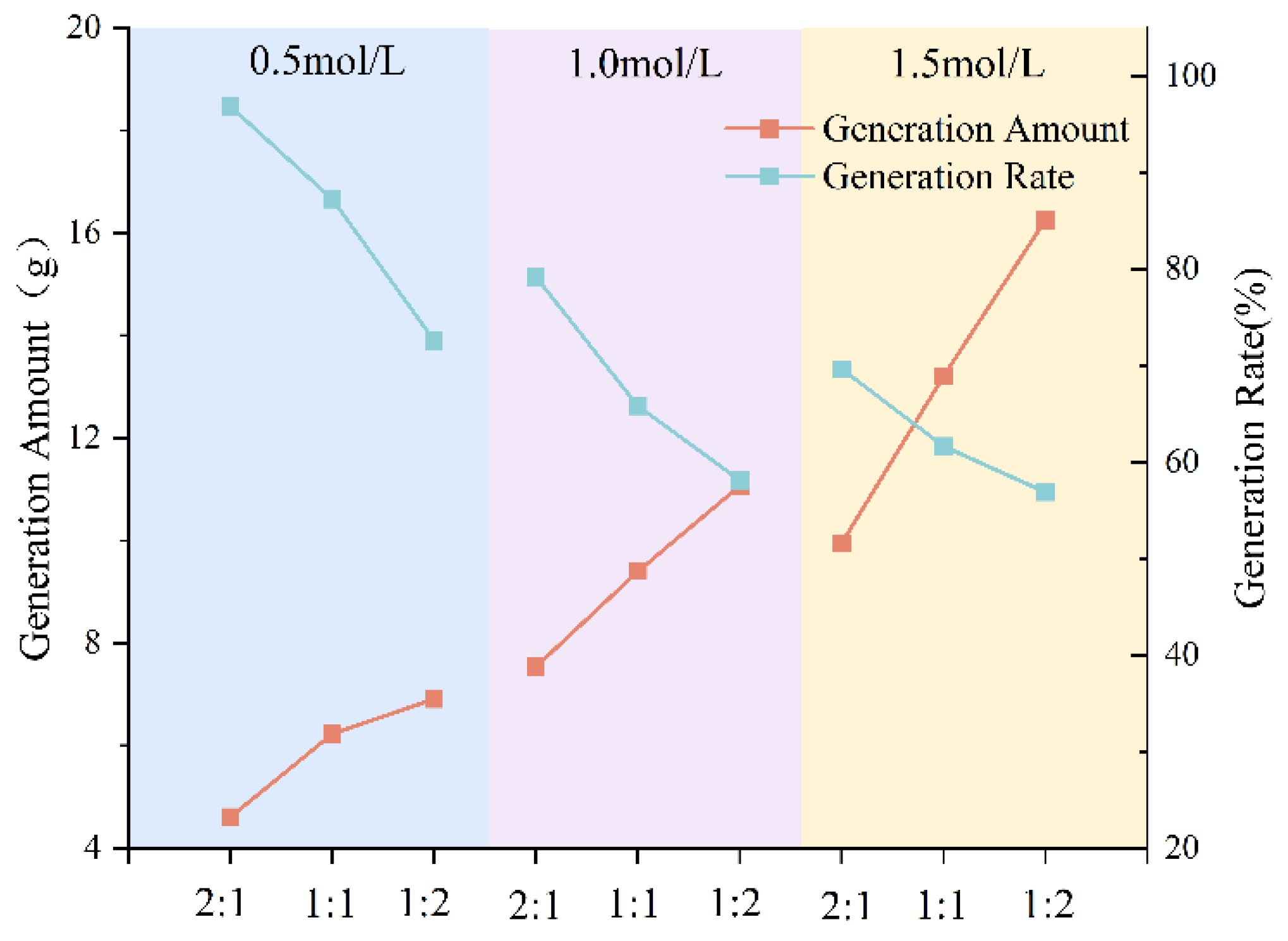
3.1.2. Effect of Grouting Solution Concentration on Precipitation Rate
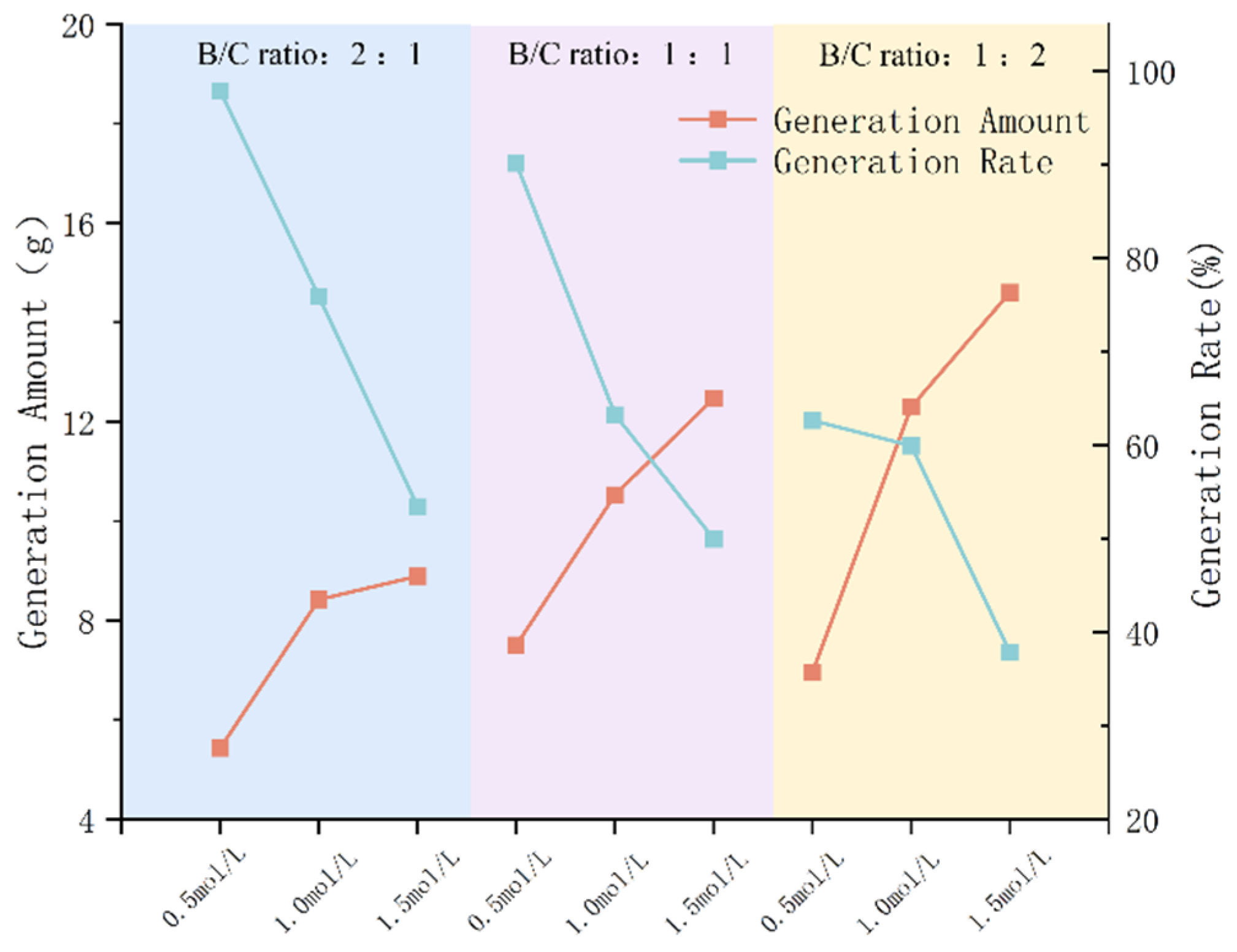
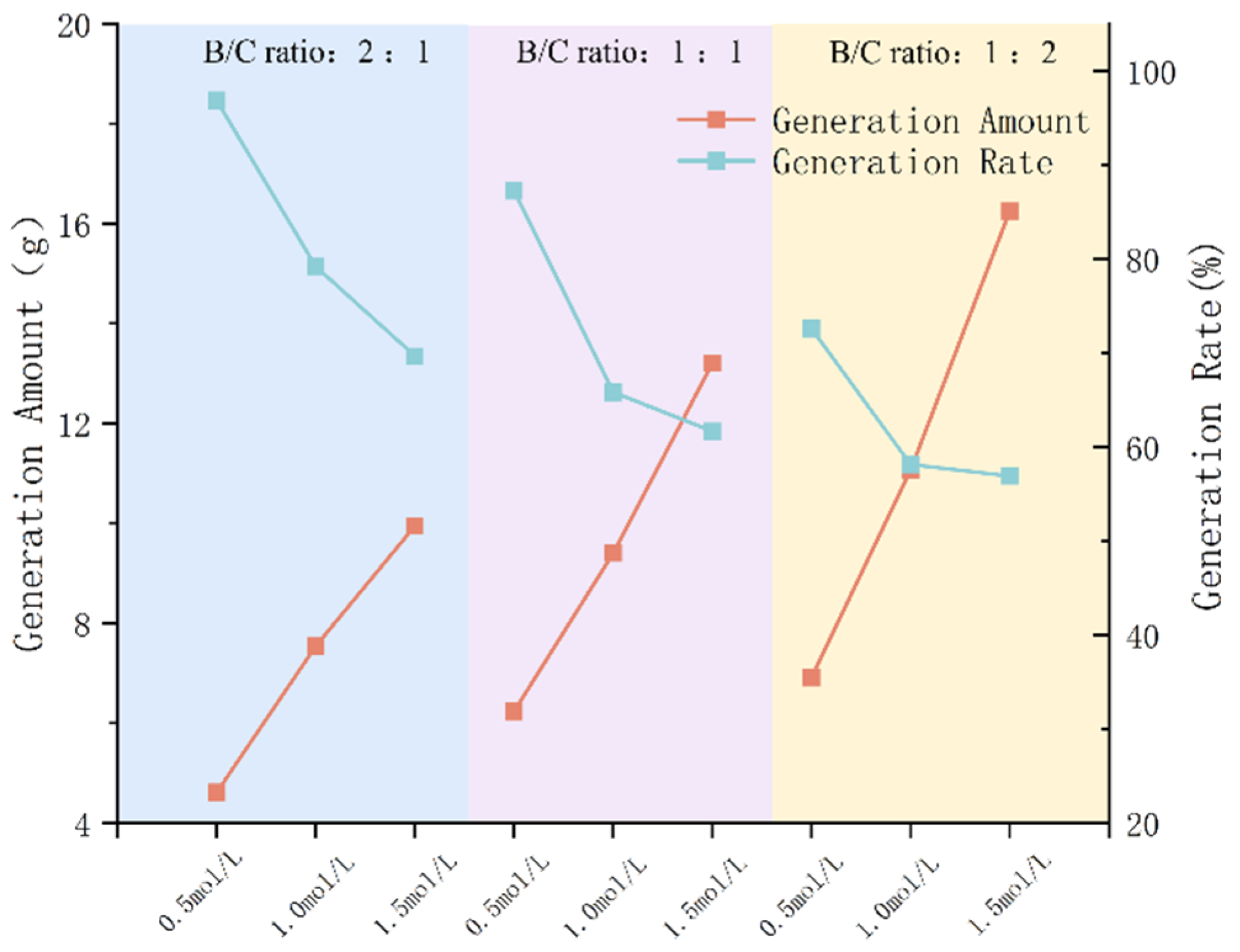
3.1.3. Impact of B/C Ratio on Precipitation Rate
3.2. Microstructural Mechanistic Analysis
3.2.1. Crystal Morphology of Three Types of Precipitates
3.2.2. The Effect of CSC and B/C Ratio on CaCO3 Crystals
3.2.3. The Effect of CSC and B/C Ratio on MgCO3 Crystals
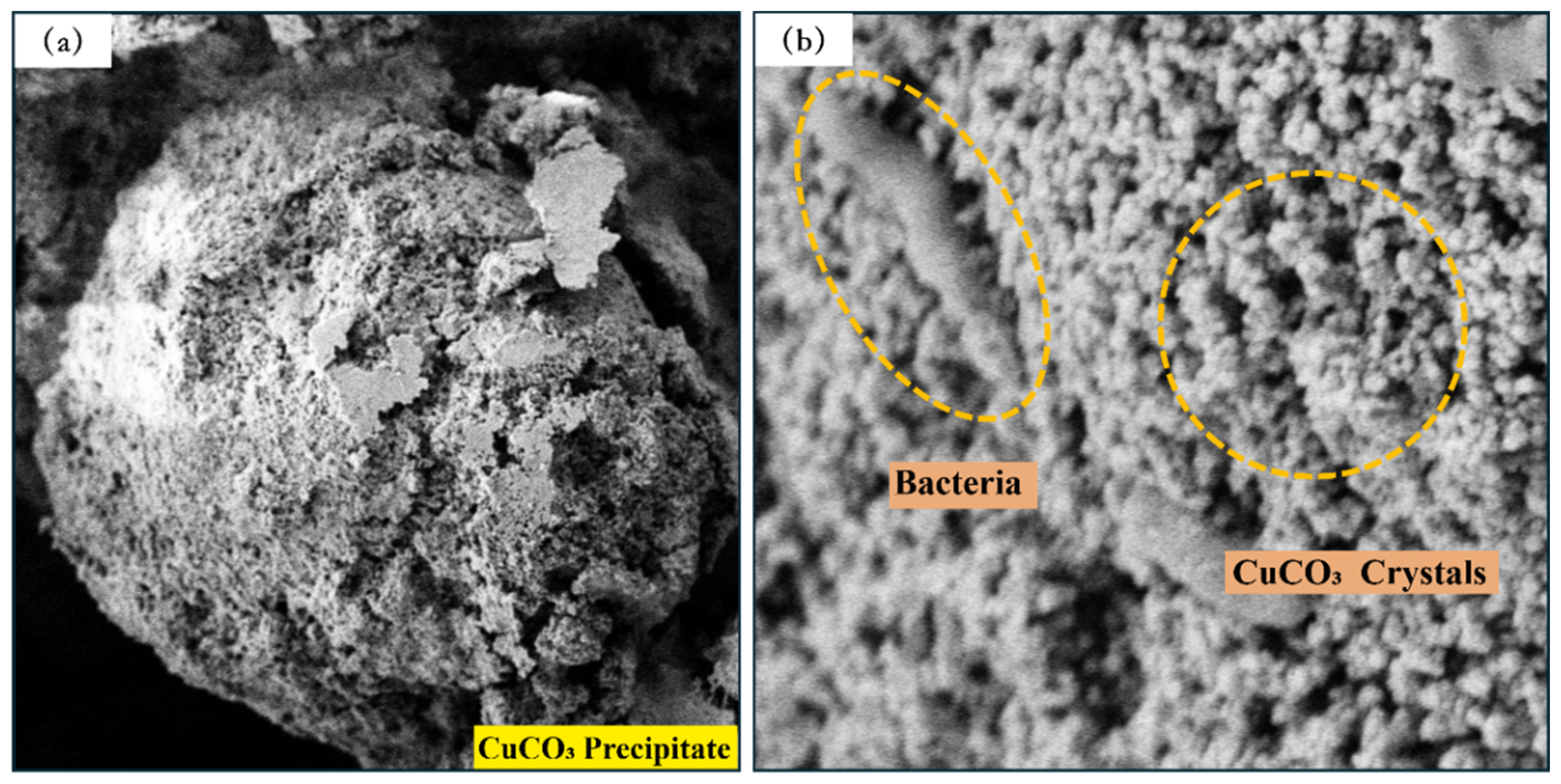
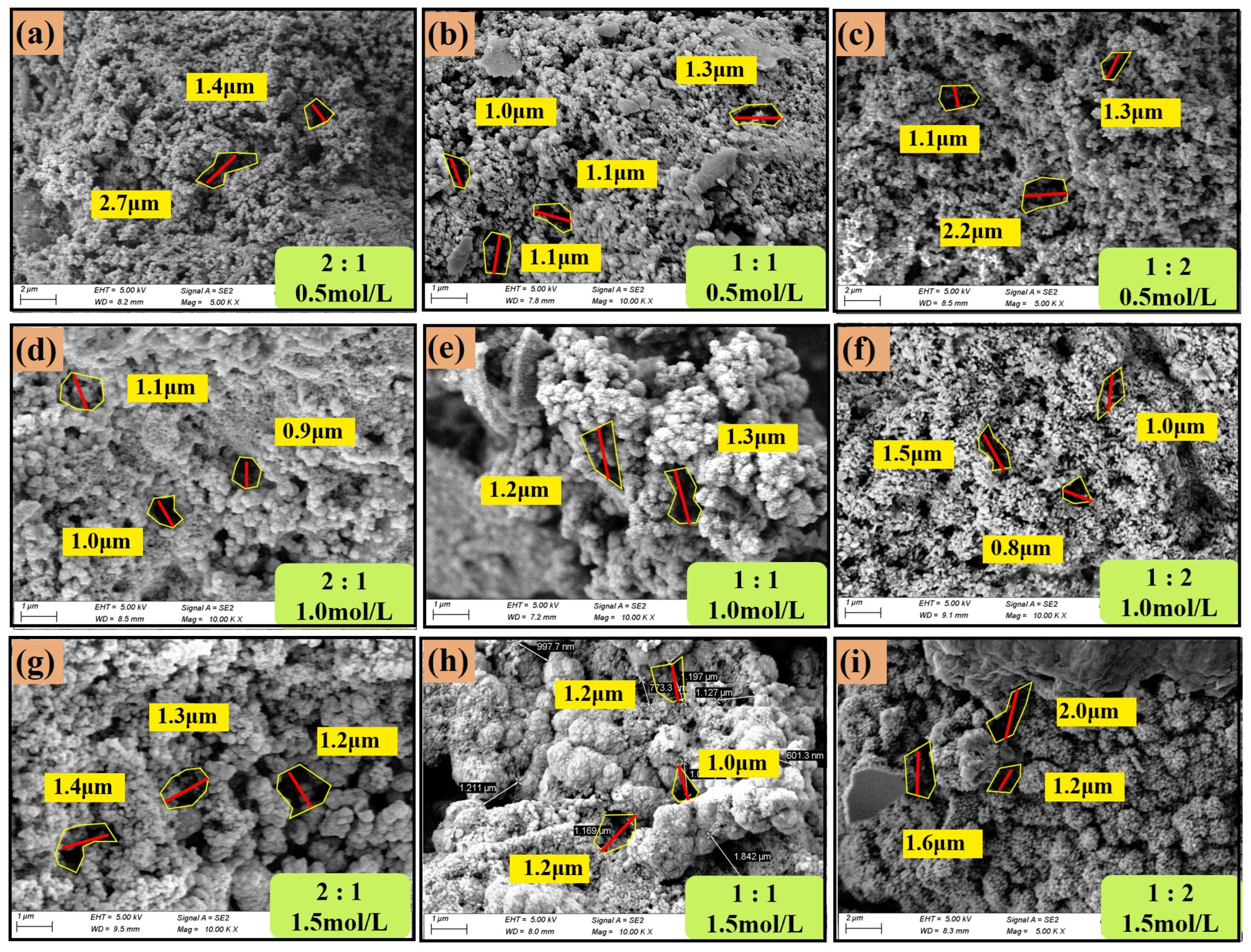
3.2.4. The Effect of CSC and B/C Ratio on CuCO3 Crystals
4. Conclusions
- (1)
- The type of grout liquid, concentration, and B/C ratio have a significant impact on the precipitation amount and rate. Among the metal sources, Mg2+ > Ca2+ > Cu2+. The CSC liquid is positively correlated with the precipitation amount and negatively correlated with the precipitation rate. The B/C ratio is positively correlated with the precipitation amount and negatively correlated with the precipitation rate. The scheme with a grout liquid concentration of 0.5 mol/L and a B/C ratio of 2:1 results in the highest production rate, which is beneficial for saving soil reinforcement resources. The scheme with a grout liquid concentration of 1.5 mol/L and a B/C ratio of 1:2 produces the most precipitation, which is beneficial for soil reinforcement effects.
- (2)
- When the CSC is 0.5 mol/L and the B/C ratio is 2:1, the CaCO3, MgCO3, and CuCO3 crystal polymers and crystals remain relatively intact, and bacteria are clearly visible. As the CSC increases and the B/C ratio decreases, excess metal ions participate in the reaction while inhibiting bacterial activity, leading to the gradual destruction of the crystals (CaCO3), crystal polymers (MgCO3, CuCO3), and spherical features. The number of bacteria on the surface sharply decreases.
- (3)
- As the concentration of the grout liquid increases and the B/C ratio decreases, the diameter of CaCO3 crystals show a decreasing trend. The MgCO3 crystals are influenced by both the grout liquid concentration and the B/C ratio. At low grout liquid concentrations, the average diameter is larger. As the B/C ratio decreases, the average diameter decreases. At higher grout liquid concentrations, the diameter of MgCO3 crystals increase, and the reduction in the B/C ratio further exacerbates this phenomenon. The diameter of CuCO3 crystals is relatively stable, around 1–2 μm.
- (4)
- This study systematically investigates the influence of multiple factors on microbial carbonate precipitation and crystal characteristics, aiming to establish optimized reaction conditions. By improving carbonate precipitation rates and quality, the approach reduces resource waste and unnecessary chemical usage, enhancing soil reinforcement effectiveness while minimizing environmental and resource impacts. The findings provide a theoretical foundation for sustainable soil remediation and ecological construction.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Jain, S. Influence of biomass and chemicals on kinetics of ureolysis-based carbonate biomineral precipitation. Environ. Earth Sci. 2024, 83, 58. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856. [Google Scholar] [CrossRef]
- Kakelar, M.M.; Yavari, M.; Yousefi, M.R.; Nimtaj, A. The influential factors in the effectiveness of microbial induced carbonate precipitation (micp) for soil consolidation. J. Hum. Environ. Health Promot. 2020, 6, 40–46. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Wang, S.; Li, C. Evaluation of calcium carbonate production and cementitious characteristics of enzymatically induced carbonate precipitation during environmental adjustment. Constr. Build. Mater. 2024, 449, 138415. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Xue, Z.-F.; Cheng, W.-C.; Wang, L.; Xie, Y.-X. Catalyzing urea hydrolysis using two-step microbial-induced carbonate precipitation for copper immobilization: Perspective of ph regulation. Front. Microbiol. 2022, 13, 1001464. [Google Scholar] [CrossRef]
- Zhao, T.; Du, H.; Shang, R. The effect of bacteria-to-calcium ratio on microbial-induced carbonate precipitation (micp) under different sequences of calcium-source introduction. Materials 2024, 17, 1881. [Google Scholar] [CrossRef]
- Sheikh, S.A.; Atmapoojya, S.L. Experimental study on factors affecting the efficiency of microbially induced carbonate precipitation in soil. Mater. Today Proc. 2022, 60, 275–280. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2018, 14, 627–638. [Google Scholar] [CrossRef]
- Chen, S.; Kang, B.; Zha, F.; Shen, Y.; Chu, C.; Tao, W. Effects of different mg/ca molar ratio on the formation of carbonate minerals in microbially induced carbonate precipitation (micp) process. Constr. Build. Mater. 2024, 442, 137643. [Google Scholar] [CrossRef]
- Jiao, Y.; Feng, Q.; Li, X. The co-effect of collagen and magnesium ions on calcium carbonate biomineralization. Mater. Sci. Eng. C 2006, 26, 648–652. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Bi, J.; Zhao, Y.; Li, Y.; Zhong, X.; Zheng, K. Influences of calcium and magnesium sources on microbially modified strongly weathered phyllite filler. Constr. Build. Mater. 2024, 416, 135118. [Google Scholar] [CrossRef]
- Su, F.; Wang, Y.; Liu, Y.; Zhang, J.; Liu, X.; Zhang, S. Factors affecting soil treatment with the microbially induced carbonate precipitation technique and its optimization. J. Microbiol. Methods 2023, 211, 106771. [Google Scholar] [CrossRef]
- Zhang, C.; Li, F.; Yang, K.; Zhou, J. The formation of calcium–magnesium carbonate minerals induced by curvibacter sp. Hj-1 under different mg/ca molar ratios. Minerals 2024, 14, 606. [Google Scholar] [CrossRef]
- Okwadha, G.D.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Qiao, S.; Zeng, G.; Wang, X.; Dai, C.; Sheng, M.; Chen, Q.; Xu, F.; Xu, H. Multiple heavy metals immobilization based on microbially induced carbonate precipitation by ureolytic bacteria and the precipitation patterns exploration. Chemosphere 2021, 274, 129661. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tang, J.; Li, K. Removal behavior of heavy metals from aqueous solutions via microbially induced carbonate precipitation driven by acclimatized sporosarcina pasteurii. Appl. Sci. 2022, 12, 9958. [Google Scholar] [CrossRef]
- Luciani, G.; Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Insights into the influence of cell concentration in design and development of microbially induced calcium carbonate precipitation (micp) process. PLoS ONE 2021, 16, e0254536. [Google Scholar] [CrossRef]
- Jarwar, M.A.; Dumontet, S.; Pasquale, V.; Chen, C. Microbial induced carbonate precipitation: Environments, applications, and mechanisms. Geomicrobiol. J. 2022, 39, 833–851. [Google Scholar] [CrossRef]
- Achal, V.; Peng, J.; Liu, Z. Influence of temperature on microbially induced calcium carbonate precipitation for soil treatment. PLoS ONE 2019, 14, e0218396. [Google Scholar] [CrossRef]
- Chen, M.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. Influence of humic acid on microbial induced carbonate precipitation for organic soil improvement. Environ. Sci. Pollut. Res. 2022, 30, 15230–15240. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Zomorodian, S.M.A.; Niazi, A.; O’Kelly, B.C. Improving sand with microbial-induced carbonate precipitation. Proc. Inst. Civ. Eng. Ground Improv. 2015, 168, 217–230. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially induced carbonate precipitation techniques for the remediation of heavy metal and trace element–polluted soils and water. Water Air Soil Pollut. 2021, 232, 268. [Google Scholar] [CrossRef]
- Hao, Z.; Su, Y.; Liu, S.; Zhang, X. Roles of bacteria and extracellular polymeric substance in calcium carbonate formation: Insights from the effects of calcium source and deposition rate on nucleation. Biochem. Eng. J. 2024, 202, 109160. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, K.; Sun, D.; Xu, W.; Wang, A.; Ma, R. A focus on ca2+ supply in microbial induced carbonate precipitation and its effect on recycled aggregate. J. Build. Eng. 2022, 51, 104334. [Google Scholar] [CrossRef]
- Tamayo-Figueroa, D.P.; Castillo, E.; Brandão, P.F.B. Metal and metalloid immobilization by microbiologically induced carbonates precipitation. World J. Microbiol. Biotechnol. 2019, 35, 58. [Google Scholar] [CrossRef]
- Sujiritha, P.B.; Vikash, V.L.; Ponesakki, G.; Ayyadurai, N.; Kamini, N.R. Microbially induced carbonate precipitation with arthrobacter creatinolyticus: An eco-friendly strategy for mitigation of chromium contamination. J. Environ. Manag. 2024, 365, 121300. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Franco, M.R.M.; Narayanasamy, R.; Balagurusamy, N. Microbially induced calcium carbonate precipitation (micp) and its potential in bioconcrete: Microbiological and molecular concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Xu, R.; Qin, H.; Liu, H.; Zhao, K. Heavy metal bioremediation using microbially induced carbonate precipitation: Key factors and enhancement strategies. Front. Microbiol. 2023, 14, 1116970. [Google Scholar] [CrossRef]
- Wu, L.; Miao, L.; Sun, X.; Wang, H. Effect of calcium sources on enzyme-induced carbonate precipitation to solidify desert aeolian sand. J. Environ. Manag. 2024, 366, 121687. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, A.I.; Ouahbi, T.; Basri, H.F.; Ong, D.E.L.; Muda, K.; Ojuri, O.O.; Flores, D.J.; Ammami, M.T. Heavy metal immobilisation with microbial-induced carbonate precipitation: A review. Geotech. Res. 2024, 11, 188–212. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, X.; Chen, X.; Huo, X.; Yu, Z.; Awoyera, P. Microbial-induced carbonate precipitation: A review on influencing factors and applications. Adv. Civ. Eng. 2021, 2021, 9974027. [Google Scholar] [CrossRef]
- Xue, Z.-F.; Cheng, W.-C.; Wang, L.; Qin, P.; Zhang, B. Revealing degradation and enhancement mechanisms affecting copper (cu) immobilization using microbial-induced carbonate precipitation (micp). J. Environ. Chem. Eng. 2022, 10, 108479. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Liu, R.; Du, Y.-J.; Bi, Y.-Z. Microbial induced carbonate precipitation for immobilizing pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, X.; Guan, W.; Liu, Y.; Xia, X.; Yuan, J.; Chen, X. Mineralization performance and crystal characteristics of microbial induced carbonate precipitation in lead–zinc tailings under multiple factors. Constr. Build. Mater. 2023, 403, 133081. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, Q.; Wang, L.; Niu, S.; Zheng, J.; Yang, M.; Yan, Y. Morphological changes of calcium carbonate and mechanical properties of samples during microbially induced carbonate precipitation (micp). Materials 2022, 15, 7754. [Google Scholar] [CrossRef]
- Wilcox, S.M.; Mulligan, C.N.; Neculita, C.M. Microbially induced calcium carbonate precipitation as a bioremediation technique for mining waste. Toxics 2024, 12, 107. [Google Scholar] [CrossRef]
- Liu, X.-j.; Fan, J.-Y.; Yu, J.; Gao, X. Solidification of loess using microbial induced carbonate precipitation. J. Mt. Sci. 2021, 18, 265–274. [Google Scholar] [CrossRef]
- Nishimura, I.; Yabiku, Y.; Shinyama, Y.; Mukunoki, T.; Matsubara, H. Ct image-based simulation of microbially induced carbonate precipitation. Comput. Geotech. 2024, 174, 106651. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Ali, A.; Su, J.; Huang, T.; Hou, C.; Li, X. Microbial-induced calcium precipitation: Bibliometric analysis, reaction mechanisms, mineralization types, and perspectives. Chemosphere 2024, 362, 142762. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Roh, Y. Microbially induced carbonate precipitation using microorganisms enriched from calcareous materials in marine environments and their metabolites. Minerals 2019, 9, 722. [Google Scholar] [CrossRef]






| Level | TCS 1 | CSC 2 | B/C Ratio 3 |
|---|---|---|---|
| 1 | CaCl2 | 0.5 mol/L | 2:1 |
| 2 | MgCl2 | 1.0 mol/L | 1:1 |
| 3 | CuCl2 | 1.5 mol/L | 1:2 |
| Test | CSC | B/C Ratio |
|---|---|---|
| 1 | 0.5 mol/L | 2:1 |
| 2 | 1:1 | |
| 3 | 1:2 | |
| 4 | 1.0 mol/L | 2:1 |
| 5 | 1:1 | |
| 6 | 1:2 | |
| 7 | 1.5 mol/L | 2:1 |
| 8 | 1:1 | |
| 9 | 1:2 |
| CSC | B/C Ratio | Average Diameter (μm) | |
|---|---|---|---|
| 1 | 0.5 mol/L | 2:1 | 27 |
| 2 | 0.5 mol/L | 1:1 | 23 |
| 3 | 0.5 mol/L | 1:2 | 20 |
| 4 | 1 mol/L | 2:1 | 15 |
| 5 | 1 mol/L | 1:1 | 10 |
| 6 | 1 mol/L | 1:2 | 9 |
| 7 | 1.5 mol/L | 2:1 | 7 |
| 8 | 1.5 mol/L | 1:1 | 3.5 |
| 9 | 1.5 mol/L | 1:2 | 3 |
| CSC | B/C Ratio | Average Diameter (μm) | Average Diameter of Spherical Polymer Aggregates (μm) | |
|---|---|---|---|---|
| 1 | 0.5 mol/L | 2:1 | 20 | 1420 |
| 2 | 0.5 mol/L | 1:1 | 14 | 1120 |
| 3 | 0.5 mol/L | 1:2 | 9 | 700 |
| 4 | 1 mol/L | 2:1 | 7 | 416 |
| 5 | 1 mol/L | 1:1 | 5 | 927 |
| 6 | 1 mol/L | 1:2 | 3.5 | 1152 |
| 7 | 1.5 mol/L | 2:1 | 11 | 1378 |
| 8 | 1.5 mol/L | 1:1 | 26 | 1495 |
| 9 | 1.5 mol/L | 1:2 | 30 | 2940 |
| CSC | B/C Ratio | Average Diameter of Spherical Polymer Aggregates (μm) | |
|---|---|---|---|
| 1 | 0.5 mol/L | 2:1 | 125.5 |
| 2 | 0.5 mol/L | 1:1 | 148.04 |
| 3 | 0.5 mol/L | 1:2 | 182.9 |
| 4 | 1 mol/L | 2:1 | 160 |
| 5 | 1 mol/L | 1:1 | 159 |
| 6 | 1 mol/L | 1:2 | 133 |
| 7 | 1.5 mol/L | 2:1 | 153 |
| 8 | 1.5 mol/L | 1:1 | 132 |
| 9 | 1.5 mol/L | 1:2 | 148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Zhang, J.; Guan, W.; Shi, G.; Ji, F.; Zhao, X.; Zhang, X.; Xia, X. Exploring Sustainable Mineralization Pathways: Multi-Factor Impacts on Microbial-Induced Carbonate Precipitation and Crystals Characteristics. Processes 2025, 13, 711. https://doi.org/10.3390/pr13030711
Xie M, Zhang J, Guan W, Shi G, Ji F, Zhao X, Zhang X, Xia X. Exploring Sustainable Mineralization Pathways: Multi-Factor Impacts on Microbial-Induced Carbonate Precipitation and Crystals Characteristics. Processes. 2025; 13(3):711. https://doi.org/10.3390/pr13030711
Chicago/Turabian StyleXie, Meng, Junhui Zhang, Weiming Guan, Guangming Shi, Fangcan Ji, Xichen Zhao, Xuewei Zhang, and Xiaocheng Xia. 2025. "Exploring Sustainable Mineralization Pathways: Multi-Factor Impacts on Microbial-Induced Carbonate Precipitation and Crystals Characteristics" Processes 13, no. 3: 711. https://doi.org/10.3390/pr13030711
APA StyleXie, M., Zhang, J., Guan, W., Shi, G., Ji, F., Zhao, X., Zhang, X., & Xia, X. (2025). Exploring Sustainable Mineralization Pathways: Multi-Factor Impacts on Microbial-Induced Carbonate Precipitation and Crystals Characteristics. Processes, 13(3), 711. https://doi.org/10.3390/pr13030711








