The Effect of Activated Carbon Support on Ru/AC Catalysts Used for the Catalytic Decomposition of Hydroxylamine Nitrate and Hydrazine Nitrate
Abstract
1. Introduction
2. Experimental Section
2.1. Material and Regents
2.2. Preparation of Ru/AC Catalysts
2.3. Characterization of the Catalysts
2.4. HAN and HN Concentrations Analysis
2.5. Activity Evaluation of the Catalysts
3. Results and Discussion
3.1. N2 Physical Adsorption
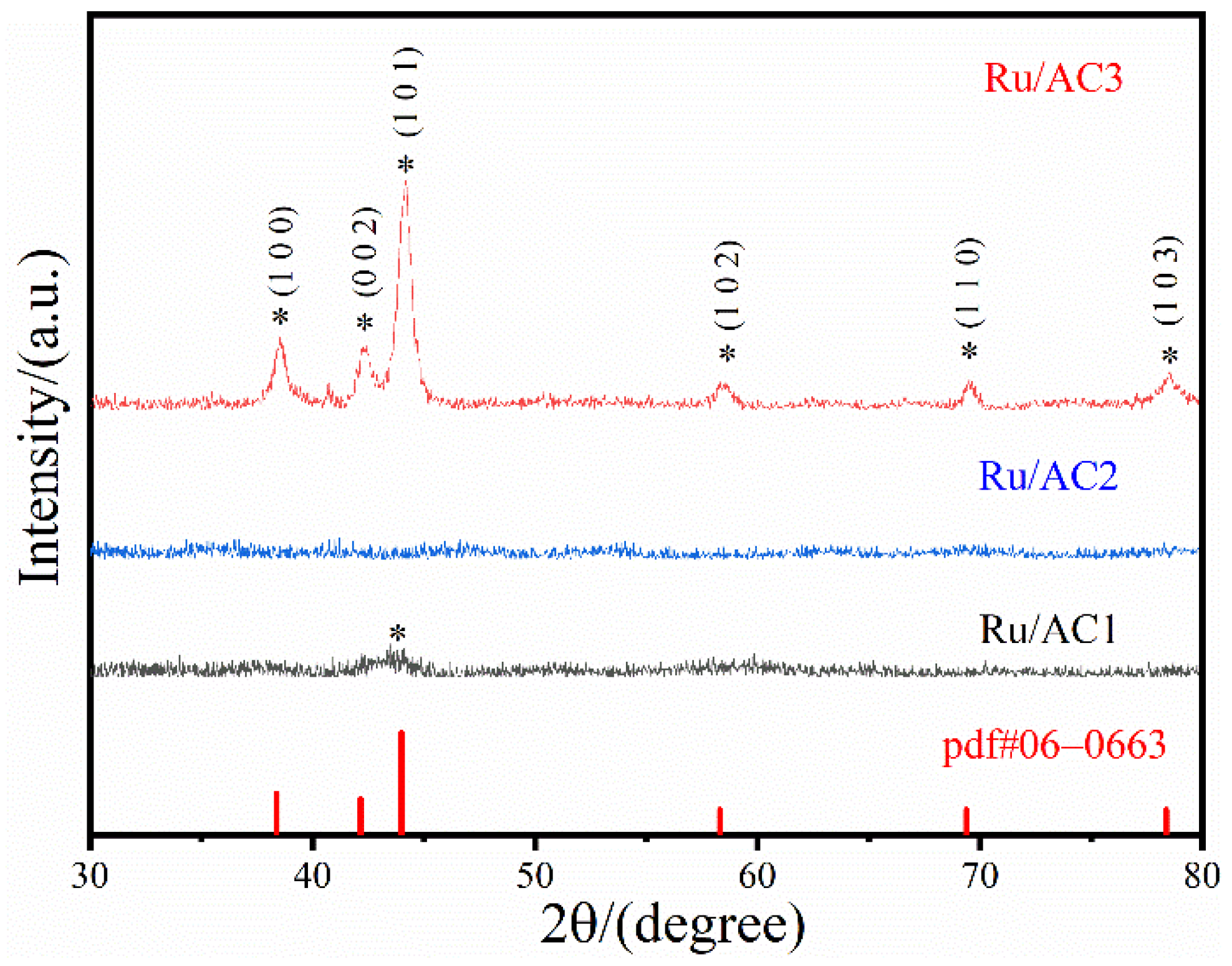
3.2. XRD
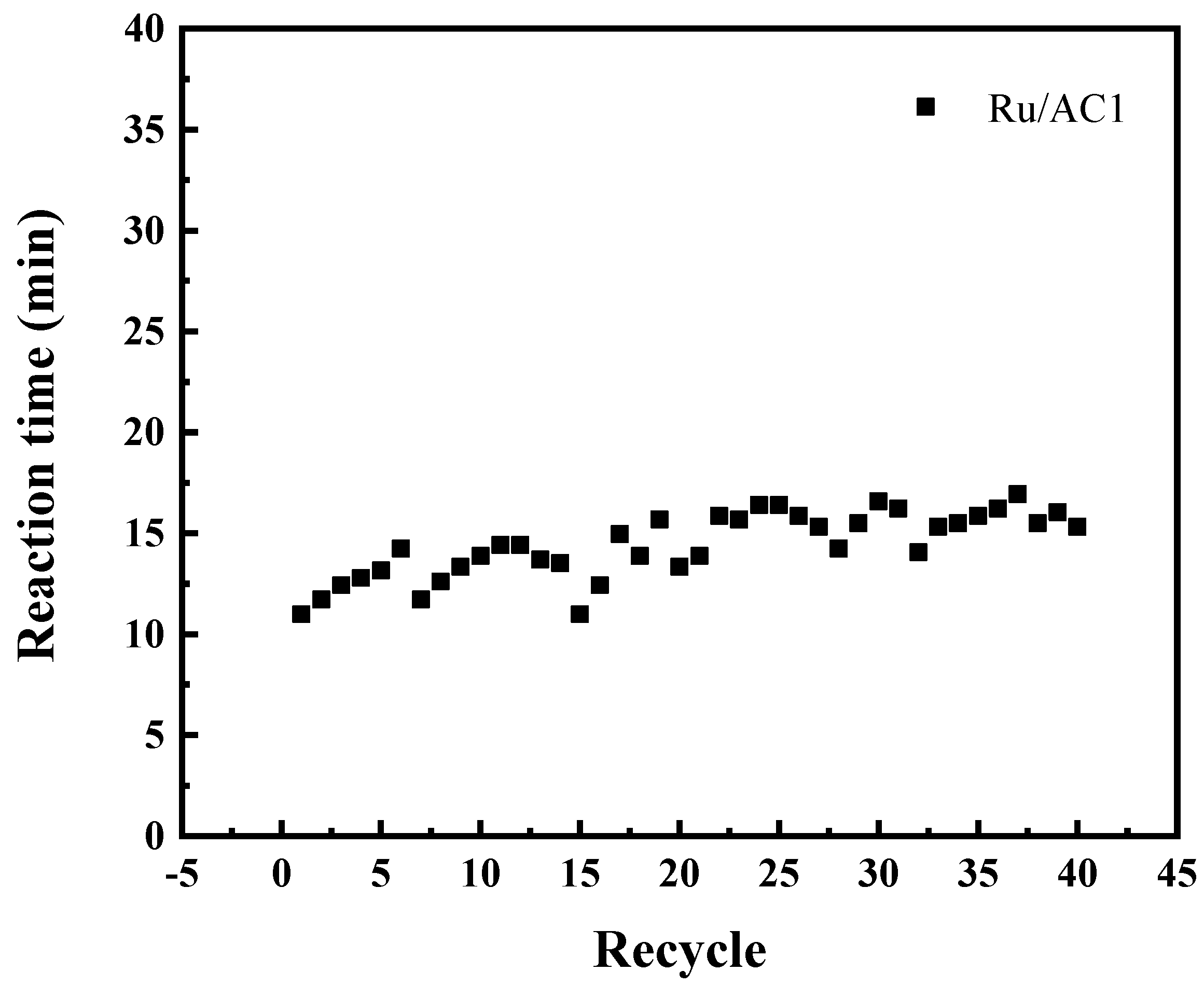
3.3. Recyclability of the Catalyst
3.4. SEM
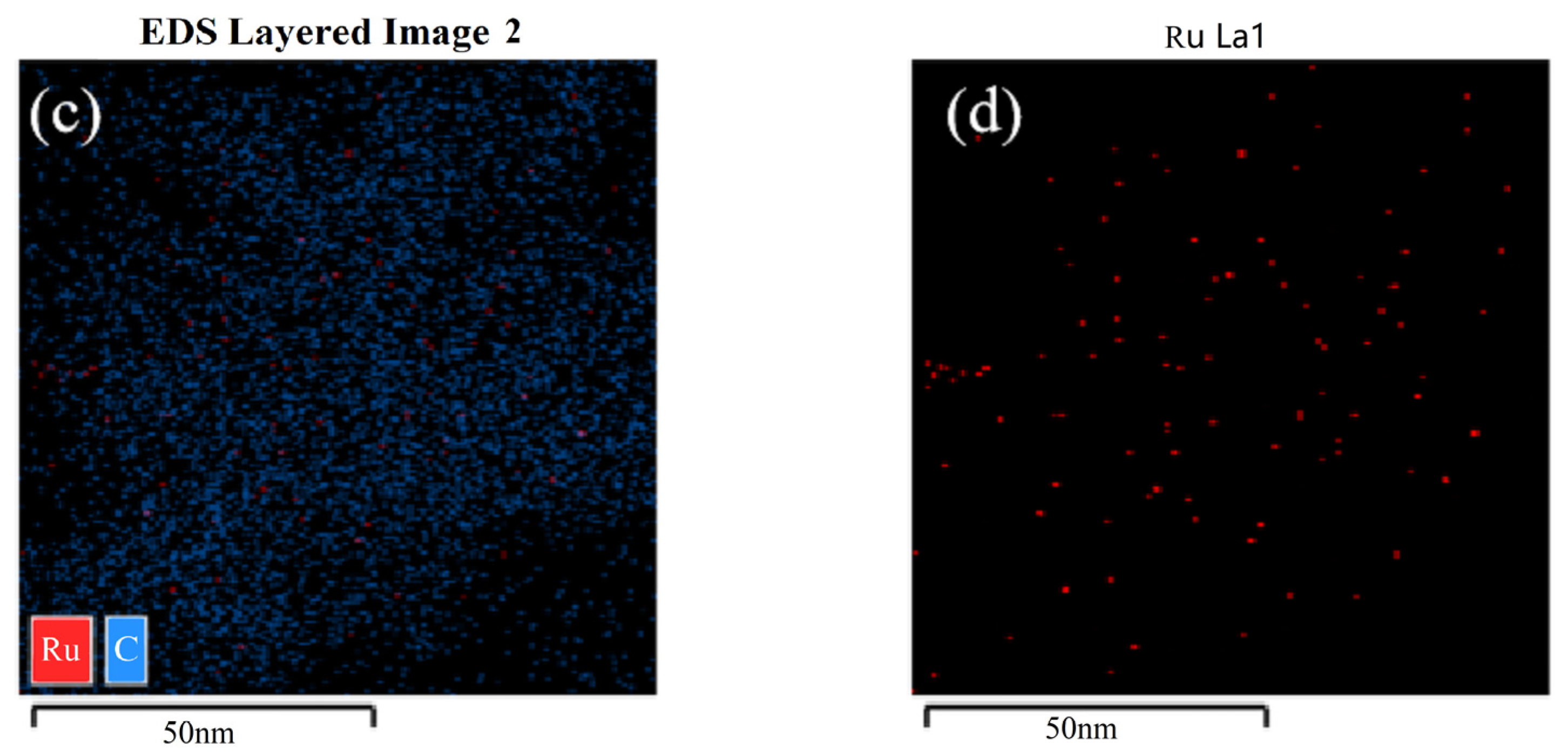
3.5. TEM
4. Conclusions
- (1)
- Smaller particle sizes reduced diffusion resistance, while larger specific surface areas enhanced Ru dispersion and increased active sites, resulting in improved catalytic performance. Ru/AC1, with its optimal structural properties, showed the best activity.
- (2)
- Catalyst deactivation was primarily caused by corrosion-induced pore structure damage and Ru loss during operation, thus reducing active site availability and structural integrity.
- (3)
- Activated carbons with higher surface areas enabled uniform Ru dispersion and smaller Ru particle sizes, significantly enhancing catalyst activity. Conversely, supports with low surface areas (e.g., AC3) resulted in larger Ru particles and poor performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taylor, R.J.; May, I.; Denniss, I.S.; Wallwork, A.L.; Hunt, G.; Hutchison, S.; Bothwell, P.; Richards, V.; Hill, N.J. The Development of Chemical Separation Technology for Advanced Purex Process. In Proceedings of the 5th International Nuclear Conference on Recycling, Conditioning and Disposal (RECOD–98), Nice, France, 25–28 October 1998; Volume 25. [Google Scholar]
- Schmieieder, H.; Petrich, G. A concept for improved Purex–process. Radiochem. Acta 1989, 48, 181–186. [Google Scholar] [CrossRef]
- Birkett, J.E.; Carrott, M.J.; Fox, O.D.; Jones, C.J.; Maher, C.J.; Roube, C.V.; Taylor, R.J.; Woodhead, D.A. Recent developments in the Purex process for nuclear fuel reprocessing: Complexant based stripping for uranium/plutonium separation. Chimia 2005, 59, 898. [Google Scholar] [CrossRef]
- He, H.; Ye, G.; Tang, H.; Zheng, W.; Li, G.; Lin, R. An advanced purex process based on salt–free reductants. Radiochim. Acta 2014, 102, 127–133. [Google Scholar]
- Harlow, D.G.; Felt, R.E.; Agnew, S.; Barney, G.S.; McKibben, J.M.; Garber, R.; Lewis, M. Technical Report on Hydroxylamine Nitrate; U.S. Department of Energy: Washington, DC, USA, 1998. [CrossRef]
- Herbst, R.S.; Baron, P.; Nilsson, M. Standard and advanced separation: Purex Processes for nuclear fuel reprocessing. In Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Woodhead Publishing: Cambridge, UK, 2011; pp. 141–175. [Google Scholar]
- Zhang, Z.; Li, B.; Chen, Q.; Chen, X.; Yan, T.; Zheng, W.; Zuo, C. Catalytic decomposition of hydroxylamine nitrate and hydrazine nitrate using Ru/ZSM-5 catalyst under mild reaction conditions. RSC Adv. 2022, 12, 4469–4474. [Google Scholar] [CrossRef]
- Gauthier-Lafaye, F.; Pourcelot, L.; Eikenberg, J.; Beer, H.; Le Roux, G.; Rhikvanov, L.; Stille, P.; Renaud, P.; Mezhibor, A. Radioisotope contaminations from releases of the Tomsk–Seversk nuclear facility (Siberia, Russia). J. Environ. Radioact. 2008, 99, 680–693. [Google Scholar] [CrossRef]
- Gerton, R.E. Accident Investigation Board Report on the May 14, 1997, Chemical Explosion at the Plutonium Reclamation Facility, Hanford Site, Richland, Washington—Summary Report; U.S. DOE Office of Environmental Restoration and Waste Management: Washington, DC, USA, 1997.
- Jiang, S.J.; Ren, F.Y. Nuclear Fuel Reprocessing Technology; Atomic Energy Press: Beijing, China, 1995. [Google Scholar]
- Ren, F.Y.; Zhou, Z.X. Foreign Nuclear Fuel Reprocessing; Atomic Energy Press: Beijing, China, 2006. [Google Scholar]
- Chang, S.W. Application of Nitrous Gas in the Conditioning of 1BP Feed Solution. J. Nucl. Radiochem. 2015, 37, 404–407. [Google Scholar]
- Wei, C.; Saraf, S.R.; Rogers, W.J.; Mannan, M.S. Thermal runaway reaction hazards and mechanisms of hydroxylamine with acid/base contaminants. Thermochim. Acta 2004, 421, 1–9. [Google Scholar] [CrossRef]
- Iwata, Y.; Koseki, H. Risk evaluation of decomposition of hydroxylamine/water solution at various concentrations. Process Saf. Prog. 2002, 21, 136–141. [Google Scholar] [CrossRef]
- Breschet, C.; Miquel, P. Improvement of the procedure used to treat highly irradiated fuels. In Proceedings of the International Solvent Extraction Conference, Lyon, France, 8–14 September 1974; London Society of Chemical Industry: London, UK, 1971; pp. 565–576. [Google Scholar]
- Bothner-By, A.; Friedman, L. The reaction of nitrous acid with hydroxylamine. J. Chem. Phys. 1952, 20, 459–462. [Google Scholar] [CrossRef]
- Koltoonov, V.S.; Marchenko, V.I. Kinetics of hydrazine oxidation by nitrous acid. J. Catal. 1967, 7, 97. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Wang, J.F.; Zhang, T.X. Power Reactor Nuclear Fuel Reprocessing Technology; China Atomic Energy Press: Beijing, China, 2013. [Google Scholar]
- Hou, X.L.; Zhang, S.Q.; Hu, J.; Wei, X.F.; Zhao, H.G. Study of plutonium valence adjustment in 1AF solution in purex process with nitrogen oxide. Nucl. Sci. Eng. 1995, 15, 165–171. [Google Scholar]
- Zhang, S.Q.; Wei, X.F.; Ye, G.A.; Zhang, X.Y.; Zhuang, W.X.; Liu, S.Y.; Fu, L.C. Research on Regulating Plutonium Valence State with N2O4 in Purex Process. Atom. Energy Sci. Technol. 1993, 72, 130–137. [Google Scholar]
- Yu, D.; Li, B.; Cao, Z.; Chen, Q.; Zuo, C.; He, T.; Yan, T.; Zheng, W. Catalytic decomposition of hydrazine nitrate hydroxylamine nitrate in radioactive nitric acid waste liquid using Ru/AC catalyst. New J. Chem. 2024, 48, 8660–8666. [Google Scholar] [CrossRef]
- Li, B.L.; Yu, D.Y.; Cao, Z.; He, T.S.; Chen, S.W.; Zheng, W.F. Effect of acid-treatment on the activated carbon of Ru/AC catalysts for catalytic decomposition of hydroxylamine nitrate and hydrazine nitrate. Appl. Catal. A Gen. 2024, 681, 119801. [Google Scholar] [CrossRef]
- Li, B.L.; He, T.S.; Zuo, C.; Cao, Z.; Yang, T.H.; Zheng, W.F. The reaction kinetics and mechanism of catalytic decomposition of hydrazine nitrate on Ru/C catalyst in nitric acid solutions. New J. Chem. 2023, 47, 7583–7587. [Google Scholar] [CrossRef]
- Cao, Z.; Li, T.C.; Li, B.L.; Chen, X.W.; Zuo, C.; Zheng, W.F. Study on the Deactivation Mechanism of Ru/C Catalysts. Processes 2024, 12, 1138. [Google Scholar] [CrossRef]
- Zhong, Z.; Aika, K. The Effect of Hydrogen Treatment of Active Carbon on Ru Catalysts for Ammonia Synthesis. J. Catal. 1998, 173, 535–539. [Google Scholar] [CrossRef]
- Aksoylu, A.; Madalena, M.; Freitas, A.; Pereira, M.R.; Figueiredo, J.L. The effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Szmigiel, D.; Komornicki, A.; Zieliński, J.; Kowalczyk, Z. Catalytic properties of small ruthenium particles deposited on carbon. Ammonia decomposition studies. Catal. Today 2003, 41, 589–591. [Google Scholar]
- Kang, M.; Bae, Y.S.; Lee, C.H. Effect of heat treatment of activated carbon supports on the loading and activity of Pt catalyst. Carbon 2005, 43, 1512–1516. [Google Scholar] [CrossRef]
- Feng, W.; Kwon, S.; Borguet, E.; Vidic, R. Adsorption of Hydrogen Sulfide onto Activated Carbon Fibers: Effect of Pore Structure and Surface Chemistry. Environ. Sci. Technol. 2005, 39, 9744–9749. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Zazo, J.A.; Casas, J.A.; Bahamonde, A.; Rodriguez, J.J. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen peroxide. Appl. Catal. A Gen. 2011, 402, 146–155. [Google Scholar] [CrossRef]
- Kowalczyk, Z.; Sentek, J.; Jodzis, S.; Mizera, E.; Góralski, J.; Paryjczak, T.; Diduszko, R. An alkali–promoted ruthenium catalyst for the synthesis of ammonia, supported on thermally modified active carbon. Catal. Lett. 1997, 45, 65–72. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Peng, J.; Liu, Z.; Jiang, Y.; Meng, M.; Zhang, W.; Ni, L. Preparation of high surface area oxidized activated carbon from peanut shell and application for the removal of organic pollutants and heavy metal ions. Water Air Soil Pollut. 2018, 229, 391. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Idris, A. Preparation and characterization of activated carbons derived from bio-solid: A review. Desalination Water Treat. 2014, 52, 4848–4862. [Google Scholar] [CrossRef]
- Januszewicz, K.; Kazimierski, P.; Klein, M.; Kardaś, D.; Łuczak, J. Activated carbon produced by pyrolysis of waste wood and straw for potential wastewater adsorption. Materials 2020, 13, 2047. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Biochar as potential precursors for activated carbon production: Parametric analysis and multi-response optimization. Environ. Sci. Pollut. Res. 2020, 27, 27480–27490. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.J.; Ying, H.J.; Cao, F.F.; Wang, Q.; Ai, N. Adsorption of congo red on mesoporous activated carbon prepared by CO2 physical activation. Chin. J. Chem. Eng. 2020, 28, 1069–1076. [Google Scholar] [CrossRef]
- Louarrat, M.; Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Blin, J.; Martin, L. Optimization of conditions for the preparation of activated carbon from olive stones for application in gold recovery. J. S. Afr. Inst. Min. Metall. 2019, 119, 297–306. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Xing, L.; Xie, Y.; Cao, H.; Minakata, D.; Zhang, Y.; Crittenden, J.C. Activated carbon-enhanced ozonation of oxalate attributed to HO· oxidation in bulk solution and surface oxidation: Effects of the type and number of basic sites. Chem. Eng. J. 2014, 245, 71–79. [Google Scholar] [CrossRef]
- Prahas, D.; Wang, M.J.; Ismadji, S.; Liu, J.C. Enhanced adsorption of quaternary amine using modified activated carbon. Water Sci. Technol. 2014, 69, 2085–2092. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, X.; Yim, W.-L.; Suryanto, B.H.R.; Zhao, C. Electrocatalytic oxygen evolution at surface oxidized multiwall carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yu, Z.; Liu, X. Surface modification of activated carbon and its effects on methane adsorption. Appl. Mech. Mater. 2013, 395–396, 605–609. [Google Scholar] [CrossRef]
- Van Pelt, A.H.; Simakova, O.A.; Schimming, S.M.; Ewbank, J.L.; Foo, G.S.; Pidko, E.A.; Hensen, E.J.; Sievers, C. Stability of functionalized activated carbon in hot liquid water. Carbon 2014, 77, 143–154. [Google Scholar] [CrossRef]
- Tangsathitkulchai, C.; Ngernyen, Y.; Tangsathitkulchai, M. Surface modification and adsorption of eucalyptus wood-based activated carbons: Effects of oxidation treatment, carbon porous structure and activation method. Korean J. Chem. Eng. 2009, 26, 1341–1352. [Google Scholar] [CrossRef]
- Zhao, H.H.; Song, H.L.; Zhao, J.; Yang, J.; Chou, L.J. The Reactivity and Deactivation Mechanism of Ru@C Catalyst over Hydrogenation of Aromatics to Cyclohexane Derivatives. ChemistrySelect 2020, 5, 4316–4327. [Google Scholar] [CrossRef]
- Lin, B.Y.; Qi, Y.C.; Guo, Y.J.; Lin, J.X.; Ni, J. Effect of potassium precursors on the thermal stability of K-promoted Ru/carbon catalysts for ammonia synthesis. Catal. Sci. Technol. 2015, 5, 2829–2838. [Google Scholar] [CrossRef]
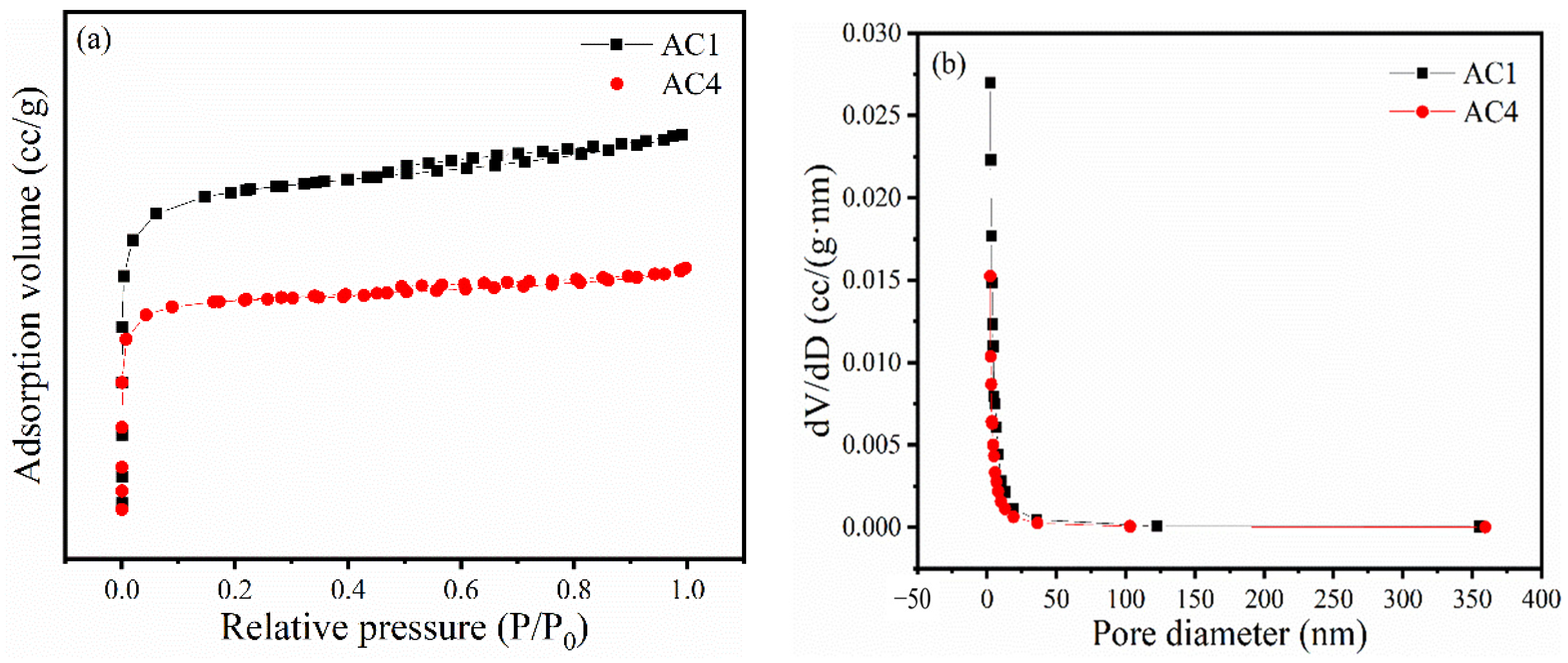
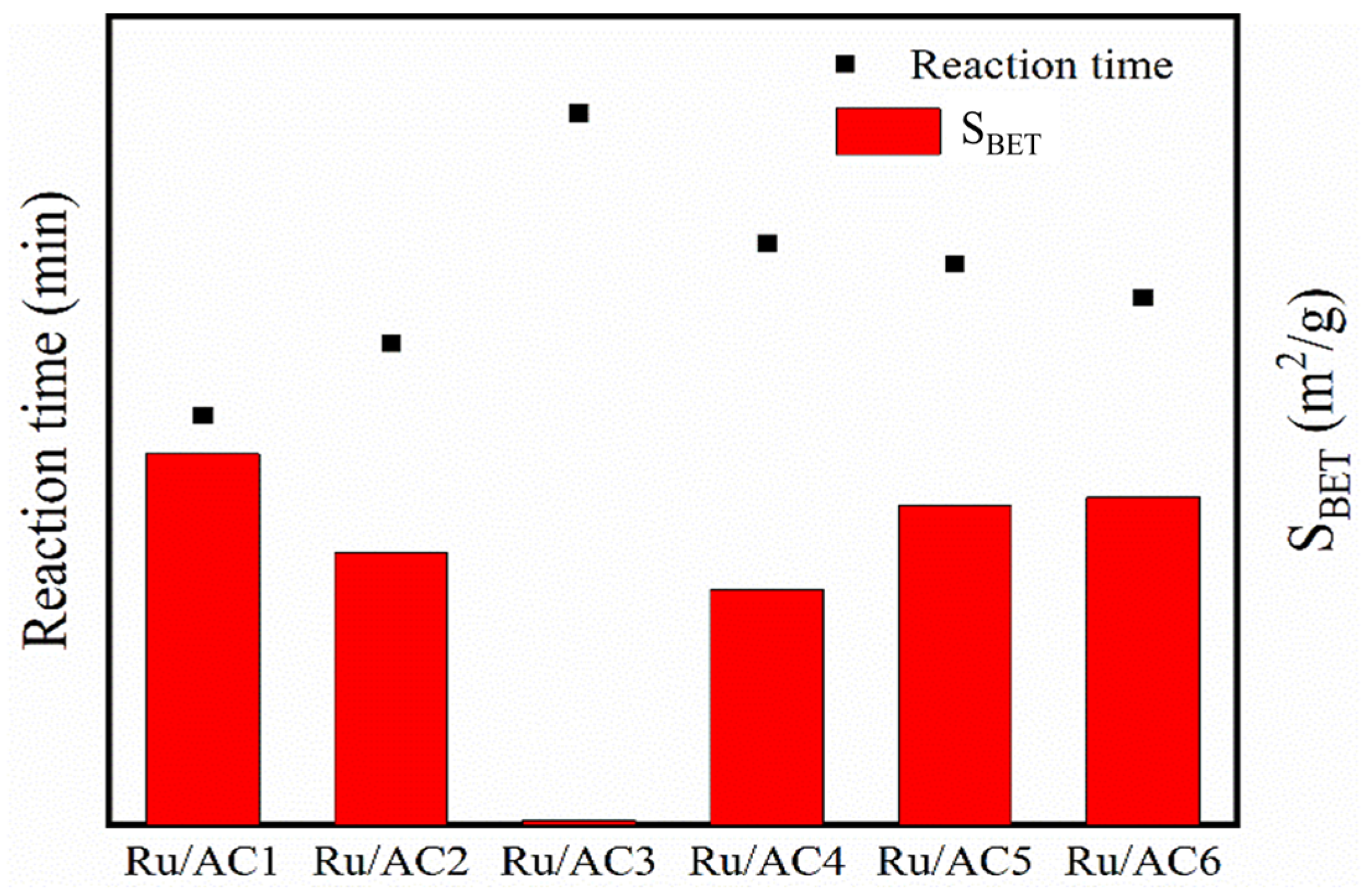


| Entry | Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| 1 | AC1 | 787.8 | 0.46 | 1.17 |
| 2 | Ru/AC1 | 665.4 | 0.39 | 1.18 |
| 3 | used Ru/AC1 | 606.4 | 0.37 | 1.25 |
| 4 | AC2 | 516.1 | 0.30 | 1.17 |
| 5 | Ru/AC2 | 488.5 | 0.29 | 1.17 |
| 6 | used Ru/AC2 | 430.6 | 0.26 | 1.19 |
| 7 | AC3 | 26.9 | 0.03 | 2.55 |
| 8 | Ru/AC3 | 8.8 | 0.02 | 5.09 |
| 9 | used Ru/AC3 | 45.8 | 0.06 | 2.60 |
| 10 | AC4 | 448.8 | 0.29 | 1.28 |
| 11 | Ru/AC4 | 421.5 | 0.25 | 1.18 |
| 12 | used Ru/AC4 | 300.8 | 0.18 | 1.19 |
| 13 | AC5 | 721.1 | 0.40 | 1.11 |
| 14 | Ru/AC5 | 572.2 | 0.33 | 1.15 |
| 15 | used Ru/AC5 | 464.9 | 0.27 | 1.17 |
| 16 | AC6 | 811.7 | 0.44 | 1.09 |
| 17 | Ru/AC6 | 587.5 | 0.32 | 1.11 |
| 18 | used Ru/AC6 | 568.2 | 0.32 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Yu, D.; He, T.; Li, T.; Zuo, C.; Li, B.; Lv, H.; Yan, T.; Zheng, W. The Effect of Activated Carbon Support on Ru/AC Catalysts Used for the Catalytic Decomposition of Hydroxylamine Nitrate and Hydrazine Nitrate. Processes 2025, 13, 641. https://doi.org/10.3390/pr13030641
Cao Z, Yu D, He T, Li T, Zuo C, Li B, Lv H, Yan T, Zheng W. The Effect of Activated Carbon Support on Ru/AC Catalysts Used for the Catalytic Decomposition of Hydroxylamine Nitrate and Hydrazine Nitrate. Processes. 2025; 13(3):641. https://doi.org/10.3390/pr13030641
Chicago/Turabian StyleCao, Zhi, Deyan Yu, Tiansheng He, Tianchi Li, Chen Zuo, Baole Li, Hongbin Lv, Taihong Yan, and Weifang Zheng. 2025. "The Effect of Activated Carbon Support on Ru/AC Catalysts Used for the Catalytic Decomposition of Hydroxylamine Nitrate and Hydrazine Nitrate" Processes 13, no. 3: 641. https://doi.org/10.3390/pr13030641
APA StyleCao, Z., Yu, D., He, T., Li, T., Zuo, C., Li, B., Lv, H., Yan, T., & Zheng, W. (2025). The Effect of Activated Carbon Support on Ru/AC Catalysts Used for the Catalytic Decomposition of Hydroxylamine Nitrate and Hydrazine Nitrate. Processes, 13(3), 641. https://doi.org/10.3390/pr13030641





