Characteristics of Resveratrol Tablets Stored Under Stress Conditions by Total Hemispherical Reflectance and Selected Pharmacopoeial Parameters †
Abstract
1. Introduction
2. Materials and Methods
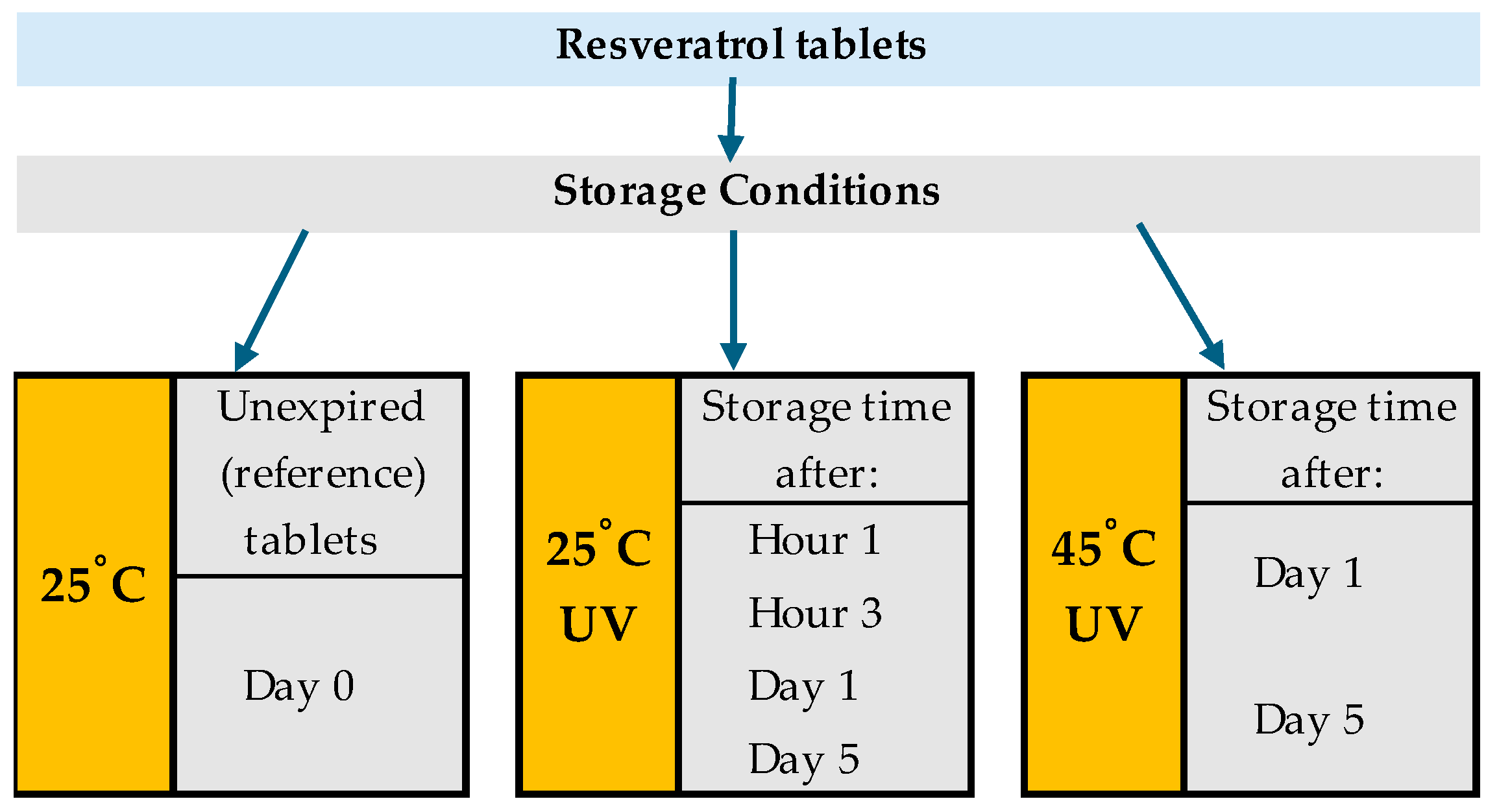
2.1. Analyzed Tablets
2.2. Quality Control of the Tablets
2.2.1. Weight Uniformity, Thickness, and Diameter
2.2.2. Hardness and Friability
2.3. Photodegradation Experiments
2.4. Directional-Hemispherical Reflectance
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Treated Tablets Containing Resveratrol
3.2. Analysis of Changes in THR Values
3.3. Changes in THR Values Between Day 0 and Day 5 of the Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Burdock, G.A.; Edwards, J.A.; Beck, M.; Bausch, J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 2009, 47, 2170–2182. [Google Scholar] [CrossRef]
- Vang, O. What is new for resveratrol? Is a new set of recommendations necessary? Ann. N. Y. Acad. Sci. 2013, 1290, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Li, S.; Yin, S.; Ding, H.; Shao, Y.; Zhou, S.; Pu, W.; Han, L.; Wang, T.; Yu, H. Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention. Cell Prolif. 2023, 56, e13346. [Google Scholar] [CrossRef]
- Morris, G.Z.; Williams, R.L.; Elliott, M.S.; Beebe, S.J. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells. Prostate 2002, 52, 319–329. [Google Scholar] [CrossRef]
- Takizawa, Y.; Nakata, R.; Fukuhara, K.; Yamashita, H.; Kubodera, H.; Inoue, H. The 4′-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PLoS ONE 2015, 10, e0120865. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Bostancıeri, N.; Elbe, H.; Eşrefoğlu, M.; Vardı, N. Cardioprotective potential of melatonin, quercetin and resveratrol in an experimental model of diabetes. Biotech. Histochem. 2022, 97, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Komorowska, J.; Wątroba, M.; Szukiewicz, D. Review of beneficial effects of resveratrol in neurodegenerative diseases such as Alzheimer’s disease. Adv. Med. Sci. 2020, 65, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Jiang, X.T.; Ma, F.F.; Han, J.; Tang, X.L. Resveratrol prevents osteoporosis s by upregulating FoxO1 transcriptional, activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. The application of resveratrol to mesenchymal stromal cell-based regenerative medicine. Stem Cell Res. Ther. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Michno, A.; Grużewska, K.; Ronowska, A.; Gul-Hinc, S.; Zyśk, M.; Jankowska-Kulawy, A. Resveratrol Inhibits Metabolism and Affects Blood Platelet Function in Type 2 Diabetes. Nutrients. 2022, 14, 1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Alsahli, M.S.M.A.; Aljohani, A.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Resveratrol, a Plant Polyphenol, and Its Role in the Therapy of Various Types of Cancer. Molecules 2022, 27, 2665. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. The Impact of Curcumin, Resveratrol, and Cinnamon on Modulating Oxidative Stress and Antioxidant Activity in Type 2 Diabetes: Moving beyond an Anti-Hyperglycaemic Evaluation. Antioxidants 2024, 13, 510. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and β*. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Youjun, D.; Huang, Y.; Lai, Y.; Ma, Z.; Wang, X.; Chen, B.; Ding, X.; Tan, Q. Mechanisms of resveratrol against diabetic wound by network pharmacology and experimental validation. Ann. Med. 2023, 55, 2280811. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Zeng, Z.; Yang, H.; He, J.; Jiang, T.; Xu, X.; Liu, J.; Li, Y.; Xiang, D.; Pan, X. Resveratrol reversed rosiglitazone administration induced bone loss in rats with type 2 diabetes mellitus. Biomed. Pharmacother. 2024, 178, 117208. [Google Scholar] [CrossRef] [PubMed]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: A randomized, placebo-controlled trial. Complement. Ther. Med. 2022, 66, 102819. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Chuah, L.H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Y.; Zhang, L.; Liu, F.; Duan, G.; Chen, S.; Long, J.; Jin, Y.; Yang, H. Recent advances in the use of resveratrol against Staphylococcus aureus infections (Review). Med. Int. 2024, 4, 67. [Google Scholar] [CrossRef]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z. Naturforsch. C. J. Biosci. 2007, 62, 189–195. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Michel, A.; Berchtold, S.; Niessner, H.; Marongiu, L.; Busch, C.; Frank, J.; Lauer, U.M.; Venturelli, S. Comparative Analysis of the Antitumor Activity of Cis- and Trans-Resveratrol in Human Cancer Cells with Different p53 Status. Molecules. 2021, 26, 5586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Dijkstra, N.E.; Sino, C.G.M.; Schuurmans, M.J.; Schoonhoven, L.; Heerdink, E.R. Medication self-management: Considerations and decisions by older people living at home. Res. Social. Adm. Pharm. 2022, 18, 2410–2423. [Google Scholar] [CrossRef]
- Funk, O.G.; Yung, R.; Arrighi, S.; Lee, S. Medication Storage Appropriateness in US Households. Innov. Pharm. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.; Shen, C.C.; Strachan, C.; Norris, P. Personal medicines storage in New Zealand. J. Prim. Health Care 2013, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2022.
- Maggi, L.; Ochoa Machiste, E.; Fasani, E.; Albini, A.; Segale, L.; Conte, U. Photostability of extended-release matrix formulations. Eur. J. Pharm. Biopharm. 2003, 55, 99–105. [Google Scholar] [CrossRef]
- Matsushima, Y.; Hattori, M.; Tanaka, A.; Furubayashi, T.; Sakane, T. Changes in Tablet Color Due to Light Irradiation: Photodegradation of the Coating Polymer, Hypromellose, by Titanium Dioxide. AAPS Pharm. Sci. Tech. 2024, 25, 26. [Google Scholar] [CrossRef]
- Silva, C.G.; Monteiro, J.; Marques, R.R.; Silva, A.M.; Martínez, C.; Canle, M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Camont, L.; Cottart, C.H.; Rhayem, Y.; Nivet-Antoine, V.; Djelidi, R.; Collin, F.; Beaudeux, J.L.; Bonnefont-Rousselot, D. Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions. Anal. Chim. Acta. 2009, 634, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; Toldo, J.M.; Rocha, W.R.; Barbatti, M. Photoisomerization pathways of trans-resveratrol. Phys. Chem. Chem. Phys. 2024, 26, 24179–24188. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, M.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Photo-induced chemical reaction of trans-resveratrol. Food Chem. 2015, 171, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.G.; Vicendo, P.; Thomas, A.H.; Lorente, C. Clearing up the photochemistry of resveratrol: Effect of the solvent. J. Photochem. Photobiol. A Chem. 2018, 367, 327–331. [Google Scholar] [CrossRef]
- Yao, Y.; Yuan, H.; Chen, C.; Liang, J.; Li, C. Study of the Antioxidant Capacity and Oxidation Products of Resveratrol in Soybean Oil. Foods 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.P.; Soares Lopes, D.P.; de Moraes Junior, R.C.; Vieira Gonçalves, C.; Pereira Rosa, L.; da Silva Rosa, F.C.; da Silva, R.A.A. Photoactivated resveratrol against Staphylococcus aureus infection in mice. Photodiagnosis Photodyn. Ther. 2019, 25, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Szulc-Musioł, B.; Sarecka-Hujar, B. The Use of Micro- and Nanocarriers for Resveratrol Delivery into and across the Skin in Different Skin Diseases-A Literature Review. Pharmaceutics. 2021, 13, 451. [Google Scholar] [CrossRef]
- Juškaitė, V.; Ramanauskienė, K.; Briedis, V. Testing of resveratrol microemulsion photostability and protective effect against UV induced oxidative stress. Acta Pharm. 2017, 67, 247–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biagini, A.; Refrigeri, N.; Caglioti, C.; Sabbatini, P.; Ticconi, S.; Ceccarelli, G.; Iannitti, R.G.; Palazzetti, F.; Fioretti, B. Accelerated Stability Testing in Food Supplements Underestimates Shelf Life Prediction of Resveratrol with Super-Arrhenius Behavior. Symmetry 2024, 16, 493. [Google Scholar] [CrossRef]
- França, L.d.M.; Pimentel, M.F.; Simões, S.d.S.; Grangeiro, S.; Prats-Montalbán, J.M.; Ferrer, A. NIR hyperspectral imaging to evaluate degradation in captopril commercial tablets. Eur. J. Pharm. Biopharm. 2016, 104, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, Y.; Mao, J.; Chen, Y.; Yin, A.; Zhao, B.; Zhang, H.; Liu, M. A Review of Pharmaceutical Robot based on Hyperspectral Technology. J. Intell. Robot. Syst. 2022, 105, 75. [Google Scholar] [CrossRef] [PubMed]
- Nishii, T.; Matsuzaki, K.; Morita, S. Real-time determination and visualization of two independent quantities during a manufacturing process of pharmaceutical tablets by near-infrared hyperspectral imaging combined with multivariate analysis. Int. J. Pharm. 2020, 590, 119871. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, L.M.; Tewari, J.; Gopinathan, N.; Boulas, P.; Cho, B.K. In-Process Control Assay of Pharmaceutical Microtablets Using Hyperspectral Imaging Coupled with Multivariate. Analysis. Anal. Chem. 2016, 88, 11055–11061. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.R.; Bhavsar, K.; Kimbahune, S.; Khandelwal, S.; Ghose, A.; Pal, A. Detection of Counterfeit Medicines Using Hyperspectral Sensing. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 6155–6158. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, S.; Deda, A.; Koprowski, R.; Banyś, A.; Błońska-Fajfrowska, B. The Use of Directional Reflectance Measurement for in vivo Assessment of Protective Properties of Cosmetics in the Infrared Radiation Range. Photochem. Photobiol. 2017, 93, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M.; Sarecka-Hujar, B. Assessment of Directional-Hemispherical Reflectance of Tablets with Cefuroxime during Storage under Elevated Temperature and Ultraviolet Radiation. Sensors 2024, 24, 630. [Google Scholar] [CrossRef] [PubMed]
- Klukkert, M.; Wu, J.X.; Rantanen, J.; Carstensen, J.M.; Rades, T.; Leopold, C.S. Multispectral UV imaging for fast and non-destructive quality control of chemical and physical tab-let attributes. Eur. J. Pharm. Sci. 2016, 90, 85–95. [Google Scholar] [CrossRef]
- França-Silva, F.; Rego, C.H.Q.; Gomes-Junior, F.G.; Moraes, M.H.D.; Medeiros, A.D.; Silva, C.B.D. Detection of Drechslera avenae (Eidam) Sharif [Helminthosporium avenae (Eidam)] in Black Oat Seeds (Avena strigosa Schreb) Using Multispectral Imaging. Sensors 2020, 20, 3343. [Google Scholar] [CrossRef]
- Kessler, R.W. Sensitivity and selectivity in optical spectroscopy and imaging: A molecular approach. In OCM 2015—Optical Characterization of Materials, Conference Proceedings; Beyerer, J., Ed.; KIT Scientific Publishing: Karlsruhe, Germany, 2015; pp. 89–102. [Google Scholar]
- Meisner, M.; Duda, P.; Szulc-Musioł, B.; Sarecka-Hujar, B. Characteristics of Commercial Effervescent Tablets Using Selected Pharmacopeial and Novel Analytical Methods. Appl. Sci. 2023, 13, 3171. [Google Scholar] [CrossRef]
- Szulc-Musioł, B.; Duda, P.; Meisner, M.; Sarecka-Hujar, B. Hyperspectral and Microtomo-graphic Analyses to Evaluate the Stability of Quercetin and Calcium Effervescent Tablets Exposed to Heat and Ultraviolet Radiation. Processes 2024, 12, 531. [Google Scholar] [CrossRef]
- Caron, S.; Herding, L.; Binyamin, Y.; Baidossi, M.; Vinetsky, Y.; Morales, A.; Hildebrandt, C.; Reoyo-Prats, R.; Faugeroux, O.; Agüero, A.; et al. A laboratory intercomparison of solar absorptance and thermal emittance measurements at room temperature. Sol. Energy Mater. Sol. Cells 2022, 238, 111579. [Google Scholar] [CrossRef]


| Stability Conditions | Weight [g] M ± SD, n = 20 | Thickness [mm] M ± SD, n = 7 | Diameter [mm] M ± SD, n = 7 |
|---|---|---|---|
| Day 0 | 0.988 ± 0.006 | 5.793 ± 0.049 | 9.234 ± 0.078 |
| Hour 1 (25 °C) | 0.984 ± 0.007 | 5.804 ± 0.111 | 9.206 ± 0.053 |
| Hour 3 (25 °C) | 0.986 ± 0.005 | 5.760 ± 0.101 | 9.189 ± 0.071 |
| Day 1 (25 °C) | 0.986 ± 0.008 | 5.746 ± 0.104 | 9.181 ± 0.035 |
| Day 5 (25 °C) | 0.987 ± 0.008 | 5.726 ± 0.054 | 9.181 ± 0.013 |
| Day 1 (45 °C) | 0.984 ± 0.009 | 5.729 ± 0.083 | 9.191 ± 0.077 |
| Day 5 (45 °C) | 0.988 ± 0.010 | 5.709 ± 0.046 | 9.211 ± 0.082 |
| p | 0.332 | 0.190 | 0.212 |
| Stability Conditions | Force Needed to Crush the Tablet [N] M ± SD, n = 7 | Hardness Factor [N/m2] M ± SD, n = 7 | Friability [%] M ± SD, n = 7 |
|---|---|---|---|
| Day 0 | 306.386 ± 33.019 | 573.093 × 104 ± 64.590 × 104 | 0.708 ± 0.014 |
| Hour 1 (25 °C) | 318.957 ± 10.396 | 596.859 × 104 ± 11.480 × 104 | 0.652 ± 0.047 |
| Hour 3 (25 °C) | 328.529 ± 12.449 | 620.725 × 104 ± 20.476 × 104 | 0.644 ± 0.064 |
| Day 1 (25 °C) | 336.386 ±17.6998 | 637.550 × 104 ± 29.192 × 104 | 0.491 ±0.038 |
| Day 5 (25 °C) | 345.071 ± 17.624 | 656.466 × 104 ± 34.449 × 104 | 0.364 ± 0.012 |
| Day 1 (45 °C) | 384.414 ± 28.381 | 730.922 × 104 ± 64.089 × 104 | 0.253 ± 0.023 |
| Day 5 (45 °C) | 398.329 ± 15.890 | 757.662 × 104 ± 33.313 × 104 | 0.207 ± 0.013 |
| p | <0.001 | <0.001 | <0.001 |
| Type of the Tablets | THR Values for Tablets Under UV and 25 °C, M ± SD | p | ||||
|---|---|---|---|---|---|---|
| Spectral Bands [nm] | Day 0 | Hour 1 | Hour 3 | Day 1 | Day 5 | |
| 335–380 | 0.104 ± 0.004 | 0.099 ± 0.004 | 0.100 ± 0.005 | 0.086 ± 0.007 | 0.085 ± 0.017 | <0.001 |
| 400–540 | 0.306 ± 0.007 | 0.301 ± 0.005 | 0.295 ± 0.024 | 0.287 ± 0.008 | 0.253 ± 0.034 | <0.001 |
| 480–600 | 0.362 ± 0.012 | 0.352 ± 0.008 | 0.343 ± 0.031 | 0.324 ± 0.010 | 0.285 ± 0.038 | <0.001 |
| 590–720 | 0.465 ± 0.012 | 0.460 ± 0.008 | 0.446 ± 0.038 | 0.447 ± 0.011 | 0.393 ± 0.051 | <0.001 |
| 700–1100 | 0.650 ± 0.010 | 0.637 ± 0.045 | 0.653 ± 0.010 | 0.654 ± 0.012 | 0.587 ± 0.072 | <0.001 |
| 1000–1700 | 0.714 ± 0.008 | 0.701 ± 0.049 | 0.716 ± 0.011 | 0.715 ± 0.011 | 0.643 ±0.071 | <0.001 |
| 1700–2500 | 0.528 ± 0.013 | 0.515 ± 0.041 | 0.529 ± 0.013 | 0.524 ± 0.016 | 0.457 ± 0.066 | <0.001 |
| Type of the Tablets | THR Values for Tablets Under UV and 45 °C, M ± SD | p | ||
|---|---|---|---|---|
| Spectral Bands [nm] | Day 0 | Day 1 | Day 5 | |
| 335–380 | 0.104 ± 0.004 | 0.077 ± 0.004 | 0.076 ± 0.013 | <0.001 |
| 400–540 | 0.306 ± 0.007 | 0.266 ± 0.014 | 0.246 ± 0.034 | <0.001 |
| 480–600 | 0.362 ± 0.012 | 0.308 ± 0.018 | 0.276 ± 0.042 | <0.001 |
| 590–720 | 0.465 ± 0.012 | 0.432 ± 0.025 | 0.393 ± 0.057 | <0.001 |
| 700–1100 | 0.650 ± 0.010 | 0.633 ± 0.027 | 0.592 ± 0.076 | <0.001 |
| 1000–1700 | 0.714 ± 0.008 | 0.693 ± 0.027 | 0.638 ± 0.079 | <0.001 |
| 1700–2500 | 0.528 ±0.013 | 0.513 ± 0.024 | 0.462 ± 0.059 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc-Musioł, B.; Sarecka-Hujar, B. Characteristics of Resveratrol Tablets Stored Under Stress Conditions by Total Hemispherical Reflectance and Selected Pharmacopoeial Parameters. Processes 2025, 13, 638. https://doi.org/10.3390/pr13030638
Szulc-Musioł B, Sarecka-Hujar B. Characteristics of Resveratrol Tablets Stored Under Stress Conditions by Total Hemispherical Reflectance and Selected Pharmacopoeial Parameters. Processes. 2025; 13(3):638. https://doi.org/10.3390/pr13030638
Chicago/Turabian StyleSzulc-Musioł, Beata, and Beata Sarecka-Hujar. 2025. "Characteristics of Resveratrol Tablets Stored Under Stress Conditions by Total Hemispherical Reflectance and Selected Pharmacopoeial Parameters" Processes 13, no. 3: 638. https://doi.org/10.3390/pr13030638
APA StyleSzulc-Musioł, B., & Sarecka-Hujar, B. (2025). Characteristics of Resveratrol Tablets Stored Under Stress Conditions by Total Hemispherical Reflectance and Selected Pharmacopoeial Parameters. Processes, 13(3), 638. https://doi.org/10.3390/pr13030638







