Abstract
This study explores an innovative topical formulation to treat alopecia by encapsulating cannabidiol (CBD) in chitosan nanoparticles. CBD, widely known for its anti-inflammatory, antioxidant, and endocannabinoid-modulating effects, shows significant potential for treating alopecia, a condition characterized by hair loss influenced by genetic, hormonal, or environmental factors. However, its low water solubility presents a significant challenge for topical applications. To address this issue, chitosan nanoparticles were synthesized using chitosan of reduced molecular mass (270 kDa) with an acetylation level of 12%, β-glycerophosphate as a crosslinking agent, and 1% glycerol to improve CBD encapsulation efficiency. Physicochemical characterization using scanning electron microscopy (SEM), zeta potential measurement, and Fourier transform infrared spectroscopy (FTIR) revealed that the β-glycerophosphate concentration impacted nanoparticle size and the electrostatic interactions between chitosan’s primary amines and phosphate groups of β-glycerophosphate. Among the tested concentrations (0.05, 0.1, 0.2, and 0.25 mol/L), 0.20 mol/L produced the smallest nanoparticles (390 nm), which were further optimized to encapsulate CBD, reaching a particle size of 227 nm. This optimized formulation may improve the solubility of CBD and enable targeted and sustained delivery to hair follicles. These findings highlight chitosan nanoparticles as a cutting-edge and scalable platform for transdermal delivery of hydrophobic bioactive compounds, presenting a promising approach for the effective management of alopecia.
Keywords:
chitosan; β-glycerophosphate; nanocomposites; hair loss; zeta potential; hydrophobic drugs 1. Introduction
Androgenetic alopecia is the predominant type of hair loss, impacting individuals of both sexes. It is characterized by the progressive reduction of hair follicles due to sensitivity to androgens, particularly dihydrotestosterone (DHT). It is estimated that approximately 50% of men and 30% of women will experience some degree of androgenetic alopecia throughout their lives, which can greatly affect the self-esteem and overall quality of life of those impacted [1]. However, the clinical management of this condition remains a challenge, since traditional treatments like minoxidil and finasteride have effectiveness limitations [2].
Despite advances in understanding the molecular mechanisms underlying androgenetic alopecia [3,4], there is still a significant gap in the availability of long-term effective and safe treatments. This gap highlights the need to explore innovative therapeutic approaches that more effectively address the disease mechanisms. One emerging approach is using biomaterials, which have shown promising potential in regenerative medicine and drug delivery [5].
Biomaterials are substances engineered to interact with living systems, serving therapeutic or diagnostic purposes. They can be categorized as natural, synthetic, or hybrid materials, depending on their chemical composition and biological response [6]. Among natural biomaterials, chitosan (CS) is distinguished by its biological characteristics, including tissue compatibility, degradability, and minimal toxicity, making it suitable for applications in tissue regeneration and drug delivery. Derived from renewable sources, chitosan also exhibits antimicrobial, wound-healing, and coagulating properties [7,8,9,10,11].
In recent years, chitosan nanoparticles have gained recognition for their capacity to improve the therapeutic effectiveness of biologically active substances. Their small particle size, which increases surface area and charge density, makes them particularly attractive for biomedical applications [12,13]. These nanoparticles are commonly synthesized through ionotropic gelation, where protonated chitosan interacts with multivalent polyanions, leading to swift gel formation through inter- and intramolecular bonds between chitosan amino groups and polyanionic compounds [14,15].
β-glycerophosphate (β-GP) is a multivalent polyanion widely used in the gelation of chitosan (CS) hydrogels. Interactions involving the phosphate groups of β-GP and the protonated amines of chitosan result in stable structures with unique properties, such as sol–gel transition under physiological conditions, particularly at 37 °C [16,17,18]. Chitosan has shown the potential to induce the proliferation of dermal papilla cells from hair follicles, making it a promising candidate for treating androgenetic alopecia [11].
Additionally, cannabidiol (CBD) has attracted considerable interest due to its strong anti-inflammatory and antioxidant effects, which can mitigate the inflammation responsible for follicle miniaturization while promoting tissue regeneration [19]. The combination of these materials leverages the synergistic action of their properties, enhancing therapeutic potential. However, the low aqueous solubility of CBD poses a challenge for its topical application, underscoring the importance of innovative strategies such as nanoparticle encapsulation to improve its bioavailability and therapeutic efficacy [20,21].
Multiple factors, including concentration, pH, molecular mass, and mixing parameters, affect the size and distribution of chitosan nanoparticles [11,13]. Thus, this research proposes the development of an innovative nanoparticulate system based on chitosan, β-glycerophosphate, glycerol, and cannabidiol (CBD) for treatment of androgenetic alopecia. This study examines the impact of β-GP concentration on nanoparticle characteristics and investigates the function of glycerol in stabilizing nanoemulsions with CBD [22,23]. The goal is to provide an effective and safe therapeutic alternative that overcomes the limitations of current treatments, contributing to an improved quality of life for patients. The innovative aspect of this research lies in developing chitosan-based nanoparticles incorporated with CBD, offering a novel therapeutic approach for future use to effectively target androgenetic alopecia. By taking advantage of the unique properties of these materials, this study seeks to overcome the limitations of current treatments and enhance patient outcomes, marking notable progress in the realm of dermatological treatments.
2. Materials and Methods
2.1. Materials
Chitosan powder, featuring an acetylation degree (AD) of 12% and a molecular weight (Mv) of 270 kDa, was provided by the Northeastern Biomaterials Evaluation and Development Laboratory—Certbio (Campina Grande, PB, Brazil). Acetic acid and glycerol were purchased from ACS Científica (São Paulo, Brazil). Cannabidiol was supplied by Prati Donaduzzi (Paraná, Brazil), and β-glycerophosphate (β-GP) was obtained from Sigma-Aldrich (Rio de Janeiro, Brazil).
2.2. Methods
2.2.1. Preparation of Chitosan Nanoparticles
The approach used to produce chitosan nanoparticles was inspired by the studies of Benamer et al. [24], Kaloti and Bohidar [25], and Smith and Satino [26]. Initially, a solution of low-molecular-weight chitosan was formulated at 2.5 mg/mL in 1% acetic acid. The mixture was magnetically stirred (100 rpm; ~25 °C) for 1 h to achieve dissolution of the chitosan.
The solution’s pH was subsequently modified to 5.5 by incrementally incorporating a β-glycerophosphate (β-GP) solution with different concentrations (Table 1). The adjustment was continuously monitored using a pH meter (Q400A, Quimis, São Paulo, Brazil) while maintaining constant stirring for an additional 1 h. Afterward, the solution underwent sonication for 30 min at a frequency of 80 Hz to optimize particle size reduction (Figure 1). The formulation that produced the smallest particle size was selected for subsequent studies.

Table 1.
Composition of the nanoparticles with different concentrations of β-GP.
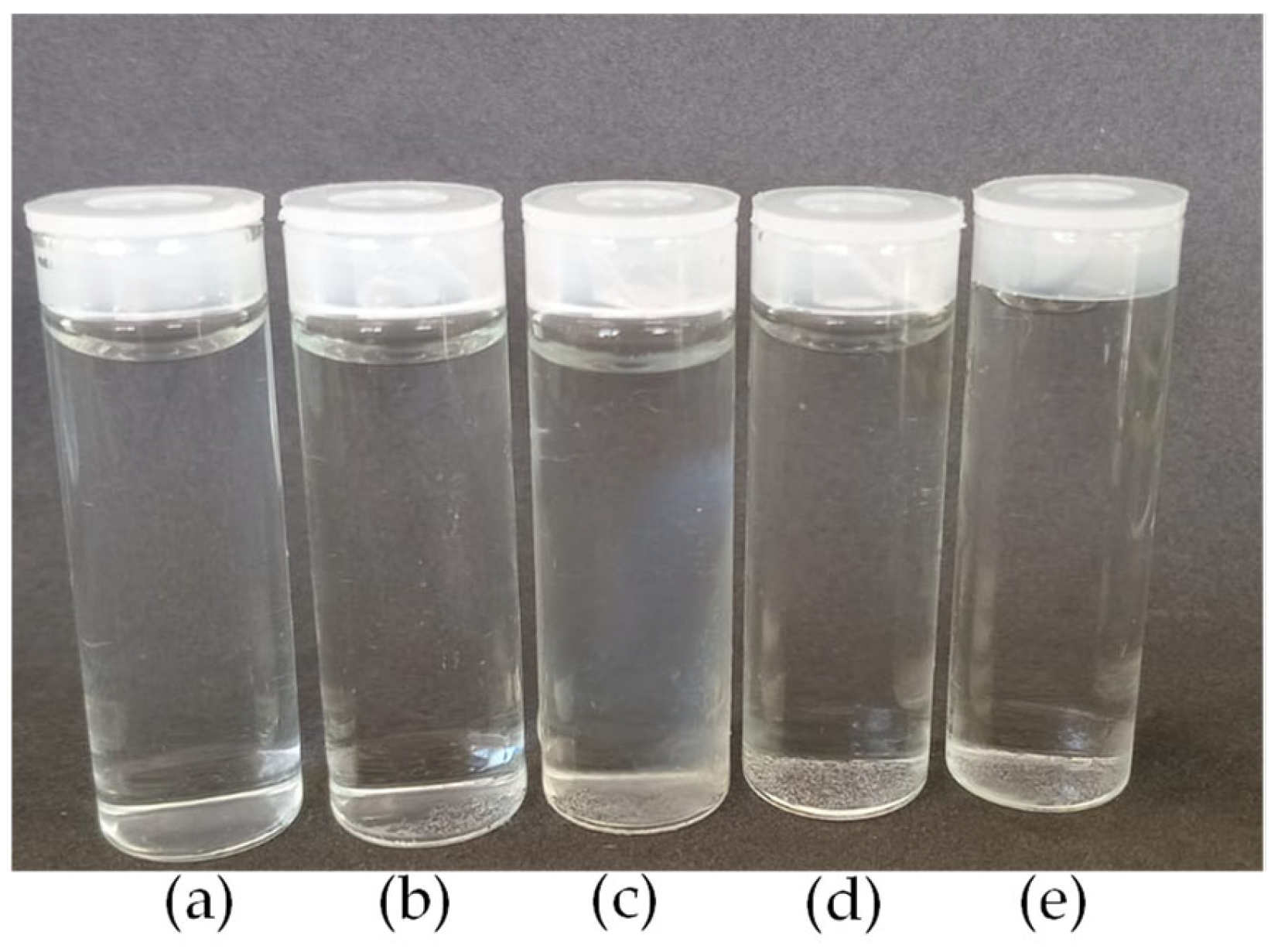
Figure 1.
Images of nanoparticles: (a) CS; (b) CS/β-GP_0.05; (c) CS/β-GP_0.10; (d) CS/β-GP_0.20; and (e) CS/β-GP_0.25.
2.2.2. Preparation of Chitosan/CBD Nanoparticles
The methodology was adapted from Kaloti and Bohidar [25], Vanti et al. [27], and Ferreira et al. [28]. Initially, solutions of chitosan nanoparticles (CS) were prepared using varying concentrations of β-glycerophosphate (β-GP). The formulation that resulted in the smallest particle size was selected for further processing, as previously reported.
After obtaining the chitosan solution, as described in the previous section, the subsequent steps were carried out for the preparation of formulations with CBD. Subsequently, 1% (v/v) glycerol (Gly) [25], was introduced into the chitosan solution to aid in the formation of a cannabidiol (CBD) nanoemulsion, improving the solubility and distribution of CBD within the solution. The solution was stirred once more at 100 rpm and maintained at 25 °C. CBD was then incorporated at 3 mg/mL [28], under continuous stirring to ensure homogeneity. Finally, the solution was subjected to sonication for 30 min at 80 Hz to further reduce particle size and optimize dispersion (CS/β-GP/Gly-CBD) (Figure 2).

Figure 2.
Image of the CS/β-GP/Gly-CBD nanoparticles.
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
The specimens, previously frozen and lyophilized for 72 h, were subjected to analysis using Fourier transform infrared spectroscopy (FTIR) at room temperature. The analyses were conducted with a Perkin Elmer Spectrum 400 spectrometer (Waltham, MA, USA). FTIR analysis was conducted to detect the characteristic bands of functional groups within the nanoparticle formulations and to verify the successful incorporation of the drug. The scanning range used was 4000 to 500 cm−1.
2.3.2. Zeta Potential (ζ)
The nanoparticle samples were analyzed for hydrodynamic diameter, polydispersity index (PDI), and zeta potential using dynamic light scattering (DLS), based on a methodology adapted from Matos et al. [29]. Measurements were carried out with a Zetasizer Nano ZS (Malvern, Worcestershire, UK). For each analysis, 5 mL of an aqueous nanoparticle suspension was utilized, and all experiments were conducted three times to guarantee reliability. The size, PDI, and zeta potential were directly assessed, with the temperature strictly controlled at 25 °C.
2.3.3. Scanning Electron Microscopy (SEM)
The morphology of the nanoparticles was analyzed using scanning electron microscopy (SEM), following an adaptation of the methodologies described by Ta et al. [29]. For this analysis, the nanoparticles were oven-dried at 37 °C, coated with a fine gold layer, and visualized using a scanning electron microscope. The gold coating is essential in SEM, as it improves the electrical conductivity of the sample, reducing surface charge accumulation and providing higher quality and resolution images, especially in non-conductive materials like the analyzed threads.
To further assess the porosity of the nanoparticles, the samples were pre-frozen, lyophilized, and subsequently analyzed using the same equipment (TESCAN, VEGA 4 model, Brno—Kohoutovice, Czech Republic). The examination was conducted under variable conditions, reaching up to 30 kV, with a high vacuum range of 7 to 150 Pa. Images were acquired with an electron beam operating at an acceleration voltage of 15 kV, a focal depth of 1 mm, and a resolution of 30 nm.
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.1. Characterization of CS/β-GP Nanoparticles
Fourier transform infrared spectroscopy (FTIR) was utilized to characterize CS/β-GP nanoparticles and examine the electrostatic interactions between chitosan (CS) and β-glycerophosphate (β-GP).
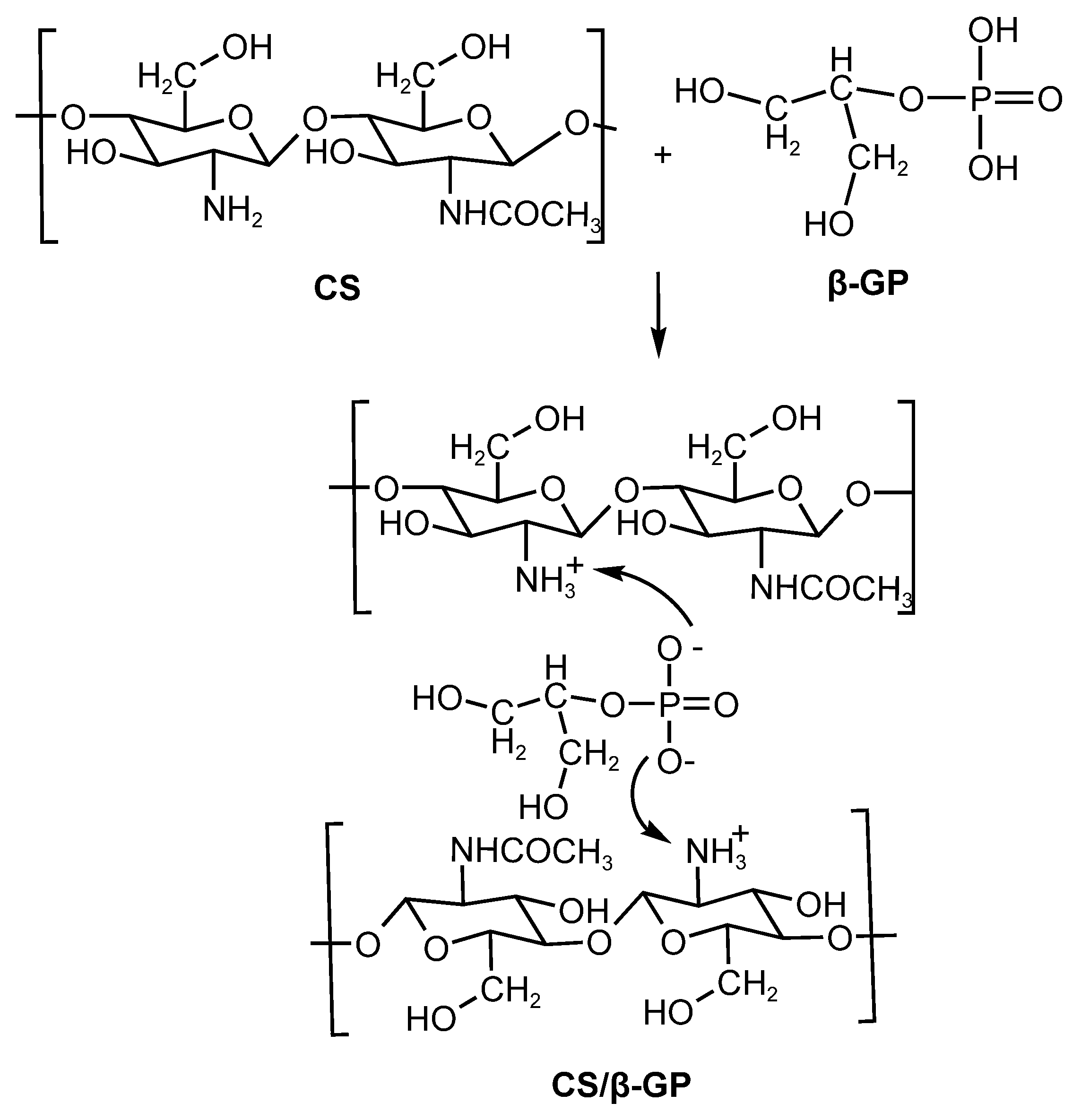
The bonding between chitosan and β-GP is mediated by the electrostatic stabilization of charged amine groups (NH3+), being facilitated by the exposure of hydrophilic glycerol groups. This system is thermosensitive, exhibiting increased viscosity and gelation at physiological temperature (37 °C). These phenomena resulted from hydrophobic interactions among chitosan chains and the formation of hydrogen bonds and ionic bridges mediated by the multivalent ionization of β-GP (Figure 3). As an additional advantage, CS/β-GP-based formulations do not require chemical crosslinking, making them promising for controlled drug delivery applications [30,31]
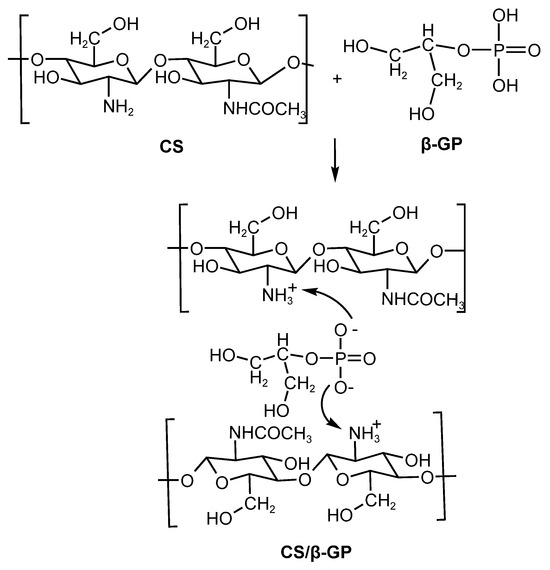
Figure 3.
Electrostatic interactions of the nanoparticles (CS/β-GP).
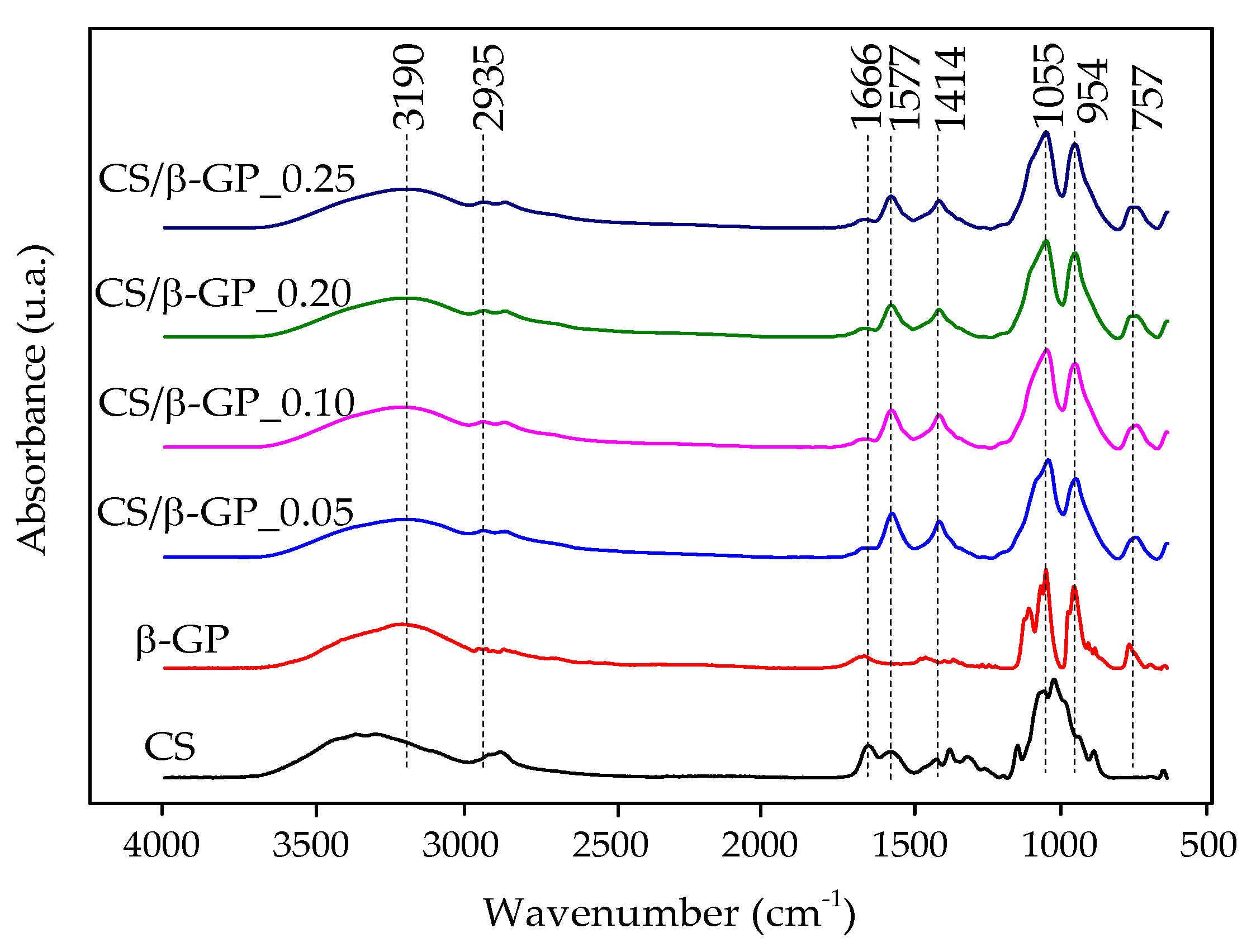
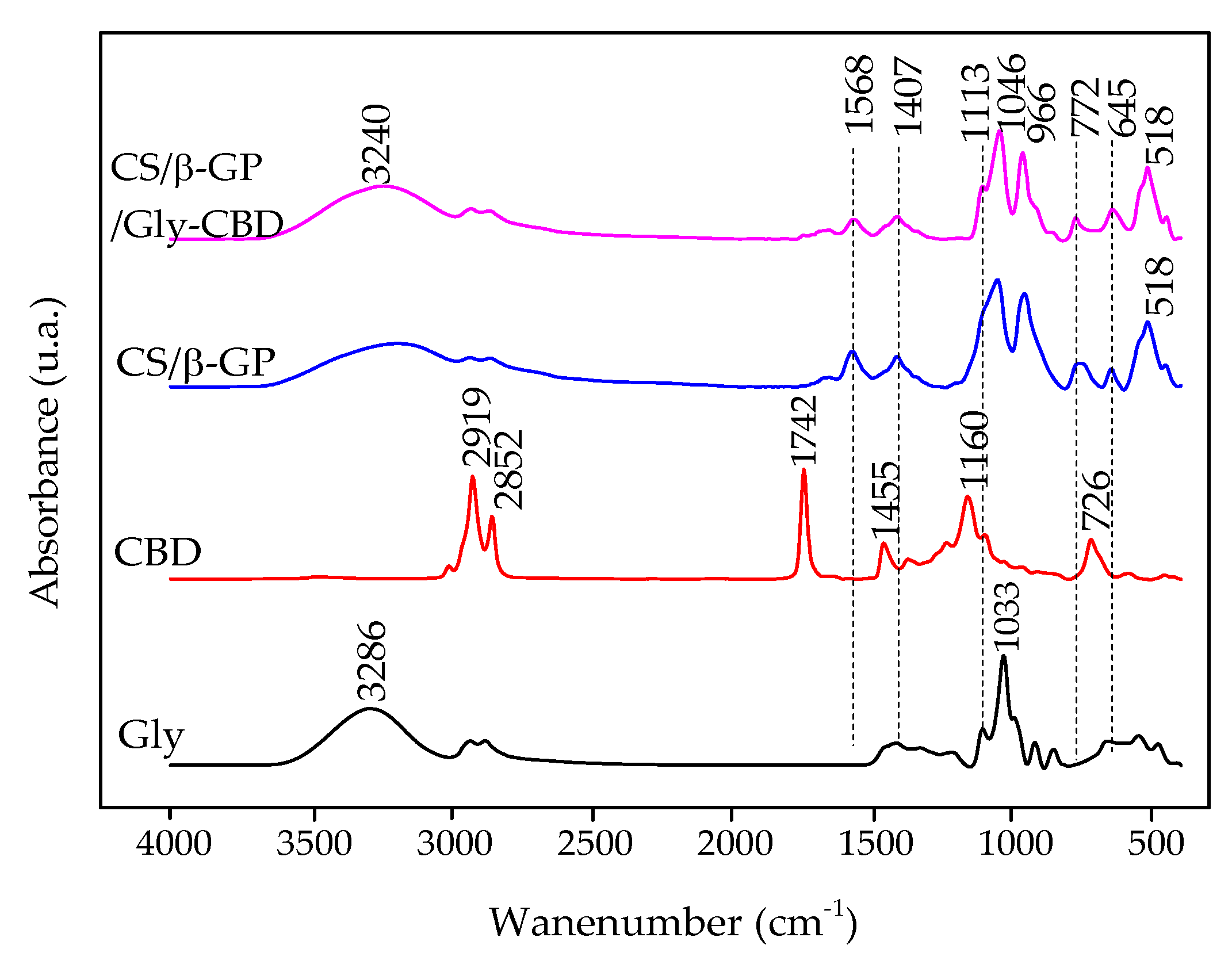
Figure 4 presents the FTIR spectra of CS/β-GP_0.05, CS/β-GP_0.1, CS/β-GP_0.20, and CS/β-GP_0.25, highlighting the decrease in the absorption peak at 1666 cm−1, attributed to the C=O bond from the amide functional group present in chitosan. This reduction indicates its interaction with the phosphate (PO) groups of β-GP [17].
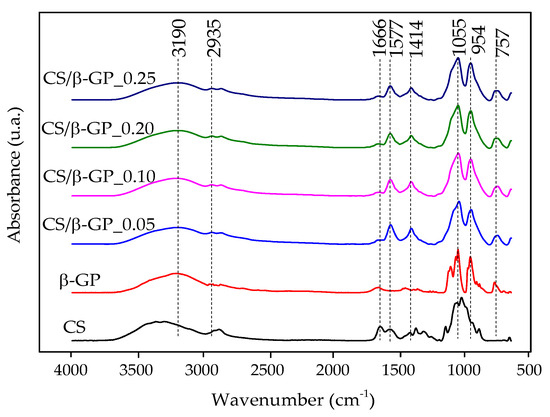
Figure 4.
FTIR spectra of chitosan (CS), β-glycerophosphate (β-GP), and chitosan/β-glycerophosphate nanoparticles (CS/β-GP).
Characteristic phosphate (PO) group bands were observed at 1055 cm−1 and 954 cm−1 in the nanoparticles. The reduced intensity of the peak at 1577 cm−1, associated with the primary amine (-NH) of chitosan, with increasing β-GP concentration, further supports evidence of electrostatic interaction between the two components. However, this band was not eliminated, suggesting that the amines were not fully neutralized as a result of the pH of the CS/β-GP solutions being approximately 5.5 [18].
The pH of 5.5 is particularly relevant for scalp applications, as it closely matches the physiological pH of the region, helping to avoid irritation and preserve the skin barrier’s integrity. These results confirm not only the formation of electrostatic interactions in the nanoparticles, but also the system’s suitability for topical applications [32].
3.1.2. Integration of CBD and Glycerol in CS/β-GP Nanoparticles
Emulsifying agents play a critical role in facilitating interactions between lipophilic components, such as cannabidiol (CBD), and encapsulating matrices. Studies by Fei et al. [33] and Demisli et al. [34] highlight the relevance of electrostatic forces in this process. Based on these findings, glycerol (Gly) was selected as an emulsifier for CBD due to its proven efficacy in stabilizing lipophilic compounds [35,36]. Additionally, glycerol is known to enhance passive dermal diffusion, increasing its relevance for topical formulations [37].
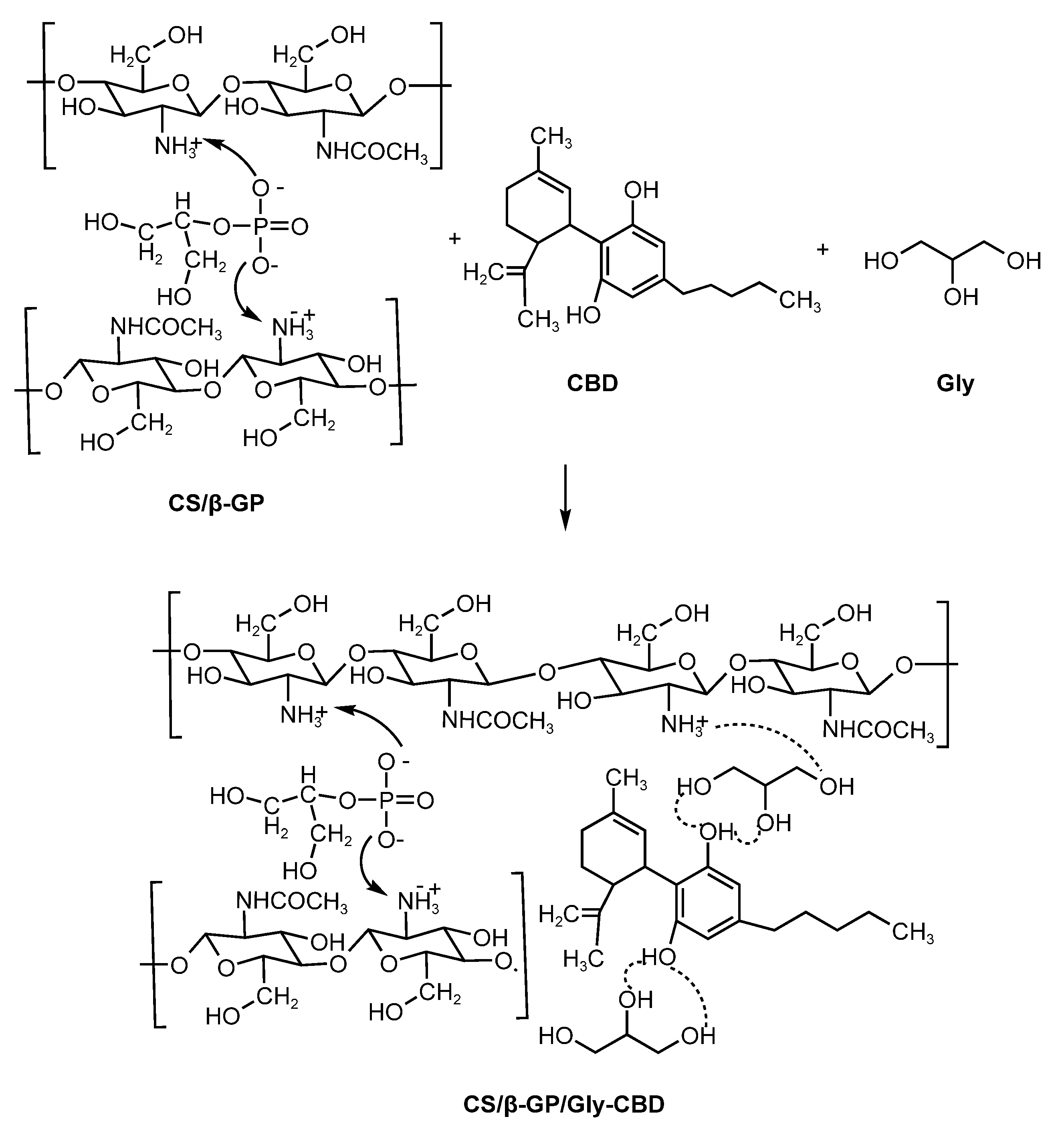
The reaction between chitosan and glycerol forms a compound with enhanced properties, including improved moisture retention and mechanical resistance. Chitosan’s functional groups (-OH and -NH₂) interact with glycerol’s -OH groups, providing significant plasticizing effects [38]. Figure 5 illustrates the electrostatic bonding between CS/β-GP nanoparticles, CBD, and glycerol, demonstrating the mechanism underlying this encapsulating matrix’s efficiency.
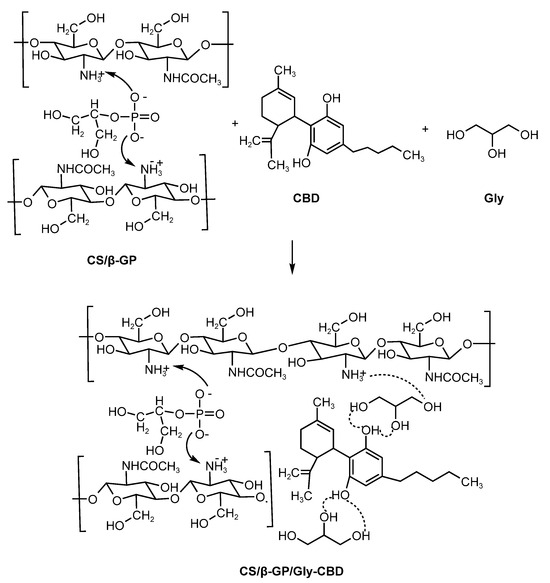
Figure 5.
Electrostatic interactions of CS/β-GP/Gly-CBD nanoparticles.
3.1.3. FTIR Analysis of CS/β-GP/Gly-CBD Nanoparticles
The FTIR spectrum of CBD (Figure 6) exhibited bands at 2919 cm−1 and 2852 cm−1, ascribed to the stretching motions of CH3 and CH2 groups within its hydrocarbon structure. Bands at 1742 cm−1 and 1455 cm−1 were associated with characteristic vibrations of the benzene skeleton, while the band at 1160 cm−1 was linked to C–O group stretching [39].
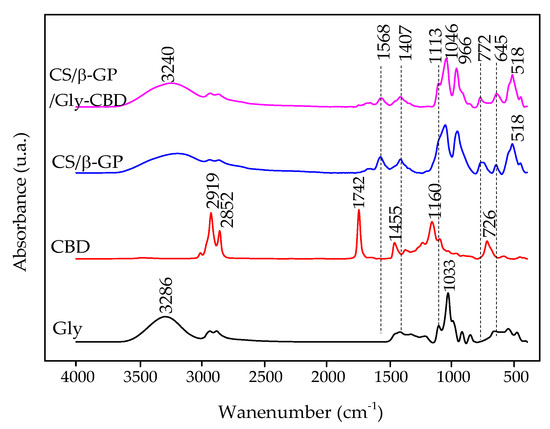
Figure 6.
FTIR spectrum of glycerol (Gly), CBD, CS/β-GP nanoparticles, and CS/β-GP/Gly-CBD nanoparticles.
In the FTIR spectrum of glycerol, an O–H stretching band was observed at 3286 cm−1, while C–H stretching vibrations were evident in the 2810–2950 cm−1 range. The bending motion of the C–O–H group was detected at 1400–1420 cm−1, while the stretching of the C–O bond in primary alcohols was prominent at 1033 cm−1 [38,40].
The FTIR analysis of CS/β-GP/Gly-CBD nanoparticles showed a decrease in the 1568 cm−1 band, attributed to chitosan’s amino groups. In the CS/β-GP spectrum, these groups were not completely neutralized. This decline was associated with electrostatic interactions involving the -OH groups of glycerol and CBD and the amino groups of chitosan [38,39]. Additionally, the 1033 cm−1 band corresponding to glycerol’s -OH group showed reduced intensity, indicating interactions with the -OH group of CBD and chitosan’s amino groups [38]. The peak at 1160 cm−1, corresponding to the -OH group of CBD, also decreased and shifted to 1113 cm−1, further supporting electrostatic interactions between the components. The attenuation of other characteristic CBD peaks corroborates its encapsulation within the nanoparticles [41] (Figure 6).
3.2. Zeta Potential (ζ)
To assess the impact of β-GP concentrations on particle size, the samples were analyzed using dynamic light scattering (DLS) and zeta potential measurements. The findings are summarized in Table 2.

Table 2.
Data on mean diameter, polydispersity index (PDI), and zeta potential (ζ) of chitosan (CS) nanoparticles and formulations containing varying concentrations of β-glycerophosphate (β-GP).
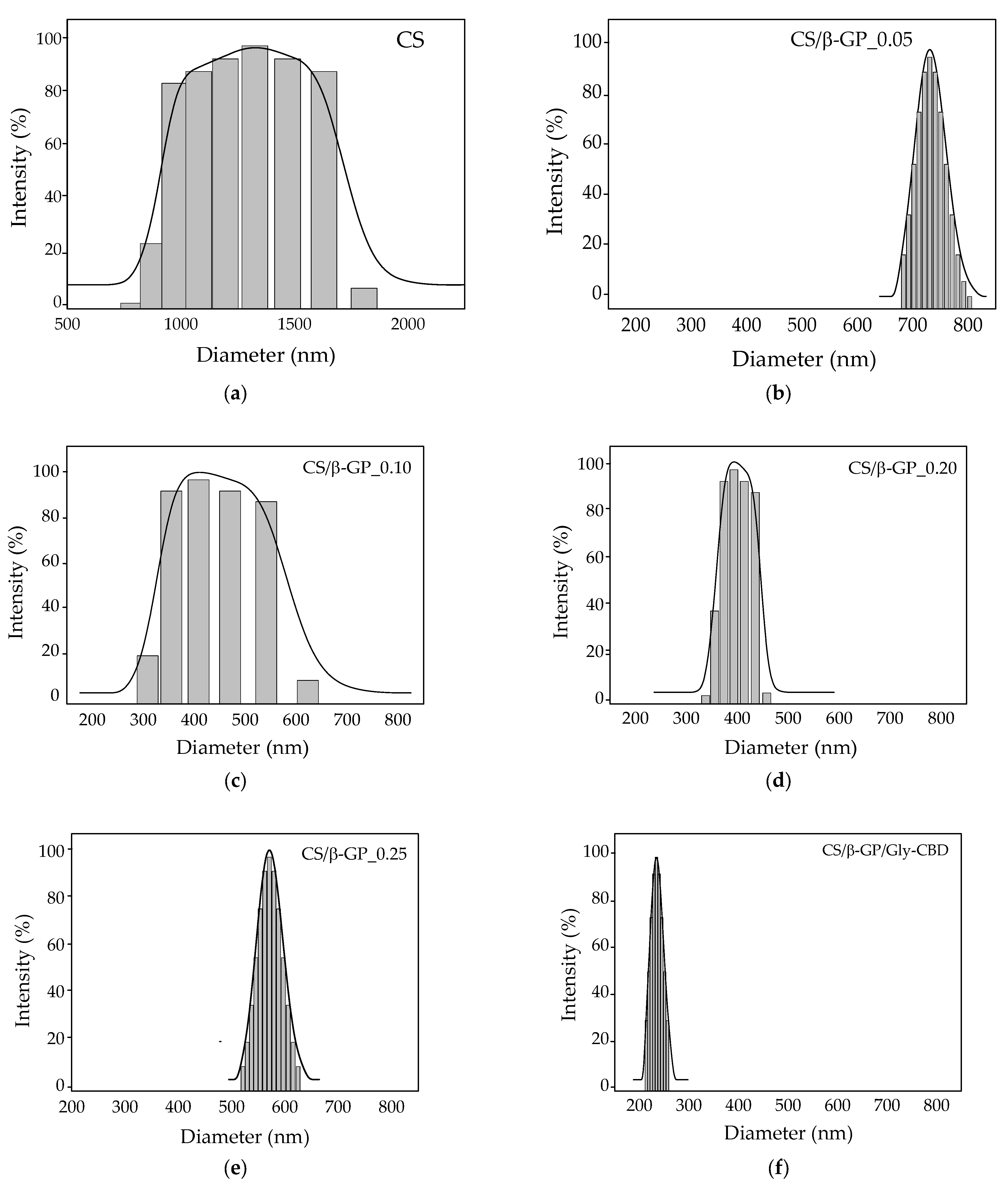
The findings in Table 2 reveal that the CS/β-GP_0.25 sample exhibited the largest particle size, indicating potential aggregate formation, as reported by Mattu et al. [42] and Sipoli, Santana, Shimojo, Azzoni and Torre [11]. In contrast, the CS/β-GP_0.20 sample presented the smallest particle size, measuring 389.80 nm, with a polydispersity index (PDI) of 0.249, a zeta potential of 5.22 mV, and a final pH of 5.5 (Figure 7). These results suggest that incorporating β-GP into chitosan reduced the zeta potential, likely due to the interaction between β-GP’s phosphate groups and the protonated amine groups (NH3+) of chitosan, leading to partial neutralization of positive charges [43]. Based on these findings, a β-GP concentration of 0.20 mol/L was selected for subsequent stages of the research. In the optimized formulation, cannabidiol (CBD) was encapsulated in CS/β-GP nanoparticles containing β-GP at 0.20 mol/L. The increased presence of carboxylate (COO−) groups from CBD contributed to the reversal of the zeta potential, which became negative, reaching −11.17 mV (Table 2), in line with the observations of Shagholani et al. [44].The reduced particle size, at 259.04 nm, was attributed to the electrostatic interactions between the carboxylate groups of CBD and the protonated amines of chitosan.
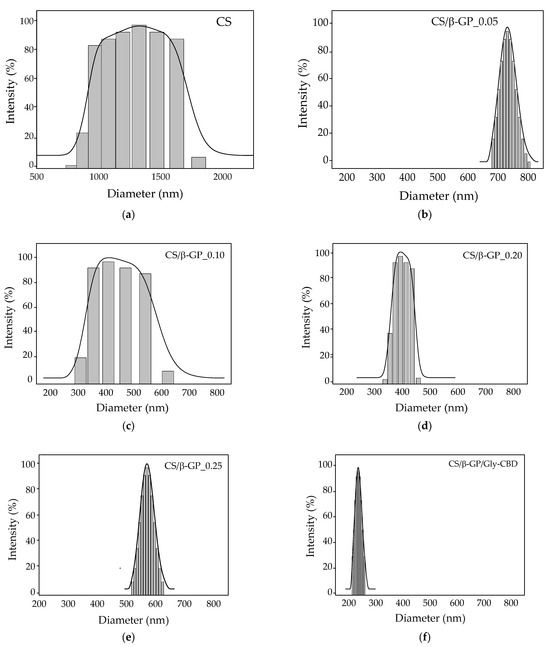
Figure 7.
Particle size distribution of nanoparticles from: (a) chitosan; (b) CS/β-GP_0.05; (c) CS/β-GP_0.10; (d) CS/β-GP_0.20; (e) CS/β-GP_0.25; (f) CS/β-GP/Gly-CBD.
Both chitosan (CS) and cannabidiol (CBD) have been shown to significantly influence hair follicles [45]. Research indicates that nanoparticles within the 228–365 nm size range are optimal for hair follicle penetration, making this size range especially suitable for therapeutic and cosmetic purposes [45,46]. Thus, the formulation of CS/β-GP nanoparticles containing CBD shows promising potential for targeted applications in hair follicles.
In multimodal particle systems, the PDI value loses its relevance as a measure of dispersion, making complementary analyses necessary to adequately characterize the particle distribution in the sample [47]. In the case of nanoparticles crosslinked with β-GP, the size distribution exhibited a multimodal profile at lower concentrations (CS/β-GP_0.05 and CS/β-GP_0.10), as illustrated in Figure 7b,c. In the CS/β-GP_0.20 formulation, β-GP initiates chitosan crosslinking; however, this process is still incomplete or heterogeneous, resulting in a curve displaying a polydispersity index (PDI) exceeding 0.4 while retaining a multimodal profile.
With increasing β-GP concentration (CS/β-GP_0.25), crosslinking becomes more efficient and homogeneous, resulting in the formation of particles with consistent sizes. This effect is evident in unimodal distributions [48]. Notably, the CS/β-GP_0.20 formulation presented smaller average particle sizes compared to CS/β-GP_0.25, as shown in Table 2. For this reason, despite exhibiting a multimodal curve, this formulation was chosen for CBD encapsulation.
The nanoparticle formulation containing CBD (CS/β-GP/Gly-CBD) exhibited a single dominant size peak, with a PDI of 0.2, which is considered monomodal, indicating a relatively homogeneous size distribution [49]. In this context, both CBD and β-GP played a fundamental role as electrostatic agents interacting with chitosan’s amine groups and enhancing the system’s stability.
At excessively high concentrations, an excess of crosslinker can promote the formation of extremely small and highly uniform particles, further reducing size variation. This phenomenon results in a narrow and unimodal distribution curve, as previously observed in studies on crosslinked nanoparticles [48]. Moreover, efficient crosslinking has been associated with greater nanoparticle stability and functionality, favoring their use in biomedical and pharmaceutical applications [50].
3.3. Scanning Electron Microscopy (SEM)
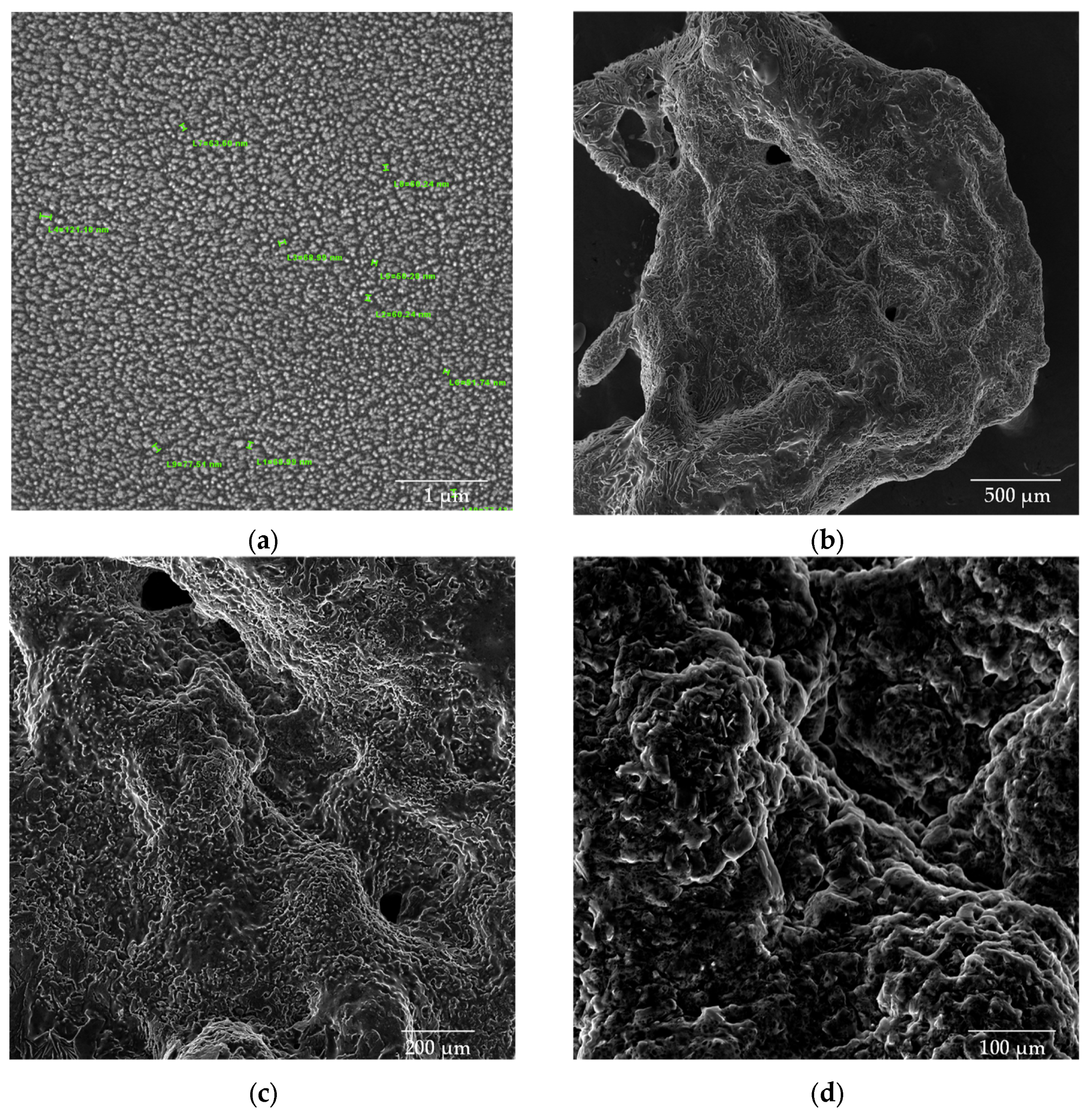
SEM images (Figure 8, Figure 9 and Figure 10) were captured to evaluate the gold-coated surface of chitosan nanoparticles, CS/β-GP nanoparticles, and chitosan nanoparticles with the addition of glycerol and CBD.
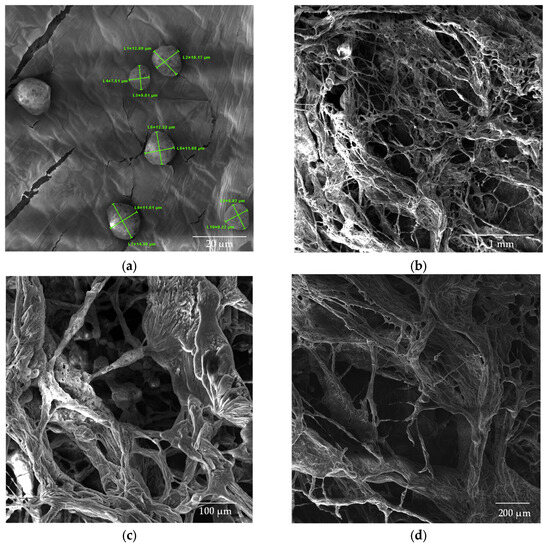
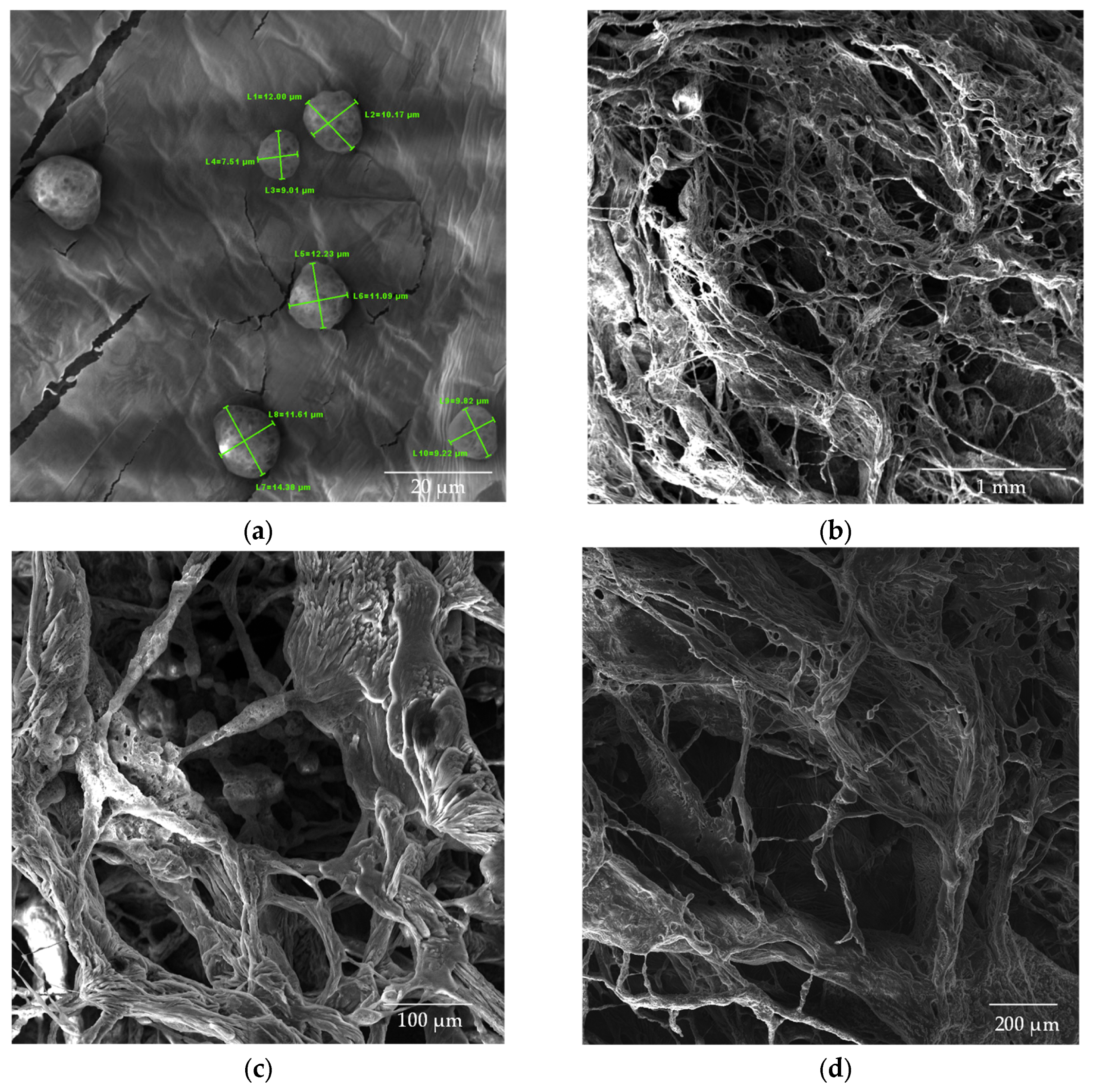
Figure 8.
SEM images of CS nanoparticles (a) dried in an oven; (b–d) lyophilized samples.
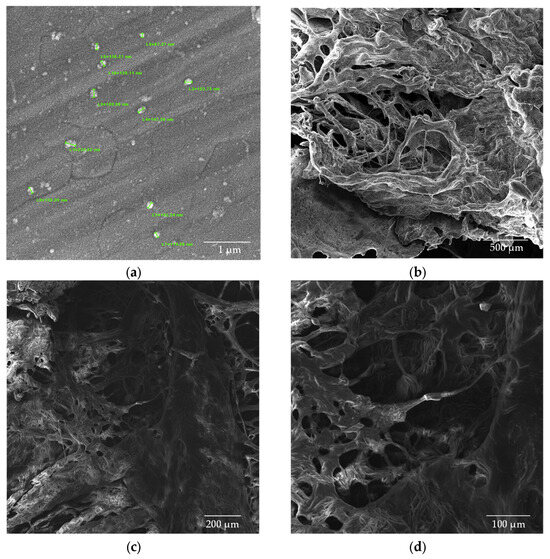
Figure 9.
SEM images of CS/β-GP_0.20 nanoparticles (a) dried in an oven; (b–d) lyophilized samples.
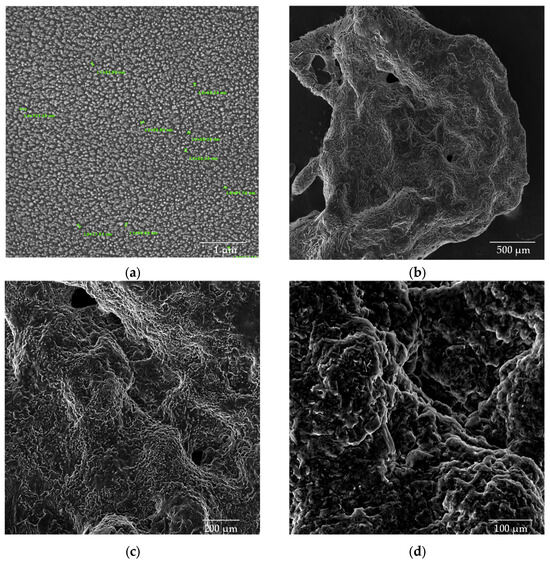
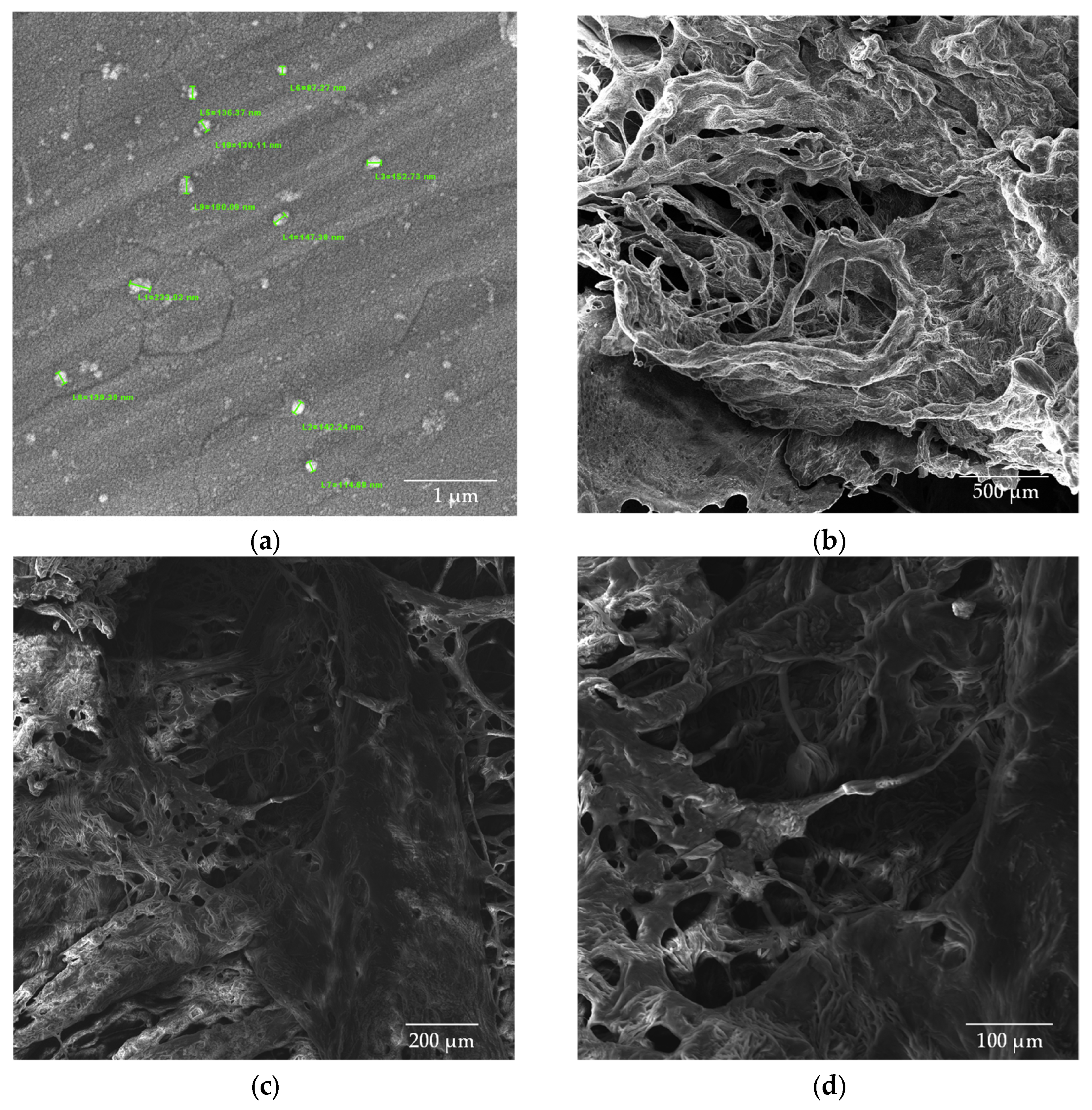
Figure 10.
SEM images of CS/β-GP/Gly-CBD nanoparticles (a) dried in an oven at a magnification of 50kx; (b) lyophilized samples at magnifications of 100x; (c) 200x; and (d) 500x.
The chitosan (CS) nanoparticles used as a control exhibited a well-defined spherical morphology, as shown in Figure 8a. The analysis of the average particle size revealed dimensions of 10.7 × 103 ± 1.87 × 103 nm for the CS solution in the absence of β-glycerophosphate (β-GP). The lyophilized chitosan samples (Figure 8b–d) exhibited a structure with larger and non-uniform pores, emphasizing the impact of the lyophilization process on nanoparticle morphology.
The introduction of β-GP into the formulation significantly reduced the size of the nanoparticles while maintaining their spherical morphology. Figure 9a shows the CS/β-GP_0.20 sample, with particles exhibiting an average size of 146.3 ± 36.9 nm. Notably, the corresponding lyophilized samples (Figure 9b–d) displayed smaller pores compared with CS nanoparticles, suggesting that β-GP also contributed to a more compact and uniform structure.
The addition of glycerol (Gly) and cannabidiol (CBD) to the CS/β-GP_0.20 formulation resulted in an even more pronounced reduction in particle size. The CS/β-GP/Gly-CBD nanoparticles demonstrated good uniformity and homogeneous dispersion, as evidenced in Figure 10a. The average particle size was reduced to 70.0 ± 19.2 nm, indicating that the additional interactions promoted by Gly and CBD charges contributed to the formation of smaller and more uniform particles. Furthermore, the lyophilized samples of this formulation (Figure 10b–d) showed no visible pores, in contrast to the previous samples. These findings corroborate previous studies, such as those by Sharma and Purkait [51] and Corveleyn and Remon [52], which emphasize the important role of plasticizers in reducing pore size in lyophilized membranes. Thus, the combination of β-GP, Gly, and CBD not only improved the uniformity of the nanoparticles but also optimized their structural properties, making them more suitable for potential therapeutic and cosmetic applications. DLS analysis tends to provide higher values than those obtained by SEM due to the consideration of the hydrodynamic diameter of the nanoparticles, the presence of aggregates, and optical artifacts intrinsic to the technique [53,54]. In this context, when comparing the values obtained in this assay with those presented in Table 2, it was noted that the mean particle size of CS/β-GP_0.20 was approximately 270% larger in the DLS analysis. For CS/β-GP/Gly-CBD particles, the difference was near 166%, indicating that the incorporation of Gly-CBD may have influenced the dispersion and stability of the formulation.
Conversely, the analysis of chitosan particles without β-GP revealed the opposite behavior, with SEM values being 144% higher than those obtained by DLS. This outcome may be attributed to the sample preparation procedure for SEM analysis, which includes solvent evaporation. This procedure can promote the agglomeration of chitosan molecules, especially in the absence of the polyanion agent (β-GP), which acts as a stabilizer and helps maintain physicochemical stability over time and under different environmental conditions [55,56].
4. Conclusions
The findings of this research highlight the effectiveness of chitosan (CS) nanoparticles combined with β-glycerophosphate (β-GP), glycerol (Gly), and cannabidiol (CBD) as a novel and promising strategy for treating androgenetic alopecia. The addition of β-GP significantly reduced the particle size while preserving their spherical morphology. Furthermore, the incorporation of Gly and CBD not only increased uniformity and dispersion but also significantly reduced the average particle size, aligning with the optimal range considered optimal for penetration into hair follicles, as evidenced by previous studies.
FTIR analysis confirmed the electrostatic interactions between the components, highlighting glycerol’s dual role as a plasticizing and emulsifying agent, along with the encapsulation efficiency of CBD. Additionally, SEM analysis revealed that freeze-dried samples containing CBD exhibited a non-porous structure, further supporting its suitability for topical application. The nanoparticles (CS/β-GP and CS/β-GP/Gly-CBD) exhibited promising cytotoxicological results, indicating their compatibility with biological systems and suitability for therapeutic applications.
The formulated system offers a promising alternative, which is potentially more effective and safer, compared to conventional treatments for androgenetic alopecia. This research lays a strong groundwork for future studies assessing the therapeutic performance and risk profile of the proposed formulation, with the goal of enhancing patients’ quality of life.
Author Contributions
Conceptualization, J.R.O., M.C.S.B., S.M.L.S. and H.N.S.; methodology, J.R.O.; M.C.S.B., A.G.B.L. and S.M.L.S.; formal analysis, M.V.L.F., A.G.B.L. and S.M.L.S.; investigation, D.S.L., M.C.S.B. and H.N.S.; resources, M.V.L.F. and S.M.L.S.; data curation, J.R.O., D.S.L., M.C.S.B. and J.M.P.Q.D.; writing—original draft preparation, J.R.O., D.S.L., M.C.S.B. and A.G.B.L.; writing—review and editing, M.C.S.B., H.N.S., D.S.L., J.M.P.Q.D. and. A.G.B.L.; supervision, M.V.L.F. and S.M.L.S.; funding acquisition, M.V.L.F., S.M.L.S., J.M.P.Q.D. and A.G.B.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their gratitude to the National Council for Scientific and Technological Development (CNPq—Brazil) and the Coordination for the Improvement of Higher Education Personnel (CAPES—Brazil) for their financial support. J.M.P.Q. Delgado is, also, grateful to the Research Unit CONSTRUCT funded by national funds through the FCT/MCTES (PIDDAC) and FCT through the individual Scientific Employment Stimulus 2020.00828.CEECIND/CP1590/CT0004, with DOI: 10.54499/2020.00828.CEECIND/CP1590/CT0004.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
We extend our thanks to the Federal University of Campina Grande (UFCG, PB, Brazil) and the Northeast Biomaterials Evaluation and Development Laboratory (CERTBIO) for their invaluable support in the execution of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ntshingila, S.; Oputu, O.; Arowolo, A.T.; Khumalo, N.P. Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Kamalasanan, K. Controlled drug delivery for alopecia: A review. J. Control. Release 2020, 325, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, F.; Tao, N.; Wang, X.; Jin, X.; Zhang, C.; Xu, C. An androgenetic alopecia remedy based on marine collagen peptide-incorporated dissolving microneedles. Int. J. Pharm. 2024, 650, 123629. [Google Scholar] [CrossRef]
- Mishra, P.; Handa, M.; Ujjwal, R.R.; Singh, V.; Kesharwani, P.; Shukla, R. Potential of nanoparticulate based delivery systems for effective management of alopecia. Colloids Surf. B Biointerfaces 2021, 208, 112050. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Ye, R.; Liu, S.; Zhu, W.; Li, Y.; Huang, L.; Zhang, G.; Zhang, Y. Synthesis, Characterization, Properties, and Biomedical Application of Chitosan-Based Hydrogels. Polymers 2023, 15, 2482. [Google Scholar] [CrossRef]
- Keegan, G.M.; Smart, J.D.; Ingram, M.J.; Barnes, L.-M.; Burnett, G.R.; Rees, G.D. Chitosan microparticles for the controlled delivery of fluoride. J. Dent. 2012, 40, 229–240. [Google Scholar] [CrossRef]
- Sipoli, C.C.; Santana, N.; Shimojo, A.A.M.; Azzoni, A.R.; Torre, L.G.d.l. Scalable production of highly concentrated chitosan/TPP nanoparticles in different pHs and evaluation of the in vitro transfection efficiency. Biochem. Eng. J. 2015, 94, 65–73. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Zhang, A.; Luo, W.; Ahmed, S.; Huang, L.; et al. Preparation of chitosan nanoparticles by ionotropic gelation technique: Effects of formulation parameters and in vitro characterization. J. Mol. Struct. 2022, 1252, 132129. [Google Scholar] [CrossRef]
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660. [Google Scholar] [CrossRef]
- Szymaǹska, E.; Sosnowska, K.; Miltyk, W.; Rusak, M.; Basa, A.; Winnicka, K. The Effect of β-Glycerophosphate Crosslinking on Chitosan Cytotoxicity and Properties of Hydrogels for Vaginal Application. Polymers 2015, 7, 2223–2244. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef]
- Moura, M.J.; Faneca, H.; Lima, M.P.; Gil, M.H.; Figueiredo, M.M. In situ forming chitosan hydrogels prepared via ionic/covalent co-cross-linking. Biomacromolecules 2011, 12, 3275–3284. [Google Scholar] [CrossRef]
- Salustiano, R.L.C.; Bortoli, S. Canabidiol: Aspectos gerais e aplicações farmacológicas. Conjecturas 2022, 22, 1157–1179. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Assadpour, E.; Rezaei, A.; Das, S.S.; Krishna Rao, B.V.; Singh, S.K.; Kharazmi, M.S.; Jha, N.K.; Jha, S.K.; Prieto, M.A.; Jafari, S.M. Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals 2023, 16, 487. [Google Scholar] [CrossRef] [PubMed]
- Mirda, E.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Earlia, N.; Maulana, A.; Idroes, G.M.; Muslem, M.; Jalil, Z. Synthesis of Chitosan-Silver Nanoparticle Composite Spheres and Their Antimicrobial Activities. Polymers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Benamer, W.; Cellesi, F.; Tirelli, N. Chitosan/β-glycerophosphate-based microparticles manufactured by laminar jet break-up technology. J. Microencapsul. 2018, 35, 407–420. [Google Scholar] [CrossRef]
- Kaloti, M.; Bohidar, H.B. Kinetics of coacervation transition versus nanoparticle formation in chitosan-sodium tripolyphosphate solutions. Colloids Surf. B Biointerfaces 2010, 81, 165–173. [Google Scholar] [CrossRef]
- Smith, G.L.; Satino, J. Hair Regrowth with Cannabidiol (CBD)-rich Hemp Extract—A Case Series. Cannabis 2021, 4, 53–59. [Google Scholar] [CrossRef]
- Vanti, G.; Grifoni, L.; Bergonzi, M.C.; Antiga, E.; Montefusco, F.; Caproni, M.; Bilia, A.R. Development and optimisation of biopharmaceutical properties of a new microemulgel of cannabidiol for locally-acting dermatological delivery. Int. J. Pharm. 2021, 607, 121036. [Google Scholar] [CrossRef]
- Ferreira, B.P.; Costa, G.; Mascarenhas-Melo, F.; Pires, P.C.; Heidarizadeh, F.; Giram, P.S.; Mazzola, P.G.; Cabral, C.; Veiga, F.; Paiva-Santos, A.C. Skin applications of cannabidiol: Sources, effects, delivery systems, marketed formulations and safety. Phytochem. Rev. 2023, 22, 781–828. [Google Scholar] [CrossRef]
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765. [Google Scholar] [CrossRef]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Physical Gelation of Chitosan in the Presence of β-Glycerophosphate: The Effect of Temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.H.; Mahdavipour, K.; Rastegari, A.; Aghsami, M.; Montazeri, H.; Faghihi, H.; Mohammadi, Z. Chitosan and its amphiphilic derivative nanoparticles loaded with Minoxidil for induction of hair growth: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2024, 259, 129122. [Google Scholar] [CrossRef]
- Fei, T.; Wan, Z.; Wang, T. Dispersing insoluble yolk low-density lipoprotein (LDL) recovered by complexing with carboxymethylcellulose (CMC) for the nanoencapsulation of hemp cannabidiol (CBD) through emulsification at neutral pH. Food Hydrocoll. 2021, 116, 106656. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of cannabidiol in oil-in-water nanoemulsions and nanoemulsion-filled hydrogels: A structure and biological assessment study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Effect of glycerol on formation, stability, and properties of vitamin-E enriched nanoemulsions produced using spontaneous emulsification. J. Colloid Interface Sci. 2013, 411, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Planchette, C.; Mercuri, A.; Arcangeli, L.; Kriechbaum, M.; Laggner, P. Self-emulsification of Lipidic Drug Delivery System in Pure Water and in Concentrated Glycerol Solution. AAPS PharmSciTech 2017, 18, 3053–3063. [Google Scholar] [CrossRef]
- Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Monti, D. Nanostructured Drug Delivery Systems for Targeting 5-α-Reductase Inhibitors to the Hair Follicle. Pharmaceutics 2022, 14, 286. [Google Scholar] [CrossRef]
- Rohaeti, E.; Laksono, E.W.; Rakhmawati, A.J.A.J.P.K. Bacterial cellulose from rice waste water and its composite which are deposited nanoparticle as an antimicrobial material. ALCHEMY J. Penelit. Kim. 2016, 12, 70–87. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Smolarkiewicz-Wyczachowski, A.; Mylkie, K.; Wujak, M.; Mlynarczyk, D.T.; Nowak, P.; Bocian, S.; Goslinski, T.; Ziegler-Borowska, M. Chitosan-based films with cannabis oil as a base material for wound dressing application. Sci. Rep. 2022, 12, 18658. [Google Scholar] [CrossRef]
- Danish, M.; Mumtaz, M.W.; Fakhar, M.; Rashid, U. Response Surface Methodology Based Optimized Purification of the Residual Glycerol from Biodiesel Production Process. Chiang Mai J. Sci. 2017, 44, 1570–1582. [Google Scholar]
- Zhang, Z.L.; Li, L.J.; Sun, D.; Wang, M.; Shi, J.R.; Yang, D.; Wang, L.H.; Zou, S.C. Preparation and properties of chitosan-based microspheres by spray drying. Food Sci. Nutr. 2020, 8, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Mattu, C.; Li, R.; Ciardelli, G. Chitosan nanoparticles as therapeutic protein nanocarriers: The effect of ph on particle formation and encapsulation efficiency. Polym. Compos. 2013, 34, 1538–1545. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Monovalent Salt Enhances Colloidal Stability during the Formation of Chitosan/Tripolyphosphate Microgels. Langmuir ACS J. Surf. Colloids 2011, 27, 10392–10399. [Google Scholar] [CrossRef] [PubMed]
- Shagholani, H.; Ghoreishi, S.M.; Sharifi, S.H. Conversion of amine groups on chitosan-coated SPIONs into carbocyclic acid and investigation of its interaction with BSA in drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 45, 373–377. [Google Scholar] [CrossRef]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef]
- Angelo, T.; El-Sayed, N.; Jurisic, M.; Koenneke, A.; Gelfuso, G.M.; Cunha-Filho, M.; Taveira, S.F.; Lemor, R.; Schneider, M.; Gratieri, T. Effect of physical stimuli on hair follicle deposition of clobetasol-loaded Lipid Nanocarriers. Sci. Rep. 2020, 10, 176. [Google Scholar] [CrossRef]
- Leong, S.S.; Ng, W.M.; Lim, J.; Yeap, S.P. Dynamic Light Scattering: Effective Sizing Technique for Characterization of Magnetic Nanoparticles. In Handbook of Materials Characterization; Sharma, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–111. [Google Scholar]
- Amin, M.K.; Boateng, J.S. Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery. Mar. Drugs 2022, 20, 156. [Google Scholar] [CrossRef]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of [(14)C]-doxorubicin. Nanotechnol. Sci. Appl. 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef]
- Sharma, N.; Purkait, M.K. Impact of synthesized amino alcohol plasticizer on the morphology and hydrophilicity of polysulfone ultrafiltration membrane. J. Membr. Sci. 2017, 522, 202–215. [Google Scholar] [CrossRef]
- Corveleyn, S.; Remon, J.P. Formulation of a lyophilized dry emulsion tablet for the delivery of poorly soluble drugs. Int. J. Pharm. 1998, 166, 65–74. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Fur Pharm. Verfahrenstechnik eV 2004, 57, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol-Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Ciro, Y.; Rojas, J.; Alhajj, M.J.; Carabali, G.A.; Salamanca, C.H. Production and Characterization of Chitosan-Polyanion Nanoparticles by Polyelectrolyte Complexation Assisted by High-Intensity Sonication for the Modified Release of Methotrexate. Pharmaceuticals 2020, 13, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).