Evaluation of Semi-Continuous Anaerobic Fermentation of Alperujo by Methanogenesis Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Anaerobic Inoculum Characterization
2.2. Anaerobic Fermentation Experimental Procedure
2.3. Chemical Analysis
2.3.1. VFA Quantification by Gas Chromatograph Analysis
2.3.2. Total and Soluble Phenolic Compounds
2.3.3. Biogas Quantification and Composition
2.4. Soluble Organic Matter Yield and Acidogenesis Ratio
2.5. Statistical Analysis
3. Results and Discussion
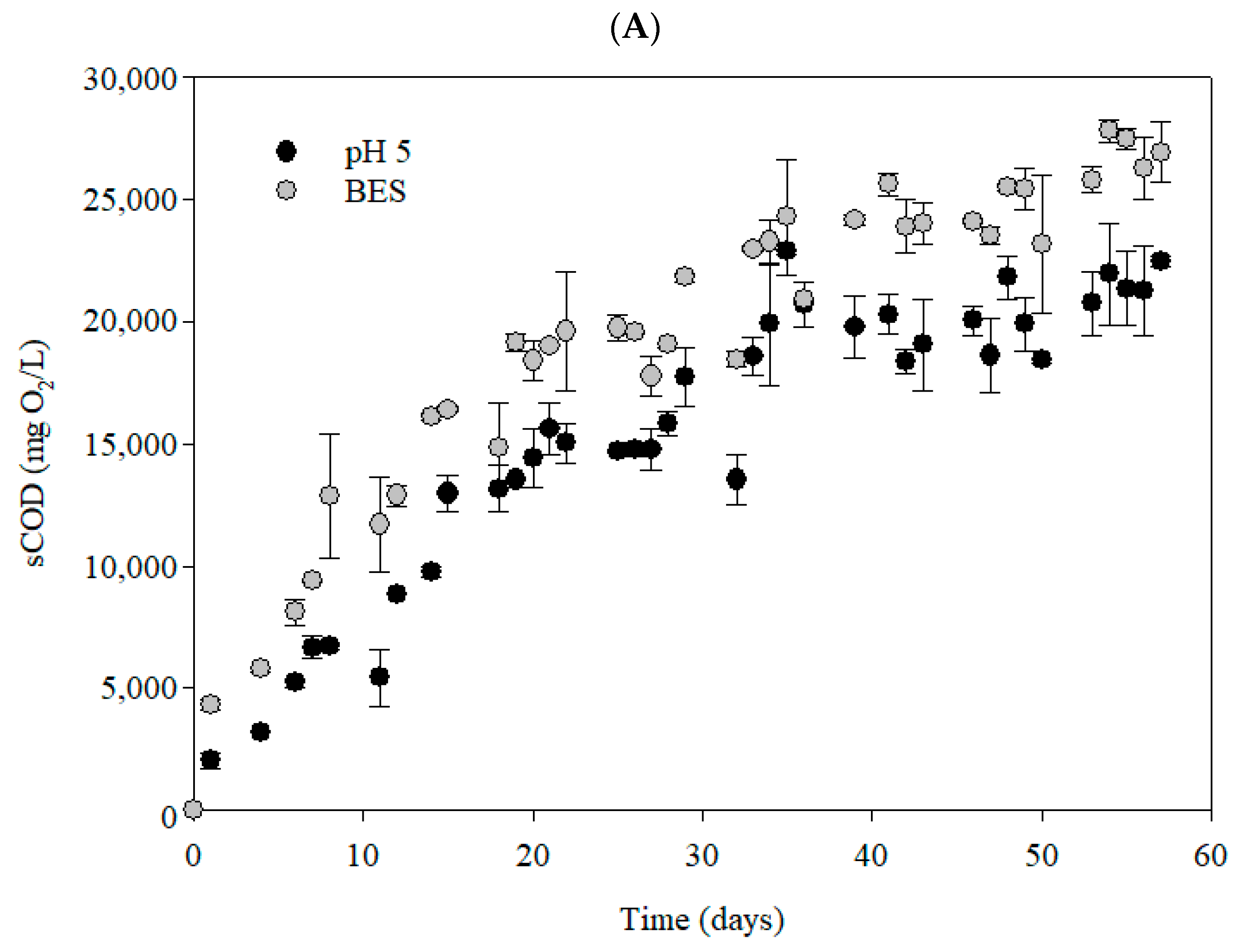
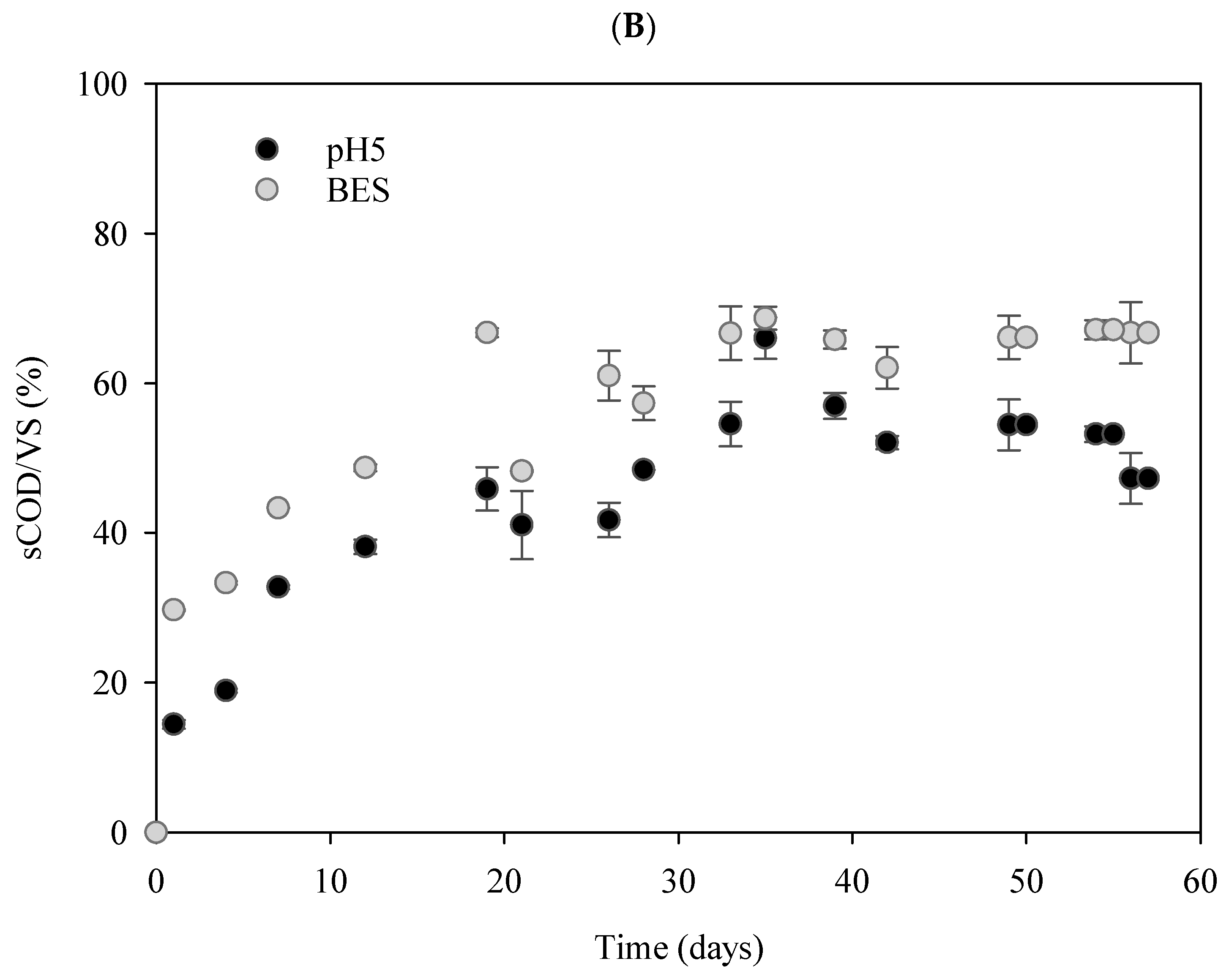
3.1. Soluble Compounds Accumulation Under Anaerobic Fermentation
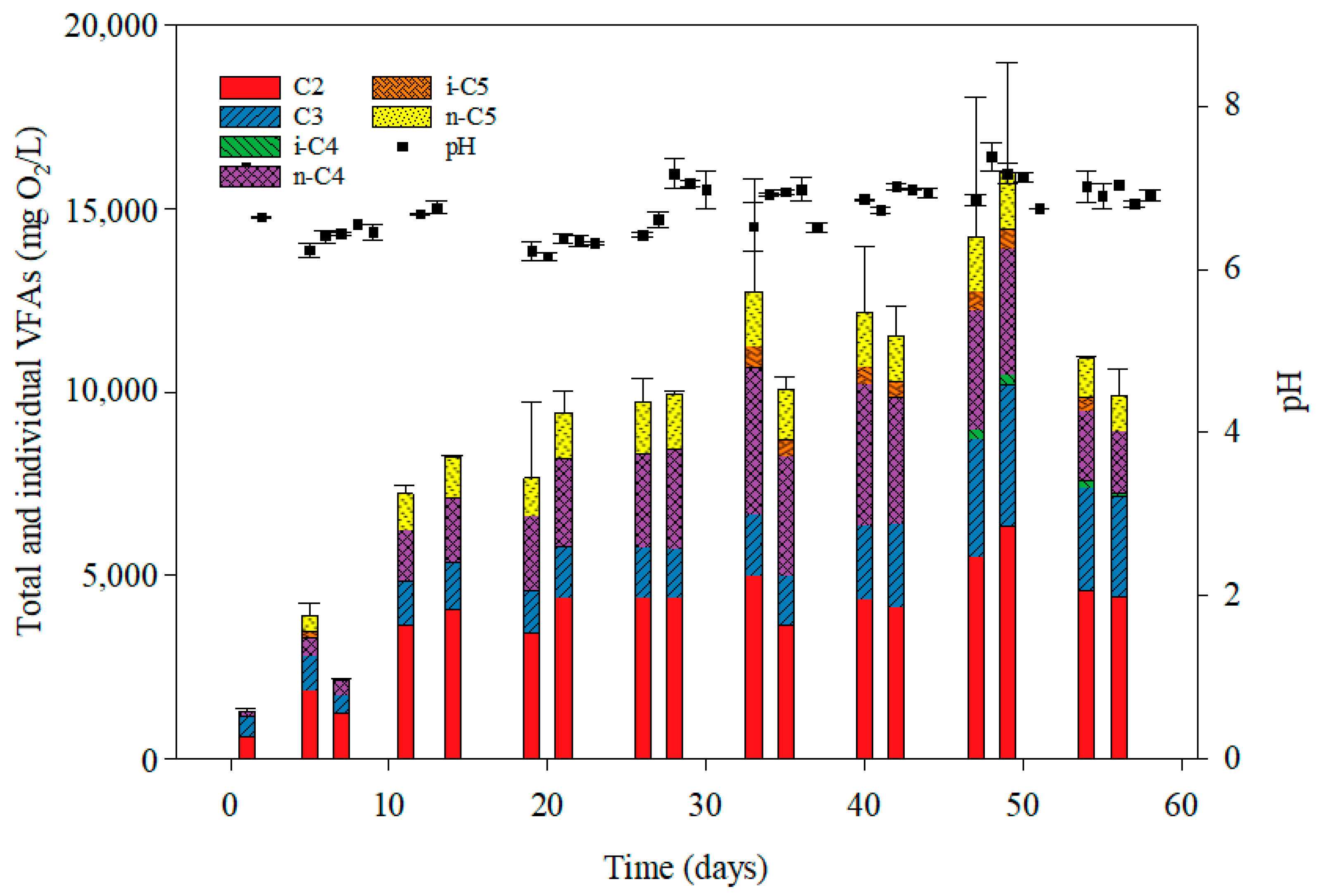
3.2. Volatile Fatty Acid Accumulation Under Anaerobic Fermentation
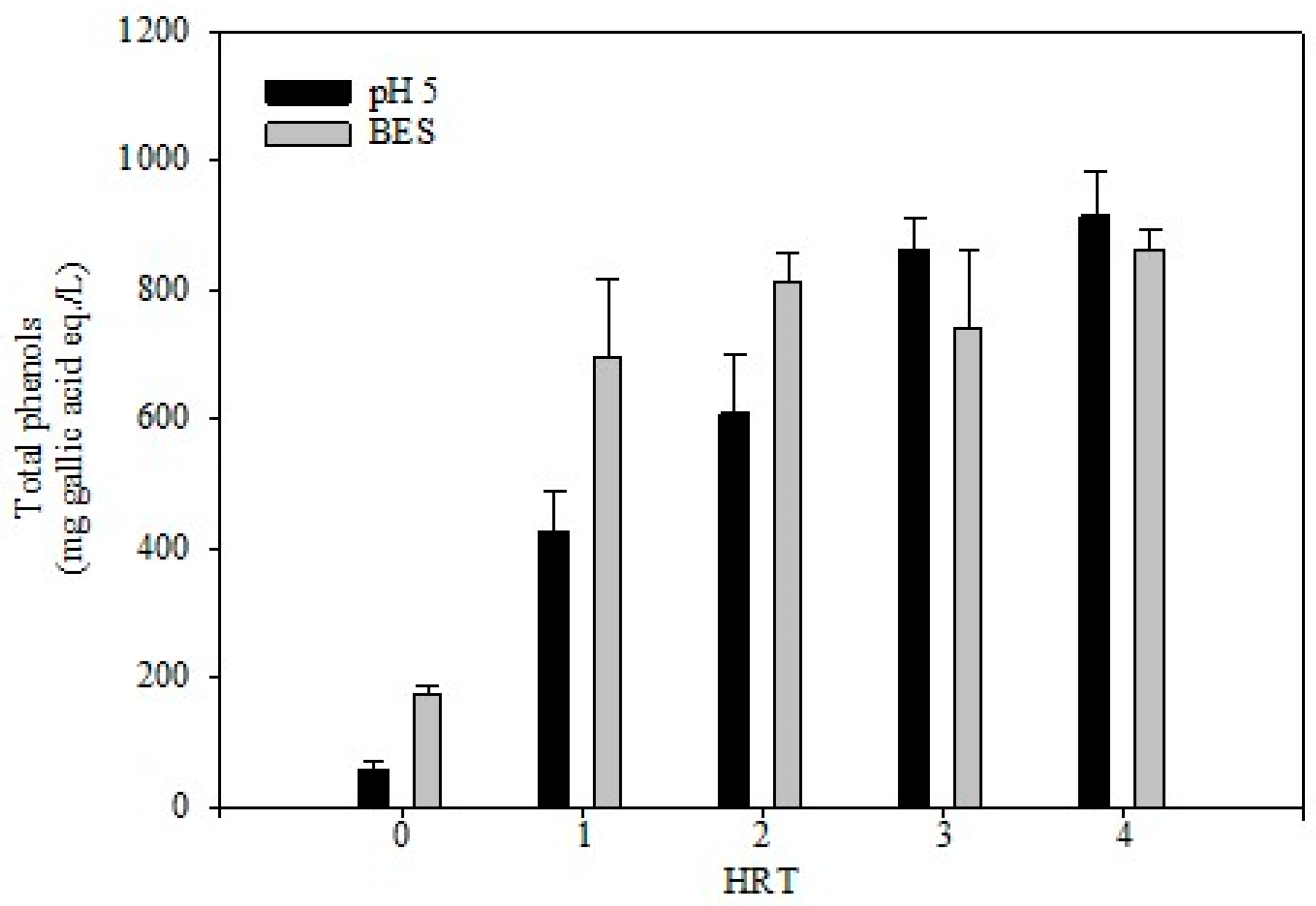
3.3. Phenolic Compounds Accumulation Under Anaerobic Fermentation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Statista. Olive Oil Production in Selected Countries Worldwide from 2020/2021 in 2023/2024. 2024. Available online: https://es.statista.com/estadisticas/1292021/produccion-de-aceite-de-oliva-en-paises-seleccionados/ (accessed on 21 January 2025).
- Fernández-Rodríguez, M.J.; de la Lama-Calvente, D.; Jiménez-Rodríguez, A.; Pino-Mejías, R.; Borja, R.; Rincón, B. Impact of soft hydrothermal pre-treatments on the olive mill solid waste characteristics and its subsequent anaerobic digestion. Biomass Convers. Biorefin. 2022, 12, 2107–2120. [Google Scholar] [CrossRef]
- Messineo, A.; Maniscalco, M.P.; Volpe, R. Biomethane recovery from olive mill residues through anaerobic digestion: A review of the state of the art technology. Sci. Total Environ. 2020, 703, 135508. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Villa-Gomez, D.; Fermoso, F.G.; Alonso-Fariñas, B.J.B. Bioproducts; Biorefining, Is anaerobic digestion a feasible alternative to the combustion of olive mill solid waste in terms of energy production? A critical review. Biofuels Bioprod. Biorefin. 2021, 15, 150–162. [Google Scholar] [CrossRef]
- European Biogas Association. Biogas Cost Reductions to Boost Sustainable Transport. 2025. Available online: https://www.europeanbiogas.eu/irena-publication-biogas-cost-reductions-boost-sustainable-transport/ (accessed on 29 January 2025).
- Annamalai, N.; Elayaraja, S.; Oleskowicz-Popiel, P.; Sivakumar, N.; Bahry, S.A. Volatile fatty acids production during anaerobic digestion of lignocellulosic biomass. In Recent Developments in Bioenergy Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 237–251. [Google Scholar]
- Chen, C.; Zhang, X.; Liu, C.; Wu, Y.; Zheng, G.; Chen, Y. Advances in downstream processes and applications of biological carboxylic acids derived from organic wastes. Bioresour. Technol. 2022, 346, 126609. [Google Scholar] [CrossRef]
- Caroca, E.; Serrano, A.; Borja, R.; Jiménez, A.; Carvajal, A.; Braga, A.F.M.; Rodriguez-Gutierrez, G.; Fermoso, F.G. Influence of phenols and furans released during thermal pretreatment of olive mill solid waste on its anaerobic digestion. Waste Manag. 2021, 120, 202–208. [Google Scholar] [CrossRef]
- Jiménez-Páez, E.; Serrano, A.; Purswani, J.; Correa-Galeote, D.; Cubero-Cardoso, J.; Fermoso, F.G. Impact on the microbial population during biological volatile fatty acid production from olive mill solid waste. Environ. Technol. Innov. 2023, 32, 103409. [Google Scholar] [CrossRef]
- Qiu, Y.; Gu, X.; Johnson, Z.; Bohutskyi, P.; Chen, S. Improving dairy manure hydrolysis and acidification through microbial community restructuring by adaptation to hyperthermophilic conditions. J. Clean. Prod. 2024, 473, 143487. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Plug-flow hydrolysis with lignocellulosic residues: Effect of hydraulic retention time and thin-sludge recirculation. Biotechnol. Biofuels Bioprod. 2023, 16, 111. [Google Scholar] [CrossRef]
- Cabrera, F.; Serrano, A.; Torres, Á.; Rodriguez-Gutierrez, G.; Jeison, D.; Fermoso, F.G. The accumulation of volatile fatty acids and phenols through a pH-controlled fermentation of olive mill solid waste. Sci. Total Environ. 2019, 657, 1501–1507. [Google Scholar] [CrossRef]
- Cubero-Cardoso, J.; Llamas, M.; Trujillo-Reyes, Á.; Fernández-Prior, Á.; Rodríguez-Gutiérrez, G. Assessing the potential of olive mill solid waste as feedstock for methane and volatile fatty acids production via anaerobic bioprocesses. New Biotechnol. 2024, 84, 77–84. [Google Scholar] [CrossRef]
- da Fonseca, Y.A.; de Camargos, A.B.; Gomes, G.S.M.; Fregulia, P.; Silva, S.Q.; Gurgel, L.V.A.; Baêta, B.E.L. Enhancing Biobased Volatile Fatty Acids Production from Olive Mill Solid Waste by Optimization of pH and Substrate to Inoculum Ratio. Processes 2023, 11, 338. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. 2021, 763, 143007. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Strazzera, G.; Valentino, F.; Gottardo, M.; Villano, M.; Matos, M.; Silva, F.; Reis, M.A.M.; Mata-Alvarez, J.; Astals, S.; et al. New insights in food waste, sewage sludge and green waste anaerobic fermentation for short-chain volatile fatty acids production: A review. J. Environ. Chem. Eng. 2022, 10, 108319. [Google Scholar] [CrossRef]
- Baird, R.B.; Rice, E.W.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewaters; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA, 2017. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Cardoso, J.C.; Bermúdez-Oria, A.; Reyes, Á.T.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G.J.A. Application of a Cold-Pressing Treatment to Improve Virgin Olive Oil Production and the Antioxidant Phenolic Profile of Its by-Products. Antioxidants 2023, 12, 1162. [Google Scholar] [CrossRef]
- Araujo, M.N.; Vargas, S.R.; Soares, L.A.; Trindade, L.F.; Fuess, L.T.; Adorno, M.A.T. Rapid method for determination of biogas composition by gas chromatography coupled to a thermal conductivity detector (GC-TCD). Int. J. Environ. Anal. Chem. 2024, 104, 8690–8707. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Mohan, S.V. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Nair, V.V.; Dhar, H.; Kumar, S.; Thalla, A.K.; Mukherjee, S.; Wong, J.W.C. Artificial neural network based modeling to evaluate methane yield from biogas in a laboratory-scale anaerobic bioreactor. Bioresour. Technol. 2016, 217, 90–99. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Technol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Goren, A.Y.; Dincer, I.; Khalvati, A. A comparative evaluation of dark fermentative bioreactor configurations for enhanced hydrogen production. Environ. Sci. Pollut. Res. 2025, 32, 2182–2209. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Kelbert, M.; Machado, T.O.; Araújo, P.H.H.; Sayer, C.; de Oliveira, D.; Maziero, P.; Simons, K.E.; Carciofi, B.A.M. Perspectives on biotechnological production of butyric acid from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2024, 202, 114717. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.-C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Wu, H.; Weimer, P.J.; Dalke, R.; Granda, C.B.; Mai, J.; Urgun-Demirtas, M. Microbial communities for valorizing biomass using the carboxylate platform to produce volatile fatty acids: A review. Bioresour. Technol. 2022, 344, 126253. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Andreolli, M.; Menini, M.; Bolzonella, D.; Lampis, S. Influence of different household Food Wastes Fractions on Volatile Fatty Acids production by anaerobic fermentation. Bioresour. Technol. 2021, 335, 125289. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Llamas, M.; Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Carboxylic acids production via anaerobic fermentation: Microbial communities’ responses to stepwise and direct hydraulic retention time decrease. Bioresour. Technol. 2022, 344, 126282. [Google Scholar] [CrossRef] [PubMed]
- Rahimieh, A.; Nosrati, M. A review on biochemistry, microbiology and thermodynamic aspects of propionate: The key intermediate in the anaerobic digestion and wastewater treatment. Desalin. Water Treat. 2024, 317, 100191. [Google Scholar] [CrossRef]
- Lenzuni, M.; Converti, A.; Casazza, A.A. From laboratory- to industrial-scale plants: Future of anaerobic digestion of olive mill solid wastes. Bioresour. Technol. 2024, 394, 130317. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the tolerance to polyphenols of the anaerobic digestion of olive wastewater through microbial adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Najla, T.; Habibi, M.; Ammar, M.H.; Abazza, L.; Mhamdi, R. Olive Fruit by-Products: From Waste Streams into a Promising Source of Value-Added Products. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Ramadan, M.F., Farag, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar]
- Caballero-Guerrero, B.; Garrido-Fernández, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernández-Prior, M.Á.; Reinhard, C.; Nyström, L.; Benítez-Cabello, A.; Arroyo-López, F.N. Antimicrobial effects of treated olive mill waste on foodborne pathogens. LWT 2022, 164, 113628. [Google Scholar] [CrossRef]
- Guo, Y.; Weber, W.J.; Yao, D.; Caixeta, L.; Zimmerman, N.P.; Thompson, J.; Block, E.; Rehberger, T.G.; Crooker, B.A.; Chen, C. Forming 4-Methylcatechol as the Dominant Bioavailable Metabolite of Intraruminal Rutin Inhibits p-Cresol Production in Dairy Cows. Metabolites 2022, 12, 16. [Google Scholar] [CrossRef]
- Soares, T.F.; Alves, R.C.; Oliveira, M.B.P.P. From Olive Oil Production to By-Products: Emergent Technologies to Extract Bioactive Compounds. Food Rev. Int. 2024, 40, 3342–3369. [Google Scholar] [CrossRef]

| Time (Days) | HRT Repetitions | CH4 (%) | CO2 (%) | H2 (%) | mL Biogas/gVS | |
|---|---|---|---|---|---|---|
| pH 5 | 0–13 | 1-HRT | 51.35 ± 4.77 | 48.65 ± 4.77 | n.d. | 15.48 ± 17.29 |
| 14–28 | 2-HRT | 11.93 ±5.89 | 87.12 ± 4.54 | 1.00 ± 1.00 | 15.79 ± 6.80 | |
| 29–42 | 3-HRT | 27.37 ± 7.44 | 72.63 ± 7.44 | n.d. | 9.28 ± 10.12 | |
| 43–57 | 4-HRT | 32.79 ± 15.01 | 67.21 ± 5.01 | n.d. | 2.88 ± 2.56 | |
| BES | 0–13 | 1-HRT | 8.69 ± 2.78 | 91.31 ± 2.78 | n.d. | 19.69 ± 15.84 |
| 14–28 | 2-HRT | 22.10 ± 9.10 | 77.89 ± 9.09 | n.d. | 25.44 ± 22.38 | |
| 29–42 | 3-HRT | 29.59 ± 0.88 | 70.40 ± 0.88 | n.d. | 34.19 ± 14.92 | |
| 43–57 | 4-HRT | 13.34 ± 1.73 | 86.66 ± 1.73 | n.d. | 23.47 ± 1.07 |
| pH 5 | BES | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (Days) | 1-HRT | 2-HRT | 3-HRT | 4-HRT | 1-HRT | 2-HRT | 3-HRT | 4-HRT |
| 0–13 Days | 14–28 Days | 29–42 Days | 43–57 Days | 0–13 Days | 14–28 Days | 29–42 Days | 43–57 Days | |
| 3,4-dihydroxyphenylglycol (mg/L) | 7.46 ± 1.33 | 7.53 ± 1.87 | 83.46 ± 10.34 | 68.04 ± 21.69 | 4.57 ± 1.09 | 17.50 ± 11.41 | 22.33 ± 1.44 | 168.78 ± 27.40 |
| Hydroxytyrosol (mg/L) | 56.37 ± 23.37 | 46.70 ± 24.14 | 72.47 ± 11.49 | 116.25 ± 11.50 | n.d. | n.d. | 30.39 ± 1.10 | 25.80 ± 1.77 |
| Tyrosol (mg/L) | 15.37 ± 7.19 | 37.57 ± 6.42 | 56.80 ± 16.11 | 38.68 ± 27.56 | n.d. | n.d. | 17.83 ± 1.00 | 30.92 ± 27.40 |
| Protocatechuic acid (mg/L) | 11.53 ± 9.46 | 48.88 ± 17.39 | 54.92 ± 50.24 | 25.81 ± 18.78 | 8.67 ± 1.00 | 18.83 ± 7.04 | 40.93 ± 7.28 | 52.66 ± 2.10 |
| 3,4-dihydroxycinnamic acid (mg/L) | 4.80 ± 0.14 | 20.32 ± 20.26 | 38.51 ± 37.15 | 54.30 ± 9.03 | n.d. | 5.59 ± 0.60 | 7.34 ± 0.48 | 6.87 ± 0.64 |
| 4-Metylcatehol (mg/L) | 8.71 ± 1.00 | 7.93 ± 0.69 | 9.03 ± 1.00 | 7.02 ± 1.11 | 80.82 ± 7.34 | 108.64 ± 18.39 | 56.44 ± 27.45 | 32.68 ± 17.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Páez, E.; Serrano, A.; Hueso, R.; Fermoso, F.G.; Cubero-Cardoso, J. Evaluation of Semi-Continuous Anaerobic Fermentation of Alperujo by Methanogenesis Inhibition. Processes 2025, 13, 600. https://doi.org/10.3390/pr13030600
Jiménez-Páez E, Serrano A, Hueso R, Fermoso FG, Cubero-Cardoso J. Evaluation of Semi-Continuous Anaerobic Fermentation of Alperujo by Methanogenesis Inhibition. Processes. 2025; 13(3):600. https://doi.org/10.3390/pr13030600
Chicago/Turabian StyleJiménez-Páez, Elena, Antonio Serrano, Rafael Hueso, Fernando G. Fermoso, and Juan Cubero-Cardoso. 2025. "Evaluation of Semi-Continuous Anaerobic Fermentation of Alperujo by Methanogenesis Inhibition" Processes 13, no. 3: 600. https://doi.org/10.3390/pr13030600
APA StyleJiménez-Páez, E., Serrano, A., Hueso, R., Fermoso, F. G., & Cubero-Cardoso, J. (2025). Evaluation of Semi-Continuous Anaerobic Fermentation of Alperujo by Methanogenesis Inhibition. Processes, 13(3), 600. https://doi.org/10.3390/pr13030600










