Extraction of Carbon Nanodots from Benzoin Resin Soot for Multifaceted Antibacterial Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Turmeric Soot
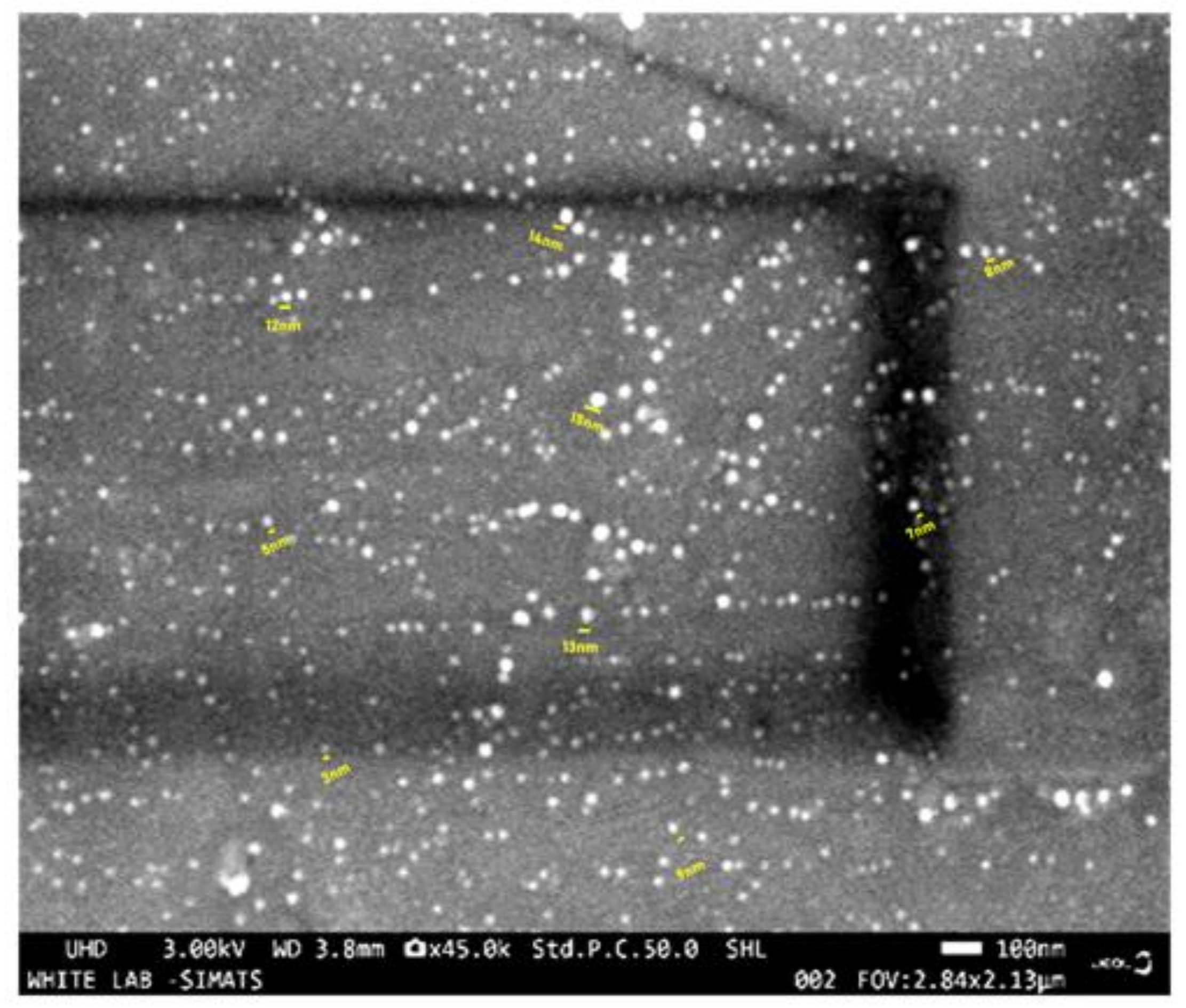
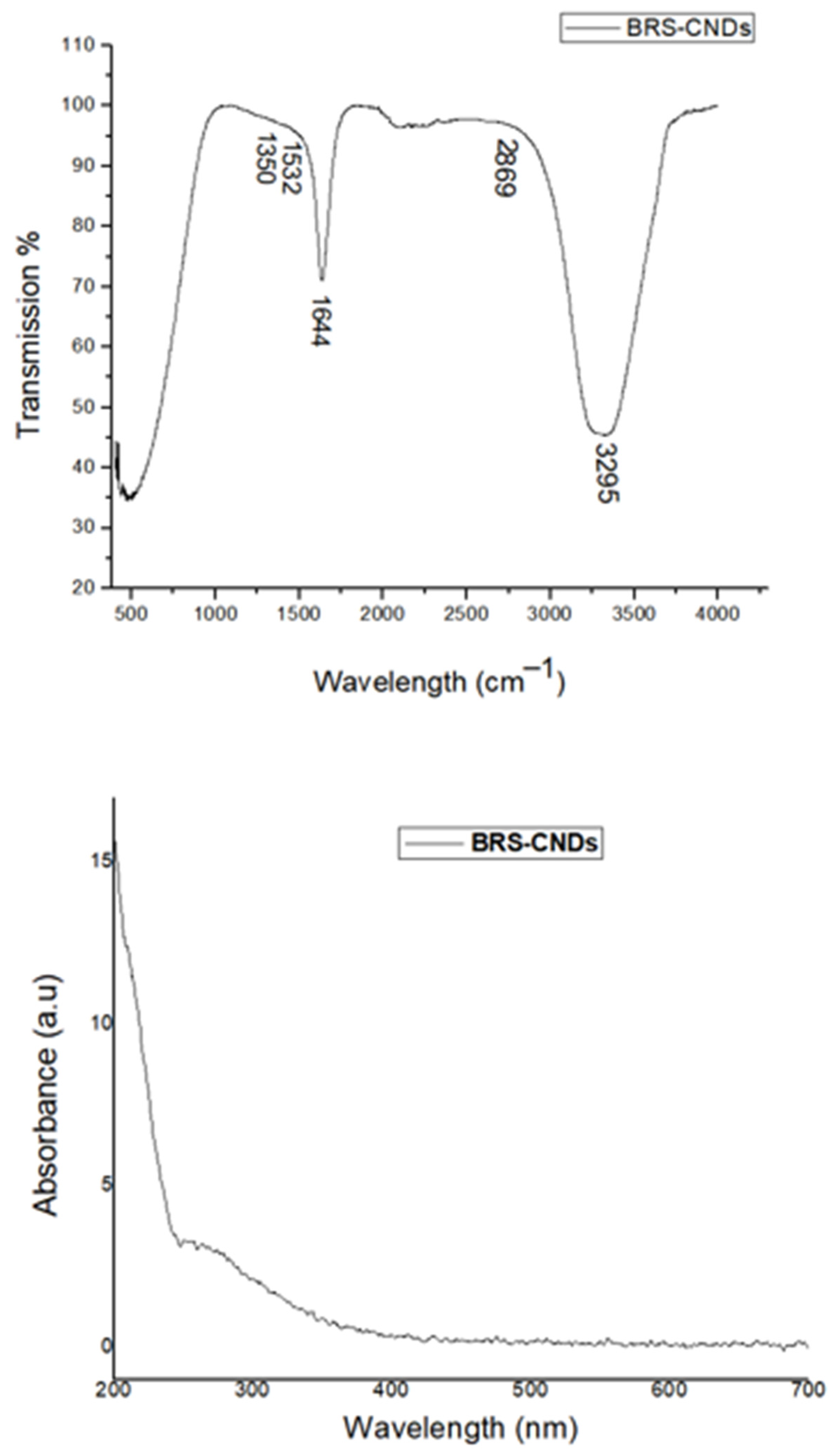
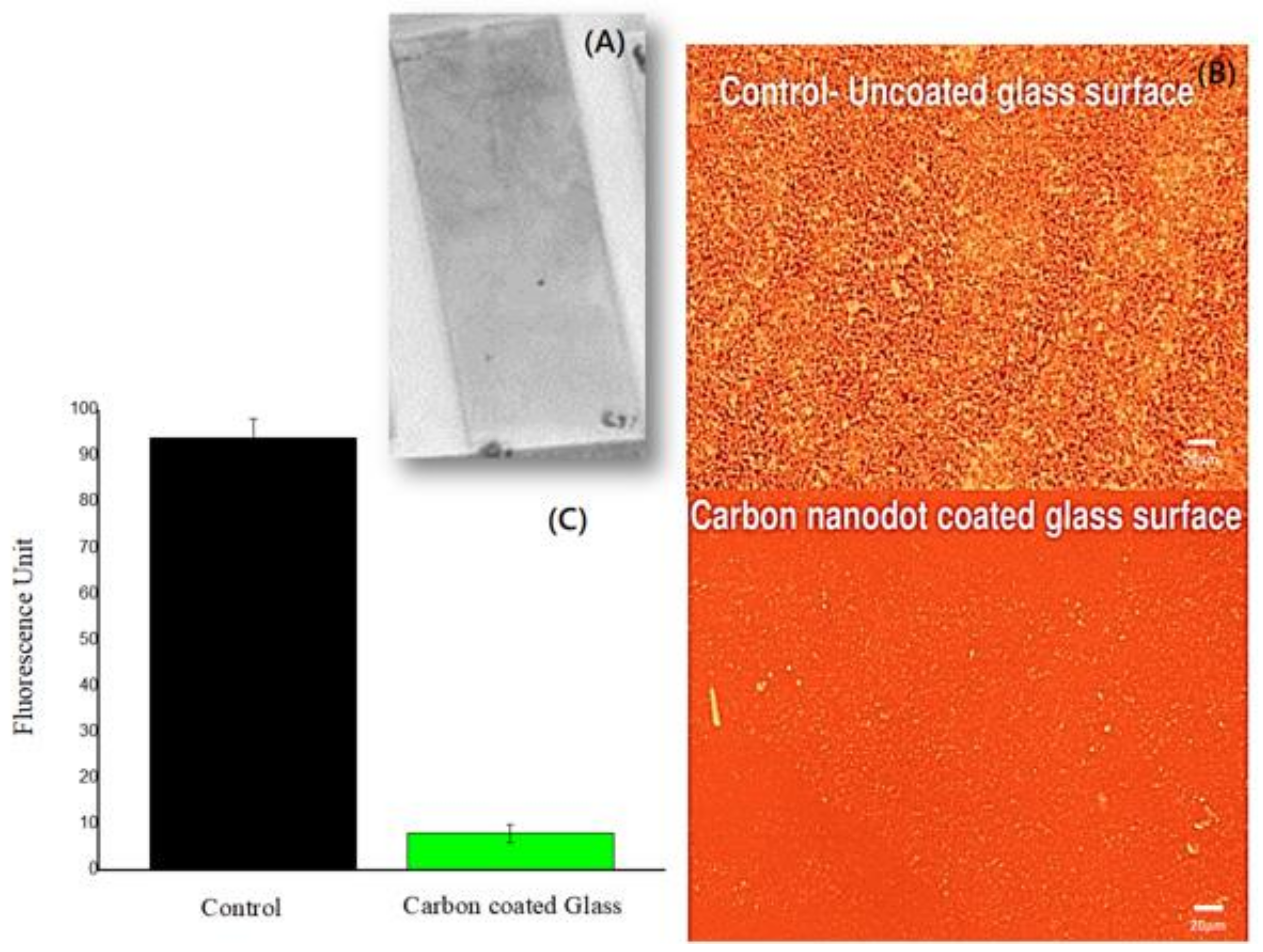
2.2. Characterization of the BRS-CNDs
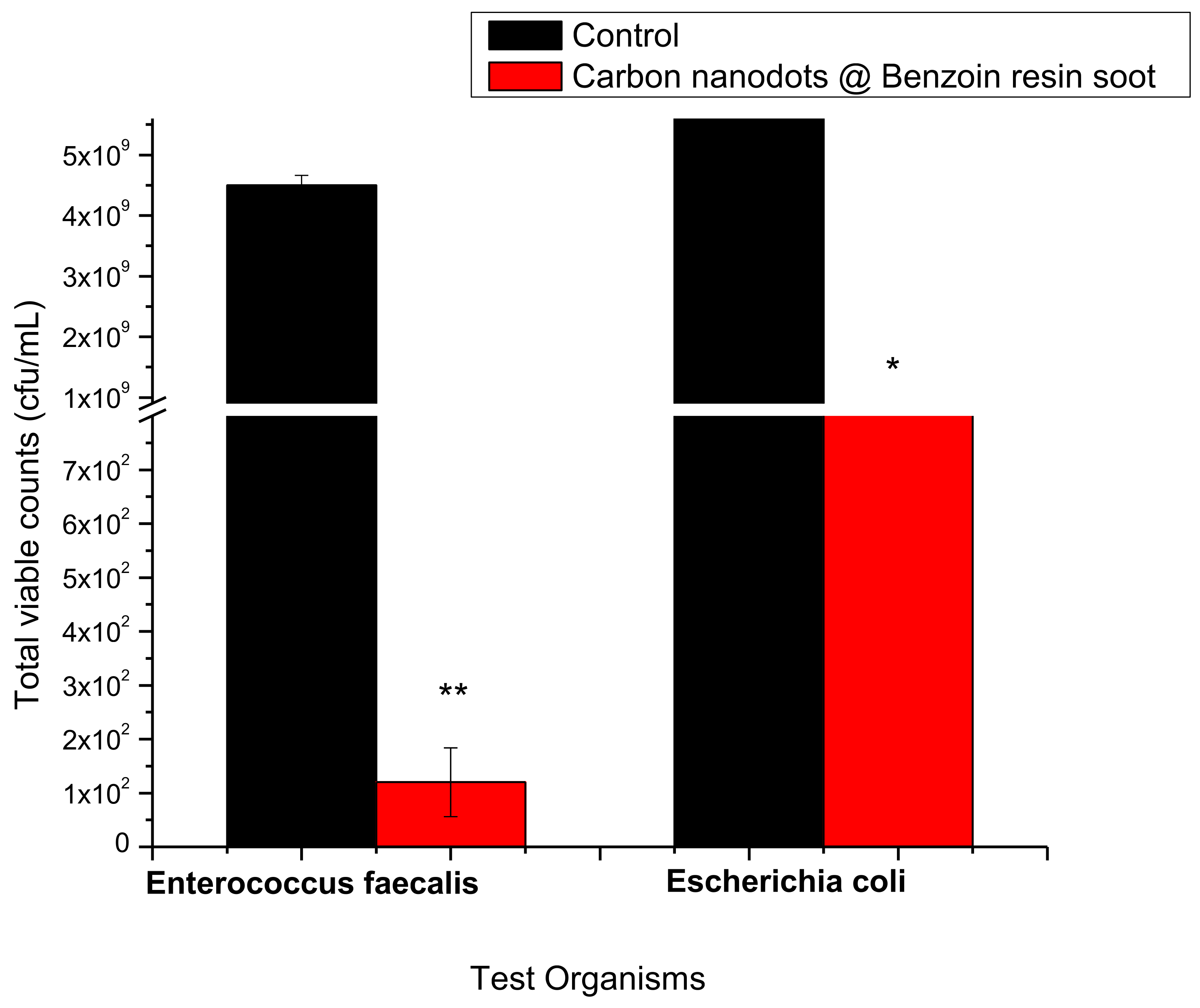
2.3. Anti-Coliform Assay
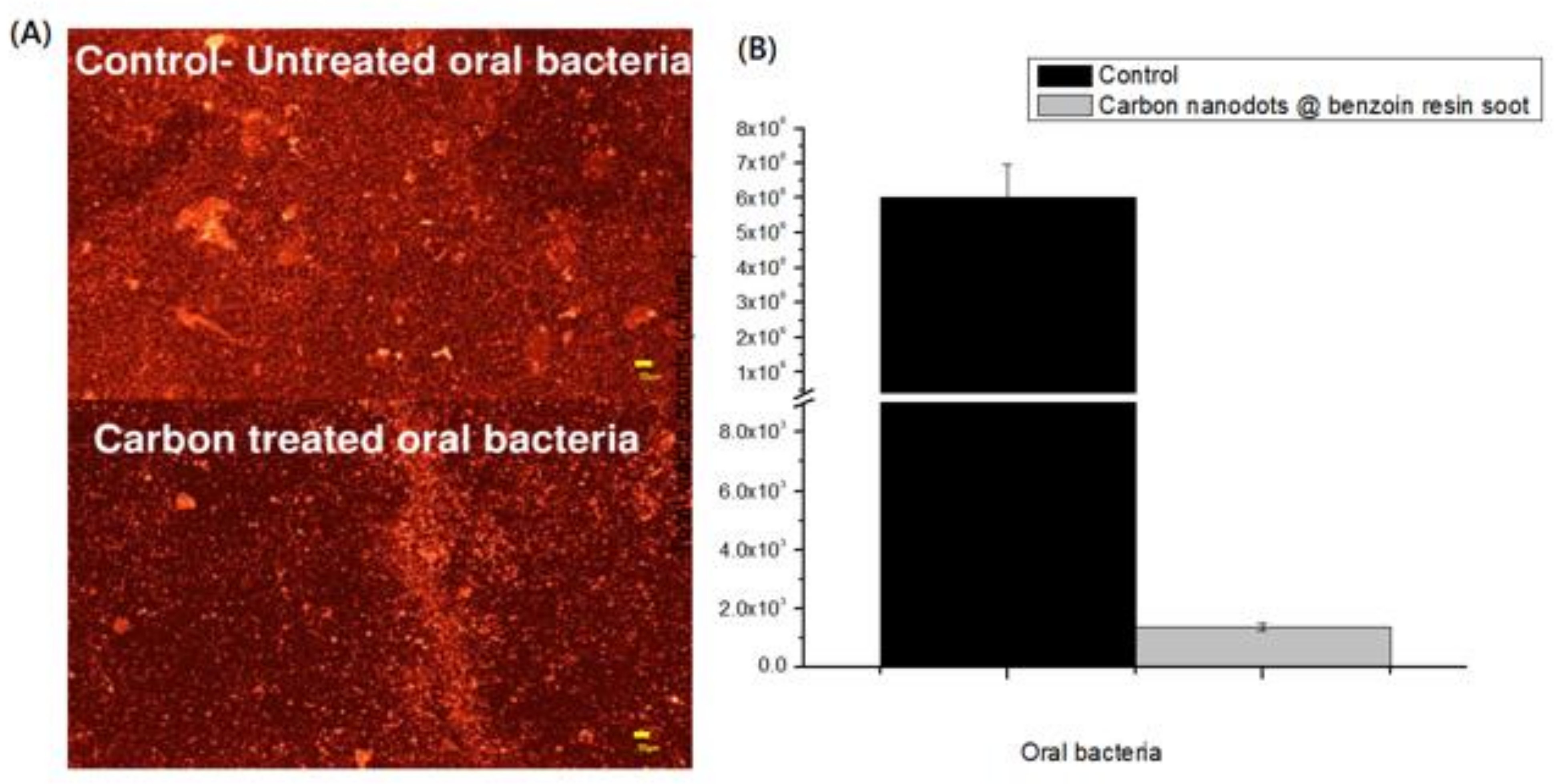
2.4. Anti-Oral Bacterial Assay
2.5. Anti-Biofilm Assay
3. Results
Characterization of Turmeric BRS-CNDs
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Institutional Reviewer Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, Q.L.; Li, B.G.; Qi, H.Y.; Gao, X.P.; Zhang, G.L. Four New Benzofurans from Seeds of Styrax Perkinsiae. Planta Med. 2005, 71, 847–851. [Google Scholar] [CrossRef]
- Coppen, J.J. Benzoin: Production, uses and international trade. Perfum. Flavorist 1999, 24, 11–24. [Google Scholar]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; American Society for Microbiology: Washington, DC, USA, 2018; pp. 521–547. [Google Scholar]
- World Health Organization. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline, Including Tuberculosis; No. WHO/EMP/IAU/2017.11; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Antibacterial Agents in Preclinical Development: An Open Access Database; No. WHO/EMP/IAU/2019.12; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Thorn, J.P.R. Royal Botanic Gardens Kew: State of the World’s Plants; Royal Botanic Gardens Kew: Richmond, UK, 2016. [Google Scholar]
- Vandebroek, I. Intercultural Health and Ethnobotany: How to Improve Healthcare for Underserved and Minority Communities? J. Ethnopharmacol. 2013, 148, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Mohagheghzadeh, A.; Faridi, P.; Shams Ardakani, M.; Ghasemi, Y. Medicinal Smokes. J. Ethnopharmacol. 2006, 108, 161–184. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Chauhan, P.S.; Nene, Y.L. Medicinal Smoke Reduces Airborne Bacteria. J. Ethnopharmacol. 2007, 114, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Klimek, H.I.; Moczulska, H.; Sieroszewski, P. Streptococcus Mutans in the Oral Cavity as a Risk Factor for Threatened Miscarriage. Ginekol. Pol. 2024, 95, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, R.; Zhang, M.; Huang, R.; Fan, C.; Zhou, S.; Qiu, J.; He, J. In Vitro Antibacterial Activity of a Novel Acid-Activated Antimicrobial Peptide against Streptococcus Mutans. Curr. Protein Pept. Sci. 2023, 25, 83–93. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, B.; Prato, M. Carbon Nanodots: Nanolights Illuminating a Bright Future. Small 2023, 19, e2304703. [Google Scholar] [CrossRef]
- Da Ros, T.; Martín, N.; Nierengarten, J.F. (Eds.) Carbon Nanostructures for Biomedical Applications; Royal Society of Chemistry: Cambridge, UK, 2021. [Google Scholar]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Valcárcel, M. Photoluminescent Carbon Dot Sensor for Carboxylated Multiwalled Carbon Nanotube Detection in River Water. Sens. Actuators B Chem. 2014, 207, 596–601. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C. A Low-Temperature Solid-Phase Method to Synthesize Highly Fluorescent Carbon Nitride Dots with Tunable Emission. Chem. Commun. 2013, 49, 8605–8607. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Song, X.; Zhao, T.; Yao, Q.; Wang, Y.; Chen, X. Synthesis of Highly Fluorescent P,O-g-C3N4 Nanodots for the Label-Free Detection of Cu2+ and Acetylcholinesterase Activity. J. Mater. Chem. C 2015, 3, 10916–10924. [Google Scholar] [CrossRef]
- Sciortino, A.; Mauro, N.; Buscarino, G.; Sciortino, L.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Cannas, M.; Messina, F. β-C3N4 Nanocrystals: Carbon Dots with Extraordinary Morphological, Structural, and Optical Homogeneity. Chem. Mater. 2018, 30, 1695–1700. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal Treatment of Hair for the Synthesis of Sustainable Carbon Quantum Dots and the Applications for Sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef]
- Gopal, J.; George, R.; Muraleedharan, P.; Khatak, H. Photocatalytic Inhibition of Microbial Adhesion by Anodized Titanium. Biofouling 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Chun, S. Autochthonous Self-Assembly of Nature’s Nanomaterials: Green, Parsimonious and Antibacterial Carbon Nanofilms on Glass. Phys. Chem. Chem. Phys. 2016, 18, 18670–18677. [Google Scholar] [CrossRef]
- Bipin, R.; Ujjwal, M. A review on characterization of carbon quantum dots. Vietnam. J. Chem. 2023, 61, 693–718. [Google Scholar]
- Chun, S.; Muthu, M.; Gansukh, E.; Thalappil, P.; Gopal, J. The Ethanopharmacological Aspect of Carbon Nanodots in Turmeric Smoke. Sci. Rep. 2016, 6, 35586. [Google Scholar] [CrossRef] [PubMed]
- Pastorova, C.; De Koster, J.B. Analytical study of free and ester bound benzoic and cinnamic acids of gum benzoin resins by GC–MS and HPLC–frit FAB–MS. Phytochem. Anal. 1997, 8, 63–73. [Google Scholar] [CrossRef]
- Atia Sharif, H.N.; Rehman, R.; Mushtaq, A.; Rashid, U.A. Review on bioactive potential of Benzoin Resin. Int. J. Chem. Biochem. Sci. 2016, 10, 106–110. [Google Scholar]
- Selvaraju, N.; Ganesh, P.S.; Palrasu, V.; Venugopal, G.; Mariappan, V. Evaluation of Antimicrobial and Antibiofilm Activity of Citrus medica Fruit Juice Based Carbon Dots against Pseudomonas aeruginosa. ACS Omega 2022, 7, 36227–36234. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Manikandan, V.; Vijayalakshmi, P.; Devanesan, S.; Salah, M.B.; Ramesh Babu, A.C.; Priyadharsan, A.; Oh, T.H.; Ragupathy, S. Synthesis and investigation on synergetic effect of activated carbon loaded silver nanoparticles with enhanced photocatalytic and antibacterial activities. Environ. Res. 2023, 233, 116431. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangavel, P.; Hasan, N.; Raja, G.; Jawhari, A.H.; Gopal, J. Extraction of Carbon Nanodots from Benzoin Resin Soot for Multifaceted Antibacterial Applications. Processes 2025, 13, 484. https://doi.org/10.3390/pr13020484
Thangavel P, Hasan N, Raja G, Jawhari AH, Gopal J. Extraction of Carbon Nanodots from Benzoin Resin Soot for Multifaceted Antibacterial Applications. Processes. 2025; 13(2):484. https://doi.org/10.3390/pr13020484
Chicago/Turabian StyleThangavel, Pranesh, Nazim Hasan, Gnanadeepam Raja, Ahmed Hussain Jawhari, and Judy Gopal. 2025. "Extraction of Carbon Nanodots from Benzoin Resin Soot for Multifaceted Antibacterial Applications" Processes 13, no. 2: 484. https://doi.org/10.3390/pr13020484
APA StyleThangavel, P., Hasan, N., Raja, G., Jawhari, A. H., & Gopal, J. (2025). Extraction of Carbon Nanodots from Benzoin Resin Soot for Multifaceted Antibacterial Applications. Processes, 13(2), 484. https://doi.org/10.3390/pr13020484









