Abstract
Current studies have paid extensive attention to the occurrence of brominated flame retardants (BFRs) in aquatic environments; however, there is a lack of exploration of BFRs in ice media in freshwater environments, and there are fewer studies on the distribution patterns and ecological risks of BFRs in different media. In order to fill this gap in the current research status, this study conducted four seasonal samplings in the Songhua River wetland in Northeast China. The distribution and risk of 14 polybrominated diphenyl ethers (PBDEs) and 22 new brominated flame retardants (NBFRs) in water, ice, sediment, and soil were analyzed using liquid–liquid extraction sample pretreatment and gas chromatography–mass spectrometry instrumentation. A total of 18, 5, 8, 19, and 18 BFRs were detected in non-ice-covered water, ice-covered water, ice, sediment, and soil, respectively. NBFRs dominated contaminant concentrations in each medium. Significant correlations were found between BFRs in ice and subglacial water, suggesting that the sources of BFRs in these two media are similar and there is an exchange between them. The ice enrichment factor (IEF) revealed the water–ice distribution mechanism of BFRs, indicating that wetland ice acts as a temporary sink for 2-(Allyloxy)-1,3,5-tribromobenzene (ATE), 1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane (α-TBECH), 1,2,5,6-Tetrabromocyclooctane (TBCO), and 2-Bromoallyl 2,4,6-tribromophenyl ether (BATE). In order to achieve dynamic equilibrium, the exchange profile of BFRs between water and sediment requires the release of BFRs into water. The risk quotient (RQ) indicated that TBCO in water and ice poses a moderate risk to aquatic organisms, and its potential impact on wetland ecology cannot be ignored.
1. Introduction
Brominated flame retardants (BFRs) are commonly used in many fields, such as construction materials, electronic devices, textiles, plastics, and garments, to reduce fire risks due to their high flame retardant efficiency, stable performance, and low price [1,2]. Polybrominated diphenyl ethers (PBDEs) are among the most widely used BFRs. As physical additives, PBDEs are not chemically integrated into the polymer matrix of the material and, therefore, escape into various environmental matrices [3,4]. Numerous studies have also reported that PBDEs in the environment are health hazards to organisms, causing neurotoxicity, immunotoxicity, genotoxicity, and reproductive toxicity due to their persistence, bioaccumulation, and toxicity [5,6,7]. Therefore, the main commercial products of PBDEs—pentabromodiphenyl ether (Penta-BDEs), octabromodiphenyl ether (Octa-BDEs), and decabromodiphenyl ether (Deca-BDEs)—have successively been listed as persistent organic pollutants (POPs) by the United Nations Stockholm Convention, and decabromodiphenyl ether was also added to the list of priority substances in China in January 2018 [1,4].
With the gradual phase-out of BFRs, a series of new brominated flame retardants (NBFRs) with similar physicochemical properties and functions as PBDEs have been introduced into the market [2]. The global production and consumption pattern is also gradually shifting from BFRs to NBFRs [3]. Research has reported that the global production of NBFRs is 100 to 180 kt per year, and the United States and China are the main producers of NBFRs [3,8]. NBFRs have similar physicochemical properties to PBDEs and, therefore, are also released into the environment where they accumulate [1]. The presence of NBFRs in various environmental media, including house dust [9], water [10], air [11], sediments [12], aquatic organisms [13], and the human body [14], has been reported in numerous studies. It has been shown that some NBFRs with similar toxicity to PBDEs also cause various human diseases, such as neurotoxicity and endocrine disruption [9]. As the production and use of NBFRs increase each year, their risks to the health of the environment and organisms cannot be ignored.
The aquatic environment is a vital ecosystem. The occurrence, fate, and risk of BFRs in aquatic environments have attracted extensive attention [15]. BFRs can enter aquatic environments through atmospheric deposition, wastewater discharge, surface runoff, and microplastic decomposition and are deposited into sediments after various transport, distribution, and degradation processes [3]. Due to their lipophilic nature, BFRs can accumulate in large quantities in various aquatic organisms, leading to oxidative stress, endocrine disruption, neurotoxicity, and genotoxicity in aquatic organisms [16,17,18], and may bioaccumulate and biomagnify in the food chain, thus posing a potential threat to aquatic ecosystems and humans [4]. Although numerous studies have focused on the occurrence and fate of BFRs in aquatic ecosystems, there are more aquatic environmental media, and BFRs have been detected in water [10], sediments [12], aquatic organisms [13], etc.; however, BFRs have not been reported in ice. In addition, current studies have focused on aquatic environments such as sewage treatment plants [19], rivers [20,21], lakes [22,23], and oceans [24,25], while the bioaccumulation, fate, and ecological risk of BFRs in wetland environments have been less studied. Wetlands are important natural ecosystems that play ecological functions of water conservation, climate regulation, environmental improvement, and maintenance of biodiversity; they are closely related to the survival and development of human beings and are known as the “kidneys of the earth” [26]. Therefore, exploring the distribution of BFRs in wetland environments can provide data for analysis of the current status of BFR pollution in wetlands, provide a reference for the establishment of relevant control systems, and be of great significance for the protection of wetland ecosystems.
Overall, there are few studies on the occurrence of BFRs in wetlands. To fill this gap, our study aimed to (1) determine the concentrations of BFRs in water, ice, soil, and sediment in the Songhua River wetland in Harbin, Heilongjiang Province; (2) reveal the partitioning of BFRs in different matrices in the wetland; and (3) conduct an ecological risk assessment of BFRs in the wetland.
2. Materials and Methods
2.1. Study Area and Sample Collection
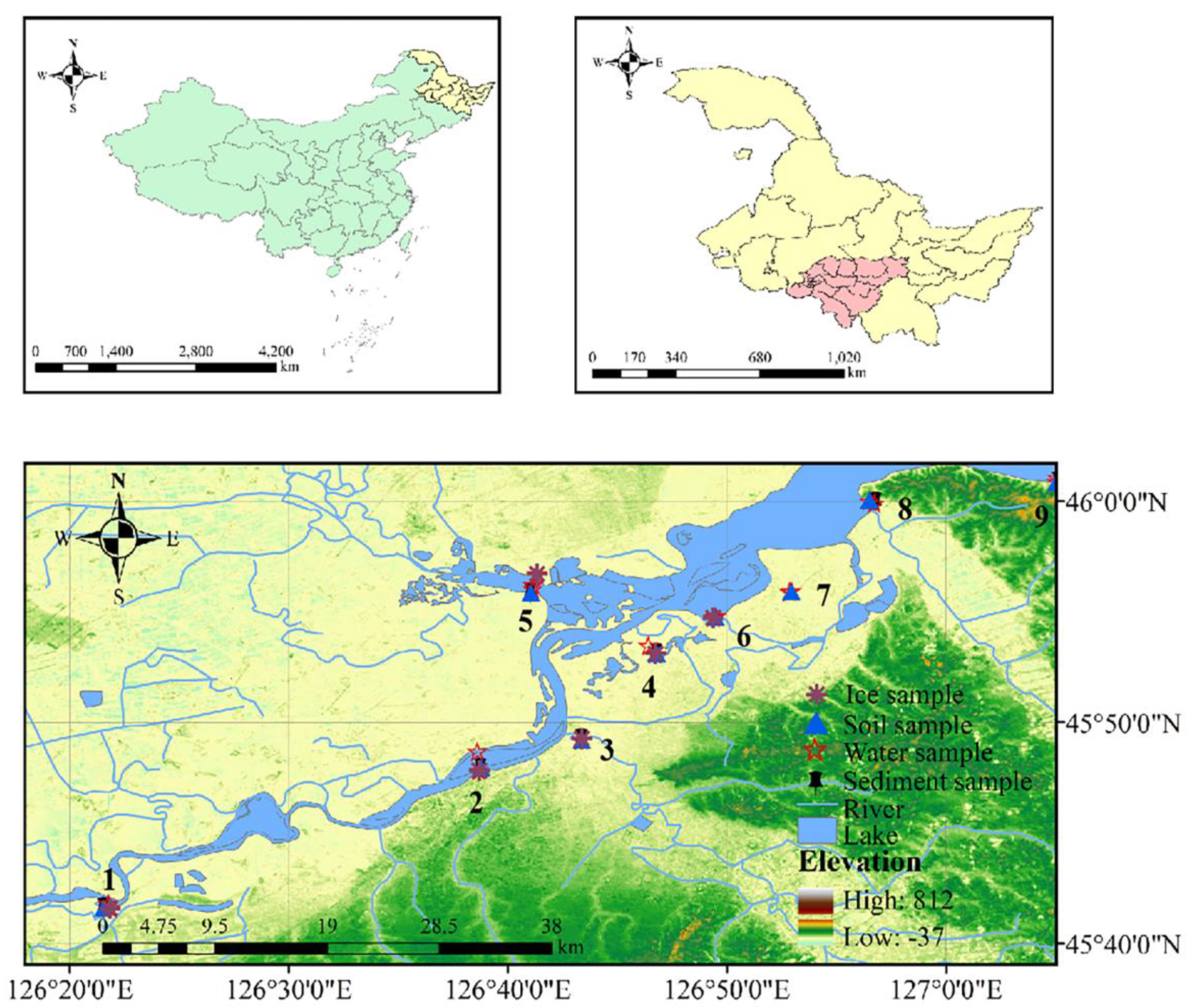
The study area is located on the south bank of the Songhua River (126°40′55″–127°14′16″ E, 45°50′48″–46°04′04″ N) in Harbin, Heilongjiang Province, China, and is an important part of the wetlands of the Songnen Plain (Figure 1). The study area extends in an east–west belt along the south bank of the Songhua River, with an east–west length of 23.5 km, the widest north–south point of 5.5 km, and a circumference of 70.3 km, with a total area of 15,319 ha. The land use types around the wetland are diverse, and the detailed categories are shown in Table S1. Based on the topographic and hydrological characteristics of the study area, the distribution of nearby towns, a review of the literature, and several field surveys, nine sampling points were selected along the course of the study area (Figure 1), two of which were located in the Ash River and the Hulan River, which receive industrial wastewater discharged by coastal factories, effluent from wastewater treatment plants, as well as domestic wastewater that is not collected by the pipeline network, which was affected by anthropogenic and industrial activities, and was in category V. The climate of the study area is a typical temperate monsoon climate, with high temperatures and rain in summer and cold and dry in winter. The water body freezes for up to 150 days between November and March. Based on climate characteristics, wetland water samples (n = 60), ice samples (n = 6), sediment samples (n = 17), and soil samples (n = 20) were collected at nine sampling sites in October 2021, January 2022, May 2022, and July 2022, respectively. The specifics of sample collection and preservation are outlined in Text S1.
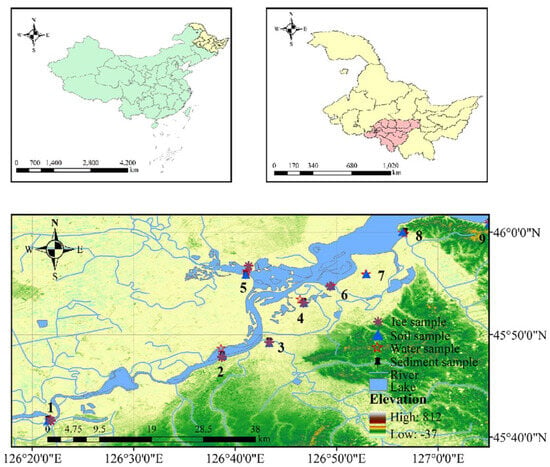
Figure 1.
Location of the sampling sites in the Songhua Wetland in Harbin, China.
2.2. Sample Analysis
2.2.1. Chemicals and Reagents
A total of 14 PBDEs and 22 NBFRs were analyzed in this study; the detailed information on the physicochemical properties of the 36 BFRs is summarized in Table S4. The standard reagents for the target BFRs were purchased from First Standard (Alta Scientific Co., Ltd., Tianjin, China). The reagents used for sample pretreatment were purchased from Dikma (Lake Forest, CA, USA) and J.T. Baker (Phillipsburg, NJ, USA), and ultrapure water was prepared using the Milli-Q ultrapure system (Thermo Scientific, Waltham, MA, USA).
2.2.2. Sample Extraction
Water samples were extracted for BFR analysis using liquid–liquid extraction. Briefly, 1 L of water sample was placed in a separatory funnel, and the sample was extracted by shaking three times with 100 mL, 50 mL, and 50 mL of dichloromethane. The extracted solution was placed into a flat-bottomed flask and dehydrated with anhydrous sodium sulphate. The extract was concentrated and dissolved gently in 1 mL isooctane under high-purity nitrogen and then stored in a refrigerator at −20 °C for subsequent instrumental analysis.
For ice samples, sealed brown glass jars containing ice were left to melt naturally at room temperature in a dark and unpolluted environment. The subsequent steps for pesticide extraction from melted water were the same as for water samples.
For sediment and soil samples, 1 g of wet sample was first mixed with 15 g of anhydrous sodium sulphate. The mixture was ground into a fine powder, mixed with 25 mL of hexane/ethyl acetate/dichloromethane mixture (1/1/1, v/v/v), and extracted by shaking for 30 min followed by centrifugation at 4000 r/min for 15 min. The extraction process was repeated twice, and the organic extracts were combined in a flat-bottomed flask. Subsequent extraction steps were the same as for the aqueous samples.
2.2.3. Instrumental Analyses
Due to the volatility and thermal stability of the BFRs, instrumental analyses of the samples were performed in dynamic MRM mode using a gas chromatograph–mass spectrometer (GC-EI-MS/MS, Agilent 7890B, Agilent 7000C, Agilent Technologies, Santa Clara, CA, USA). The detection principle of the instrument is that the GC section vaporizes the sample and then separates the brominated flame retardants in the chromatographic column according to the difference in physicochemical properties using carrier gas. Then, the MS section ionizes the separated components, the mass analyzer separates the ions according to the mass-to-charge ratio, and the detector records and generates the mass spectra for qualitative and quantitative analysis. The samples were separated by high-purity helium (99.999%) fed into an Agilent 122-5511 (Agilent Technologies, Santa Clara, CA, USA) column (15 m × 250 μm × 0.1 μm) at a constant flow rate of 2.25 mL/min. The sample injection volume was 2 μL and the temperature of the injection port was 300 °C. A glass fiber liner tube was used. The temperature of the gas chromatography column was determined by a temperature program and consisted of holding at 80 °C for 1 min, ramping up to 300 °C at 22 °C/min, and holding for 6 min. The instrument’s parameters for analysis of the 36 BFRs are given in Table S5. For subsequent data processing, Agilent Qualitative Navigator B.08.00 was used for qualitative processing of the BFRs, and Agilent MassHunter Quantitative Analysis 10.1 was used for quantitative processing of the BFRs.
2.2.4. Total Organic Carbon Analysis
Total organic carbon (TOC) is a central element in the analysis of the environmental behavior of organic pollutants in soil, sediment, and other media. It has strong adsorption properties, which affect the migration and diffusion of organic pollutants. TOC participates in various chemical reactions and plays a key role in the degradation and transformation of organic pollutants [27,28,29]. In order to investigate the effect of TOC on BFRs in sediments and soils, this study examined the TOC content in sediment samples and soil samples using a total organic carbon analyzer (Multi N/C 3100, Analytik Jena AG, Jena, TH, Germany). The procedure was as follows: the sediment samples and soil samples were dried at 60 °C until they reached constant weight; they were then ground into powder, sieved to about 70 mg of sediment and soil, and finally heated to 1150 °C to measure TOC.
2.3. Quality Assurance and Quality Control
To minimize procedural contamination, all the glassware were soaked overnight in a washing solution and then rinsed with deionized water. They were then washed with dichloromethane and n-hexane, baked at 120 °C, and stored appropriately. During the assay, parallel samples were used to confirm the accuracy and reliability of the assay, with three parallel samples for each sample. The experiment included experimental blank and solvent blank controls for quality control, where the samples were replaced with Milli-Q water (Thermo Scientific, Waltham, MA, USA) and 4 g of anhydrous sodium sulfate and baked at 400 °C. Blank samples were extracted, purified, concentrated, and instrumentally analyzed at the same time as the actual samples to assess the influence of environmental background on the measurement results. A series of 8-point standard calibration curves ranging from 0.5 to 100 ng/mL were established to quantify BFRs, and all correlation coefficients (r2) exceeded 0.99. All results for soil and sediment are reported on a dry weight (dw) basis.
2.4. Theory and Calculations
2.4.1. Ice Enrichment Factor
Contaminant enrichment in ice has been reported in the literature [30,31], and may be the result of contaminant exchange between water and ice and atmospheric deposition. The ice enrichment factor (IEF) was calculated as a ratio of the concentration of contaminants in ice to water based on modifying the enrichment factor (EF). The IEF was employed to investigate the partitioning and enrichment of BFRs between ice and subglacial water. Detailed information on the IEF is provided in Text S2 of the Supplementary Information.
2.4.2. Fugacity Fraction
The fugacity method has been used in numerous studies to investigate the partitioning of pollutants between water and sediments [32]. We used the fugacity fraction (ff) to analyze the partitioning of BFRs between sediments and water. The detailed ff calculation procedure is provided in Text S3.
2.4.3. Risk Quotient
Risk quotient (RQ) is a well-established assessment method for evaluating the latent risk of organic contaminants in aquatic environments and has been used in numerous studies [33,34]. We applied the RQ method to assess the risk of BFRs in water, ice, and sediment. Text S4 provides detailed information on the RQ method.
2.5. Data Analysis
The basic property information for the target BFRs was simulated using EPI Suite Version 4.1 (USEPA, 2022) to establish and optimize instrumental detection methods. The topographic map of the study area was prepared using ArcGIS 10.7 (Esri, Redlands, CA, USA). Data were statistically analyzed and graphically plotted using IBM SPSS 25.0 (IBM, Armonk, NY, USA) statistical software and Origin 2021 (OriginLab, Northampton, MA, USA). The Shapiro–Wilk test for homogeneity of variance was used. The correlation between the two groups was assessed using the Spearman correlation coefficient, while differences among multiple groups were tested with the Kruskal–Wallis test, considering p < 0.05 as statistically significant.
3. Results and Discussion
3.1. Concentration of BFRs in Wetlands
3.1.1. BFRs in Water
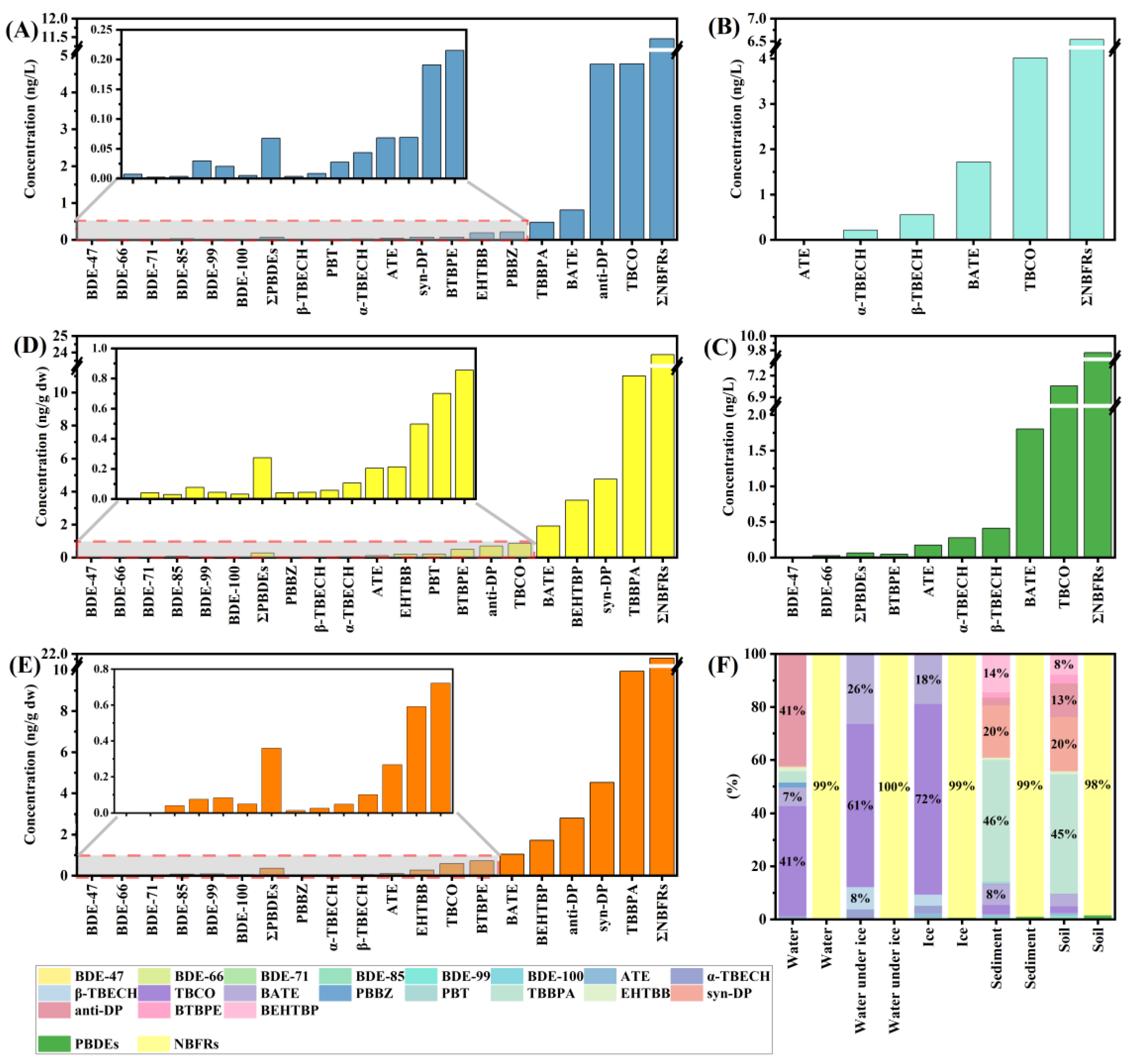
Due to climatic factors, the study area is frozen from November to March; therefore, the water samples collected in each of the four seasons were categorized into non-ice-covered water samples and ice-covered water samples for discussion in this study. Concentrations of BFRs detected in the wetland water samples are presented in Table S6 and Figure 2A,B. A total of 18 BFRs were detected in the non-ice-covered water samples, of which six were PBDEs and 12 were NBFRs. The concentration of ΣPBDEs was 0.068 ng/L. The concentration of detected NBFRs was larger than that of PBDEs, and the concentration of ΣNBFRs was 11 ng/L, accounting for 99% of the concentration of ΣBFRs (Figure 2F). By comparison, we found that the concentration of NBFRs was much higher than that of PBDEs in the water of the Songhua River wetland during the non-freezing period, and there was a difference of two orders of magnitude in their concentration. This suggests that following the banning of some traditional BFR products, NBFRs are gradually becoming the major BFR contaminants in aquatic environments. This conclusion has also been reached in numerous other studies [35,36]. As can be seen in Figure 2A, the BFRs with the highest concentrations in non-ice-covered water samples were Anti-Dechlorane Plus (anti-DP) and 1,2,5,6-Tetrabromocyclooctane (TBCO), both with a concentration of 4.8 ng/L, each accounting for 41% of the concentration of ΣBFRs. Anti-DP is a high-volume product in flame retardants that has been used for nearly half a century, and many studies have confirmed its prevalence around the world [37,38]. In 2023, Dechlorane Plus (DP) was classified as a persistent organic pollutant by the Stockholm Convention (UNEP 2023) [39]. TBCO is widely used as an alternative to hexabromocyclododecane (HBCD) [40,41]. Compared to other BFRs, anti-DP and TBCO were detected at higher concentrations, suggesting that they are used more often in and around the study area.
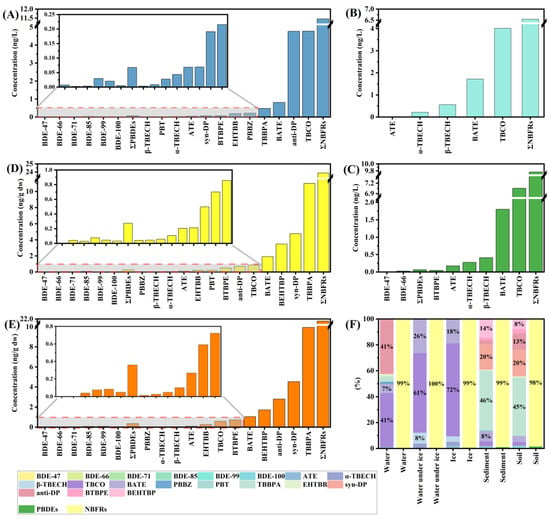
Figure 2.
Concentrations and distributions of BFRs in wetland non-ice-covered water (A), ice-covered water (B), ice (C), sediment (D), and soil (E) samples, and the relative contributions of each BFR, PBDEs, and NBFRs to ∑BFRs of the wetland (F).
A total of five BFRs were detected in the water in the Songhua River wetland during the ice-cover period; these were 2-(Allyloxy)-1,3,5-tribromobenzene (ATE) (0.035 ng/L), 1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane (α-TBECH) (0.22 ng/L), 1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane (β-TBECH) (0.55 ng/L), TBCO (4.0 ng/L), and 2-Bromoallyl 2,4,6-tribromophenyl ether (BATE) (1.7 ng/L). The concentration of ΣNBFRs was 6.5 ng/L. Similar to the results of the non-ice-covered water, the most prevalent compound in ice-covered water was TBCO, which accounted for 61% of the ΣBFRs. In addition, BATE was also detected in high proportions, accounting for 26% of the concentration of ΣBFRs. It has been found that BATE not only crosses the blood–brain barrier to a greater extent than other BFRs, but it may also affect neuronal function [42]. Moreover, BATE has been detected in numerous environments and animals [43,44], and it may be a potential risk to wetland environments.
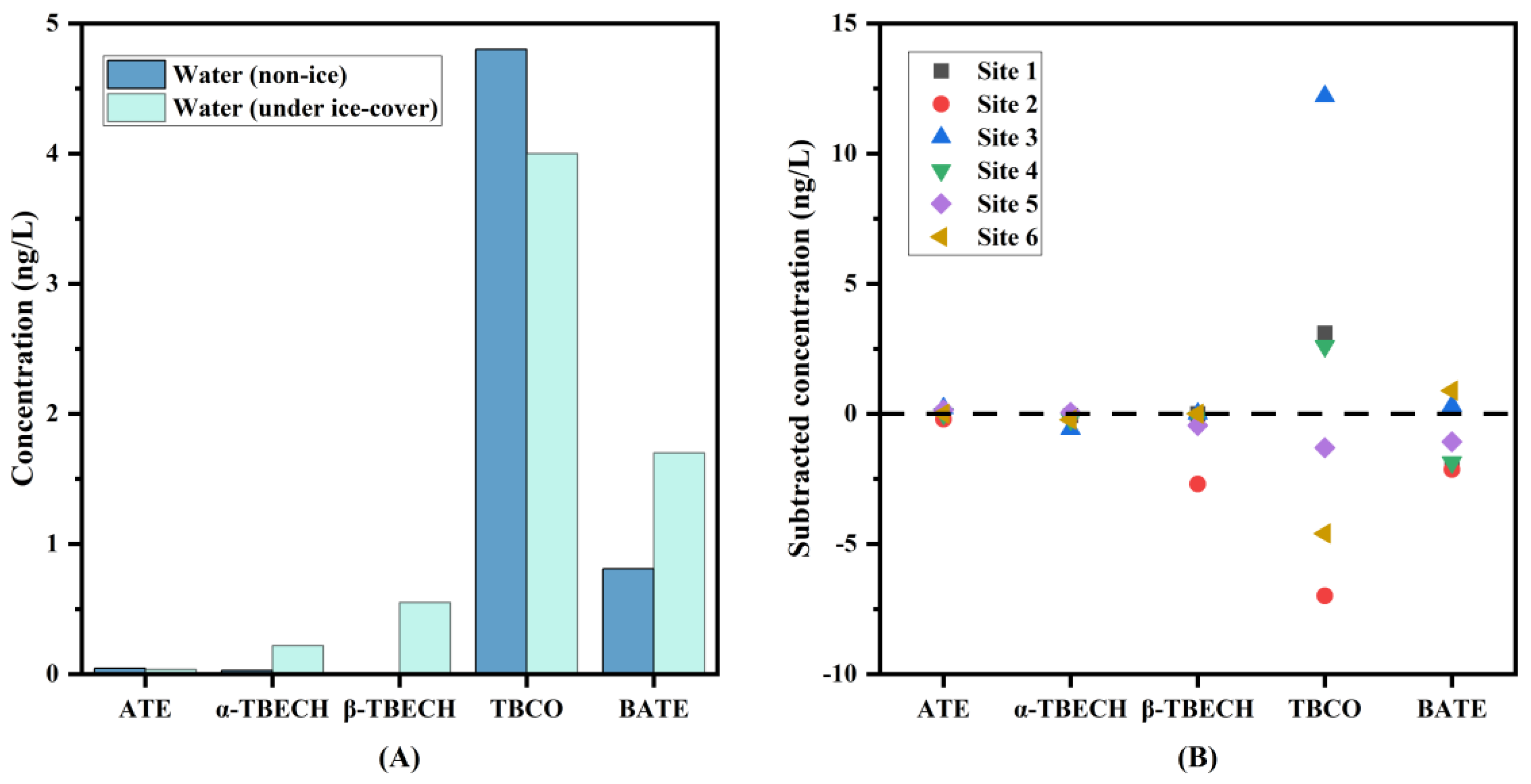
To further explore the distribution of BFRs in water, the concentrations of commonly detected BFRs in water samples collected during ice-covered and non-ice-covered periods were compared. As shown in Figure 3, the concentrations of BFRs were generally higher in ice-covered than in non-ice-covered water (except for TBCO). The same results were found at different sampling sites, except for TBCO at sites 1, 3, and 4 and BATE at site 6, which may be due to their physicochemical properties. The finding that BFRs are more concentrated in water during winter has also been reported in other studies, which may be due to lower Kaw of BFRs; the low precipitation in winter, which has a less diluting effect on pollutants in water; lower microbial activity during winter, which has a reduced effect on pollutants degradation; and the resuspension of contaminants in sediments, etc. [35,36]. Although there were differences in the concentration levels of BFRs co-detected in the water during the ice-covered and non-ice-covered periods, overall, there were no significant differences in the total BFR concentrations between all seasons (Mann–Whitney test, p > 0.05) (Figure S1). Also, there were no significant differences in total BFR concentrations between sampling sites (Mann–Whitney test, p > 0.05) (Figure S2).
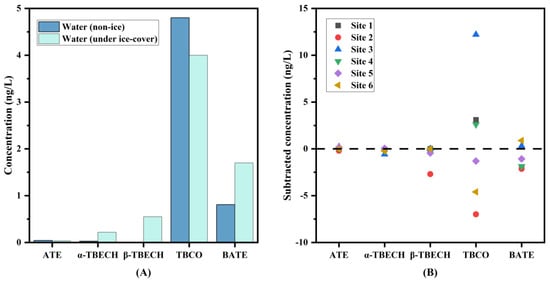
Figure 3.
Comparison of BFR concentrations between non-ice-covered water and ice-covered water in the wetland. (A): Comparison of individual BFRs (the columns represent the average values). (B): Subtracted BFR concentration in water samples in the wetland (data are obtained by subtracting the concentrations of BFRs in ice-covered water and non-ice-covered water).
3.1.2. BFRs in Ice
Ice in aqueous environments is an important medium for pollutant enrichment, and numerous studies have reported the occurrence of pollutants in ice [30,45]. This study focused on the occurrence of BFRs in wetland ice. Among the 36 target BFRs, only eight were detected. The two PBDEs detected were 1,1′-Oxybis(2,4-dibromobenzene) (BDE-47) (0.040 ng/L) and 1,2-Dibromo-4-(2,4-dibromophenoxy)benzene (BDE-66) (0.025 ng/L), and the six NBFRs were ATE (0.17 ng/L), α-TBECH (0.28 ng/L), β-TBECH (0.41 ng/L), TBCO (7.1 ng/L), BATE (1.8 ng/L), and 1,1′-[1,2-Ethanediylbis(oxy)]bis(2,4,6-tribromobenzene) (BTBPE) (0.047 ng/L) (Table S6 and Figure 2C). As with the results of the water samples, the concentration of ΣNBFRs continued to dominate the concentration of ΣBFRs in ice (99%), with TBCO and BATE accounting for larger proportions (72%, 18%) (Figure 2F). This is similar to the results detected in ice-covered water bodies. Some studies have shown that pollutants in the aqueous environment are transported and distributed between the water and ice phases to reach an equilibrium state [31,45]. The results of BFRs detected in ice were similar to those of water in the ice-covered phase, which is likely to be the result of the migration of BFRs between water and ice. In addition, in order to explore the distribution of BFRs at different sampling sites, the concentration levels of BFRs at different sampling sites were examined by the Mann–Whitney method, and it was found that there were no significant differences (p > 0.05).
3.1.3. BFRs in Sediments
The accumulation of BFRs in sediments is shown in Table S6 and Figure 2D. Due to the lipophilic and hydrophobic natures of BFRs, a large number of BFRs were detected in the wetland sediment samples, with a total of 19 species detected, including six PBDEs and 13 NBFRs. Six PBDEs were detected at concentrations ranging from 0.029 to 0.077 ng/g dw, with a ΣPBDE concentration of 0.27 ng/g dw, while the 13 NBFRs were detected at concentrations ranging from 0.042 to 11 ng/g dw, with a ΣNBFR concentration of 24 ng/g dw. The ΣNBFR concentration accounted for 99% of the ΣBFR concentration (Figure 2F). Tetrabromobisphenol A (TBBPA) was the BFR with the highest concentration in the sediment samples (mean: 11 ng/g dw), at 46%. TBBPA is widely used as a reactive flame retardant in printed circuit boards and as an additive in many types of polymers; its toxicity data, such as acute toxicity, immunotoxicity, neurotoxicity, nephrotoxicity, and hepatotoxicity, have been reported in numerous studies [46,47,48]. The presence of TBBPA at high concentrations in sediments is highly likely to pose a serious threat to the biosafety of the aquatic environment. In addition, Syn-Dechlorane Plus (syn-DP) and Bis(2-ethylhexyl) 3,4,5,6-tetrabromophthalate (BEHTBP) (mean values: 4.7 and 3.5 ng/g dw) also accounted for a high proportion (20% and 14%) of the concentrations of ΣBFRs. Syn-DP was found to have higher concentrations of BFRs compared to the other target BFRs because of the tendency of DP to accumulate in sediments through surface runoff and atmospheric deposition due to its high hydrophobicity [37,39]. Moreover, numerous studies have reported that DP has a significant bioconcentration effect and a strong biomagnification capacity along the food chain [49,50,51]. Therefore, DP stored in sediments may pose a greater ecological risk. BEHTBP is a major component of BFRs that is often detected in atmospheric and indoor dusty media [52,53,54] but is rarely reported in sediments. Nevertheless, de Jourdan et al. explored the fate of NBFRs in aquatic environments through an aquatic mesocosm experiment and found that BEHTBP is persistent in particle and sediment compartments in the environment [55]. The toxicity of BEHTBP has also been characterized [56]. In conclusion, the ecological risk of BFRs in sediments cannot be ignored.
Numerous environmental factors, such as organic matter content and chemical properties, affect the accumulation and distribution of contaminants in sediments [57]. In this study, TOC levels in sediment were measured at each sampling point, which ranged from 0.29 to 1.7%. In this study, the correlation between TOC and BFR concentration was analyzed. Figure S3 shows that the concentrations of PBDEs and NBFRs in the wetland sediment samples from each site were positively and significantly (p < 0.05) correlated with the TOC levels. This correlation suggests that the TOC level in the sediment affects the distribution of BFRs in the sediment; this has been found in some studies [58,59]. Similar to the water, there was no significant difference (Mann–Whitney test, p > 0.05) in the total concentration of BFRs between seasons and sites.
3.1.4. BFRs in Bank Soil
A total of 18 BFRs, including six PBDEs and 12 NBFRs (Table S6 and Figure 2E), were detected in soil samples from wetland banks. The concentration of each PBDE detected ranged from 0.039 to 0.083 ng/g dw, with a ΣPBDEs concentration of 0.36 ng/g dw. The concentration of each NBFR detected ranged from 0.013 to 9.9 ng/g dw, with a Σ NBFR concentration of 22 ng/g dw. As in the other media, ΣNBFRs still accounted for a large proportion of the ΣBFR concentration (98%, Figure 2F). Clearly, the target contaminant with the highest concentration in the wetland soil samples was TBBPA, which accounted for 45% of the total target BFRs, followed by syn-DP (20%), anti-DP (13%), and BEHTBP (8%). It is worth noting that the main contaminants detected in the soil samples were similar to those in the sediment samples (except for anti-DP). Therefore, a correlation analysis was performed for the 18 BFRs that were jointly detected in soil and sediment samples. The Spearman correlation coefficient matrix heatmap of the individual concentrations of different BFRs is shown in Figure S4A. Significant positive correlations (p < 0.05) were found between the concentrations of different BFRs in both sediment and soil samples. There was also a strong overall positive correlation between concentrations in different seasons and at different sampling sites (Figure S4B,C). Other studies have also found positive correlations between contaminant concentrations in sediments and soils in aquatic environments [30]. This positive correlation indicates a statistically significant similarity in the sources of BFRs in wetland sediments and soils. In addition, in the same way as for sediments, there was a positive correlation between the concentrations of BFRs in soil and the level of TOC (Figure S5). However, again, there was no significant spatial or temporal variation in the distribution of BFRs in soil (Mann–Whitney test, p > 0.05).
3.2. Migration and Exchange of BFRs Between Different Media
3.2.1. Exchange Between Water and Ice
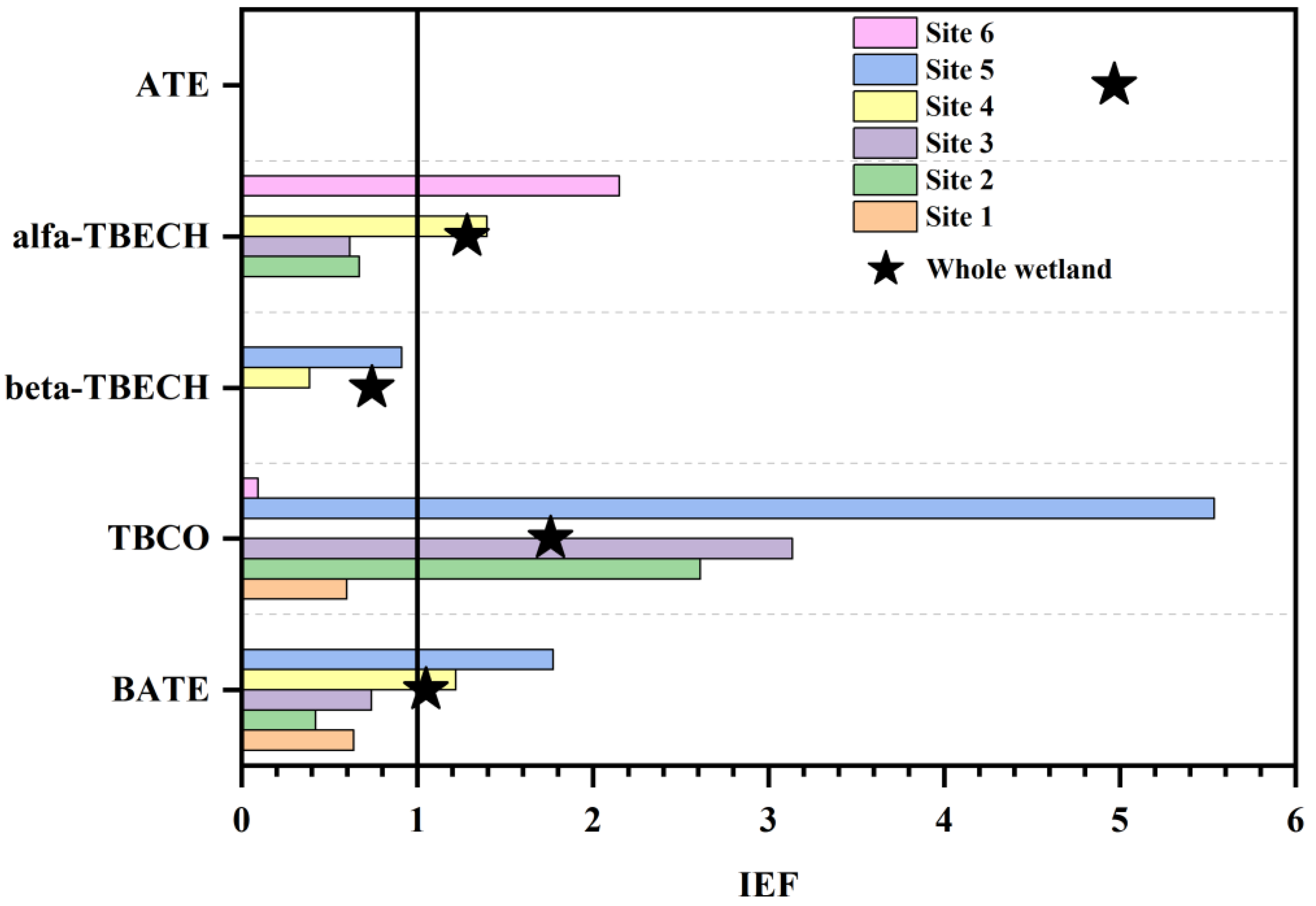
Pollutants can be enriched in ice through migration from water and atmospheric deposition, and studies have reported the enrichment of pollutants in ice and explored the exchange mechanisms between ice and water [60]. In contrast, the ice–water partitioning pattern of BFRs in aquatic environments remains to be explored. In order to analyze the migration and partitioning of BFRs between ice and water, the IEF of five NBFRs jointly detected in ice and ice-covered water collected in winter were calculated, and the results were summarized and plotted in Figure 4. Analyzing the IEF for each compound, it was found that only β-TBECH had an IEF value less than 1 (0.74), which indicates that its content in water under ice is significantly higher than in ice. At this time, ice is a secondary release source of β-TBECH. The IEF values of ATE, α-TBECH, TBCO, and BATE were all greater than 1 (1.05–4.97), which indicates that their tendency to be enriched in ice was stronger; thus, ice acted as a sink for them. Overall, BFRs were more enriched in wetland ice. This finding is similar to the environmental behavior of pollutants in ice reported in other studies. Numerous scholars have found that the concentration of organic pollutants in Arctic Ocean sea ice is higher than in seawater under the ice [61,62]. The same observation was found in aquatic freshwater environments, i.e., the concentration of PAH in river ice and wetland ice is higher than the concentration of PAH in subglacial water [31,63]. Moreover, some studies found that the ability of ice to enrich pollutants was affected by water salinity and concluded that the concentration of pollutants was higher in freshwater ice [64,65]. In addition to water–ice exchange, atmospheric deposition and particle retention are also important pathways through which pollutants enter ice [66]. Organic pollutants in aquatic environments commonly undergo water–air exchange; however, during the freezing period, the ice cover on the upper layer of the water surface blocks the water–air exchange behavior of pollutants, which makes organic contaminants in water migrate into the ice cover at the same time that the organic pollutants in the atmosphere also enter the ice layer through wet and dry deposition. Snowfall, atmospheric particulate deposition, and sea salt aerosol deposition are all important sources of organic pollutants in ice [67,68]. Entry of organic pollutants into ice through particle trapping is a process that occurs mainly during suspension freezing of ice crystals. Turbulent removal and filtration of suspended sediments by progressively frozen ice crystals results in entrapment of fine particles in the newly formed ice [69,70]. Afterward, due to the high affinity of many trace organic contaminants in this fine sediment, particle-reactive contaminants can be entrained from the particulate matter into the ice through an efficient pathway [71]. Suspension freezing occurs in shallow shelf waters at depths typically <50 m. The study area is a typical shallow shelf water with a maximum depth of 10 m. The easy trapping of particles in the water during the freezing of ice crystals may be one of the reasons why BFR concentrations are higher in ice than in ice-covered water. In addition to the above reasons, the physicochemical properties of BFRs and the fractionation between water and particles are also important factors that may affect the results of water–ice exchange [66].
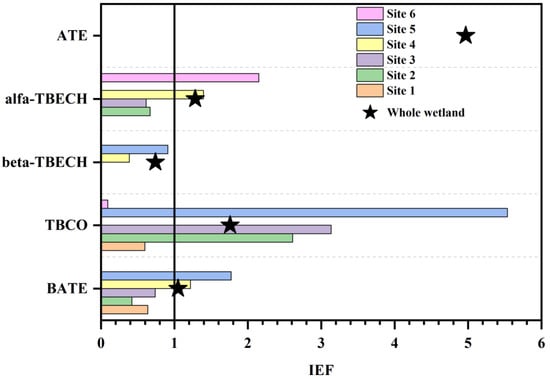
Figure 4.
The IEF of BFRs co-collected from ice and ice-covered water.
In addition, to further investigate the exchange of BFRs in the water–ice medium, correlation analyses were conducted for both the concentrations of individual BFRs between ice and water and the concentrations of BFRs at different sites. As shown in Figure S6, the overall positive correlation (p < 0.05) between both the concentrations of different individual BFRs and the concentrations of BFRs at different sites indicated that the sources of these BFRs were similar in both ice and water under ice and that the levels of BFRs were similar at each site, with the results being statistically significant.
3.2.2. Exchange Between Sediment and Water
The partitioning exchange of organic pollutants between water and sediments is a widespread environmental behavior [32]. The current study did not explore the pattern of exchange of BFRs between water and sediment. Fugacity fraction (ff) is a well-established theoretical calculation method used in many studies. This analysis adopted the fugacity fraction (ff) to elucidate the migration of BFRs between sediments and water in wetlands. The results of the ff calculations for BFRs detected in both water and sediment samples collected at each sampling site are plotted in Figure S7. The ff of the target BFRs detected at each site is greater than 0.5, indicating that BFRs are more likely to migrate in water than to be retained in sediment. Under these circumstances, wetland sediment is a secondary source of release of BFRs. This may be due to the fact that the hydrophobic and lipophilic characteristics of BFRs make the pre-existing concentrations of BFRs in the sediment higher than that in water, and in order to reach an equilibrium state, the BFRs enriched in the sediment are released into the water. In addition, the adsorption potential of the sediments and the physicochemical properties of the compounds are also responsible for the migration of BFRs into water [72]. The ratio of contaminant concentration in water versus sediment (Cwater/Csediment) may provide an indication of contaminant exchange behavior and the potential for resuspension; thus, combined with the ff, this ratio can further assess contaminant exchange [32]. Figure S8 summarizes the relationship between Cwater/Csediment and ff for BFRs co-detected in water and sediment. The Cwater/Csediment of all BFRs except 1,2,4-Tribromo-5-(2,4-dibromophenoxy)benzene (BDE-99), 1,3,5-Tribromo-2-(2,4-dibromophenoxy)benzene (BDE-100), β-TBECH, 1,2,3,4,5-Pentabromo-6-methylbenzene (PBT), syn-DP, and BEHTBP have significant positive correlations with ff. This is easy to understand as the release of these BFRs from sediments increases their concentration in water, leading to an increase in the Cwater/Csediment ratio; a ff > 0.5 also confirms the migration of BFRs from sediments to water. In summary, both the fugacity approach and the Cwater/Csediment ratio combined with ff suggested that wetland sediment is a secondary source of release of BFRs.
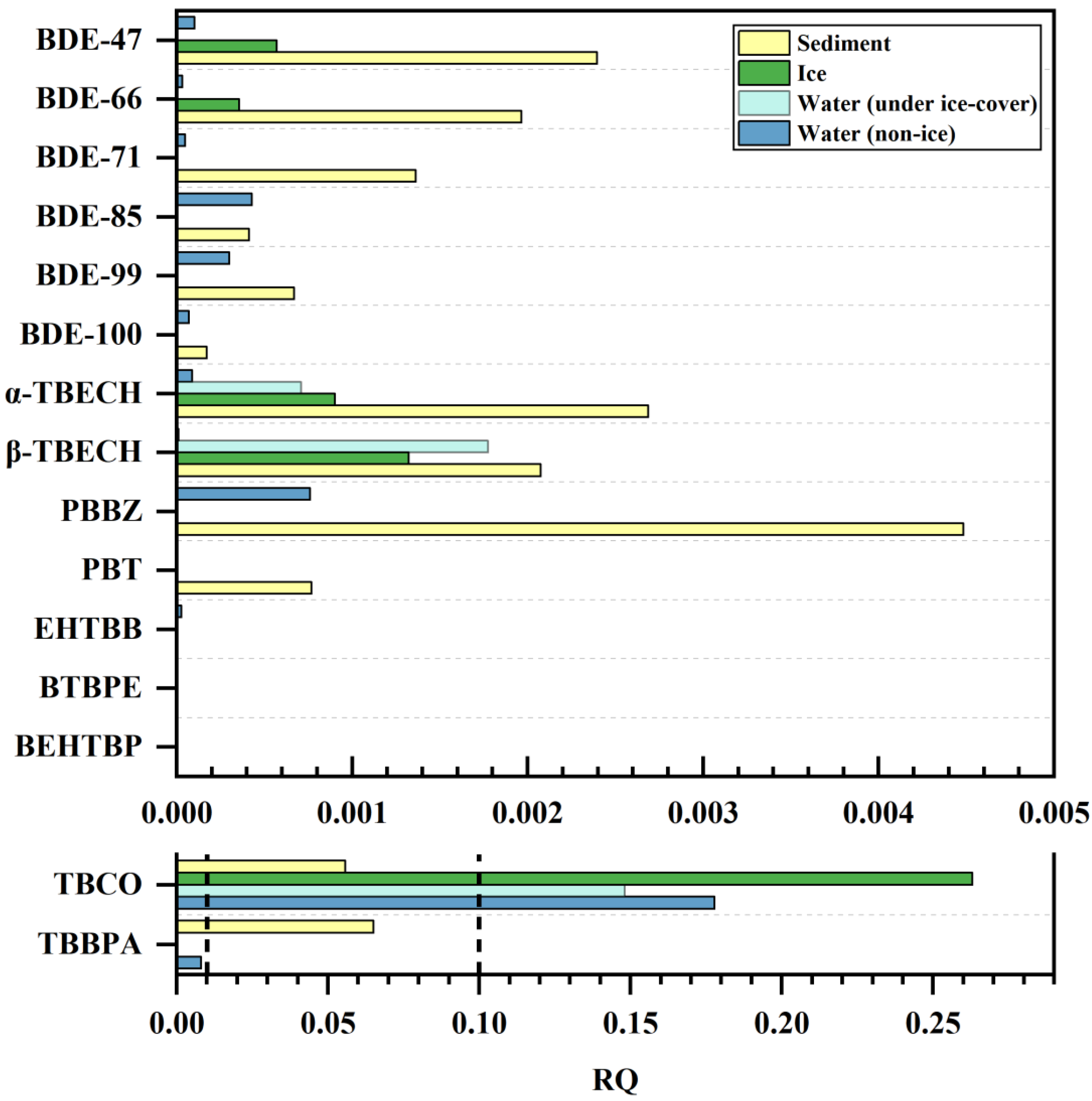
3.3. Risk Assessment
The increasing production and use of BFRs, especially NBFRs, has led to their detection in various environmental media as well as in organisms. Numerous studies have reported that BFRs bioaccumulate, concentrate, and amplify along the food chain and can have acute or chronic toxic effects in organisms, including hepatotoxicity, endocrine disruption toxicity, and developmental toxicity [73]. Therefore, the enrichment of BFRs in aquatic environments poses potential risks to the ecological environment and human health. In order to assess the ecological risk of BFRs in wetland aquatic environments, toxicity data from aquatic ecosystem organisms were used in this study to calculate the risk quotient (RQ) values for BFRs detected in water, ice, and sediments (the results are summarized in Table S7 and Figure 5). Of the 19 detected BFRs, ATE, BATE, syn-DP, and anti-DP lacked toxicity data and were not subjected to risk calculations; toxicity data for PBT, α-TBECH, and β-TBECH were found in the literature [74,75], and toxicity data for the remaining 12 BFRs were found in the US Environmental Protection Agency’s ECOTOX database. It is clear from Figure 5 that apart from TBCO and TBBPA, the RQ values of the remaining 13 BFRs in water, ice, and sediment are all less than 0.01, suggesting that they have no potential risk to the wetland ecosystem. In contrast, TBBPA and TBCO concentrations in sediment are associated with low risk (0.01 < RQ < 0.1), and TBCO concentrations in water and ice are associated with medium risk (0.1 < RQ < 1). Multiple studies have reported that TBCO has a significant effect on the reproductive capacity and embryonic development of aquatic organisms [76,77]. In addition, the European Food Safety Authority (EFSA)’s Food Chain Contamination Panel concluded that TBCO has a high bioaccumulation potential [78], and TBCO is listed as a potential acute toxicant of aquatic organisms [41]; therefore, the potential impacts of TBCO on wetland ecology cannot be ignored. Current toxicity study data on BFRs, especially NBFRs, are insufficient; additionally, most of the current toxicity studies are focused on simple systems in the laboratory and do not pay attention to the complexity of the environment and the diversity of pollutants [79,80]. Although most of the target compounds in this study do not present ecological risks in wetlands, photodegradation, biodegradation, and other transformation processes impact the toxic effects of BFRs [74]. Therefore, the mechanisms of toxicity and the toxic effects of BFRs on the actual environment still need to be investigated, taking into account the synergistic and cumulative toxic effects of multiple BFRs as well as the ecological risks associated with the effects of their bioconcentration, accumulation, and amplification in different food chains.
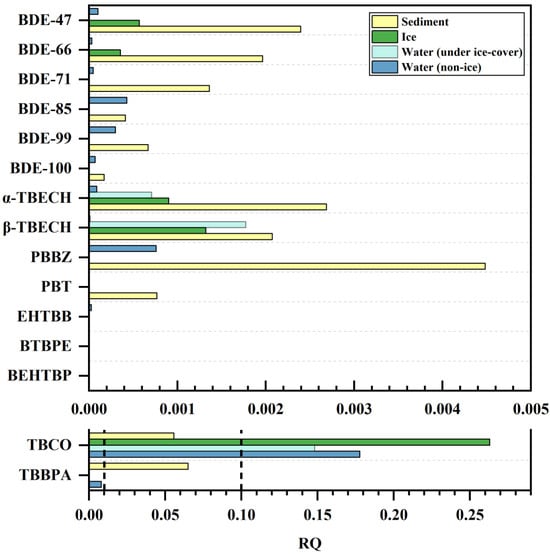
Figure 5.
The RQ of BFRs in water, ice, and sediments.
4. Conclusions
The fate and risks of 36 BFRs in a wetland environment were studied. It was found that the concentrations of NBFRs were always significantly higher than those of PBDEs in non-ice-covered water, ice-covered water, ice, soil, and sediments. The complexity and diversity of BFRs were higher in non-glacial water, sediments, and soil. Most of the BFRs were found to be present at higher concentrations in ice-covered water than in non-ice-covered water, and there was a significant positive correlation between the concentrations of BFRs in subglacial water and ice. Further exploration of the water–ice partitioning pattern of BFRs using the IEF revealed that a variety of BFRs were more enriched in ice, which may also be influenced by atmospheric deposition and particle trapping. Water–sediment exchange suggests that sediment is a secondary release source of BFRs. RQ was used to assess the potential ecological risk of BFRs to wetland aquatic organisms, and it was found that most of the BFRs did not currently present a risk; however, TBCO was associated with moderate risk. Additionally, the ecological risk from the cumulative toxicity of multiple BFRs and the effects of their bioconcentration, accumulation, and amplification in the food chain cannot be underestimated. In conclusion, this study detected and analyzed the contamination of BFRs in multiple media in wetlands and focused on the enrichment of BFRs in ice, providing new insights into the occurrence of contaminants in ice in freshwater environments. However, this study did not investigate the transport of BFRs through ice in sufficient depth, for example, the transport of BFRs at different depths in ice. More studies are needed in the future to fully understand the behavior of organic pollutants in ice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13020423/s1, Text S1: Sample collection and preservation; Text S2: Ice enrichment factor calculation; Text S3: Fugacity Fraction Calculation; Text S4: Risk quotient calculation; Table S1. The category of land use of data in the Songhua River alluvial plain, obtained from the GlobeLand 30 system (download from https://www.webmap.cn/main.do?method=index); Table S2. Location of the sampling sites in the Wetland in Harbin, China; Table S3. Type and number of samples collected at different sampling times and sites; Table S4. Detailed information on the properties of target substances (calculated by EPI Suite, Version 4.1), MF: molecular formula; MW: molecular weight; Table S5. GC-MS/MS detection parameters of target BFRs, including the optimized retention time (RT), transitions, and collision energy (CE); Table S6. BFRs concentrations in the wetland, the unit is ng/L for water and ice samples, ng/g dw for sediment and soil samples; Table S7. The predicted no-effect concentration (PNEC), measured concentration of BFRs (MEC), and maximum risk quotient (RQ) for aquatic organism; Figure S1. Concentrations of BFRs in wetland water, sediment and soil in different seasons, and the relative contribution of each BFR; Figure S2. Concentrations of BFRs in wetland water, ice, sediment and soil at different sampling sites, and the relative contribution of each BFR; Figure S3. Heat map of Speaman correlation coefficient matrix of PBDEs and NBFRs with TOC in sediment samples from each site (* p < 0.05); Figure S4. (A): Heat map of Speaman’s correlation coefficient matrix for individual BFRs in sediment and soil (* p < 0.05); (B,C): Linear regression plots of BFRs between sediment and soil for different seasons and sampling sites; Figure S5. Heat map of Speaman correlation coefficient matrix of PBDEs and NBFRs with TOC in soil samples from each site (* p < 0.05); Figure S6. (A): Heat map of Speaman correlation coefficient matrix for individual BFRs in ice and subglacial water (* p < 0.05); (B): Linear regression of BFRs in ice and subglacial water at different sampling sites; Figure S7. The fugacity fraction (ff) of BFRs co-detected in non-ice-covered water and sediment at different sampling sites; Figure S8. The relationship between the value of ff and the concentration ratio of water and sediment (Cwater/Csediment).
Author Contributions
Conceptualization, B.M. and M.-S.W.; Methodology, J.-W.J., M.-S.W., Z.-F.Z. and Y.-F.L.; Investigation, B.M., F.C., Z.-Z.Z. and S.-S.J.; Resources, B.M. and S.-S.J.; Data curation, X.-M.L., J.-W.J., F.C., Z.-Z.Z. and M.-S.W.; Writing—original draft, X.-M.L.; Writing—review & editing, Z.-F.Z. and Y.-F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Heilongjiang Provincial Natural Science Foundation of China (LH2021E096), the Open Project of Heilongjiang Cold Region Wetland Ecology and Environment Research Key Laboratory, Harbin University (No. 201911), and the Open Project of the National Engineering Research Center for Safe Disposal and Resources Recovery of Sludge (No. Z2024B003).
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, R.; Lin, L.; Li, H.; Liu, S.; Xu, X.; Xu, Y.; Jin, X.; Yuan, Y.; Wang, Z. Occurrence, Bioaccumulation, Fate, and Risk Assessment of Novel Brominated Flame Retardants (NBFRs) in Aquatic Environments—A Critical Review. Water Res. 2021, 198, 117168. [Google Scholar] [CrossRef]
- Iqbal, M.; Syed, J.H.; Katsoyiannis, A.; Malik, R.N.; Farooqi, A.; Butt, A.; Li, J.; Zhang, G.; Cincinelli, A.; Jones, K.C. Legacy and Emerging Flame Retardants (FRs) in the Freshwater Ecosystem: A Review. Environ. Res. 2017, 152, 26–42. [Google Scholar] [CrossRef]
- Li, M.; Gong, X.; Tan, Q.; Xie, Y.; Tong, Y.; Ma, J.; Wang, D.; Ai, L.; Gong, Z. A Review of Occurrence, Bioaccumulation, and Fate of Novel Brominated Flame Retardants in Aquatic Environments: A Comparison with Legacy Brominated Flame Retardants. Sci. Total Environ. 2024, 939, 173224. [Google Scholar] [CrossRef]
- Wang, N.; Lai, C.; Xu, F.; Huang, D.; Zhang, M.; Zhou, X.; Xu, M.; Li, Y.; Li, L.; Liu, S.; et al. A Review of Polybrominated Diphenyl Ethers and Novel Brominated Flame Retardants in Chinese Aquatic Environment: Source, Occurrence, Distribution, and Ecological Risk Assessment. Sci. Total Environ. 2023, 904, 166180. [Google Scholar] [CrossRef] [PubMed]
- Andvik, C.; Haug, T.; Lyche, J.L.; Borgå, K. Emerging and Legacy Contaminants in Common Minke Whale from the Barents Sea. Environ. Pollut. 2023, 319, 121001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, S.; Lu, C.; Jiang, L.; Zhou, S.; Fu, M.; Zhang, W.; Lin, K.; Zhou, B. Exploring the Environmental Fate of Novel Brominated Flame Retardants in a Sediment-Water-Mudsnail System: Enrichment, Removal, Metabolism and Structural Damage. Environ. Pollut. 2020, 265, 114924. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.; Qu, J.; You, H.; Liu, D. New Understanding of Novel Brominated Flame Retardants (NBFRs): Neuro(Endocrine) Toxicity. Ecotoxicol. Environ. Saf. 2021, 208, 111570. [Google Scholar] [CrossRef] [PubMed]
- Zuiderveen, E.A.R.; Slootweg, J.C.; de Boer, J. Novel Brominated Flame Retardants—A Review of Their Occurrence in Indoor Air, Dust, Consumer Goods and Food. Chemosphere 2020, 255, 126816. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.-N.; Zhang, L.-H.; Meng, B.; Li, Y.-F.; Xiao, H.; Egorovich, K.V.; Nikolaevna, P.N.; Zhang, Z.-F.; Tang, Z.-H. Brominated Flame Retardants in Road Dust and Green Belt Soil from Harbin, China: Contamination Characteristics, Sources and Health Risks. Environ. Chem. Ecotoxicol. 2024, 6, 229–235. [Google Scholar] [CrossRef]
- Suzuki, G.; Matsukami, H.; Michinaka, C.; Hashimoto, S.; Nakayama, K.; Sakai, S.-I. Emission of Dioxin-like Compounds and Flame Retardants from Commercial Facilities Handling Deca-BDE and Their Downstream Sewage Treatment Plants. Environ. Sci. Technol. 2021, 55, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xiong, S.; Wang, P.; Yang, R.; Pei, Z.; Li, Y.; Zhang, Q.; Jiang, G. Novel Brominated and Organophosphate Flame Retardants in the Atmosphere of Fildes Peninsula, West Antarctica: Continuous Observations from 2011 to 2020. J. Hazard. Mater. 2022, 440, 129776. [Google Scholar] [CrossRef] [PubMed]
- Vauclin, S.; Mourier, B.; Dendievel, A.-M.; Marchand, P.; Vénisseau, A.; Morereau, A.; Lepage, H.; Eyrolle, F.; Winiarski, T. Temporal Trends of Legacy and Novel Brominated Flame Retardants in Sediments along the Rhône River Corridor in France. Chemosphere 2021, 271, 129889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Gao, Y.; Chen, H.; Cui, J.; Yu, Y.; Ma, S. Identification and Occurrence of TBBPA and Its Debromination and O-Methylation Transformation Products in Sediment, Fish and Whelks from a Typical e–Waste Dismantling Site. Sci. Total Environ. 2022, 833, 155249. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, A.; Navarro, I.; Sanz, P.; de los Ángeles Martínez, M. Organophosphate Compounds, Polybrominated Diphenyl Ethers and Novel Brominated Flame Retardants in European Indoor House Dust: Use, Evidence for Replacements and Assessment of Human Exposure. J. Hazard. Mater. 2020, 382, 121009. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, H.; Gong, Y.; Huang, T. New Brominated Flame Retardant Decabromodiphenyl Ethane (DBDPE) in Water Sediments: A Review of Contamination Characteristics, Exposure Pathways, Ecotoxicological Effects and Health Risks. Environ. Pollut. 2023, 334, 122121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, W.; Lei, L.; Guo, Y.; Yang, L.; Han, J.; Zhou, B. Bioconcentration and Developmental Neurotoxicity of Novel Brominated Flame Retardants, Hexabromobenzene and Pentabromobenzene in Zebrafish. Environ. Pollut. 2021, 268, 115895. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Zhu, B.; Li, F.; Fu, K.; Guo, Y.; Men, J.; Han, J.; Zhang, W.; Yang, L.; et al. Multi- and Transgenerational Developmental Impairments Are Induced by Decabromodiphenyl Ethane (DBDPE) in Zebrafish Larvae. Environ. Sci. Technol. 2023, 57, 2887–2897. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qv, M.; Dai, D.; Wang, X.; Zhu, L. Toxic Responses of Freshwater Microalgae Chlorella Sorokiniana Due to Exposure of Flame Retardants. Chemosphere 2023, 310, 136808. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, K.; Ma, L.-X.; Sun, S.-J.; Jia, L.-R.; Yuan, A.-N.; Shen, J.-M.; Qi, H.; Zhang, A.-P. Deca-BDE and Alternative Halogenated Flame Retardants in a Wastewater Treatment Plant in Harbin (2009–2016): Occurrence, Temporal Trends, Seasonal Variation, and Fate. Sci. Total Environ. 2018, 625, 1156–1163. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N.; Li, J.; Zhang, G. Distribution, Congener Profile, and Risk of Polybrominated Diphenyl Ethers and Dechlorane plus in Water and Sediment from Two Tributaries of the Chenab River, Pakistan. Arch. Environ. Contam. Toxicol. 2015, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cristale, J.; García Vázquez, A.; Barata, C.; Lacorte, S. Priority and Emerging Flame Retardants in Rivers: Occurrence in Water and Sediment, Daphnia Magna Toxicity and Risk Assessment. Environ. Int. 2013, 59, 232–243. [Google Scholar] [CrossRef]
- Sutton, R.; Chen, D.; Sun, J.; Greig, D.J.; Wu, Y. Characterization of Brominated, Chlorinated, and Phosphate Flame Retardants in San Francisco Bay, an Urban Estuary. Sci. Total Environ. 2019, 652, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Venier, M.; Dove, A.; Romanak, K.; Backus, S.; Hites, R. Flame Retardants and Legacy Chemicals in Great Lakes’ Water. Environ. Sci. Technol. 2014, 48, 9563–9572. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Möller, A.; Ahrens, L.; Sturm, R.; Ebinghaus, R. Brominated Flame Retardants in Seawater and Atmosphere of the Atlantic and the Southern Ocean. Environ. Sci. Technol. 2011, 45, 1820–1826. [Google Scholar] [CrossRef]
- Möller, A.; Xie, Z.; Cai, M.; Zhong, G.; Huang, P.; Cai, M.; Sturm, R.; He, J.; Ebinghaus, R. Polybrominated Diphenyl Ethers vs Alternate Brominated Flame Retardants and Dechloranes from East Asia to the Arctic. Environ. Sci. Technol. 2011, 45, 6793–6799. [Google Scholar] [CrossRef] [PubMed]
- Erwin, K.L. Wetlands and Global Climate Change: The Role of Wetland Restoration in a Changing World. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Avramidis, P.; Nikolaou, K.; Bekiari, V. Total Organic Carbon and Total Nitrogen in Sediments and Soils: A Comparison of the Wet Oxidation-Titration Method with the Combustion-Infrared Method. Agric. Agric. Sci. Procedia 2015, 4, 425–430. [Google Scholar] [CrossRef]
- Bisutti, I.; Hilke, I.; Raessler, M. Determination of Total Organic Carbon—An Overview of Current Methods. TrAC Trends Anal. Chem. 2004, 23, 716–726. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Lacorte, S.; Barceló, D. Sampling of Water, Soil and Sediment to Trace Organic Pollutants at a River-Basin Scale. Anal. Bioanal. Chem. 2006, 386, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-M.; Zhang, Z.-Z.; Xiao, M.-Y.; Meng, B.; Kolodeznikov, V.E.; Petrova, N.N.; Mukhin, V.V.; Liu, B.-F.; Zhang, Z.-F. Screening and Quantification of Pesticides in Wetland Water, Ice, Sediment and Soil: Occurrence, Transport and Risk Assessment. Environ. Res. 2024, 263, 120143. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Min, X.-Z.; Xiao, M.-Y.; Xie, W.-X.; Li, W.-L.; Cai, M.-G.; Xiao, H.; Zhang, Z.-F. Multimedia Distribution, Dynamics, and Seasonal Variation of PAHs in Songhua Wetland: Implications for Ice-Influenced Conditions. Chemosphere 2024, 354, 141641. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Hough, R.; Yates, K.; Osprey, M.; Kerr, C.; Cooper, P.; Coull, M.; Zhang, Z. Effects of Season and Sediment-Water Exchange Processes on the Partitioning of Pesticides in the Catchment Environment: Implications for Pesticides Monitoring. Sci. Total Environ. 2020, 698, 134228. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Dou, Y.; Cong, J.; Sun, H.; Wang, L.; Duan, Z. Ecological Risk Assessment for Typical Organophosphorus Pesticides in Surface Water of China Based on a Species Sensitivity Distribution Model. Sci. Total Environ. 2024, 913, 169805. [Google Scholar] [CrossRef] [PubMed]
- Tholley, M.S.; George, L.Y.; Fu, M.; Qiao, Z.; Wang, G.; Ling, S.; Peng, C.; Zhang, W.; Ye, C.; Liu, F.; et al. Occurrence, Spatial Distribution, and Risk Assessment of Brominated Flame Retardants in Farmland Soils of Typical Provinces in China. Chemosphere 2023, 313, 137356. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhen, X.; Wang, X.; Li, Y.; Sun, X.; Tang, J. Legacy and Novel Halogenated Flame Retardants in Seawater and Atmosphere of the Bohai Sea: Spatial Trends, Seasonal Variations, and Influencing Factors. Water Res. 2020, 184, 116117. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhen, X.; Wang, X.; Zhang, D.; Sun, L.; Tang, J. Spatio-Temporal Variations and Input Patterns on the Legacy and Novel Brominated Flame Retardants (BFRs) in Coastal Rivers of North China. Environ. Pollut. 2021, 283, 117093. [Google Scholar] [CrossRef]
- Schuster, J.K.; Harner, T.; Sverko, E. Dechlorane Plus in the Global Atmosphere. Environ. Sci. Technol. Lett. 2021, 8, 39–45. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Zhang, H.; Wang, T.; Sun, H.; Zheng, S.; Li, Y.; Liang, Y.; Jiang, G. Sources and Environmental Behaviors of Dechlorane Plus and Related Compounds—A Review. Environ. Int. 2016, 88, 206–220. [Google Scholar] [CrossRef]
- Huang, C.; Zeng, Y.; Guan, K.; Qi, X.; Liu, Y.; Lu, Q.; Wang, S.; Luo, X.; Mai, B. Occurrence, Composition, and Spatial Distribution of Dechlorane plus in Surface Sediments of Black-Odorous Urban Rivers across China. Environ. Sci. Pollut. Res. 2024, 31, 17472–17480. [Google Scholar] [CrossRef] [PubMed]
- Devoy, C.; Raza, Y.; Kleiner, M.; Jones, P.D.; Doering, J.A.; Wiseman, S. The Brominated Flame Retardant, 1,2,5,6-Tetrabromocyclooctane (TBCO), Causes Multigenerational Effects on Reproductive Capacity of Japanese Medaka (Oryzias Latipes). Chemosphere 2023, 313, 137561. [Google Scholar] [CrossRef] [PubMed]
- Entai, Y.; Bei, W.E.N.; Honglin, H.; Shuzhen, Z. Analytical Methods, Environmental Behaviors and Toxicological Effects of 1, 2, 5, 6-Tetrabromocyclooctane (TBCO). Hjkxyj 2021, 34, 2008–2017. [Google Scholar] [CrossRef]
- Kharlyngdoh, J.B.; Pradhan, A.; Asnake, S.; Walstad, A.; Ivarsson, P.; Olsson, P.-E. Identification of a Group of Brominated Flame Retardants as Novel Androgen Receptor Antagonists and Potential Neuronal and Endocrine Disrupters. Environ. Int. 2015, 74, 60–70. [Google Scholar] [CrossRef]
- Recke, R.; Vetter, W. Synthesis and Characterization of 2,3-Dibromopropyl-2,4,6-Tribromophenyl Ether (DPTE) and Structurally Related Compounds Evidenced in Seal Blubber and Brain. Environ. Sci. Technol. 2007, 41, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, M.; Harner, T.; Webster, G.M.; Sverko, E.; Cheng, Y. Legacy and Current-Use Flame Retardants in House Dust from Vancouver, Canada. Environ. Pollut. 2012, 169, 175–182. [Google Scholar] [CrossRef]
- Kelly, A.; Lannuzel, D.; Rodemann, T.; Meiners, K.M.; Auman, H.J. Microplastic Contamination in East Antarctic Sea Ice. Mar. Pollut. Bull. 2020, 154, 111130. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Yan, S.; Zhang, W.; Li, Y.; Han, D. A Review of Status of Tetrabromobisphenol A (TBBPA) in China. Chemosphere 2016, 148, 8–20. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Z.; Chen, H.; Han, Y.; Xiang, M.; Chen, X.; Ma, R.; Wang, Z. Tetrabromobisphenol A: Disposition, Kinetics and Toxicity in Animals and Humans. Environ. Pollut. 2019, 253, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yin, N.; Faiola, F. Tetrabromobisphenol A (TBBPA): A Controversial Environmental Pollutant. J. Environ. Sci 2020, 97, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, L.; Wang, J.; Wu, J.; Mai, B.; Dai, J. Accumulation Pattern of Dechlorane Plus and Associated Biological Effects on Rats after 90 d of Exposure. Chemosphere 2013, 90, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Wang, Q.; Ban, X.; Zhang, H.; Li, J.; Yuan, G.-L. Aging Affects Isomer-Specific Occurrence of Dechlorane plus in Soil Profiles: A Case Study in a Geographically Isolated Landfill from the Tibetan Plateau. Sci. Total Environ. 2023, 878, 163119. [Google Scholar] [CrossRef]
- Niu, N.; Feng, L.; Lin, Y.; Li, X.; Zhang, D.; Yao, S. The Sources of Dechlorane Plus (DP) in Surface Sediment from Bohai Sea and the Northern Part of the Yellow Sea, China: Evidence from the Fractional Abundance of Anti-DP ( f Anti ) Combined with Lignin Biomarker. Reg. Stud. Mar. Sci. 2020, 39, 101437. [Google Scholar] [CrossRef]
- Li, W.-L.; Liu, L.-Y.; Song, W.-W.; Zhang, Z.-F.; Qiao, L.-N.; Ma, W.-L.; Li, Y.-F. Five-Year Trends of Selected Halogenated Flame Retardants in the Atmosphere of Northeast China. Sci. Total Environ. 2016, 539, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, W.-L.; Liu, L.-Y.; Zhang, Z.-F.; Zhu, N.-Z.; Song, W.-W.; Ma, W.-L.; Li, Y.-F. Levels, Distribution and Human Exposure of New Non-BDE Brominated Flame Retardants in the Indoor Dust of China. Environ. Pollut. 2014, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, H.; Liu, X.; Lin, T.; Guo, T.; Wang, W.; Guo, Z.; Yao, Z. Characterizing Novel Brominated Flame Retardants in the Coastal Atmosphere of the Northern Bohai and Yellow Seas. Atmos. Environ. 2023, 314, 120125. [Google Scholar] [CrossRef]
- de Jourdan, B.P.; Hanson, M.L.; Muir, D.C.G.; Solomon, K.R. Environmental Fate of Three Novel Brominated Flame Retardants in Aquatic Mesocosms. Environ. Toxicol. Chem. 2013, 32, 1060–1068. [Google Scholar] [CrossRef]
- Knudsen, G.A.; Sanders, J.M.; Birnbaum, L.S. Disposition of the Emerging Brominated Flame Retardant, Bis(2-Ethylhexyl) Tetrabromophthalate, in Female Sprague Dawley Rats: Effects of Dose, Route and Repeated Administration. Xenobiotica 2017, 47, 245–254. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.-F.; Liu, L.-Y.; Zhu, F.-J.; Ma, W.-L. National-Scale Monitoring of Historic Used Organochlorine Pesticides (OCPs) and Current Used Pesticides (CUPs) in Chinese Surface Soil: Old Topic and New Story. J. Hazard. Mater. 2023, 443, 130285. [Google Scholar] [CrossRef]
- Xiong, S.; Hao, Y.; Li, Y.; Yang, R.; Pei, Z.; Zhang, Q.; Jiang, G. Accumulation and Influencing Factors of Novel Brominated Flame Retardants in Soil and Vegetation from Fildes Peninsula, Antarctica. Sci. Total Environ. 2021, 756, 144088. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Zhong, L.; Gu, W.; Liang, M.; Wang, L.; Wang, Z.; Shi, L.; Sun, S. Occurrence and Accumulation Characteristics of Legacy and Novel Brominated Flame Retardants in Surface Soil and River Sediments from the Downstream of Chuhe River Basin, East China. Environ. Sci. Pollut. Res. 2023, 30, 97416–97425. [Google Scholar] [CrossRef] [PubMed]
- Nemirovskaya, I.A. The Content and Composition of Organic Compounds in the Snow-Ice Cover of the White Sea. Geochem. Int. 2009, 47, 393–404. [Google Scholar] [CrossRef]
- Pućko, M.; Stern, G.A.; Burt, A.E.; Jantunen, L.M.; Bidleman, T.F.; Macdonald, R.W.; Barber, D.G.; Geilfus, N.-X.; Rysgaard, S. Current Use Pesticide and Legacy Organochlorine Pesticide Dynamics at the Ocean-Sea Ice-Atmosphere Interface in Resolute Passage, Canadian Arctic, during Winter-Summer Transition. Sci. Total Environ. 2017, 580, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Blum, J.D.; Douglas, T.A.; Steffen, A. Frost Flowers Growing in the Arctic Ocean-Atmosphere–Sea Ice–Snow Interface: 2. Mercury Exchange between the Atmosphere, Snow, and Frost Flowers. J. Geophys. Res. Atmos. 2012, 117, D00R10. [Google Scholar] [CrossRef]
- Cong, L.; Fang, Y.; He, M.; Wang, X.; Kannan, N.; Li, D. Ice Phase as an Important Factor on the Seasonal Variation of Polycyclic Aromatic Hydrocarbons in the Tumen River, Northeastern of China. Environ. Sci. Pollut. Res. 2010, 17, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.; Halsall, C.; Thomas, M.; France, J.; Kaiser, J.; Graf, C.; Leeson, A.; Wynn, P. Mechanistic Insight into the Uptake and Fate of Persistent Organic Pollutants in Sea Ice. Environ. Sci. Technol. 2019, 53, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pućko, M.; Stern, G. Transport and Transformation of Contaminants in Sea Ice. In Sea Ice: Third Edition; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 472–491. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Zhang, Y.; Tian, J.; Song, L.; Han, J.; Yu, J.; Zhang, Y. Spatial Distribution and Ecological Risks of Polycyclic Aromatic Hydrocarbons in Sea Ice and Seawater from Northern Liaodong Bay, China. Mar. Pollut. Bull. 2022, 174, 113319. [Google Scholar] [CrossRef] [PubMed]
- Pućko, M.; Stern, G.A.; Barber, D.G.; Macdonald, R.W.; Warner, K.-A.; Fuchs, C. Mechanisms and Implications of α-HCH Enrichment in Melt Pond Water on Arctic Sea Ice. Environ. Sci. Technol. 2012, 46, 11862–11869. [Google Scholar] [CrossRef] [PubMed]
- Pućko, M.; Stern, G.A.; Macdonald, R.W.; Rosenberg, B.; Barber, D.G. The Influence of the Atmosphere-Snow-Ice-Ocean Interactions on the Levels of Hexachlorocyclohexanes in the Arctic Cryosphere. J. Geophys. Res. Ocean. 2011, 116, C02035. [Google Scholar] [CrossRef]
- Dethleff, D.; Kuhlmann, G. Entrainment of Fine-Grained Surface Deposits into New Ice in the Southwestern Kara Sea, Siberian Arctic. Cont. Shelf Res. 2009, 29, 691–701. [Google Scholar] [CrossRef]
- Reimnitz, E.; Barnes, P.W.; Weber, W.S. Particulate Matter in Pack Ice of the Beaufort Gyre. J. Glaciol. 1993, 39, 186–198. [Google Scholar] [CrossRef]
- Pfirman, S.L.; Eicken, H.; Bauch, D.; Weeks, W.F. The Potential Transport of Pollutants by Arctic Sea Ice. Sci. Total Environ. 1995, 159, 129. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Poot, A.; Lange, H.J.D.; Velzeboer, I.; Harmsen, J.; Noort, P.C.M. van. Estimation of In Situ Sediment-to-Water Fluxes of Polycyclic Aromatic Hydrocarbons, Polychlorobiphenyls and Polybrominated Diphenylethers. Environ. Sci. Technol. 2010, 44, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Huang, Q.; Pan, Y.; Lin, L.; Liu, S.; Li, H.; Xu, X. Novel Brominated Flame Retardants (NBFRs) in a Tropical Marine Food Web from the South China Sea: The Influence of Hydrophobicity and Biotransformation on Structure-Related Trophodynamics. Environ. Sci. Technol. 2022, 56, 3147–3158. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chenguang, L.; Shiqi, Y.; Yanlei, Z.; Fengmin, L. A Review on Ecotoxicity of Novel Brominated Flame Retardants (NBFRs). Asian J. Ecotoxicol. 2023, 18, 212–223. [Google Scholar] [CrossRef]
- Wang, N.; He, L.; Sun, X.; Bu, Y. The Potential Environmental Behavior and Risks of TBECH Transformation Initiated by Reactive Oxygen Species in Natural Waters and AOPs. Ecotoxicol. Environ. Saf. 2021, 228, 113055. [Google Scholar] [CrossRef]
- Devoy, C.; Raza, Y.; Jones, P.D.; Doering, J.A.; Wiseman, S. Japanese Medaka (Oryzias Latipes) Exposed via Maternal Transfer to the Brominated Flame Retardant, 1,2,5,6-Tetrabromocyclooctane (TBCO), Experience Decreased Fecundity and Impaired Oocyte Maturation. Aquat. Toxicol. 2023, 265, 106761. [Google Scholar] [CrossRef]
- Van Essen, D.; Devoy, C.; Miller, J.; Jones, P.D.; Wiseman, S. Effects of the Brominated Flame Retardant, TBCO, on Development of Zebrafish (Danio Rerio) Embryos. Chemosphere 2021, 266, 129195. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Emerging and Novel Brominated Flame Retardants (BFRs) in Food. EFSA J. 2012, 10, 2908. [Google Scholar] [CrossRef]
- Ling, S.; Zhou, S.; Tan, J.; Lu, C.; Fu, M.; Peng, C.; Zhang, W.; Hu, S.; Lin, K.; Zhou, B. Brominated Flame Retardants (BFRs) in Sediment from a Typical e-Waste Dismantling Region in Southern China: Occurrence, Spatial Distribution, Composition Profiles, and Ecological Risks. Sci. Total Environ. 2022, 824, 153813. [Google Scholar] [CrossRef]
- Xie, Y.; Li, M.; Ma, J.; Gong, X.; Tong, Y.; Wang, D.; Ai, L.; Gong, Z. Occurrence and Distribution of Legacy and Novel Brominated Flame Retardants in River and Sediments in Southwest China: A Seasonal Investigation. Environ. Res. 2024, 262, 119842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).