Synergistic Effect of Ultrasound and Osmotic Pretreatment on the Drying Kinetics and Antioxidant Properties of Satkara (Citrus macroptera): A Novel Preservation Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Ultrasound-Osmotic Pretreatment (US-OP) Process
2.4. Drying of Satkara
2.5. Drying Kinetics
2.5.1. Determination of Moisture Content
2.5.2. Mathematical Modeling of Drying Data
2.5.3. Evaluation of Mathematical Models
2.5.4. Determination of the Activation Energy (Ea)
2.6. Quality Assessment of Satkara
2.6.1. Preparation of Sample Extracts
2.6.2. Determination of Vitamin C (Ascorbic Acid) Content
2.6.3. Determination of Total Phenolic Content
2.6.4. Determination of Total Flavonoid Content
2.6.5. Antioxidant Activity Assessment
2.7. Statistical Analysis
3. Results and Discussion
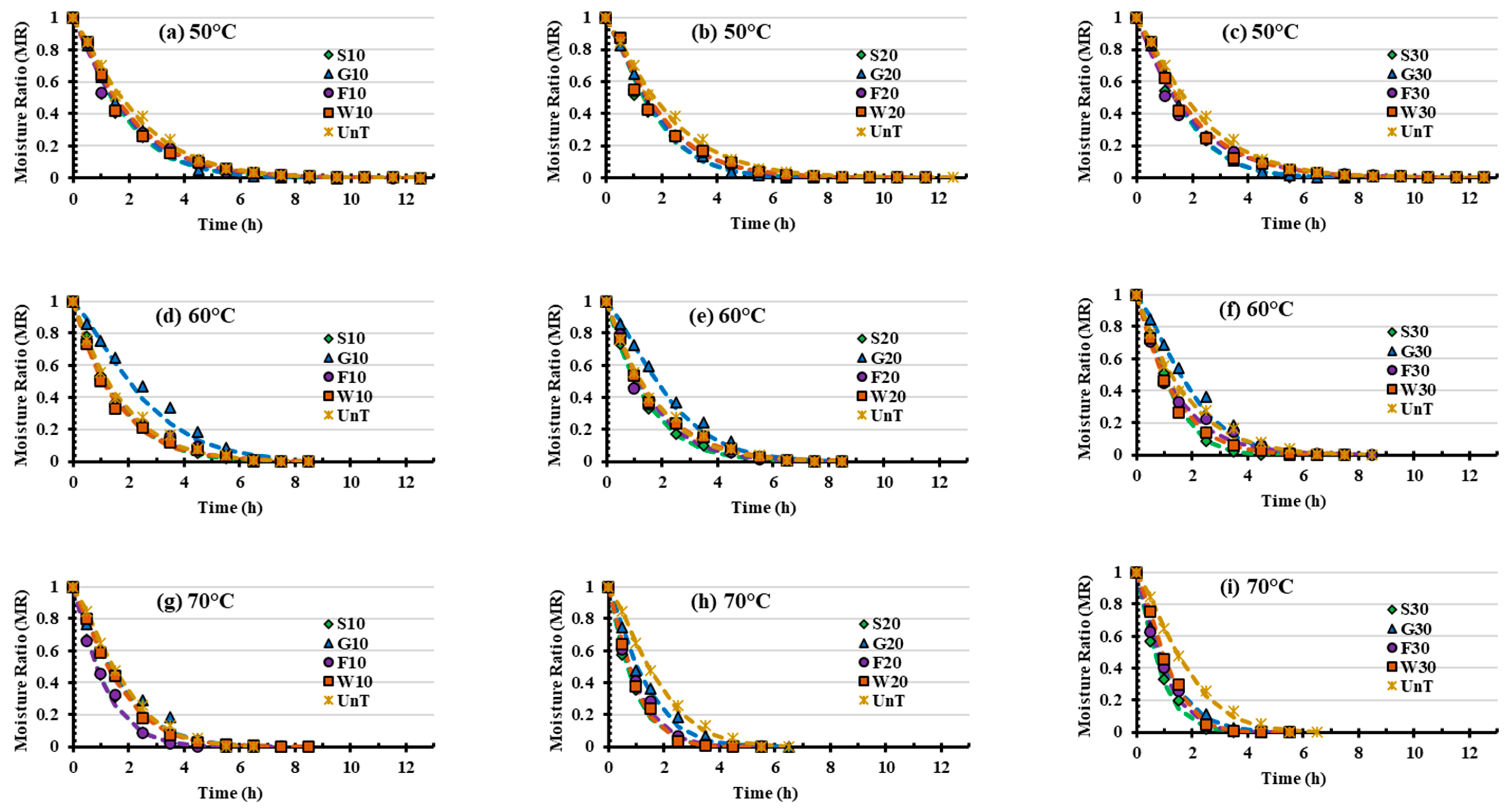
3.1. Drying Characteristics of Ultrasound-Osmotic Pretreated Satkara
3.2. Fitting and Evaluation of Mathematical Models
3.3. Quality Evaluation of Dried Satkara
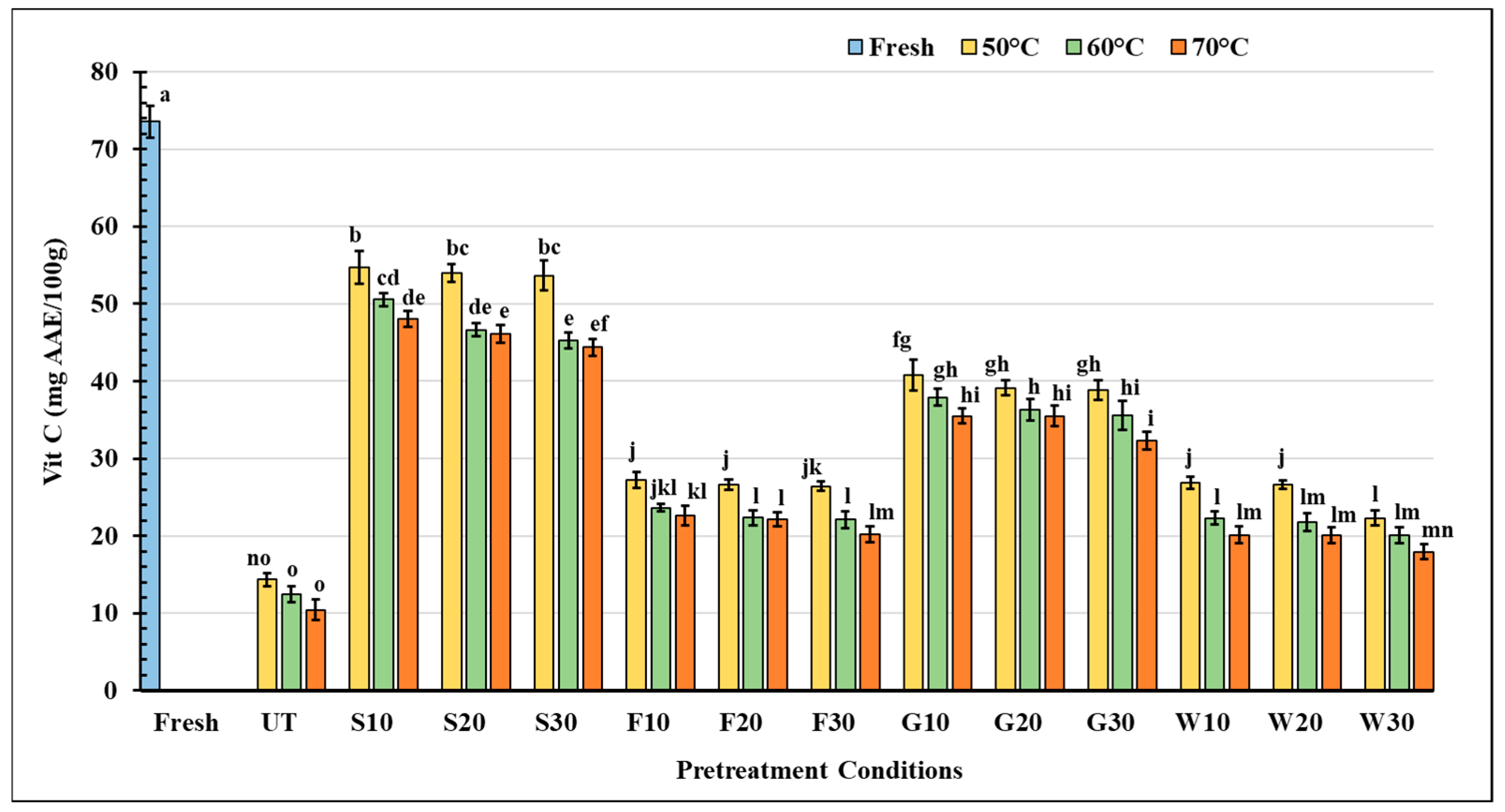
3.3.1. Vitamin C (Ascorbic Acid) Content
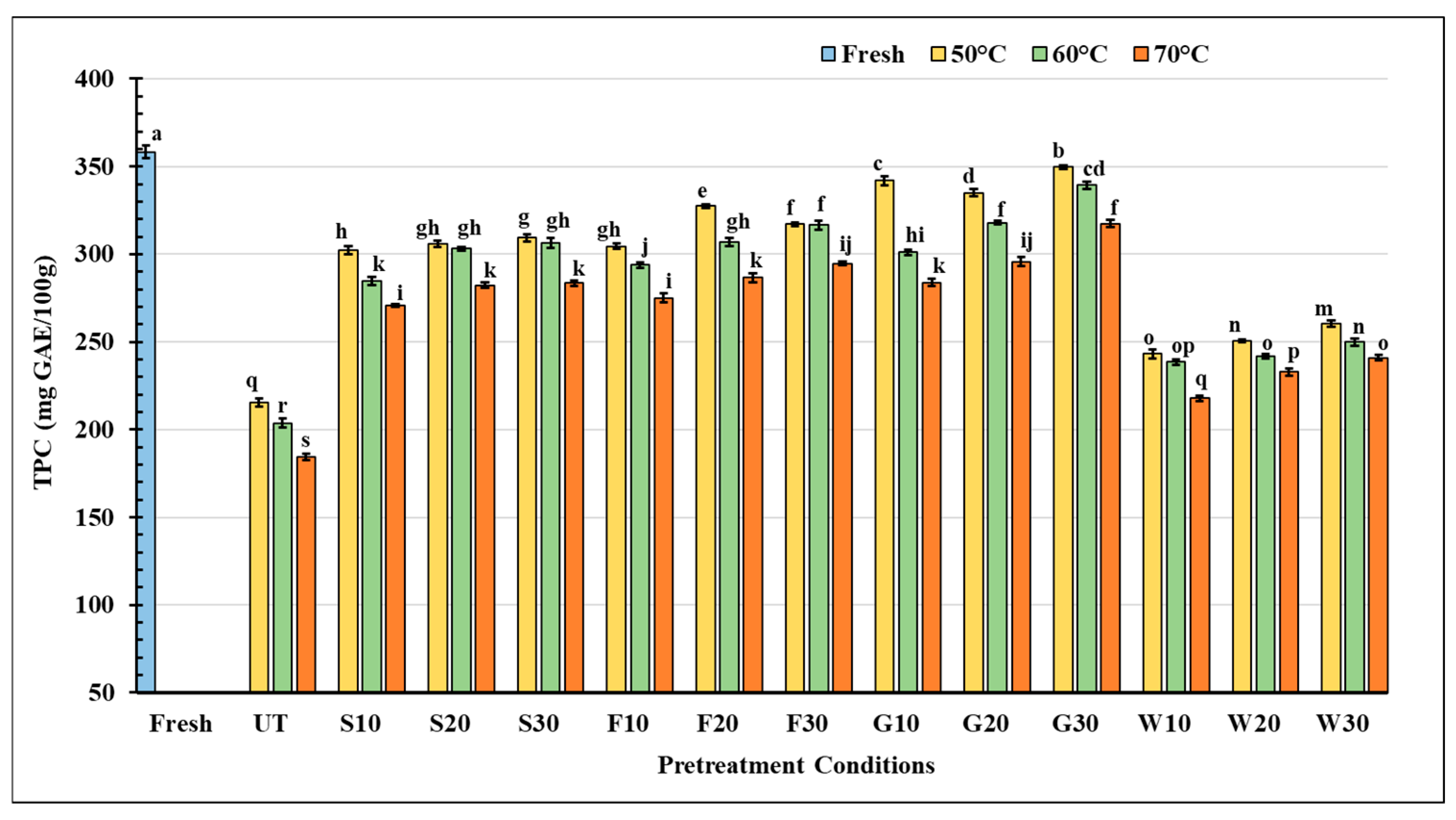
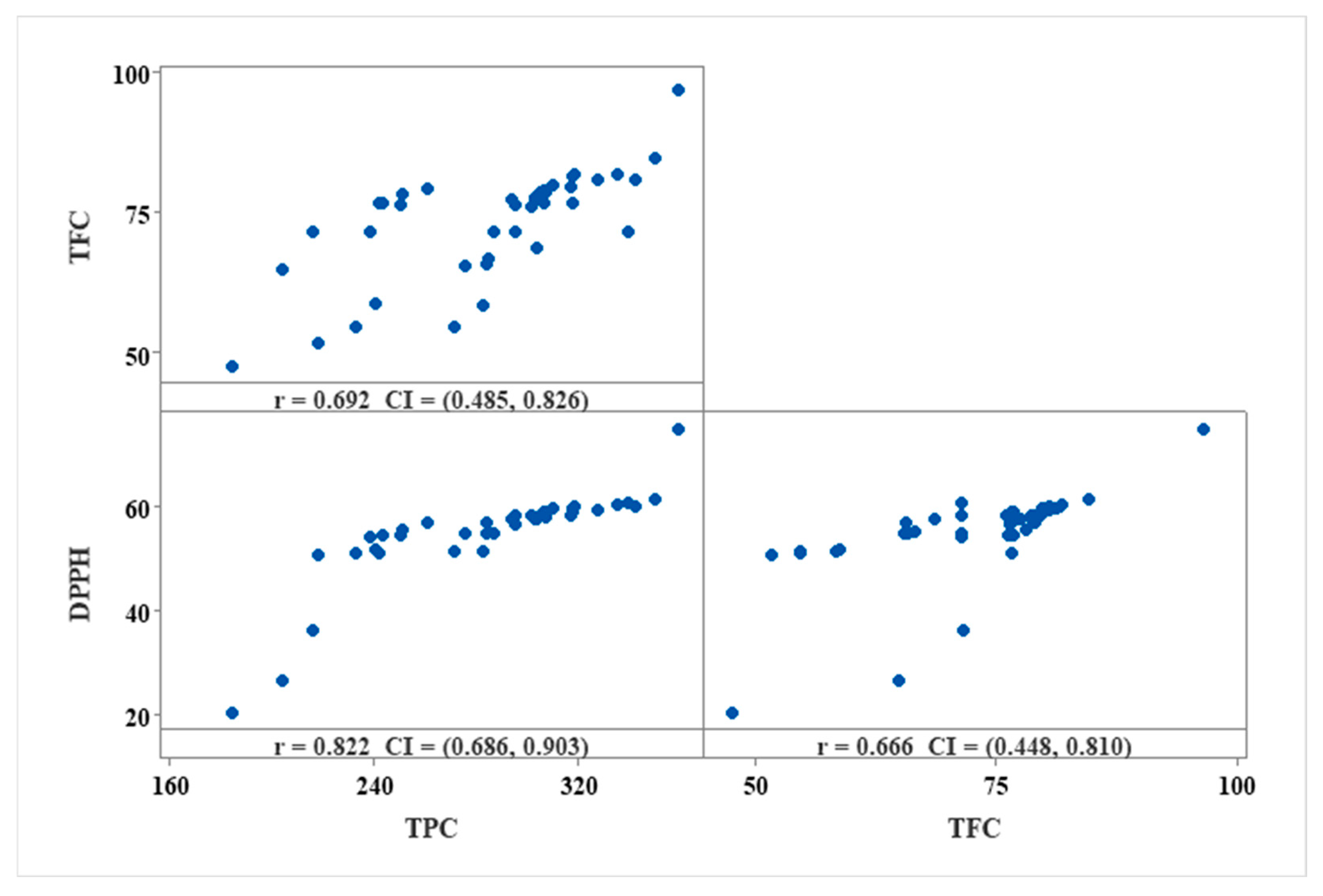
3.3.2. Total Phenolic Content (TPC)
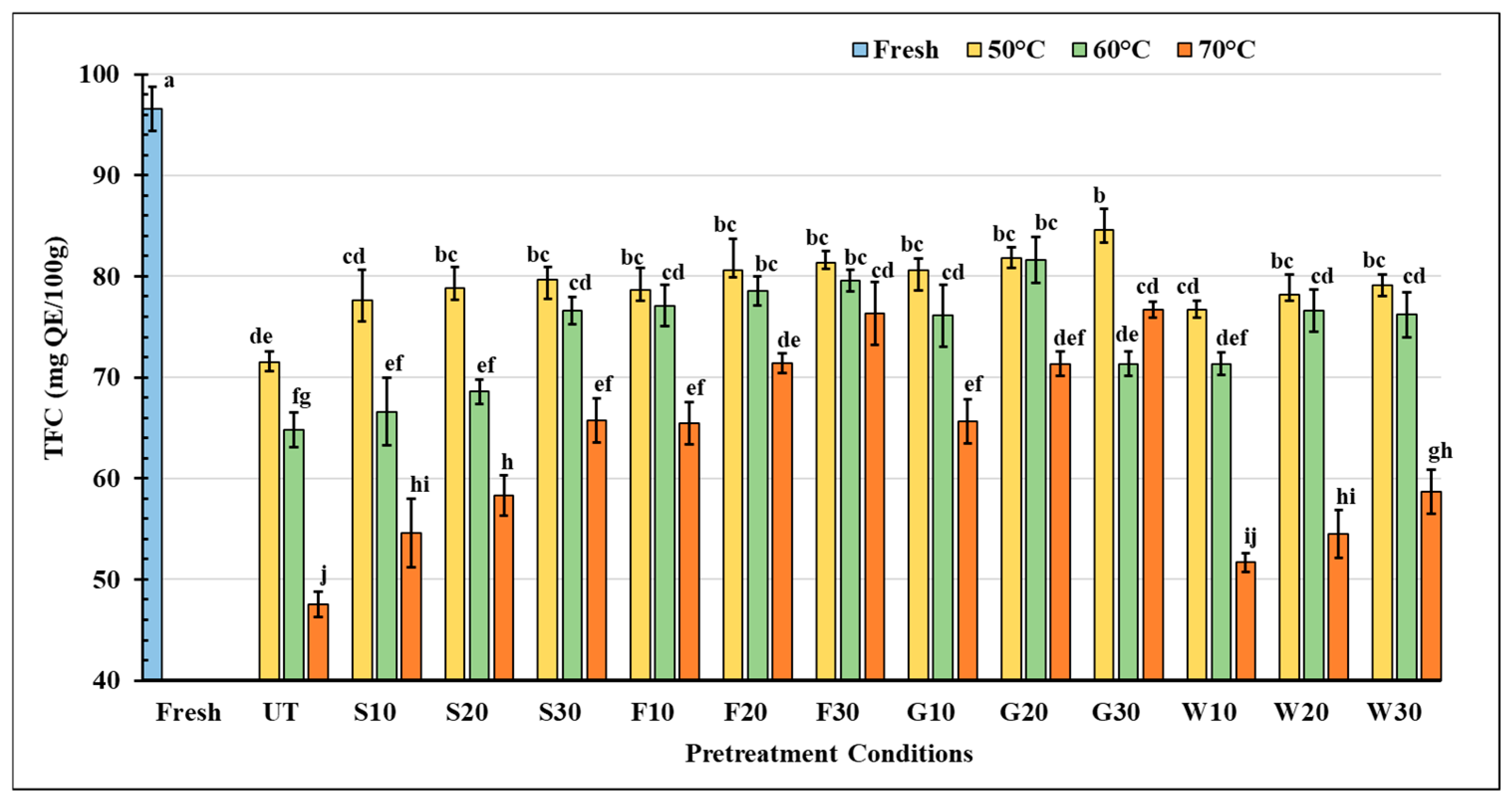
3.3.3. Total Flavonoid Content (TFC)
3.3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar Bhattacharjya, D.; Pujirahayu, N.; Suzuki, T.; Katayama, T. Chemical constituents of whole fruit of Citrus macroptera and their antioxidant activity. J. For. Biomass Util. Soc. 2020, 15, 29–38. [Google Scholar]
- Upadhaya, A.; Chaturvedi, S.S.; Tiwari, B.K. Utilization of wild Citrus by Khasi and Garo tribes of Meghalaya. Ind. J. Tradit. Knowl. 2016, 15, 121–127. [Google Scholar]
- Hazarika, T.K.; Singh, T.S. Wild edible fruits of Manipur, India: Associated traditional knowledge and implications to sustainable livelihood. Genet. Resour. Crop Evol. 2018, 65, 319–332. [Google Scholar] [CrossRef]
- Aktar, K.; Foyzun, T. Phytochemistry and Pharmacological Studies of Citrus macroptera: A Medicinal Plant Review. Evid. Based Complement. Alternat. Med. 2017, 2017, 9789802. [Google Scholar] [CrossRef] [PubMed]
- Amani, T.; Surenthar, M.; Shanmugam, R. Anti-inflammatory and Antioxidant Activity of Cucumis sativus and Citrus macroptera Herbal Formulation: An In-Vitro Study. Cureus 2024, 16, e51818. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.; Mahjabin, T.; Khan, M.R.M.; Hossain, M.; Sadia, U.; Zzaman, W. Effect of cooking methods on the nutritional quality of selected vegetables at Sylhet City. Heliyon 2023, 9, e21709. [Google Scholar] [CrossRef]
- Hasan, M.M.; Roy, P.; Alam, M.; Hoque, M.M.; Zzaman, W. Antimicrobial activity of peels and physicochemical properties of juice prepared from indigenous citrus fruits of Sylhet region, Bangladesh. Heliyon 2022, 8, e09948. [Google Scholar] [CrossRef]
- Roy, M.; Bulbul, M.A.I.; Hossain, M.A.; Shourove, J.H.; Ahmed, S.; Sarkar, A.; Biswas, R. Study on the drying kinetics and quality parameters of osmotic pre-treated dried Satkara (Citrus macroptera) fruits. J. Food Meas. Charact. 2022, 16, 471–485. [Google Scholar] [CrossRef]
- Xu, B.; Sylvain Tiliwa, E.; Yan, W.; Roknul Azam, S.M.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res. Int. 2022, 152, 110744. [Google Scholar] [CrossRef]
- Zzaman, W.; Biswas, R.; Hossain, M.A. Application of immersion pre-treatments and drying temperatures to improve the comprehensive quality of pineapple (Ananas comosus) slices. Heliyon 2021, 7, e05882. [Google Scholar] [CrossRef]
- Hossain, M.A.; Talukder, S.; Uz Zaman, A.; Sarkar, A.; Yasin, M.; Biswas, R. Effective drying processes for Taikor (Garcinia pedunculata Roxb.) fruit by ultrasound-assisted osmotic pretreatment: Analysis of quality and kinetic models. Ultrason. Sonochem. 2024, 103, 106784. [Google Scholar] [CrossRef] [PubMed]
- Prithani, R.; Dash, K.K. Mass transfer modelling in ultrasound assisted osmotic dehydration of kiwi fruit. Innov. Food Sci. Emerg. Technol. 2020, 64, 102407. [Google Scholar] [CrossRef]
- Kucner, A.; Klewicki, R.; Sójka, M. The Influence of Selected Osmotic Dehydration and Pretreatment Parameters on Dry Matter and Polyphenol Content in Highbush Blueberry (Vaccinium corymbosum L.) Fruits. Food Bioproc. Technol. 2013, 6, 2031–2047. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Nsor-Atindana, J.; Wang, Y.C. Use of Ultrasound Pretreatment in Drying of Fruits: Drying Rates, Quality Attributes, and Shelf-Life Extension. Drying Technol. 2011, 29, 1611–1621. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Ma, H.; Qu, W.; Zhou, C.; Wu, B.; Yang, X. Influence of ultrasound pretreatments on diffusion coefficients, texture and colour of osmodehydrated sweet potato (Ipomea batatas). Int. J. Food Sci. Technol. 2017, 52, 888–896. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, S.; Tarafdar, A.; Nema, P.K. Ultrasonic and osmotic pretreatments followed by convective and vacuum drying of papaya slices. J. Sci. Food Agric. 2021, 101, 2264–2272. [Google Scholar] [CrossRef]
- Gül, O.; Açikgöz, N.; Gül, L.B. Effect of ultrasound assisted osmotic dehydration pre-treatment on drying kinetics and some functional properties of pumpkin (Cucurbita moschata). GIDA—J. Food 2022, 47, 874–888. [Google Scholar]
- Jiang, J.; Zhang, M.; Devahastin, S.; Yu, D. Effect of ultrasound-assisted osmotic dehydration pretreatments on drying and quality characteristics of pulsed fluidized bed microwave freeze-dried strawberries. LWT 2021, 145, 111300. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, M.; Mujumdar, A.S.; Yu, D. Influence of ultrasonic-assisted osmotic dehydration pretreatment on hot air-assisted radio frequency drying of bitter gourd. Food Biosci. 2024, 59, 103923. [Google Scholar] [CrossRef]
- Ramallo, L.A.; Mascheroni, R.H. Quality evaluation of pineapple fruit during drying process. Food Bioprod. Process. 2012, 90, 275–283. [Google Scholar] [CrossRef]
- Hossain, M.A.; Dey, P.; Joy, R.I. Effect of osmotic pretreatment and drying temperature on drying kinetics, antioxidant activity, and overall quality of taikor (Garcinia pedunculata Roxb.) slices. Saudi J. Biol. Sci. 2021, 28, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Hossain, M.A.; Zzaman, W. Thin layer modeling of drying kinetics, rehydration kinetics and color changes of osmotic pre-treated pineapple (Ananas comosus) slices during drying: Development of a mechanistic model for mass transfer. Innov. Food Sci. Emerg. Technol. 2022, 80, 103094. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Osmotic dehydration with sorbitol combined with hot air convective drying of apple cubes. J. Food Sci. Technol. 2017, 54, 3152–3160. [Google Scholar] [CrossRef] [PubMed]
- Korese, J.K.; Achaglinkame, M.A. Convective drying of Gardenia erubescens fruits: Effect of pretreatment, slice thickness and drying air temperature on drying kinetics and product quality. Heliyon 2024, 10, e25968. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.C.; Singh, R.P. Thin-Layer Drying Studies on Short-Grain Rough Rice; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1977. [Google Scholar]
- Ayensu, A. Dehydration of food crops using a solar dryer with convective heat flow. Sol. Energy 1997, 59, 121–126. [Google Scholar] [CrossRef]
- Westerman, P.W.; White, G.M.; Ross, I.J. Relative Humidity Effect on the High-Temperature Drying of Shelled Corn. Trans. ASAE 1973, 16, 1136–1139. [Google Scholar] [CrossRef]
- Yagcioglu, A.D.A.C.F. Drying characteristic of laurel leaves under different conditions. In Proceedings of the 7th International Congress on Agricultural Mechanization and Energy, Adana, Turkey, 26–27 May 1999; pp. 565–569. [Google Scholar]
- Castro, A.M.; Mayorga, E.Y.; Moreno, F.L. Mathematical modelling of convective drying of fruits: A review. J. Food Eng. 2018, 223, 152–167. [Google Scholar] [CrossRef]
- Hossain, M.A.; Evan, M.S.S.; Moazzem, M.S.; Roy, M.; Zzaman, W. Response Surface Optimization for Antioxidant Extraction from Jackfruit (Artocarpus heterophyllus Lam.) Seed and Pulp. J. Sci. Res. 2020, 12, 397–409. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Pinna, I.; Fenu, P.M.; Agabbio, M. Effect of Drying Conditions and Storage Period on Polyphenolic Content, Antioxidant Capacity, and Ascorbic Acid of Prunes. J. Agric. Food Chem. 2004, 52, 4780–4784. [Google Scholar] [CrossRef]
- Ahmed, S.; Jubair, A.; Hossain, M.A.; Hossain, M.M.; Azam, M.S.; Biswas, M. Free radical-scavenging capacity and HPLC-DAD screening of phenolic compounds from pulp and seed of Syzygium claviflorum fruit. J. Agric. Food Res. 2021, 6, 100203. [Google Scholar] [CrossRef]
- Taşkin, O.; İzli, G.; İzli, N. Convective drying kinetics and quality parameters of European Cranberrybush. Tarim Bilim. Derg. 2018, 24, 349–358. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A. Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod. Process. 2013, 91, 495–506. [Google Scholar] [CrossRef]
- Garcia-Noguera, J.; Oliveira, F.I.P.; Gallão, M.I.; Weller, C.L.; Rodrigues, S.; Fernandes, F.A.N. Ultrasound-Assisted Osmotic Dehydration of Strawberries: Effect of Pretreatment Time and Ultrasonic Frequency. Dry. Technol. 2010, 28, 294–303. [Google Scholar] [CrossRef]
- Ispir, A.; Toǧrul, I.T. Osmotic dehydration of apricot: Kinetics and the effect of process parameters. Che. Eng. Res. Des. 2009, 87, 166–180. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, Z.; Yu, F.; Yang, Z. Effect of ultrasound on heat pump drying characteristics of pea seeds. Int. J. Food Eng. 2018, 14, 20180204. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; An, K.; Zou, B.; Yang, W. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT 2021, 138, 110653. [Google Scholar] [CrossRef]
- Yildiz, G.; İzli, G. Influence of microwave and microwave-convective drying on the drying kinetics and quality characteristics of pomelo. J Food Process. Preserv. 2019, 43, e13812. [Google Scholar] [CrossRef]
- Thao, B.T.T.; Vo, T.T.K.; Tran, T.Y.N.; Le, D.T.; Tran, T.T.; Bach, L.G.; Dao, T.P. Application of mathematical techniques to study the moisture loss kinetics and polyphenol degradation kinetics of mango (Mangifera indica L.) slices during heat pump drying by pilot equipment. LWT 2023, 176, 114454. [Google Scholar] [CrossRef]
- Meisami-asl, E.; Rafiee, S.; Keyhani, A.; Tabatabaeefar, A. Determination of Suitable Thin Layer Drying Curve Model for Apple Slices (variety-Golab). Plant Omics 2010, 3, 103–108. [Google Scholar]
- López, J.; Uribe, E.; Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Gonzalez, E.; Di Scala, K. Effect of air temperature on drying kinetics, vitamin c, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety óneil. Food Bioproc. Technol. 2010, 3, 772–777. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Chen, G. Recent advances of novel thermal combined hot air drying of agricultural crops. Trends Food Sci. Technol. 2016, 57, 132–145. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; da Silva, G.M.; Monteiro, S.S.; André, A.M. Effect of ultrasound pre-treatment on the kinetics and thermodynamic properties of guava slices drying process. Innov. Food Sci. Emerg. Technol. 2020, 66, 102507. [Google Scholar] [CrossRef]
- Kaveh, M.; Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Taghinezhad, E.; Moghimi, M.B.F. The effect of ultrasound pre-treatment on quality, drying, and thermodynamic attributes of almond kernel under convective dryer using ANNs and ANFIS network. J. Food Process Eng. 2018, 41, e12868. [Google Scholar] [CrossRef]
- Bai, J.W.; Gao, Z.J.; Xiao, H.W.; Wang, X.T.; Zhang, Q. Polyphenol oxidase inactivation and vitamin C degradation kinetics of Fuji apple quarters by high humidity air impingement blanching. Int. J. Food Sci. Technol. 2013, 48, 1135–1141. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chem. 2009, 117, 647–653. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Retention of Vitamin C in Drying Processes of Fruits and Vegetables—A Review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P.; Harris, N.D. Effect of Sucrose on Oxygen Uptake of Ascorbic Acid in a Closed Aqueous System. J. Agric. Food Chem. 1993, 41, 259–262. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Hall, R.D.; Capanoglu, E.A. Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2016, 56, S110–S129. [Google Scholar] [CrossRef]
- Cao, X.; Islam, M.N.; Zhong, S.; Pan, X.; Song, M.; Shang, F.; Nie, H.; Xu, W.; Duan, Z. Drying kinetics, antioxidants, and physicochemical properties of litchi fruits by ultrasound-assisted hot air-drying. J. Food Biochem. 2020, 44, e13073. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Rayaguru, K.; Nayak, P.K. Ultra-sonicated vacuum drying’s effect on antioxidant activity, TPC, TFC and color of elephant apple slices: Drying of elephant apple. Food Biosci. 2020, 36, 100629. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, Q.; Wang, T.; Xu, Y.; Zang, Z.; Wan, F.; Yue, Y.; Huang, X. Effect of Ultrasonic Pretreatment on the Far-Infrared Drying Process and Quality Characteristics of Licorice. Foods 2023, 12, 2414. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Perussello, C.A.; Zhang, Z.; Kerry, J.P.; Tiwari, B.K. Impact of ultrasound and blanching on functional properties of hot-air dried and freeze-dried onions. LWT 2018, 87, 102–111. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Wang, Q.; Sun, C.; Xi, H. Drying characteristics, microstructure, glass transition temperature, and quality of ultrasound-strengthened hot air drying on pear slices. J. Food Process. Preserv. 2019, 43, e13899. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Degradation kinetics of total phenolic compounds, capsaicinoids and antioxidant activity in red pepper during hot air and infrared drying process. Int. J. Food Sci. Technol. 2016, 51, 842–853. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Li, M.; Pare, P.W.; Zhang, J.; Kang, T.; Zhang, Z.; Yang, D.; Wang, K.; Xing, H. Antioxidant capacity connection with phenolic and flavonoid content in Chinese medicinal herbs. Rec. Nat. Prod. 2018, 12, 239–250. [Google Scholar] [CrossRef]
- Alonzo-Macías, M.; Cardador-Martínez, A.; Mounir, S.; Montejano-Gaitán, G.; Allaf, K. Comparative Study of the Effects of Drying Methods on Antioxidant Activity of Dried Strawberry (Fragaria Var. Camarosa). J. Food Res. 2013, 2, 92–107. [Google Scholar] [CrossRef]
- García-Moreira, D.P.; Hernández-Guzmán, H.; Pacheco, N.; Cuevas-Bernardino, J.C.; Herrera-Pool, E.; Moreno, I.; López-Vidaña, E.C. Solar and Convective Drying: Modeling, Color, Texture, Total Phenolic Content, and Antioxidant Activity of Peach (Prunus persica (L.) Batsch) Slices. Processes 2023, 11, 1280. [Google Scholar] [CrossRef]
- Chobot, M.; Kozłowska, M.; Ignaczak, A.; Kowalska, H. Development of drying and roasting processes for the production of plant-based pro-healthy snacks in the light of nutritional trends and sustainable techniques. Trends Food Sci. Technol. 2024, 149, 104553. [Google Scholar] [CrossRef]
- Yulni, T.; Agusta, W.; Jayanegara, A.; Alfa, M.N.; Hartono, L.K.; Mariastuty, T.E.P.; Hermansyah, H.D.; Fauziah, P.Y.; Anggraeni, D.; Lintang, M.M.J. Unveiling the Influence of Osmotic Pretreatment on Dried Fruit Characteristics: A Meta-Analysis Approach. Prev. Nutr. Food Sci. 2024, 29, 178. [Google Scholar] [CrossRef]

| Group Symbols | Pretreatment Conditions |
|---|---|
| UT | Neither osmotic nor sonication treatment was involved |
| S10 | 10% Sucrose solution with 10 min sonication |
| G10 | 10% Glucose solution with 10 min sonication |
| F10 | 10% Fructose solution with 10 min sonication |
| W10 | Water with 10 min sonication |
| S20 | 10% Sucrose solution with 20 min sonication |
| G20 | 10% Glucose solution with 20 min sonication |
| F20 | 10% Fructose solution with 20 min sonication |
| W20 | Water with 20 min sonication |
| S30 | 10% Sucrose solution with 30 min sonication |
| G30 | 10% Glucose solution with 30 min sonication |
| F30 | 10% Fructose solution with 30 min sonication |
| W30 | Water with 30 min sonication |
| Name of the Model | Equation | References |
|---|---|---|
| Page | MR = exp (−ktn) | [25] |
| Newton | MR = exp (−kt) | [26] |
| Handerson and Pabis | MR = a exp (−kt) | [27] |
| Logarithmic | MR = a exp (−kt) + c | [28] |
| Tem. | Pre-Treatment | Page Model | Newton Model | Henderson and Pabis Model | Logarithmic Model | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | n | R2 | RMSE | χ2 | k | R2 | RMSE | χ2 | k | a | R2 | RMSE | χ2 | a | k | c | R2 | RMSE | χ2 | ||
| 50 °C | UT | 0.3682 | 1.1714 | 0.997 | 0.0184 | 0.0004 | 0.5354 | 0.9717 | 0.0561 | 0.0034 | 0.5672 | 0.7782 | 0.8338 | 0.1358 | 0.0213 | 1.2414 | 0.7686 | 0.0938 | 0.8889 | 0.111 | 0.0154 |
| S10 | 0.4863 | 1.1592 | 0.9891 | 0.0352 | 0.0015 | 0.6459 | 0.9829 | 0.044 | 0.0021 | 0.6867 | 0.8092 | 0.8739 | 0.1195 | 0.0175 | 0.9636 | 0.7048 | 0.0813 | 0.971 | 0.0573 | 0.0045 | |
| G10 | 0.4404 | 1.2226 | 0.998 | 0.0155 | 0.0003 | 0.679 | 0.9586 | 0.0696 | 0.0053 | 0.7371 | 0.7114 | 0.7287 | 0.1783 | 0.0382 | 1.0268 | 0.6539 | 0.0718 | 0.969 | 0.0603 | 0.0049 | |
| F10 | 0.4767 | 1.0749 | 0.9899 | 0.0322 | 0.0012 | 0.552 | 0.9913 | 0.0298 | 0.0009 | 0.564 | 1.0900 | 0.9856 | 0.0384 | 0.0017 | 1.011 | 0.7065 | 0.078 | 0.9665 | 0.0586 | 0.0044 | |
| W10 | 0.4418 | 1.1033 | 0.9939 | 0.0254 | 0.0007 | 0.5365 | 0.9929 | 0.0273 | 0.0008 | 0.5427 | 0.9525 | 0.9837 | 0.0413 | 0.002 | 1.1025 | 0.7305 | 0.0597 | 0.9681 | 0.0579 | 0.0042 | |
| S20 | 0.4761 | 1.2626 | 0.9842 | 0.0431 | 0.0023 | 0.7652 | 0.9486 | 0.0779 | 0.0067 | 0.8604 | 1.5348 | 0.7116 | 0.1844 | 0.0425 | 1.1615 | 1.158 | 0.0633 | 0.5693 | 0.2254 | 0.0726 | |
| G20 | 0.4437 | 1.3198 | 0.9974 | 0.0181 | 0.0004 | 0.7823 | 0.9207 | 0.0998 | 0.0111 | 0.8878 | 0.6220 | 0.5112 | 0.2477 | 0.0767 | 1.1185 | 0.8002 | 0.0803 | 0.9427 | 0.0848 | 0.008 | |
| F20 | 0.4482 | 1.1698 | 0.9889 | 0.0349 | 0.0014 | 0.6148 | 0.9844 | 0.0414 | 0.0019 | 0.6417 | 0.8385 | 0.91 | 0.0995 | 0.0117 | 1.197 | 0.8781 | 0.0766 | 0.9287 | 0.0886 | 0.0102 | |
| W20 | 0.4340 | 1.1798 | 0.9883 | 0.0356 | 0.0015 | 0.6105 | 0.9835 | 0.0423 | 0.0019 | 0.6313 | 0.8609 | 0.9261 | 0.0894 | 0.008 | 1.0542 | 0.6915 | 0.0551 | 0.9768 | 0.0501 | 0.0032 | |
| S30 | 0.4699 | 1.297 | 0.9886 | 0.0372 | 0.0018 | 0.7678 | 0.9454 | 0.0814 | 0.0075 | 0.8717 | 0.6728 | 0.5938 | 0.2219 | 0.0633 | 1.0719 | 0.677 | 0.0253 | 0.9847 | 0.043 | 0.0028 | |
| G30 | 0.4431 | 1.3398 | 0.9982 | 0.0153 | 0.0003 | 0.8033 | 0.9142 | 0.1044 | 0.01 | 0.9131 | 0.6101 | 0.4889 | 0.2548 | 0.0812 | 1.2474 | 0.9317 | 0.0784 | 0.887 | 0.1198 | 0.0205 | |
| F30 | 0.5144 | 1.0213 | 0.9885 | 0.0334 | 0.0013 | 0.5249 | 0.989 | 0.0327 | 0.0012 | 0.5165 | 1.0685 | 0.9765 | 0.0479 | 0.0026 | 1.1746 | 0.9339 | 0.0856 | 0.9263 | 0.0847 | 0.009 | |
| W30 | 0.4575 | 1.0948 | 0.9937 | 0.0256 | 0.0008 | 0.5397 | 0.9934 | 0.0263 | 0.0007 | 0.5377 | 1.0158 | 0.9945 | 0.0241 | 0.0007 | 1.1808 | 0.7542 | 0.0365 | 0.9653 | 0.0603 | 0.0045 | |
| 60 °C | UT | 0.5564 | 1.0823 | 0.9934 | 0.0264 | 0.0009 | 0.6905 | 0.9787 | 0.0476 | 0.0028 | 0.753 | 0.7234 | 0.7759 | 0.1545 | 0.0292 | 1.2577 | 0.9502 | 0.0752 | 0.8728 | 0.1164 | 0.0187 |
| S10 | 0.5865 | 1.1187 | 0.9916 | 0.0306 | 0.0012 | 0.7481 | 0.9824 | 0.0442 | 0.0022 | 0.8076 | 0.7654 | 0.8276 | 0.1385 | 0.024 | 0.9803 | 0.8173 | 0.078 | 0.9754 | 0.0524 | 0.0039 | |
| G10 | 0.2855 | 1.2933 | 0.9816 | 0.0475 | 0.0028 | 0.5698 | 0.8323 | 0.143 | 0.0227 | 0.6962 | 0.5663 | 0.1176 | 0.3282 | 0.1346 | 1.0399 | 0.4755 | 0.094 | 0.9416 | 0.0845 | 0.0102 | |
| F10 | 0.6072 | 1.0397 | 0.9937 | 0.0255 | 0.0008 | 0.6983 | 0.9883 | 0.0347 | 0.0013 | 0.7529 | 0.7534 | 0.8399 | 0.1286 | 0.0202 | 0.9206 | 0.6269 | 0.0412 | 0.9889 | 0.0338 | 0.0016 | |
| W10 | 0.6488 | 1.0256 | 0.9962 | 0.0199 | 0.0005 | 0.7124 | 0.9945 | 0.0238 | 0.0006 | 0.7528 | 0.8112 | 0.9118 | 0.0954 | 0.0111 | 0.967 | 0.7417 | 0.0448 | 0.9921 | 0.0285 | 0.0011 | |
| S20 | 0.6793 | 1.0681 | 0.9967 | 0.0187 | 0.0004 | 0.7966 | 0.9926 | 0.0278 | 0.0009 | 0.8476 | 0.7678 | 0.8694 | 0.117 | 0.0167 | 1.0122 | 0.8053 | 0.0306 | 0.9941 | 0.024 | 0.0009 | |
| G20 | 0.3225 | 1.343 | 0.9929 | 0.0301 | 0.0011 | 0.6299 | 0.8833 | 0.122 | 0.0165 | 0.743 | 0.6012 | 0.3387 | 0.2902 | 0.1053 | 1.1892 | 0.6781 | 0.0887 | 0.9035 | 0.1112 | 0.0177 | |
| F20 | 0.5885 | 1.1209 | 0.9847 | 0.041 | 0.002 | 0.7597 | 0.9765 | 0.0503 | 0.0028 | 0.8208 | 0.7285 | 0.7957 | 0.1482 | 0.0269 | 0.8859 | 0.7126 | 0.0802 | 0.9631 | 0.063 | 0.0055 | |
| W20 | 0.5738 | 1.029 | 0.9963 | 0.0199 | 0.0005 | 0.7328 | 0.9802 | 0.0461 | 0.0023 | 0.8009 | 0.7028 | 0.7578 | 0.1612 | 0.0318 | 1.0454 | 0.7655 | 0.055 | 0.9802 | 0.0461 | 0.0029 | |
| S30 | 0.7070 | 1.3689 | 0.9997 | 0.0067 | 0.001 | 1.138 | 0.9193 | 0.1021 | 0.1191 | 1.2716 | 0.6584 | 0.5869 | 0.2309 | 0.0711 | 1.495 | 1.633 | 0.0717 | 0.6477 | 0.2133 | 0.0728 | |
| G30 | 0.3728 | 1.3979 | 0.9855 | 0.0432 | 0.0024 | 0.7758 | 0.8303 | 0.1478 | 0.0246 | 0.9363 | 0.5421 | 0.1483 | 0.331 | 0.1409 | 1.0485 | 0.6131 | 0.0861 | 0.9457 | 0.0836 | 0.0105 | |
| F30 | 0.6922 | 1.031 | 0.99 | 0.0316 | 0.0012 | 0.7843 | 0.9852 | 0.0385 | 0.0016 | 0.8417 | 0.7427 | 0.8394 | 0.1269 | 0.0197 | 0.8782 | 0.8223 | 0.0962 | 0.9608 | 0.0627 | 0.0054 | |
| W30 | 0.7446 | 1.0786 | 0.9971 | 0.018 | 0.0004 | 0.8346 | 0.9947 | 0.0242 | 0.0007 | 0.8496 | 0.9346 | 0.9801 | 0.047 | 0.0028 | 1.4476 | 1.4305 | 0.0528 | 0.7587 | 0.1636 | 0.0382 | |
| 70 °C | UT | 0.4226 | 1.3762 | 0.9975 | 0.0179 | 0.0004 | 0.7987 | 0.8956 | 0.1156 | 0.015 | 0.9356 | 0.5931 | 0.3715 | 0.2837 | 0.1035 | 1.1375 | 0.7897 | 0.0737 | 0.9402 | 0.0875 | 0.0115 |
| S10 | 0.8289 | 1.194 | 0.9965 | 0.0201 | 0.0005 | 1.124 | 0.9592 | 0.0684 | 0.0053 | 1.212 | 0.7146 | 0.7514 | 0.167 | 0.0367 | 0.9023 | 0.982 | 0.1083 | 0.9419 | 0.0817 | 0.01 | |
| G10 | 0.5041 | 1.2468 | 0.9846 | 0.0423 | 0.0022 | 0.8586 | 0.8891 | 0.1135 | 0.0143 | 1 | 0.5291 | 0.3132 | 0.2825 | 0.0998 | 0.9513 | 0.6403 | 0.0868 | 0.9635 | 0.0651 | 0.0061 | |
| F10 | 0.8402 | 1.1676 | 0.9949 | 0.0237 | 0.0007 | 1.0955 | 0.9647 | 0.0625 | 0.0043 | 1.1384 | 0.8244 | 0.8761 | 0.1171 | 0.0171 | 1.3652 | 1.5453 | 0.0677 | 0.7882 | 0.153 | 0.0335 | |
| W10 | 0.5305 | 1.2481 | 0.9992 | 0.0101 | 0.0001 | 0.8139 | 0.9529 | 0.0754 | 0.0063 | 0.8723 | 0.7390 | 0.7609 | 0.1699 | 0.0353 | 1.01 | 0.8774 | 0.0746 | 0.9481 | 0.0792 | 0.0086 | |
| S20 | 1.0899 | 1.1465 | 0.9949 | 0.0239 | 0.0008 | 1.3631 | 0.9723 | 0.0558 | 0.0036 | 1.4712 | 0.7128 | 0.7803 | 0.157 | 0.0329 | 1.4807 | 1.8634 | 0.0376 | 0.6742 | 0.1912 | 0.0585 | |
| G20 | 0.6565 | 1.2495 | 0.9938 | 0.027 | 0.0009 | 1.0134 | 0.9351 | 0.0872 | 0.0086 | 1.1465 | 0.6018 | 0.5259 | 0.2358 | 0.0715 | 0.9745 | 0.8573 | 0.0763 | 0.9727 | 0.0566 | 0.0048 | |
| F20 | 0.9587 | 1.1876 | 0.9906 | 0.0328 | 0.0014 | 1.2914 | 0.9494 | 0.0878 | 0.0067 | 1.4279 | 0.6524 | 0.6552 | 0.1984 | 0.0525 | 0.8679 | 1.115 | 0.1224 | 0.9346 | 0.0865 | 0.012 | |
| W20 | 1.0066 | 1.267 | 0.9968 | 0.0196 | 0.0005 | 1.4201 | 0.9435 | 0.082 | 0.0077 | 1.5382 | 0.6911 | 0.7084 | 0.1862 | 0.0463 | 0.9821 | 1.0802 | 0.0438 | 0.9836 | 0.0442 | 0.0031 | |
| S30 | 1.1726 | 1.1969 | 0.995 | 0.0243 | 0.0008 | 1.4863 | 0.9659 | 0.0635 | 0.0047 | 1.6211 | 0.7192 | 0.7639 | 0.167 | 0.0391 | 0.9426 | 1.3694 | 0.0828 | 0.9713 | 0.0582 | 0.0059 | |
| G30 | 0.8482 | 1.1571 | 0.9972 | 0.0181 | 0.0004 | 1.0923 | 0.9689 | 0.06 | 0.004 | 1.1981 | 0.7182 | 0.7464 | 0.171 | 0.039 | 1.1122 | 1.2672 | 0.0745 | 0.9401 | 0.0831 | 0.0111 | |
| F30 | 0.9828 | 1.2434 | 0.9941 | 0.0263 | 0.0009 | 1.36 | 0.9447 | 0.0808 | 0.0075 | 1.474 | 0.6998 | 0.7164 | 0.183 | 0.0446 | 0.9162 | 1.2316 | 0.119 | 0.9345 | 0.0879 | 0.0124 | |
| W30 | 0.7603 | 1.4666 | 0.9977 | 0.0174 | 0.0004 | 1.3655 | 0.8732 | 0.129 | 0.019 | 1.5315 | 0.5948 | 0.4661 | 0.2648 | 0.0935 | 0.9892 | 0.9819 | 0.0827 | 0.43 | 0.0816 | 0.0106 | |
| Pretreatment Condition | Ea (kJ/mol) | A (min−n) | R2 |
|---|---|---|---|
| UT | 16.66 | 1.59 | 0.61 |
| S10 | 24.49 | 8.37 | 0.97 |
| G10 | 15.75 | 1.16 | 0.54 |
| F10 | 26.05 | 8.94 | 0.98 |
| W10 | 18.7 | 2.51 | 0.64 |
| S20 | 38.08 | 13.42 | 0.99 |
| G20 | 17.56 | 5.56 | 0.78 |
| F20 | 34.91 | 12.16 | 0.96 |
| W20 | 38.61 | 13.49 | 0.95 |
| S30 | 42.06 | 14.89 | 0.99 |
| G30 | 29.44 | 9.98 | 0.54 |
| F30 | 29.78 | 10.41 | 0.64 |
| W30 | 23.6 | 8.08 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.A.; Shaha, L.C.; Romen, T.I.; Sarkar, A.; Biswas, R.; Ahmed, S.; Islam, M.A.; Muntasir, F.; Patwary, M.A.; Morais, R.M.S.C.; et al. Synergistic Effect of Ultrasound and Osmotic Pretreatment on the Drying Kinetics and Antioxidant Properties of Satkara (Citrus macroptera): A Novel Preservation Strategy. Processes 2025, 13, 384. https://doi.org/10.3390/pr13020384
Hossain MA, Shaha LC, Romen TI, Sarkar A, Biswas R, Ahmed S, Islam MA, Muntasir F, Patwary MA, Morais RMSC, et al. Synergistic Effect of Ultrasound and Osmotic Pretreatment on the Drying Kinetics and Antioxidant Properties of Satkara (Citrus macroptera): A Novel Preservation Strategy. Processes. 2025; 13(2):384. https://doi.org/10.3390/pr13020384
Chicago/Turabian StyleHossain, Mohammad Afzal, Limon Chandra Shaha, Tasnim Islam Romen, Animesh Sarkar, Rahul Biswas, Shafi Ahmed, Md. Atiqual Islam, Fahim Muntasir, Md. Amjad Patwary, Rui M. S. C. Morais, and et al. 2025. "Synergistic Effect of Ultrasound and Osmotic Pretreatment on the Drying Kinetics and Antioxidant Properties of Satkara (Citrus macroptera): A Novel Preservation Strategy" Processes 13, no. 2: 384. https://doi.org/10.3390/pr13020384
APA StyleHossain, M. A., Shaha, L. C., Romen, T. I., Sarkar, A., Biswas, R., Ahmed, S., Islam, M. A., Muntasir, F., Patwary, M. A., Morais, R. M. S. C., & Morais, A. M. M. B. (2025). Synergistic Effect of Ultrasound and Osmotic Pretreatment on the Drying Kinetics and Antioxidant Properties of Satkara (Citrus macroptera): A Novel Preservation Strategy. Processes, 13(2), 384. https://doi.org/10.3390/pr13020384















