Strategies for Improving the Techno-Functional and Sensory Properties of Bean Protein
Abstract
1. Introduction
2. Bean Proteins vs. Soy and Pea Proteins
2.1. Chemical Composition
2.2. Protein Digestibility
2.3. Secondary Structure
| Variety | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | Authors | |
|---|---|---|---|---|---|---|
| Black Bean | Isolated protein | 7.5 ± 0.0 | 39 ± 0.0 | 21.7 ± 0.0 | 31.8 ± 0.0 | [47] |
| Bambara Bean | 15.47 ± 0.52 | 67.34 ± 2.24 | 5.6 ± 0.19 | 11.59 ± 0.38 | [48] | |
| Winged Bean | 15.38 | 37.46 | 31.67 | 15.38 | [49] | |
| Faba Bean | 19.7 | 41.9 | 12.2 | 26.2 | [50] | |
| Soy | Isolated protein | 15.46 | 46.15 | 30.78 | 7.69 | [49] |
| 13 | 50 | 22 | 15 | [51] | ||
| 18 | 42 | 22 | 18 | [52] | ||
| Concentrated protein | 18 | 42 | 22 | 18 | ||
| Pea | Isolated protein | 10.43 ± 0.18 | 24.59 ± 1.13 | 44.28 ± 0.98 | 15.66 ±0.84 | [53] |
| Concentrated protein | 21 | 46 | 18 | 15 | [52] | |
| 13.91 ± 0.49 | 53.29 ± 0.72 | 21.47 ± 0.35 | 11.33 ± 0.12 | [54] | ||
2.4. Techno-Functional Properties
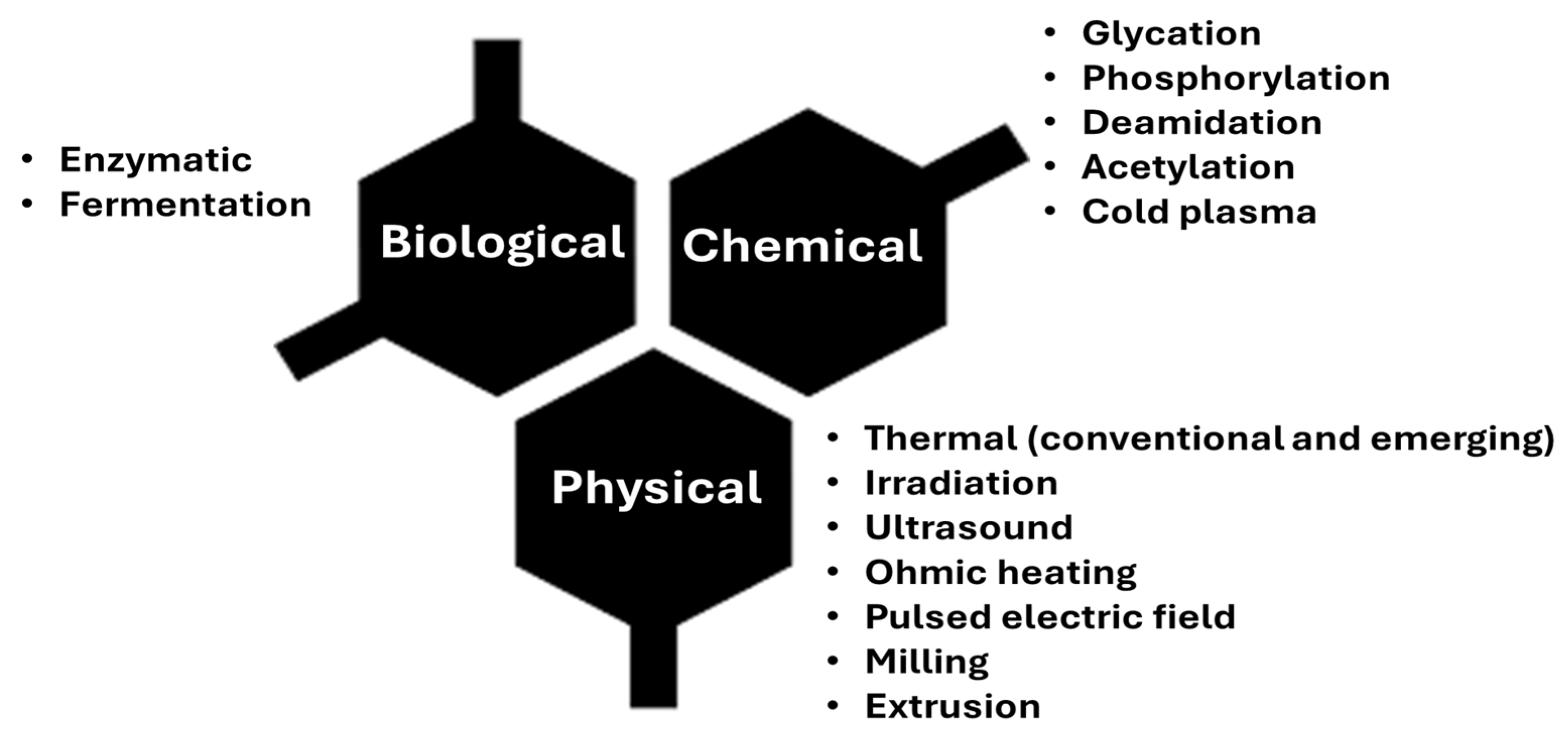
3. Strategies for Improving the Techno-Functional and Sensory Characteristics of Bean Protein
- (i)
- Mild thermal conditions promote protein unfolding, leading to an intermediate globular state that enhances its functionality. However, under extreme conditions, irreversible changes occur in the molecular structure, promoting denaturation and the aggregation of disulfide, hydrophobic, and electrostatic bonds, resulting in a decrease in its functionality [56].
- (ii)
- Gamma irradiation causes cross-linking, protein fragmentation, and physical aggregation. The hydroxyl radicals and superoxide anions formed during the process are responsible for altering the primary, secondary, tertiary, and quaternary structures of the proteins [70].
- (iii)
- Electron beam irradiation is conducted using an electron microscope that employs a heated filament or field emission as an electron source. High-energy irradiated electrons are capable of affecting chemical and molecular bonds in the protein structure, leading to their unfolding and denaturation, resulting in alterations in their functional properties and bioactivity [16].
- (iv)
- Ultraviolet (UV) light can be absorbed by aromatic amino acids such as tryptophan, tyrosine, and phenylalanine. Therefore, it can be induced that UV irradiation is also capable of chemically modifying the protein structure, and consequently, its techno-functional properties [28].
- (v)
- Pulsed electric field is a non-thermal method that applies short, repetitive pulses of high voltage to a material placed between two electrodes [71]. This pulse can unfold the protein structure and increase its interaction with the solvent, improving solubility and, consequently, its techno-functional properties [72,73,74].
- (vi)
- High-pressure treatment acts by breaking electrostatic and hydrophobic interactions and forming new bonds, which can lead to aggregation and, consequently, gelatinization [7,38]. This treatment does not cause significant damage to nutritional value, aroma, color, flavor, or vitamin content, as it only breaks non-covalent bonds in the matrix [75,76,77].
- (vii)
- Sonication is a process that applies frequencies greater than 20 kHz in an ultrasonic bath, aiming to disperse particles in the medium [78]. It generates regions with mechanical waves under high temperature and pressure, which can induce and accelerate chemical reactions. These reactions, in turn, can modify the secondary structure and partially denature the tertiary and quaternary structures, without significantly altering the primary structure, by breaking non-covalent bonds [48,79,80].
- (viii)
- Extrusion is a combination of mechanical forces, heat, and pressure that can induce unfolding, denaturation, and aggregation of protein molecules, which enhances their functionality [81].
- (ix)
- (x)
- (xi)
- (xii)
- (xiii)
- Phosphorylation involves the addition of phosphate groups to the primary sequence of the protein, which alters its functionality by enabling more interactions between protein molecules through phosphorylated regions [91].
- (xiv)
- Acylation involves the conversion of compounds containing active hydrogens, such as -OH, -SH, and -NH, into esters, thioesters, and amines through the use of acylation reagents [92,93]. This conversion alters the electric charges on the surface of the molecule, which in turn affects its solubility and consequently its functional properties [92,93].
- (xv)
- (xvi)
- Acidic or alkaline treatments can induce structural and functional changes in proteins. In a basic environment, proteins are denatured and unfolded, exposing hydrophilic groups and sulfhydryl groups within the structure. This exposure promotes increased interaction between molecules [96].
- (xvii)
- Enzymatic hydrolysis is achieved through the cleavage of peptide bonds using enzymes. Enzymatic cross-linking, on the other hand, despite being classified separately from hydrolysis, is obtained by enzymatic formation of covalent bonds using transglutaminase. This enzyme catalyzes the acyl transfer reaction between the γ-carboxamide group of glutamine bound to the protein and lysine [97].
- (xviii)
- (xix)
- pH-induced complexation facilitates the interaction between plant proteins and polysaccharides, protein–protein, protein–phenolic, or protein–surfactant systems, thereby influencing their techno-functional characteristics. These proteins can electrostatically interact with oppositely charged molecules, forming soluble or insoluble complexes that may alter their properties [70].
- (xx)
| Varieties | Treatment | Modifications | References |
|---|---|---|---|
| Beans (black, navy, kidney, and pinto) | Beans boiled in ultrapure water at a ratio of 1:15 (w.v−1) for 90 min | Increase in WRC. Higher viscosity in black and common beans. Increased digestibility of starch and proteins. Decreased paste property. | [103] |
| Beans roasted for 20 min in an air fryer at 165 °C | Greater ORC. Loss of paste properties. | ||
| Vicilin isolated from kidney bean | 95 °C for 15–30 min | Improved S, EC, and FS (at neutral pH), although the FFC decreased. Gradually decreased the levels of total and exposed free sulfhydryls. Gradually increased the hydrophobic surface. | [107] |
| 95 °C for 60–120 min | From 30 to 60 min, there was no significant change in the free sulfhydryl content. However, after 120 min, the decrease was more pronounced. The hydrophobic surface, EC, FFC, and their stabilities gradually decreased. | ||
| White bean | Beans boiled for 50 min | Decreased the amount of globulins, phaseolin, lipoxygenase enzyme, aspartic acid, glutamic acid, arginine, and leucine. Increased the amount of low-molecular-weight isoforms, heat shock proteins (HSPs), tyrosine, lysinoalanine, and methionine. | [104] |
| Mung bean | Lyophilization: −30°C/48h | Porous protein. Elastic gel with better GC.Good S, WRC, and ORC. | [81] |
| Spray dryer: 185 °C inlet and 90 °C outlet | Protein in the form of wrinkled crystals. Elastic gel with better GC. Smaller particle size; good EC and ES. Higher amount of β-sheet. | ||
| Drying in oven: 50 °C/24 h | Protein in the form of compact crystals. Aggregated gel. | ||
| Proteins from kidney (P. vulgaris L.), red (P. angularis), and mungo (Phaseolus aureus) beans | 95 °C for 30 min | Increased S and CE. | [108] |
| Faba bean protein | 75–175 °C for 60 min | Increased WRC and decreased S. | [109] |
| Common bean (Phaseolus vulgaris L.) | Boiling for 15 or 120 min | Decreased S. After 120 min, increased WRC and ORC. Decreased EC but increased ES. | [110] |
| Azuki bean (Vigna angularis) | At 25.0, 32.5, 40.0, 55.0, and 70.0 °C (15–40 min) | The higher the temperature, the greater the water retention capacity (WRC) (g/100 g). | [111] |
| Cultivars of carioca beans: BRSMG Madrepérola (MA), TAA Dama (DA), BRS Notável (NO), IAC Imperador (IM), TAA Gol (GOL), TAA Bola Cheia (BC) | 60 °C/8–12 h | It reduced the S and increased the WRC. It reduced the ORC of the “BC” and “MA” flours but increased it in the “GOL” and “IM” flours. | [112] |
| 60 °C/8–12 h | The thermal treatment reduced the EC and ES of most flours. | ||
| Bambara bean (Vigna subterrânea (L) green) | 50 °C, 70 °C, 80 °C, and 100 °C for 10 min | The highest EC was observed at 80 °C at pH 9, and the highest ES was at pH 4. Hydrophobicity decreased between 50 and 80 °C. S decreased at temperatures between 70 and 100 °C. | [48] |
| Black bean (Vigna cylindrica (L.)) | Roasting—180 °C for 20 min; Cooking—Boiling for 30 min; Autoclaving—120 °C for 5 min | Increase in WRC. No significant difference in ORC. | [113] |
| Proteins from Boer bean, lentil, and chickpea | Bromelain | High hydrophobicity, thus exhibiting WRC and ORC. | [105] |
| Alkaline hydrolysis | Good EC, good FFC, and effective inhibitory action on lipid oxidation in emulsions. | ||
| Mung bean protein | Ultrasound at 114 W, 222 W, 330 W, 438 W, and 546 W power for 20 min | Increased the content of aromatic and hydrophobic amino acids. Decreased α-helix content and increased β-sheet and β-turn content. | [82] |
| Ultrasound at 546 W power for 20 min | Significantly decreased the protein particle size (290.13 nm), exhibited a lower zeta potential (−36.37 mV), and reduced hydrophobic surface (367.95 A.U.), in addition to increasing antioxidant activity. | ||
| Mung bean protein | Alkaline treatment | Higher S and free thiol content, smaller particle size, and reduced hydrophobic surface. | [114] |
| Kidney bean cultivars (P. vulgaris L.) | Papain at a concentration of 0.01 g/10 g of protein isolate for 30 and 60 min | Increased S, WRC, ORC, EC, and ES. The FFC was directly proportional to the hydrolysis time. | [106] |
| Black bean protein | 20 kHz, 150–450 W, 12–24 min | Increase in S. | [48] |
| Faba bean protein | 20 kHz, 50–75% amplitude, 15–30 min | Increase in FFC. | [50] |
| Kidney bean protein | 200–600 MPa, 15 min | Increase in WRC, FFC, and EC. | [75] |
| Protein isolated from mung bean (MB), black bean (BB), azuki bean (AB), rice bean (RB), kidney bean (KB), speckled kidney bean (SKB), cowpea (CP) | pH 3.0, 5.0, 7.0, and 9.0 | All isolates showed lower WRC and ORC at pH 5.0, with no capacity at pH 9.0 due to complete solubility. FFC was lower at pH 5.0 due to reduced S. KB and SKB exhibited the highest foam formation properties. FS was highest at pH 3.0. The highest EC was found at pH 7.0 and 9.0. ES was directly proportional to the increase in pH. | [107] |
| Jack bean | Acylation using acetic acid and succinic anhydride | Improved WRC. ORC increased with acetylation but decreased with succinylation. Maximum EC was observed at pH 10. ES was higher in the pH range of 4–10. FFC and FS increased with higher protein concentrations. | [95] |
4. Final Considerations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein Demand: Review of Plant and Animal Proteins Used in Alternative Protein Product Development and Production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Otero, D.M.; Da Rocha Lemos Mendes, G.; Da Silva Lucas, A.J.; Christ-Ribeiro, A.; Ribeiro, C.D.F. Exploring Alternative Protein Sources: Evidence from Patents and Articles Focusing on Food Markets. Food Chem. 2022, 394, 133486. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-Quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations: Sustainability, Challenge and Innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Präger, L.; Simon, J.C.; Treudler, R. Food Allergy—New Risks through Vegan Diet? Overview of New Allergen Sources and Current Data on the Potential Risk of Anaphylaxis. J. Dtsch. Derma Gesell 2023, 21, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.C.D.S.; Dos Santos, J.; Batista, L.F.; Rodrigues, J.M.M.D.O.; Simiqueli, A.A.; Pires, A.C.D.S.; Minim, V.P.R.; Minim, L.A.; Vidigal, M.C.T.R. Technical-Functional and Surface Properties of White Common Bean Proteins (Phaseolus vulgaris L.): Effect of pH, Protein Concentration, and Guar Gum Presence. Food Res. Int. 2024, 192, 114809. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.H.; Oliveira, L.D.C.; Melo, D.D.S.; Bakalis, S.; Cristianini, M. Modification of Protein Concentrate from Carioca Bean (Phaseolus vulgaris L.) by Dynamic High-Pressure Technology: Structural and Techno-Functional Properties. Innov. Food Sci. Emerg. Technol. 2024, 97, 103823. [Google Scholar] [CrossRef]
- Tarahi, M.; Abdolalizadeh, L.; Hedayati, S. Mung Bean Protein Isolate: Extraction, Structure, Physicochemical Properties, Modifications, and Food Applications. Food Chem. 2024, 444, 138626. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-S.; Shuai, S.; Fitzgerald, R. Mung Bean Proteins and Peptides: Nutritional, Functional and Bioactive Properties. Food Nutr. Res. 2018, 62. [Google Scholar]
- FAO. Countries by Commodity: Bean; FAO: Rome, Italy, 2024. [Google Scholar]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Bean Processing: Thermal and Non-Thermal Processing on Chemical, Antinutritional Factors, and Pharmacological Properties. Molecules 2023, 28, 5431. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, Physicochemical Characteristics and Functional Properties of Mung Bean Protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Liu, C.; Pei, R.; Heinonen, M. Faba Bean Protein: A Promising Plant-Based Emulsifier for Improving Physical and Oxidative Stabilities of Oil-in-Water Emulsions. Food Chem. 2022, 369, 130879. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.A.; Prudêncio, S.H.; Fernandes, K.F. Changes in the Functional Properties and Antinutritional Factors of Extruded Hard-to-Cook Common Beans (Phaseolus vulgaris, L.). J. Food Sci. 2010, 75, C286–C290. [Google Scholar] [CrossRef]
- Ramírez-Cárdenasi, L.; Leonel, A.J.; Costa, N.M.B. Effect of Domestic Processing on Nutrient and Antinutritional Factor Content in Different Cultivars of Common Beans. Ciênc. Tecnol. Aliment. 2008, 28, 200–213. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Fan, M.; Qian, H.; Wang, L. Recent Advances in Mung Bean Protein: From Structure, Function to Application. Int. J. Biol. Macromol. 2024, 273, 133210. [Google Scholar] [CrossRef]
- Thomsen, J.; Rao, J.; Chen, B. Faba Bean Protein: Chemical Composition, Functionality, Volatile Compounds, and Applications in Food Production. Trends Food Sci. Technol. 2025, 156, 104863. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, Y.; Shang, Z.; Ma, L.; Xu, Y.; Zhang, L.; Chen, Y. Structural and Functional Modification of Miscellaneous Beans Protein by High-Intensity Ultrasound: Mechanism, Processing, and New Insights. Food Hydrocoll. 2024, 151, 109774. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Wang, Q.; Yan, J.; Han, S.; Ma, C.; Ma, P.; Liu, X.; McClements, D.J. Future Foods: Alternative Proteins, Food Architecture, Sustainable Packaging, and Precision Nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 6423–6444. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Future Foods: A Manifesto for Research Priorities in Structural Design of Foods. Food Funct. 2020, 11, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.V.; Oliveira, M.G.D.A.; Rosa, J.C.; Costa, N.M.B. Nutritional Quality and Chemical Score of Amino Acids from Different Protein Sources. Ciênc. Tecnol. Aliment. 2006, 26, 179–187. [Google Scholar] [CrossRef]
- Reynaud, Y.; Buffière, C.; Cohade, B.; Vauris, M.; Liebermann, K.; Hafnaoui, N.; Lopez, M.; Souchon, I.; Dupont, D.; Rémond, D. True Ileal Amino Acid Digestibility and Digestible Indispensable Amino Acid Scores (DIAASs) of Plant-Based Protein Foods. Food Chem. 2021, 338, 128020. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of Antinutritional Factors in Food Proteins on the Digestibility of Protein and the Bioavailability of Amino Acids and on Protein Quality. Br. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Aubry, L.; Kristiawan, M.; Jebalia, I.; Dupont, D.; Guillevic, M.; Germain, A.; Chesneau, G.; Sayd, T.; Talens, P.; et al. Fava Bean (Vicia faba L.) Protein Concentrate Added to Beef Burgers Improves the Bioaccessibility of Some Free Essential Amino Acids after in Vitro Oral and Gastrointestinal Digestion. Food Res. Int. 2024, 177, 113916. [Google Scholar] [CrossRef] [PubMed]
- Palander, S.; Laurinen, P.; Perttilä, S.; Valaja, J.; Partanen, K. Protein and Amino Acid Digestibility and Metabolizable Energy Value of Pea (Pisum sativum), Faba Bean (Vicia faba) and Lupin (Lupinus angustifolius) Seeds for Turkeys of Different Age. Anim. Feed. Sci. Technol. 2006, 127, 89–100. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef]
- Liu, Y.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Modifying the Functional Properties of Egg Proteins Using Novel Processing Techniques: A Review. Comp. Rev. Food Sci. Food Safe 2019, 18, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, S.M.; Fadel, H.H.M.; Bekhit, M.A.; Edris, A.E.; Ahmed, M.Y.S. Effect of Substitution of Soy Protein Isolate on Aroma Volatiles, Chemical Composition and Sensory Quality of Wheat Cookies. Int. J. Food Sci. Technol. 2009, 44, 1705–1712. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Auer, J. Assessing the Digestibility and Estimated Bioavailability/ Bioaccessibility of Plant-Based Proteins and Minerals from Soy, Pea, and Faba Bean Ingredients. LWT 2024, 197, 115893. [Google Scholar] [CrossRef]
- Çabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Guillin, F.M.; Gaudichon, C.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Airinei, G.; Khodorova, N.; Benamouzig, R.; Pomport, P.-H.; Martin, J.; Calvez, J. Real Ileal Amino Acid Digestibility of Pea Protein Compared to Casein in Healthy Humans: A Randomized Trial. Am. J. Clin. Nutr. 2022, 115, 353–363. [Google Scholar] [CrossRef]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein Digestibility-Corrected Amino Acid Scores (PDCAAS) for Soy Protein Isolates and Concentrate: Criteria for Evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Mariotti, F.; Pueyo, M.E.; Tomé, D.; Mahé, S. The Bioavailability and Postprandial Utilisation of Sweet Lupin (Lupinus albus)-Flour Protein Is Similar to That of Purified Soyabean Protein in Human Subjects: A Study Using Intrinsically 15N-Labelled Proteins. Br. J. Nutr. 2002, 87, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal Digestion of Dairy and Soy Proteins in Infant Formulas: An in Vitro Study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.E.; Moraru, C.I. Effect of High Pressure Processing and Heat Treatment on in Vitro Digestibility and Trypsin Inhibitor Activity in Lentil and Faba Bean Protein Concentrates. LWT 2021, 152, 112342. [Google Scholar] [CrossRef]
- Ayala-Rodriguez, V.A. Nutritional Quality of Protein Flours of Fava Bean (Vicia faba L.) and in Vitro Digestibility and Bioaccesibility. Food Chem. 2022, 14, 100303. [Google Scholar] [CrossRef] [PubMed]
- Rivera Del Rio, A.; Boom, R.M.; Janssen, A.E.M. Effect of Fractionation and Processing Conditions on the Digestibility of Plant Proteins as Food Ingredients. Foods 2022, 11, 870. [Google Scholar] [CrossRef]
- Liu, L.H.; Hung, T.V.; Bennett, L. Extraction and Characterization of Chickpea ( Cicer arietinum ) Albumin and Globulin. J. Food Sci. 2008, 73, C299–C305. [Google Scholar] [CrossRef]
- Wang, X.-S. Characterization, Amino Acid Composition and in Vitro Digestibility of Hemp (Cannabis sativa L.) Proteins. Food Chem. 2008, 107, 11–18. [Google Scholar] [CrossRef]
- Wardah, W. Protein Secondary Structure Prediction Using Neural Networks and Deep Learning: A Review. Comput. Biol. Chem. 2019, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.L. Applications of de Novo Designed Peptides. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–86. ISBN 978-0-08-100736-5. [Google Scholar]
- Sasidharan, S.; Ramakrishnan, V. Aromatic Interactions Directing Peptide Nano-Assembly. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 130, pp. 119–160. ISBN 978-0-323-99229-9. [Google Scholar]
- Ahn, J.-M.; Kassees, K.; Lee, T.-K.; Manandhar, B.; Yousif, A.M. Strategy and Tactics for Designing Analogs: Biochemical Characterization of the Large Molecules ✩. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 66–115. ISBN 978-0-12-803201-5. [Google Scholar]
- Smith, L.J.; Fiebig, K.M.; Schwalbe, H.; Dobson, C.M. The Concept of a Random Coil: Residual Structure in Peptides and Denatured Proteins. Fold. Des. 1996, 1, R95–R106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Ngui, S.P.; Nyobe, C.E.; Bakwo Bassogog, C.B.; Nchuaji Tang, E.; Minka, S.R.; Mune Mune, M.A. Influence of pH and Temperature on the Physicochemical and Functional Properties of Bambara Bean Protein Isolate. Heliyon 2021, 7, e07824. [Google Scholar] [CrossRef]
- Makeri, M.U.; Abdulmannan, F.; Ilowefah, M.A.; Chiemela, C.; Bala, S.M.; Muhammad, K. Comparative Physico-Chemical, Functional and Structural Characteristics of Winged Bean [Psophocarpus Tetragonolobus DC] and Soybean [Glycine Max.] Protein Isolates. Food Meas. 2017, 11, 835–846. [Google Scholar] [CrossRef]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High Intensity Ultrasound Treatment of Faba Bean (Vicia faba L.) Protein: Effect on Surface Properties, Foaming Ability and Structural Changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Wang, R.; Sui, X.; Qi, B.; Han, F.; Li, Y.; Jiang, L. Secondary Structure and Subunit Composition of Soy Protein In Vitro Digested by Pepsin and Its Relation with Digestibility. BioMed Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Mao, B.; Singh, J.; Hodgkinson, S.; Farouk, M.; Kaur, L. Conformational Changes and Product Quality of High-Moisture Extrudates Produced from Soy, Rice, and Pea Proteins. Food Hydrocoll. 2024, 147, 109341. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Yang, Y.; Zhang, L.; Li, J.; Wang, S. Functional Properties and Structural Characteristics of Phosphorylated Pea Protein Isolate. Int. J. Food Sci. Technol. 2020, 55, 2002–2010. [Google Scholar] [CrossRef]
- Tang, J. Comparative Studies on Enhancing Pea Protein Extraction Recovery Rates and Structural Integrity Using Ultrasonic and Hydrodynamic Cavitation Technologies. LWT 2024, 200, 116130. [Google Scholar] [CrossRef]
- Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in Food Systems—Bionanomaterials, Conventional and Unconventional Sources, Functional Properties, and Development Opportunities. Polymers 2021, 13, 2506. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of Processing on the Chemistry and Functionality of Food Proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–45. ISBN 978-0-08-100722-8. [Google Scholar]
- Gundogan, R.; Can Karaca, A. Physicochemical and Functional Properties of Proteins Isolated from Local Beans of Turkey. LWT 2020, 130, 109609. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Konakbayeva, D.; Rajabzadeh, A.R.; Legge, R.L. Functional Properties of Navy Bean (Phaseolus vulgaris) Protein Concentrates Obtained by Pneumatic Tribo-Electrostatic Separation. Food Chem. 2019, 283, 101–110. [Google Scholar] [CrossRef]
- Krause, M.; Sørensen, J.C.; Petersen, I.L.; Duque-Estrada, P.; Cappello, C.; Tlais, A.Z.A.; Di Cagno, R.; Ispiryan, L.; Sahin, A.W.; Arendt, E.K.; et al. Associating Compositional, Nutritional and Techno-Functional Characteristics of Faba Bean (Vicia faba L.) Protein Isolates and Their Production Side-Streams with Potential Food Applications. Foods 2023, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Aguirre, M.G.; Rodríguez-Miranda, J.; Falfán-Cortes, R.N.; Hernández-Santos, B. Physicochemical and Techno-Functional Properties of Mixtures of Michigan Bean Protein Concentrate (Phaseolus vulgaris L): Maltodextrin. Food Meas. 2023, 17, 1844–1851. [Google Scholar] [CrossRef]
- Mundi, S.; Aluko, R.E. Physicochemical and Functional Properties of Kidney Bean Albumin and Globulin Protein Fractions. Food Res. Int. 2012, 48, 299–306. [Google Scholar] [CrossRef]
- Li, W.; Shu, C.; Yan, S.; Shen, Q. Characteristics of Sixteen Mung Bean Cultivars and Their Protein Isolates. Int. J. Food Sci. Technol. 2010, 45, 1205–1211. [Google Scholar] [CrossRef]
- Subagio, A. Characterization of Hyacinth Bean (Lablab purpureus (L.) Sweet) Seeds from Indonesia and Their Protein Isolate. Food Chem. 2006, 95, 65–70. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effects of Soluble Aggregates Sizes on Rheological Properties of Soybean Protein Isolate under High Temperature. LWT 2023, 182, 114793. [Google Scholar] [CrossRef]
- Othmeni, I.; Karoui, R.; Blecker, C. Impact of pH on the Structure, Interfacial and Foaming Properties of Pea Protein Isolate: Investigation of the Structure—Function Relationship. Int. J. Biol. Macromol. 2024, 278, 134818. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean—French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of Wheat, Soybean, Rice, and Pea Protein Properties for Effective Applications in Food Products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid. Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on Plant Protein-Polysaccharide Complex Coacervation, and the Functionality and Applicability of Formed Complexes: Review on Plant Protein-Polysaccharide Complex Coacervation. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.-H.; Sun, D.-W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Zhang, Q.H.; Tabilo-Munizaga, G. Pulsed Electric Fields in Food Processing; CRC Press: Lacaster, CA, USA, 2001. [Google Scholar]
- Shams, R.; Manzoor, S.; Shabir, I.; Dar, A.H.; Dash, K.K.; Srivastava, S.; Pandey, V.K.; Bashir, I.; Khan, S.A. Pulsed Electric Field-Induced Modification of Proteins: A Comprehensive Review. Food Bioprocess. Technol. 2024, 17, 351–383. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.-H.; Wen, Q.-H.; He, F.; Xu, F.-Y.; Chen, B.-R.; Zeng, X.-A. Combination of Pulsed Electric Field and pH Shifting Improves the Solubility, Emulsifying, Foaming of Commercial Soy Protein Isolate. Food Hydrocoll. 2023, 134, 108049. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, R.; Hu, J.; Luan, Y.; Liu, R.; Ge, Q.; Yu, H.; Wu, M. Moderate Pulsed Electric Field-Induced Structural Unfolding Ameliorated the Gelling Properties of Porcine Muscle Myofibrillar Protein. Innov. Food Sci. Emerg. Technol. 2022, 81, 103145. [Google Scholar] [CrossRef]
- Ahmed, J. Effect of High Pressure Treatment on Functional, Rheological and Structural Properties of Kidney Bean Protein Isolate. LWT 2018, 91, 191–197. [Google Scholar] [CrossRef]
- Baskıncı, T.; Gul, O. Modifications to Structural, Techno-Functional and Rheological Properties of Sesame Protein Isolate by High Pressure Homogenization. Int. J. Biol. Macromol. 2023, 250, 126005. [Google Scholar] [CrossRef]
- Vidotto, D.C.; Mantovani, R.A.; Tavares, G.M. High-Pressure Microfluidization of Whey Proteins: Impact on Protein Structure and Ability to Bind and Protect Lutein. Food Chem. 2022, 382, 132298. [Google Scholar] [CrossRef]
- Feng, H.; Barbosa-Canovas, G.; Weiss, J. (Eds.) Ultrasound Technologies for Food and Bioprocessing; Food Engineering Series; Springer New York: New York, NY, USA, 2011; ISBN 978-1-4419-7471-6. [Google Scholar]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical Properties of Ultrasound Treated Soy Proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Z.; Miao, Z.; Liu, J.; Liu, J.; Xu, X.; Wu, Y.; Qi, B.; Yan, J. Ultrasound Heat Treatment Effects on Structure and Acid-Induced Cold Set Gel Properties of Soybean Protein Isolate. Food Biosci. 2021, 39, 100827. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized Mung Bean Protein as a Sustainable Food Source: Effects of Extrusion on Its Physical, Textural and Protein Quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591. [Google Scholar] [CrossRef]
- Liu, F.-F.; Li, Y.-Q.; Sun, G.-J.; Wang, C.-Y.; Liang, Y.; Zhao, X.-Z.; He, J.-X.; Mo, H.-Z. Influence of Ultrasound Treatment on the Physicochemical and Antioxidant Properties of Mung Bean Protein Hydrolysate. Ultrason. Sonochem. 2022, 84, 105964. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Y.; Li, Z.; Wang, C.; Zhang, D.; Wang, L. Effect of Different Milling Methods on Physicochemical and Functional Properties of Mung Bean Flour. Front. Nutr. 2023, 10, 1117385. [Google Scholar] [CrossRef]
- Tan, L.; Hua, X.; Yin, L.; Jia, X.; Liu, H. Effect of Corona Discharge Cold Plasma on the Structure and Emulsification Properties of Soybean Protein Isolate. Food Hydrocoll. 2024, 156, 110337. [Google Scholar] [CrossRef]
- Tolouie, H. Cold Atmospheric Plasma Manipulation of Proteins in Food Systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Du, J.; Han, C.; Yu, D. Effect of Cold Plasma Treatment of Sunflower Seed Protein Modification on Its Structural and Functional Properties and Its Mechanism. Food Hydrocoll. 2024, 155, 110175. [Google Scholar] [CrossRef]
- Alfaro-Diaz, A.; Urías-Silvas, J.E.; Loarca-Piña, G.; Gaytan-Martínez, M.; Prado-Ramirez, R.; Mojica, L. Techno-Functional Properties of Thermally Treated Black Bean Protein Concentrate Generated through Ultrafiltration Process. LWT 2021, 136, 110296. [Google Scholar] [CrossRef]
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of Enzymatic Hydrolysis and Ultrafiltration on Physicochemical and Functional Properties of Faba Bean Protein. Cereal Chem. 2019, 96, 725–741. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Z.; Cheng, K.-W.; Shan, F.; Ren, G.-X.; Chen, F.; Wang, M. Inhibitory Effect of Mung Bean Extract and Its Constituents Vitexin and Isovitexin on the Formation of Advanced Glycation Endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Tang, C.-H.; Sun, X.; Foegeding, E.A. Modulation of Physicochemical and Conformational Properties of Kidney Bean Vicilin (Phaseolin) by Glycation with Glucose: Implications for Structure–Function Relationships of Legume Vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Jafarzadeh, S.; Ibarz, A. Modified Mung Bean Protein: Optimization of Microwave-Assisted Phosphorylation and Its Functional and Structural Characterizations. LWT 2021, 151, 112119. [Google Scholar] [CrossRef]
- El-Adawy, T.A. Functional Properties and Nutritional Quality of Acetylated and Succinylated Mung Bean Protein Isolate. Food Chem. 2000, 70, 83–91. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O. The Acylated Protein Derivatives of Canavalia Ensiformis (Jack Bean): A Study of Functional Characteristics. LWT—Food Sci. Technol. 2006, 39, 918–929. [Google Scholar] [CrossRef]
- Hamada, J.S.; Swanson, B. Deamidation of Food Proteins to Improve Functionality. Crit. Rev. Food Sci. Nutr. 1994, 34, 283–292. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of Succinylation and Deamidation on Functional Properties of Oat Protein Isolate. Food Chem. 2009, 114, 127–131. [Google Scholar] [CrossRef]
- Choe, U.; Chang, L.; Ohm, J.-B.; Chen, B.; Rao, J. Structure Modification, Functionality and Interfacial Properties of Kidney Bean (Phaseolus vulgaris L.) Protein Concentrate as Affected by Post-Extraction Treatments. Food Hydrocoll. 2022, 133, 108000. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring Colloidal Oat and Faba Bean Protein Particles via Enzymatic Modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef]
- Khorsandi, A.; Shi, D.; Stone, A.K.; Bhagwat, A.; Lu, Y.; Xu, C.; Das, P.P.; Polley, B.; Akhov, L.; Gerein, J.; et al. Effect of Solid-state Fermentation on the Protein Quality and Volatile Profile of Pea and Navy Bean Protein Isolates. Cereal Chem. 2024, 101, 131–143. [Google Scholar] [CrossRef]
- Nie, Y.; Liu, Y.; Jiang, J.; Xiong, Y.L.; Zhao, X. Rheological, Structural, and Water-Immobilizing Properties of Mung Bean Protein-Based Fermentation-Induced Gels: Effect of pH-Shifting and Oil Imbedment. Food Hydrocoll. 2022, 129, 107607. [Google Scholar] [CrossRef]
- Allameh, A.; Fazel, M.; Sheikhan, N.; Goli, M. Formation and Physicochemical Properties of Freeze-Dried Amyloid-Like Fibrils from Pinto Bean Protein: Amyloid-Like Fibrils from Pinto Bean Protein. Int. J. Anal. Chem. 2024, 2024, 5571705. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Zhang, Y.-H.; Wen, Q.-B.; Huang, Q. Formation of Amyloid Fibrils from Kidney Bean 7S Globulin (Phaseolin) at pH 2.0. J. Agric. Food Chem. 2010, 58, 8061–8068. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Osorno, J.M.; Ohm, J.-B.; Chen, B.; Rao, J. Modification of Physicochemical, Functional Properties, and Digestibility of Macronutrients in Common Bean (Phaseolus vulgaris L.) Flours by Different Thermally Treated Whole Seeds. Food Chem. 2022, 382, 132570. [Google Scholar] [CrossRef] [PubMed]
- Deb-Choudhury, S.; Cooney, J.; Brewster, D.; Clerens, S.; Knowles, S.O.; Farouk, M.M.; Grosvenor, A.; Dyer, J.M. The Effects of Blanching on Composition and Modification of Proteins in Navy Beans (Phaseolus vulgaris). Food Chem. 2021, 346, 128950. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, Y.; Shi, B.; Dia, V.P. Alcalase and Bromelain Hydrolysis Affected Physicochemical and Functional Properties and Biological Activities of Legume Proteins. Food Struct. 2021, 27, 100178. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Shivhare, U.S.; Gill, B.S. Physico-Chemical and Functional Properties of Native and Hydrolyzed Kidney Bean (Phaseolus vulgaris L.) Protein Isolates. Food Res. Int. 2015, 76, 11–18. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Mata, A.; Corke, H.; Gan, R.-Y.; Fang, Y. Physicochemical and pH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocoll. 2021, 112, 106288. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ma, C.-Y. Heat-Induced Modifications in the Functional and Structural Properties of Vicilin-Rich Protein Isolate from Kidney (Phaseolus vulgaris L.) Bean. Food Chem. 2009, 115, 859–866. [Google Scholar] [CrossRef]
- Tang, C.-H.; Sun, X.; Yin, S.-W. Physicochemical, Functional and Structural Properties of Vicilin-Rich Protein Isolates from Three Phaseolus Legumes: Effect of Heat Treatment. Food Hydrocoll. 2009, 23, 1771–1778. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; Van Der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Fernández-Fraguas, C. Effect of Thermal and High-Pressure Processing on the Thermo-Rheological and Functional Properties of Common Bean (Phaseolus vulgaris L.) Flours. LWT 2020, 127, 109325. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Colnaghi, B.G.; Silva, E.Z.D.; Gouvêa, I.R.; Vieira, R.L.; Augusto, P.E.D. Modelling the Effect of Temperature on the Hydration Kinetic of Adzuki Beans (Vigna angularis). J. Food Eng. 2013, 118, 417–420. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Morais, D.K.; De Berse, R.S.; Bassinello, P.Z.; Caliari, M.; Soares Júnior, M.S. Functional, Thermal, and Pasting Properties of Cooked Carioca Bean (Phaseolus vulgaris L.) Flours. Appl. Food Res. 2022, 2, 100027. [Google Scholar] [CrossRef]
- Le, N.L.; Le, T.T.H.; Nguyen, N.T.M.; Vu, L.T.K. Impact of Different Treatments on Chemical Composition, Physical, Anti-Nutritional, Antioxidant Characteristics and in Vitro Starch Digestibility of Green-Kernel Black Bean Flours. Food Sci. Technol. 2022, 42, e31321. [Google Scholar] [CrossRef]
- Liu, F.-F.; Li, Y.-Q.; Wang, C.-Y.; Zhao, X.-Z.; Liang, Y.; He, J.-X.; Mo, H.-Z. Impact of pH on the Physicochemical and Rheological Properties of Mung Bean (Vigna radiata L.) Protein. Process Biochem. 2021, 111, 274–284. [Google Scholar] [CrossRef]

| Amino Acid (g·100 g−1) | Bean | Pea | Soy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Alanine | 5.07 | 3.97 | 3.2 | 4.59 | 5.4 | 3.2 | 4.5 | 2.8 | 4.2 |
| Arginine * | 7.01 | 8.96 | 7.2 | 7.98 | 8.4 | 5.9 | 7.8 | 4.8 | 8.0 |
| Aspartic Acid | 10.17 | 9.28 | 8.7 | 10.50 | 11.9 | ND | 11.8 | ND | 12.1 |
| Cysteine | ND | 1.26 | 0.5 | 1.54 | 1.0 | 0.2 | 1.2 | 0.2 | 1.4 |
| Glutamic Acid | 19.50 | 15.67 | 14.0 | 17.34 | 16.4 | 12.9 | 20.5 | 12.4 | 20.4 |
| Glycine | 3.60 | 3.95 | 3.2 | 4.33 | 4.0 | 2.8 | 4.4 | 2.7 | 4.2 |
| Histidine * | 2.48 | 2.61 | 2.0 | 2.94 | 2.4 | 1.6 | 2.5 | 1.5 | 2.7 |
| Isoleucine * | 5.47 | 3.67 | 3.4 | 4.25 | 4.4 | 2.3 | 4.9 | 1.9 | 4.3 |
| Leucine * | 9.33 | 6.57 | 6.5 | 6.70 | 7.6 | 5.7 | 5.6 | 5.0 | 7.8 |
| Lysina * | 6.82 | 5.97 | 4.7 | 7.20 | 6.7 | 4.7 | 5.6 | 3.4 | 6.5 |
| Methionine * | 1.29 | 0.52 | 0.6 | 0.72 | 0.9 | 0.3 | 1.4 | 0.3 | 1.4 |
| Phenylalanine * | 6.79 | 3.98 | 3.3 | 4.83 | 5.7 | 3.7 | 5.5 | 3.2 | 5.4 |
| Proline | 4.06 | 4.27 | 4.3 | 5.00 | 4.4 | 3.1 | 4.9 | 3.3 | 5.3 |
| Serine | 2.47 | 4.28 | 4.2 | 4.75 | 5.4 | 3.6 | 5.2 | 3.4 | 5.7 |
| Threonine * | 1.77 | 2.96 | 3.0 | 3.40 | 3.8 | 2.5 | 3.9 | 2.3 | 3.6 |
| Tyrosine | 2.99 | 3.16 | 2.5 | 3.37 | 4.0 | 2.6 | 3.9 | 2.2 | 4.1 |
| Valine * | 6.72 | 3.41 | 3.6 | 3.97 | 4.9 | 2.7 | 5.1 | 2.2 | 4.5 |
| Tryptophan * | ND | ND | 0.4 | ND | 0.9 | ND | 1.3 | ND | 1.0 |
| References | [25,26] | [25,27,28] | [27,28,29] | ||||||
| Variety | WRC | ORC | EC | ES | LCG | FFC | FS | Authors | |
|---|---|---|---|---|---|---|---|---|---|
| Akkus bean (Phaseolus vulgaris) | Isolated protein | 190% | 410% | 468.4 ± 0.8 g·g−1 | 164.2 ± 12.9 min | 90 ± 0 g·L−1 | 91 ± 1% | 65.9% | [57] |
| Bombay bean (Phaseolus coccineous) | 190% | 400% | 468.5 ± 0.4 g·g−1 | 62.3 ± 6.0 min | 80 ± 0 g·L−1 | 76 ± 0% | 96% | ||
| Gembos bean (Phaseolus vulgaris) | 180% | 540% | 435.4 ± 0.6 g·g−1 | 60.1 ± 1.5 min | 100 ± 0 g·L−1 | 81 ± 2% | 81.4% | ||
| Hinis bean (Phaseolus vulgaris) | 210% | 470% | 467.8 ± 0.8 g·g−1 | 60.5 ± 1.1 min | 90 ± 0 g·L−1 | 72 ± 2% | 97.2% | ||
| Simav bean (Phaseolus vulgaris) | 200% | 400% | 402.7 ± 1.4 g·g−1 | 135.4 ± 3.8 min | 90 ± 0 g·L−1 | 83 ± 6% | 84.3% | ||
| White bean (Phaseolus vulgaris) | Isolated protein | 189.3 ± 1.9% | 300.3 ± 1.5% | 68.4 ± 0.6% | 96.0 ± 0.4% | ND | 50.0% | 23.3% | [58] |
| Faba bean (Vicia faba L.) | Globulins | 149.80 ± 0.61% | 65.32 ± 0.33% | ND | ND | ND | 18.06 ± 2.41% | 70.00 ± 8.66% | [59] |
| Albumins | ND | 229.13 ± 4.42% | ND | ND | ND | 133.33 ± 15.73% | 39.79 ± 4.09% | ||
| Michigan bean (Phaseolus vulgaris L.) | Flour | 2.3 g·g−1 ** | 2.1 g·g−1 ** | 39.60 ± 0.00% | ND | 4.00 ± 0.00% | 58.16 ± 0.06% | 30% | [60] |
| Concentrated protein | 4.5g·g−1 ** | 2.1g·g−1 ** | 13.33 ± 0.01% | ND | 14.00 ± 0.00% | 20.06 ± 0.07% | 5% | ||
| Kidney bean (P. vulgaris L.) | Globulin | 2.56 ± 0.06 mL.g−1 | 1.87 ± 0.06 mL·g−1 | ND | 95% ** | 6.00 ± 0.00% | 76% | 75% ** | [61] |
| Albumin | 3.40 ± 0.10 mL·g−1 | 2.37 ± 0.12 mL·g−1 | ND | 48% ** | 16.00 ± 0.00% | 100% | 70% ** | ||
| Mung bean | Isolated protein | 1.03 ± 0.09 2.78 ± 0.04 g·g−1 | 1.00 ± 0.12 3.38 ± 0.12g·g−1 | 1.77 ± 0.02 3.30 ± 0.05 g.g−1 | ND | ND | 33.00 ± 2.20 67.50 ± 1.04% | 20.00 ± 4.00 56.00 ± 3.98% | [62] |
| Hyacinth bean (Lablab purpureus) | Isolated protein | 321 ± 12.2% | 254 ± 0.2% | 534 ± 4.5 m2.g−1 | 2.7 ± 0.1 h | ND | 232 ± 12.2 mL·g−1 | 2.3 ± 0.2 min | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.E.G.; Azevedo, P.Z.; Matos, J.d.S.; Wischral, D.; Rigolon, T.C.B.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Strategies for Improving the Techno-Functional and Sensory Properties of Bean Protein. Processes 2025, 13, 371. https://doi.org/10.3390/pr13020371
Costa JEG, Azevedo PZ, Matos JdS, Wischral D, Rigolon TCB, Stringheta PC, Martins E, Campelo PH. Strategies for Improving the Techno-Functional and Sensory Properties of Bean Protein. Processes. 2025; 13(2):371. https://doi.org/10.3390/pr13020371
Chicago/Turabian StyleCosta, Juliana Eloy Granato, Paula Zambe Azevedo, Jessica da Silva Matos, Daiana Wischral, Thaís Caroline Buttow Rigolon, Paulo César Stringheta, Evandro Martins, and Pedro Henrique Campelo. 2025. "Strategies for Improving the Techno-Functional and Sensory Properties of Bean Protein" Processes 13, no. 2: 371. https://doi.org/10.3390/pr13020371
APA StyleCosta, J. E. G., Azevedo, P. Z., Matos, J. d. S., Wischral, D., Rigolon, T. C. B., Stringheta, P. C., Martins, E., & Campelo, P. H. (2025). Strategies for Improving the Techno-Functional and Sensory Properties of Bean Protein. Processes, 13(2), 371. https://doi.org/10.3390/pr13020371






