Abstract
The development of Pickering interfacial catalysts for organic reactions in water is of great importance to the development of green chemistry. In this study, amphiphilic hydrochar was prepared by a simple urea-modified hydrothermal carbonization with cellulose as an environmentally benign carbon source. It was found that the addition of urea could not only promote the carbonization of cellulose but also introduce N atoms to the final hydrochar material and tune the amphiphilicity of the hydrochar. Palladium nanoparticles supported on the amphiphilic N-doped hydrochar exhibited high activity in the Suzuki reaction in aqueous media. It can be seen that amphiphilic hydrochar can effectively stabilize Pickering emulsion, increase interface surface area, and further accelerate the Suzuki reaction.
1. Introduction
Organic synthesis in water has attracted a lot of attention due to the severe pollution caused by hazardous organic solvents. However, most organic substrates are insoluble in water, thus preventing the transformation in the aqueous phase and leading to low reaction efficiency [1]. Recently, Pickering emulsions have been demonstrated as an ideal strategy to enhance mass transfer in aqueous systems and alternatives to environmentally hazardous phase transfer agents [2,3]. Pickering interfacial catalysts are fabricated by the anchoring of metal nanoparticles with catalytic activity on amphiphilic solid particles. In a Pickering interfacial catalysis system, the huge interface area of Pickering emulsions and active particles placed at the interface between two immiscible liquids could enhance mass transfer efficiency, thus promoting catalytic activity [4].
To date, a variety of Pickering interfacial catalysts have been developed on silica [5,6], zeolites [7,8], carbon materials [9,10,11], polymers [12,13,14], cellulose nanofibers [15], and other materials [16,17,18]. Carbon materials are seen as one of the ideal Pickering emulsifier candidates for their stability and convenient surface modification. Carbon materials are stable under alkaline or acidic environments and can be applied in most reactions. In addition, their surface properties can be tuned by atom doping or combining with other materials. Pristine carbon materials are hydrophobic and unsuitable for Pickering interfacial catalysts. Common methods to obtain amphiphilic carbon material include (1) combinations with hydrophilic materials (silica, cellulose, etc.) [19,20,21], (2) inorganic acid treatment [22], and (3) doping with heteroatoms (N, O, B, etc.) [23,24]. Even in method (2), the introduction of hydrophilic atoms is crucial for the fabrication of amphiphilic carbon material. For example, Ji Wen reported a Pickering emulsion system stabilized by carbon nanotubes (CNTs) treated with a HNO3 and H2SO4 solution [22]. It was found that oxygen-containing functional groups (-COOH, -OH) introduced by acid treatment are important for the stabilizing of Pickering emulsions. Honglei Fan et al. reported the synthesis of superamphiphilic carbon by doping B and F during the carbonization of sawdust. Adding the element B could enhance the hydrophilicity of biochar materials, and the oleophilicity of carbon was kept simultaneously and superamphiphilic carbon was obtained [25]. Doping with hydrophilic atoms seems a promising method for the preparation of amphiphilic carbon materials.
Although many scholars have conducted a lot of excellent research on carbon-based Pickering interface catalysis, research on the utilization of hydrochar for Pickering interface catalysis is rarely explored. The preparation of hydrochar (hydrothermal carbonization) employs a lower energy consumption compared to pyrolysis at high temperatures (usually up to 800 °C) and better suits the principles of green chemistry [26,27]. Hydrochar has been applied in various areas, including the environment [28], catalysis [29], and electronics [28]. Most studies on hydrochar have focused on adsorption capacity and metal–support interactions [27,30] rather than wettability and Pickering emulsion stabilization capacity. In fact, due to the hydrothermal process, hydrochar is rich in oxygenated functional groups [31,32,33], which would make it more hydrophilic than pyrolyzed carbon material and more suitable for Pickering interface catalysis. Therefore, we decided to investigate the application of hydrochar in interfacial catalysis.
The Suzuki coupling reaction is a fundamental reaction for C-C bond formation to synthesize complex molecules, and has been applied in the synthesis of a variety of intermediates, pharmaceuticals, and fine chemical products and other high-technology materials in industries [34]. On the other hand, palladium (Pd) catalysts have been regarded as powerful catalysts for C-C coupling reactions, including Suzuki [35], Heck [36] and Sonogashira [37]. Compared with other transition metals, palladium (Pd) catalysts possess the unique ability to activate reaction substrates via Pd–carbon bond formation. Therefore, Pd nanoparticles (NPs) loaded on different support materials have been widely applied in Suzuki reactions [38,39].
Inspired by the fabrication of amphiphilic carbon material, N-doped hydrochar was developed. In this work, amphiphilic hydrochar was prepared via a urea-modified hydrothermal procedure and then developed as an efficient emulsifier for Pickering emulsion catalysis. It was found that urea can not only affect the hydrothermal carbonization process of cellulose and induce the formation of a uniform spherical hydrothermal carbon nanosphere, but also increase the hydrophilicity of the obtained hydrochar material. The Suzuki reaction in the aqueous phase was catalyzed by amphiphilic hydrochar-supported Pd NPs. A high reaction efficiency was achieved owing to the enlarged interface area.
2. Materials and Methods
2.1. Materials
All chemicals were purchased from commercial sources and used without further treatment. Micro-cellulose with an approximate length of 60 μm, palladium chloride (PdCl2), sodium formate (HCOONa), aryl halide, 0.23 mmol phenylboronic acid and potassium carbonate (K2CO3) was purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China) and used as received.
2.2. Preparation of Carbon Materials
Hydrochar (HC) and N-doped hydrochar (HNC) were prepared via the hydrothermal carbonization of cellulose. Total amounts of 3 g α-cellulose and 0.5 g urea were added to 30 mL deionized water and stirred for 10 min at room temperature before being transferred into a 100 mL autoclave. Then, the mixture was heated at 200 °C for 8 h. The obtained material was separated by filtration, washed with deionized water and ethanol several times, and dried at 80 °C for 24 h. The obtained samples were denoted as HNC.
HC was synthesized by some procedures without urea.
2.3. Pd Catalyst Preparation
A total of 50 mg PdCl2 was dissolved in 10 mL ethanol and the mixture was sonicated for 30 min before catalyst preparation. Then, 100 mg HNC and 0.2 mL 5 mg/mL PdCl2 solution were added to an agate mortar, ground manually for 20 min, and then rested for 24 h. Then, 60 mg sodium formate was added to the mixture and ground for 30 min and rested for another 24 h. The obtained catalyst (Pd/HNC) was washed repeatedly with distilled water (3 × 15 mL) and ethanol (3 × 15 mL) before drying in a vacuum oven at 25 °C for 12 h. Pd/HC was synthesized by the same procedure with HC as the catalyst support.
2.4. Characterization of Catalysts
Catalyst morphologies were characterized by SEM (Gemini Sigma 300 microscope, Oberkochen, Germany) and TEM (PHILIPS Tecnai 12 microscope, Amsterdam, The Netherlands). X-ray photoelectron spectroscopy (XPS) measurements were performed on a Nexsa spectrometer (Thermo Fisher Scientific, Shanghai, China). The water contact angles (WCAs) of compressed HC and HNC were characterized on a interface parameter test instrument (Kino SL200KS, Boston, MA, USA). A drop of water was dropped on the compressed samples and then imaged by a camera. The size of the Pickering emulsion drops was also characterized by a BX53M (Olympus Corp., Shanghai, China) Microscope images of oil-in-water emulsions were taken by the microscope.
2.5. Suzuki Reaction
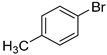
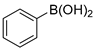
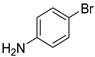
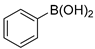
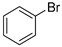
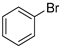
In total, 0.2 mmol aryl halide, 0.23 mmol phenylboronic acid, 0.4 mmol K2CO3, 3 mL deionized water, and 50 mg catalysts were added to a 15 mL flask. The flask was purged with Ar three times. The flask was placed in an oil bath and heated to 80 °C for 8 h. The reaction yield was determined by GC.
3. Results and Discussion
The Pd/HNC catalysts were synthesized using a simple and convenient process of the hydrothermal carbonization and chemical reduction of Pd ions before being utilized in Suzuki reactions (Figure 1). Cellulose, an abundant biomass on Earth, was chosen as the carbon precursor. The urea was attributed to the N source.
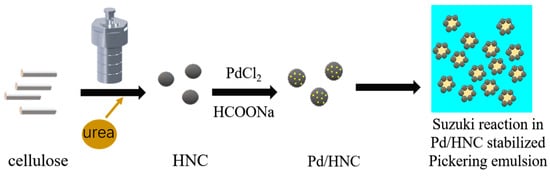
Figure 1.
Schematic diagram of the preparation of Pd/HNC for Suzuki reaction.
3.1. Catalyst Characterization
Initially, pristine cellulose, HC, and HNC were characterized with SEM. As shown in Figure 2, the cellulose was rod-like (Figure 2a) with a length of about 90 μm. After hydrothermal carbonization, the HC was composed of miscellaneous strips, blocks, and debris with different sizes ranging from 10 μm to 90 μm (Figure 2b and Figure S2). Interestingly, the morphological feature of HNC was much more uniform, with a spherical structure with a size of about 400 nm (Figure 2c and Figure S1), indicating that urea could promote the hydrothermal carbonization process of cellulose and benefit the formation of carbon nanospheres. The effect of urea on hydrochar structure was further investigated by XRD.

Figure 2.
SEM images of pristine (a) cellulose, (b) HC, and (c) HNC.
The powder X-ray diffraction (XRD) spectrum is shown in Figure 3a. No obvious difference was found between the XRD spectra of cellulose and HC, indicating the cellulose crystal phase structure of sucrose was preserved during hydrothermal treatment without the addition of urea. In HNC, a broad characteristic peak was found at 2θ = 24.2° and 2θ = 44°corresponding to a (002) diffraction of diffracted amorphous carbon (PDF#01–0604). The results provide further evidence that urea can promote the hydrothermal carbonization of cellulose. As for Pd/HNC, peaks at 2θ = 40°, 2θ = 47°, and 2θ = 69° assignable to the (111), (200), and (220) crystal planes of metallic Pd (PDF#01–1310) were observed. There is a huge difference in the FT-IR spectra of HC and HNC (Figure 3b). The peaks at 3430 cm−1 and 1080 cm−1 in HC are related to the vibration of –OH and the stretching vibration of C–O-C, respectively. As for the FT-IR spectra of HNC, there is a much weaker peak at 3440 cm−1, indicating the –OH groups might be destroyed during hydrothermal carbonization and the addition of urea could promote the hydrothermal carbonization process. This is consistent with the XRD and SEM results. The peaks at 1600–1700 cm−1 could be assigned to C-N bending, indicating that N was successfully introduced.
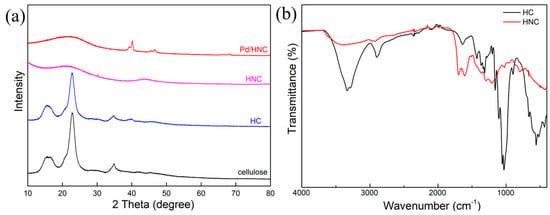
Figure 3.
(a) XRD patterns of cellulose, HC, HNC, and Pd/HNC, and (b) FT-IR spectra of HC and HNC.
The transmission electron microscopy (TEM) images of Pd/HNC show that most Pd NPs were uniformly distributed on the carbon surface (Figure S3a). The uniform distribution of Pd NPs could benefit the contact of Pd atoms with reactants. Further high-resolution (HR)TEM imaging revealed a highly integrated nanostructure of Pd NPs (Figure S3b). The lattice spacing of 0.20 nm corresponding to (111) of the Pd can be perceived. As shown in the element mappings (Figure 4), N atoms were successfully introduced to hydrochar via the hydrothermal process and distributed uniformly on the HNC surface. Furthermore, Pd atoms were also detected and thus we can confirm that a N-doped hydrochar-supported Pd NP catalyst was successfully prepared.
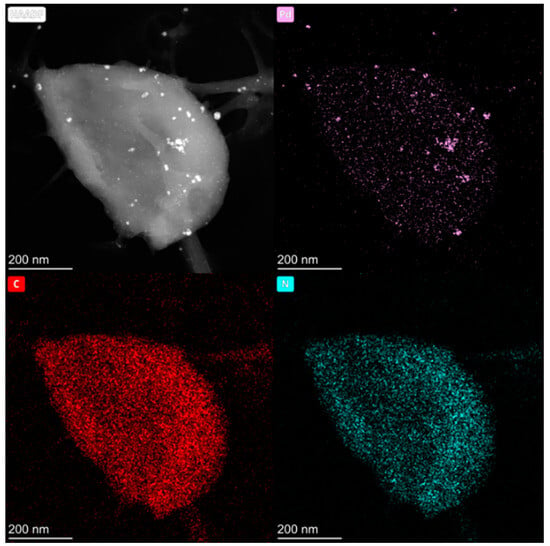
Figure 4.
EDX mapping of Pd/HNC.
The surface wettability of HC and HNC was tested by contact angle experiments. It was found that HNC was more hydrophilic than HC. The contact angle of HC was 57° (Figure 5a), while that of HNC was 38° (Figure 5b). Notably, the contact angle of HC was smaller than 90°, which could have been because of –OH groups, as confirmed by FT-IR. In HNC, the amounts of –OH groups decreased and the hydrochar material should therefore have been more hydrophobic, but the contact angle of HNC showed a smaller value, indicating that N doping could tune the wettability of hydrochar.
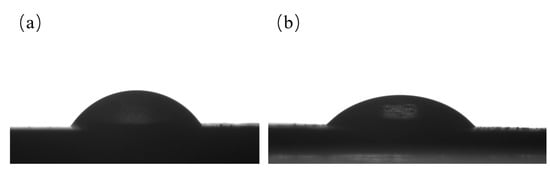
Figure 5.
WCAs of water on the surface of (a) HC and (b) HNC.
Furthermore, the emulsifying capacity of HC and HNC was examined in a toluene/water system. As shown in Figure 6a,b, the emulsion stabilized by HC was unstable and a rapid separation between the water and oil phases was observed. However, the Pickering emulsion stabilized by HNC showed better stability for about 12 h. This observation indicates that HNC is a suitable support for Pickering interfacial catalysts. The morphology of emulsion droplets stabilized by HNC was characterized by an optical microscope. The optical microscopy images showed that the droplets had an average diameter of 150 μm.
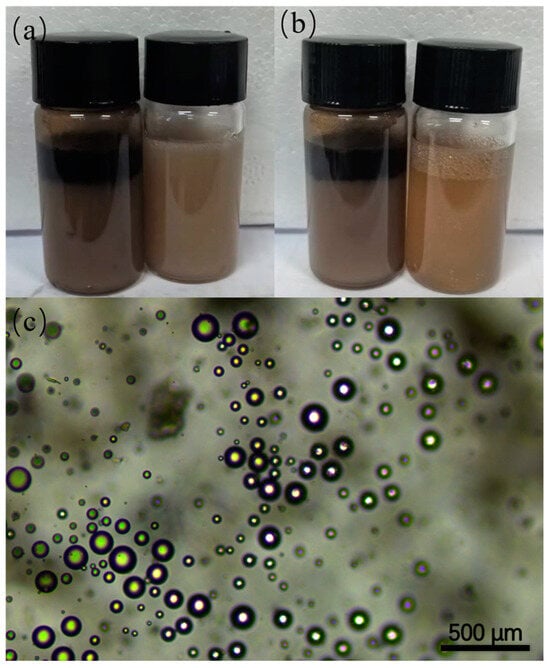
Figure 6.
Digital photos of the Pickering emulsion solution stabilized by HC (left) and HNC (right) taken at (a) 0 h and (b) 24 h. (c) Micrograph image of the Pickering emulsion solution stabilized by HNC.
Pd/HNC was further characterized with X-ray photoelectron spectroscopy (XPS). As seen in the wide scan spectra (Figure 7a), Pd/HNC consists of Pd, C, and O, which is also confirmed by the EDX spectrum (Figure 4). However, the peaks of N and Pd are not obvious because of their low contents. It can be seen that Pd mainly exists in the Pd0 state. A small amount of Pd was oxidized and existed in the Pd2+ state. The peaks at 335.8 eV and 339.9 eV were assigned to the Pd 3d5/2 and Pd 3d3/2 transitions, respectively. Four peaks were found in the C 1s spectrum, located at 284.7 eV, 286.3 eV, and 288.1 eV corresponding to sp2C, sp3C, and C–N species, respectively (Figure 7c). The N 1s spectrum of Pd/NFBC is shown in Figure 7d. Only two components can be deconvoluted from the spectrum, representing pyridinic N (399.2 eV) and graphitic N (400.8 eV).
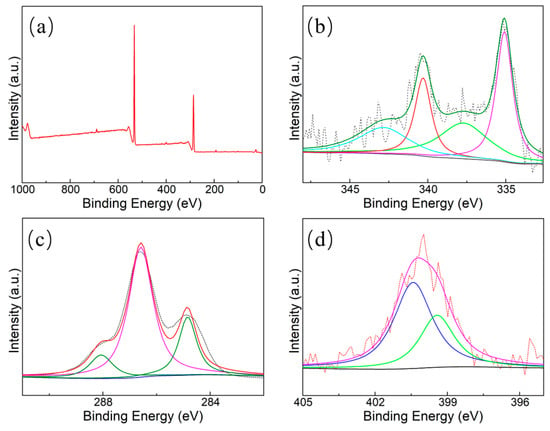
Figure 7.
XPS spectra of (a) full spectra, (b) Pd 3d, and (c) C 1s of Pd/HNC, and (d) N 1s of Pd/HNC.
3.2. Catalyst Tests
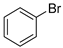
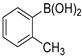
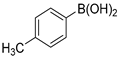
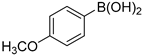
The applicability of Pd/HNC in the Suzuki reaction was further evaluated in water. Hazardous phase transfer agents are usually employed for the Suzuki reaction in water. Otherwise, lower yields will be achieved. Encouragingly, as shown in Figure 7a, Pd/HNC exhibited high activity in the Suzuki reaction and a final yield of 93% was achieved in 8 h. In contrast, only a 39% yield of biphenyl was achieved with Pd/HC as the catalyst within the same time (Figure 8). The higher yield of Pd/HNC could be attributed to the formation of a Pickering emulsion by Pd/HNC (Figure S5), which would increase the water-reactant interface with Pd/HNC oriented at the interface, and then increase the chance of contact between the reactants and the catalyst. For further demonstration, more Suzuki reactions were performed using Pd/HNC and great yields were achieved (Table 1).
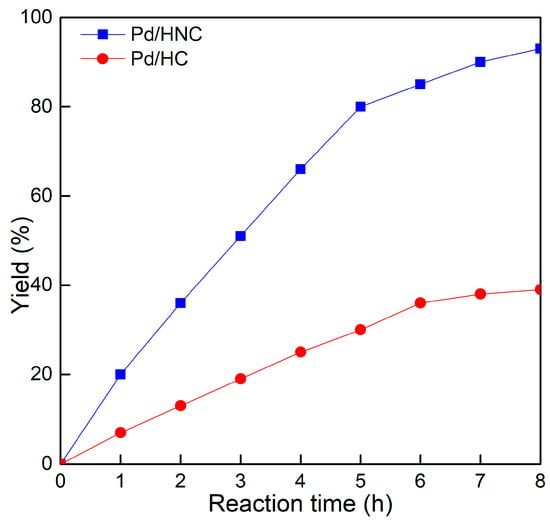
Figure 8.
Plotting of biphenyl yield as a function of reaction time.

Table 1.
Suzuki reactions catalyzed by Pd/HNC 1.
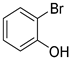
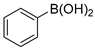
A mechanism of the Suzuki reaction with Pd/HNC can be proposed based on the above results and previous studies (Scheme 1). First, an organopalladium intermediate is formed by the oxidative addition of bromobenzene. Then, phenyl boronic acid is alkalized by K2CO3 and further transferred to Pd atoms. Finally, a C-C bond is formed by a reductive elimination step to give the final product, and the original Pd (0) species is also regenerated. In the reaction mixture, the alkalization of phenyl boronic acid occurs in water while bromobenzene is insoluble in water, so the main hindrance of the reaction is the mass transfer between two phases. As can be seen in Figure 6a,b, HC could not form a Pickering emulsion while HNC showed great emulsion stabilization capacity and could connect with both phases. Therefore, the mass transformation in the Pd/HNC-catalyzed Suzuki reaction was promoted and led to high activity. Hence, the formation of an emulsion is crucial to enhance the catalytic activity of a Pd catalyst.
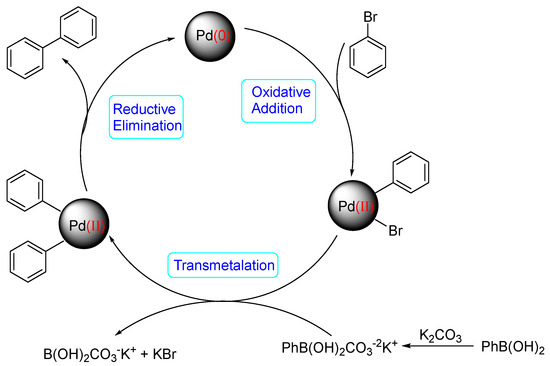
Scheme 1.
Proposed mechanistic pathway for the Suzuki reaction catalyzed by Pd/HNC.
3.3. Catalyst Reusability
The reusability of Pd/HNC was further investigated in the Suzuki reaction. In a typical procedure, the catalyst was recovered by centrifugation after each run. As presented in Figure 9, Pd/HNC could be reused six times without an obvious decrease in biphenyl yields. After the sixth cycle, the yield of biphenyl still reached 88%. The reused Pd/HNC was characterized by TEM. Aggregation of Pd NPs was observed (Figure S4), which would resulted in a decrease in the specific surface area and catalytic activity of Pd/HNC. Furthermore, the emulsifying capacity was studied. The optical microscopy images showed stabilized Pickering emulsion droplets with sizes of 100–150 μm. It can be seen that the recycling process had little effect on the emulsifying capacity of HNC.
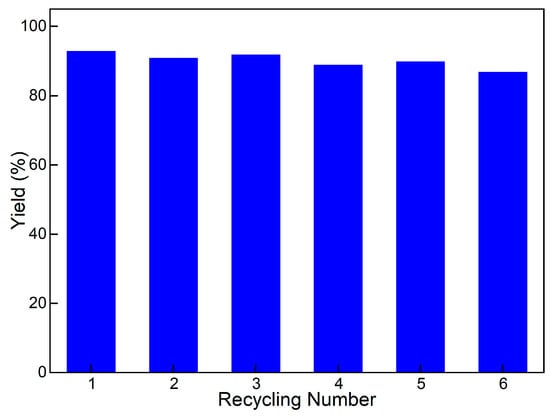
Figure 9.
Recycling performance of Pd/HNC.
4. Conclusions
In summary, a Pickering interfacial catalyst was fabricated by the urea-mediated hydrothermal carbonation of cellulose and further deposition of Pd NPs. The addition of urea can not only promote the hydrothermal process so that the final carbon material has a uniform spherical shape with a size of about 400 nm, but also introduce nitrogen atoms into the hydrothermal carbon material. The final N-doped hydrochar could stabilize the Pickering emulsion and thus enhance the Suzuki reaction efficiency in water. The design of new amphiphilic hydrochar materials is expected to shed new light on the development of high-performance Pickering emulsion catalysis systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13020339/s1, Figure S1: SEM images of HNC, Figure S2: SEM images of HC, Figure S3: (a) TEM and (b) HRTEM images of Pd/HNC, Figure S4: TEM images of reused Pd/HNC, Figure S5: Optimal images of Pickering emulsion in Suzuki reaction stabilized by Pd/HNC, Figure S6: Optimal images of Pickering emulsion stabilized by Pd/HNC after reused for 6 times in Suzuki reaction.
Author Contributions
Conceptualization, D.L. and F.M.; methodology, Z.W. and Y.W.; software, D.L.; validation, Z.W. and Y.W.; formal analysis, Z.W. and Y.W.; investigation, D.L. and F.M.; resources, J.C. and Z.L.; data curation, D.L. and F.M.; writing—original draft preparation, D.L.; writing—review and editing, F.M.; visualization, D.L.; supervision, F.M.; project administration, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Research Project of Anhui Educational Committee (Grant No. 2022AH050847), the Scientific Research Foundation for High-level Talents of Anhui University of Science and Technology (13210574), and the Open Research Fund Program of Anhui Provincial International Joint Research Center of Modern Environmental Engineering, Anhui University of Science and Technology (Grant No. XDHJGC2022007), and the Opening Foundation of Anhui Key Laboratory of Explosive Energy Utilization and Control (No. BP20240101).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Authors Dandan Li, Feichao Miao, Jinhua Chen and Zhibing Liu were employed by the company Anhui Key Laboratory of Explosive Energy Utilization and Control. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, L.; Zhang, S.; Liu, X.; Ge, X. Recent advances in water-mediated multiphase catalysis. Curr. Opin. Colloid Interface Sci. 2023, 65, 101691. [Google Scholar] [CrossRef]
- Guzmán, E. Pickering Emulsions in Catalytic Processes. ChemCatChem 2024, 16, e202400856. [Google Scholar] [CrossRef]
- Chang, F.; Vis, C.M.; Ciptonugroho, W.; Bruijnincx, P.C.A. Recent developments in catalysis with Pickering Emulsions. Green Chem. 2021, 23, 2575–2594. [Google Scholar] [CrossRef]
- Li, D.-d.; Jiang, J.-z.; Cai, C. Palladium nanoparticles anchored on amphiphilic Janus-type cellulose nanocrystals for Pickering interfacial catalysis. Chem. Commun. 2020, 56, 9396–9399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q.; Guo, J.-L.; Liang, R.-H.; Wang, F.-X.; Li, Z.-K.; Liu, Y.; Ying, A. Two birds with one stone: A magneto-optical dual-stimuli-responsive intelligent Pickering emulsion for efficient conversion of glucose to 5-hydroxymethylfurfural. Chem. Eng. J. 2024, 479, 147757. [Google Scholar] [CrossRef]
- Li, C.; Pi, Y.; Liu, S.; Feng, J.; Zhang, X.; Li, S.; Tan, R. Phosphotungstate-Functionalized Mesoporous Janus Silica Nanosheets for Reaction-Controlled Pickering Interfacial Catalysis. ACS Sustain. Chem. Eng. 2021, 9, 13501–13513. [Google Scholar] [CrossRef]
- Lv, G.; Wang, T.; Chen, Y.; Wang, J.; Zou, X.; Lu, J.; Wang, F.; Zhang, X.; Zhai, Y. Hydrophobized hollow TS-1 zeolite as pickering interfacial catalyst for selective oxidation reactions. Colloids Surf. A 2022, 633, 127842. [Google Scholar] [CrossRef]
- Richards, K.D.; Evans, R.C. Light-responsive Pickering emulsions based on azobenzene-modified particles. Soft Matter 2022, 18, 5770–5781. [Google Scholar] [CrossRef]
- Ni, L.; Yu, C.; Xie, Y.; Wei, Q.; Liu, D.; Tan, X.; Ding, Y.; Qiu, J. pH-Switchable Pickering miniemulsion enabled by carbon quantum dots for quasi-homogenized biphasic catalytic system. Chem. Commun. 2023, 59, 3261–3264. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, Z.; An, J.; Li, Z.; Shi, L.; Shan, Y. Preparation and Emulsifying Properties of Carbon-Based Pickering Emulsifier. Processes 2023, 11, 1070. [Google Scholar] [CrossRef]
- Fan, H.; Chen, B.; Chen, C.; Zhang, Z.; Song, J.; Yang, G.; Han, B. Superamphiphilic carbon from sawdust activated by oxygen/argon mixtures promoting the oxidation of benzyl alcohol in Pickering emulsion. Green Chem. 2021, 23, 6341–6348. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, H.; Shao, X.; Chen, J.; Cheng, Y.; Chen, M.; Jiang, Y.; Chen, X.; Ke, Q.; Wang, Y.; et al. Pickering Emulsions Stabilized by Linear Amphiphilic Polymers for Enhancing the Selective Oxidation of Cinnamyl Alcohol. ACS Appl. Polym. Mater. 2024, 6, 10083–10089. [Google Scholar] [CrossRef]
- Rayees, R.; Gani, A.; Noor, N.; Ayoub, A.; Ashraf, Z.U. General approaches to biopolymer-based Pickering emulsions. Int. J. Biol. Macromol. 2024, 267, 131430. [Google Scholar] [CrossRef]
- Wang, L.; Mohamad Ali, B.; Yu, S.; Liang, S.; Huang, Q.; Zhang, H.; Zhu, L.; Wang, J. Dumbbell-shaped polymeric nanoparticles with one-side anchored Pd-clusters for efficient catalytic hydrogenation at biphasic interfaces. J. Catal. 2024, 432, 115444. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Chen, S.; Yang, H.; Liu, K.; Pang, B.; Xu, T.; Si, C. Microencapsulated phase change material through cellulose nanofibrils stabilized Pickering emulsion templating. Adv. Compos. Hybrid Mater. 2023, 6, 149. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Ding, Y.; Xiong, D.; Li, Z.; Wang, H.; Qiu, J.; Xuan, X.; Wang, J. Zr-Based MOF-Stabilized CO2-Responsive Pickering Emulsions for Efficient Reduction of Nitroarenes. Langmuir 2024, 40, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Liu, J.; Song, W.; Xu, D.; Wang, Z.; Xie, Y. CO2-Switchable Hierarchically Porous Zirconium-Based MOF-Stabilized Pickering Emulsions for Recyclable Efficient Interfacial Catalysis. Materials 2023, 16, 1675. [Google Scholar] [CrossRef]
- Yin, L.; Gao, K.; Mao, X.; Hu, Y. Lipase B from Candida antarctica immobilized on amphiphilic Janus halloysite nanosheet and application in biphasic interface conversion. Food Chem. 2024, 437, 137787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhu, X.; Hung, C.-T.; Wang, P.; Elzatahry, A.; Al-Khalaf, A.A.; Hozzein, W.N.; Zhang, F.; Li, X.; Zhao, D. Spatial isolation of carbon and silica in a single Janus mesoporous nanoparticle with tunable amphiphilicity. J. Am. Chem. Soc. 2018, 140, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yu, C.; Wei, Q.; Chang, J.; Qiu, J. Decoupling the role of carbon counterparts in Pickering emulsifier for an enhanced selective oxidation of benzyl alcohol. Green Chem. 2020, 22, 5711–5721. [Google Scholar] [CrossRef]
- Chen, G.; Han, J.; Niu, Z.; She, P.; Li, L.; Guan, B.; Yu, J. Regioselective Surface Assembly of Mesoporous Carbon on Zeolites Creating Anisotropic Wettability for Biphasic Interface Catalysis. J. Am. Chem. Soc. 2023, 145, 9021–9028. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yu, C.; Ni, L.; Wei, Q.; Yao, X.; Qiu, J. Fundamental mechanistic insights into in-situ reduction of Pd2+ coupling with benzyl alcohol oxidation at oil–water interface. Chem. Eng. Sci. 2024, 299, 120506. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Z.; Hou, M.; Song, J.; Yang, G.; Han, B. Fabrication of Superamphiphilic Carbon Using Lignosulfonate for Enhancing Selective Hydrogenation Reactions in Pickering Emulsions. ACS Appl. Mater. Interfaces 2021, 13, 25234–25240. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Zhao, H.; Gao, Y.; Zhang, B.; Xu, X.; Yue, Q.; Leiviskä, T.; Jin, B.; Chen, B.; Gao, B. Accelerated selective oxidation of benzyl alcohol to benzaldehyde via a self-catalyzed Pickering emulsion microreactor. J. Mater. Chem. A 2024, 12, 25381–25392. [Google Scholar] [CrossRef]
- Fan, H.; Wang, Q.; Liu, H.; Han, B.; Liu, H.; Yang, G. Surface Engineering of Biochar Toward Simultaneously Generating Superamphiphilicity and Catalytic Activity for Strengthening Pickering Interfacial Catalysis. ChemSusChem 2024, 17, e202400248. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-García, P.; Chaparro-Garnica, J.; Navlani-García, M.; Cazorla-Amorós, D. Exploring the Effects Behind the Outstanding Catalytic Performance of PdAg Catalysts Supported on Almond Shell-Derived Activated Carbon Towards the Dehydrogenation of Formic Acid. ChemCatChem 2024, 16, e202400160. [Google Scholar] [CrossRef]
- Sun, S.; Peng, X.; Guo, X.; Chen, X.; Liu, D. Boosting Solvent-Free Aerobic Oxidation of Benzylic Compounds into Ketones over Au-Pd Nanoparticles Supported by Porous Carbon. Catalysts 2024, 14, 158. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zheng, M.; Xiao, Y.; Hu, H.; Liang, Y.; Liu, Y.; Dong, H. Preparation of High-Performance Porous Carbon Materials by Citric Acid-Assisted Hydrothermal Carbonization of Bamboo and Their Application in Electrode Materials. Energy Fuels 2022, 36, 9303–9312. [Google Scholar] [CrossRef]
- Pereira, G.R.; Lopes, R.P.; Wang, W.; Guimarães, T.; Teixeira, R.R.; Astruc, D. Triazole-functionalized hydrochar-stabilized Pd nanocatalyst for ullmann coupling. Chemosphere 2022, 308, 136250. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ding, L.; Leghari, A.; Hungwe, D.; Gao, M.; Gao, Y.; Zhang, Y.; Chen, X.; Wang, F. Ferric sludge derived pyrolyzed-hydrochar supported iron catalysts for catalytic cracking of toluene. Chem. Eng. J. 2024, 491, 152001. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Q.; Abou-Elwafa, S.F.; Alshehri, M.A.; Zhang, T. Hydrothermal Carbonization Technology for Wastewater Treatment under the “Dual Carbon” Goals: Current Status, Trends, and Challenges. Water 2024, 16, 1749. [Google Scholar] [CrossRef]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sustain. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Li, K.; Yu, S.; Li, Q.; Zhang, Y.; Zhou, H. Selective hydrodeoxygenation of guaiacol to cyclohexanol using activated hydrochar-supported Ru catalysts. Front. Chem. Sci. Eng. 2024, 18, 50. [Google Scholar] [CrossRef]
- Liu, L.; Yang, C.-J.; Li, Z.-L.; Gu, Q.-S.; Liu, X.-Y. The first-row transition metal-catalysed enantioconvergent radical Suzuki–Miyaura C(sp3)–C coupling of racemic alkyl halides. Green Chem. 2024, 26, 2525–2533. [Google Scholar] [CrossRef]
- Almaradhi, M.A.; Hassan, H.M.A.; Alhumaimess, M.S. Fe3O4-carbon spheres core–shell supported palladium nanoparticles: A robust and recyclable catalyst for suzuki coupling reaction. Chin. J. Chem. Eng. 2022, 51, 75–85. [Google Scholar] [CrossRef]
- Rohani, S.; Mohammadi Ziarani, G.; Badiei, A.; Ziarati, A.; Luque, R. Mesoporous Hierarchically Hollow Flower-Like CoAl-LDH@N,S-doped Graphene@Pd Nanoarchitectures for Heck Couplings. Catal. Lett. 2019, 149, 2984–2993. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Lahcini, M.; Bousmina, M.; Garcia, H.; El Kadib, A. Pd embedded in chitosan microspheres as tunable soft-materials for Sonogashira cross-coupling in water-ethanol mixture. Green Chem. 2015, 17, 1893–1898. [Google Scholar] [CrossRef]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Misztalewska-Turkowicz, I.; Maj, J.; Leśniewska, B.; Wojtulewski, S.; Zgłobicka, I.; Wilczewska, A.Z. Polymer-Covered Magnetic Nanoparticles as a Palladium Pickering Interfacial Catalyst for the Suzuki–Miyaura Reaction Performed in a Water Environment. J. Phys. Chem. Lett. 2023, 127, 19937–19946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).