2.1. Petrophysical Volumetric Model
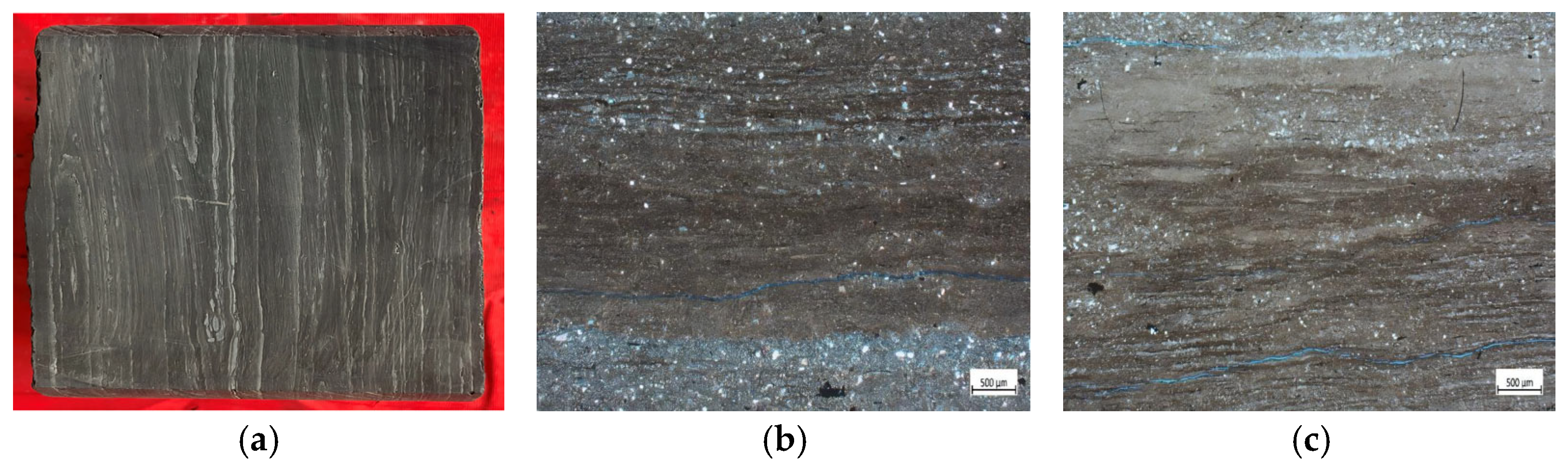
The target interval in this study primarily consists of laminated and massive shales. Laminated shales average 45.8% quartz, 47.4% clay, 4.3% feldspar, 2.0% calcite, and 0.5% siderite. Massive shales average 37.7% quartz, 53.3% clay, 5.8% feldspar, 1.2% calcite, and 1.5% siderite. Quartz content is slightly higher in laminated shales, while clay content is slightly lower. The clay minerals are predominantly mixed-layer illite/smectite (I/S), illite, kaolinite, and chlorite, with relative contents of 51.7% I/S, 21.5% illite, 10% kaolinite, and 17% chlorite, indicating the highest proportion of I/S. The laminated shale was examined using a Leica DM4P polarizing microscope from Germany. Light-colored laminae on a millimeter scale were observed distributed within the dark shale (
Figure 1a). Meanwhile, interlayer fractures and well-developed bedding fractures are visible (
Figure 1b,c). The thin-section photographs clearly reveal the presence of bedding fractures.
Clay minerals are primarily illite, chlorite, smectite, and kaolinite. The general chemical formula for illite is K
0.65Al
2(Si
3.35Al
0.65)O
10(OH)
2·mH
2O, for chlorite it is (Mg,Fe,Al)
6(Al,Si)
4O
10(OH)
8, for smectite it is (Al, Mg)
2(Si
4O
10)(OH)
2·4H
2O, and for kaolinite it is Al
4(Si
4O
10)(OH)
8 [
9]. The chemical formulas indicate that silicates in clay minerals contain hydroxyl groups (OH), and the number of interlayer water molecules (m) in illite is controlled by burial depth-induced compaction and associated pore fluid expulsion processes, evolving dynamically. The increase in mudstone density is a continuous process, transitioning from predominantly physical compaction to chemical compaction. During physical compaction, mechanical pressure from overlying strata rearranges and packs sedimentary particles, significantly increasing rock density by directly reducing pore space. Chemical compaction, on one hand, increases rock mass per unit volume by transforming low-density minerals like smectite into high-density illite and chlorite. On the other hand, this transformation process itself expels interlayer water and causes mineral volume contraction, further reducing the total volume. Additionally, silica and other substances released during transformation precipitates in residual pores forming authigenic cements, eventually filling the pores completely. Physical and chemical compaction work synergistically, driving mudstones to become increasingly dense and hard [
8,
10]. Consequently, the clay fraction in shale needs to be divided into dry clay, hydroxyl groups and bound water, and clay interlayer pores. The shale inorganic pores, including clay interlayer pores, pyrite intercrystalline pores, bedding fractures, and micro-fractures, were observed using a Quanta 450 field-emission scanning electron microscope from FEI, the Netherlands (
Figure 2a). Kerogen mainly consists of solid hydrocarbon compounds containing organic pores. These organic pores span a range of scales from nanometers to micrometers (
Figure 2b), and kerogen is often associated with clay minerals (
Figure 2c). Organic pores are generally considered oil-wet and contain adsorbed oil that is difficult to mobilize; thus, the kerogen fraction is divided into solid kerogen and organic pores.
Argillaceous sandstone porosity models consider bound and movable fluids. In contrast, continental shales differ in that crystalline minerals like quartz and feldspar are clay-sized particles, clay content is high, and clays contain substantial hydroxyl groups and bound water. Furthermore, inorganic and organic pores contain both adsorbed and free fluids, characterizing the fluid occurrence state in continental shales. Shale pores are classified by pore diameter into micropores (<2 nm), mesopores (2–50 nm), and macropores (>50 nm). Previous shale porosity models were modeled based on bound water, bound fluids, organic pores, and movable fluids without considering the pore diameter of organic and inorganic pores and the occurrence state of shale oil [
4]. Therefore, this study establishes a rock physics volume model for shale considering the shale framework (dry clay, crystalline minerals, kerogen) and fluid components (surface-adsorbed fluids, bound fluids, and free fluids) (
Figure 3), where crystalline minerals include quartz, feldspar, calcite, and dolomite.
Crystalline minerals, clay, and kerogen in shale possess distinct density and neutron matrix values. Different literature sources provide varying density and neutron values for clay and kerogen, showing slight differences for specific study areas and target intervals [
11,
12].
2.2. Review of Shale Porosity Logging Evaluation
Compensation neutron logging utilizes an isotope neutron source (americium-beryllium source) to emit fast neutrons into the formation in the wellbore, then employs two thermal neutron detectors at different source distances (long and short). Since hydrogen atoms have the largest scattering cross-section for neutrons, elastic scattering between hydrogen atoms and neutrons significantly slows the neutrons, converting them to low-energy thermal neutrons. These thermal neutrons are detected and used to analyze hydrogen atom abundance, which is proportional to the number of scattered thermal neutrons received. In shale formations, hydrogen atoms are present not only in pore fluids (oil/water) but also in kerogen, clay minerals, and bound water. Consequently, kerogen, clay minerals, and bound water contribute to the compensated neutron log value. The neutron log reading in shale oil reservoirs is thus greater than the effective porosity and does not reflect the total porosity value.
Shale oil reservoirs are typically undercompacted and characterized by high formation pressure. This condition leads to an increase in the compressional wave (P-wave) slowness reading from acoustic logs. Therefore, when using P-wave slowness for porosity evaluation in shale oil reservoirs, formation pressure corrections must be applied. However, due to the inherently low porosity of shale reservoirs (usually less than 10%), this correction can introduce significant errors into the corrected acoustic log data. For this reason, this paper investigates the use of density logs and nuclear magnetic resonance (NMR) logs for porosity assessment.
Density logging is a porosity log that measures the intensity of gamma rays Compton-scattered by formation elements from a gamma source to investigate the density properties of the formation. Different elements (e.g., oxygen, silicon, calcium) in the rock scatter gamma rays with varying intensities; higher atomic number elements absorb gamma rays more strongly, resulting in higher measured density values. Using density logs to evaluate porosity in shale oil reservoirs requires determining the matrix mineral density values. However, unlike sandstone reservoirs, shale oil reservoirs include kerogen and clay minerals as part of the rock matrix. The key challenge lies in the variable amount of bound water associated with clay minerals, causing the matrix density values of clay minerals to change with differences in burial depth and compaction.
Nuclear magnetic resonance (NMR) logging involves polarizing the atomic nuclei (primarily hydrogen nuclei) of formation media using a magnetic field, followed by the application of pulsed high-frequency electromagnetic waves to induce resonance absorption. The energy released from the resonance absorption of the formation media is then measured to identify the pore spaces and occurrence states of hydrogen nuclei. This technique can distinguish hydrogen nuclei in different pore spaces, such as clay-bound water, capillary-bound water, and free water. Additionally, due to the differences in NMR characteristics of hydrogen nuclei in oil, gas, and water, fluid types can be identified. In contrast, neutron logging measures the hydrogen index of all hydrogen nuclei in the formation and cannot differentiate the occurrence states of fluids. In shale oil reservoirs, two-dimensional NMR (T1–T2) logging is typically applied with a minimum echo spacing (TE) of 0.2 ms, enabling the measurement of fluids in clay interlayer pores, organically adsorbed oil, and large organic/inorganic pores. However, it cannot detect signals from kerogen, clay-bound water, and hydroxyl groups.
In summary, among conventional logs, density logging is suitable for total porosity evaluation in shale oil reservoirs. Two-dimensional NMR logging not only evaluates porosity but also identifies the occurrence states of fluids in different pore spaces [
12] (
Figure 4).
2.3. Shale Porosity Evaluation Method Using Density Logs
For the evaluation of shale porosity using density logs, the process begins by calculating the shale matrix using the crystalline mineral content and clay mineral content obtained from lithoscopic logging. Then, the dry weight organic carbon content derived from lithoscopic logging is converted into kerogen volume. Finally, a petrophysical volumetric model is applied to establish the density log response equation, thereby determining the shale porosity.
The measurement signals of lithoscopic logging are gamma rays induced by neutrons interacting with different elements in the formation. Through spectrum analysis and the oxygen closure technique, the dry-weight fractions of matrix elements are obtained. Based on this, an optimization program is used to derive the mineral content and the (dry-weight) total organic carbon content in the formation from the elemental concentrations.
The density matrix value of the inorganic matrix is calculated as follows:
where
represents the matrix density (g/cm3);
represents the number of crystalline mineral types;
represents the number of clay mineral types;
represents the mass percentage of the i-th crystalline mineral (%);
represents the mass percentage of the j-th clay mineral (%);
represents the density log matrix parameter for the i-th crystalline mineral (g/cm3);
represents the density log matrix parameter for the j-th clay mineral (g/cm3).
The conversion of total organic carbon (TOC) from weight percentage to kerogen volume is a critical step in the petrophysical evaluation of shale reservoirs, as it enables the accurate quantification of kerogen content required for logging analysis. The formula for converting TOC mass percentage to kerogen volume is [
4]:
where
represents the kerogen density parameter (g/cm3);
k represents the conversion coefficient, k = 1.2;
represents the logged density value (g/cm3);
represents the kerogen volume content (%).
Since kerogen density increases with thermal maturity (Ro) and can be determined accordingly [
6], and given the lack of locally derived kerogen volume parameters, a constant conversion factor (
k = 1.2) was adopted from the literature [
4].
Based on the shale petrophysical volumetric model (
Figure 3), the shale is divided into inorganic mineral matrix, kerogen volume, and porosity, yielding the density log response equation:
where
represents the matrix density (g/cm3);
represents the logged density value (g/cm3);
represents the kerogen volume content (%);
represents the kerogen density parameter (g/cm3);
represents the complex fluid density parameter (g/cm3);
represents the porosity (%).
The study area is located in the Sichuan Basin, China, and targets the Lianggaoshan Formation. The organic-rich shale in the Lianggaoshan Formation is widely distributed, thick, highly mature, and possesses strong hydrocarbon generation capacity, indicating significant exploration potential (
Figure 5). As burial depth increases, the matrix values of clay minerals increase due to compaction. If a constant matrix value is used for clay when calculating porosity from density logs, the low porosity values of shale may lead to significant errors in the derived porosity.
The entire rock system was treated as a multi-component mixture consisting of crystalline minerals, clay minerals, kerogen, and pores. First, the total organic carbon (TOC) was converted into kerogen content using Equation (2). Next, the shale porosity derived from core analysis was integrated with the weight fractions of crystalline and clay minerals obtained from lithology scanning logs, while the density log reading was used as the observed measurement. Subsequently, a constrained optimization algorithm was applied to Equation (3) to determine the matrix density of the clay minerals. Finally, the total shale porosity was calculated using Equation (5).
In addition to conventional logging, Well ZX1 was also logged with lithology scanning and 2D T1-T2 NMR (CMR-NG) tools. The cored interval spans 1885.5–1912.6 m. Porosity data were obtained from six plug samples using helium porosity measurements combined with 2D NMR (
Table 1, where mineral contents are presented as weight percentages). The thermal maturity (R
o) of the Lianggaoshan Formation in this well is 1.19. Based on the thermal maturity–kerogen density model [
6], the kerogen density was determined to be 1.339 g/cm
3. Substituting TOC, log density, and kerogen density into Equation (2) yields the kerogen volume (V
ker). Core analysis of shale from this interval provided 11 water saturation (S
w) measurements, ranging from 32.83% to 56.56%, with an average of 46.43%. In the target zone of this block, the oil density is 0.86 g/cm
3 and the formation water density is 1.02 g/cm
3. Using Equation (4), the average fluid density for the block is calculated as 0.9343 g/cm
3. Substituting this value into Equation (3) allows the equation to be simplified as follows:
The primary mechanism for the increase in clay mineral density with depth is diagenetic evolution, particularly the transformation of smectite to illite. During this process, exchangeable cations and significant amounts of adsorbed water are expelled from the interlayers of the smectite structure. Concurrently, elements such as silicon, aluminum, and potassium undergo reorganization to form illite, which exhibits higher density and greater structural stability. This mineralogical transformation results in a marked increase in the intrinsic density of the clay minerals.
The dominant clay minerals in the target interval of the study area are illite and chlorite. Their weight percentages can be derived from lithology scanning logs (
Table 1). Since the matrix density of clay minerals is variable, it is necessary to determine the density matrix values for these two clay types. Although compaction occurs simultaneously, it primarily reduces the total rock volume and porosity, with limited contribution to altering the intrinsic density of the mineral crystals themselves. Therefore, the density matrix values of crystalline minerals remain essentially constant. The density values for different crystalline minerals are listed in
Table 2.
The weight percentage of each crystalline mineral was obtained from lithology scanning logs, and its corresponding density matrix value is known. The total weight percentage of crystalline minerals in the rock was calculated by summing the weight percentages of individual minerals. Using Equation (7), the average density of the crystalline minerals was then determined (
Table 3).
The sum of the weight percentages of the clay minerals chlorite and illite from
Table 1 was substituted into Equation (1) to calculate the average clay matrix density (
ρcl), yielding values in the range of 2.812–2.941 g/cm
3 (
Table 3). Although Equation (8) can be used to determine the matrix density values of chlorite (
ρchlorite) and illite (
ρillite), both parameters remain unknown, resulting in a nonlinear optimization problem. Given that the matrix density values of chlorite and illite in the target interval of the study area are known to exceed 2.67 g/cm
3, a constrained grid search method was employed to solve the problem.
The target interval of the study area, ranging from 1500 to 2500 m in depth, lies within the mesodiagenetic stage. The intrinsic mineral densities of illite and chlorite are not consistently distinct; their ranges overlap depending on chemical composition. Chlorite density is primarily controlled by the Fe/(Fe + Mg) ratio. Mg-rich chlorite exhibits densities close to or slightly below 2.67 g/cm3 (typically 2.65–2.70 g/cm3). In continental shales, pyrite formation during diagenesis favors the development of Fe-rich chlorite, whose density can rise significantly to 2.75–3.00 g/cm3; under normal (non-iron-enriched) conditions, chlorite density typically fluctuates between 2.67 and 2.75 g/cm3. Illite density is governed by crystallographic ordering and interlayer potassium occupancy. At these depths, smectite-to-illite transformation is active, and newly formed illite evolves from disordered to ordered stacking, resulting in a gradual density increase from about 2.75 g/cm3 to 2.82 g/cm3, commonly ranging from 2.75 to 2.82 g/cm3. Consequently, the two minerals share an overlapping density interval (e.g., 2.75–2.78 g/cm3).
The observed increase in clay-mineral density with depth mainly results from the reduction in interlayer porosity. Compaction and cementation decrease the total rock porosity, thereby raising the bulk density—this is not caused by a change in the intrinsic density of the minerals. Mineralogical transformations also contribute; for example, the conversion of smectite to denser illite or the development of chlorite coatings increases the volumetric proportion of higher-density phases, which likewise elevates the bulk density.
To address the parameter estimation problem for the nonlinear Equation (8), a constrained grid-search method was applied using six observational datasets. The multi-mineral rock model, when applied to a combination of well logs, allows the solution for the volumes of minerals and fluids in the formation [
13]. Conventionally, default clay-matrix density values of
ρchlorite = 2.67 g/cm
3 and
ρillite = 2.78 g/cm
3 are used. However, because the goal here is to determine the matrix density values of clay minerals excluding interlayer pore fluids, the core objective is to identify the optimal parameter combination that minimizes the model-fitting error within the specified parameter range of 2.67–3.5 g/cm
3 for both minerals, subject to the constraint (
ρillite −
ρchlorite) < 0.11 g/cm
3.
Constraints are crucial for the solution. In the algorithm, the limitation on the separation between the matrix densities of illite and chlorite serves as the constraint condition. This constraint is directly enforced via conditional statements at each computational step, ensuring that only parameter combinations fully satisfying all conditions are evaluated and compared. Consequently, the optimization search is strictly confined to the feasible parameter space. The grid-search approach is intuitive and systematic. First, the continuous parameter range is discretized with a step size of 0.01, generating a grid that covers all possible
ρillite and
ρchlorite values. Subsequently, each point in the grid is traversed. For points that satisfy both the bound constraints and the relative constraint, the corresponding model predictions are computed and compared with observational data, yielding the five best-fitting solutions along with their errors (
Figure 6).
This algorithm offers several advantages. First, it guarantees global optimality within the discretized space, avoiding the risk of local minima inherent in gradient-based methods. Second, the logic is transparent and easy to verify, providing stable and reliable computations. Third, constraints are handled directly and flexibly, clearly illustrating how they shape the feasible solution space. Furthermore, the method yields rich information beyond a single optimum, such as the error topography over the entire parameter space, ranking of sub-optimal solutions, and quantitative analysis of how constraint strictness affects the results—all of which support a deeper understanding of the problem and robust decision-making.
Five sets of dry-clay matrix parameter combinations were obtained, with a maximum absolute error of 1.4 × 10−5, mean absolute error of 4.0 × 10−6, and root-mean-square error (RMSE) of 6.0 × 10−6. All five sets are of acceptable quality. Based on the diagenetic trends of chlorite and illite described above, the parameter pair ρchlorite = 3.32 g/cm3 and ρillite = 2.89 g/cm3 was selected and substituted into Equation (5) to compute porosity using the variable-matrix-density approach.