1. Introduction
Chemical pollution of water and soil ecosystems can occur naturally, such as through the destruction of rocks, volcanic activity, and forest fires. However, the majority of pollution is caused by human activity, including metalworking, mining, the pharmaceutical and oil industries, and agriculture [
1,
2,
3,
4,
5]. The main sources of these pollutants are pharmaceuticals, herbicides, solvents, polycyclic aromatic hydrocarbons, plasticizers, heavy metals, organochlorine compounds, and other toxic substances. These substances enter natural ecosystems primarily from anthropogenic sources, causing irreparable harm to biota [
6]. As a result, the challenge of finding effective methods to clean natural ecosystems of a wide range of toxins is a significant concern so as to mitigate associated risks.
In recent years, adsorption processes have been successfully employed to remove inorganic and organic contaminants from the aquatic environment and sequester them in soils [
7]. The benefits of adsorption technologies include the relative simplicity of using sorption materials, considering the possible desorption of adsorbed substances, the multiple reuses of adsorbents, and waste-free production. Additionally, these technologies demonstrate high efficiency even when dealing with toxicants at low and ultra-low concentrations [
8,
9]. Furthermore, the cost of sorbents is relatively low, and they have a wide range of potential pollutants that can be adsorbed. From the perspective of “green chemistry,” clay minerals are promising sorbents for both inorganic and organic toxins. These are aqueous aluminosilicate materials that possess high adsorption capacities. Both naturally and chemically modified clay minerals are widely used as adsorbents [
10].
The ability of natural clay minerals to adsorb non-ionic organic compounds and metal-containing anions is very low, as the surface of these minerals is hydrophilic and has a negative charge [
11,
12]. However, changing the surface character of these clays from hydrophilic to hydrophobic through modification with surfactants provides an excellent opportunity to increase their sorption capacity for a wide range of hydrophobic organic substances.
One of the major concerns regarding the widespread use of organoclays for environmental restoration is their potential toxicity [
13,
14,
15]. Due to the presence of organic modifiers, organoclays may interfere with natural biodegradation processes, negatively affecting bacterial and plant communities in aquatic and soil environments. Some organoclays based on quaternary ammonium and cetylpyridinium derivatives are known to exhibit antimicrobial activity [
16,
17,
18]. The toxicity of organoclays depends largely on the properties of the organic matter used for modification. The most toxic modifiers are often those with hydroxyl groups, while the least toxic are those with long alkyl chains. It should be noted that these findings are based on limited empirical evidence and further research is needed to understand the toxicity patterns of these materials [
19]. The systemic toxic effects of organoclays synthesized using different surfactants have not been well studied [
17,
20,
21,
22].
Determining the general level of toxicity of environmental objects solely on the basis of chemical analysis methods is currently difficult due to the huge variety of potential toxicants and the lack of standardization of many of them. This problem is solved by using the biotesting method, in which the toxicity level is determined using biological objects. The use of biotesting has a number of other advantages over chemical analysis—for example, the ability to detect unstable compounds or quantitatively determine ultra-low concentrations of pollutants based on physiological responses, as well as considering the combined effect of pollutants.
To assess the toxicity of environmental samples, special organisms called test objects are used. Toxicity is measured by biotesting, which involves recording changes in specific biological indicators of the test object. The criterion for toxicity is a reliable change in one or more of these indicators, which allows us to make a conclusion about the toxicity of a sample. Among the test parameters for biotesting, the most frequently used are the survival rate of individuals, behavioral reactions, changes in morphological characteristics, as well as enzymatic and metabolic activity of organisms [
23]. A wide variety of organisms can be used as test objects: bacteria, protozoa, fungi, algae, plant organisms, microscopic crustaceans, etc. [
24].
The aim of this study is to investigate the toxic effects of natural and modified bentonite clay minerals in combination with various surfactants through biotesting using soil microorganisms, agricultural plants, and special bacteria from the Ecolum biotest.
2. Materials and Methods
2.1. The Composition and Characteristics of the Natural Clays
To synthesize organoclays, we used natural bentonite from the Sarigyugh deposit in the Republic of Armenia, kindly provided by the “Bento Group Minerals” company (Moscow, Russia). The mineral contains over 85% montmorillonite. According to the producer, the mineral has the following oxide content: SiO2—58.3%, Al2O3—14.3%, Fe2O3—4.4%, MgO—3.6%, Na2O—2.3%, K2O—1.2%, CaO—2.1%. The cation exchange capacity of the mineral is about 105 mg-eq/100 g.
2.2. Synthesis of Organoclays Using Different Types of Surfactants
The synthesis of organoclays (modification of natural bentonite with surfactants of various natures) was carried out according to the method suggested in our previous work [
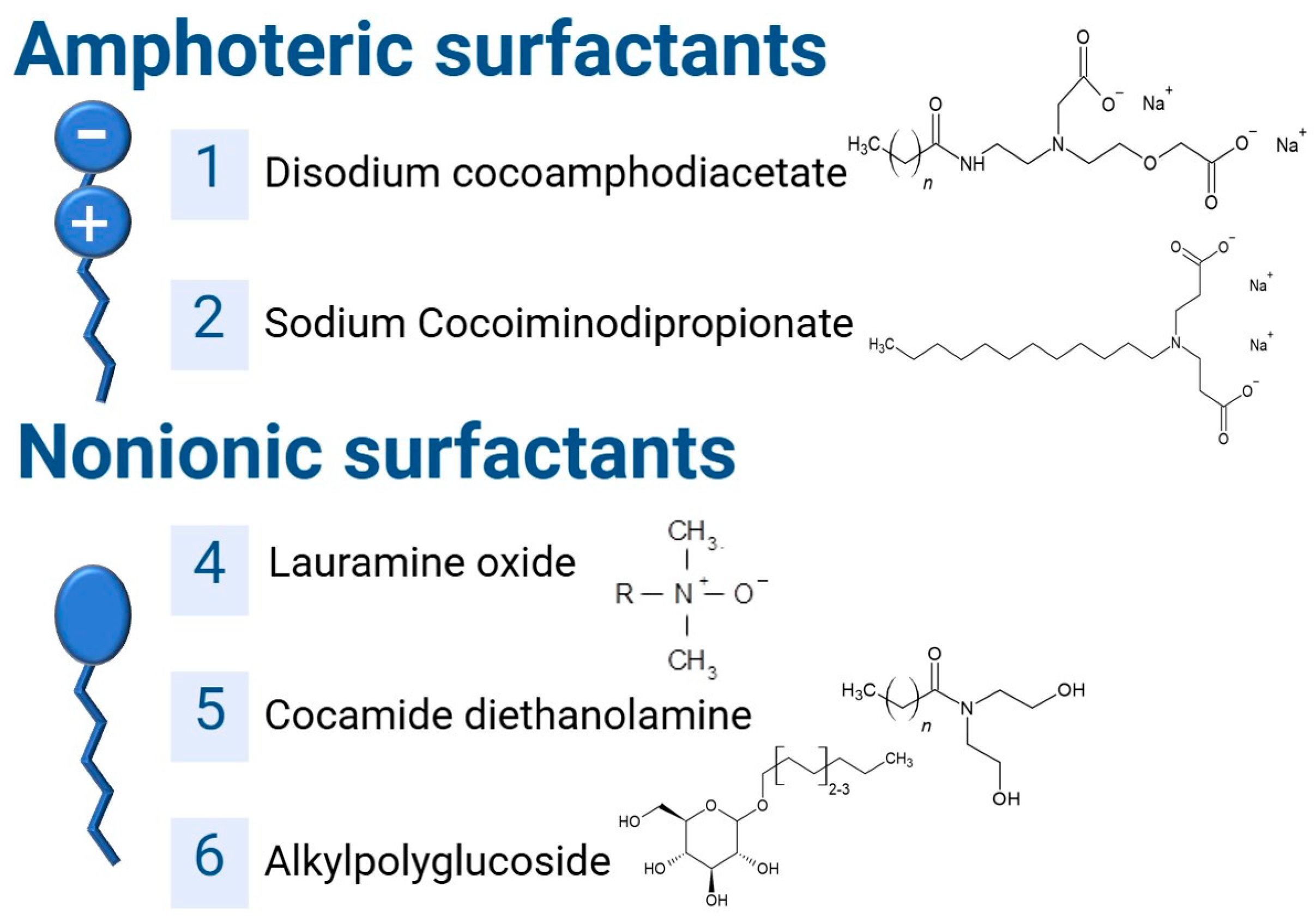
25]. The surfactants used in this study are shown in
Figure 1.
2.3. Determination of the Elemental Composition of the Natural Bentonite and Synthesized Organoclays
Samples of the original bentonite and synthesized organoclays were analyzed using elemental analysis with a Sundy SDCHN636 CHN analyzer («Hunan Sundy Science and Technology Co», Changsha, China) [
25].
2.4. FTIR Spectroscopy
FTIR spectra of the samples were obtained using an FSM-2202 infrared Fourier spectrometer (INFRASPEC, St. Petersburg, Russia). Spectral analysis was performed in the range of 4000–400 cm
−1 in transmission mode with a resolution of 4 cm
−1 and four scans. The Norton-Beer average apodization method was used [
26].
2.5. Evaluation of the Toxic Effect of the Natural Bentonite and Synthesized Organoclays on Autochthonous Soil Microorganisms In Vitro
Microorganisms were extracted from gray forest soil (Haplic Luvisol) at a depth of 0–20 cm. The collected soil was cleaned from plant residues and other impurities, ground in a mortar, and passed through a sieve with a 3 mm pore diameter.
To extract microorganisms, 9 mL of sterile water was added to a 1 g prepared soil sample and ground thoroughly in a porcelain mortar for 10 min to desorb cells from the soil surface [
27]. To transfer the maximum number of bacterial cells from the solid phase to solution after grinding, the suspension was shaken thoroughly on an S-3M.A10 orbital shaker (SIA “ELMI”, Riga, Latvia) at 200 rpm for 30 min. Then, 1 mL of the resulting suspension was transferred from the flask using a sterile pipette to a test tube containing 9 mL of sterile water and mixed using an MX-S vortex mixer (DLab, Beijing, China). After that, 1 mL of this mixture was transferred to another test tube in a similar manner. A total of four dilutions were carried out.
Before starting work, the laboratory glassware and media for microorganisms were sterilized in a VK-75-01 steam sterilizer (TZMOI, Moscow, Russia).
The following media were used in the experiments:
HFM-agar medium (hydrolyzed fish meal) (State Research Center for Applied Microbiology and Biotechnology, Obolensk, Russia). Composition (g·L−1): pancreatic hydrolysate of fish meal—24; baker’s yeast extract—2; sodium chloride—4; microbiological agar—0.0 ± 2.0.
GRM-broth medium (State Scientific Center for Applied Microbiology and Biotechnology, Obolensk, Russia). Composition (g·L−1): pancreatic hydrolysate of fish meal—18.0; sodium chloride—2.0.
Soil microorganisms were seeded and cultivated in a liquid nutrient medium in shaking flasks. 100 mL of the liquid medium was added to the flasks and autoclaved. Then microorganisms were added to a flask containing the HFM medium and cultivated in a Biosan ES-20/60 shaker-incubator (BioSan, Riga, Latvia) at 180 rpm at 37 °C for 48 h.
Cultivation of microorganisms from soil samples was conducted on a liquid nutrient medium using experimental samples to investigate the toxicity of organoclays towards soil microflora. The experiment was performed in triplicate. The following samples were employed:
Nutrient medium;
Bentonite (control);
Organoclay with amphoteric surfactant (sodium cocoiminodipropionate);
Organoclay with amphoteric surfactant (disodium cocoamphodiacetate);
Organoclay with nonionic surfactant (lauramine oxide);
Organoclay with nonionic surfactant (cocamide diethanolamine);
Organoclay with nonionic surfactant (alkylpolyglucoside).
2.6. CFU (Colony-Forming Unit) Count
Sowing was performed using the standard method on a dense agar medium with experimental samples to count colonies and determine the titer of microorganisms [
28].
The bentonite and organoclay samples were added to the nutrient medium immediately before it was poured into a sterile Petri dish. The amount of clay used was 1% and 3%, based on the volume of the medium (20 mL per Petri dish). This corresponds to 0.2 g and 0.6 g of clay, respectively. After the clay had been added, the soil microorganisms were then seeded.
The colony-forming units were counted using a formula [
27]:
where A is CFU/mL; ∑c is the total number of colonies in all dilution plates; n is the number of plates; d is the dilution factor of the test sample; and V is the volume of sample inoculated on the plate, in mL.
2.7. Determination of Biomass
The toxicity of the organoclays in the study was also determined by measuring the biomass of soil microorganisms. To do this, 20 mL of the GRM broth medium was added to culture tubes and autoclaved for 40 min at 121 °C. One or three percent volume of an aqueous extract of bentonite or organoclay was added to each tube, along with 1 mL of an inoculum suspension of soil microorganisms. The tubes were then shaken for 58 h in a Biosan ES-20/60 incubator at 28 to 30 °C. Samples were taken and the biomass quantified after 8, 24, and 48 h of bacterial growth. The contents of each tube (1 mL) were transferred to Eppendorf tubes and centrifuged at 10,000 revolutions per minute for 6 min at room temperature in a 5417R centrifuge. After centrifuging, the supernatant was removed from the Eppendorf tube and centrifuged again for one minute to remove any remaining liquid. The resulting sediment was weighed, and the previously measured mass of the empty tube was subtracted from the total mass to obtain the net mass.
2.8. Evaluation of the Toxic Effect of the Original Bentonite and Synthesized Organoclays on Agricultural Plants
For biotesting, small seeds such as watercress, radishes, mustard, and others are usually used. These seeds have a limited supply of nutrients, making them more susceptible to external factors. Watercress (
Lepidium sativum L.) is a type of vegetable that has increased sensitivity to pollution. It has rapid seed germination and a near 100% germination rate. This makes it convenient for use in bioindication tests, as the effect of stressors on a large number of plants can be studied in a small space (Petri dish) in a short period of time [
29,
30]. Radish (
Raphanus sativus L.) is also often used as a test object for studying soil pollution, which is due to its increased sensitivity to the presence of pollutants, high seed germination energy and early maturity of the crop [
31].
The experiment with two test cultures—watercress (
Lepidium sativum L.) and radish (
Raphanus sativus L.)—was carried out in triplicate to investigate the phytotoxicity of organoclays on agricultural plants. 10 g of the studied sorbents were placed in Petri dishes, and 20 watercress seeds and radish seeds were sown in each dish. The plants were grown at room temperature (25 ± 2 °C) with constant humidity (60 ± 2%) by adding distilled water and illuminated by a phytolamp (16/8 h light-dark cycle, FFP—35 μmol·s
−1, 660 nm, 24 V) for a week. After 72 h, germinated seeds were counted to determine germination energy (%). Germination energy is one of the indicators of the sowing qualities of crop seeds, showing the percentage of normally germinated seeds over 3 days. After seven days from sowing, root and stem lengths were measured, and germination rates (%) (similar to germination energy, this is the ratio of the number of seeds that germinated during a 7-day period, to the total number of seeds) and total plant mass were determined according to the procedure [
32]. The experiment was performed in triplicate. The same samples as in
Section 2.5 were used for the experiment. Instead of a nutrient medium, we used distilled water.
2.9. Evaluation of the Toxic Effect of the Natural Bentonite and Synthesized Organoclays Using the “Ecolum” Biotest
To evaluate the toxic properties of the developed organoclays, the Ecolum biosensor from the Biotox-10 series was used (Lomonosov Moscow State University, Moscow, Russia) [
33]. Lyophilized luminescent bacterial preparations were used as a test object for the biosensor. This method is based on measuring the change in bioluminescence intensity of the biosensor when exposed to a substance, compared to a control. A decrease in bioluminescence indicates a toxic effect. The toxicity of the sample is determined by measuring the decrease in bioluminescence over a 30 min period [
28].
3. Results
3.1. Elemental Composition of the Natural Bentonite and Synthesized Organoclays
The results of the elemental analysis of bentonite and organoclays are presented in
Table 1. The data obtained indicate an increase in carbon content in organoclays compared to their original mineral form, which correlates with the number of carbon atoms in the hydrocarbon radicals of corresponding surfactants. This can be seen as evidence of successful intercalation of the surfactants into the mineral structure.
3.2. FTIR Spectroscopy
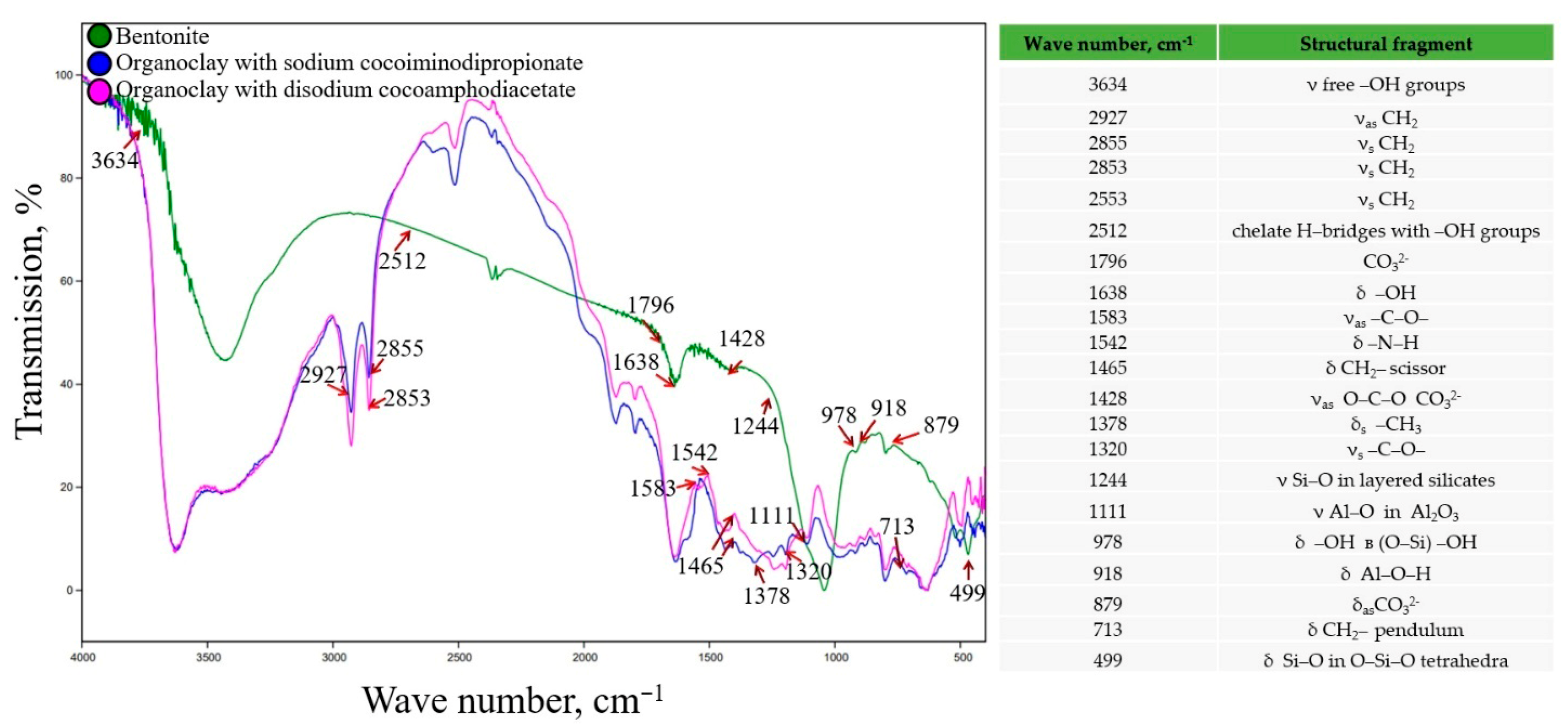
The infrared spectrum of the original bentonite contains several characteristic absorption bands (
Figure 2). These are absorption bands at 978 and 1197 cm
−1, which correspond to free and associated forms of Si–OH groups. The absorption band at 1638 cm
−1 is due to the stretching and deformation vibrations of the OH group. The bands in the range of 798–838 cm
−1 are caused by the vibrations of the Si-O bond in tetrahedral molecules, while the bands in the region 426–439 cm
−1 correspond to vibrations of this bond within the tetrahedron [
34]. The bands between 3000 and 3700 cm
−1 represent the stretching and deformation of OH groups in free or bound water molecules. The band at 2512 cm
−1 may indicate the formation of chelate hydrogen bridges with OH groups. Characteristic absorption bands of Al-O groups in Al
2O
3 (1111 cm
−1), stretching vibrations of Si-O-Al bonds (645–669 cm
−1), and bands associated with O-C-O vibrations in carbonate ions CO
32− (1197 cm
−1) were also observed [
35].
The FTIR spectra of organoclays synthesized using bentonite and amphoteric surfactants contain both absorption bands characteristic of the original mineral and a number of changes. A decrease in the number of free -OH groups is observed, which is likely due to the absorption of surfactant molecules on the bentonite surface and the displacement of previously located water molecules. Absorption bands at 1465, 2853–2927, and 1378 cm
−1 correspond to CH
2 and CH
3 vibrations, respectively, indicating the presence of organic compounds in the mineral structure. Hydrophobic alkyl chains of surfactants can interact with the lipid membranes of cells, potentially damaging them and increasing their toxicity. The longer and more saturated these alkyl chains are, the greater the likelihood of these effects occurring. The appearance of absorption bands at 1542, 1320, and 1583 cm
−1 may be due to the presence of amino groups and carboxylate acid residues (
Figure 2).
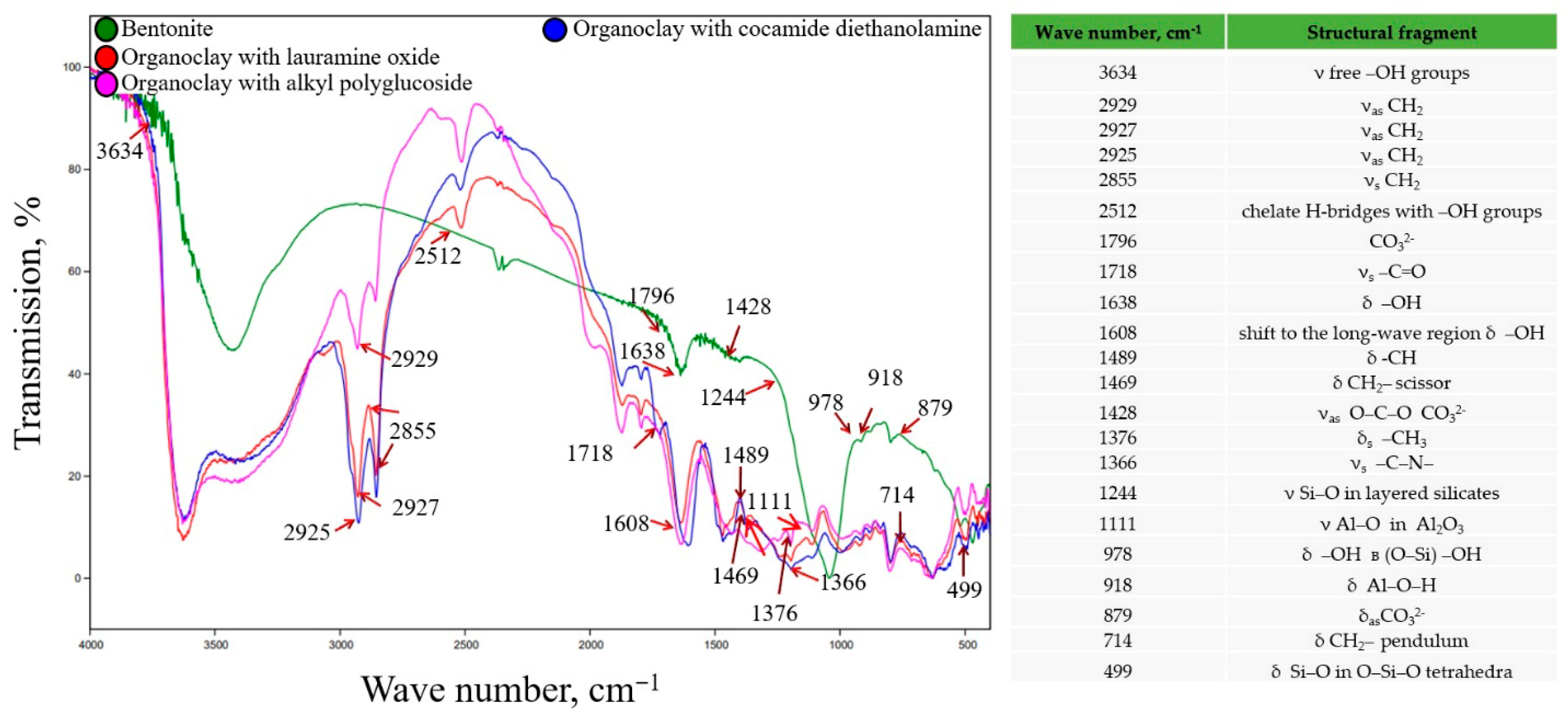
For organoclays synthesized with non-ionic surfactants, a similar picture is characteristic as in the synthesis of organoclays with amphoteric surfactants (
Figure 3).
A shift to the long-wave region of hydroxyls at 1608 cm−1, carbonyls -C=O at 1718 cm−1 and absorption bands at 2855–2929 cm−1, belonging to asymmetric and symmetric stretching vibrations of CH2 groups of adsorbed surfactants, is also observed.
3.3. Toxicity of Original Bentonite and Synthesized Organoclays Based on Colony-Forming Test
To quantitatively assess the effect of the sorbents under study, a colony-forming assay in vitro was performed using the internal addition of bentonite and organoclays at concentrations of 1% and 3% by volume of the medium. The results of this experiment allowed us to determine the number of colonies and calculate the CFU/mL (
Table 2).
The maximum number of colonies was observed in the control variant (bentonite)—10.0 ± 0.4 × 106 CFU/mL (1%) and 8.0 ± 0.5 × 106 CFU/mL (3%). All organoclays inhibit bacterial growth and reduce the number of CFU compared to the original bentonite. The degree of reduction in each case is different and is determined by the surfactant used for modification. The minimum number of CFU corresponds to organoclay with sodium cocoiminodipropionate—2.2 ± 0.6 × 106 CFU/mL (1%)/3.0 ± 0.1 × 106 CFU/mL (3%) and organoclay with lauramine oxide—3.4 ± 0.8 × 106 CFU/mL (1%)/2.4 ± 0.8 × 106 CFU/mL (3%). The maximum number of CFU and, accordingly, minimal toxicity was demonstrated when introducing organoclay with alkyl polyglucoside—8.2 ± 0.4 × 106 CFU/mL (1%)/7.6 ± 0.4 × 106 CFU/mL (3%).
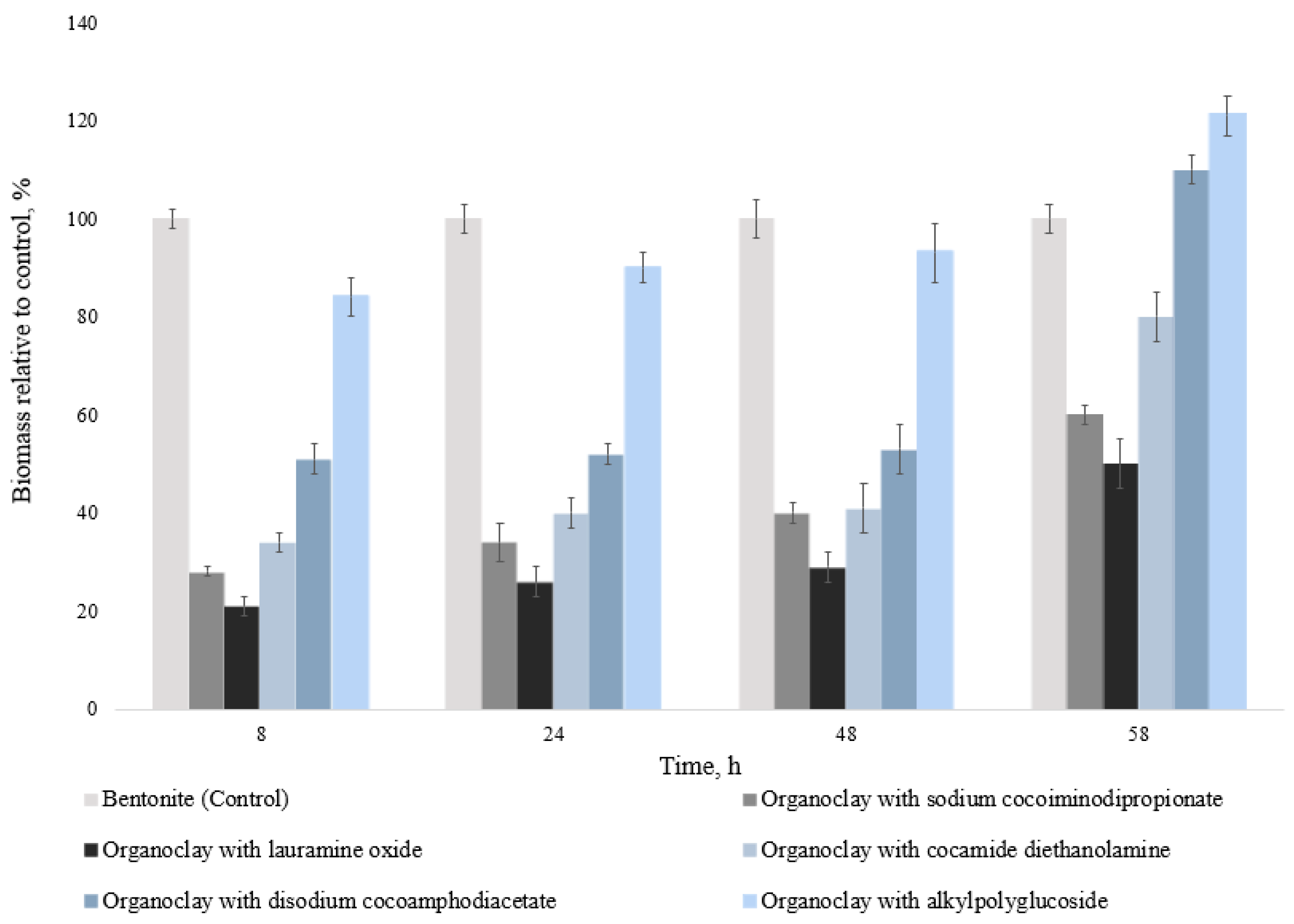
3.4. Evaluation of the Influence of Synthesized Organoclays on the Biomass of Microorganisms
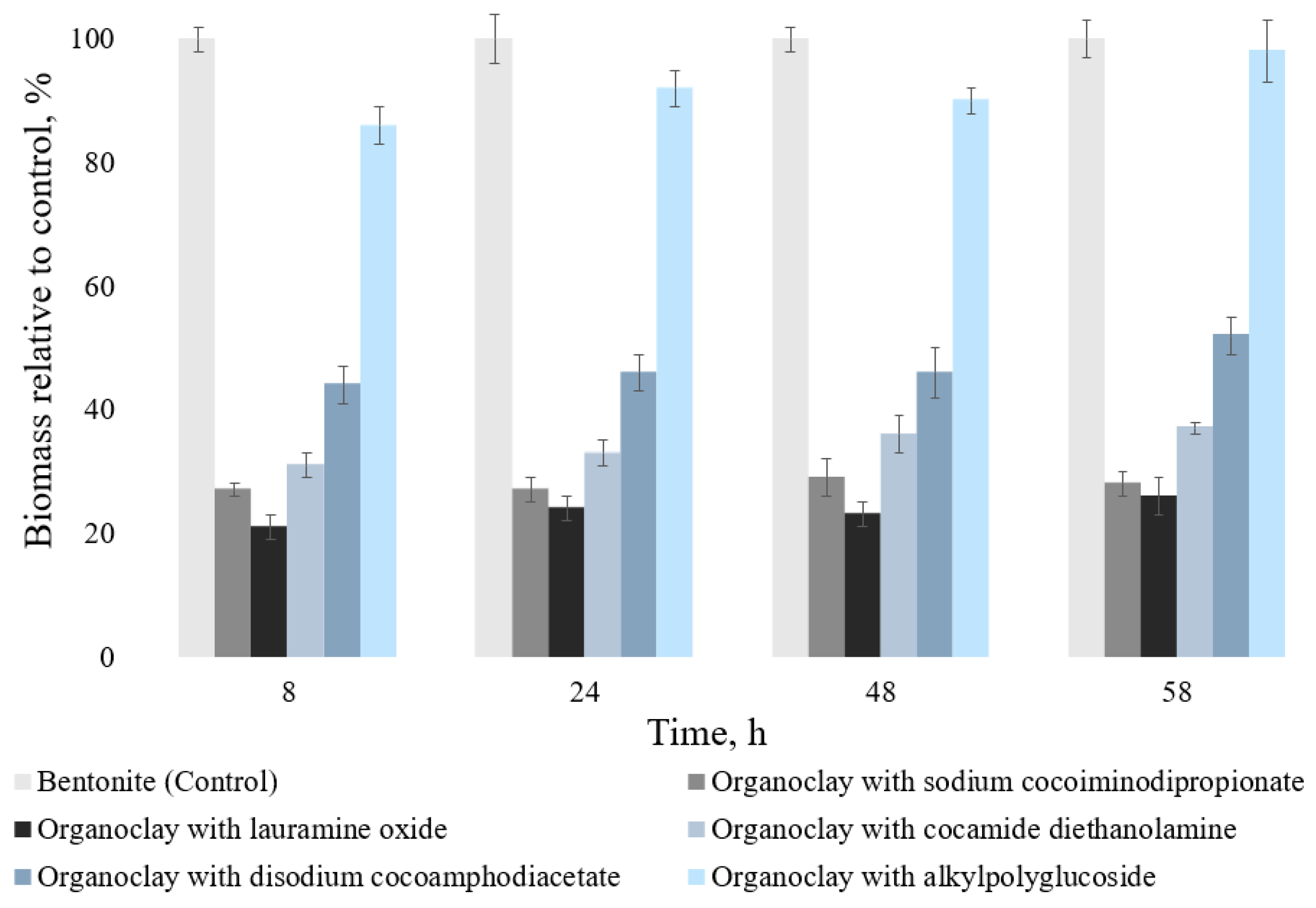
To assess the growth of soil microorganisms, the biomass yield was determined. The results are shown in
Figure 4 and
Figure 5.
It was found that, in general, the biomass increase correlates with the data on the change in the number of CFU microorganisms from the gray forest soil. When 1% of the studied organoclays were added, the biomass of microorganisms decreased relative to the control (bentonite—100%) by 2–79%, depending on the cultivation time and the type of surfactant selected for modification. The minimum biomass values correspond to organoclay with lauramine oxide—21 ± 2% (8 h of cultivation), 26 ± 3% (58 h of cultivation). The maximum biomass values are observed in the variant of organoclay with alkylpolyglucoside—86 ± 3% (8 h of cultivation), 98 ± 5% (58 h of cultivation).
When organoclays were added at a concentration of 3% of the total medium volume, the biomass of microorganisms decreased by 7–79%, depending on cultivation time. However, for organoclays with disodium cocoamphodiacetate and alkylpolyglucoside after 58 h of cultivation, biomass exceeded the control values by 10% and 21%, respectively (
Figure 5).
Thus, the minimum values of biomass and maximum toxicity are characteristic of organoclay with lauramine oxide—21 ± 2% (8 h of cultivation), 50 ± 5% (58 h of cultivation), the maximum (minimum toxicity)—for organoclay with alkylpolyglucoside—84 ± 4% (8 h of cultivation), 121 ± 4% (58 h of cultivation).
3.5. Evaluation of the Toxic Effect of the Original Bentonite and Synthesized Organoclays on Agricultural Plants
To investigate the toxic effects of the synthesized organoclays, a phytotoxicity test was conducted using watercress (
Lepidium sativum L.) and radish (
Raphanus sativus L.) as test organisms. The results of the study are presented in
Table 3.
Statistically significant (according to Student’s t-test (p < 0.05)) decreases in germination energy and capacity of watercress were not found in any of the organoclay samples compared to the control: germination energy increased by 3 ± 1%–7 ± 1%, and germination rate increased by 3 ± 1%–11 ± 2%, relative to the control (no statistically significant decrease in germination was observed for organoclays with lauramine oxide or the product of the interaction between organoclay and sodium cocoiminodipropionate—a decrease of 4 ± 1%) In the case of radishes, no statistically significant decrease in germination energy was observed; however, a decrease in germination rate was seen for organoclay containing sodium cocoiminodipropionate by 11 ± 2%. Other organoclay variations had no significant impact on the sowing quality of radish seeds.
Germination energy reflects the initial stage of seed activation. When clays are modified, surfactants bind to the surface, replacing hydrophilic groups. Biocompatible functional groups (carboxylates, polyelectrolyte chains, glycosidic residues) of surfactants likely form more stable and biocompatible organomineral complexes that improve water retention and gas exchange, creating more favorable conditions for seed germination. Germination is a parameter that includes not only the onset of germination but also the successful continuation of growth. Organoclays can influence germination energy by improving moisture and microbiological conditions, but they do not always promote stable growth once germination begins. This means that seeds may begin to germinate more easily, but subsequent seedlings may die due to other factors. Germination also depends on temperature, pH, oxygen availability, nutrient and toxin concentrations, and interactions with microflora [
37]. Modified clay can create a microenvironment unfavorable for long-term growth. Germination energy characterizes only the initial stage, ignoring subsequent stages of development. Therefore, these indicators likely vary for the same organoclays.
3.5.1. Morphometric Characteristics of Test Objects in the Presence of Bentonite and Organoclays
After 7 days from the moment of sowing the seeds, the length of the roots and hypocotyl of the seedlings was measured. The results are presented in
Table 4.
A statistically significant (according to Student’s t-test (p < 0.05)) decrease in the root length of watercress compared to the control was observed for organoclay with sodium cocoiminodipropionate: 61% lower compared to the control, organoclay with lauramine oxide—by 85%, organoclay with cocamide diethanolamine—by 49%; a statistically significant increase in root length was characteristic of organoclay with disodium cocoamphodiacetate—by 33%, organoclay with alkylpolyglucoside—by 31% compared to the control. A decrease in the hypocotyl length of watercress was observed only in the case of organoclay with sodium cocoiminodipropionate: 12.5% lower than the control. The other variants increased the hypocotyl length by 26–79% compared to the original bentonite.
In the case of radish, a statistically significant decrease in root length compared to the control was observed for organoclay with sodium cocoiminodipropionate: by 25%, organoclay with lauramine oxide—by 62%, organoclay with cocamide diethanolamine—by 25%. A decrease in the length of radish hypocotyl was observed for organoclay with lauramine oxide—by 23%, organoclay with cocamide diethanolamine—by 14%, organoclay with disodium cocoamphodiacetate—by 20%.
Thus, depending on which test object indicator we consider when studying toxicity, organoclays exhibit varying degrees of toxic properties.
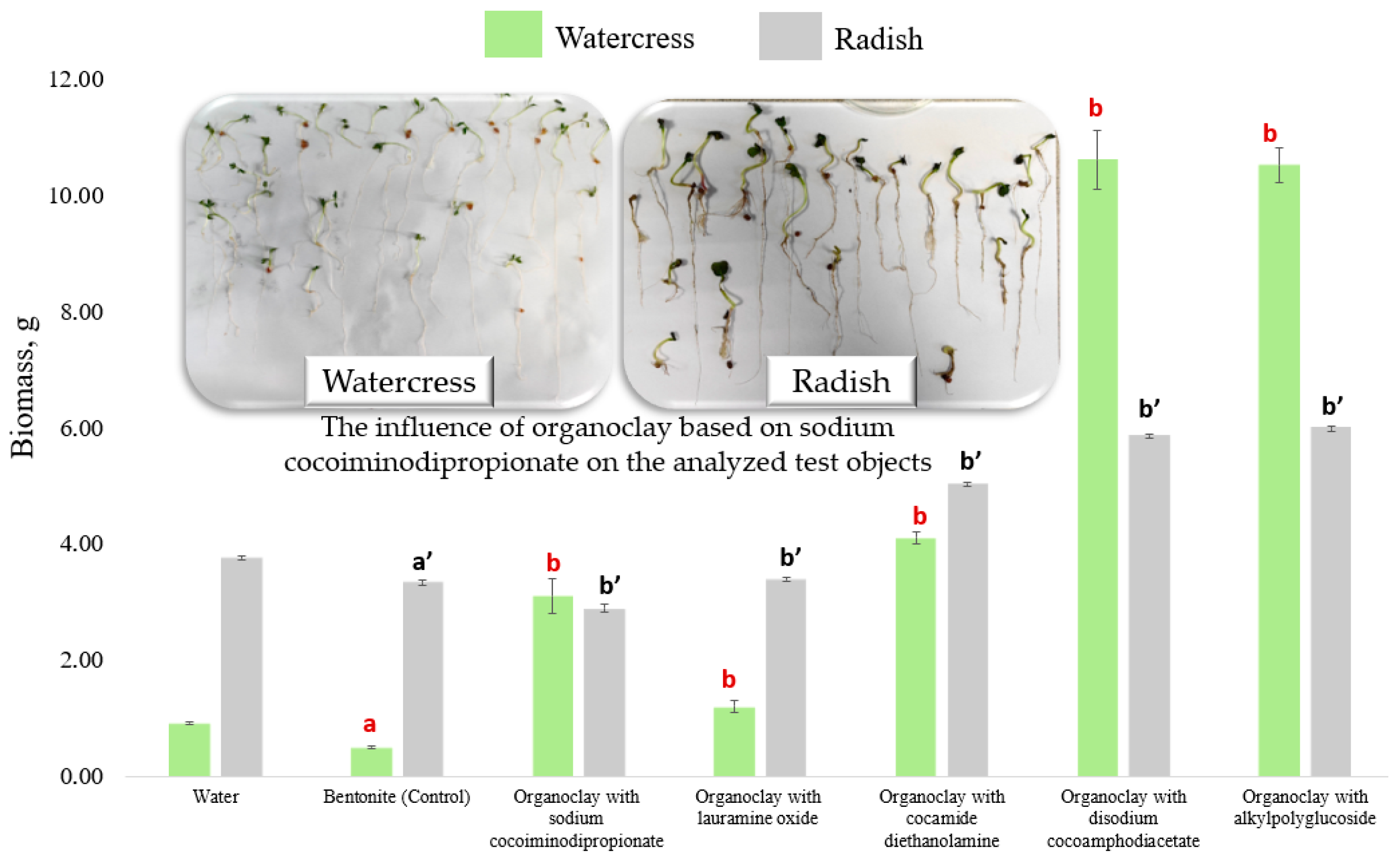
3.5.2. Biomass of Test Object Sprouts in the Presence of Bentonite and Organoclays
Analysis of the seedling biomass measured on the 7th day of cultivation showed a similar trend in the influence of the added sorbents as the analysis of morphometric characteristics (
Figure 6).
A statistically significant decrease in the biomass of watercress seedlings was observed for organoclays with sodium cocoiminodipropionate, compared to the control. The decrease was 57% for organoclay with bentonite and 91% for organoclay with lauramine oxide. The biomass of watercress was also significantly lower for organoclay containing cocamide diethanolamine, by 28% compared to the control group. On the other hand, a statistically significant increase in the biomass was observed for watercress when using organoclays containing disodium cocoamphodiacetate and alkylpolyglucoside. The increase was 10% and 28%, respectively, compared to the control.
For radish, there is a statistically significant decrease in the biomass of sprouts for all variants of synthesized organoclays, ranging from 22% to 68% lower than the control, depending on the type of organics used for modification. The lowest biomass value was observed for organoclay containing lauramine oxide, which was 68% lower than the control.
Phytotesting of the synthesized sorbents revealed that, based on the data on the seed germination of watercress, none of the organoclays were toxic. In the case of radish, the maximum toxicity compared to the control (original bentonite), according to the germination data, was exhibited by organoclay with sodium cocoiminodipropionate. The other organoclay variants had a negligible effect (within the permitted error range) on the changes in the seed germination rates of both radish and watercress. Therefore, using the example of the synthesized organoclays, it can be concluded that the seed germination is insensitive to external chemical influences.
According to the data on changes in morphometric characteristics based on the reduction in the length of watercress roots, the maximum toxic effect was observed when applying organoclays with lauramine oxide and sodium cocoiminodipropionate. No toxicity was observed for organoclays containing disodium cocoamphodiacetate or alkylpolyglucoside, as these substances stimulated the growth of watercress root lengths. However, the toxic effect relative to watercress hypocotyl lengths was only observed in the case of sodium cocoiminodipropionate organoclay. Other adsorbent variants resulted in increased hypocotyl lengths. For radish, the highest toxicity was observed with lauramine oxide organoclay based on data from both root and hypocotyl measurements.
Analysis of the biomass of watercress and radish sprouts revealed that organoclays containing lauramine oxide exhibited the highest level of toxicity. Organoclays with disodium cocoamphodiacetate and alkylpolyglucoside, on the other hand, exhibited a stimulating effect on the growth of watercress biomass.
Based on the study of test parameters in phytotesting, it was established that radish is the most sensitive test organism in these experiments. It is noticeably affected by toxic substances, showing significant morphological and morphometric changes (
Figure 6).
Due to the different biological characteristics of plants, the selected test subjects, watercress and radish, may respond differently to the analyzed organoclays. Radish seeds are larger and fleshy, germinate faster, but they may be more sensitive to soil conditions [
38,
39]. The energy required for seed germination depends on the reserve of nutrients and the rate at which they are mobilized within the seed. These processes occur at different intensities and rates in radishes and watercress [
40]. The root and shoot systems of these plants develop with different growth vigor due to genetic differences, which affect root length, biomass, and other parameters. Additionally, the response to organoclays may vary depending on interactions between roots, micronutrients, substrate structure, and water holding capacity.
3.6. Determination of Integral Toxicity of Organoclays by Biosensor Method
For each organoclay, the toxicity of aqueous extracts was measured (solid-to-liquid phase ratio 1:10) from three pairs of “control/experimental” samples using the Ecolum biosensor test system [
29]. The toxicity was assessed by comparing the relative differences in bioluminescence intensity between the control and experimental samples, and by calculating a toxicity index (T) using Formula (2).
where I
k—the luminescence intensity of the control sample of bacteria, and I
o—the luminescence intensity of bacteria after adding an aqueous extract of the test sample.
If the toxicity index is higher than 20%, the sample is considered to be toxic. If it is more than 50%, it is considered to be highly toxic. The results are presented in
Table 5.
This method has established that the following organoclays are toxic: organoclay with sodium cocoiminodipropionate and organoclay with lauramine oxide. A slight increase in the toxicity index is observed for organoclay with disodium cocoamphodiacetate, organoclay with cocamide diethanolamine, and organoclay with alkyl polyglucoside. The original bentonite is not toxic to luminescent bacteria.
Based on the results obtained through microbiological, phytotoxic, and instrumental methods, we can conclude that organoclays based on lauramine oxide exhibit the highest level of toxicity, while those based on alkylpolyglucoside exhibit minimal or no toxicity. Lauramine oxide is an amphoteric surfactant that contains a cationic (positive charge) amine oxide group and a hydrophobic alkyl tail. The positive charge on the amine oxide attracts the negatively charged cell membranes, disrupting their integrity and leading to cell breakdown and death or damage. In contrast, alkylpolyglucoside lacks a charged group and interacts with cell membranes more gently, without destroying them or causing intracellular damage.
4. Conclusions
Based on the experiments conducted to assess the toxicity of synthesized organoclays relative to pure bentonite using microbiological, phytotoxic and instrumental (biosensor) methods, it can be concluded that organoclay with lauramine oxide and, depending on the method of assessing toxicity and the test parameter, organoclay with sodium cocoiminodipropionate (microbiological experiments, phytotesting—assessment of sowing qualities, assessment of morphometric characteristics) have an increased toxic effect on test objects. The biosensor results align with the phytotoxicity data, indicating moderate toxicity levels for certain organoclays. This comprehensive assessment helps in understanding the environmental impact of these organoclays, highlighting formulations that are less toxic or even beneficial for plant growth. The minimum toxic effect, depending on the method of assessing toxicity and the test parameter, is manifested by organoclay with alkylpolyglucoside (for all tests) and organoclay with disodium cocoamphodiacetate (phytotesting—assessment of germination (radish), assessment of morphometric characteristics, biosensor method). This comprehensive assessment helps in understanding the environmental impact of these organoclays, highlighting formulations that are less toxic or even beneficial for plant growth.