1. Introduction
Acetonitrile is an important chemical raw material [
1] that is widely used as an extraction solvent in organic synthesis [
2], the petroleum industry, and pharmaceuticals [
3]. To ensure the purity of acetonitrile, during the process, the separation of acetonitrile–water mixtures is required. However, acetonitrile–water mixtures form a typical azeotropic system, with an azeotropic temperature of approximately 76 °C at atmospheric pressure and an azeotropic composition of 16% water and 84% acetonitrile by mass [
4]. Conventional distillation methods cannot effectively separate them [
4,
5,
6], necessitating specialized techniques. To address this, various separation methods have been developed, including heterogeneous azeotropic distillation, extractive distillation, pervaporation, and extractive distillation [
7,
8,
9]. Among these, extractive distillation is widely applied due to its high energy efficiency and operational flexibility.
In the extractive distillation process, selecting an appropriate entrainer is crucial for the design. Traditional organic entrainers, such as ethylene glycol (EG) [
10,
11], dimethyl sulfoxide (DMSO) [
10,
12], and glycerol [
1], are commonly used for the separation of acetonitrile and water. For example, DMSO can effectively disrupt the key hydrogen bonding interactions in the acetonitrile–water azeotropic system, thereby increasing the relative volatility of acetonitrile and breaking the azeotropic behavior. However, the practical application of these entrainers is limited by their volatility, high toxicity, and difficulties in recovery.
In the acetonitrile and water system, the addition of salt-based extractants can improve the separation of an azeotropic mixture through the salt effect [
13]. However, in practical operations, using solid salts as extractants alone presents challenges such as solid salt handling, corrosion of metals, and crystallization, which may lead to pipeline blockages. As a novel type of solvent, ionic liquids (ILs) possess unique properties, including negligible volatility, near-zero vapor pressure, and the ability to form various combinations of asymmetric organic cations with organic or inorganic anions [
14,
15,
16,
17,
18]. These features make ILs particularly attractive as entrainers in separation processes, as they can enhance selectivity and efficiency in systems such as acetonitrile + water [
16,
18,
19,
20,
21].
Li et al. [
22] validated through COSMO-RS calculations and experimental measurements that [Emim][OAc] and [Emim][Pro] are effective entrainers for the separation of acetonitrile and water. Fang et al. [
19] and Li et al. [
20] measured the isobaric VLE data for the ternary system of acetonitrile + water + [C
4mim][BF
4] at atmospheric pressure to investigate the influence of ILs on the phase behavior of the mixture. Their results showed that [C
4mim][BF
4] has a limited effect on enhancing the relative volatility of acetonitrile. But its aqueous solution exhibits lower viscosity [
23,
24] and surface tension [
25], which can improve mass transfer performance and enhance operational efficiency in industrial applications. Meanwhile, another study by Li et al. [
16] demonstrated that [C
4mim][Cl] and [C
6mim][Cl] exhibit superior separation performance for acetonitrile, effectively eliminating the azeotrope of the acetonitrile + water system.
However, most previous studies have focused on atmospheric pressure systems, while the VLE behavior of acetonitrile + water + ILs under reduced pressures remains largely unexplored. Theoretically, operating under reduced pressure can further improve the separation efficiency of acetonitrile and even eliminate the azeotropic behavior, offering a new pathway for more efficient industrial separations. Moreover, existing research has primarily centered on ternary systems, with limited data available for binary systems such as acetonitrile + ILs, which are essential for understanding the fundamental interactions between acetonitrile and ILs.
In this work, three ILs ([C
4mim][Cl], [C
6mim][Cl], and [C
4mim][BF
4]) were selected as entrainers. Chloride and tetrafluoroborate anions were chosen because Cl
− exhibits stronger hydrophilicity and hydrogen-bonding ability than BF
4− [
26], allowing the evaluation of anion effects on phase behavior and selectivity. The [C
4mim]
+ and [C
6mim]
+ cations were selected as representative imidazolium cations with medium-length alkyl chains, providing a balance between polarity and viscosity. Previous studies have shown that for the acetonitrile + water system, [Emim]
+ exhibits a stronger salting-out effect than [Bmim]
+ [
18], motivating us to further investigate the performance of [C
4mim]
+ and [C
6mim]
+.
The VLE data of the binary systems (acetonitrile + ILs) were adopted from the literature [
27] to explore the interaction mechanisms between ILs and acetonitrile. Meanwhile, the VLE data of the ternary systems (acetonitrile + water + ILs) were determined under different reduced pressures. The combination of these three ILs allows comparison of the effects of cation alkyl chain length and anion type on separation performance, enriching the available literature data, particularly for VLE behavior under reduced pressure. Furthermore, the NRTL model [
16,
18,
19,
20] and e-NRTL model [
28,
29,
30], whose accuracy and applicability have been validated in many previous studies, were employed to correlate and predict the experimental data, providing a quantitative description of the thermodynamic behavior of the acetonitrile–water–ILs systems. The results of this study are significant for understanding the separation behavior of binary and ternary systems under reduced pressure, elucidating the effect of pressure on relative volatility, and providing valuable insights for the design of industrial separation processes.
2. Experimental Section
2.1. Chemicals
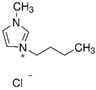
The chemical reagents used in this work included water, acetonitrile, and three ILs. The chemical structures and basic properties of the used ILs are presented in
Table 1.
Ultrapure water (conductivity < 0.06 μS/cm, resistivity 18.2
at 25 °C) was used without further purification. Acetonitrile (mass fraction ≥ 0.999) was analyzed by gas chromatography (A91 Pro, Panna, Changzhou, China), and no significant impurities were detected, so it was used without further purification. The ILs (mass fraction > 0.990) were degassed using a rotary evaporator (DZFY-1, Kexingyiqi, Shanghai, China) and dehydrated in a vacuum drying oven (DHG-9023A, Jinghongsh, Shanghai, China; pressure 0–5 kPa) at 353.15 K for at least 24 h [
20] before use. The water content in the chemical reagents and ILs was determined using a coulometric Karl Fischer titrator (Model KF 831, Metrohm AG, Herisau, Switzerland). The basic specifications of the chemicals used in the experiment are listed in
Table 2.
2.2. Apparatus and Procedure
The VLE data for binary and ternary mixtures were measured using a glass equilibrium apparatus (self-designed and constructed in-house), as described in the literature [
32]. A schematic diagram of the apparatus is provided in
Figure S1 of the Supplementary Material (SI). The setup consisted of a vacuum system, a thermostatic bath, and a measurement system. Prior to each experiment, the equilibrium cell was prepared and loaded, and temperature and pressure measurements were calibrated and corrected to ensure reliable VLE data (see
Supplementary Material for details).
A liquid sample with a known total amount nT and the total mole fraction zi of each component was injected into the equilibrium cell (volume 45 cm3). Typically, about 18 cm3 of liquid was injected, leaving a vapor phase volume of approximately 27 cm3. To remove dissolved gases and volatile impurities, the liquid sample was subjected to freeze–degas–thaw cycles. The sample was rapidly frozen in a liquid nitrogen bath, after which the gases in the equilibrium system were evacuated until the pressure dropped below 7 Pa. The frozen sample was then thawed in a warm water bath. This procedure was typically repeated three times to ensure effective removal of residual gases. The total mass and composition of the sample were checked before and after degassing, showing negligible changes, indicating that the procedure did not affect the final VLE measurements. The equilibrium cell was subsequently immersed in a thermostatted water bath, where the temperature was maintained within a tolerance of ±0.05 K using a precision temperature controller. Equilibrium was deemed reached when the temperature (T) and pressure (P) remained stable for a predefined duration (typically 1 h). The equilibrium T, P, nT, zi, liquid phase volume were recorded. The vapor-phase volume was obtained as the difference between the total cell volume and the equilibrium liquid volume (). For each temperature, measurements were repeated three times to ensure reliability. The quality of the experimental data was rigorously validated through three key checks: (1) equilibrium confirmation: temperature and pressure were monitored continuously, with readings considered stable if their fluctuations were within ±1.0% over a 5 min interval; (2) reproducibility assessment: triplicate measurements at each temperature yielded standard deviations of <0.5% for pressure and <0.02 K for temperature, confirming data consistency; (3) degassing effectiveness: repeated freeze–thaw cycles were confirmed to remove virtually all dissolved gases and volatile impurities, as evidenced by negligible changes in sample mass and composition (<0.1% relative deviation) before and after degassing. Collectively, these validation steps ensured the accuracy and reliability of the collected VLE data. The compositions of the liquid and vapor phases, and other properties, were determined using a thermodynamic model.
For each component
i, mass-balance requires that
where
and
are the vapor and liquid phase mole numbers, and
and
are the corresponding mole fractions in the vapor and liquid phases, respectively. The moles in liquid and vapor phases, and liquid phase composition can be calculated by Equation (2), Equation (3), and Equation (4), respectively.
where
is the vapor-phase fraction, defined by
where
is the molar volume of the vapor phase, the experiments were conducted at low pressures. Under these conditions, the
was calculated using the ideal gas law (
). The initial compositions were calculated using mass-balance and phase-fraction relations and then iteratively updated via the NRTL model to satisfy VLE conditions (see
Figure S2 for details).
For acetonitrile + IL systems, the volatility of the IL was negligible, and thus the vapor phase was essentially composed entirely of acetonitrile, which simplified the calculations. The ILs used in the experiment were recovered at the end using a rotary evaporator for cost-saving purposes.
The reliability of the apparatus was validated using both pure component and acetonitrile (1) + H
2O (2) binary system. The saturated vapor pressure data of the acetonitrile was measured and compared with literature data [
33,
34,
35,
36]. The results are in excellent agreement with literature data. Subsequently, the binary mixture experimental VLE data were compared to literature values [
37]. As shown in
Figure S3, the experimental and literature data exhibit excellent agreement in both the total pressure–liquid phase composition (P −
x) and pressure-vapor-phase composition (P −
y) correlations, confirming the accuracy and reliability of the present measurements. More details can be found in
Tables S1 and S2, and Figure S4 in the Supplementary Material.
2.3. Uncertainty in Experiment
The uncertainties are primarily determined by the recorded data of the temperature, pressure, and composition (
T,
P, and
x1). The calculation method of the uncertainties is based on the literature [
38,
39]. The deviation in the liquid phase composition is mainly from the uncertainty of the balance and the molar fraction values used in the calculation process. The electronic balance had a precision of ±0.1 mg. The deviation in the vapor phase composition primarily results from errors in the measured temperature, pressure, and liquid composition.
The temperature fluctuations of the thermostatic water bath were measured as
0.05 K. The standard uncertainty for the water bath temperature (
) is calculated as
. The accuracy of the mercury thermometer is ±0.02 K, and the standard uncertainty of the thermometer (
) is calculated as
The standard deviation (s) of temperature measurements over three repetitions was included, with a typical value of
s = 0.02 K. Therefore, the standard uncertainty of the temperature measurement is
The uncertainty in the measurement of the mercury column height is ±0.02 mm, and the pressure corresponding to each mm of the mercury column is 1.36 kPa. Therefore, the uncertainty in pressure is
In addition to the instrument uncertainty, the overall pressure uncertainty also includes contributions from variations in the sample composition (mole fractions), temperature fluctuations, and other experimental factors. The combined standard uncertainties were used in the calculation of all reported thermodynamic properties and are consistently reported in the table footnotes.
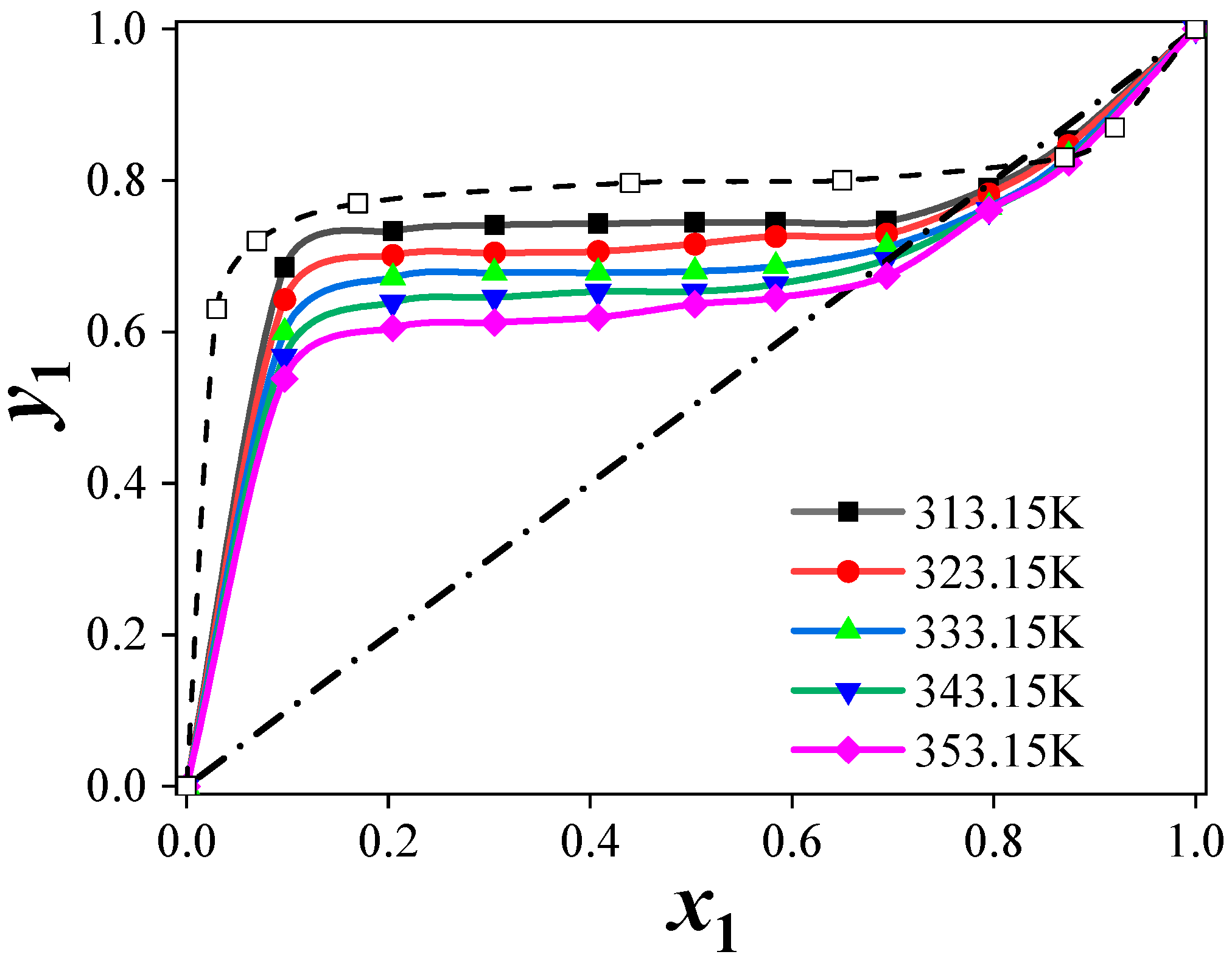
4. Conclusions
This study analyzed VLE data for three binary systems and three ternary systems (acetonitrile + water + IL), which were experimentally measured using a static isothermal VLE apparatus. The data were correlated and predicted using the NRTL and e-NRTL models. The main findings are summarized as follows:
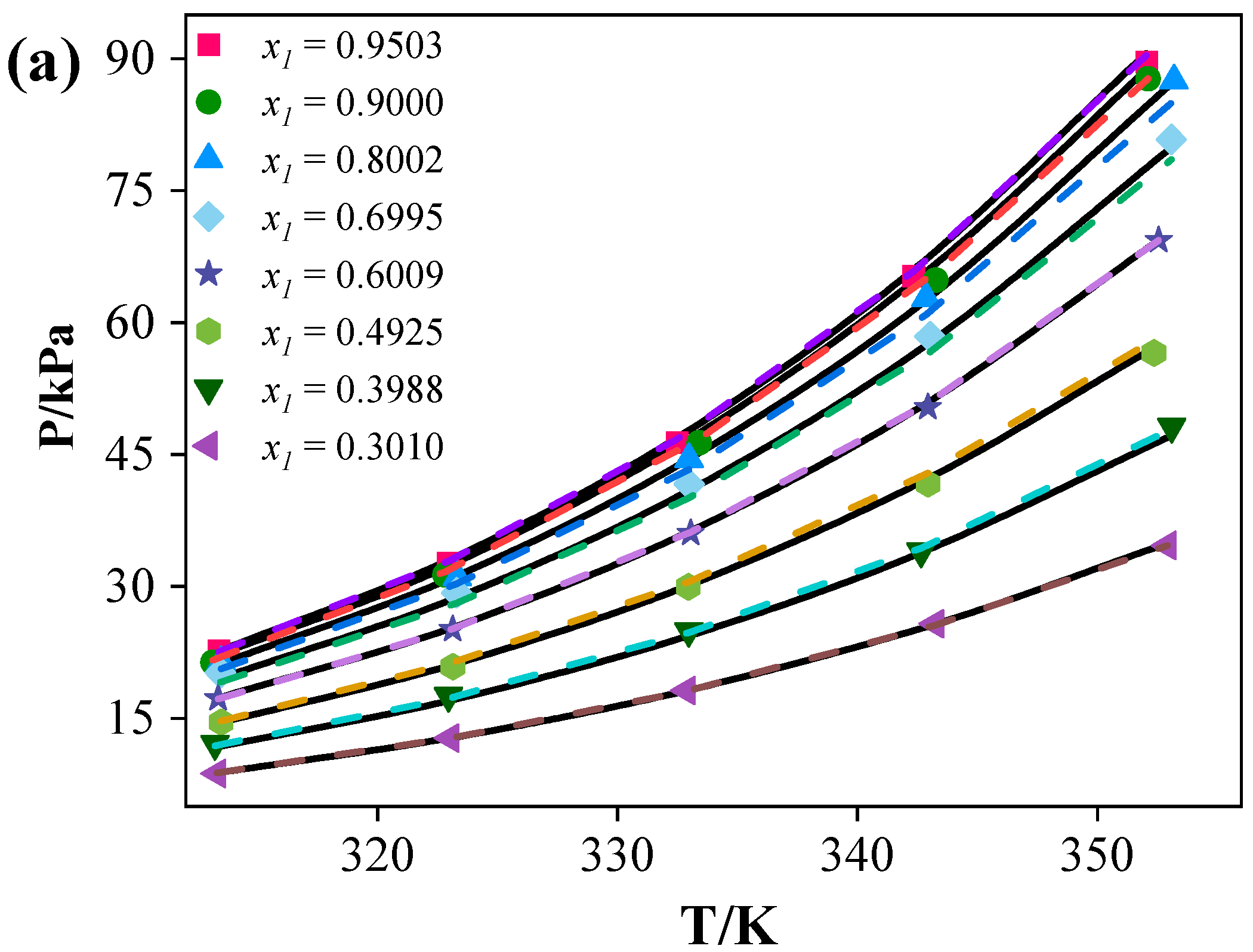
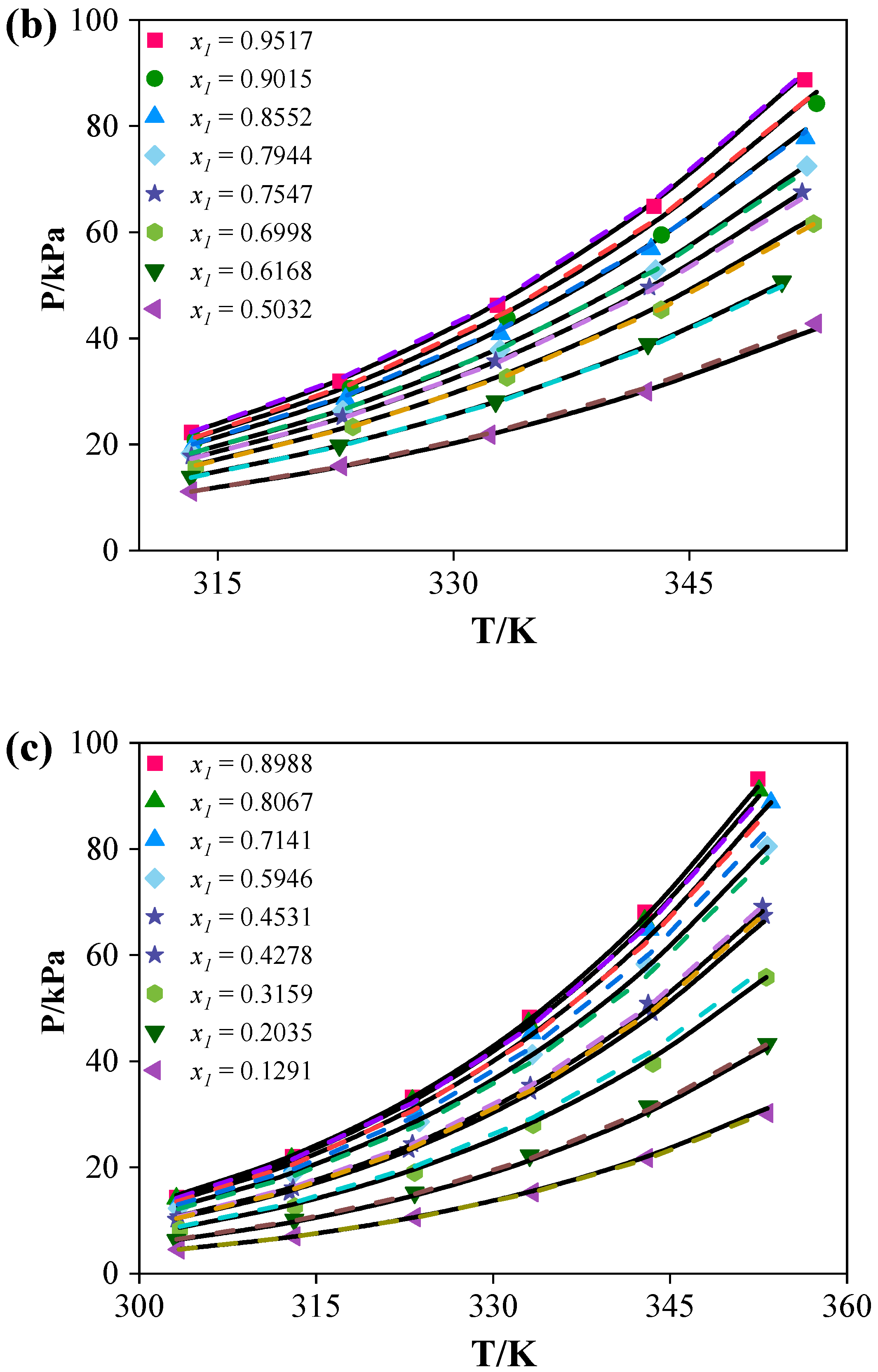
(1) For the binary systems, the vapor pressure data exhibited negative deviations, with [C4mim][BF4] exerting the strongest influence on acetonitrile. Both the NRTL and e-NRTL models provided good correlations of the binary data. The NRTL model yielded average relative pressure deviations of 1.19% ([C4mim][Cl]), 1.22% ([C4mim][BF4]), and 1.60% ([C6mim][Cl]), whereas the e-NRTL model resulted in deviations of 1.54% ([C4mim][Cl]), 1.36% ([C4mim][BF4]), and 2.88% ([C6mim][Cl]).
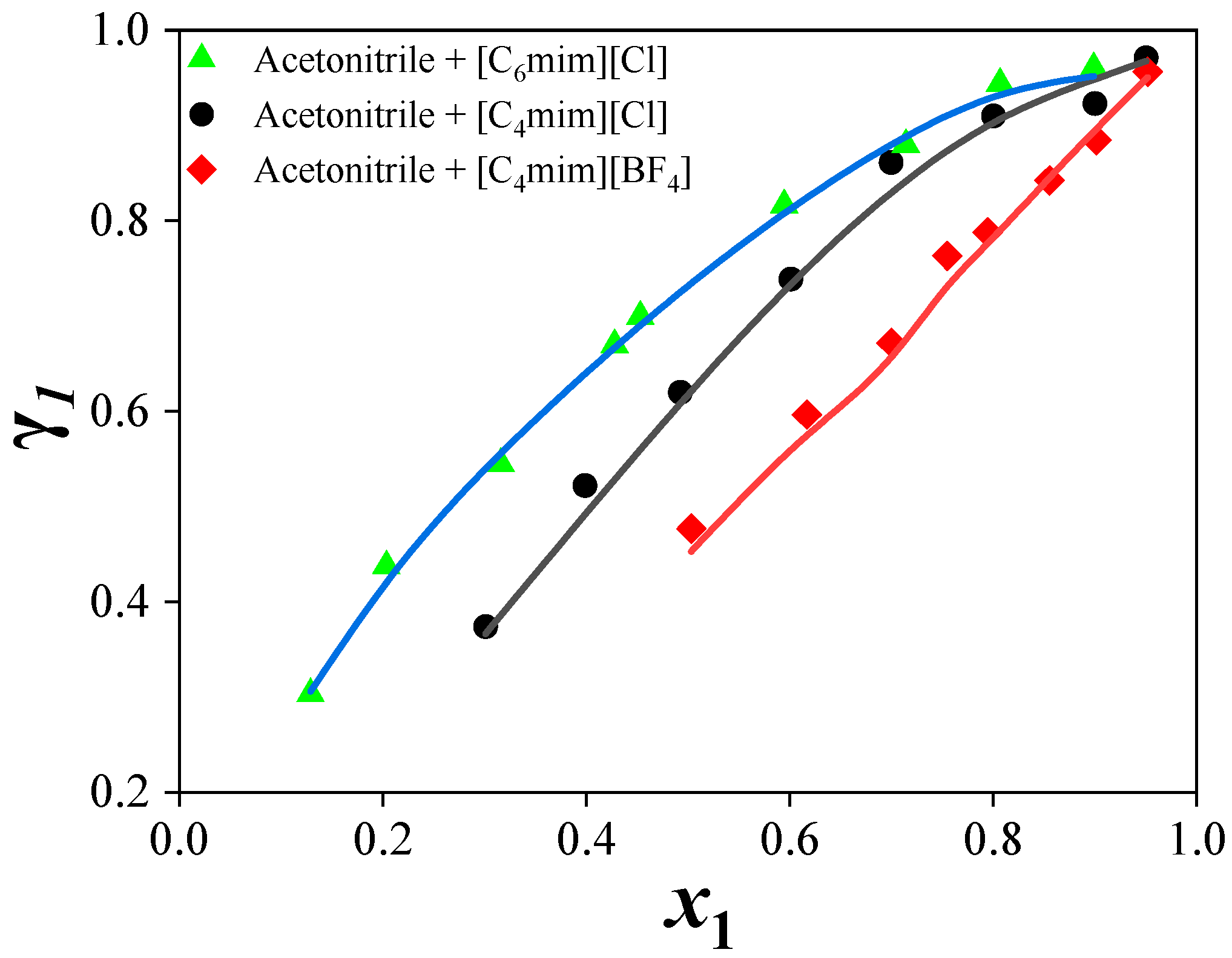
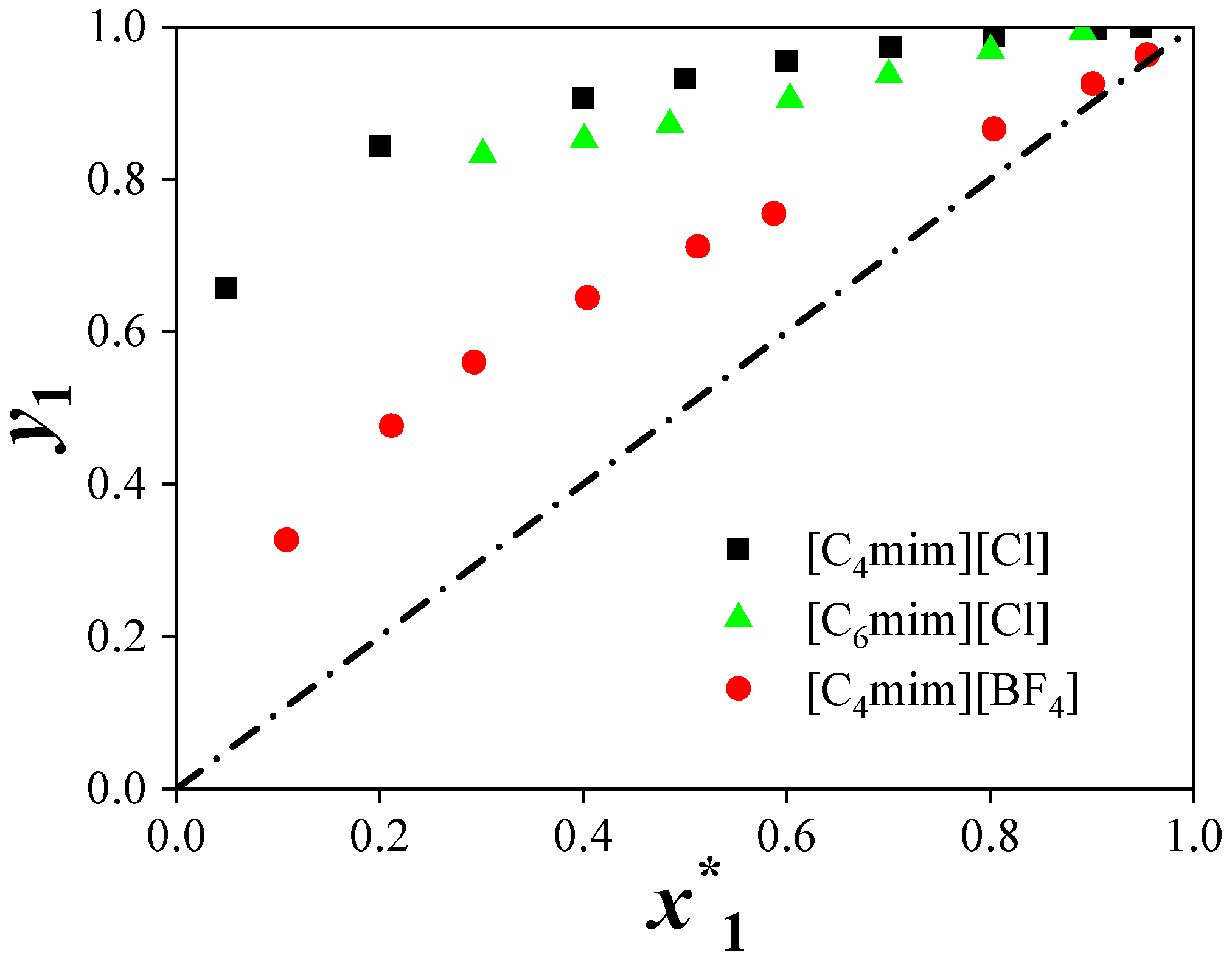
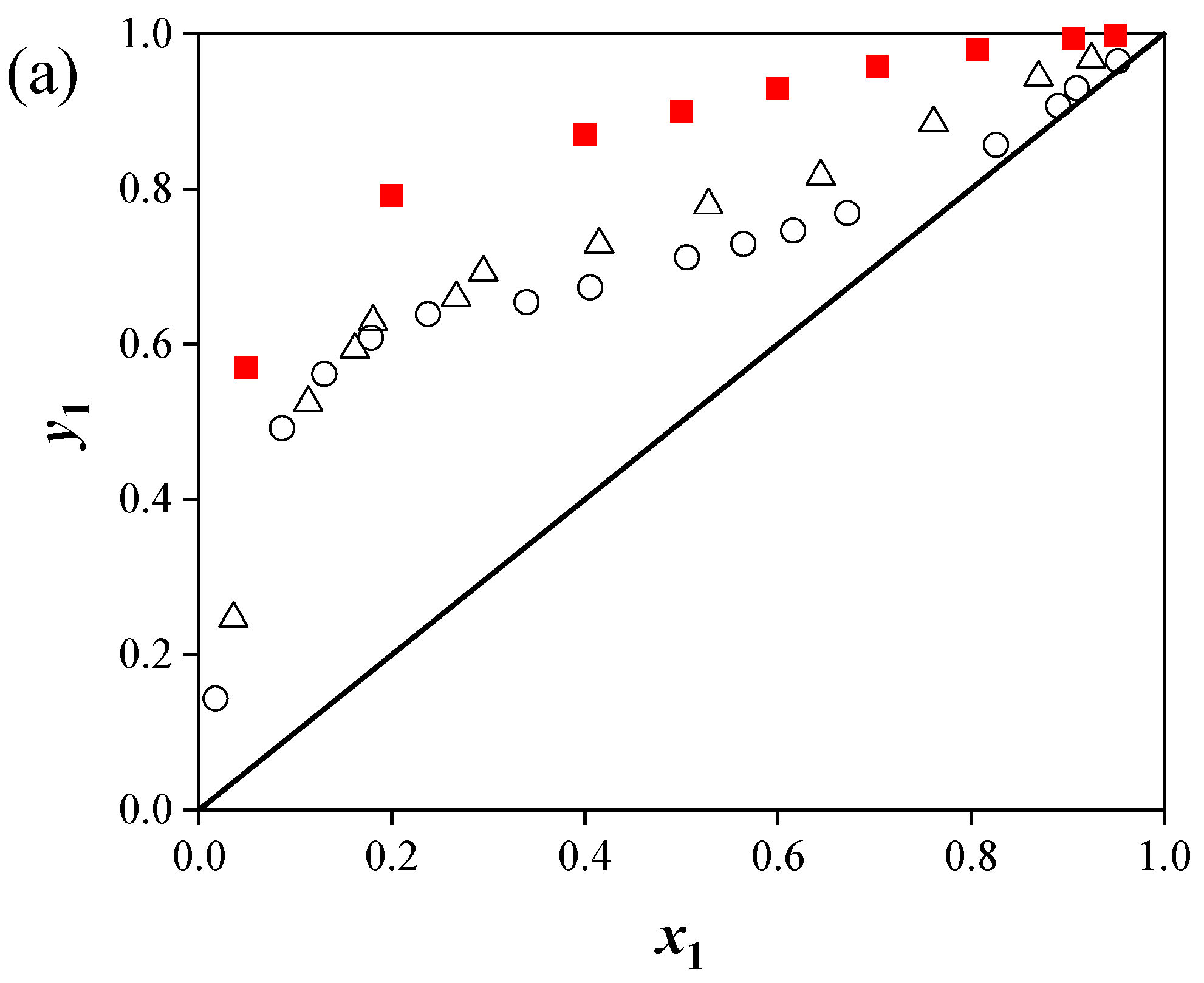
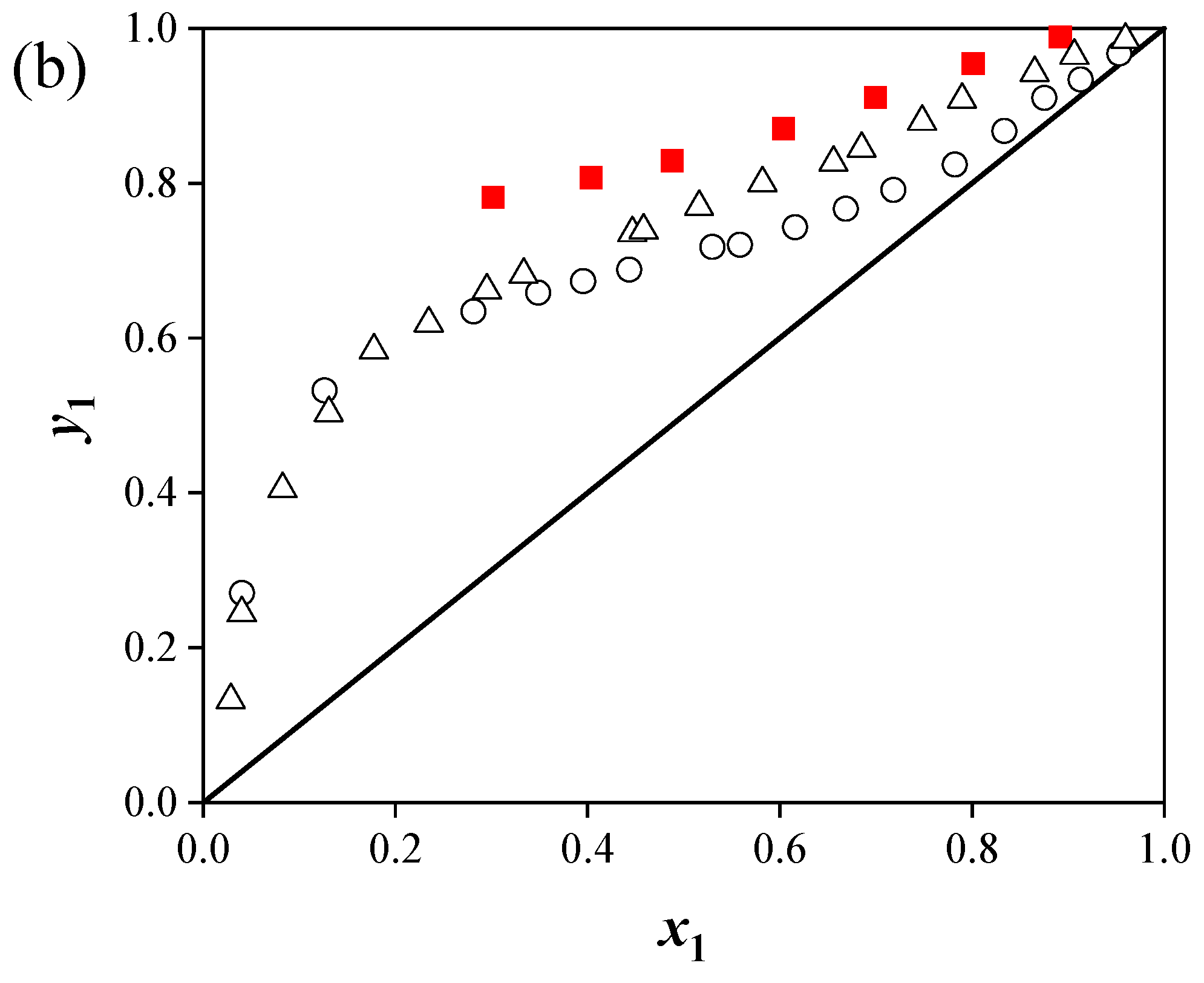
(2) Predictions for the ternary systems showed average relative pressure deviations of 9.11% ([C4mim][Cl]), 8.85% ([C4mim][BF4]), and 9.86% ([C6mim][Cl]). The addition of ILs increased the relative volatility of acetonitrile by disrupting the strong interactions between acetonitrile and water, thereby enhancing separation efficiency. The separation performance of the ILs followed the order: [C4mim][Cl] > [C6mim][Cl] > [C4mim][BF4].
(3) The effect of ILs on acetonitrile activity coefficients and phase behavior depended on both cation and anion structures. Chloride-based ILs with shorter alkyl chains provided higher separation efficiency.
These results provide valuable guidance for selecting ILs for acetonitrile + water separation and offer a theoretical basis for designing IL-based separation processes.