Unlocking Refractory Gold: Synergistic Pretreatment Strategies for High-Efficiency Thiosulfate Leaching
Abstract
1. Introduction
2. Experimental Work
2.1. Materials and Methods
2.2. Leaching Experiments
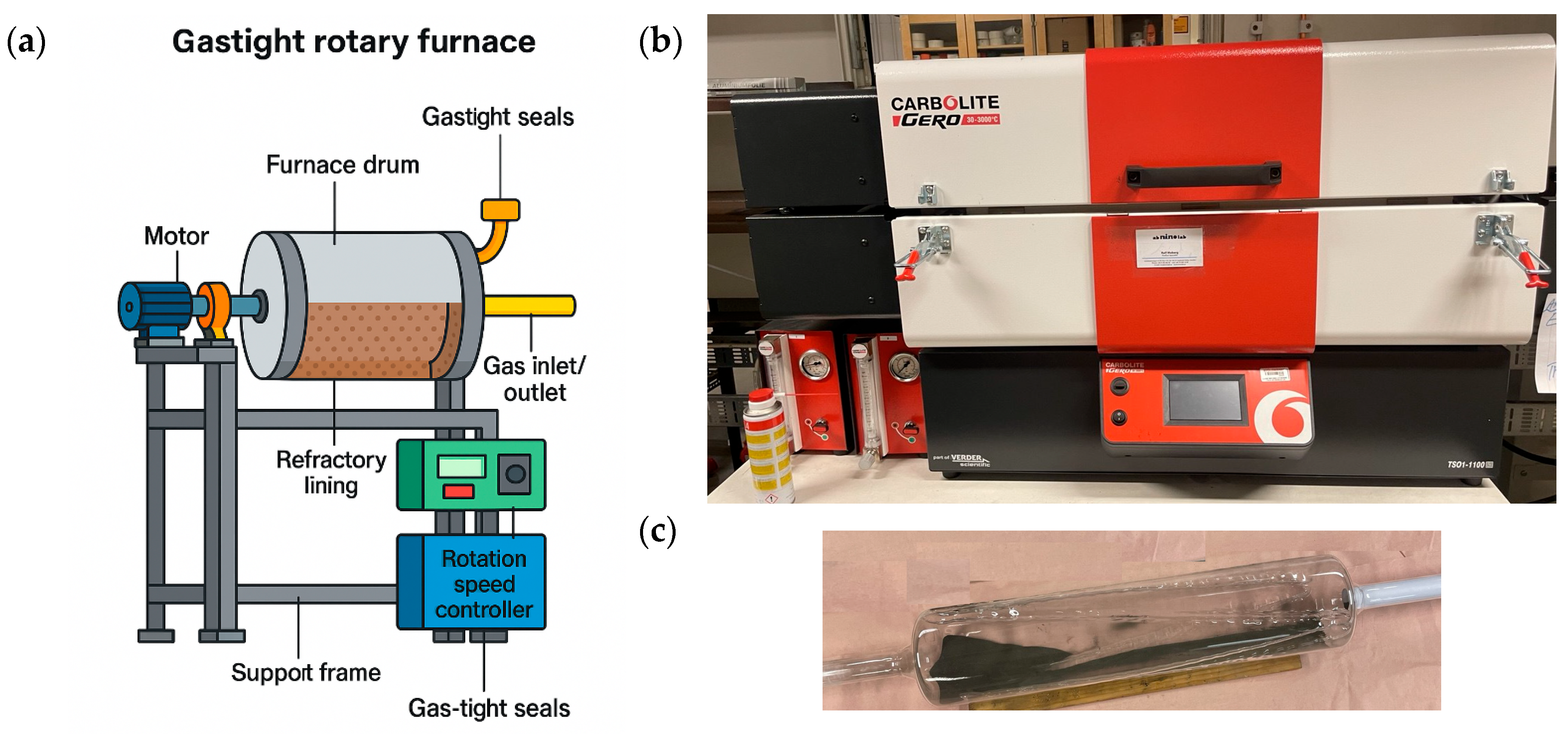
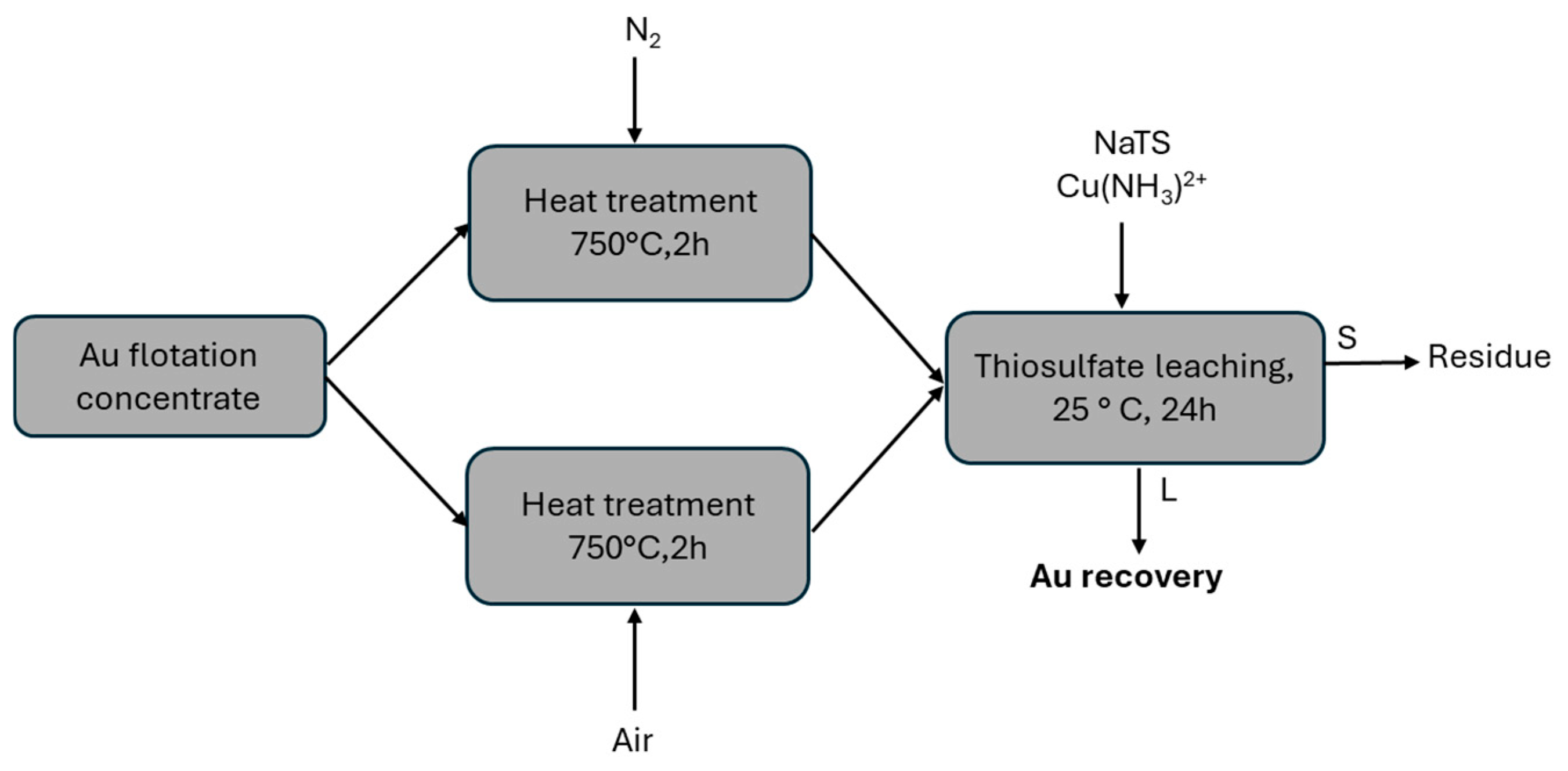
2.3. Pre-Treatment Techniques
2.3.1. Grinding
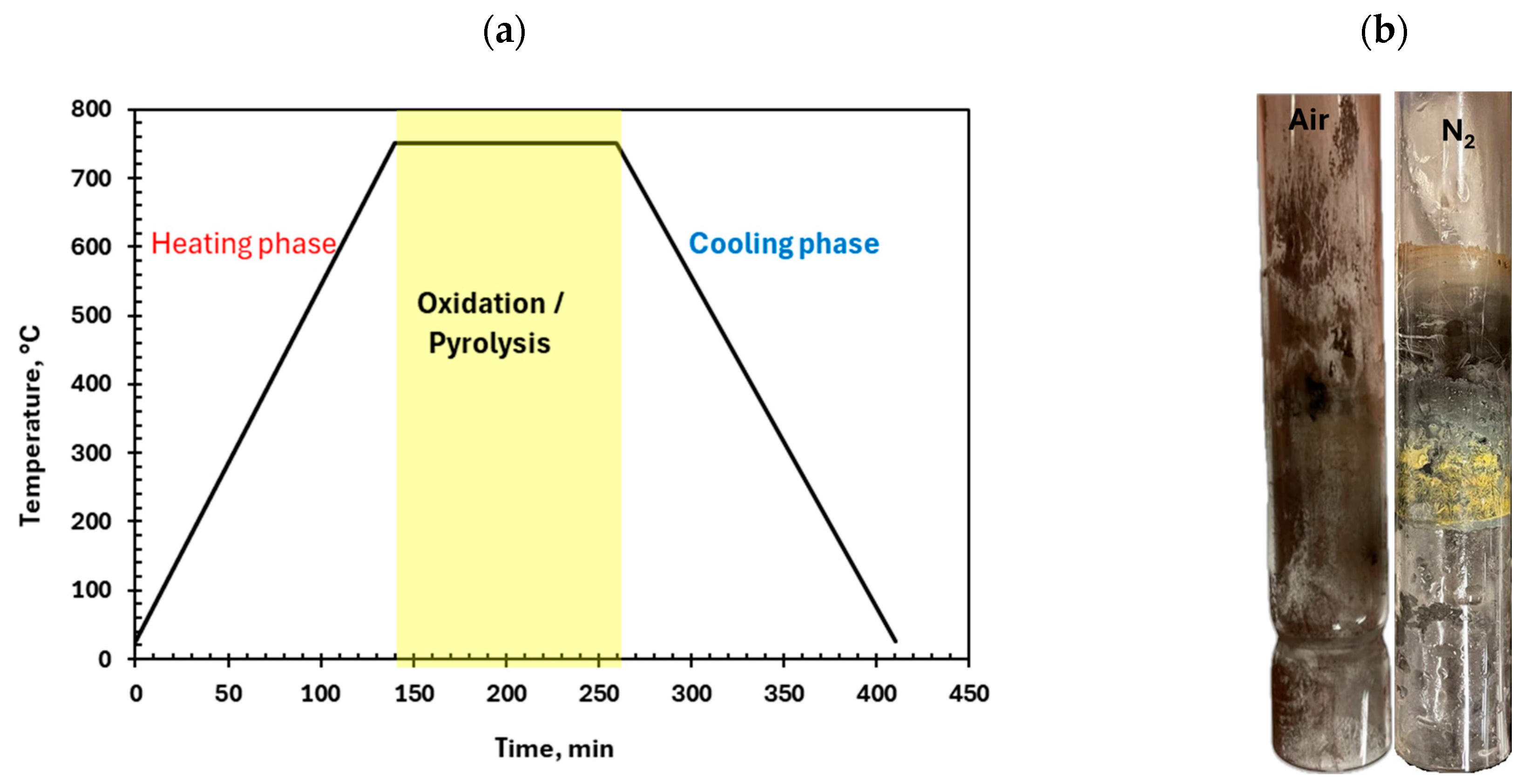
2.3.2. Heat Treatment Experiments
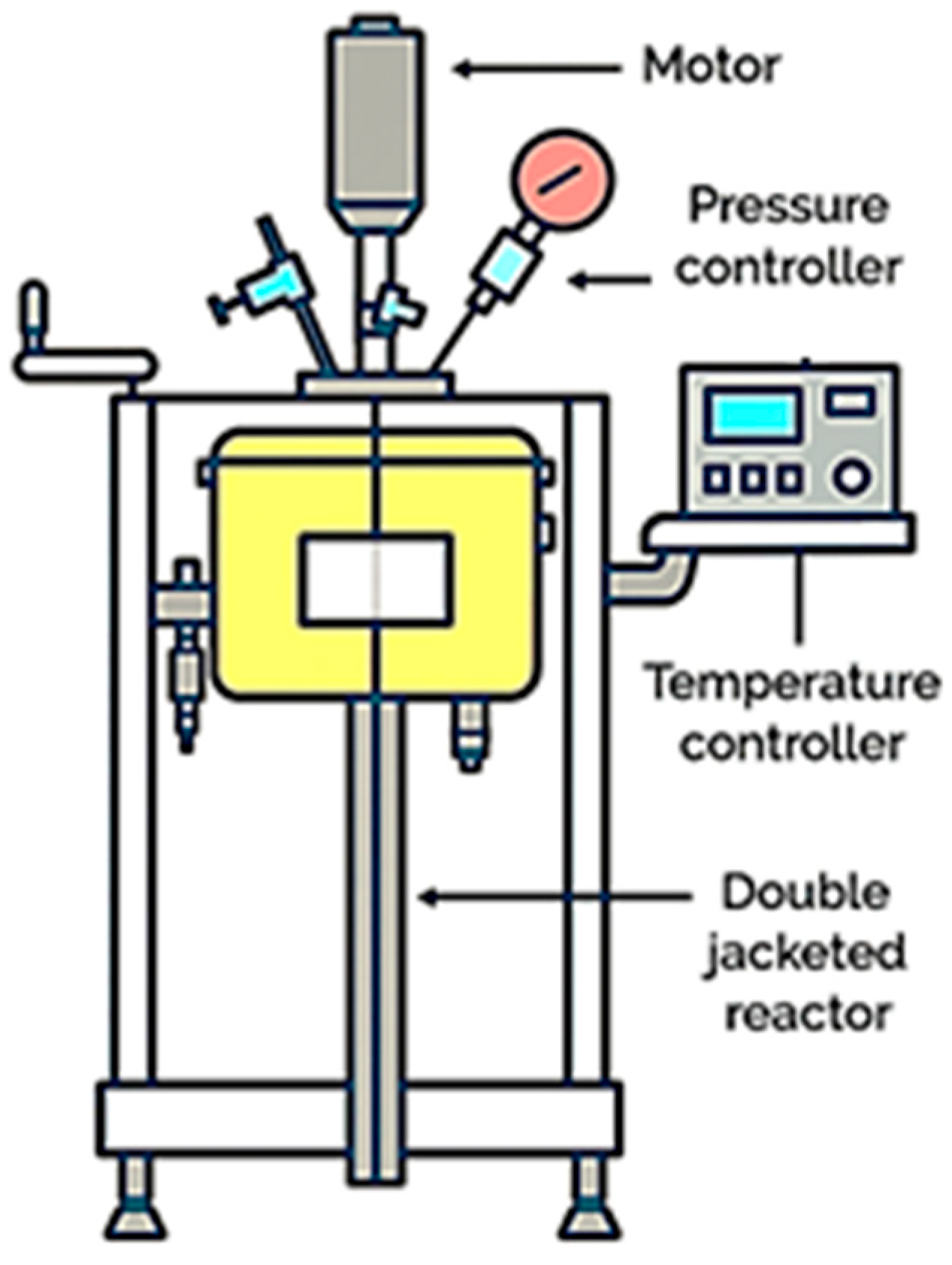
2.3.3. Pressure Oxidative Leaching
2.3.4. Ammoniacal Oxidative Leaching
3. Results and Discussion
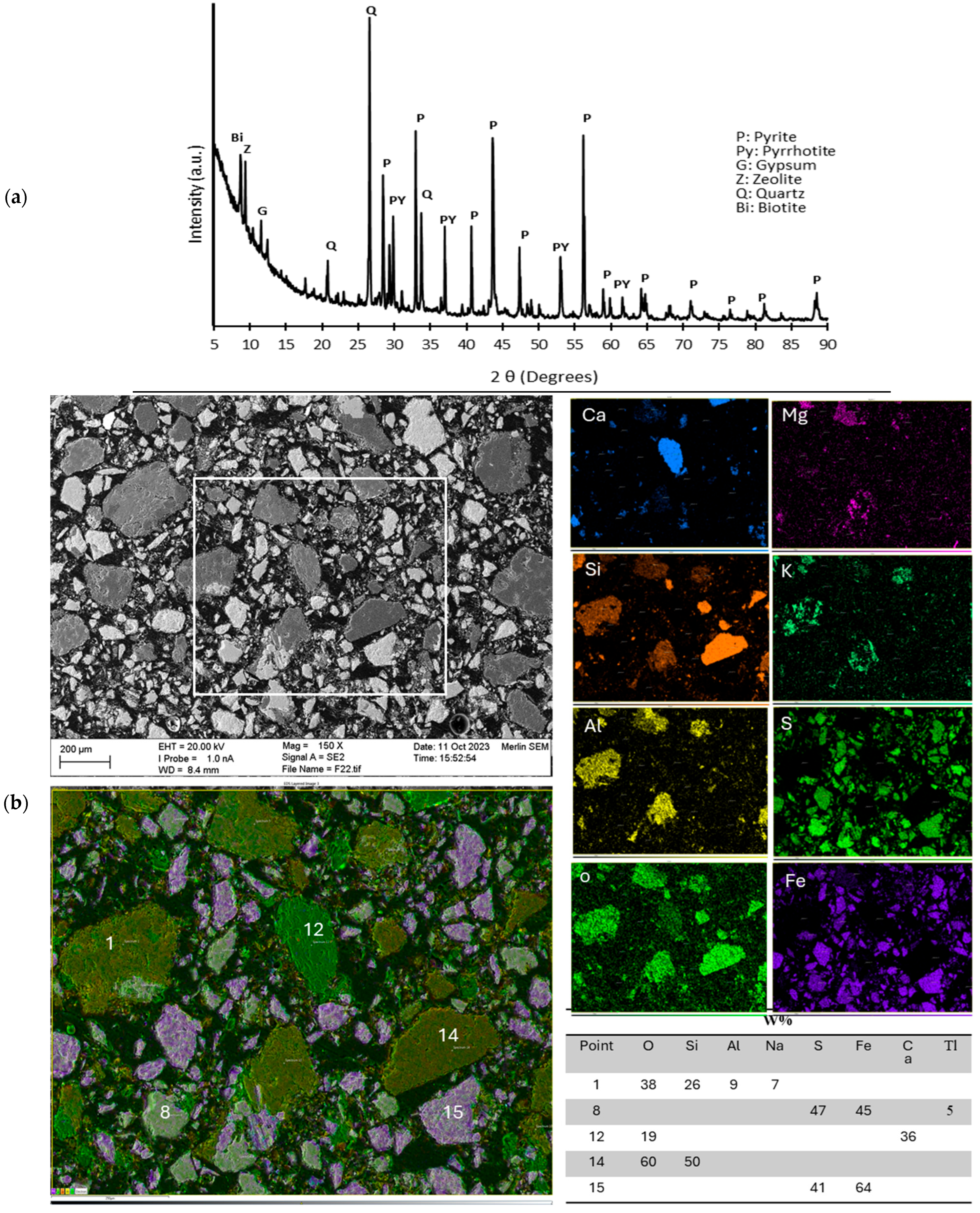
3.1. Characterization of Sample
3.2. Direct Cyanidation Test for Feed Material
- Anodic (on steel surface):
- Cathodic (on pyrite surface):
3.3. Pretreatment Strategies for Enhanced Gold Liberation and Recovery
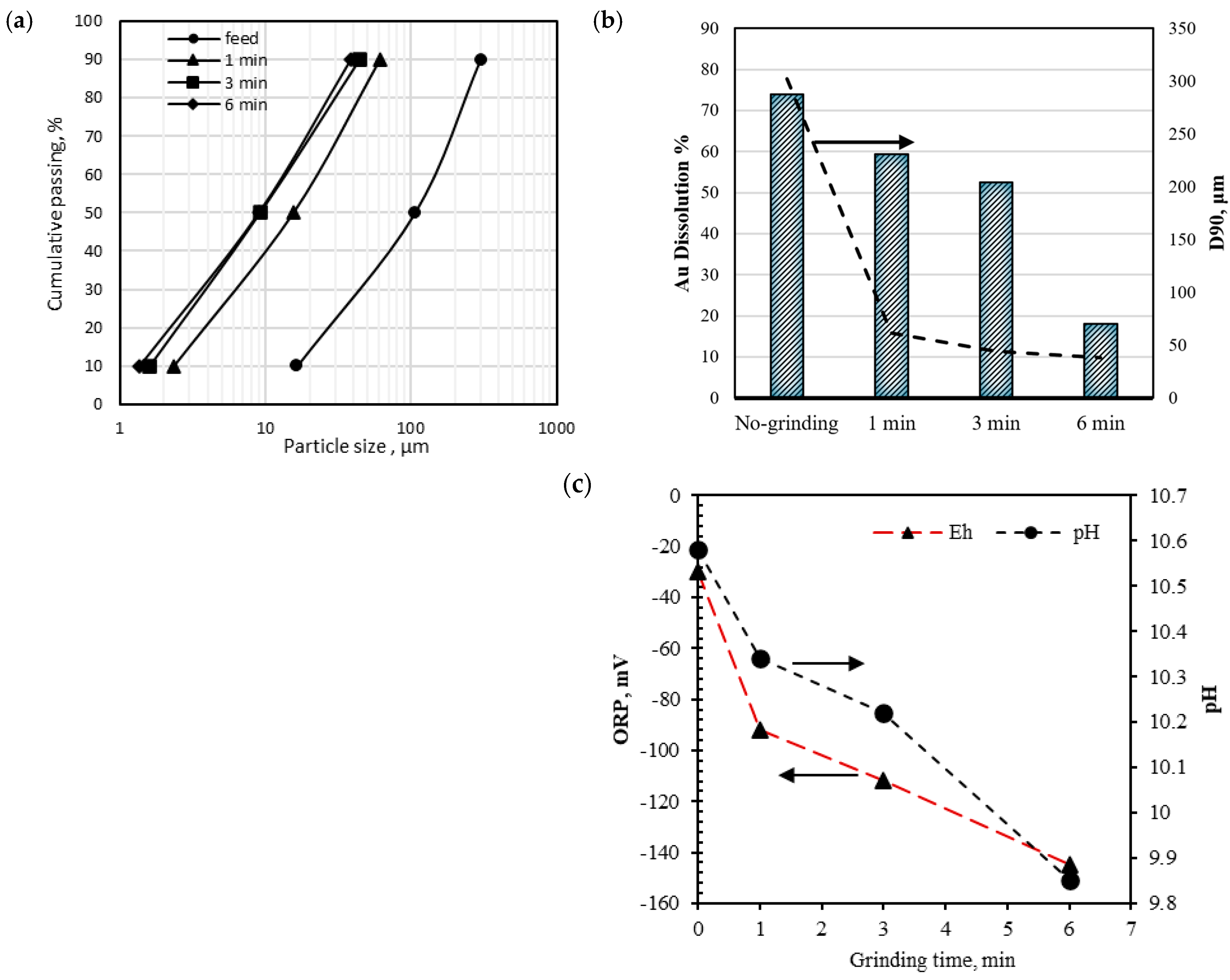
3.3.1. Pre-Treatment with Ultrafine Grinding (UFG)
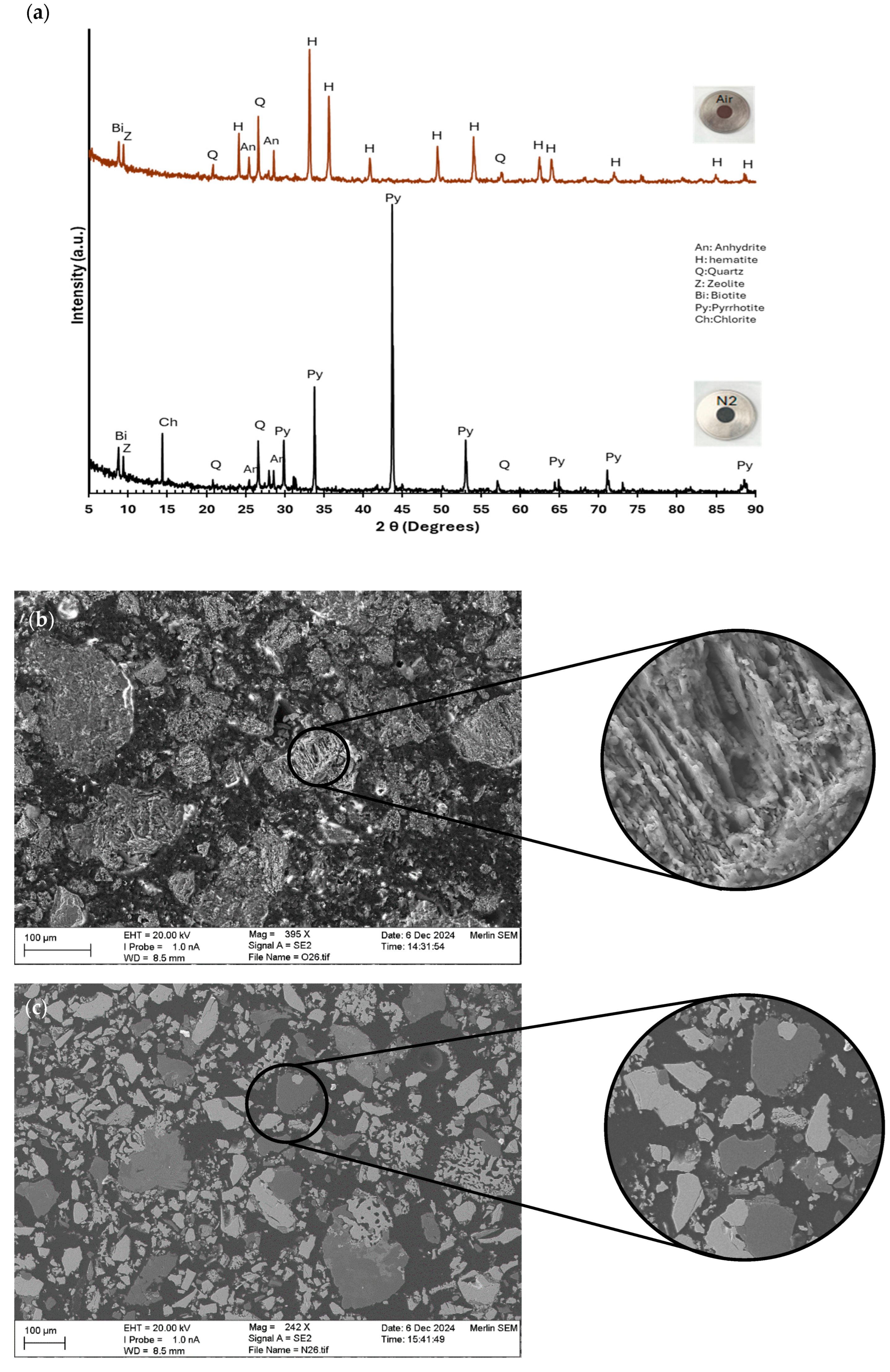
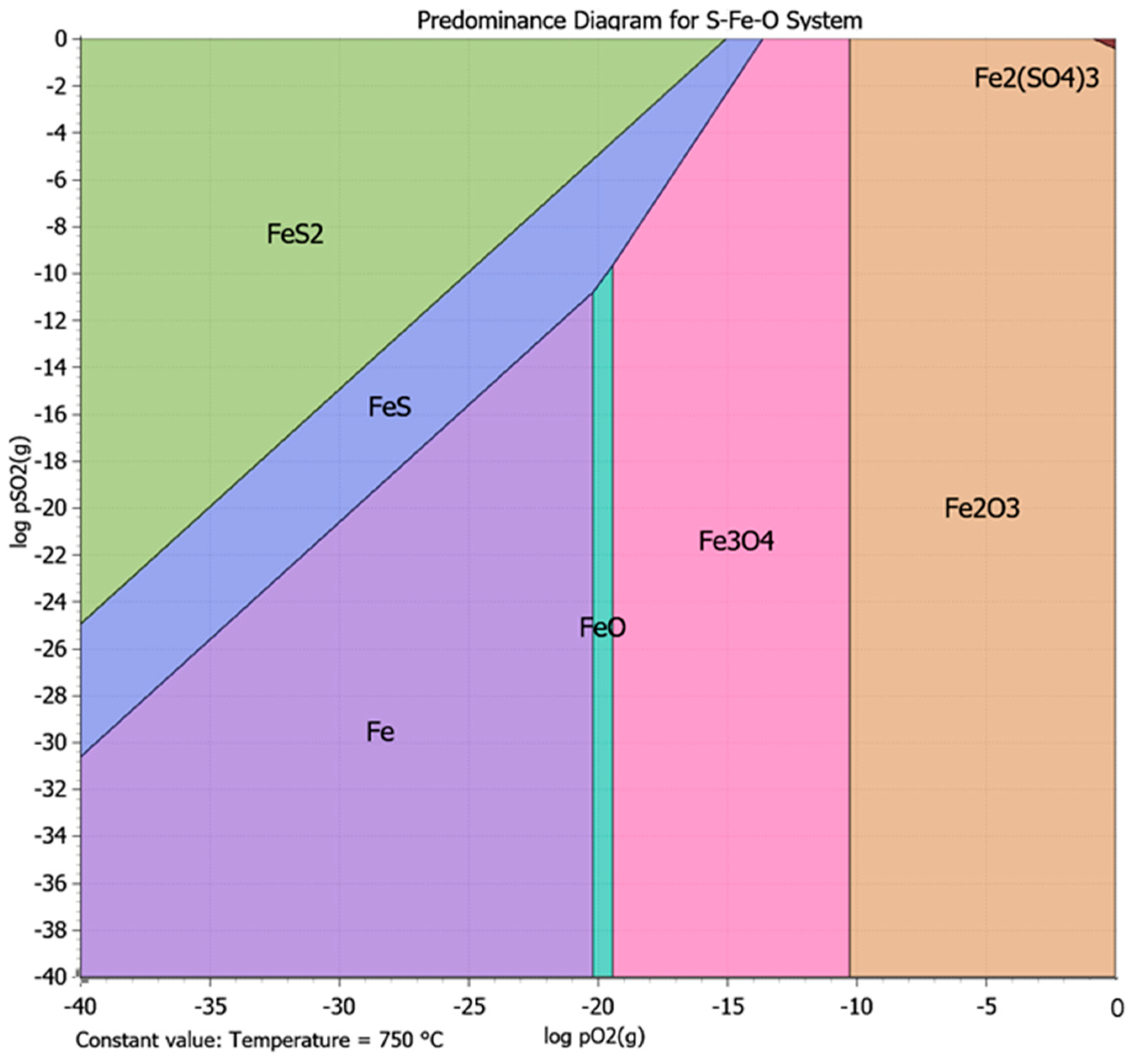
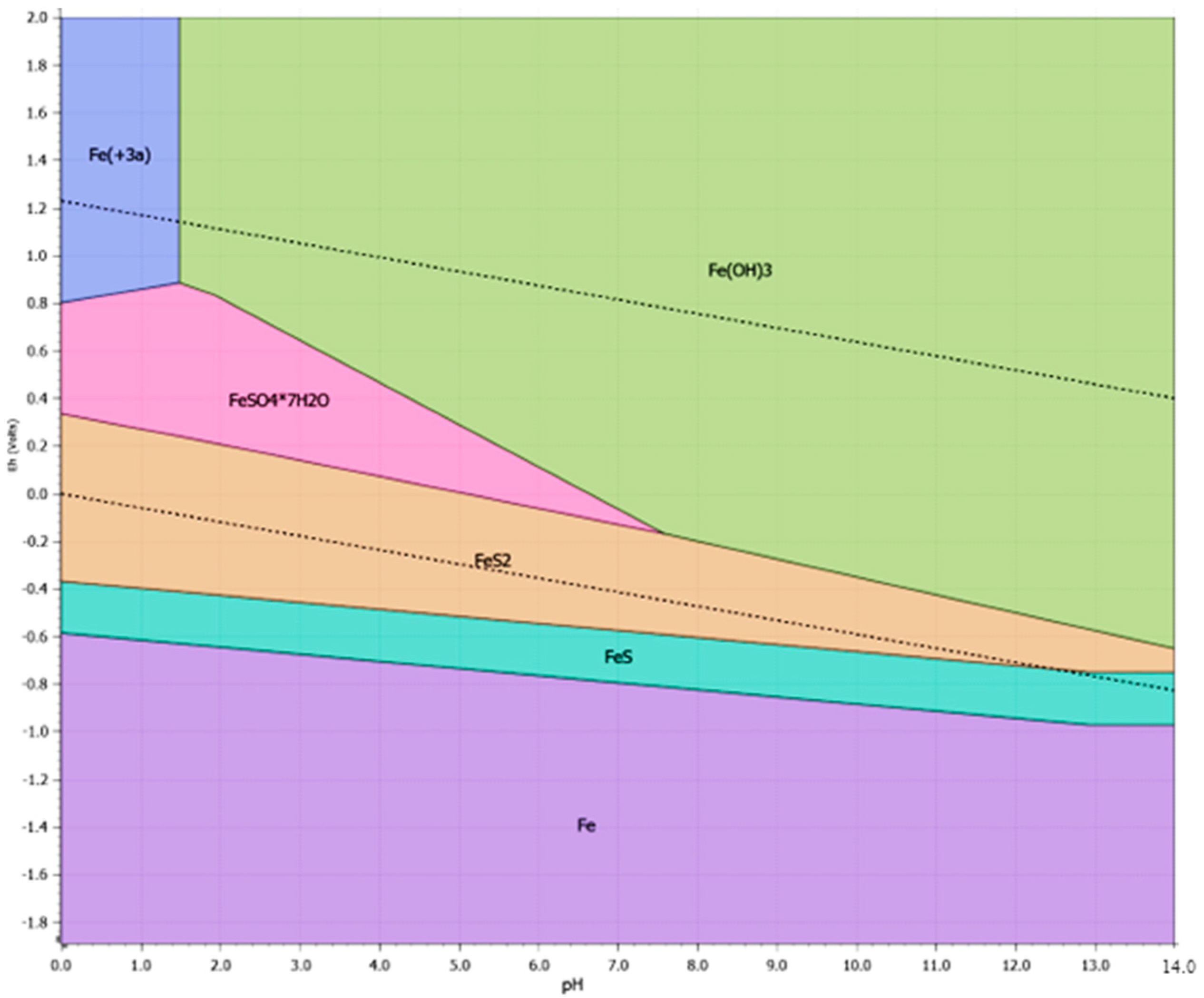
3.3.2. Pre-Treatment with Heat Treatment
3.3.3. Pre-Treatment with Pressure Oxidative Leaching (POX)
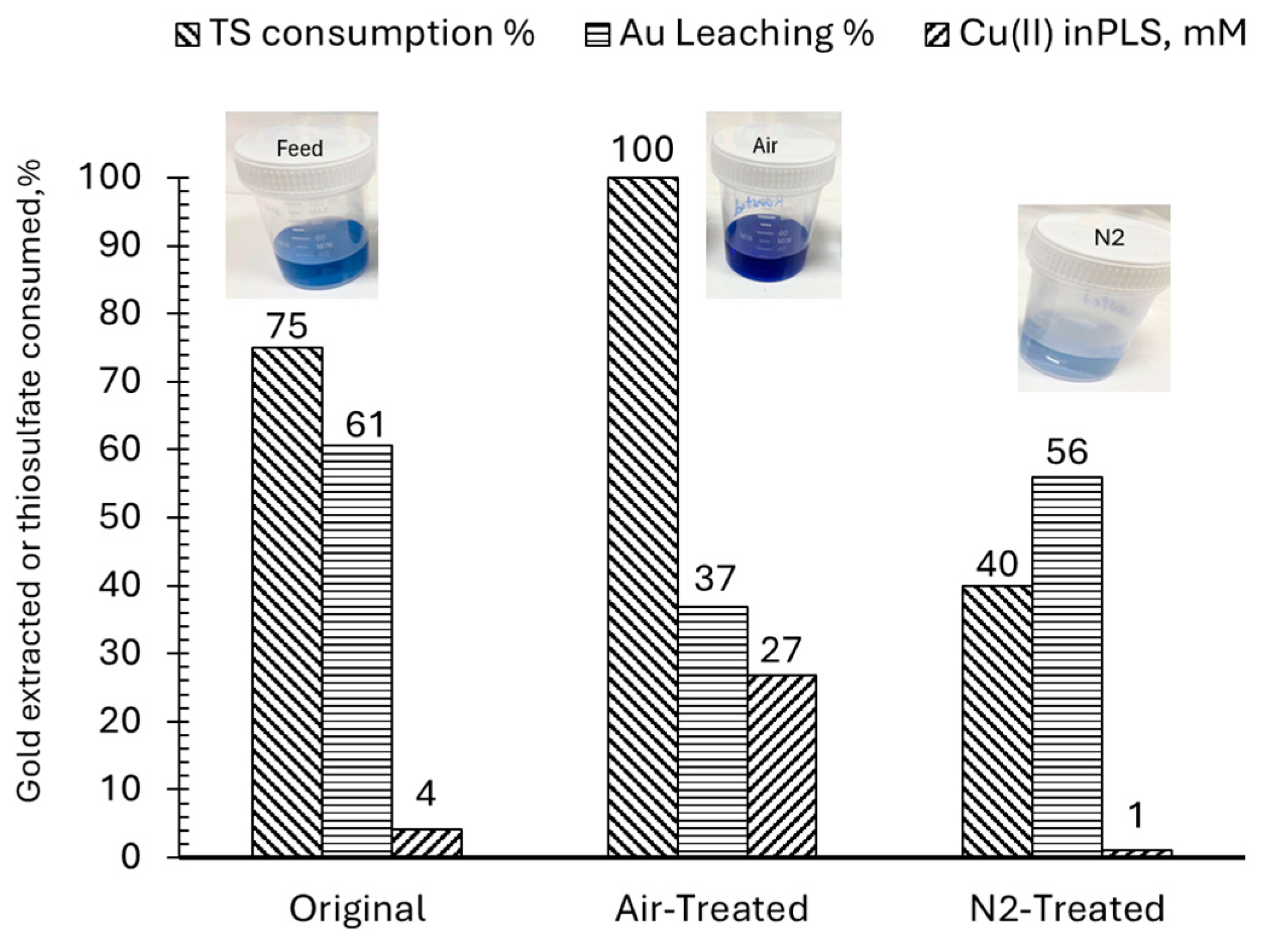
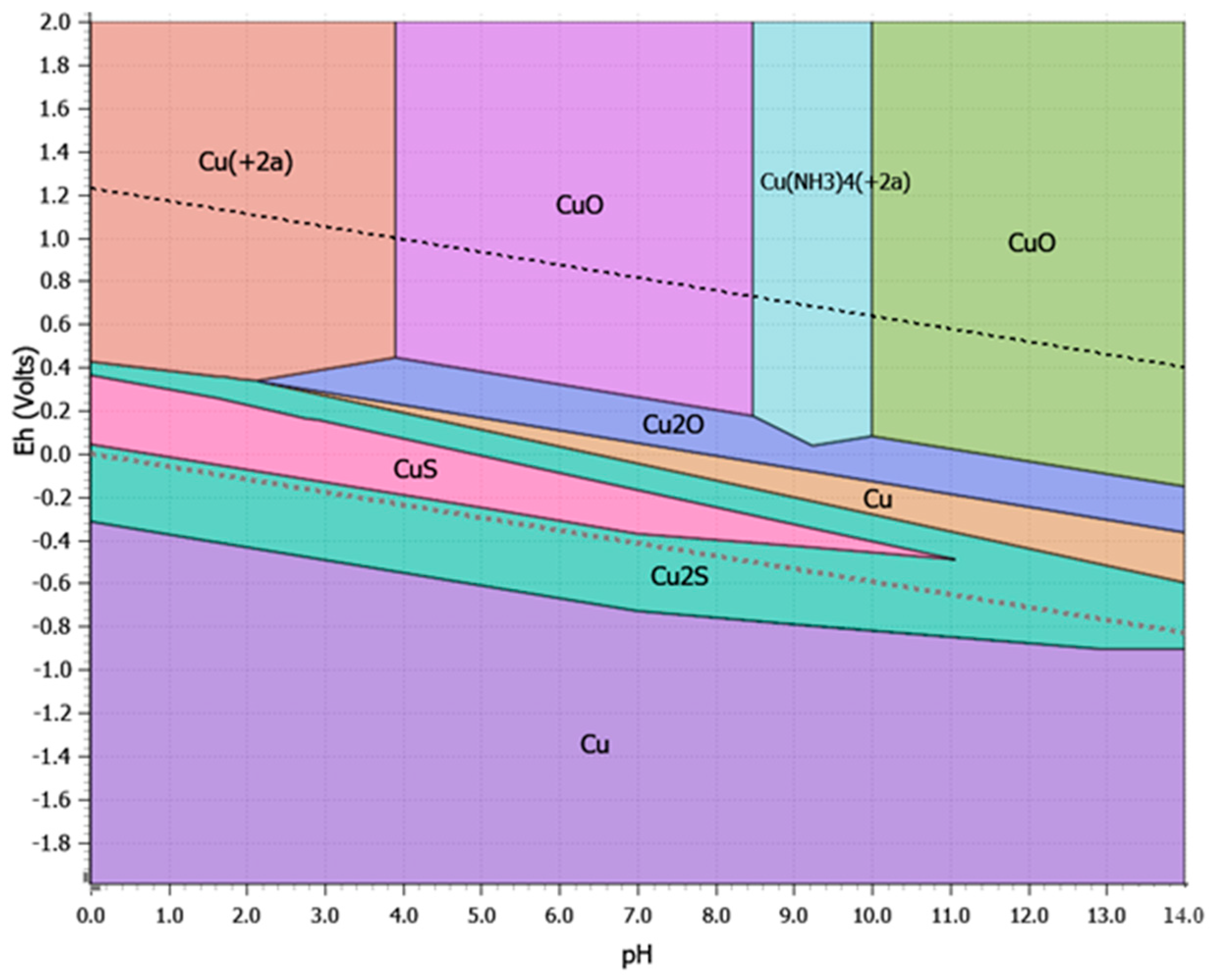
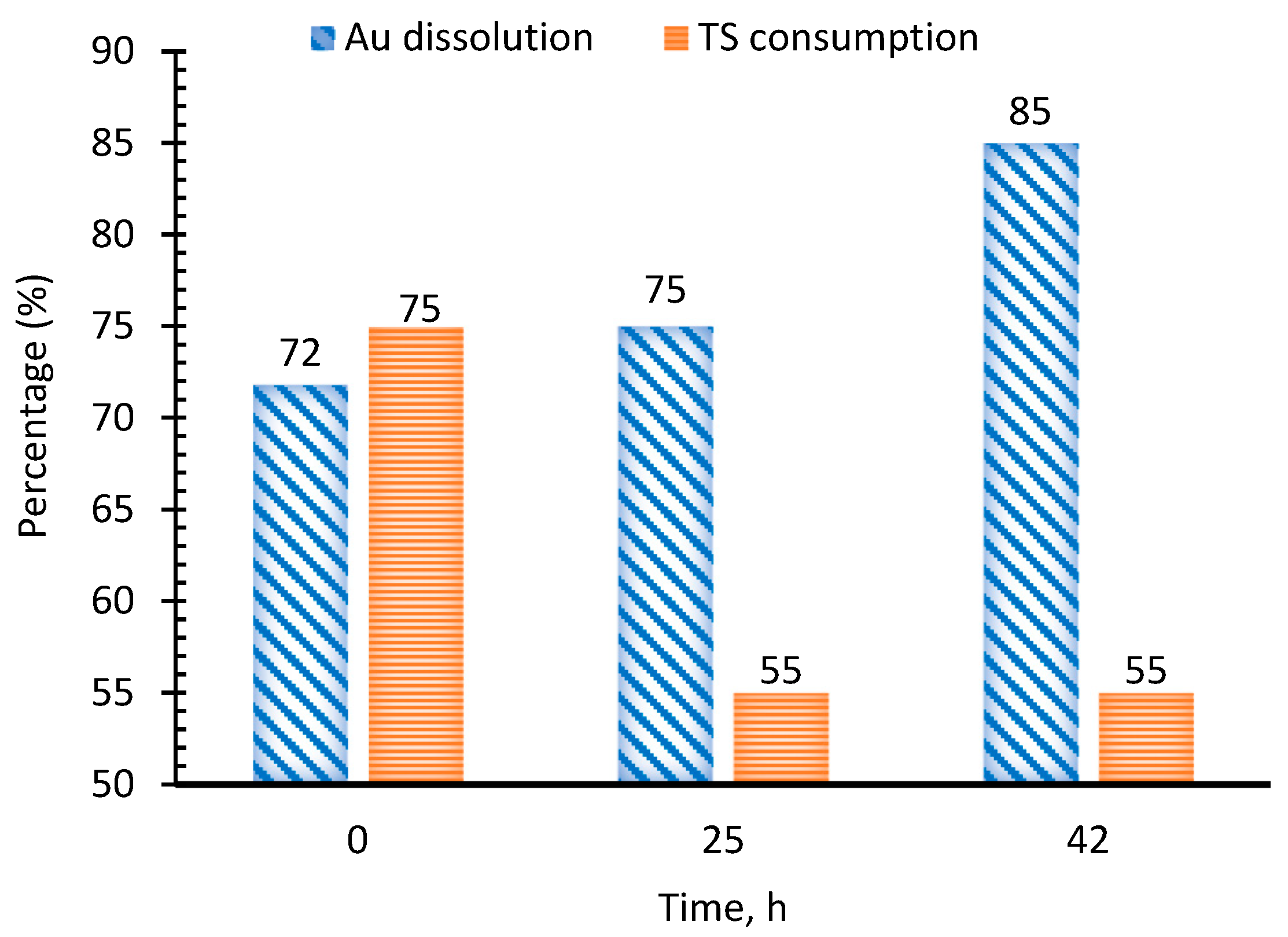
3.3.4. Pretreatment with Ammoniacal Oxidative Leaching
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsden, J.; House, I. The Chemistry of Gold Extraction; SME: Englewood, CO, USA, 2006. [Google Scholar]
- Breuer, P.L.; Dai, X.; Jeffrey, M.I. Leaching of gold and copper minerals in cyanide deficient copper solutions. Hydrometallurgy 2005, 78, 156–165. [Google Scholar] [CrossRef]
- Muir, D.M. A review of the selective leaching of gold from oxidised copper–gold ores with ammonia–cyanide and new insights for plant control and operation. Miner. Eng. 2011, 24, 576–582. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Income, K.; Boonpo, S.; Kruatian, T.; Sooksamiti, P.; Kungwankunakorn, S. Gold Recovery from Copper-Gold Tailings by Ammoniacal Thiosulphate Leaching; Current Applied Science and Technology: Bangkok, Thailand, 2021; pp. 735–750. [Google Scholar]
- Xie, F.; Chen, J.-N.; Wang, J.; Wang, W. Review of gold leaching in thiosulfate-based solutions. Trans. Nonferrous Met. Soc. China 2021, 31, 3506–3529. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J. Oxidative pre-treatment in thiosulphate leaching of sulphide gold ores. Int. J. Miner. Process. 2010, 94, 28–34. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.; Salinas-Maldonado, R.G.; García-Cerón, A.; Flores-Badillo, J.; Cerecedo-Sáenz, E.; Toro, N.; Saldana, M.; Gálvez, E.; Gutiérrez-Amador, M.P.; Salinas-Rodríguez, E. A Comparative Study of Cyanide and Thiosulfate for Silver Leaching from Tailings: A Kinetics Approach. Processes 2025, 13, 1522. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulfate as an alternative lixiviant to cyanide for gold ores. In Developments in Mineral Processing; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 541–560. [Google Scholar]
- Espitia, S.L.M.; Lapidus, G.T. Arsenic removal strategy in the processing of an arsenopyritic refractory gold ore. Hydrometallurgy 2021, 203, 105628. [Google Scholar] [CrossRef]
- Celep, O.; Altinkaya, P.; Yazici, E.Y.; Deveci, H. Thiosulphate leaching of silver from an arsenical refractory ore. Miner. Eng. 2018, 122, 285–295. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A Review of Thiosulfate Leaching of Gold: Focus on Thiosulfate Consumption and Gold Recovery from Pregnant Solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Yang, Y.-b.; Zhang, X.; Xu, B.; Li, Q.; Jiang, T.; Wang, Y.-x. The Effects of Common Associated Sulfide Minerals on Thiosulfate Leaching of Gold. In Rare Metal Technology 2015; Neelameggham, N.R., Alam, S., Oosterhof, H., Jha, A., Dreisinger, D., Wang, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 79–86. [Google Scholar]
- Feng, D.; Van Deventer, J.S.J. Effect of sulfides on gold dissolution in ammoniacal thiosulfate medium. Metall. Mater. Trans. B 2003, 34, 5–13. [Google Scholar] [CrossRef]
- Mhandu, T.J. Ammonium Thiosulfate Leaching of Arsenic-Bearing Refractory Gold Ores. Ph.D. Thesis, Hokkaido University, Sapporo, Japan, 2024. [Google Scholar]
- Yu, L.; Li, S.; Liu, Q.; Deng, J.; Luo, B.; Liang, Y.; Zhao, L.; Lai, H. Gold recovery from refractory gold concentrates by pressure oxidation pre-treatment and thiosulfate leaching. Physicochem. Probl. Miner. Process. 2019, 55, 537–551. [Google Scholar]
- Qin, H.; Guo, X.; Tian, Q.; Yu, D.; Zhang, L. Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Nazari, A.M.; Ghahreman, A.; Bell, S. A comparative study of gold refractoriness by the application of QEMSCAN and diagnostic leach process. Int. J. Miner. Process. 2017, 169, 35–46. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ramsay, J.; Ghahreman, A. A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources. J. Clean. Prod. 2021, 296, 126457. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Perrot, F.; Morris, C.; Rea, S.; Benvie, B.; Austin, P.; Hackl, R. Evaluation of submerged bio-oxidation concept for refractory gold ores. Hydrometallurgy 2014, 141, 117–125. [Google Scholar] [CrossRef]
- Mubarok, M.Z.; Winarko, R.; Chaerun, S.K.; Rizki, I.N.; Ichlas, Z.T. Improving gold recovery from refractory gold ores through biooxidation using iron-sulfur-oxidizing/sulfur-oxidizing mixotrophic bacteria. Hydrometallurgy 2017, 168, 69–75. [Google Scholar] [CrossRef]
- Rogozhnikov, D.; Karimov, K.; Shoppert, A.; Dizer, O.; Naboichenko, S. Kinetics and mechanism of arsenopyrite leaching in nitric acid solutions in the presence of pyrite and Fe(III) ions. Hydrometallurgy 2021, 199, 105525. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Fang, Y.; Cabrera, A.R.; Peng, C.; López-Valdivieso, A. Roasting temperature effect on the recovery of refractory gold and silver in pyrite concentrates. J. Min. Metall. Sect. B Metall. 2021, 57, 235–243. [Google Scholar] [CrossRef]
- Ballo, I.; Hein, K.A.; Guindo, B.; Sanogo, L.; Ouologuem, Y.; Daou, G.; Traore, A. The Syama and Tabakoroni goldfields, Mali. Ore Geol. Rev. 2016, 78, 578–585. [Google Scholar] [CrossRef]
- Runkel, M.; Sturm, P. Pyrite roasting, an alternative to sulphur burning. J. S. Afr. Inst. Min. Metall. 2009, 109, 491–496. [Google Scholar]
- Lampinen, M.; Laari, A.; Turunen, I. Ammoniacal thiosulfate leaching of pressure oxidized sulfide gold concentrate with low reagent consumption. Hydrometallurgy 2015, 151, 1–9. [Google Scholar] [CrossRef]
- Xia, C. Associated Sulfide Minerals in Thiosulfate Leaching of Gold: Problems and Solutions; Queen’s University: Kingston, ON, Canada, 2008. [Google Scholar]
- Boduen, A.; Zalesov, M.; Melamud, V.; Grigorieva, V.; Bulaev, A. Combined Bacterial and Pressure Oxidation for Processing High-Sulfur Refractory Gold Concentrate. Processes 2023, 11, 3062. [Google Scholar] [CrossRef]
- Marchbank, A.R.; Thomas, K.G.; Dreisinger, D.; Fleming, C. Gold Recovery from Refractory Carbonaceous Ores by Pressure Oxidation and Thiosulfate Leaching; Park Gold Corporation: Washington, DC, USA, 1996. [Google Scholar]
- Wan, R.Y.; LeVier, K.M.; Clayton, R.B. Hydrometallurgical Process for the Recovery Of Precious Metal Values from Precious Metal Ores with Thiosulfate Lixiviant; Newmont Gold Co.: Denver, CO, USA, 1994. [Google Scholar]
- Ellis, S.; Gao, M. Development of ultrafine grinding at kalgoorlie consolidated gold mines. Min. Metall. Explor. 2003, 20, 171–177. [Google Scholar] [CrossRef]
- Mahlangu, T.; Sumaili, F.; Ayizi, D.N.; Sindani, B.; Mande, P.; Du Toit, G.; Verster, M.; Mogashoa, S.; Lotz, P. Kibali Gold Mine sulphide concentrate treatment–understanding the preoxidation of sulphide concentrates. Miner. Process. Extr. Metall. 2020, 129, 74–86. [Google Scholar] [CrossRef]
- Smith, P.; Osman, R.; Franzmann, D.; Johnson, N.; Boreham, C. Mineral Resource and Reserve Estimate for the Sukari Gold Project. Egypt; Internal Technical Report; Centamin Egypt Ltd.: Mount Pleasant, Australia, 2017. [Google Scholar]
- Celep, O.; Yazici, E.; Kuzu, M.; Deveci, H. Effect of ultra-fine grinding on extraction of gold and silver from a refractory flotation tailings by cyanide leaching. In Proceedings of the 26 th International Mining Congress and Exhibition of Turkey, Antalya, Turkey, 16–19 April 2019. [Google Scholar]
- Gómez Santiago, M.; Martínez-Ponce, M.Á.; Ortiz Lara, N.; Escudero García, R.; Cholico-González, D. Ultrafine grinding of a refractory ore and its effect on the gold and silver leaching. MRS Adv. 2025, 10, 868–873. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Li, Q.; Jiang, T.; Zhang, X.; Li, G. Effect of common associated sulfide minerals on thiosulfate leaching of gold and the role of humic acid additive. Hydrometallurgy 2017, 171, 44–52. [Google Scholar] [CrossRef]
- Feng, D.; van Deventer, J.S.J. The effect of sulphur species on thiosulphate leaching of gold. Miner. Eng. 2007, 20, 273–281. [Google Scholar] [CrossRef]
- Mhandu, T.J.; Park, I.; Jeon, S.; Hamatsu, S.; Elakneswaran, Y.; Ito, M.; Hiroyoshi, N. A Pretreatment of Refractory Gold Ores Containing Sulfide Minerals to Improve Gold Leaching by Ammonium Thiosulfate: A Model Experiment Using Gold Powder and Arsenic-Bearing Sulfide Minerals. Metals 2023, 13, 1357. [Google Scholar] [CrossRef]
- Liu, X.; Xu, B.; Min, X.; Li, Q.; Yang, Y.; Jiang, T.; He, Y.; Zhang, X. Effect of Pyrite on Thiosulfate Leaching of Gold and the Role of Ammonium Alcohol Polyvinyl Phosphate (AAPP). Metals 2017, 7, 278. [Google Scholar] [CrossRef]
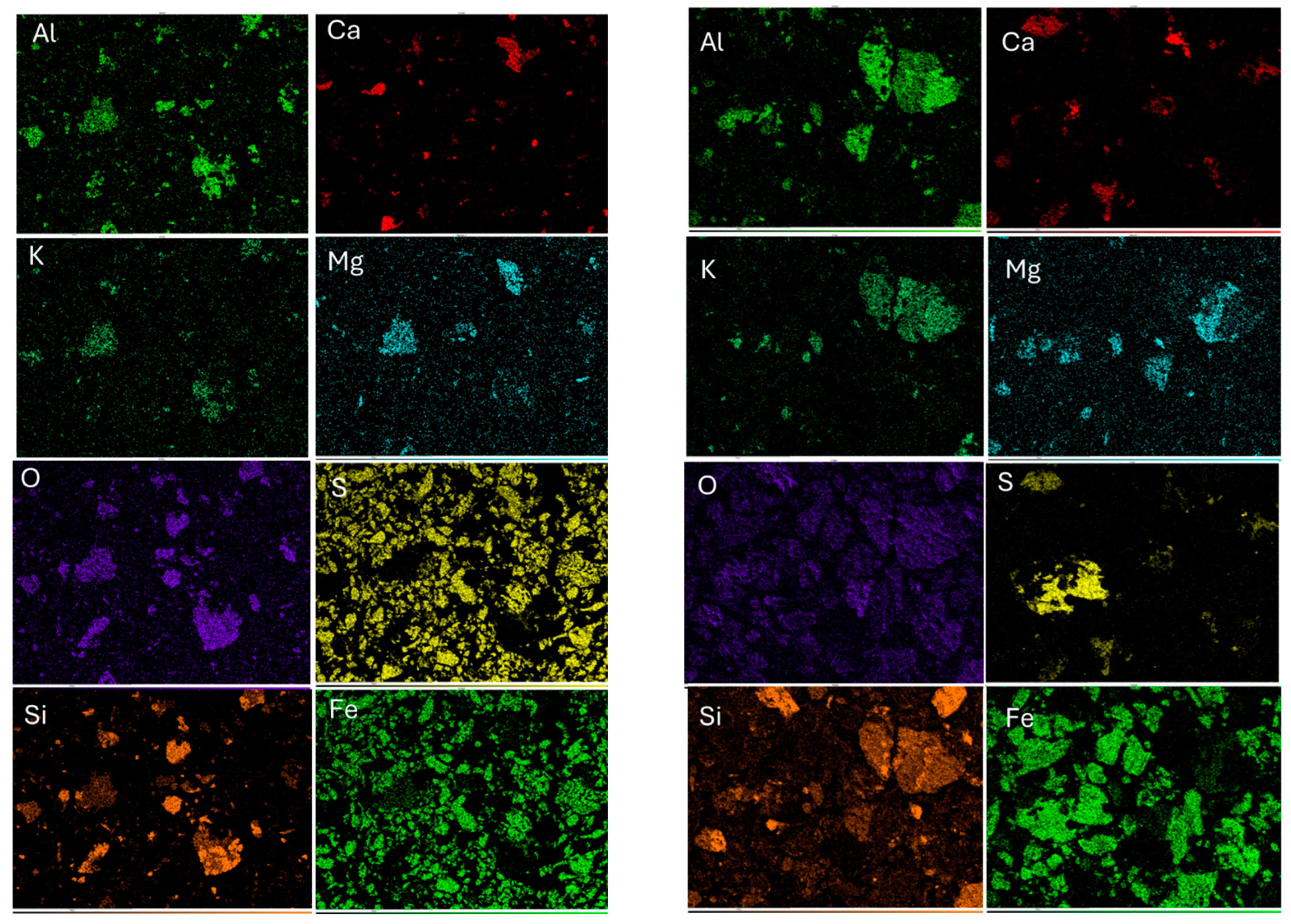
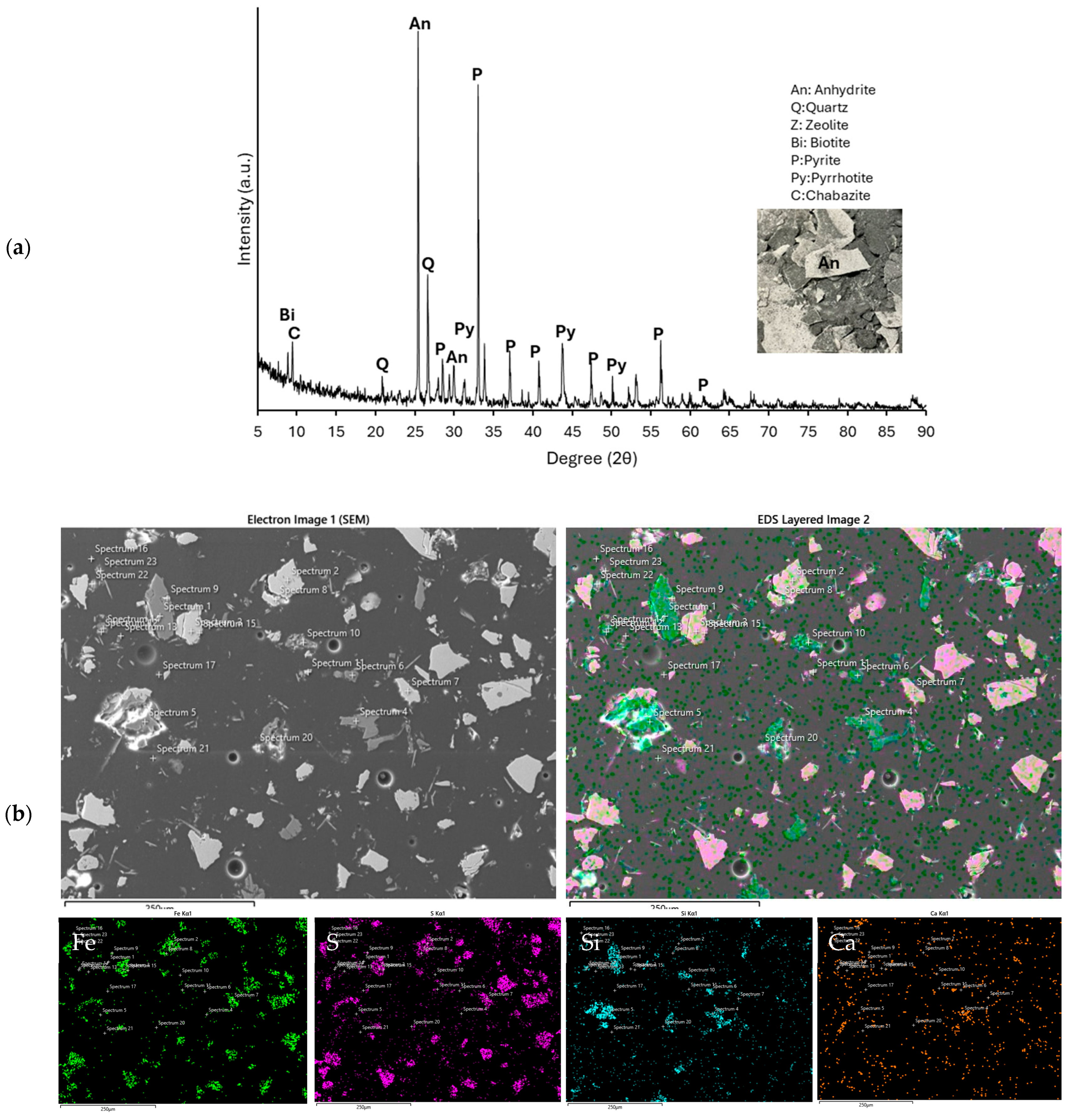
| Element | Al | Si | S | Ca | Fe | Cu | Zn | Mg | Ti | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | W% | W% | W% | W% | W% | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg |
| Feed | 1.5 | 4.2 | 13.1 | 1.4 | 23.8 | 3721 | 1438 | 4850 | 695.7 | 103 |
| Phases | Exposed Gold | Encapsulated in Sulfides | Encapsulated in Oxides | Encapsulated in Silicates | Total |
|---|---|---|---|---|---|
| Content g/t | 26.0 | 3.9 | 1.0 | 1.0 | 32.0 |
| Distribution% | 81.3 | 12.3 | 3.3 | 3.1 | 100.0 |
| No-Grinding | Grinding Time | |||
|---|---|---|---|---|
| 1 min | 3 min | 6 min | ||
| D10 | 16.5 | 2.36 | 1.60 | 1.37 |
| D50 | 107 | 15.6 | 9.16 | 9.02 |
| D90 | 302 | 61.3 | 44.2 | 38.6 |
| BET, m2/g | 0.70 | 1.64 | 3.22 | 5.65 |
| ELM. | Al | Si | S | Ca | Fe | K | Mg | Zn | As | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | |
| Feed | 3.0 | 8.0 | 12.2 | 1.34 | 22.9 | 4738 | 8109 | 1718 | 87.2 | 510.3 |
| PL leachate | 0.24 | 0.14 | 3.18 | 0.15 | 22.1 | 406.8 | 1485 | 207.3 | 65.1 | 12.6 |
| PL residue | 2.04 | 14.01 | 23.4 | 4.10 | 30.9 | 4064 | 6632 | 221.6 | 1631 | 1140 |
| TS residue | 2.10 | 13.40 | 22.8 | 2.00 | 31.9 | 3970 | 5670 | 223.8 | 1161 | 725 |
| Pretreatment Method | Key Conditions | Gold Leaching Efficiency (%) | Thiosulfate Consumption (kg t−1) | Main Observations |
|---|---|---|---|---|
| Ultra-fine grinding | 6 min grinding; 8× increase in BET surface area | 18.5 | 54.4 | Excessive grinding accelerated thiosulfate decomposition and Cu(I) passivation; severe decline in gold recovery. |
| Roasting | 750 °C, 2 h, air atmosphere 750 °C, 2 h, Inert atmosphere | 37.0 56.0 | 64.0 25.6 | Pyrite–pyrrhotite converted to hematite; no significant liberation of gold; limited benefit from thermal treatment. |
| Pressure Oxidative Leaching (POX) | 190 °C, 10 bar O2 | 40.0 | 32.0 | Dissolved ~33% of solids; high reagent and energy consumption and low gold recovery. |
| Oxidative ammoniacal pre-leaching | 0.4 M NH3 + 10 mM Cu2+, 42 h | 85.0 | 29.0 | Enhanced gold liberation, lower reagent use, and improved leaching kinetics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javanshir, S.; Sundqvist Öqvist, L.; Strandkvist, I.; Engström, F. Unlocking Refractory Gold: Synergistic Pretreatment Strategies for High-Efficiency Thiosulfate Leaching. Processes 2025, 13, 3760. https://doi.org/10.3390/pr13123760
Javanshir S, Sundqvist Öqvist L, Strandkvist I, Engström F. Unlocking Refractory Gold: Synergistic Pretreatment Strategies for High-Efficiency Thiosulfate Leaching. Processes. 2025; 13(12):3760. https://doi.org/10.3390/pr13123760
Chicago/Turabian StyleJavanshir, Sepideh, Lena Sundqvist Öqvist, Ida Strandkvist, and Fredrik Engström. 2025. "Unlocking Refractory Gold: Synergistic Pretreatment Strategies for High-Efficiency Thiosulfate Leaching" Processes 13, no. 12: 3760. https://doi.org/10.3390/pr13123760
APA StyleJavanshir, S., Sundqvist Öqvist, L., Strandkvist, I., & Engström, F. (2025). Unlocking Refractory Gold: Synergistic Pretreatment Strategies for High-Efficiency Thiosulfate Leaching. Processes, 13(12), 3760. https://doi.org/10.3390/pr13123760







