Artificial Intelligence in Rice Quality and Milling: Technologies, Applications, and Future Prospects
Abstract
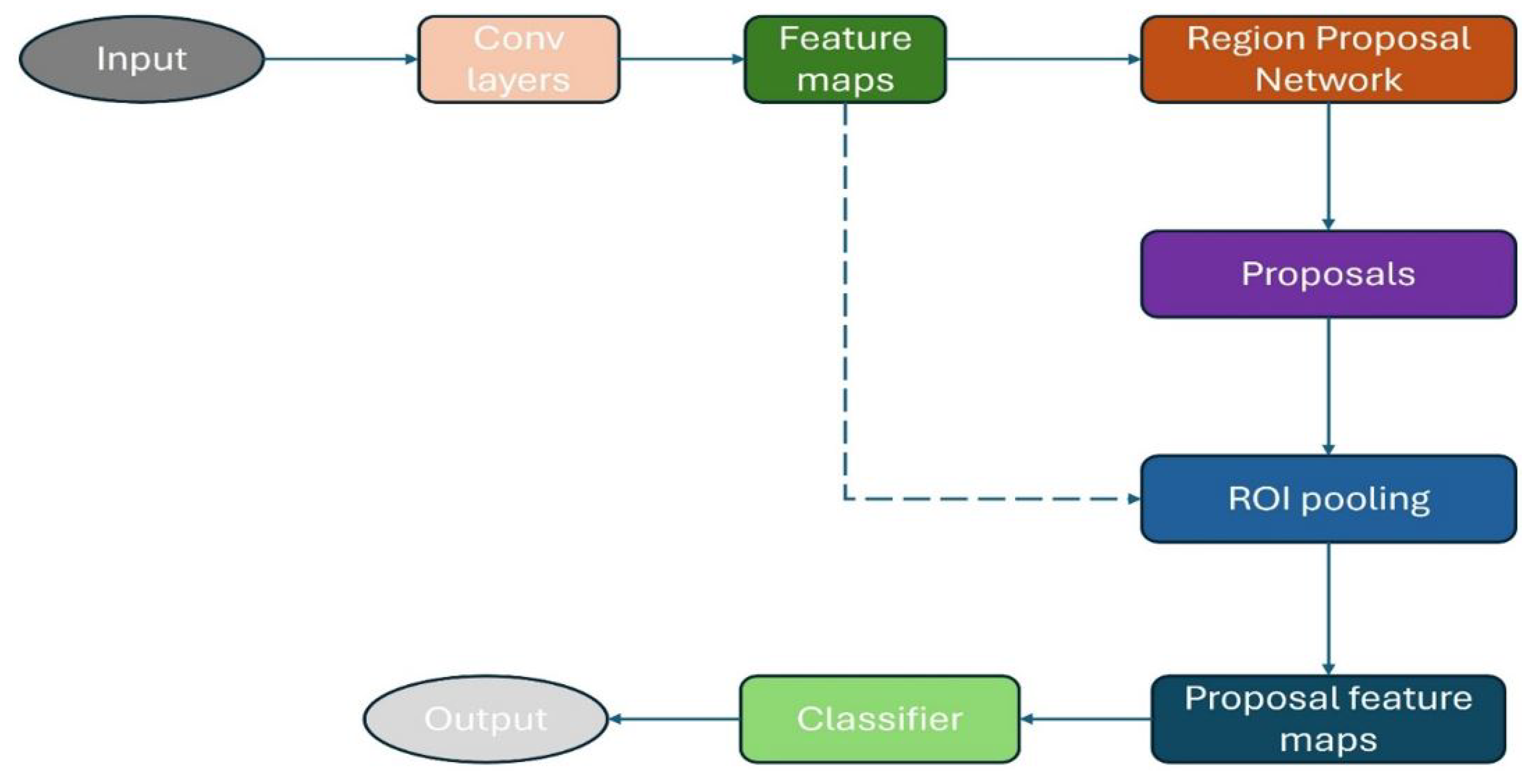
1. Introduction
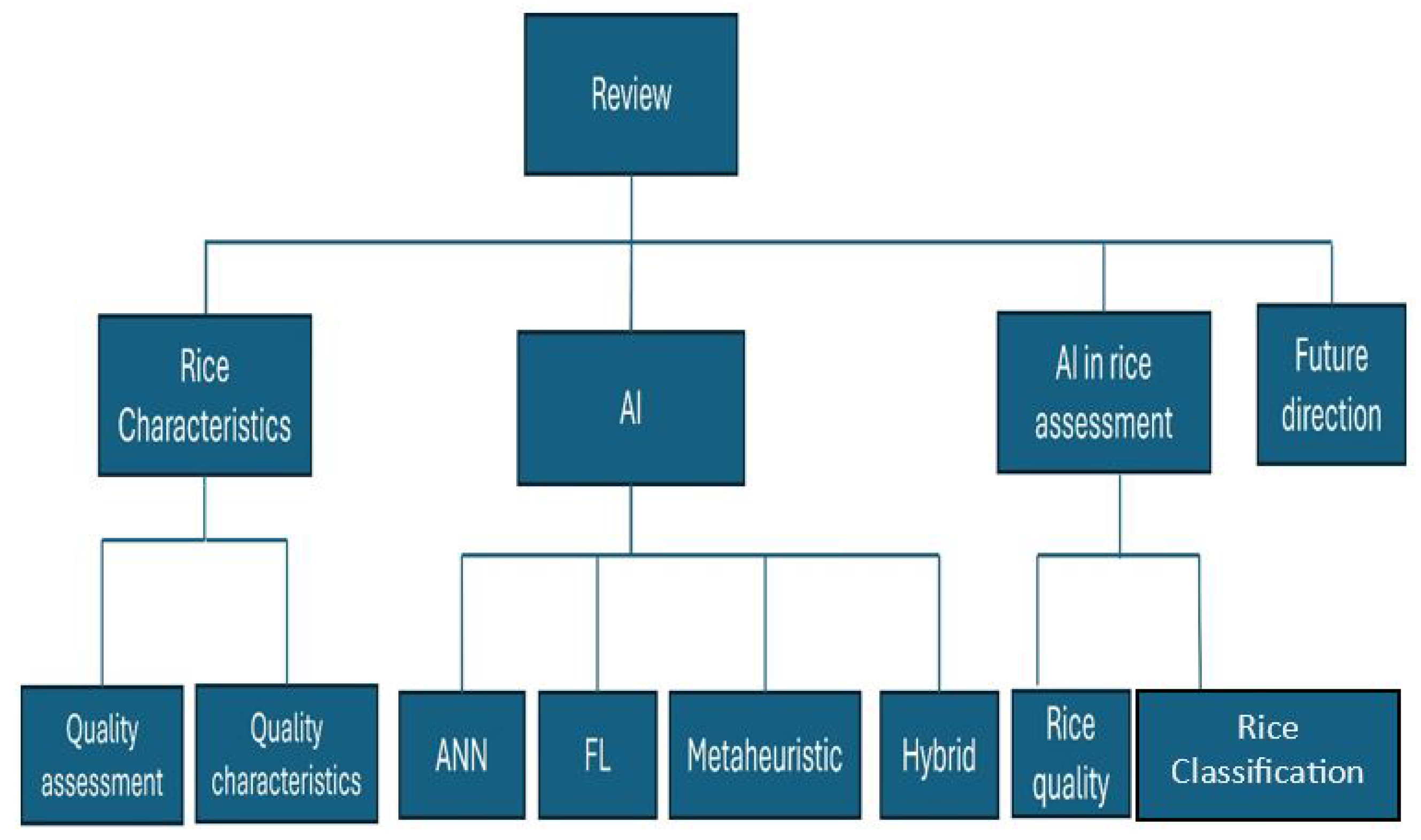
1.1. Aim and Scope of the Study
- (i)
- Which AI-based sensing and analytical technologies have been used to assess rice-grain quality and morphology?
- (ii)
- How do these technologies compare in terms of performance, data requirements, and practicality for real-time applications?
- (iii)
- What are the limitations, challenges, and future research directions in implementing AI-based quality assessment at industrial scale?
1.2. Major Contribution
2. Materials and Methods
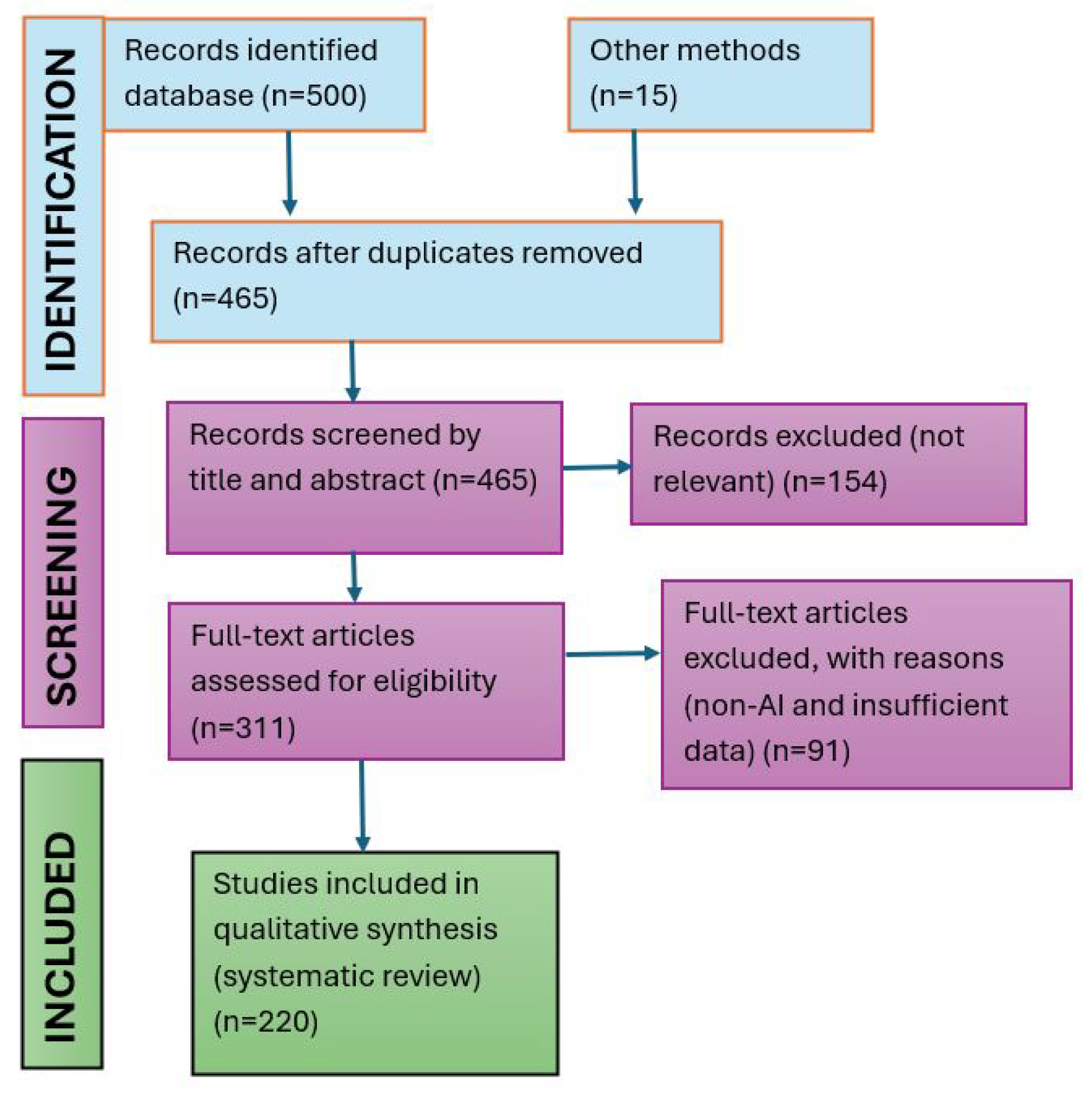
2.1. Systematic Review Statement
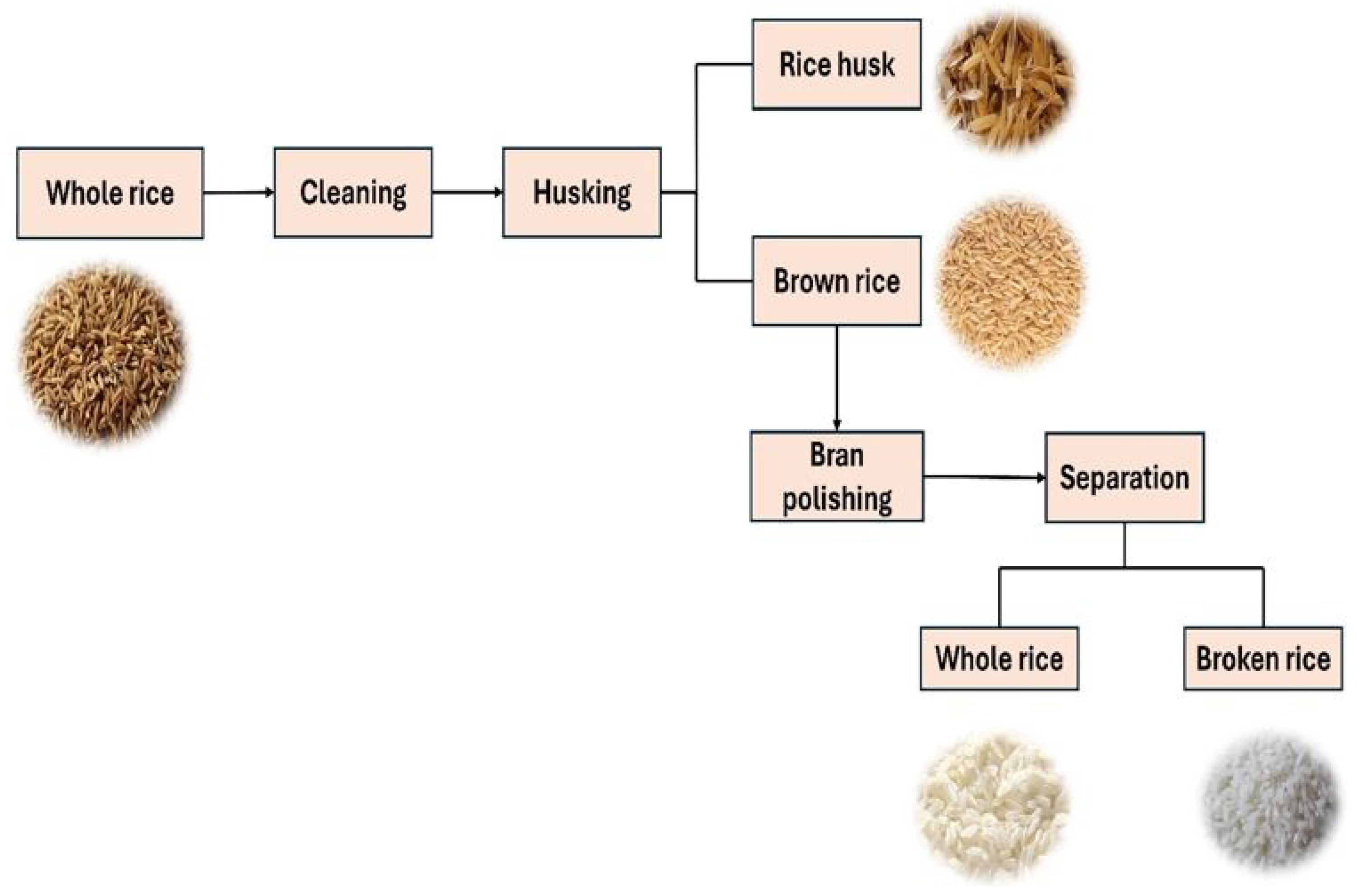
2.2. Agronomic Importance and Processing
2.3. Structure and Chemical Composition
2.4. Biotic and Abiotic Factors Affecting Grain Quality
2.5. Quality Assessment of Rice Grain Techniques
2.6. Traditional Assessment Methods in Rice Milling
Milling Quality
2.7. Physical Properties Assessed
Grain Color
2.8. Non-Destructive Techniques for Rice Quality Assessment
2.8.1. Machine Vision
2.8.2. Spectroscopy
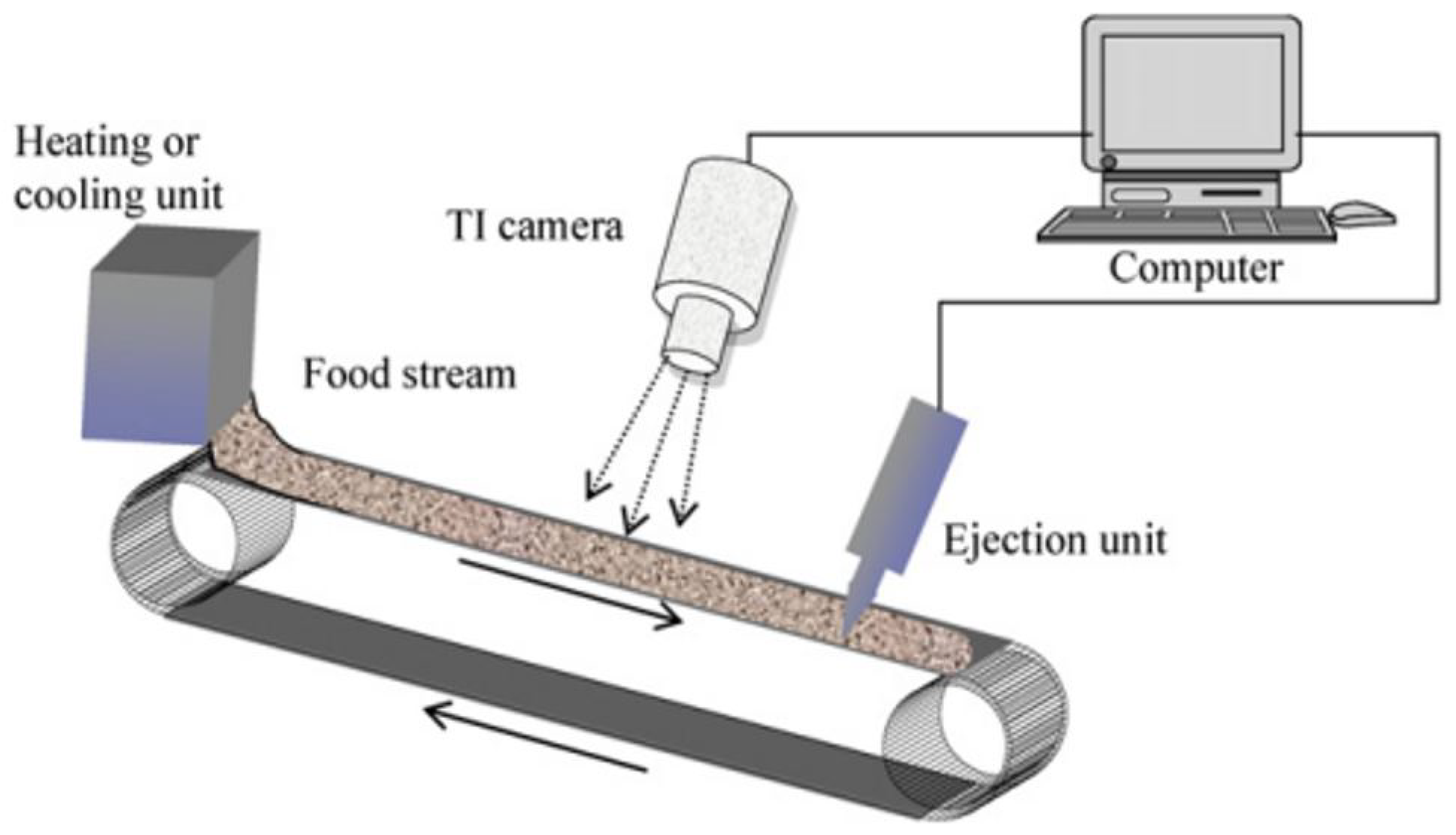
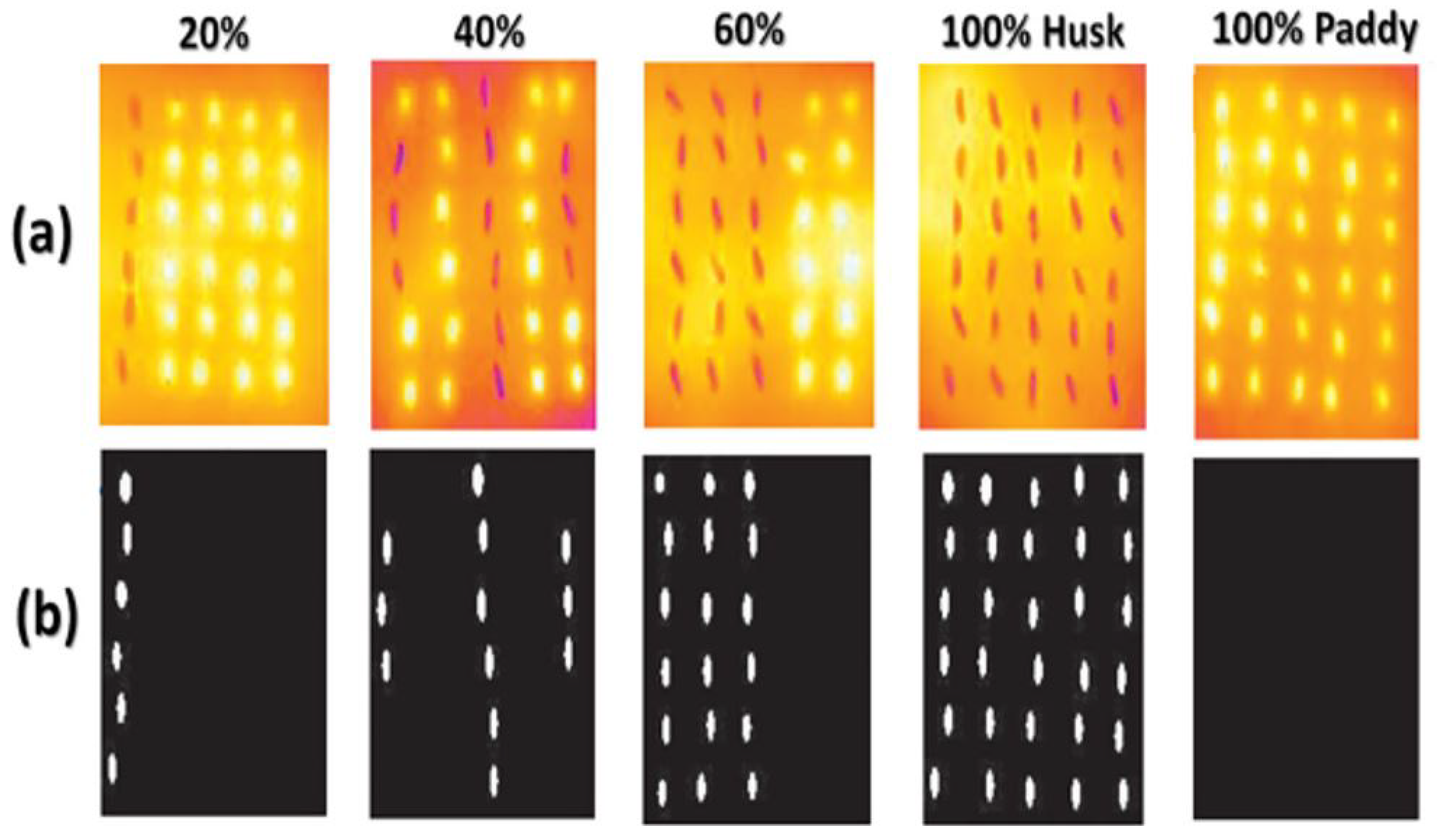
2.8.3. Thermal Images of Paddy Seeds
2.8.4. Hyperspectral Imaging
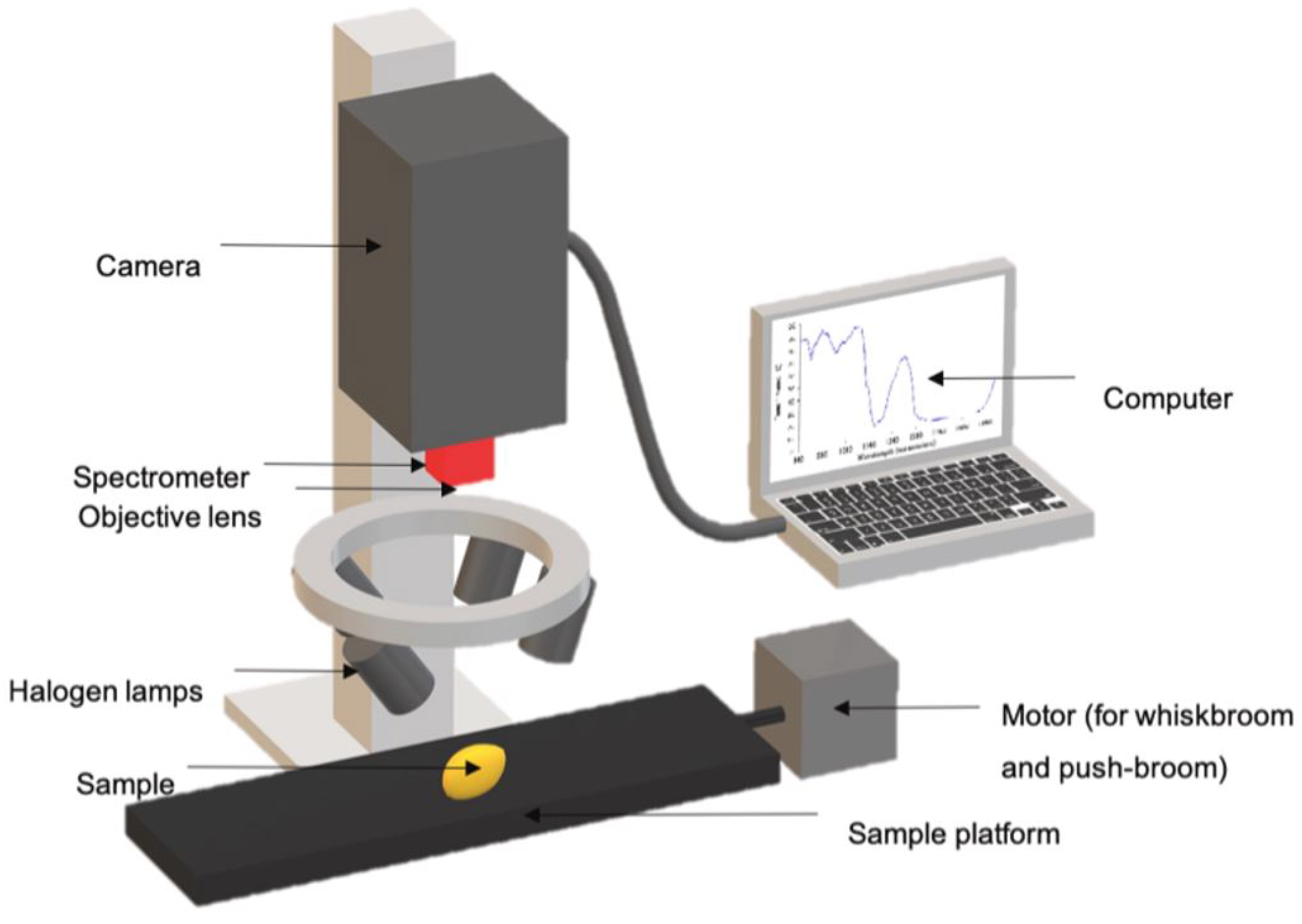
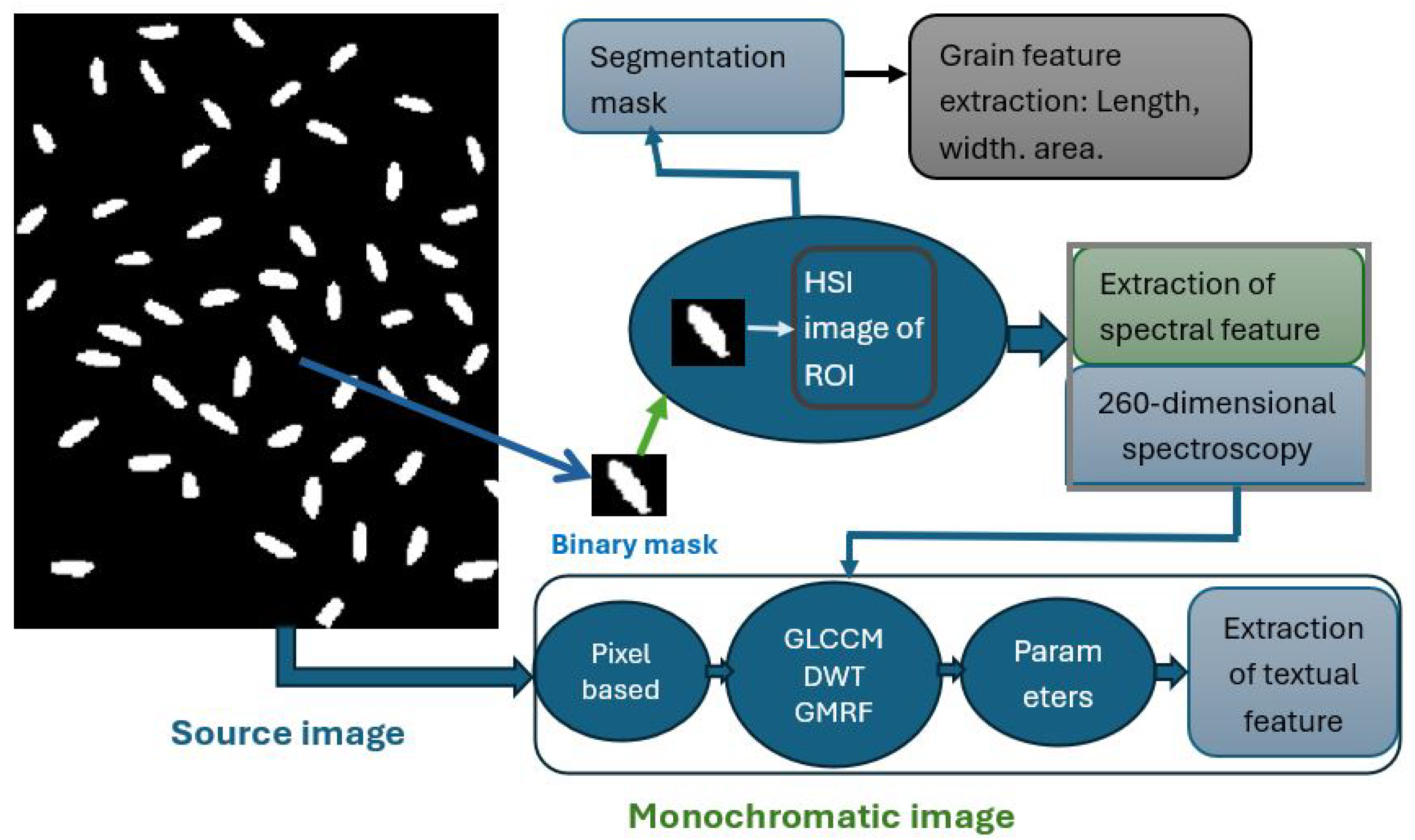
2.8.5. Artificial Intelligence
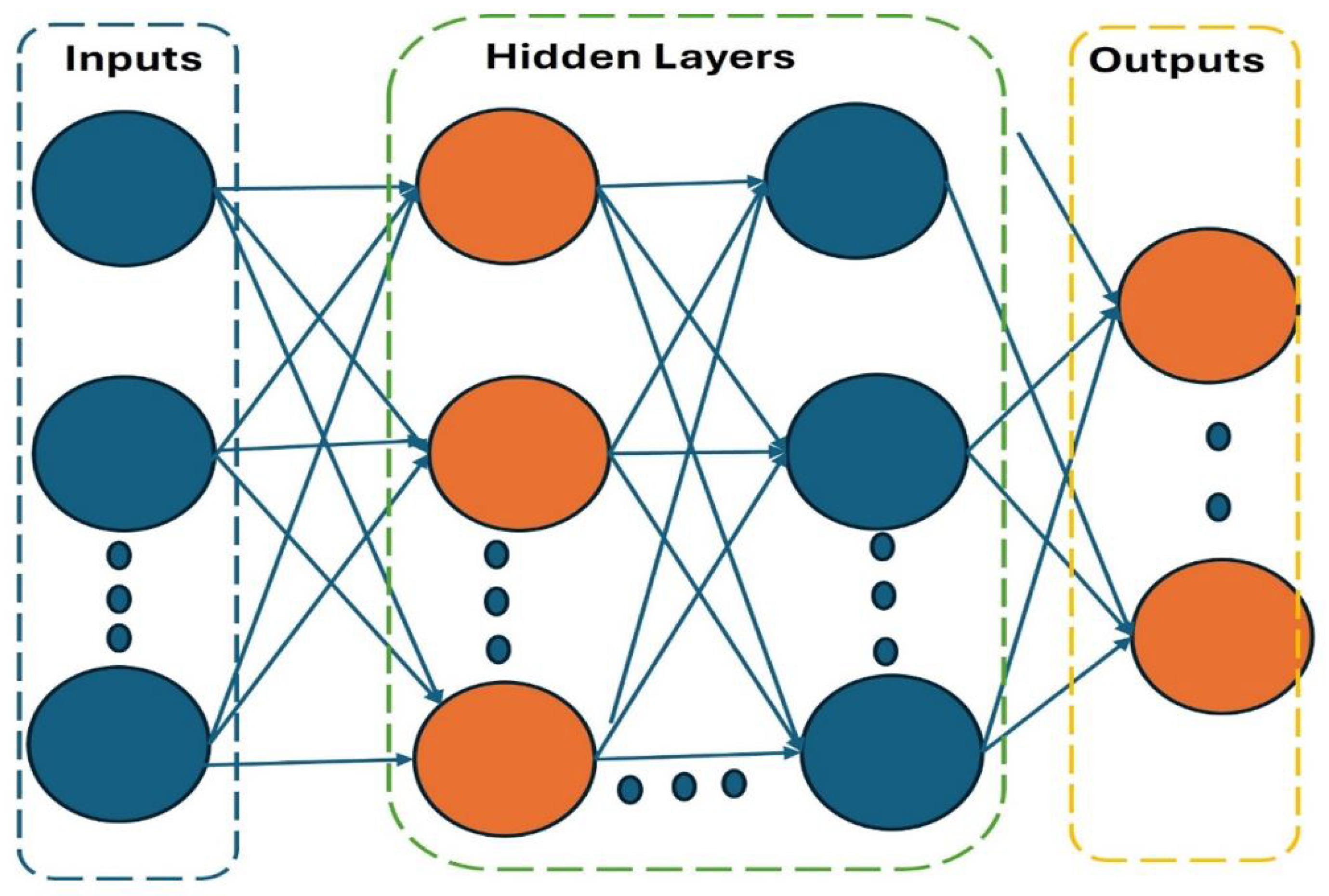
2.8.6. Machine Learning
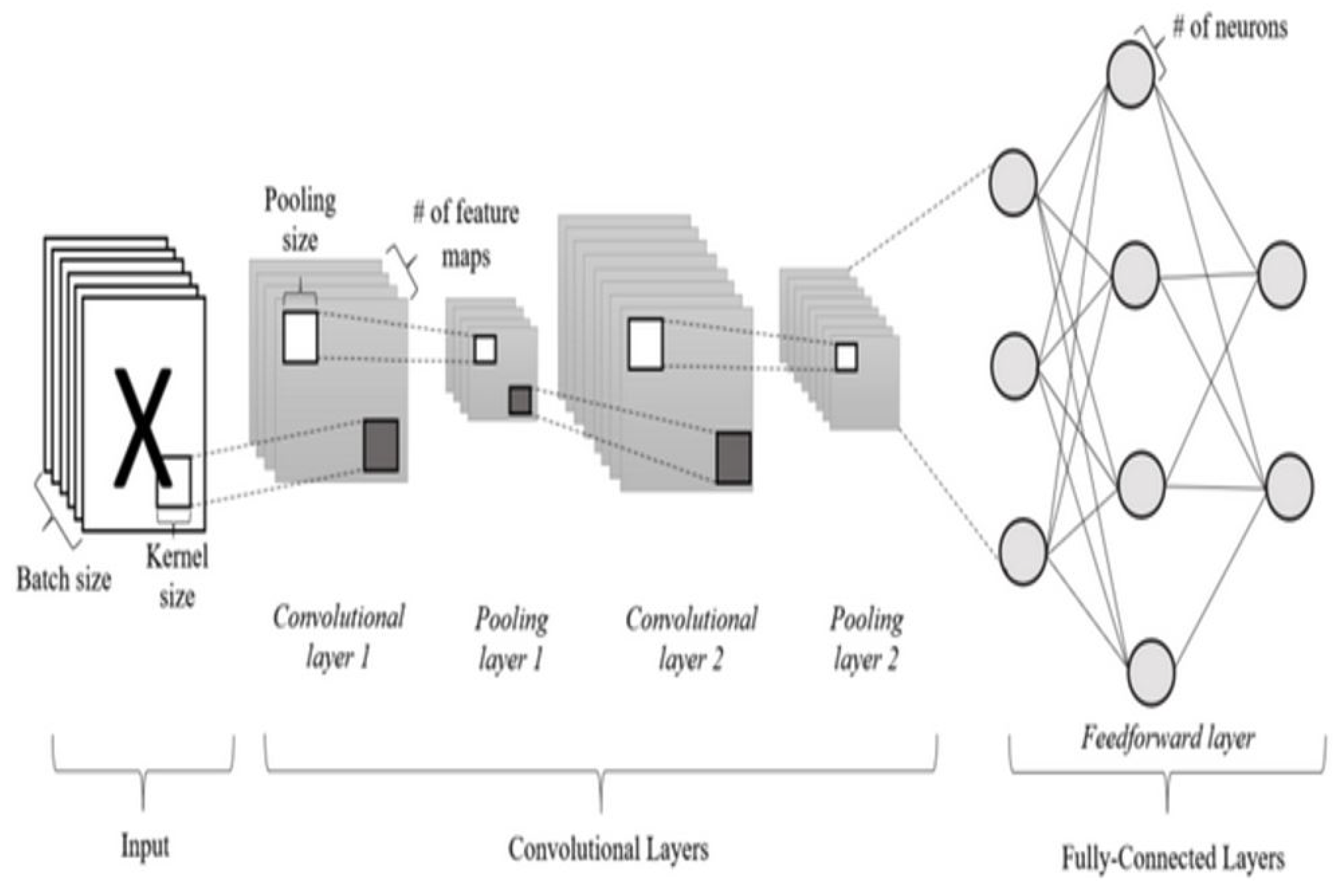
2.8.7. Deep Learning
| Prediction Model | Technique | Objective | Main Outcomes | Reference |
|---|---|---|---|---|
| AlexNet architecture | Computer vision | Rice grading classification | Accuracy 98%, sensitivity 97%, specificity 96% | [154] |
| DNN, CNN, ANN | Visible imaging | Classification of rice varieties | ANN 99%, DNN 99%, CNN 100% accuracy | [155] |
| PCA, PLS, ANN, LS-SVM, BPNN | Multispectral imaging | Classify rice cultivars and detect adulteration | BPNN reached 92% accuracy | [19] |
| MLP neural network | CCD cameras | Rice grading classification | 55.93% (Fajr), 84% (Tarom), 82% (Shiroodi); binary 86–95% | [156] |
| LR, LDA, k-NN, SVM | Machine vision | Rice seed classification | SVM: 90.61% (group1), 82% (group2), 83% combined; InceptionResNetV2: 95% | [157] |
| BPNN | Digital camera | Classifying paddy seeds | Colour–shape–texture model 95.2%, proposed method 97% | [158] |
| ANN | E-nose, NIR | Rice quality traits | Classification 98%; aroma prediction –0.98 | [71] |
| ANN, MLR | Biochemical composition | Rice quality prediction | MLR = 0.27–0.96; ANN = 0.98 (train), 0.88 (val), 0.90 overall | [20] |
| MLR | NIRS | Grain weight, amylose, brown rice weight | = 0.67–0.85 | [159] |
| PLSR, LS-SVM, ICA | IR | Rice quality prediction | = 0.89–0.98 | [160] |
| LeNet, GoogLeNet, RF, LR, SVM, ResNet | Hyperspectral | Variety identification | Accuracy 86% | [161] |
| PLSDA, SIMCA, RF, KNN, SVM, PCA | Hyperspectral | Variety identification | Accuracy 80–100% | [162] |
| ResNet, VGG, EfficientNet, MobileNet | Imaging | Rice grain classification | EfficientNet 99.67%; MobileNet fastest (2556s) | [163] |
| SVM | Imaging | Chalkiness | Indica 98.5%, Japonica 97.6% | [164] |
| PCANet | Hyperspectral imaging | Rice classification | Train 98%, predict 98.57% | [128] |
| BP-ANN | IR | Rice grades | = 95.45% | [165] |
| SVM, LR, RF, LeNet, GoogLeNet, ResNet | NIR | Variety identification | ResNet best: 86% | [161,165] |
| ANN, SVM, BN | Computer vision | Milled rice grain classification | ANN 98%, SVM 98%, DT 97%, BN 96% | [166] |
| ANFIS, SVM, KNN | Imaging | Grading of Basmati rice | Accuracy > 98% (broken/whole) | [167] |
| SVM + GA, KNN | Geometric properties | Grain quality analysis | Accuracy 92%→93%; SVM best; k-NN 88% | [168] |
| YOLOv7 | Video | Rice seed counting | mAP 99%; tracking 100% accuracy, 83% precision | [161] |
| MSIA, CNN | Hyperspectral | Rice quality | Accuracy, precision, recall, F1 = 99% | [169] |
| CNN | Imaging | Early disease detection | Accuracy 97.70% | [170] |
| YOLOv5, RCNN, RetinaNet, SSD, Cascade RCNN | ED imaging | Yield traits | YOLOv5: Precision 98.94%, Recall 97.91% (filled); 90.96/94.94% (unfilled) | [171] |
| PCA-KNN, SPA-KNN, PCA-LS-SVM, SPA-LS-SVM | Raman spectroscopy | Classification | SPA-LS-SVM: 94% | [172] |
| Correlation analysis | VIS-NIR | Chalkiness index | = 0.89 | [173] |
| SVM | NIR imaging | Colored rice inspection | Broken 99%, chalkiness 96.3%, damaged 93% | [174] |
| AlexNet | NI-myRIO vision | Variety classification | Accuracy 98%, sensitivity 97%, specificity 96.4% | [154] |
| CNN models | Imaging | Damage classification | EfficientNet-B0 up to 100% accuracy | [175] |
| Fuzzy logic | Computer vision | Whitening performance | Accuracy 89.2% | [166] |
| Mask R-CNN | Imaging | Impurity and broken rate | Accuracy +6.13% (broken), +9.19% (impurities) | [159] |
| CNN | E-nose hyperspectral | Rice quality difference | Accuracy 98.07% | [176] |
| Logistic regression | Computer vision | Sorting broken/chalky grains | Correlation | [177] |
| ResNet34, ResNet50 | Imaging | Classification and quality | ResNet50 > 99.85% (six varieties) | [178] |
| YOLOX | Imaging | Rice disease identification | mAP 95.58% | [179] |
| YOLOv5s-CBAM-DMLHea | Imaging | Weedy rice identification | mAP@0.5 = 98.9%; inference 4 ms; 28% fewer computations | [180] |
2.8.8. CNN
2.8.9. Instance Segmentation
2.8.10. You Only Look Once (YOLO)
2.8.11. Other Learning Methods
3. Implementation and Limitations of Current AI Techniques in Rice Quality Assessment
4. Conclusions
5. Future Works
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. PRISMA Checklist
References
- Dorling, D. World population prospects at the UN: Our numbers are not our problem? In The Struggle for Social Sustainability; Policy Press: Bristol, UK, 2021; pp. 129–154. [Google Scholar]
- Pomeroy, J.; Jose, D.; Tyler, A.; Bloxham, P.; Culling, J. The Future of Food: Can We Meet the Needs of 9bn People? Free to View Report; HSBC Global Research: London, UK, 2023. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Asma, J.; Subrahmanyam, D.; Krishnaveni, D. The global lifeline: A staple crop sustaining two thirds of the world’s population. Agric. Arch. 2023, 2, 15–18. [Google Scholar] [CrossRef]
- Abdo, A.I.; Tian, M.; Shi, Z.; Sun, D.; Abdel-Fattah, M.K.; Zhang, J.; Wei, H.; Abdeen, M.A. Carbon footprint of global rice production and consumption. J. Clean. Prod. 2024, 474, 143560. [Google Scholar] [CrossRef]
- Zafar, S.; Jianlong, X. Recent advances to enhance nutritional quality of rice. Rice Sci. 2023, 30, 523–536. [Google Scholar] [CrossRef]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Shahbandeh, M. Total Global Rice Consumption 2008/09–2024/25. Statista. Available online: https://www.statista.com/statistics/255977/total-global-rice-consumption/ (accessed on 10 November 2025).
- Bairagi, S.; Demont, M.; Custodio, M.C.; Ynion, J. What drives consumer demand for rice fragrance? Evidence from South and Southeast Asia. Br. Food J. 2020, 122, 3473–3498. [Google Scholar] [CrossRef]
- Mané, I.; Bassama, J.; Ndong, M.; Mestres, C.; Diedhiou, P.M.; Fliedel, G. Deciphering urban consumer requirements for rice quality gives insights for driving the future acceptability of local rice in Africa: Case study in the city of Saint-Louis in senegal. Food Sci. Nutr. 2021, 9, 1614–1624. [Google Scholar] [CrossRef]
- Wahyudi, A.; Kuwornu, J.K.M.; Gunawan, E.; Datta, A.; Nguyen, L.T. Factors influencing the frequency of consumers’ purchases of locally-produced rice in Indonesia: A Poisson regression analysis. Agriculture 2019, 9, 117. [Google Scholar] [CrossRef]
- Li, D.; Shen, M.; Li, D.; Yu, X. Green apple recognition method based on the combination of texture and shape features. In Proceedings of the IEEE International Conference on Mechatronics and Automation (ICMA), Takamatsu, Japan, 6–9 August 2017. [Google Scholar]
- Mao, S.; Wang, B.; Tang, Y.; Qian, F. Opportunities and Challenges of Artificial Intelligence for Green Manufacturing in the Process Industry. Engineering 2019, 5, 995–1002. [Google Scholar] [CrossRef]
- Mavani, N.R.; Ali, J.M.; Othman, S.; Hussain, M.A.; Hashim, H.; Rahman, N.A. Application of Artificial Intelligence in Food Industry—A Guideline. Food Eng. Rev. 2022, 14, 134–175. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Marandi, R.; Khanzadeh, M.; Tian, W.; Smith, B.; Bian, L. From in-situ monitoring toward high-throughput process control: Cost-driven decision-making framework for laser-based additive manufacturing. J. Manuf. Syst. 2019, 51, 29–41. [Google Scholar] [CrossRef]
- Aznan, A.; Viejo, C.G.; Pang, A.; Fuentes, S. Review of technology advances to assess rice quality traits and consumer perception. Food Res. Int. 2023, 172, 113105. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Nishad, S.; Biswas, D.; Roy, S. A comprehensive review on artificial intelligence assisted technologies in food industry. Food Biosci. 2023, 56, 103231. [Google Scholar] [CrossRef]
- Addanki, M.; Patra, P.; Kandra, P. Recent advances and applications of artificial intelligence and related technologies in the food industry. Appl. Food Res. 2022, 2, 100126. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Liu, C.; Zheng, L. Nondestructive Detection of Authenticity of Thai Jasmine Rice Using Multispectral Imaging. J. Food Qual. 2021, 2021, 6642220. [Google Scholar] [CrossRef]
- Sampaio, P.S.; Almeida, A.S.; Brites, C.M. Use of artificial neural network model for rice quality prediction based on grain physical parameters. Foods 2021, 10, 3016. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, H. Effects of quality characteristics on milled rice produced under different milling conditions. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 643–649. [Google Scholar] [CrossRef]
- Qiu, X.; Pang, Y.; Yuan, Z.; Xing, D.; Xu, J.; Dingkuhn, M.; Li, Z.; Ye, G. Genome-wide association study of grain appearance and milling quality in a worldwide collection of Indica rice germplasm. PLoS ONE 2015, 10, e0145577. [Google Scholar] [CrossRef]
- Yadav, B.K.; Jindal, V.K. Changes in head rice yield and whiteness during milling of rough rice (Oryza sativa L.). J. Food Eng. 2008, 86, 113–121. [Google Scholar] [CrossRef]
- Espinel, R.; Herrera-Franco, G.; Rivadeneira García, J.L.; Escandón-Panchana, P. Artificial intelligence in agricultural mapping: A review. Agriculture 2024, 14, 1071. [Google Scholar] [CrossRef]
- He, Y.; Fan, B.; Sun, L.; Fan, X.; Zhang, J.; Li, Y.; Suo, X. Rapid appearance quality of rice based on machine vision and convolutional neural network research on automatic detection system. Front. Plant Sci. 2023, 14, 1190591. [Google Scholar] [CrossRef]
- Zhou, J.; Zeng, S.; Chen, Y.; Kang, Z.; Li, H.; Sheng, Z. A method of polished rice image segmentation based on YO-LACTS for quality detection. Agriculture 2023, 13, 182. [Google Scholar] [CrossRef]
- Son, S.; Kim, D.; Choi, M.C.; Lee, J.; Kim, B.; Choi, C.M.; Kim, S. Weight interpretation of artificial neural network model for analysis of rice (Oryza sativa L.) with near-infrared spectroscopy. Food Chem. X 2022, 15, 100430. [Google Scholar] [CrossRef] [PubMed]
- Ilo, B.; Rippon, D.; Singh, Y.; Shenfield, A.; Zhang, H. Real-Time Rice Milling Morphology Detection Using Hybrid Framework of YOLOv8 Instance Segmentation and Oriented Bounding Boxes. Electronics 2025, 14, 3691. [Google Scholar] [CrossRef]
- Atwell, B.J.; Wang, H.; Scafaro, A.P. Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sci. 2014, 215, 48–58. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Morishima, H.; Kadowaki, K. Diversity in the Oryza genus. Curr. Opin. Plant Biol. 2003, 6, 139–146. [Google Scholar] [CrossRef]
- Wei, X.; Huang, X. Origin, taxonomy, and phylogenetics of rice. In Rice: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–29. [Google Scholar]
- Zhu, D.; Zheng, X.; Yu, J.; Chen, M.; Li, M.; Shao, Y. Effects of Starch Molecular Structure and Physicochemical Properties on Eating Quality of Indica Rice with Similar Apparent Amylose and Protein Contents. Foods 2023, 12, 3535. [Google Scholar] [CrossRef] [PubMed]
- Kowsalya, P.; Sharanyakanth, P.S.; Mahendran, R. Traditional rice varieties: A comprehensive review on its nutritional, medicinal, therapeutic and health benefit potential. J. Food Compos. Anal. 2022, 114, 104742. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Luo, X.; Zeng, X. Pasting, cooking, and digestible properties of Japonica rice with different amylose contents. Int. J. Food Prop. 2022, 25, 936–947. [Google Scholar] [CrossRef]
- Śliwińska-Bartel, M.; Burns, D.T.; Elliott, C. Rice fraud a global problem: A review of analytical tools to detect species, country of origin and adulterations. Trends Food Sci. Technol. 2021, 116, 36–46. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Ferrero, A. Meeting the challenges of global rice production. Paddy Water Environ. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Singh, P.K.; Venkatesan, K.; Swarnam, T.P. Rice genetic resources in tropical islands. In Biodiversity and Climate Change Adaptation in Tropical Islands; Elsevier: Amsterdam, The Netherlands, 2018; pp. 355–384. [Google Scholar]
- Mwakyusa, L.; Dixit, S.; Herzog, M.; Heredia, M.C.; Madege, R.R.; Kilasi, N.L. Flood-tolerant rice for enhanced production and livelihood of smallholder farmers of Africa. Front. Sustain. Food Syst. 2023, 7, 1244460. [Google Scholar] [CrossRef]
- Yuan, S.; Linquist, B.A.; Wilson, L.T.; Cassman, K.G.; Stuart, A.M.; Pede, V.; Miro, B.; Saito, K.; Agustiani, N.; Aristya, V.E. Sustainable intensification for a larger global rice bowl. Nat. Commun. 2021, 12, 7163. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Adviento-Borbe, M.A.; Hill, J.E.; Six, J.; van Kessel, C.; Linquist, B.A. Yield-Scaled Global Warming Potential of Annual Nitrous Oxide and Methane Emissions from Continuously Flooded Rice in Response to Nitrogen Input. Agric. Ecosyst. Environ. 2013, 177, 10–20. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Shen, N.; Tan, J.; Wang, W.; Xue, W.; Wang, Y.; Huang, L.; Yan, G.; Song, Y.; Li, L. Long-term changes of methane emissions from rice cultivation during 2000–2060 in China: Trends, driving factors, predictions and policy implications. Environ. Int. 2024, 191, 108958. [Google Scholar] [CrossRef]
- Eyarkai Nambi, V.; Manickavasagan, A.; Shahir, S. Rice milling technology to produce brown rice. In Brown Rice; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 3–21. [Google Scholar]
- Carcea, M.; Turfani, V.; Narducci, V.; Melloni, S.; Galli, V.; Tullio, V. Stone milling versus roller milling in soft wheat: Influence on products composition. Foods 2019, 9, 3. [Google Scholar] [CrossRef]
- Roy, P.; Orikasa, T.; Okadome, H.; Nakamura, N.; Shiina, T. Processing conditions, rice properties, health and environment. Int. J. Environ. Res. Public Health 2011, 8, 1957–1976. [Google Scholar] [CrossRef]
- Xiao, Y.; Jia, F.; Meng, X.; Han, Y. Breakpoint Planning Method for Rice Multibreak Milling. Foods 2023, 12, 1864. [Google Scholar] [CrossRef]
- Liu, K.; Cao, X.; Bai, Q.; Wen, H.; Gu, Z. Relationships between physical properties of brown rice and degree of milling and loss of selenium. J. Food Eng. 2009, 94, 69–74. [Google Scholar] [CrossRef]
- Muchlisyiyah, J.; Shamsudin, R.; Kadir Basha, R.; Shukri, R.; How, S.; Niranjan, K.; Onwude, D. Parboiled rice processing method, rice quality, health benefits, environment, and future perspectives: A review. Agriculture 2023, 13, 1390. [Google Scholar] [CrossRef]
- Chen, F.; Lu, Y.; Pan, L.; Fan, X.; Li, Q.; Huang, L.; Zhao, D.; Zhang, C.; Liu, Q. The underlying physicochemical properties and starch structures of indica rice grains with translucent endosperms under low-moisture conditions. Foods 2022, 11, 1378. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Man, H.C. Rice for food security: Revisiting its production, diversity, rice milling process and nutrient content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Cornejo-Ramírez, Y.I.; Martínez-Cruz, O.; Del Toro-Sánchez, C.L.; Wong-Corral, F.J.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J. Características estructurales de almidones y sus propiedades funcionales. CYTA J. Food 2018, 16, 1003–1017. [Google Scholar] [CrossRef]
- Zhang, H.; Jang, S.G.; Lar, S.M.; Lee, A.R.; Cao, F.Y.; Seo, J.; Kwon, S.W. Genome-wide identification and genetic variations of the starch synthase gene family in rice. Plants 2021, 10, 1154. [Google Scholar] [CrossRef]
- Siregar, S.; Nurhikmat, A.; Amdani, R.Z.; Hatmi, R.U.; Kobarsih, M.; Kusumaningrum, A.; Karim, M.A.; Dameswari, A.H.; Siswanto, N.; Siswoprayogi, S. Estimation of proximate composition in rice using ATR-FTIR spectroscopy and Chemometrics. ACS Omega 2024, 9, 32760–32768. [Google Scholar] [CrossRef]
- Vici, G.; Perinelli, D.R.; Camilletti, D.; Carotenuto, F.; Belli, L.; Polzonetti, V. Nutritional properties of rice varieties commonly consumed in Italy and applicability in gluten free diet. Foods 2021, 10, 1375. [Google Scholar] [CrossRef]
- Manzoor, A.; Pandey, V.K.; Dar, A.H.; Fayaz, U.; Dash, K.K.; Shams, R.; Ahmad, S.; Bashir, I.; Fayaz, J.; Singh, P. Rice bran: Nutritional, phytochemical, and pharmacological profile and its contribution to human health promotion. Food Chem. Adv. 2023, 2, 100296. [Google Scholar] [CrossRef]
- Müller, A.; Nunes, M.T.; Maldaner, V.; Coradi, P.C.; de Moraes, R.S.; Martens, S.; Leal, A.F.; Pereira, V.F.; Marin, C.K. Rice drying, storage and processing: Effects of post-harvest operations on grain quality. Rice Sci. 2022, 29, 16–30. [Google Scholar] [CrossRef]
- Jayaprakash, G.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application. Polymers 2022, 14, 3003. [Google Scholar] [CrossRef]
- Samaranayake, M.D.W.; Abeysekera, W.K.S.M.; Hewajulige, I.G.N.; Somasiri, H.P.P.S.; Mahanama, K.R.R.; Senanayake, D.M.J.B.; Premakumara, G.A.S. Fatty acid profiles of selected traditional and new improved rice varieties of Sri Lanka. J. Food Compos. Anal. 2022, 112, 104686. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Q.W.; Kim, H.; Kim, H.J. Changes in the chemical, physical, and sensory properties of rice according to its germination rate. Food Chem. 2022, 388, 133060. [Google Scholar] [CrossRef]
- Maftoon Azad, N.; Alizadeh, A.; Kazemiyan Jahromi, A.; Ehsan Torkamani, A.; Baghaei, S.; Mirazimi Abarghuei, F. Effects of Thermodynamic Properties of Rice and Ambient Conditions on Moisture Migration during Storage at Naturally Ventilated Warehouses. Arab. J. Chem. 2023, 16, 104761. [Google Scholar] [CrossRef]
- Peng, B.; He, L.; Tan, J.; Zheng, L.; Zhang, J.; Qiao, Q.; Wang, Y.; Gao, Y.; Tian, X.; Liu, Z. Effects of Rice Aging on Its Main Nutrients and Quality Characters; Canadian Center of Science and Education: Toronto, ON, Canada, 2019. [Google Scholar]
- Rodrigues, D.M.; Coradi, P.C.; Teodoro, L.P.R.; Teodoro, P.E.; dos S. Moraes, R.; Leal, M.M. Monitoring and predicting corn grain quality on the transport and post-harvest operations in storage units using sensors and machine learning models. Sci. Rep. 2024, 14, 6232. [Google Scholar] [CrossRef]
- de Moraes, R.S.; Coradi, P.C.; Nunes, M.T.; Leal, M.M.; Müller, E.I.; Teodoro, P.E.; Flores, E.M.M. Thick layer drying and storage of rice grain cultivars in silo-dryer-aerator: Quality evaluation at low drying temperature. Heliyon 2023, 9, e17962. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, N.; Zhu, W.; Wang, D.; Jiang, M.; Qu, C.; Li, Y.; Zou, Z. A Heat and Mass Transfer Model of Peanut Convective Drying Based on a Two-Component Structure. Foods 2023, 12, 1823. [Google Scholar] [CrossRef]
- Ilias, I.A.; Wagiran, A.; Azizan, K.A.; Ismail, I.; Samad, A.F.A. Irreversibility of the cell wall modification acts as a limiting factor in desiccation tolerance of Oryza sativa ssp. Indica cv MR303. Plant Stress 2024, 12, 100463. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, N.; Ding, J.; Zhang, Y.; Liu, X.; Zhang, H. Effect of germination temperature on hierarchical structures of starch from brown rice and their relation to pasting properties. Int. J. Biol. Macromol. 2020, 147, 965–972. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, L.Á.; Abhilasha, A.; Singh, J.; Elias, M.C.; Colussi, R. Rice Germination and Its Impact on Technological and Nutritional Properties: A Review. Rice Sci. 2022, 29, 201–215. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Boue, S.M.; Goufo, P. Health-promoting germinated rice and value-added foods: A comprehensive and systematic review of germination effects on brown rice. Crit. Rev. Food Sci. Nutr. 2023, 63, 11570–11603. [Google Scholar] [CrossRef]
- Plasek, B.; Lakner, Z.; Temesi, Á. Factors That Influence the Perceived Healthiness of Food—Review. Nutrients 2020, 12, 1881. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wai, P.P.; Koirala, P.; Bromage, S.; Nirmal, N.P.; Pandiselvam, R.; Nor-Khaizura, M.A.R.; Mehta, N.K. Food product quality, environmental and personal characteristics affecting consumer perception toward food. Front. Sustain. Food Syst. 2023, 7, 1222760. [Google Scholar] [CrossRef]
- Aznan, A.; Gonzalez Viejo, C.; Pang, A.; Fuentes, S. Rapid assessment of rice quality traits using low-cost digital technologies. Foods 2022, 11, 1181. [Google Scholar] [CrossRef]
- Dixon, W.R.; Morales-Contreras, B.E.; Kongchum, M.; Xu, Z.; Harrell, D.; Moskowitz, H.R.; Wicker, L. Aroma, Quality, and Consumer Mindsets for Shelf-Stable Rice Thermally Processed by Reciprocal Agitation. Foods 2020, 9, 1559. [Google Scholar] [CrossRef]
- Maleki, C.; Oliver, P.; Lewin, S.; Liem, G.; Keast, R. Preference Mapping of Different Water-to-Rice Ratios in Cooked Aromatic White Jasmine Rice. J. Food Sci. 2020, 85, 1576–1585. [Google Scholar] [CrossRef]
- Sultana, S.; Faruque, M.; Islam, M.R. Rice grain quality parameters and determination tools: A review on the current developments and future prospects. Int. J. Food Prop. 2022, 25, 1063–1078. [Google Scholar] [CrossRef]
- Qadir, N.; Wani, I.A. Physical properties of four rice cultivars grown in Indian temperate region. Appl. Food Res. 2023, 3, 100280. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, F.; Ren, J.; Jiang, F.; Zhao, N.; Du, S.k. Structural analysis, nutritional evaluation, and flavor characterization of parched rice made from proso millet. Food Chem. X 2023, 19, 100784. [Google Scholar] [CrossRef]
- Yi, Z.; Zhuohua, Z.; Likui, F.; Yunjun, Z.; Geng, Z. Impact of milling on the sensory quality and flavor profile of an aromatic rice variety produced in Chongqing. J. Cereal Sci. 2024, 116, 103844. [Google Scholar] [CrossRef]
- Ali, F.; Jighly, A.; Joukhadar, R.; Niazi, N.K.; Al-Misned, F. Current status and future prospects of head rice yield. Agriculture 2023, 13, 705. [Google Scholar] [CrossRef]
- Prom-U-Thai, C.; Rerkasem, B. Rice quality improvement: A review. Agron. Sustain. Dev. 2020, 40, 64. [Google Scholar] [CrossRef]
- Hebishy, E.; Buchanan, D.; Rice, J.; Oyeyinka, S.A. Variation in amylose content in three rice variants predominantly influences the properties of sushi rice. J. Food Meas. Charact. 2024, 18, 4545–4557. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Z.; Zhang, Y.; Li, H.; Pei, H.; Yang, H. Characteristics of Damage to Brown Rice Kernels under Single and Continuous Mechanical Compression Conditions. Foods 2024, 13, 1069. [Google Scholar] [CrossRef]
- Venkatesan, S.; Udhaya Nandhini, D.; Senthilraja, K.; Prabha, B.; Jidhu Vaishnavi, S.; Eevera, T.; Somasundaram, E.; Balakrishnan, N.; Raveendran, M.; Geethalakshmi, V. Traditional cultivars influence on physical and engineering properties of rice from the cauvery deltaic region of Tamil Nadu. Appl. Sci. 2023, 13, 5705. [Google Scholar] [CrossRef]
- Singh, N.; Singh, H.; Kaur, K.; Singh Bakshi, M. Relationship between the degree of milling, ash distribution pattern and conductivity in brown rice. Food Chem. 2000, 69, 147–151. [Google Scholar] [CrossRef]
- Dhankhar, P.; Hissar, T. Rice Milling. IOSR J. Eng. 2014, 4, 34–42. [Google Scholar] [CrossRef]
- Cruz, N.D.; Khush, G. Rice grain quality evaluation procedures. Aromat. Rices 2000, 3, 15–28. [Google Scholar]
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 2014, 9, e85106. [Google Scholar] [CrossRef] [PubMed]
- Thissa Marasingha, M.M.M.; Samarakoon, E.R.J.; Senarathne, B.M.K.; Samarasinghe, H.G.A.S. Comparative Assessment of Grain Quality Characteristics and Cooking Parameters of White Rice (Oryza sativa Indica and Oryza sativa Japonica) Varieties Cultivated in Sri Lanka. Eng. Proc. 2024, 67, 58. [Google Scholar]
- Ren, D.; Ding, C.; Qian, Q. Molecular bases of rice grain size and quality for optimized productivity. Sci. Bull. 2023, 68, 314–350. [Google Scholar] [CrossRef]
- Sharma, A.; Jaiswal, H.K. Heterosis for yield and grain quality parameters in basmati rice (Oryza sativa L.). Electron. J. Plant Breed. 2020, 11, 1106–1115. [Google Scholar] [CrossRef]
- Tam, B.P.; Tu, P.T.B.; Pha, N.T. Identification of medium-grain rice based on GS3, a gene linked to rice grain size. Indones. J. Biotechnol. 2024, 29, 82–90. [Google Scholar] [CrossRef]
- Yin, C.; Li, H.; Li, S.; Xu, L.; Zhao, Z.; Wang, J. Genetic dissection on rice grain shape by the two-dimensional image analysis in one japonica × indica population consisting of recombinant inbred lines. Theor. Appl. Genet. 2015, 128, 1969–1986. [Google Scholar] [CrossRef]
- Arikit, S.; Wanchana, S.; Khanthong, S.; Saensuk, C.; Thianthavon, T.; Vanavichit, A.; Toojinda, T. QTL-seq identifies cooked grain elongation QTLs near soluble starch synthase and starch branching enzymes in rice (Oryza sativa L.). Sci. Rep. 2019, 9, 8328. [Google Scholar] [CrossRef]
- Lin, Z.; Zheng, D.; Zhang, X.; Wang, Z.; Lei, J.; Liu, Z.; Li, G.; Wang, S.; Ding, Y. Chalky part differs in chemical composition from translucent part of japonica rice grains as revealed by a notched-belly mutant with white-belly. J. Sci. Food Agric. 2016, 96, 3937–3943. [Google Scholar] [CrossRef]
- Singh, N.; Sodhi, N.S.; Kaur, M.; Saxena, S.K. Physico-chemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels. Food Chem. 2003, 82, 433–439. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zheng, M.; Hu, R.; Dong, J.; Zhou, L.; Liu, W.; Liu, D.; Yang, W. Genes controlling grain chalkiness in rice. Crop J. 2024, 12, 979–991. [Google Scholar] [CrossRef]
- Kumar, A.; Thomas, J.; Gill, N.; Dwiningsih, Y.; Ruiz, C.; Famoso, A.; Pereira, A. Molecular mapping and characterization of QTLs for grain quality traits in a RIL population of US rice under high nighttime temperature stress. Sci. Rep. 2023, 13, 4880. [Google Scholar] [CrossRef]
- Aznan, A.; Gonzalez Viejo, C.; Pang, A.; Fuentes, S. Computer vision and machine learning analysis of commercial rice grains: A potential digital approach for consumer perception studies. Sensors 2021, 21, 6354. [Google Scholar] [CrossRef]
- Cuevas, R.P.; Pede, V.O.; McKinley, J.; Velarde, O.; Demont, M. Rice grain quality and consumer preferences: A case study of two rural towns in the Philippines. PLoS ONE 2016, 11, e0150345. [Google Scholar] [CrossRef]
- Paul, H.; Nath, B.C.; Golam, M.; Bhuiyan, K. Effect of Degree of Milling on Rice Grain Quality. J. Agric. Eng. 2019, 42, 69–76. [Google Scholar]
- Bergman, C.J. Rice end-use quality analysis. In Rice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–337. [Google Scholar]
- Sun, H.; Xue, J.; Song, Y.; Wang, P.; Wen, Y.; Zhang, T. Detection of fruit tree diseases in natural environments: A novel approach based on stereo camera and deep learning. Eng. Appl. Artif. Intell. 2024, 137, 109148. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, X.; Gao, H.; He, T.; Lv, Z.; Zhangzhong, L. When Crops Meet Machine Vision: A Review and Development Framework for a Low-Cost Nondestructive Online Monitoring Technology in Agricultural Production. Agric. Commun. 2024, 2, 100029. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Mao, H.; Abomohra, A.E.F. Applications of non-destructive technologies for agricultural and food products quality inspection. Sensors 2019, 19, 846. [Google Scholar] [CrossRef]
- Olorunfemi, B.O.; Nwulu, N.I.; Adebo, O.A.; Kavadias, K.A. Advancements in machine visions for fruit sorting and grading: A bibliometric analysis, systematic review, and future research directions. J. Agric. Food Res. 2024, 16, 101154. [Google Scholar] [CrossRef]
- Narendra, V.G.; Hareesh, K.S. Prospects of computer vision automated grading and sorting systems in agricultural and food products for quality evaluation. Int. J. Comput. Appl. 2010, 1, 1–12. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Farahmandfar, R. Effect of hulling and milling on the physical properties of rice grains. Int. Agrophysics 2008, 22, 353–359. [Google Scholar]
- Singathala, H.; Malla, J.; Lekkala, P. Quality Analysis and Classification of Rice Grains using Image Processing Techniques. Int. Res. J. Eng. Technol. 2023, 10, 311–315. [Google Scholar]
- Vu, H.; Duong, V.N.; Nguyen, T.T. Inspecting rice seed species purity on a large dataset using geometrical and morphological features. In Proceedings of the ACM International Conference Proceeding Series, Danang City, Vietnam, 6–7 December 2018; pp. 321–328. [Google Scholar]
- Jeong, E.; Abdellaoui, N.; Lim, J.; Seo, J.A. The presence of a significant endophytic fungus in mycobiome of rice seed compartments. Sci. Rep. 2024, 14, 23367. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Ali, S.; Li, H.; Ouyang, Q.; Wu, X.; Hashim, M.M.; Chen, Q. Recent advances in assessing qualitative and quantitative aspects of cereals using nondestructive techniques: A review. Trends Food Sci. Technol. 2021, 116, 815–828. [Google Scholar] [CrossRef]
- Hussain, N.; Sun, D.W.; Pu, H. Classical and emerging non-destructive technologies for safety and quality evaluation of cereals: A review of recent applications. Trends Food Sci. Technol. 2019, 91, 598–608. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. TrAC Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Kawamura, S.; Natsuga, M.; Takekura, K.; Itoh, K. Development of an automatic rice-quality inspection system. Comput. Electron. Agric. 2003, 40, 115–126. [Google Scholar] [CrossRef]
- Osborne, B.; Mertens, B.; Thompson, M.; Fearn, T. The authentication of Basmati rice using near infrared spectroscopy. J. Near Infrared Spectrosc. 1993, 1, 77–83. [Google Scholar] [CrossRef]
- Kumar, D.; Jevin Christy, D.; Sakthibalan, S.; Srivind, J.; Kesavan, K.; Eevera, T.; Thilagar, S.H. Thermal imaging of paddy seeds for quality assessment. J. Trop. Agric. 2024, 62, 111–121. [Google Scholar]
- Gowen, A.A.; Tiwari, B.K.; Cullen, P.J.; McDonnell, K.; O’Donnell, C.P. Applications of thermal imaging in food quality and safety assessment. Trends Food Sci. Technol. 2010, 21, 190–200. [Google Scholar] [CrossRef]
- ElMasry, G.; ElGamal, R.; Mandour, N.; Gou, P.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Emerging thermal imaging techniques for seed quality evaluation: Principles and applications. Food Res. Int. 2020, 131, 109025. [Google Scholar] [CrossRef]
- Lutz, É.; Coradi, P.C. Applications of New Technologies for Monitoring and Predicting Grains Quality Stored: Sensors, Internet of Things, and Artificial Intelligence. Measurement 2022, 188, 110609. [Google Scholar] [CrossRef]
- Danno, A.; Miyazato, M.; Ishiguro, E. Quality evaluation of agricultural products by infrared imaging method. Mem. Fac. Agric. Kagoshima Univ. 1980, 16, 157–164. [Google Scholar]
- Jamil, N.; Bejo, S.K. Husk Detection Using Thermal Imaging Technology. Agric. Agric. Sci. Procedia 2014, 2, 128–135. [Google Scholar] [CrossRef]
- Ginesu, G.; Giusto, D.D.; Margner, V.; Meinlschmidt, P. Detection of foreign bodies in food by thermal image processing. IEEE Trans. Ind. Electron. 2004, 51, 480–490. [Google Scholar] [CrossRef]
- Bejo-Khairunniza, S.; Azman, N.; Jamil, N. Paddy grading using thermal imaging technology. Int. Food Res. J. 2016, 23, S245. [Google Scholar]
- Aviara, N.A.; Liberty, J.T.; Olatunbosun, O.S.; Shoyombo, H.A.; Oyeniyi, S.K. Potential application of hyperspectral imaging in food grain quality inspection, evaluation and control during bulk storage. J. Agric. Food Res. 2022, 8, 100288. [Google Scholar] [CrossRef]
- An, D.; Zhang, L.; Liu, Z.; Liu, J.; Wei, Y. Advances in infrared spectroscopy and hyperspectral imaging combined with artificial intelligence for the detection of cereals quality. Crit. Rev. Food Sci. Nutr. 2023, 63, 9766–9796. [Google Scholar] [CrossRef]
- Saha, D.; Manickavasagan, A. Machine learning techniques for analysis of hyperspectral images to determine quality of food products: A review. Curr. Res. Food Sci. 2021, 4, 28–44. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, H.; Sun, D.W. Hyperspectral Imaging Technique for Evaluating Food Quality and Safety during Various Processes: A Review of Recent Applications. Trends Food Sci. Technol. 2017, 69, 25–35. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Ahmad, W.; Man, Z.; Duan, Y. Identification of rice storage time based on colorimetric sensor array combined hyperspectral imaging technology. J. Stored Prod. Res. 2020, 85, 101523. [Google Scholar] [CrossRef]
- Weng, S.; Tang, P.; Yuan, H.; Guo, B.; Yu, S.; Huang, L.; Xu, C. Hyperspectral imaging for accurate determination of rice variety using a deep learning network with multi-feature fusion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118237. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, L.; Li, H.; Rao, Z.; Ji, H. Nondestructive identification of barley seeds varieties using hyperspectral data from two sides of barley seeds. J. Food Process Eng. 2021, 44, e13769. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, Q.; Huang, M. Semi-supervised classification of rice seed based on hyperspectral imaging technology. In Proceedings of the ASABE Annual International Meeting, Quebec, QC, Canada, 13–16 July 2014; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2014. [Google Scholar]
- Wang, L.; Liu, D.; Pu, H.; Sun, D.W.; Gao, W.; Xiong, Z. Use of hyperspectral imaging to discriminate the variety and quality of rice. Food Anal. Methods 2015, 8, 515–523. [Google Scholar] [CrossRef]
- Li, B.; Zhao, M.; Zhou, Y.; Hou, B.; Zhang, D. Detection of Waxed Rice Using Visible-Near Infrared Hyperspectral Imaging. J. Food Nutr. Res. 2016, 4, 267–275. [Google Scholar]
- Zhao, C.; Lee, W.S.; He, D. Immature green citrus detection based on colour feature and sum of absolute transformed difference (SATD) using colour images in the citrus grove. Comput. Electron. Agric. 2016, 124, 243–253. [Google Scholar] [CrossRef]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Teixido-Orries, I.; Marín, S. Hyperspectral imaging for the classification of individual cereal kernels according to fungal and mycotoxins contamination: A review. Food Res. Int. 2022, 155, 111102. [Google Scholar] [CrossRef]
- Ullrich, K.; von Elling, M.; Gutzeit, K.; Dix, M.; Weigold, M.; Aurich, J.C.; Wertheim, R.; Jawahir, I.S.; Ghadbeigi, H. AI-based optimisation of total machining performance: A review. CIRP J. Manuf. Sci. Technol. 2024, 50, 40–54. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Zhu, L.; Spachos, P.; Pensini, E.; Plataniotis, K.N. Deep learning and machine vision for food processing: A survey. Curr. Res. Food Sci. 2021, 4, 233–249. [Google Scholar] [CrossRef]
- Naik, N.K.; Subbarao, M.V.; Sethy, P.K.; Behera, S.K.; Panigrahi, G.R. Machine learning with analysis-of-variance-based method for identifying rice varieties. J. Agric. Food Res. 2024, 18, 101397. [Google Scholar] [CrossRef]
- Mana, A.A.; Allouhi, A.; Hamrani, A.; Rahman, S.; el Jamaoui, I.; Jayachandran, K. Sustainable AI-based production agriculture: Exploring AI applications and implications in agricultural practices. Smart Agric. Technol. 2024, 7, 100416. [Google Scholar] [CrossRef]
- Meshram, V.; Patil, K.; Meshram, V.; Hanchate, D.; Ramkteke, S.D. Machine learning in agriculture domain: A state-of-art survey. Artif. Intell. Life Sci. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Mahesh, B. Machine Learning Algorithms—A Review. Int. J. Sci. Res. 2020, 9, 381–386. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Bansal, M.; Goyal, A.; Choudhary, A. A comparative analysis of K-nearest neighbor, genetic, support vector machine, decision tree, and long short term memory algorithms in machine learning. Decis. Anal. J. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Dhaliwal, D.S.; Williams, M.M. Sweet corn yield prediction using machine learning models and field-level data. Precis. Agric. 2024, 25, 51–64. [Google Scholar] [CrossRef]
- Taherdoost, H. Deep Learning and Neural Networks: Decision-Making Implications. Symmetry 2023, 15, 1723. [Google Scholar] [CrossRef]
- Taye, M.M. Understanding of Machine Learning with Deep Learning: Architectures, Workflow, Applications and Future Directions. Computers 2023, 12, 91. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Sitokonstantinou, V.; Koukos, A.; Drivas, T.; Kontoes, C.; Papoutsis, I.; Karathanassi, V. A scalable machine learning pipeline for paddy rice classification using multi-temporal sentinel data. Remote Sens. 2021, 13, 1769. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J. Unsupervised deep clustering via adaptive GMM modeling and optimization. Neurocomputing 2021, 433, 199–211. [Google Scholar] [CrossRef]
- Gambella, C.; Ghaddar, B.; Naoum-Sawaya, J. Optimization problems for machine learning: A survey. Eur. J. Oper. Res. 2021, 290, 807–828. [Google Scholar] [CrossRef]
- Archana, R.; Jeevaraj, P.E. Deep learning models for digital image processing: A review. Artif. Intell. Rev. 2024, 57, 11. [Google Scholar] [CrossRef]
- Attri, I.; Awasthi, L.K.; Sharma, T.P.; Rathee, P. A review of deep learning techniques used in agriculture. Ecol. Inform. 2023, 77, 102217. [Google Scholar]
- Tien, P.W.; Wei, S.; Darkwa, J.; Wood, C.; Calautit, J.K. Machine Learning and Deep Learning Methods for Enhancing Building Energy Efficiency and Indoor Environmental Quality – A Review. Energy AI 2022, 10, 100198. [Google Scholar] [CrossRef]
- Jeyaraj, P.R.; Asokan, S.P.; Samuel Nadar, E.R. Computer-Assisted Real-Time Rice Variety Learning Using Deep Learning Network. Rice Sci. 2022, 29, 489–498. [Google Scholar]
- Koklu, M.; Cinar, I.; Taspinar, Y.S. Classification of rice varieties with deep learning methods. Comput. Electron. Agric. 2021, 187, 106285. [Google Scholar] [CrossRef]
- Fayyazi, S.; Abbaspour-Fard, M.H.; Rohani, A.; Monadjemi, S.A.; Sadrnia, H. Identification and classification of three Iranian rice varieties in mixed bulks using image processing and MLP neural network. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Kiratiratanapruk, K.; Temniranrat, P.; Sinthupinyo, W.; Prempree, P.; Chaitavon, K.; Porntheeraphat, S.; Prasertsak, A. Development of paddy rice seed classification process using machine learning techniques for automatic grading machine. J. Sens. 2020, 2020, 7041310. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Mali, S.N. Identification of paddy varieties based on novel seed angle features. Comput. Electron. Agric. 2016, 123, 415–422. [Google Scholar] [CrossRef]
- Wu, J.G.; Shi, C.H. Prediction of grain weight, brown rice weight and amylose content in single rice grains using near-infrared reflectance spectroscopy. Field Crops Res. 2004, 87, 13–21. [Google Scholar] [CrossRef]
- Shao, Y.; Cen, Y.; He, Y.; Liu, F. Infrared spectroscopy and chemometrics for the starch and protein prediction in irradiated rice. Food Chem. 2011, 126, 1856–1861. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, C.; Jia, L.; Tang, Q.; Gao, L.; Zhao, G.; Qi, H. Identification of Rice Seed Varieties Based on Near-Infrared Hyperspectral Imaging Technology Combined with Deep Learning. ACS Omega 2022, 7, 4735–4749. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, C.; Liu, F.; Nie, P.; He, Y. Rice seed cultivar identification using near-infrared hyperspectral imaging and multivariate data analysis. Sensors 2013, 13, 8916–8927. [Google Scholar] [CrossRef]
- Farahnakian, F.; Sheikh, J.; Farahnakian, F.; Heikkonen, J. A comparative study of state-of-the-art deep learning architectures for rice grain classification. J. Agric. Food Res. 2024, 15, 100890. [Google Scholar] [CrossRef]
- Sun, C.; Liu, T.; Ji, C.; Jiang, M.; Tian, T.; Guo, D.; Wang, L.; Chen, Y.; Liang, X. Evaluation and analysis the chalkiness of connected rice kernels based on image processing technology and support vector machine. J. Cereal Sci. 2014, 60, 426–432. [Google Scholar] [CrossRef]
- Chen, K.; Huang, M. Prediction of milled rice grades using Fourier transform near-infrared spectroscopy and artificial neural networks. J. Cereal Sci. 2010, 52, 221–226. [Google Scholar] [CrossRef]
- Zareiforoush, H.; Minaei, S.; Alizadeh, M.R.; Banakar, A. Qualitative classification of milled rice grains using computer vision and metaheuristic techniques. J. Food Sci. Technol. 2016, 53, 118–131. [Google Scholar] [CrossRef]
- Mandal, D. Adaptive neuro-fuzzy inference system based grading of basmati rice grains using image processing technique. Rom. J. Inf. Sci. Technol. 2019, 22, 19. [Google Scholar] [CrossRef]
- Ramdhani, Y.; Alamsyah, D.P. Enhancing Sustainable Rice Grain Quality Analysis with Efficient SVM Optimization Using Genetic Algorithm. E3S Web Conf. 2023, 426, 01035. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, Q.; Wei, H.; Shi, Y. An efficient multiscale integrated attention method combined with hyperspectral system to identify the quality of rice with different storage periods and humidity. Comput. Electron. Agric. 2023, 213, 108259. [Google Scholar] [CrossRef]
- Debnath, O.; Saha, H.N. An IoT-based intelligent farming using CNN for early disease detection in rice paddy. Microprocess. Microsyst. 2022, 94, 104631. [Google Scholar] [CrossRef]
- Sun, M.; Huang, S.; Lu, Z.; Wang, M.; Zhang, S.; Yang, K.; Tang, B.; Yang, W.; Huang, C. A novel method for intelligent analysis of rice yield traits based on LED transmission imaging and cloud computing. Measurement 2023, 217, 113017. [Google Scholar] [CrossRef]
- Tian, F.; Tan, F.; Li, H. An rapid nondestructive testing method for distinguishing rice producing areas based on Raman spectroscopy and support vector machine. Vib. Spectrosc. 2020, 107, 103017. [Google Scholar] [CrossRef]
- Saha, K.K.; Al Riza, D.F.; Ogawa, Y.; Suzuki, T.; Sugimoto, T.; Kondo, N. Assessment of chalkiness index of Sake rice using transmission imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 275, 121149. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, J.; Guo, W.; Bu, R.; Zheng, Z.; Chen, Y.; Yang, Z.; Lin, R. Colored rice quality inspection system using machine vision. J. Cereal Sci. 2019, 88, 87–95. [Google Scholar] [CrossRef]
- Moses, K.; Miglani, A.; Kankar, P.K. Deep CNN-based damage classification of milled rice grains using a high-magnification image dataset. Comput. Electron. Agric. 2022, 195, 106811. [Google Scholar]
- Shi, Y.; Yuan, H.; Xiong, C.; Zhang, Q.; Jia, S.; Liu, J.; Men, H. Improving performance: A collaborative strategy for the multi-data fusion of electronic nose and hyperspectral to track the quality difference of rice. Sens. Actuators B Chem. 2021, 333, 129546. [Google Scholar] [CrossRef]
- Fan, F.; Chen, H.; Gao, Y.; Mou, T. Quantitative detection and sorting of broken kernels and chalky grains in milled rice using computer vision algorithms. J. Food Eng. 2024, 383, 112225. [Google Scholar] [CrossRef]
- Razavi, M.; Mavaddati, S.; Koohi, H. ResNet deep models and transfer learning technique for classification and quality detection of rice cultivars. Expert Syst. Appl. 2024, 247, 123276. [Google Scholar] [CrossRef]
- Pan, J.; Wang, T.; Wu, Q. RiceNet: A Two Stage Machine Learning Method for Rice Disease Identification. Biosyst. Eng. 2023, 225, 25–40. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, T.; Gao, F.; Zhang, R.; Seng, X. YOLOv5s-CBAM-DMLHead: A lightweight identification algorithm for weedy rice (Oryza sativa f. spontanea) based on improved YOLOv5. Crop Prot. 2023, 172, 106342. [Google Scholar] [CrossRef]
- Krichen, M. Convolutional neural networks: A survey. Computers 2023, 12, 151. [Google Scholar] [CrossRef]
- Naranjo-Torres, J.; Mora, M.; Hernández-García, R.; Barrientos, R.J.; Fredes, C.; Valenzuela, A. A review of convolutional neural network applied to fruit image processing. Appl. Sci. 2020, 10, 3443. [Google Scholar] [CrossRef]
- Ang, K.M.; El-Kenawy, E.S.M.; Abdelhamid, A.A.; Ibrahim, A.; Alharbi, A.H.; Khafaga, D.S.; Tiang, S.S.; Lim, W.H. Optimal design of convolutional neural network architectures using teaching–learning-based optimization for image classification. Symmetry 2022, 14, 2323. [Google Scholar] [CrossRef]
- Hafiz, A.M.; Bhat, G.M. A Survey on Instance Segmentation: State of the Art. Int. J. Multimed. Inf. Retr. 2020, 9, 171–189. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Gong, C.; Chen, Y.; Yu, H. Applications of deep learning for dense scenes analysis in agriculture: A review. Sensors 2020, 20, 1520. [Google Scholar] [CrossRef]
- Champ, J.; Mora-Fallas, A.; Goëau, H.; Mata-Montero, E.; Bonnet, P.; Joly, A. Instance segmentation for the fine detection of crop and weed plants by precision agricultural robots. Appl. Plant Sci. 2020, 8, e11373. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, H.; Niu, Y.; Zhou, Z.; Bu, Y.; Zheng, W. Segmentation of cotton leaves based on improved watershed algorithm. In Proceedings of the 9th IFIP WG 5.14 International Conference, CCTA 2015, Beijing, China, 27–30 September 2015; Springer: Berlin/Heidelberg, Germany, 2015; pp. 425–436. [Google Scholar]
- Pham, V.H.; Lee, B.R. An Image Segmentation Approach for Fruit Defect Detection Using K-Means Clustering and Graph-Based Algorithm. Vietnam J. Comput. Sci. 2015, 2, 25–33. [Google Scholar] [CrossRef]
- Clement, J.; Novas, N.; Gazquez, J.A.; Manzano-Agugliaro, F. An active contour computer algorithm for the classification of cucumbers. Comput. Electron. Agric. 2013, 92, 75–81. [Google Scholar] [CrossRef]
- Ma, J.; Du, K.; Zhang, L.; Zheng, F.; Chu, J.; Sun, Z. A Segmentation Method for Greenhouse Vegetable Foliar Disease Spots Images Using Color Information and Region Growing. Comput. Electron. Agric. 2017, 142, 110–117. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, G.; Wang, Z.; Li, E.; Liang, Z. Instance segmentation of apple flowers using the improved Mask R–CNN model. Biosyst. Eng. 2020, 193, 264–278. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Y.; Zou, X.; Wang, C. Three-dimensional reconstruction of guava fruits and branches using instance segmentation and geometry analysis. Comput. Electron. Agric. 2021, 184, 106107. [Google Scholar] [CrossRef]
- Carranza-García, M.; Torres-Mateo, J.; Lara-Benítez, P.; García-Gutiérrez, J. On the performance of one-stage and two-stage object detectors in autonomous vehicles using camera data. Remote Sens. 2020, 13, 89. [Google Scholar] [CrossRef]
- Hassan, E.; El-Rashidy, N.; Talaa, F.M. Review: Mask R-CNN Models. Nile J. Commun. Comput. Sci. 2022, 3, 17–27. [Google Scholar] [CrossRef]
- Hoogenboom, G. Contribution of agrometeorology to the simulation of crop production and its applications. Agric. For. Meteorol. 2000, 103, 137–157. [Google Scholar] [CrossRef]
- Sapkota, R.; Ahmed, D.; Karkee, M. Comparing YOLOv8 and Mask R-CNN for instance segmentation in complex orchard environments. Artif. Intell. Agric. 2024, 13, 84–99. [Google Scholar] [CrossRef]
- Wang, S.; Sun, G.; Zheng, B.; Du, Y. A crop image segmentation and extraction algorithm based on Mask RCNN. Entropy 2021, 23, 1160. [Google Scholar] [CrossRef]
- Afzaal, U.; Bhattarai, B.; Pandeya, Y.R.; Lee, J. An instance segmentation model for strawberry diseases based on mask R-CNN. Sensors 2021, 21, 6565. [Google Scholar] [CrossRef]
- Osorio, K.; Puerto, A.; Pedraza, C.; Jamaica, D.; Rodríguez, L. A Deep Learning Approach for Weed Detection in Lettuce Crops Using Multispectral Images. AgriEngineering 2020, 2, 471–488. [Google Scholar] [CrossRef]
- Safonova, A.; Guirado, E.; Maglinets, Y.; Alcaraz-Segura, D.; Tabik, S. Olive tree biovolume from UAV multi-resolution image segmentation with Mask R-CNN. Sensors 2021, 21, 1617. [Google Scholar] [CrossRef] [PubMed]
- Soviany, P.; Ionescu, R.T. Optimizing the Trade-off between Single-Stage and Two-Stage Object Detectors Using Image Difficulty Prediction. arXiv 2018, arXiv:1803.08707. [Google Scholar]
- Hussain, M.; He, L.; Schupp, J.; Lyons, D.; Heinemann, P. Green fruit segmentation and orientation estimation for robotic green fruit thinning of apples. Comput. Electron. Agric. 2023, 207, 107734. [Google Scholar] [CrossRef]
- Seol, J.; Kim, J.; Son, H.I. Field evaluations of a deep learning-based intelligent spraying robot with flow control for pear orchards. Precis. Agric. 2022, 23, 712–732. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, G.; Li, C.; Li, D. DCF-YOLOv8: An Improved Algorithm for Aggregating Low-Level Features to Detect Agricultural Pests and Diseases. Agronomy 2023, 13, 2012. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J. Vegetable disease detection using an improved YOLOv8 algorithm in the greenhouse plant environment. Sci. Rep. 2024, 14, 4261. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Nie, Z.; Yang, H.; Yu, S. A Lightweight YOLOv8 Tomato Detection Algorithm Combining Feature Enhancement and Attention. Agronomy 2023, 13, 1824. [Google Scholar] [CrossRef]
- Jahangirlou, M.R.; Morel, J.; Akbari, G.A.; Alahdadi, I.; Soufizadeh, S.; Parsons, D. Combined use of APSIM and logistic regression models to predict the quality characteristics of maize grain. Eur. J. Agron. 2023, 142, 126629. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Sun, Y.; Zhang, C.; Wang, M.; Wang, L. A New Three-Way Incremental Naive Bayes Classifier. Electronics 2023, 12, 1730. [Google Scholar] [CrossRef]
- Bhargava, A.; Bansal, A. Fruits and vegetables quality evaluation using computer vision: A review. J. King Saud-Univ.-Comput. Inf. Sci. 2021, 33, 243–257. [Google Scholar] [CrossRef]
- Chuquimarca, L.E.; Vintimilla, B.X.; Velastin, S.A. A review of external quality inspection for fruit grading using CNN models. Artif. Intell. Agric. 2024, 14, 1–20. [Google Scholar] [CrossRef]
- Mahamat, A.A.; Boukar, M.M.; Leklou, N.; Celino, A.; Obianyo, I.I.; Bih, N.L.; Stanislas, T.T.; Savastanos, H. Decision Tree Regression vs. Gradient Boosting Regressor Models for the Prediction of Hygroscopic Properties of Borassus Fruit Fiber. Appl. Sci. 2024, 14, 7540. [Google Scholar] [CrossRef]
- Esmaili, M.; Aliniaeifard, S.; Mashal, M.; Vakilian, K.A.; Ghorbanzadeh, P.; Azadegan, B.; Seif, M.; Didaran, F. Assessment of adaptive neuro-fuzzy inference system (ANFIS) to predict production and water productivity of lettuce in response to different light intensities and CO2 concentrations. Agric. Water Manag. 2021, 258, 107201. [Google Scholar] [CrossRef]
- Tomar, V.; Bansal, M.; Singh, P. Metaheuristic Algorithms for Optimization: A Brief Review. Eng. Proc. 2023, 59, 238. [Google Scholar]
- Singh, A.; Raj, K.; Meghwar, T.; Roy, A.M. Efficient Paddy Grain Quality Assessment Approach Utilizing Affordable Sensors. AI 2024, 5, 686–703. [Google Scholar] [CrossRef]
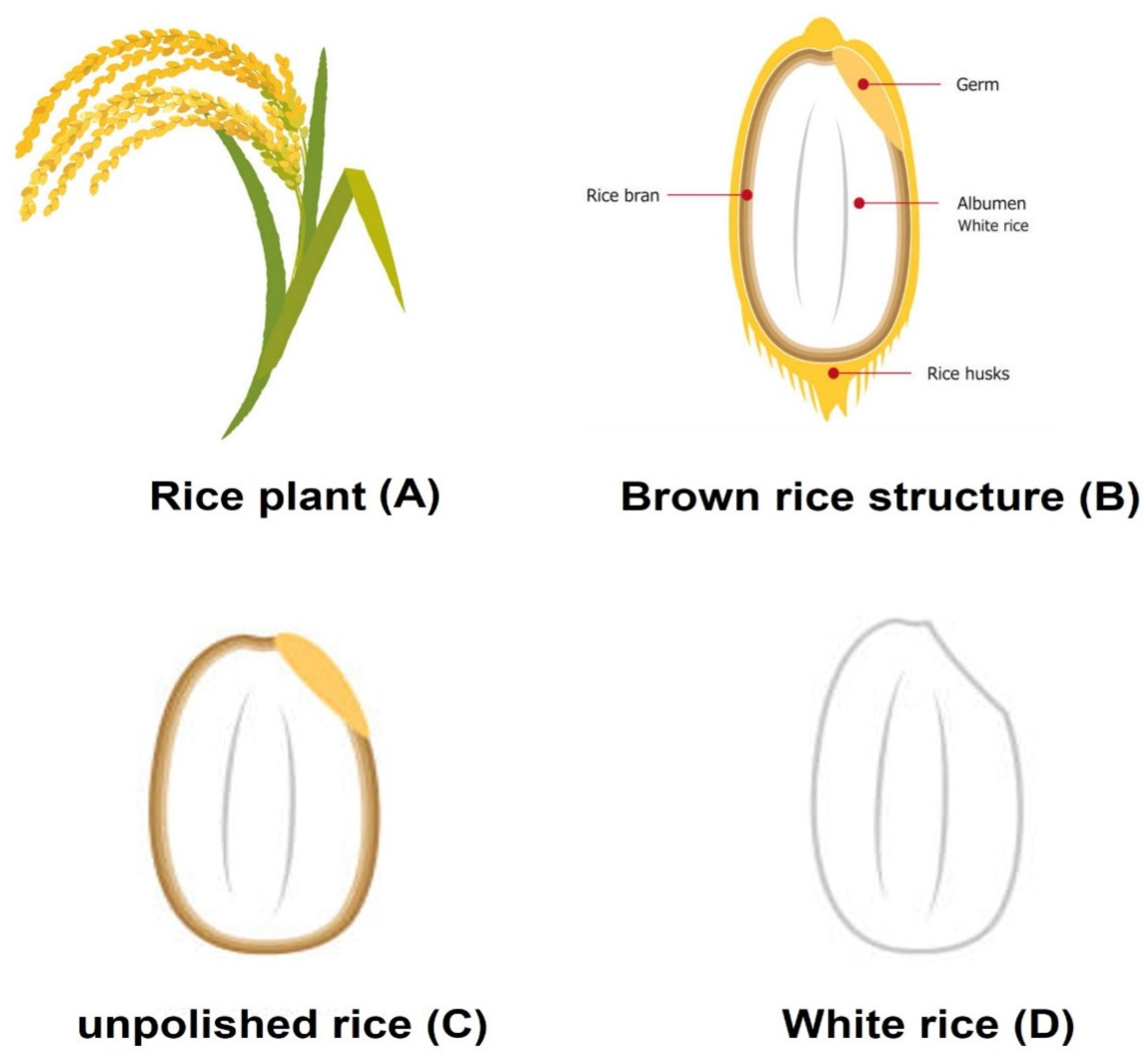
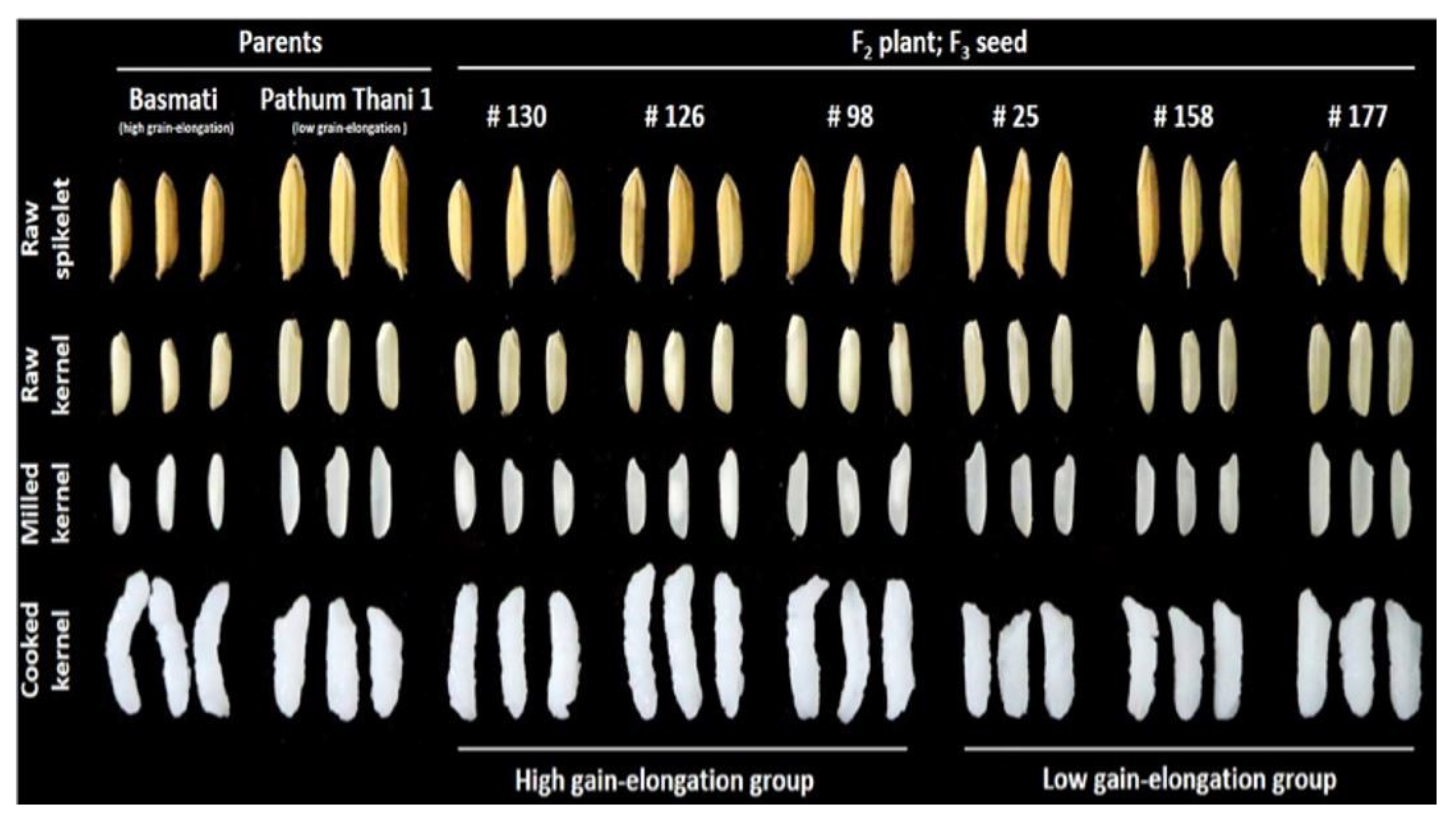
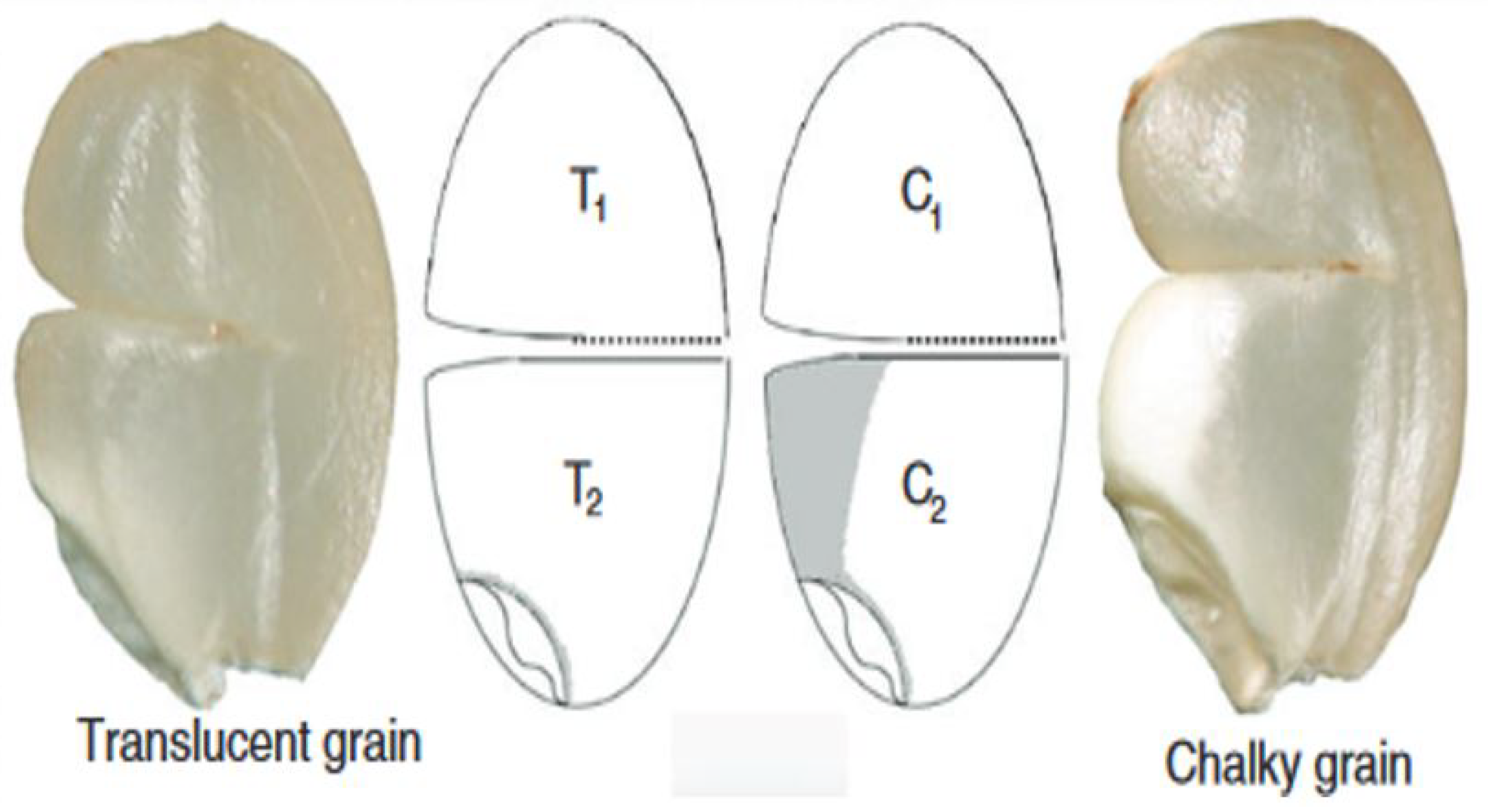
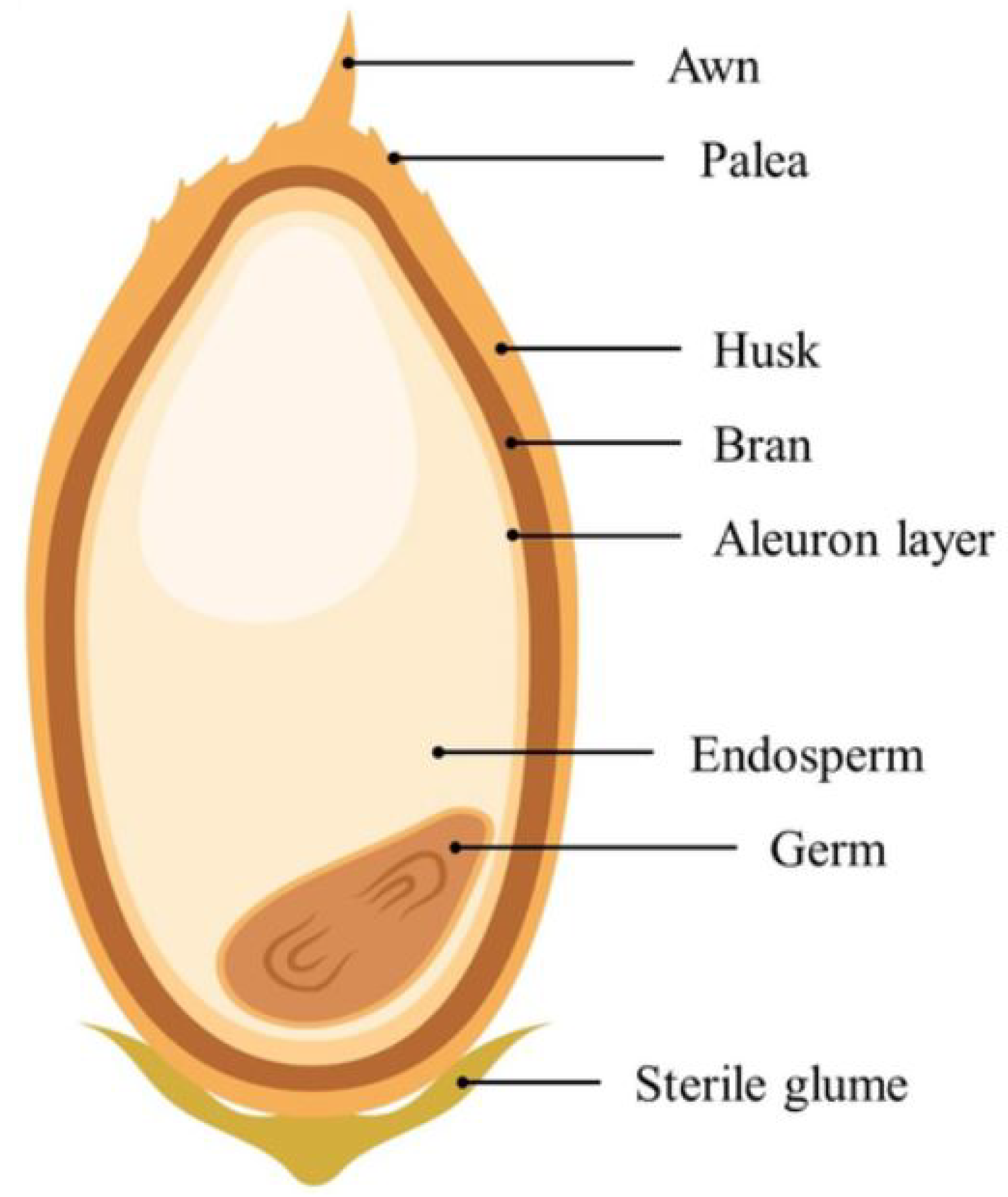
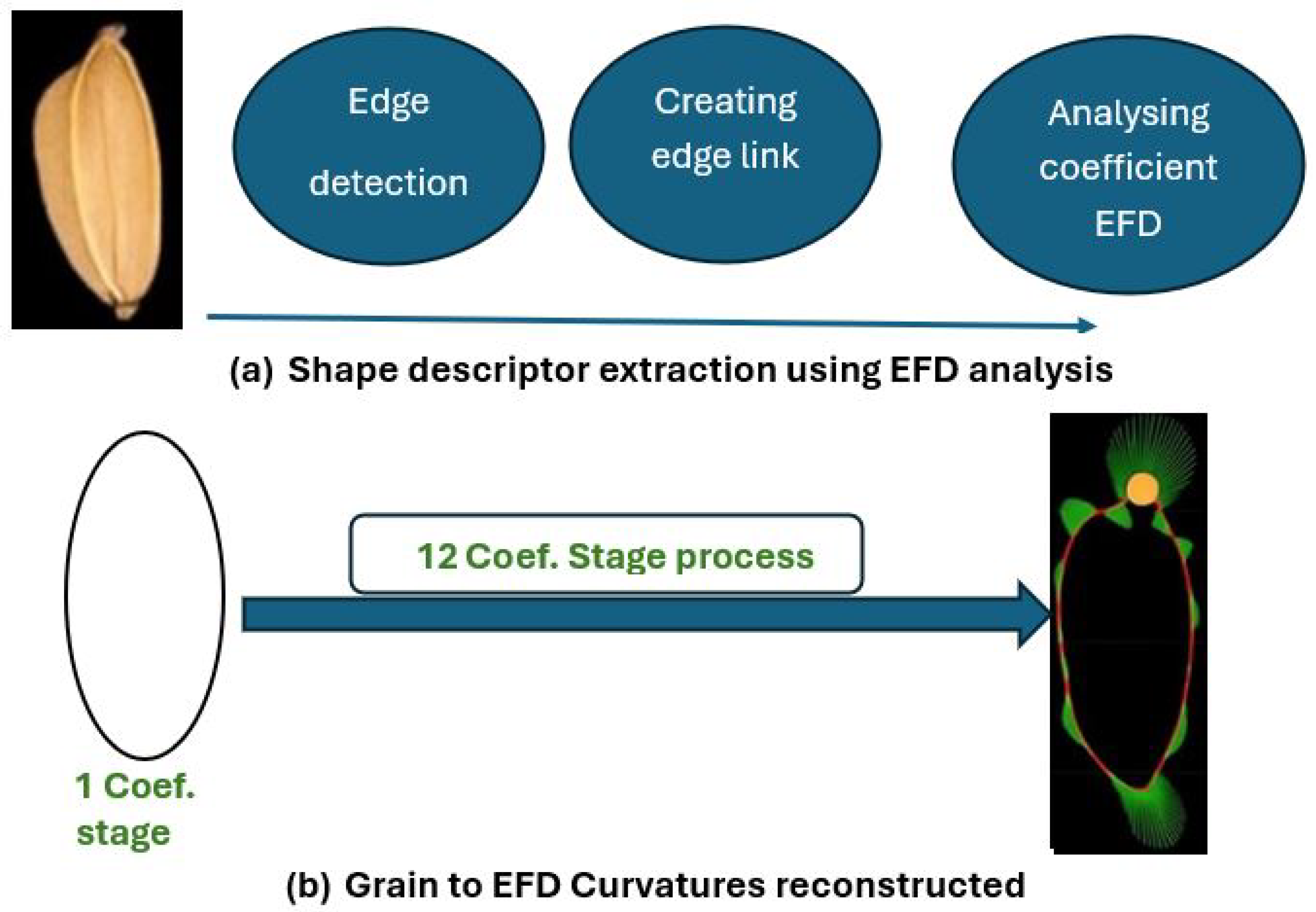
| Grain Type | Length (mm) | Scale |
|---|---|---|
| Very long | ≥7.50 | 1 |
| Long | to | 3 |
| Medium | to | 5 |
| Short | ≤5.50 | 7 |
| Grain Type | Length (mm) | Scale |
|---|---|---|
| Slender | ≥3.0 | 1 |
| Medium | to | 5 |
| Bold | ≤2.0 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilo, B.; Badjona, A.; Singh, Y.; Shenfield, A.; Zhang, H. Artificial Intelligence in Rice Quality and Milling: Technologies, Applications, and Future Prospects. Processes 2025, 13, 3731. https://doi.org/10.3390/pr13113731
Ilo B, Badjona A, Singh Y, Shenfield A, Zhang H. Artificial Intelligence in Rice Quality and Milling: Technologies, Applications, and Future Prospects. Processes. 2025; 13(11):3731. https://doi.org/10.3390/pr13113731
Chicago/Turabian StyleIlo, Benjamin, Abraham Badjona, Yogang Singh, Alex Shenfield, and Hongwei Zhang. 2025. "Artificial Intelligence in Rice Quality and Milling: Technologies, Applications, and Future Prospects" Processes 13, no. 11: 3731. https://doi.org/10.3390/pr13113731
APA StyleIlo, B., Badjona, A., Singh, Y., Shenfield, A., & Zhang, H. (2025). Artificial Intelligence in Rice Quality and Milling: Technologies, Applications, and Future Prospects. Processes, 13(11), 3731. https://doi.org/10.3390/pr13113731








