1. Introduction
In the context of global efforts to proactively address climate change and promote carbon neutrality [
1], the reduction of carbon emissions in the industrial sector and the sustainable development of agriculture have become a research focus. Lime kilns are a common piece of industrial equipment, accounting for approximately 250–300 Mt of CO
2 emissions annually worldwide, of which China contributes over 60% due to its extensive lime production capacity. The exhaust gas from these kilns contains a significant amount of carbon dioxide, typically 20–35% by volume, accompanied by low temperatures favorable for capture and utilization. When carbon dioxide is released directly into the atmosphere, it contributes to the greenhouse effect, which is a major cause of global climate change [
2]. Additionally, the direct emissions from lime kilns represent a waste of valuable resources.
Concurrently, the role of carbon dioxide gas fertilizer in promoting crop growth has become increasingly prominent in the process of developing agricultural facilities [
3]. Controlled CO
2 enrichment can increase photosynthetic efficiency and yield by 20–30% in C3 crops under suitable environmental conditions. The rational application of this fertilizer has thus emerged as a critical strategy to enhance crop yield and quality.
The transformation of industrial tail gas from a lime kiln into carbon dioxide gas fertilizer, with subsequent application in agriculture, fulfills two crucial objectives. Firstly, it addresses the demand for energy conservation and emission reduction in industrial contexts. Secondly, it aligns with the agricultural industry’s need for effective gas fertilizers. This approach establishes an innovative model that fosters a symbiotic relationship between industry and agriculture, thereby promoting mutually beneficial outcomes.
In recent years, with the advent of CCUS technology, lime kiln tail gas resource utilization has garnered significant traction in academic discourse [
4,
5,
6], though it persists in facing considerable challenges in practical application. These challenges encompass the treatment of tail gas impurities, the precise application of gas fertilizer, and the promotion of industrialization [
7].
Compared with existing reviews focusing mainly on CO2 capture or greenhouse fertilization separately, this work uniquely integrates the technological, agricultural, and industrial dimensions of lime kiln flue gas utilization. Consequently, conducting extensive research on the preparation of carbon dioxide fertilizer from lime kiln industrial tail gas and its agricultural application, along with the exploration of effective industrialization models, holds great practical and strategic significance. Such efforts not only facilitate the large-scale implementation of carbon recycling technologies but also bridge the gap between industrial emission reduction and agricultural productivity enhancement. Moreover, this approach exemplifies a circular carbon pathway that integrates carbon capture, utilization, and smart agricultural practices, thereby contributing to both economic growth and environmental sustainability.
2. CO2 Gas Fertilizer Preparation and Application
2.1. CO2-Based Fertilizer Preparation and Agricultural Performance
Recent studies have explored multiple pathways for synthesizing CO
2-based fertilizers derived from industrial flue gas, with notable differences in reaction mechanisms, nutrient integration, and agricultural performance. Chemical absorption using amine-based solvents remains the most mature technology, providing high CO
2 capture efficiency (>90%) and stable gas purity for fertilization applications [
8]. However, its high regeneration energy and solvent degradation remain challenges.
In contrast, solid adsorption using amine-functionalized porous materials or metal–organic frameworks (MOFs) offers a low-energy alternative with strong cyclic stability and easier integration into decentralized greenhouse systems [
9]. Carbonate-based mineralization, although slower in kinetics, provides permanent CO
2 fixation and nutrient co-release, which can improve soil buffering capacity and long-term fertility [
10].
Performance comparisons indicate that direct CO
2 gas fertilization enhances C3 crop yields by 15–30%, while mineralized and adsorbent-derived CO
2 fertilizers show better performance in soil quality improvement and pH stabilization. The hybridization of chemical absorption with adsorption–desorption cycles is emerging as a promising strategy for scalable and energy-efficient CO
2 utilization in protected agriculture [
11].
2.2. Progress of CCUS Research in China
Carbon capture, utilization, and storage (CCUS) is the process of separating CO
2 from industrial exhaust gases (e.g., biogas, coal-fired flue gas, iron and steel plant exhaust, cement plant exhaust, etc.) or directly from the atmosphere. The separated CO
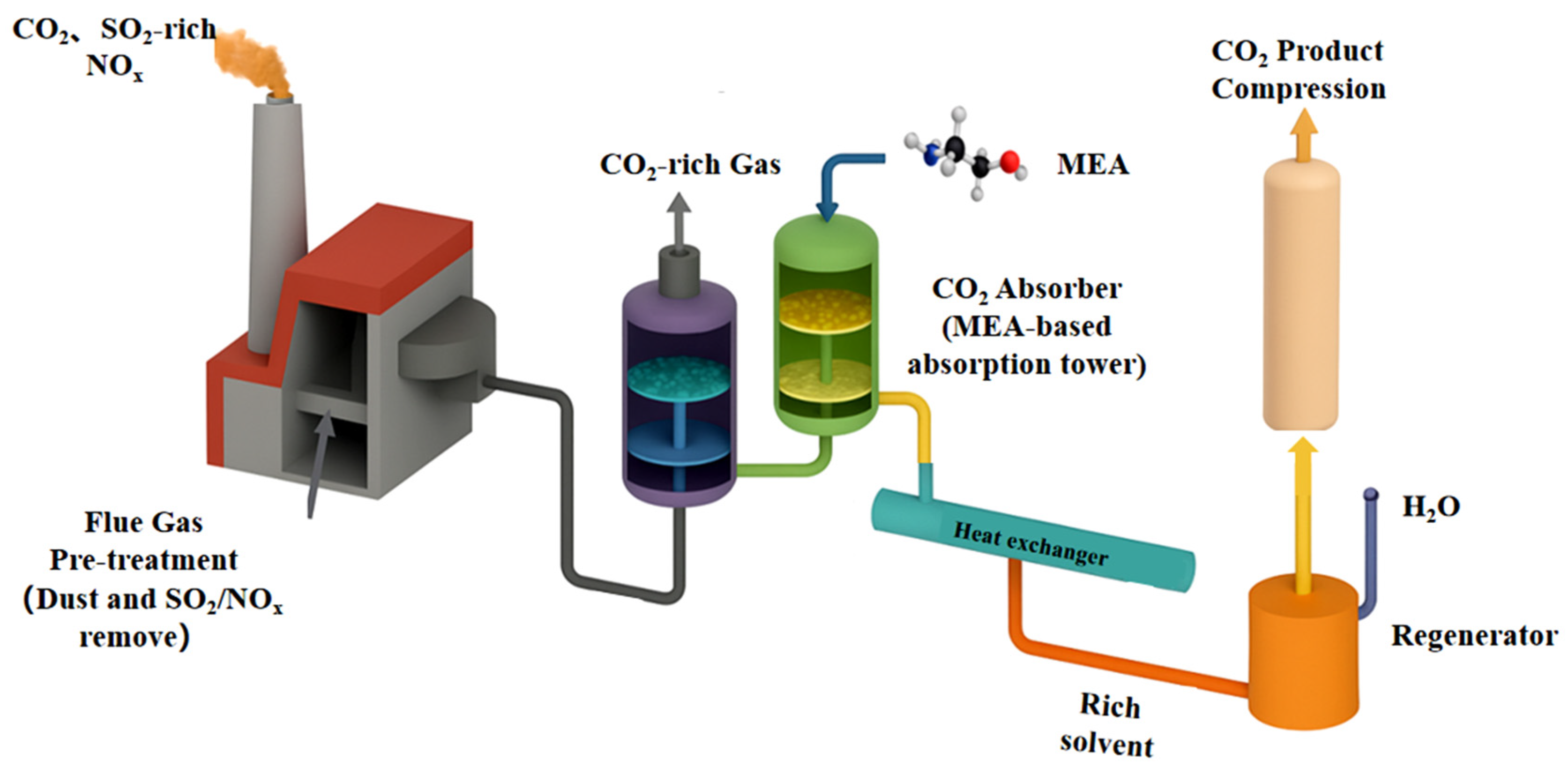
2 is then utilized or injected into the ground, where it is stored permanently. The development of CCUS technology in China has shown significant progress across various domains. With respect to capture technology, the current development of various capture technologies, including chemical absorption, physical adsorption, and membrane separation technology, among others, differs. The current status of their technological development is summarized in
Figure 1. The chemical absorption method of post-combustion capture is currently the most mature capture technology. It can be used in most existing coal-fired power plants for large-scale decarbonization. The chemical absorption CO
2 capture technology has been commercially applied in foreign countries, and large-scale industrial demonstrations are being carried out in China. These include the 150,000-ton carbon capture and sequestration demonstration project of the Guohua Jinjie Power Plant and the one-million-ton industrial demonstration of low energy consumption CO
2 capture, oil drive, and sequestration of a coal-fired power plant carried out in the Longdong Energy Base of Huaneng. In China, large-scale industrial demonstrations are also underway, including the 150,000-tonne carbon capture and storage demonstration project at Guohua Jinjie Power Plant and the million-ton coal-fired power plant demonstration project at Huaneng Longdong Energy Base [
12,
13]. Physical adsorption for post-combustion capture is currently in the industrial demonstration stage, while chemical adsorption and membrane separation are still in the laboratory basic research and pilot stage. Pre-combustion capture systems are more complex, and only the physical absorption method of pre-combustion capture is currently in the stage of commercial application. The Integrated Gasification Combined Cycle (IGCC) technology is a typical system suitable for pre-combustion carbon capture. Oxyfuel combustion is a promising carbon capture technology due to its higher CO
2 concentration, which facilitates capture (approximately 90–95 percent). The present generation of carbon capture technologies, which includes post-combustion capture by chemical absorption, pre-combustion capture by physical absorption, and oxygen-enriched combustion, has been adequately developed. However, these technologies are still limited by cost and energy consumption. Second-generation carbon capture technologies (e.g., novel absorption and adsorption technologies, gas membrane separation technologies, etc.) and emerging direct air capture technologies for distributed source CO
2 emissions are still in the laboratory research and development or small-scale testing stage. When the technology development is mature, the energy consumption and cost of capture will be reduced by more than 30 percent compared with the first-generation technologies. It is expected to achieve the large-scale promotion and application around 2035 [
14,
15].
Biogas, which typically contains 19–45% CO
2, 2–7% H
2O, and trace amounts of H
2 and H
2S, among other components, is currently subject to pressure change adsorption (PVAD) or membrane separation due to its elevated partial pressure of CO
2. Conversely, the chemical absorption method is typically employed in the context of coal-fired flue gas (~11% CO
2, ~2–7% H
2O, and trace amounts of NO
x and SO
x, etc.), building materials plant exhaust (~19% CO
2 and trace amounts of NO
x and SO
x, etc.), and iron and steel plant exhaust (~15% CO
2 and trace amounts of SO
x, etc.). The rationale behind this choice is the relatively low partial pressures of CO
2 in these sources. However, it is anticipated that advancements in adsorption and membrane separation technologies will facilitate the separation of CO
2 from sources with low partial pressures of CO
2. However, with the advancement of adsorption, separation, and membrane separation technologies, it is anticipated that the separation of CO
2 from emission sources with low partial pressure of CO
2 can be accomplished [
16]. In regard to the utilization and storage technologies, the commercial application of CO
2 uranium extraction by ground leaching has been established. Similarly, the industrial demonstration of CO
2-enhanced oil recovery (CO
2-EOR) has been achieved, along with the completion of pilot tests for CO
2-enhanced saltwater recovery (CO
2-EWR) and CO
2-driven coalbed methane (CO
2-ECBM). At present, mineralization is undergoing industrial testing, while CO
2-enhanced natural gas extraction and enhanced shale gas extraction technology remain in the preliminary research phase.
2.3. Lime Kiln Tail Gas Characteristics and Treatment Challenges
The current state of affairs reveals that China’s predominant sources of CO
2 emissions encompass biogas, flue gas (generated from pulverized coal combustion, waste incineration, IGCC, NCGG, and cement kiln flue gas), syngas, and ambient air. The components of these typical emission sources are summarized in
Table 1.
As illustrated in the table, the CO2 concentration in the cement kiln (lime kiln exhaust) is comparatively elevated when benchmarked against other CO2 emission sources. This attribute renders it a candidate for direct recycling, thereby establishing a tangible foundation for its conversion into products such as gas fertilizers. From an industrial production perspective, the high concentration of CO2 signifies that relatively pure CO2 can be obtained without the need for complicated enrichment processes in tail gas treatment and resource recovery. This, in turn, results in a significant reduction in recovery costs and an enhancement in recovery efficiency. Moreover, the temperature of industrial lime kiln tail gas is typically below 100 °C, a property that renders low-temperature physisorption materials a focal point of research in the domain of CO2 capture technology. In comparison with high-temperature tail gas, low-temperature tail gas does not necessitate supplementary cooling apparatus during treatment, thereby decreasing energy consumption and equipment expenditures. However, this approach concomitantly imposes limitations on the applicability of certain tail gas treatment technologies that necessitate elevated temperature conditions.
In addition to CO2, the exhaust gas contains various impurities, including SO2, NOx, dust, and other volatile organic compounds. Among the aforementioned elements, the concentrations of SO2 and NOx, though comparatively negligible, exert a more substantial influence on the subsequent CO2 capture and purification process. SO2 reacts with the adsorbent, decreasing its CO2 adsorption capacity and introducing impurities into the later stage of gas fertilizer preparation, thereby affecting product quality. NOx pollutes the environment and may also have a side reaction with CO2 under certain conditions, interfering with the conversion and utilization of CO2. Consequently, effective purification measures are imperative to eliminate these impurities during the treatment of lime kiln tail gas, ensuring the purity of CO2 and the quality of subsequent gas fertilizer products.
2.4. Tail Gas Pre-Treatment
2.4.1. Dust Removal
Mechanical de-dusting is a process that utilizes physical principles, such as gravity settling and inertial forces, to remove larger particles of dust from exhaust gases. Common mechanical dedusting devices include gravity settling chambers and cyclone separators [
17]. The gravity settling chamber is designed to facilitate the natural settling of dust particles by allowing exhaust gas to flow slowly within a larger space. This process utilizes the gravitational forces to ensure the dust settles under its own weight. The device’s design is characterized by its simplicity and cost-effectiveness. However, its dust removal efficiency is relatively limited, typically effective only for particles larger than 50 μm. The cyclone separator utilizes centrifugal force to dislodge dust particles from the gas stream. The dusty gas rotates at a high speed in the cyclone, and the dust is thrown to the wall under the effect of centrifugal force. Subsequently, the dust falls down along the wall to the bottom and is discharged. The cyclone separator has been demonstrated to exhibit superior efficacy in the removal of dust particles within the size range of 5–50 μm. It is characterized by its substantial handling capacity and its compact equipment design. However, its capacity to remove fine dust particles is comparatively constrained. Electrostatic dust removal is a process that utilizes electric field force to charge dust particles, which are then adsorbed on the electrode, thereby facilitating their removal. In the electrostatic precipitator, a strong electric field is formed between the electrodes by high-voltage direct current. The dust present in the exhaust gas is charged in the electric field, and the positively or negatively charged dust moves to the electrode that is opposite to its own charge and is adsorbed. Finally, the dust is dislodged from the electrodes by means of vibration, and the process continues until the dust is collected and processed. Electrostatic dust removal efficiency is high, with a maximum of 99% removal capacity, and it effectively removes fine dust particles with a diameter less than 1 μm.
However, the initial equipment investment is substantial, and the operational and maintenance requirements are onerous. The bag dust collector is a type of dust collector that functions by intercepting dust particles through a filter medium. The bag filter is equipped with a fiber filter bag. When exhaust gas passes through the filter bag, dust is intercepted, and the gas is discharged after being purified. Filter bags are fabricated from a variety of materials, including polyester fiber and glass fiber, among others. The selection of appropriate filter bags is contingent upon the nature of the exhaust gas. Bag filters demonstrate high removal efficiency for dust particles between 0.5 and 5 μm, exhibiting stable dust removal efficiency and straightforward operation. However, the filter bag must be replaced at regular intervals, and the operational cost is significant. In practice, a combination of dust removal methods is typically employed, contingent upon the nature of the dust in the tail gas, concentration, and processing requirements. This combination typically involves the utilization of dust removal methods such as the following: first, the removal of larger particles of dust by means of a cyclone, and subsequently, the use of a bag dust or electrostatic precipitator to further remove fine dust. This approach is adopted with the objective of enhancing the overall dust removal efficiency.
2.4.2. Desulphurization and Denitrification
The limestone–gypsum process is a prevalent method for wet FGD. In this process, limestone is ground into a slurry that functions as an absorbent. The slurry is then in countercurrent contact with the exhaust gas in an absorption tower. SO2 reacts with limestone slurry to produce calcium sulfite, which is oxidized to calcium sulfate in the absorption tower and finally crystallizes to form gypsum. The limestone–gypsum process has been demonstrated to exhibit high desulfurization efficiency, with levels that exceed 95%, and relies on technology that has already been thoroughly developed. Furthermore, it ensures stable operation. Furthermore, gypsum, a by-product of desulfurization, has the potential for comprehensive utilization in a variety of fields, including the construction industry. Nevertheless, this method is also associated with challenges, including equipment corrosion, scaling, and high capital and operating costs.
One alternative is dry desulfurization, such as the activated carbon adsorption method. This method uses the adsorption properties of activated carbon to remove SO
2 in the tail gas. Activated carbon is characterized by a porous structure and a substantial specific surface area [
18]. The process of SO
2 adsorption and subsequent chemical reactions occur on the surface of activated carbon, resulting in the generation of sulfuric acid and other substances. These substances become immobilized on the activated carbon surface. The activated carbon adsorption process is characterized by its simplicity and the absence of wastewater discharge. However, the capacity of the activated carbon to adsorb is limited, necessitating periodic regeneration or replacement, which in turn leads to elevated operating costs. Semi-dry FDG, exemplified by circulating fluidized bed FDG, involves the preparation of a slurry comprising lime powder and water. This slurry is then sprayed into the circulating fluidized bed reactor, where it undergoes a complete mixture and reaction with the exhaust gas. In the reactor, lime slurry reacts with SO
2 to generate calcium sulfite and calcium sulfate. The high mass and heat transfer characteristics of the circulating fluidized bed are leveraged to enhance the reaction. Semi-dry desulfurization combines the advantages of wet and dry methods, exhibiting high desulfurization efficiency (up to 80–90%), relatively low investment and operating costs, and no wastewater discharge. However, it requires sophisticated equipment and is challenging to operate. Selective catalytic reduction (SCR) is among the most prevalent denitrification technologies. In the SCR reactor, ammonia (NH
3) functions as a reducing agent. Catalysts, such as vanadium and titanium, facilitate the reaction of ammonia with NO
x in the exhaust gas, reducing it to nitrogen and water. SCR denitrification efficiency is high, with reported values exceeding 90%, and it effectively controls NO
x emissions. However, the catalyst is more costly, and the reaction requires temperatures in the range of 300–400 °C. Selective non-catalytic reduction (SNCR) is a process that occurs at elevated temperatures (850–1100 °C) in the presence of a reductant (e.g., urea) in the tail gas. The reduction in urea at high temperatures results in the generation of ammonia and NO
x, leading to a reaction that reduces nitrogen and water. The SNCR process is characterized by its simplicity and relatively low investment costs. However, its denitrification efficiency is typically in the range of 50–70%, which is considered suboptimal. The amount of reductant injected during the process is also challenging to control, which may result in issues such as ammonia escape [
19].
2.5. CO2 Capture Technology
Carbon dioxide (CO
2) capture represents the initial stage in the CCUS (carbon capture, utilization, and storage) process. CCUS encompasses technologies that facilitate the separation of CO
2 from industrial production, fuel utilization, or the atmosphere. The primary categories of CO
2 capture include pre-combustion capture, post-combustion capture, oxygen-enriched combustion, and chemical chain capture technologies for CO
2 emissions from stationary sources, as well as direct air capture for CO
2 emissions from distributed sources [
20]. For carbon dioxide (CO
2) emissions from industrial tail gas stationary sources, such as biogas, coal-fired power generating units, and iron and steel building materials industries, only post-combustion capture technologies are generally available. These technologies include CO
2 capture in coal-fired flue gases using liquid amine solutions, including monoethanolamide (MEA) and diethanolamine (DEA), as well as chemical absorption of flue gas CO
2 [
21,
22].
2.5.1. Absorption Techniques
Gas absorption represents a pivotal technology that is frequently employed following combustion to extract carbon dioxide from the flue gas of a power plant [
23,
24,
25]. In this process, the gas undergoes initial cooling and is directed to the base of a carbon dioxide absorber, where it comes into contact with a solution comprising a chemical that absorbs carbon dioxide (
Figure 2). Amine scrubbing is a widely utilized process in the context of carbon capture, exhibiting efficacy when the flue gas is devoid of nitrogen and sulfur. The process entails the use of scrubbing solvents, which encompass chemical absorbents, such as amines, amino acid salts, carbonates, ionic liquids, and ammonia, as well as physical absorbents, including selexol, recitsol, and purisol, among others. MEA, for instance, exhibits a chemical absorption capacity for carbon dioxide. In an absorption column, carbon dioxide is absorbed by the MEA solution to form a carbon dioxide-rich solution. This solution is then passed to a stripping column, where it is heated to approximately 150 °C to release the carbon dioxide. The MEA can then be recycled. MEA demonstrates an absorption efficiency of over 90% for CO
2, yet the scale and energy demands of the regeneration process contribute to increased installation costs. A further advantage of aqueous potassium carbonate is that it functions as a chemisorption adsorbent. This type of adsorbent has certain advantages over amine-based adsorbents, including low toxicity, slow decomposition, and easy regeneration. The adsorption rate of this adsorbent can be enhanced by adding promoters such as diethanolamine and piperazine [
26]. During the process of ammonia scrubbing, carbon dioxide (CO
2) present in the flue gas undergoes a reaction with ammonia (NH
3), resulting in the formation of products such as NH
4HCO
3. This method has been demonstrated to exhibit several key advantages, including its capacity to conserve energy, its high rate of carbon dioxide capture, its resistance to degradation during the absorption and regeneration processes, and its tolerance to the presence of oxygen in flue gas. Additionally, the resulting ammonium bicarbonate can be utilized as a fertilizer for agricultural purposes [
27].
2.5.2. Adsorption Techniques
Adsorption is defined as the process of capturing CO
2 by an adsorbent, thereby achieving separation. Adsorbents are classified into two categories: physical and chemical. Carbonaceous materials are a class of physical adsorbents that possess several advantageous properties, including high stability, heat and chemical resistance, low synthesis cost, a large specific surface area, a large pore volume, and light weight [
28]. In recent years, the physical adsorption of CO
2 by activated carbon has attracted considerable attention due to the low energy required for its regeneration [
29,
30,
31]. The adsorption behavior of CO
2, N
2, and CH
4 on carbon porous materials is related to their physical properties. Specifically, CO
2 is more readily adsorbed on the adsorbent active sites due to its linear shape and smaller kinetic diameter. A variety of desorption methodologies can be employed to facilitate the removal of adsorbed carbon dioxide. These methodologies include direct heating at temperatures ranging from 150 to 200 degrees Celsius, the purging of the bed with heated nitrogen gas, and indirect heating techniques, among others. Additionally, research is underway to investigate the efficacy of indirect heating and electro pendulum adsorption methods. In addition to activated carbon, other materials such as carbon nanotubes, graphene, zeolites, and metal–organic frameworks have been the focus of extensive research for their potential in CO
2 adsorption. For instance, nitrogen-doped bamboo carbon nanotubes encapsulated with transition metal (nickel, cobalt, and iron) salts exhibited enhanced CO
2 adsorption at varying temperatures and pressures [
32]. Treated graphene nanosheets demonstrated efficacy in capturing CO
2 [
33,
34]. 13X and 5A zeolites exhibited favorable CO
2 adsorption properties, which could be further enhanced by coating with palladium/silver ions or amine-functionalized modification [
35]. Metal–organic frameworks demonstrated proficiency inCO
2 uptake due to their distinctive structure and properties, and their adsorption capacity could be augmented by impregnation with a heterocyclic ligand [
36,
37].
2.5.3. Electrochemical Separation
The electrochemical process for the separation of carbon dioxide (CO
2) from exhaust gas streams comprises two stages. Initially, CO
2 is captured and released into a liquid electrolyte medium. Subsequently, the electrolyte is regenerated through an ionic conduction pathway. The efficacy of this process must be assessed in terms of current capacity, efficiency, and electrochemical energy consumption. A variety of electrochemical cell designs and technological approaches give rise to discrepancies in energy consumption and efficiency. For instance, gas-fed cells attain energy efficiency primarily contingent on the movement of hydroxide ions, while processes employing bipolar membrane electrodialysis (BPMED) exhibit heightened current efficiency [
38,
39]. Researchers have persistently investigated the enhancement of current efficiency and energy consumption of electrochemical cells through experimental means. For instance, Eisaman et al. employed a bipolar membrane electrolysis (BPMED) system to remove carbon dioxide (CO
2) from a bicarbonate solution [
40], while Nagasawa et al. applied a triple-membrane configuration to recover CO
2 from a sodium bicarbonate solution [
41].
2.5.4. Carbon Capture Based on Natural Inclusions
Biological methods for CO2 capture have been shown to have several advantages, including being environmentally friendly, fast-growing, lipid-rich, and photosynthesizing. For instance, microalgae have been utilized for the absorption of carbon dioxide. Some species of microalgae demonstrate resistance to toxic gases, and their lipid content and conversion to biodiesel through the trans-esterification process are crucial for CO2 capture. The tolerance of different microalgae to CO2 concentrations exhibited variation, with some microalgae attaining their maximum cell growth mass at CO2 concentrations of approximately 10–20%. Furthermore, the process of mineralization, wherein dissolved carbon dioxide (CO2) and metal ions interact to form stable carbonates within subsurface rock formations, constitutes an additional method of carbon capture. Although this process requires a greater temporal investment to establish stable carbonate minerals in sandstones, it is the most stable mode of carbon capture. Biocatalysts, such as carboxylases, which react with CO2 under mild conditions, play an important role in mitigating atmospheric CO2 accumulation.
2.5.5. CO2 Capture Based on Membrane Separation
Membrane screens are utilized in the separation of molecules based on their molecular size and are employed in a variety of applications, including CO
2 separation. The membranes currently in use include inorganic membranes, mixed matrix membranes, hollow fiber gas–liquid membrane contactors, facilitated transport membranes, and polymer gas permeation membranes. Polymer membranes are preferred due to their cost-effectiveness, stability, and high flux. In contrast, ceramic membranes are effective when used in combination with zeolites; however, they present manufacturing challenges. The selection of membrane is contingent upon its permeability and selectivity, with the incorporation of amine functional groups serving to augment the membrane’s capacity to capture CO
2 [
42]. In the domain of membrane capture technology, the gas mixture is separated into permeate and retention streams through the use of a semipermeable barrier. The membrane mechanisms that facilitate CO
2 separation include non-transportable and transportable membranes. For instance, the incorporation of polyethylene glycol plasticizers into low permeability glass polymer membranes enhances CO
2 permeability and CO
2/N
2 selectivity. In addition, copolymer membranes composed of polyvinylidene fluoride exhibit enhanced selectivity for CO
2 over N
2 and superior permeability [
43].
2.5.6. CO2 Capture Based on Cryogenic Separation
The low-temperature process of CO
2 capture involves the compression and cooling of the flue gas to a temperature slightly above the temperature at which the CO
2 becomes a solid. The pressurization of the CO
2 precipitate then forms a liquid CO
2 phase and a gaseous nitrogen stream. This process is conducted under conditions of low temperature and high pressure, with the capacity to achieve a CO
2 recovery rate of approximately 90–95% from the flue gas. Low-temperature systems are available in the form of distillation column separations and internally cooled flash separations. These are energy-intensive distillation processes; however, they are capable of handling high concentrations of CO
2 gas and achieving high efficiencies. Nevertheless, the process under discussion is incapable of directly capturing CO
2 from high-temperature, high-emission flue gases. At present, it is utilized for the separation and purification of gas mixtures containing 30–90% CO
2. The primary challenge in developing low-temperature CO
2 capture technology is the implementation of low-temperature refrigeration, which facilitates the separation of high-purity CO
2 through the effective utilization of cold energy [
44].
2.5.7. Comparative Performance of CO2 Capture Techniques
Recent studies have demonstrated notable differences among CO
2-capture technologies in terms of energy consumption, CO
2 purity, and scalability. Chemical absorption using amine-based solvents can achieve CO
2 purities above 99%, but typically requires regeneration energies in the range of 3.5–4.0 GJ t
−1 CO
2 [
45]. In contrast, adsorption-based systems employing solid sorbents consume less energy (approximately 2.0–2.5 GJ t
−1 CO
2) and offer superior modular integration, although their capture efficiencies of 80–90% depend strongly on sorbent surface stability [
46]. Membrane separation processes present moderate energy demands (about 2.5–3.0 GJ t
−1 CO
2) and high scalability potential, yet typically deliver CO
2 purities below 95% [
47].
Among various emission sources, lime-kiln flue gas is particularly advantageous for CO
2 capture due to its relatively high CO
2 concentration (approximately 20–35%) and low exit temperature (120–180 °C). These characteristics markedly reduce the energy required for cooling and compression, in comparison with power-plant flue gas (~12–15% CO
2, 300–350 °C) or steelmaking gas (~15–20% CO
2, 200–250 °C). As a result, lime-kiln emissions are inherently more energy-efficient and economically viable for CO
2 capture and subsequent agricultural utilization [
48].
In practical greenhouse trials, CO
2 enrichment to 800–1000 ppm has been shown to increase tomato and cucumber yields by 25–35%, lettuce by 20–30%, and strawberry by 15–25% relative to ambient conditions [
49]. For cereal crops such as wheat and rice, moderate CO
2 concentrations of 600–800 ppm have resulted in grain-weight and photosynthetic-efficiency improvements of about 10–20% [
50]. These findings confirm the strong agronomic potential of purifying lime-kiln CO
2 for use as a fertilizer gas, particularly within controlled-environment agriculture settings where gas distribution can be precisely regulated.
2.6. CO2 Purification and Concentration
The captured carbon dioxide gas contains a trace amount of impurities, including incompletely removed SO2, NOx, CO, and moisture. These impurities require further purification and concentration to ensure the desired purity level of the gas. The purification process typically involves the use of adsorption, distillation, and other methods. The adsorption method involves the use of an adsorbent to purify impurities. This process utilizes activated carbon, molecular sieve, and other adsorbents that are incorporated into the adsorption tower. During the process, carbon dioxide gas passes through the adsorption tower, where the impurities are adsorbed by the adsorbent, leading to the purification of the carbon dioxide. Conversely, distillation employs the disparity in boiling points of the components to effectuate the separation of impurities from carbon dioxide gas through a series of evaporation and condensation processes. The process of achieving concentration entails the application of compression and cooling techniques, thereby increasing the concentration of carbon dioxide to the level that meets the standard criteria for utilization as a gas fertilizer. Multi-stage compression is employed to reduce the volume of the carbon dioxide gas to a specific pressure, and subsequently, it is liquefied through cooling to achieve the desired concentration. In practical applications, the impurity is removed by Pressure Swing Adsorption (PSA) technology, which utilizes the difference in adsorption capacity of the adsorbent to the gas under different pressures to adsorb the impurity under high pressure and desorb the impurity under low pressure. This process realizes the purification and concentration of carbon dioxide. The process entails the liquefaction of carbon dioxide through multi-stage compression and cooling, resulting in a concentration of carbon dioxide that exceeds 99%, thereby meeting the criteria for gas fertilizer.
2.7. Gas Fertilizer Storage and Transportation
The stability of gas fertilizers during storage and transportation is a critical factor that impacts the efficacy of their application. The utilization of phase change materials in the domains of storage and transportation offers a novel solution to this challenge.
The EG/DEG-based absorption system demonstrates remarkable CO2 sequestration efficacy at low temperatures. The findings indicated that the system retained its capacity for CO2 sequestration at a rate of over 90% when exposed to temperatures as low as −10 °C. The EG/DEG-based absorption system utilizes the phase change properties of phase change materials at low temperatures to immobilize the CO2 in the system to form stable compounds or solutions, thereby achieving efficient CO2 sequestration. The system’s stability at low temperatures is notable, as it effectively mitigates leakage and loss of CO2, thereby ensuring the reliability of long-distance transportation and long-term gas fertilizer storage.
The microencapsulation technology further enhances the controllability of the gas fertilizer. The employment of sodium alginate to encapsulate CO2 droplets enables the precise modulation of the release rate of CO2, which can be adjusted within the range of 0.1–1.0 ppm/h. This method is particularly well-suited to the demands of precise fertilization. Sodium alginate, a natural polymer material, exhibits favorable biocompatibility and degradability. Through the meticulous regulation of sodium alginate concentration, the coating process, and other variables, the structural and functional characteristics of the microcapsules can be tailored to achieve precise modulation of the CO2 release rate. This ensures that the crops receive the optimal supply of CO2 at various growth stages, enhancing the efficacy of gas fertilization.
2.8. Air Fertilizer Application
Presently, the enhancement of CO
2 levels in greenhouses can be accomplished through the utilization of CO
2 cylinder gas, organic composting, the combustion of organic matter, and chemical reactions [
51]. Carbon dioxide (CO
2) cylinder gas is characterized by its safety, cleanliness, and controllable concentration. However, during winter months, the vaporization of CO
2 can effectively reduce greenhouse temperatures. This phenomenon can pose significant logistical challenges, as the transportation of cylinders can be cumbersome and costly. Organic composting is an economical approach; however, the CO
2 concentration and timing of application are challenging to regulate, thereby constraining its practical application [
52,
53,
54]. The organic combustion method has been demonstrated to be a source of toxic and harmful gases, and it has been observed to present safety hazards. The chemical reaction method typically utilizes sulfuric acid and ammonium bicarbonate to generate CO
2. However, sulfuric acid is a hazardous chemical, which hinders its promotion. Consequently, an effective CO
2 aerosol fertilization method ought to be capable of standardized, large-scale production in protected agriculture, while also aligning with the national strategy of energy conservation and emission reduction. Li et al. developed a slow-release system based on microporous diffusion tubes, which have been shown to stabilize the concentration of CO
2 in greenhouses within the range of 800–1000 ppm. The system gradually releases CO
2 into greenhouses through microporous diffusion tubes, thereby achieving a relatively stable control of the concentration of CO
2. However, in practical applications, the system may experience uneven distribution of CO
2, which can impede the ability to meet the CO
2 demand of crops in different areas of the greenhouse.
The integration of adsorption carriers with intelligent control devices to achieve uniform spatial distribution through multi-point deployment holds significant potential. The adsorption carrier has the capacity to store a specific quantity of CO2, which it can then release in accordance with the real-time demand within the greenhouse. The intelligent control device is capable of monitoring the CO2 concentration, temperature, humidity, and other environmental parameters within the greenhouse in real time. Based on these measurements, the device can automatically adjust the amount of CO2 released and the timing of its release, ensuring that it is always aligned with the specific growth demands of the crop. Multi-point deployment facilitates the uniform distribution of CO2 throughout the greenhouse, enhancing the utilization efficiency of gas fertilizers and creating a more favorable CO2 environment for crop growth.
3. Current Status of Research on the Application of CO2 Gas Fertilizer in Agriculture
3.1. Effect of CO2 Fertilization on Crop Growth
A substantial body of research has demonstrated that an appropriate increase in CO2 concentration exerts a significant effect on the growth of C3 crops. C3 crops are distinguished by their initial products of CO2 fixation during photosynthesis, which are three-carbon compounds. This characteristic renders them sensitive to changes in CO2 concentration.
At the level of physiological response, Kimball demonstrated through meta-analysis that the photosynthetic rate of tomatoes increased by 40% and water use efficiency increased by 27% when the CO
2 concentration was doubled [
55]. This phenomenon can be attributed, primarily, to the fact that an increase in the CO
2 concentration furnishes greater quantities of raw materials for photosynthesis. This, in turn, strongly promotes the photosynthetic electron transfer and carbon assimilation processes, thereby increasing the photosynthetic rate. Concurrently, elevated atmospheric carbon dioxide levels can stimulate stomatal opening in crops, thereby reducing water loss through evaporation and enhancing water-use efficiency. This phenomenon is of particular significance for agricultural production in regions experiencing limited water availability.
With respect to the enhancement of yield, trials conducted at Wageningen University in the Netherlands yielded significant results. The application of CO
2 enrichment (800 ppm) to cucumbers resulted in a 22–35% increase in yield [
56]. The provision of an adequate supply of CO
2 resulted in optimal conditions for the photosynthesis of the crop, leading to a substantial accumulation of photosynthesis products and establishing a sufficient material foundation for the crop’s growth. This, in turn, effectively augmented the crop yield [
56].
With respect to the pursuit of quality enhancement, the research outcomes of the Chinese Academy of Agricultural Sciences (CAAS) are noteworthy. The findings of the study indicated that CO
2 fertilization of spinach resulted in an 18% increase in VC content and a 15% increase in soluble sugars [
57]. This outcome can be attributed to the promotion of carbon metabolism processes within the crop by CO
2 fertilization, thereby enabling the synthesis of additional organic substances. Consequently, this enhanced the crop’s quality, particularly in terms of taste and nutritional value, thereby satisfying consumer demand for high-quality agricultural products.
However, the majority of extant studies in this area have utilized pure CO2 gas sources. In practice, gas sources such as industrial tail gas from lime kilns contain trace amounts of pollutants such as SO2 and NOx. These pollutants are likely to exert long-term effects on crops during extended CO2 fertilization. For instance, SO2 has been shown to induce stomatal closure in crop leaves, thereby impeding the conventional operation of photosynthesis. Similarly, NOx has been observed to disrupt the physiological and metabolic processes of crops, potentially leading to adverse effects on their growth, development, yield, and quality. A number of studies have demonstrated that low concentrations of NOx have the capacity to inhibit the activity of certain key enzymes in crops over an extended period of time. This inhibition can affect the absorption and utilization of nutrients, thereby influencing the growth and development of crops. Consequently, a systematic investigation into the long-term impacts of trace pollutants in industrial tail gas on crops is imperative to comprehensively evaluate the feasibility and safety of using such tail gas as a CO2 fertilizer source.
3.2. Precision Application Techniques
In the context of greenhouse agriculture, the application of CO2 gas fertilizer poses a significant challenge due to the uneven spatial and temporal distribution of CO2 concentration. The distribution of carbon dioxide (CO2) gas in greenhouses is often challenging due to the intricate airflow patterns, the obstruction of crops, and the positioning of the CO2 gas source. These factors contribute to the inability to achieve uniform distribution, resulting in areas of the crop receiving insufficient CO2. This, in turn, significantly impacts the efficacy of gas fertilization.
A multi-node monitoring system based on the Internet of Things (IoT) was developed by Japanese scholars to optimize airflow organization with the help of CFD simulation [
58]. The monitoring system employs IoT technology, systematically integrating multiple sensor nodes within the greenhouse. These nodes facilitate the real-time observation of critical environmental variables, including CO
2 concentration, temperature, and humidity. A CFD (Computational Fluid Dynamics) simulation can be utilized to analyze the airflow movement inside the greenhouse in depth. This analysis can then be used to optimize the location of the CO
2 air source and the airflow organization. This optimization is based on the simulation results, and it can effectively improve the distribution uniformity of CO
2 in the greenhouse.
In this regard, the proposed “six-direction” deployment scheme, in conjunction with online monitoring, offers a novel solution to the problem, thereby establishing a more refined concentration control model. The “six-orientation” deployment scheme, which involves the delivery of CO2 to the greenhouses from multiple angles and locations, has been shown to effectively mitigate the issue of dead spots in CO2 distribution. This approach ensures that crops within each area receive an adequate supply of CO2, thereby enhancing their growth and development. Online monitoring systems have been developed to obtain real-time CO2 concentration data in the greenhouse, thereby providing an accurate and timely basis for concentration regulation. The integration of the “Six Directions” layout program with online monitoring facilitates the dynamic adjustment of the amount and location of CO2 release, contingent on the actual conditions in the greenhouse, including fluctuations in CO2 concentration during varying time periods and under diverse weather conditions. For instance, during daylight hours when there is ample light and vigorous photosynthesis by the crop, the release rate of CO2 can be appropriately increased. Conversely, during nocturnal hours or under low-light conditions, the release rate is reduced to avoid unnecessary resource consumption. Consequently, a more sophisticated concentration regulation model can be formulated to ensure the precise application of CO2 gas fertilizer, enhance its utilization efficiency, reduce production costs, and simultaneously provide an optimal solution for crop cultivation.
4. Exploration of Industrialization Mode
Presently, the global market for CO
2 gas fertilizer is predominantly concentrated in North America and Europe, where the application technology for this fertilizer is relatively mature and the market size is substantial. In contrast, China is still in the demonstration stage in this field. Although relevant research and practice have been carried out, a gap remains when compared with developed countries [
59].
The OCAP system in the Netherlands exemplifies a successful implementation, facilitating the transportation of CO2 from the refinery to 4500 hectares of greenhouses through the utilization of pipelines. This development has effectively established an interface between industrial CO2 emissions and agricultural production, resulting in an annual carbon reduction of 300,000 tons. The implementation of large-scale pipeline delivery has been demonstrated to reduce transportation costs of CO2 and enhance the efficiency of gas fertilizer supply, thereby achieving substantial emission reductions.
In China, a trial conducted by Bao steel to utilize steel mill tail gas for leafy vegetable gas fertilizer also achieved some results, with a cost reduction of 40% [
60]. This trial provides a useful reference for the resource utilization of tail gas CO
2 in the steel industry in China and proves the economic feasibility of preparing gas fertilizer from industrial tail gas.
The tripartite collaboration model of “enterprise-scientific research-farmer” fully takes into account the distributed nature of Chinese facility-based agriculture. Enterprises are responsible for supplying industrial tail gas CO2 gas source and related equipment, scientific research institutes provide technical support and R&D innovation, and farmers are responsible for the practical application of gas fertilizer and crop cultivation. Through the integration of localized production of adsorption carriers and agricultural channels, transportation costs can be effectively reduced and the economic efficiency of the whole industrial chain can be improved. For example, in the localized production of adsorbent carriers, local resources and production conditions can be used to reduce transportation and intermediate link costs; in the integration of agricultural channels, the promotion and sales costs of gas fertilizer can be reduced by cooperating with existing agricultural sales channels, so that the gas fertilizer can reach farmers more conveniently, and the industrialization and promotion of the technology can be promoted.
5. Research Perspectives
5.1. Scale-Up Process for Preparation of Low-Cost Adsorbent Materials
Despite the development of numerous high-performance adsorbent materials, cost remains a significant constraint in their practical applications. In the future, further study is necessary to expand the understanding of the scale-up preparation process of low-cost adsorbent materials with the objective of reducing the production cost of adsorbent materials. A concerted effort should be made to utilize more economical raw materials, optimize preparation methods, and enhance production efficiency. These measures will facilitate the large-scale industrial production of adsorbent materials [
61]. Concurrently, it is imperative to prioritize the environmental sustainability of the preparation process to avert the emergence of novel environmental contamination issues.
5.2. Mechanisms of Long-Term Effects of Tail Gas Impurities on Crop Quality
In addition to CO
2, industrial exhaust also contains impurities such as SO
2 and NO
x. The mechanism of the long-term effects of these impurities on crop quality is not yet fully elucidated [
62]. In the future, a comprehensive study of the effects of exhaust impurities on crop growth and development, physiological metabolism, and quality formation will require long-term field experiments and indoor simulation experiments. By analyzing the accumulation and transformation process of impurities in crops, as well as their effects on gene expression and enzyme activities, the mechanism of their action will be revealed. This will provide a theoretical basis for developing targeted countermeasures.
5.3. Development of a Digital Twin-Based System for Precise Regulation of Air Fertilization
Digital twin technology, as an emerging intelligent control tool, has broad application prospects in agricultural CO
2 utilization. In future greenhouse systems, digital twin models can create a real-time virtual replica of crop growth and CO
2 fertilization dynamics. The system operates by continuously acquiring multi-source data, including environmental parameters such as temperature, humidity, light intensity, CO
2 concentration, and air velocity, as well as crop physiological indicators such as photosynthetic rate, stomatal conductance, and biomass accumulation, together with equipment operation data such as CO
2 injection rate, valve position, and fan speed. These real-time datasets serve as input for model calibration and predictive simulation, enabling bidirectional interaction between the physical greenhouse and its virtual counterpart [
63,
64]. Key control parameters include CO
2 release rate (mg·m
−2·s
−1), release duration, spatial distribution uniformity, and energy consumption per unit yield. Optimization algorithms such as model predictive control (MPC) or adaptive feedback regulation can then adjust CO
2 dosing based on predicted crop demand and environmental fluctuation [
65].
By integrating sensing, simulation, and control, the digital twin enables precise CO
2 management, improving gas fertilizer utilization efficiency by up to 20–30% and supporting stable, high-quality crop production [
66]. This approach provides a foundation for developing intelligent, self-optimizing greenhouse systems that link industrial CO
2 recycling with smart agriculture.
6. Critical Perspectives and Future Outlook
The conversion of lime-kiln flue gas into CO2-based fertilizer forms a key link between industrial emission reduction and sustainable agriculture. While research on CO2 capture and fertilization has progressed rapidly, most studies treat these systems independently. Integrating capture efficiency, purification quality, and plant response is essential to position CO2 fertilization as a circular-carbon strategy that transforms industrial waste into agricultural value.
Advances in chemical absorption, solid adsorption, and membrane separation have improved CO2 recovery efficiency, with amine-based systems remaining dominant and emerging hybrid sorbents showing energy advantages. Controlled CO2 fertilization effectively enhances photosynthesis and yield in C3 crops, yet existing studies are largely short-term and idealized. The roles of impurities (SO2, NOx), humidity, and soil chemistry are insufficiently examined, and unified evaluation standards are lacking. Moreover, limited techno-economic analyses constrain the scalability and cross-disciplinary relevance of current findings.
Future work should focus on developing durable and low-cost sorbents that can adapt to variable flue-gas compositions, as well as conducting long-term and comprehensive studies on the interactions among impurities, crops, and soils. Integration of CO2 distribution control, environmental monitoring, and digital-twin feedback systems can improve precision fertilization and sustainability. Coupling these technologies with carbon-accounting and trading frameworks will expand CO2 fertilization from an agronomic tool to a core component of industrial carbon management, supporting both emission reduction and food-production goals.
7. Conclusions
The utilization of industrial tail gas from lime kilns for the production of CO2 gas fertilizer, followed by its application in agriculture, has emerged as a promising “industry-agriculture” synergistic model for emission reduction. In terms of CO2 recovery, adsorption technologies have shown remarkable progress; however, the development of high-efficiency, low-cost adsorbent materials and the optimization of CO2 fertilization systems remain critical research priorities.
In agricultural applications, CO2 fertilization has demonstrated significant benefits in enhancing crop growth, yield, and quality. Nevertheless, further studies are needed to elucidate the long-term effects of tail gas impurities on crop physiology and soil ecosystems, as well as to improve the precision and uniformity of CO2 distribution within controlled environments.
From an industrialization perspective, several domestic and international practices, such as OCAP in the Netherlands and pilot-scale projects in China, have validated the feasibility of CO2 resource utilization. Moving forward, research should focus on scalable preparation of cost-effective adsorbents, mechanistic understanding of impurity interactions, and the establishment of cross-industry carbon sink trading frameworks. Additionally, integrating CO2 utilization with digital twin-based precision control systems, greenhouse environment monitoring, and intelligent irrigation management will extend the application of this technology to smart agriculture, carbon recycling, and even urban carbon management. Such advancements will accelerate the deep integration of industrial emission reduction with sustainable agricultural production, thereby supporting the achievement of global carbon neutrality goals.