FTIR is an important means to study the chemical structure of coal, which can quantitatively characterize the degree of coalification to a certain extent [
23].
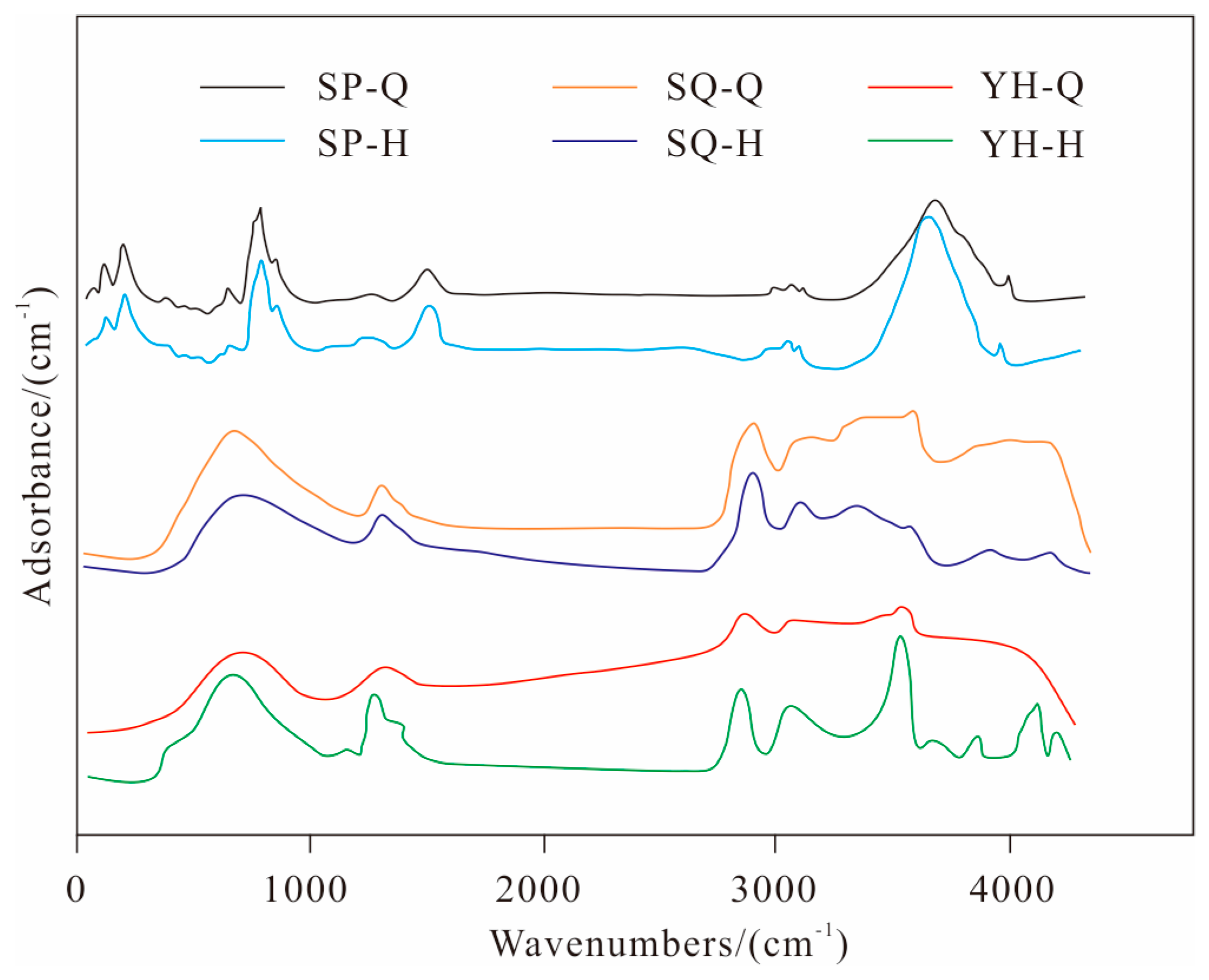
Figure 2 shows the FTIR of raw coal and fractured coal samples from Shaping, Shaqu, and Yonghong coal mines. It can be seen that the peak profile type of coal samples changes to varying degrees before and after HPWI treatment.
Due to the phenomenon of overlapping infrared spectral peaks, it is necessary to use infrared overlapping spectral band peaking technology to improve the accuracy of quantification of infrared spectral peaks [
21,
22,
23]. In this paper, Origin8.5 software was used to carry out infrared peaking fitting, ensuring that the fitting coefficient reached the standard of 99.9% or more, and the attribution of each functional group was determined by each fitting peak position after fitting. Among them, the changes of coal chemical structure and before and after HPWI treatment were analyzed mainly from three aspects: oxygen-containing functional group (infrared spectrum 1000–1800 cm
−1), aliphatic hydrocarbon (infrared spectrum 2800–3000 cm
−1), and hydrogen bond (infrared spectrum 3000–3700 cm
−1).
3.2.1. Oxygen Functional Groups in Coal (1000–1800 cm−1)
Oxygen in coal mainly exists in the form of water, inorganic oxygen-containing compounds, and oxygen-containing functional groups, among which the oxygen in the form of oxygen-containing functional groups has a greater impact on the properties of coal [
30,
31]. The oxygen-containing functional groups in coal mainly include carboxyl, carbonyl, hydroxyl, etheroxy, methoxy, and other types. Among them, hydroxyl and ether oxygen are the main functional groups. In the range of 1000–1800 cm
−1, it mainly includes the infrared absorption fluctuations of the ether–oxygen bond, carboxyl group, and carbonyl group oxygen-containing functional groups, as well as the deformation vibration of CH
3, C=C, and C=O stretching vibration.
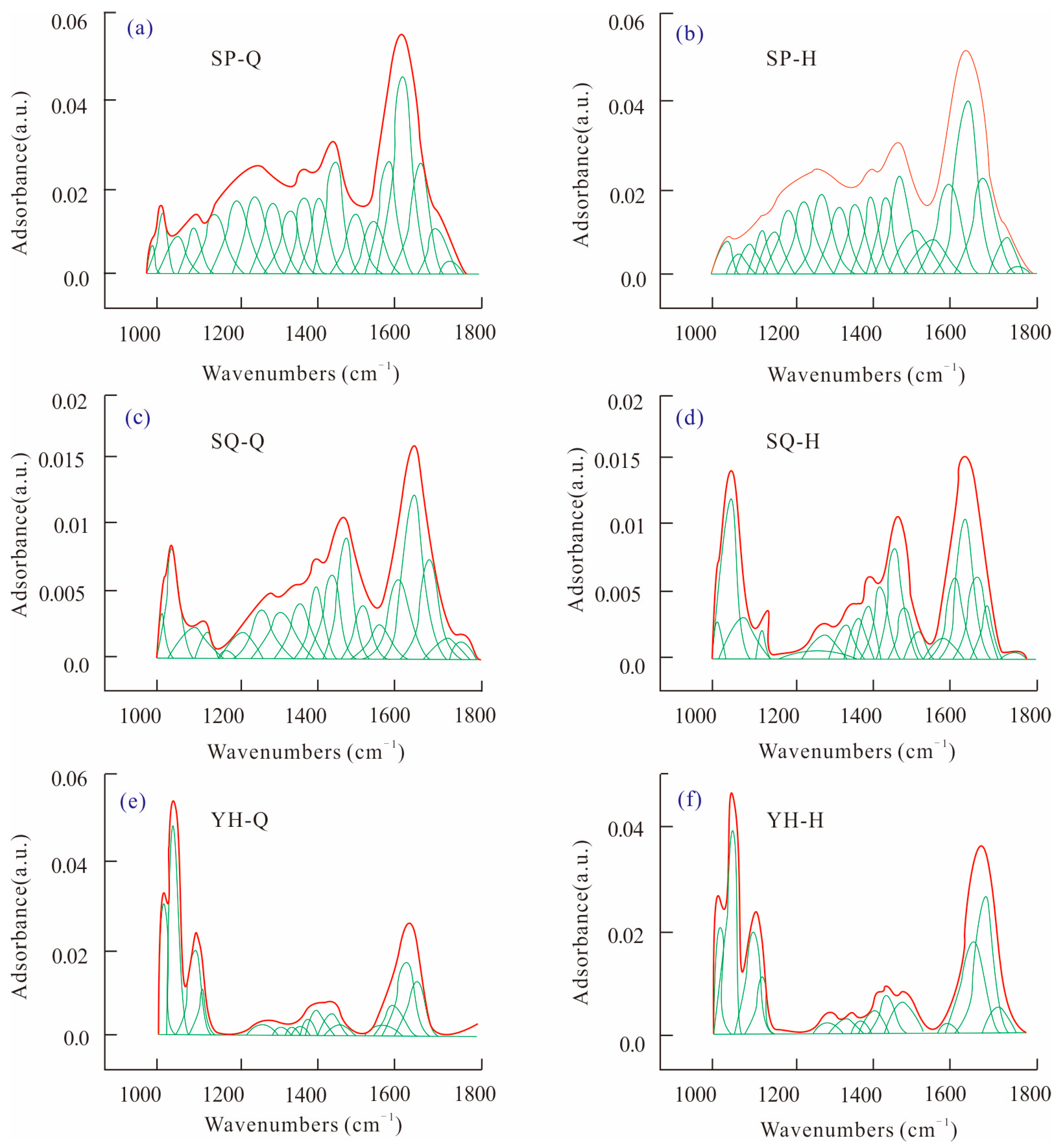
The oxygen-containing functional groups of coal samples were fitted by peak separation before and after HPWI treatment, as shown in
Figure 3. The regression coefficients were all above 99.9%, indicating a good degree of fitting. According to the peak area, the content of oxygen-containing functional groups in Shaping coal is obviously higher than that in Shaqu coal and Yonghong coal, and the content of oxygen-containing functional groups in Shaqu coal is the lowest.
The peak fitting results of the three groups of coal samples were statistically analyzed, and the relative contents of each oxygen-containing functional group in the coal samples were obtained, as shown in
Table 2. The ‘relative content’ values represent the percentage of the total peak area. The ash content of Yonghong coal is the highest, while the ash content of Shaping coal is the least. Among the three kinds of coal samples, the phenols, alcohols, ethers, phenoxy groups, C–O stretching vibration in ester, and C=C vibration of aromatic hydrocarbons in Yonghong anthracite are obviously higher, indicating that oxygen is mainly in the form of non-active oxygen in coal with high metamorphism. This is consistent with previous experimental results [
32].
The type and content of functional groups in coal will be changed by HPWI treatment. In the 1000–1800cm−1 coal sample, the absorption peaks are mainly C–O single bond, C=O double bond, and C=C double bond. The peak area content is more than 60% of the total area. After HPWI treatment, the ash content in coal changes to different degrees. The content of Ash in Shaqu coal and Yonghong coal decreases by 2.29% and 27.91%, while it increases by 297.87% in Shaping coal. The C–O bond content in Shaping coal and Yonghong coal decreases by 6.32% and 15.19%, while the C–O bond content in Shaqu coal increases by 50.96%, which reflects that the HPWI treatment changes the content of phenols, alcohols, ethers, phenoxy groups, and esters in coal to different degrees. The content of C=O in Shaping coal and Yonghong coal increases by 2.44% and 27.84%, respectively.
The change of ash, C–O, and C=O content in coal is caused by the change of aromatic hydrocarbon and oxygen-containing group in coal structure and mineral content in coal matrix after HPWI treatment. According to the C=O stretching vibration in coal and the weak peak at 2840 cm−1, it can be inferred that there are aldehyde and ketone groups in the molecular structure of coal. After HPWI treatment, the intensity of the C=O stretching vibration changes, and the content of aldehyde and ketone groups in coal changes.
3.2.2. Aliphatic Hydrocarbons in Coal (2800–3000 cm−1)
There are three kinds of fatty substances in coal, i.e., methyl, methylene, and methylene. In the infrared spectrum, the adipose stretching vibration range is 2990–2800 cm−1, and there are three large absorption peaks at 2953 cm−1, 2921 cm−1, and 2851 cm−1. The absorption peak at 2953 cm−1 reflects the antisymmetric CH3 stretching vibration in coal, and the absorption peak at 2921 cm−1 is the antisymmetric CH2 stretching vibration in coal. The absorption peak at 2851 cm−1 is a symmetric CH2 stretching vibration.
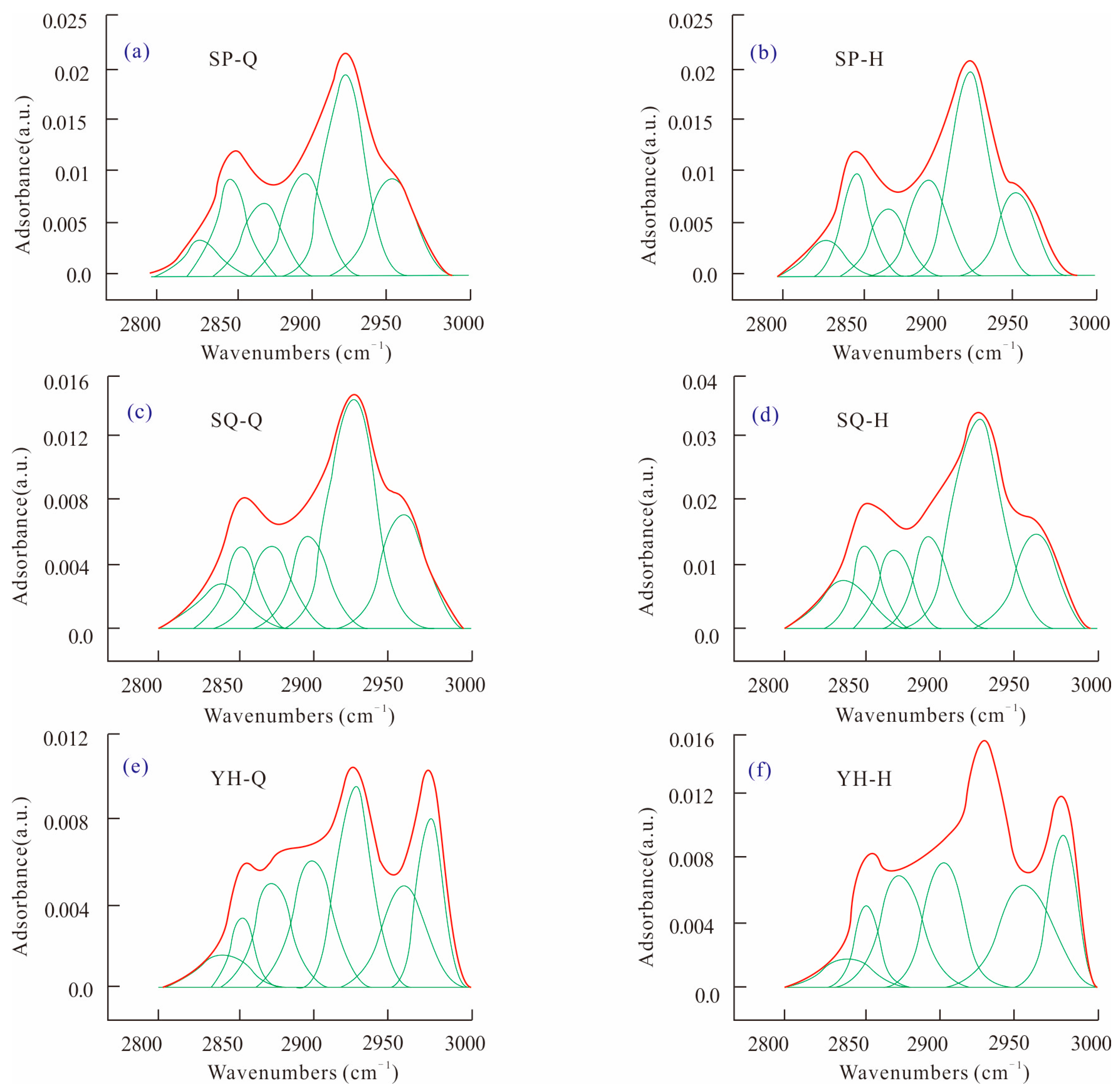
The absorption peak of 2800–3000 cm
−1 is the absorption range of C-H in the FTIR spectrum of the fat chain and lipid ring. The aliphatic hydrocarbons in coal mainly include methyl group, methylene group, and methylene group. The fitting spectra of Shaping, Shaqu, and Yonghong coal samples between 2800 and 3000 cm
−1 before and after HPWI treatment are shown in
Figure 4.
The peak fitting results of the three groups of coal samples were statistically analyzed, and the relative contents of each aliphatic hydrocarbon in the coal samples were obtained, as shown in
Table 3. It can be seen that asymmetric CH
2 and CH
3 stretching vibrations are the two main absorption peaks of coals. Yonghong coal is mainly dominated by antisymmetric RCH
3, followed by antisymmetric R
2CH
2, and Shaping coal and Shaqu coal are mainly dominated by antisymmetric R
2CH
2. Among them, the objection R
2CH
2 of Yonghong coal is much smaller than that of Shaping coal and Shaqu coal, the objection RCH
3 content increases with the increase in metamorphism degree, and the objection RCH
3 of Yonghong coal is much larger than that of Shaping coal and Shaqu coal. The aliphatic hydrocarbon content of Yonghong coal is significantly different from that of Shaping coal and Shaqu coal, indicating that during the evolution of coal, the straight chain part of fat will decrease and the number of branched chains will increase.
After HPWI treatment, the infrared absorbance increases significantly, but the peak shape change is not very obvious. The R2CH2 contents increase by 19.75% and 12.5% in Shaping coal and Shaqu coal, while decreasing by 6.48% in Yonghong coal. The RCH3 content increases by 21.11% in Yonghong coal, while it decreases by 19.09% and 24.01% in Shaping coal and Shaqu coal. These changes imply that the branched degree of the alkane chain decreases and the order is better after HPWI treatment. The branched chain of aliphatic hydrocarbon increases, and the branched chain decreases in the coal molecules of Shaping fractured coal and Shaqu fractured coal. However, the branched chain of aliphatic hydrocarbon increases, and the branched chain decreases in the coal molecules of the Yonghong fractured coal.
The change of the content of asymmetric methylene and asymmetric methylene in the three groups of coal samples indicates the change of the degree of H shedding in coals. The change trend of aliphatic hydrocarbons in Yonghong coal is different from that of Shaping coal and Shaqu coal, because the evolution of Yonghong anthracite has matured, and the chemical properties of coal have changed greatly compared with those of middle and low-rank coal [
21,
23]. In addition, the peak area of Yonghong coal and Shaping coal decreased after HPWI treatment, indicating that the alkane in coal decreased, while the peak area of Shaqu coal increased after HPWI treatment, indicating that the alkane in coal increased.
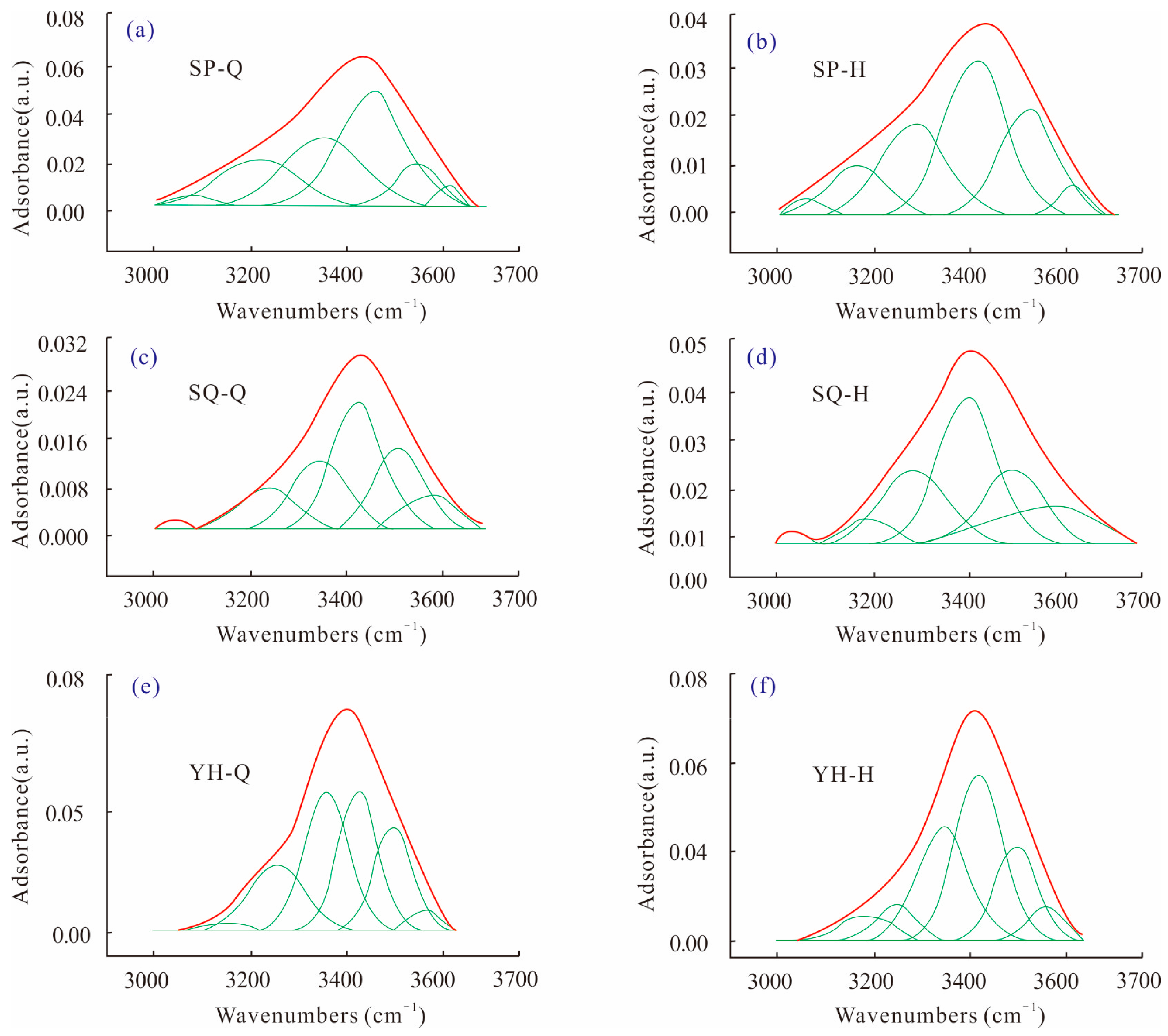
3.2.3. Hydrogen Bonds in Coal (3000–3700 cm−1)
The most important functional group in hydrogen bonding is the hydroxyl group, which is the main non-covalent bond for the construction of coal macromolecular structure and is most closely related to the surface properties of coal. Among them, the polymer that accounts for a large proportion of the total hydroxyl group is the specific manifestation of the association structure in coal structure. According to relevant literature [
33,
34,
35], there are six types of hydroxyl groups in coal. The fitting spectra of coal samples between 3000 and 2800 cm
−1 before and after HPWI treatment in Shaping, Shaqu, and Yonghong coals are shown in
Figure 5.
The peak fitting results of the three groups of coal samples were statistically analyzed, and the relative content of each hydrogen bond in the coal samples was obtained, as shown in
Table 4. It can be seen that the hydroxy-ether–oxygen bond and hydroxy–hydroxy–hydrogen bond are the two main components of coal. Among them, Yonghong coal has the most hydroxy–ether–oxygen bond and no hydroxy–N bond, and Shaping coal and Shaqu coal have the most hydroxy–hydroxy–hydrogen bond. With the increase in coal rank, the content of hydroxy–N bond gradually decreases and disappears, and the content of hydroxy–hydroxy-hydrogen bond gradually decreases, indicating that the hydroxy–N bond and hydroxy–hydroxy–hydrogen bond have been destroyed in the coal evolution process. With the increase in coal rank, the number of ring-associated hydroxyl groups decreases, which is related to the continuous shedding of hydroxyl groups during coal evolution. The content of the hydroxy–ether–oxygen bond in Yonghong coal is the highest because there are more oxygen elements in the form of ether oxygen in Yonghong coal, which increases the probability of the hydroxy–ether–oxygen bond.
After HPWI treatment, the content of cyclic associated hydroxy–hydrogen bond decreases by 41.25%, 63.92% and 65.86% in Shaping, Shaqu, and Yonghong coals, and the content of free hydroxyl group increases by 57.92%, 58.42%, and 93.71%. And the content of the hydroxy–ether–oxygen bond decreases in Shaping coal and Yonghong coal, but increases in Shaqu coal, which is consistent with the change trend of the ether–oxygen bond after HPWI described above. The content of the hydroxy–N bond and hydroxy–hydroxyl–hydrogen bond decreases in Shaping coal, but increases in Shaqu coal and Yonghong coal. The content of the hydroxy–P hydrogen bond increases in Shaqu coal, but decreases in Shaqu coal and Yonghong coal. The differences in the variation trend of Shaping coal after HPWI are caused by the low evolution degree of low-rank coal.
After HPWI treatment, the changes of hydrogen bonds in different coal ranks are different, because the interaction mechanism between hydrogen bonds and water in different coal ranks is different, and the sensitivity to water is also different. As pointed out by Kang et.al. [
36] in their study on the structure and spectral properties of typical hydrogen-bonded organic crystals under high pressure, high pressure causes the formation of new hydrogen bonds, while the original hydrogen bonds are broken, and the hydrogen bond network is rearranged, which leads to the change of crystal structure symmetry. It can be inferred that the size and type of intramolecular and intermolecular hydrogen bonds in coal samples change when the hydroxyl group transitions from a free state to a bound state under HPWI treatment. The high-pressure action of water makes the arrangement of coal molecules denser and more orderly, and changes the configuration of coal molecules. Hydrogen bonds with weak bond energy in coal molecules will break under high pressure, and the interaction between water and coal molecules will also lead to the formation of some new bonds.