Towards Carbon-Neutral Hydrogen: Integrating Methane Pyrolysis with Geothermal Energy
Abstract
1. Introduction and State-of-the-Art (Chronological)
1.1. Why Turquoise Hydrogen Now?
1.2. State-of-the-Art Chronology
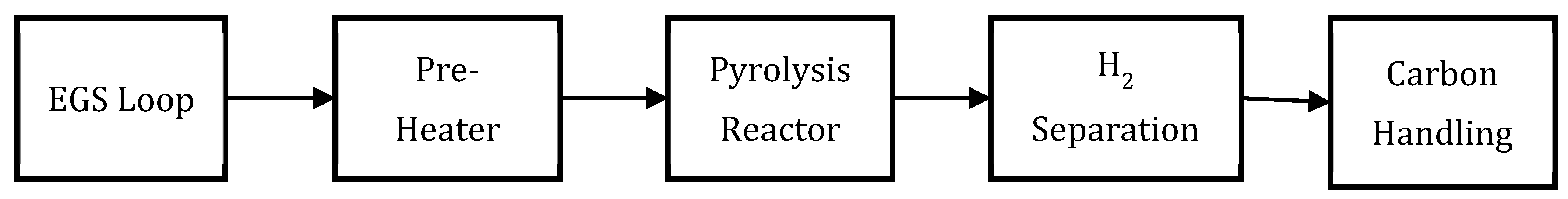
2. Concept and Real-World Anchors (EGS → Pyrolysis)
2.1. Process Concept and Duty Split
2.2. Heat Integration Logic
- ▪
- Use the EGS to supply base-load sensible heat to the largest heat-capacity streams (fresh methane, recycled flow, and, where applicable, the molten medium for isothermal hold).
- ▪
- ▪
2.3. Why Geothermal Energy?
2.4. Reactor Options and Operating Envelopes
2.5. Hydrogen Separation and Recycle
2.6. Carbon Handling and Value Preservation
2.7. Controls, Start-Up, and Operability
2.8. Site–EGS Coupling and Reporting Guidance
- ▪
- ▪
- For reproducibility, the study should report heat curves, major equipment sizes, duty splits between EGS and trim heat, catalyst or melt compositions, carbon QA/QC metrics, and separation or pressure targets. These disclosures should be aligned with best practices from recent turquoise-hydrogen techno-economic analyses (TEAs) and standard process design methodology [20,21,33].
3. Scalability: High-Pressure Design, Thermal Management, and Carbon Separation
3.1. High-Pressure Reactor Design
- ▪
- Kinetic/equilibrium guidance (design checks):
3.2. Thermal Management
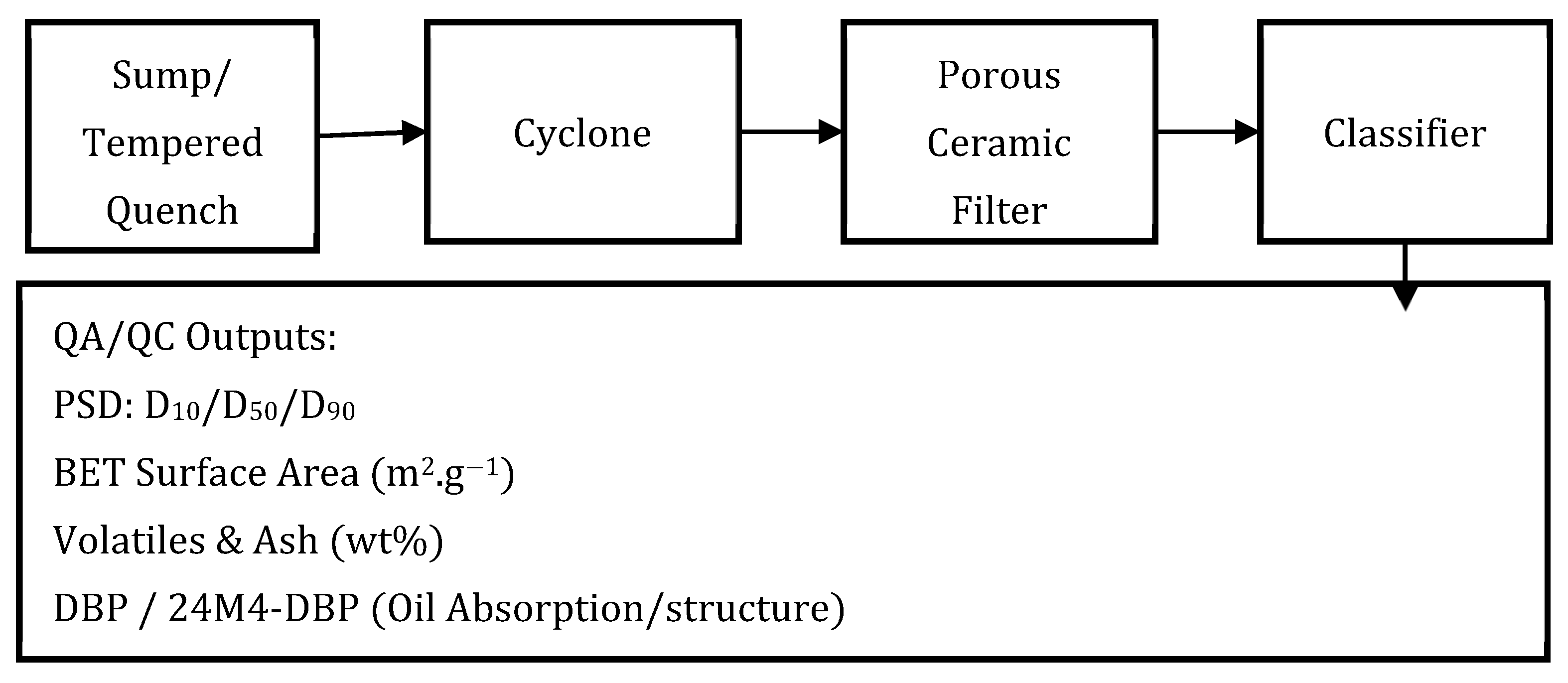
3.3. Carbon Separation and Handling
3.4. Practical Design Rules (Ready for the Methods Box)
- ▪
- Pressure and Temperature Setting: Start FEED with 10 to 25 bar, 600 to 900 °C; verify and ; close with recycling.
- ▪
- ▪
- ▪
- Carbon PSD: cyclone = 2 to 10 µm; filter face velocity = 1 to 3 cm s−1; protect compressors/separators with polishing filters [33].
- ▪
4. Techno-Economic Analysis (TEA)
4.1. Scope and Cases
- (i)
- EGS + electric, with geothermal input providing a base-load preheat/isothermal hold and electricity covering the final ΔT and transients.
- (ii)
- Solar–thermal + electric, where a solar field (with optional storage) provides preheat and electricity trims to the setpoint.
- (iii)
- Electric-only, where all of the duty comes from electric heaters.
4.2. Cost Structure
- (i)
- CAPEX (installed):
- ▪
- The heat supply includes EGS wells with surface heat exchangers and tie-ins, or, alternatively, a purchased-heat interface. In the solar-assisted case, this also covers a solar collector field and thermal storage, while electric trim heaters and power distribution systems are required across all configurations [20,21,33].
- ▪
- ▪
- ▪
- ▪
- (ii)
- OPEX (annual):
- ▪
- ▪
- Consumables consist of the catalyst or melt make-up, filtration media, inert gases, and water for quench or utility purposes.
- ▪
- (iii)
- Throughput-linked stoichiometry:
- (iv)
- Heat and power:
4.3. Revenue and Policy Levers
- ▪
- H2 product: The off-take price of hydrogen depends on the delivery pressure, product purity, and contract duration. The compression costs scale with the target pressure and the requirements of pipeline or storage specifications.
- ▪
- Carbon co-product: This is the revenue from carbon increases when the product has a tighter particle size distribution (PSD) and low volatile or ash content. Specialty carbon black (CB) grades command a premium compared to commodity carbon. The overall revenue from carbon can be expressed as follows:
- ▪
- Carbon credits/policy: Additional revenue can be captured by stacking production credits or applying market-based carbon pricing where eligible. The sensitivity of the levelized cost of hydrogen (LCOH) to these incentives is particularly strong when the power carbon intensity (CI) is low and the carbon sale value is high [3,4,5]. Cases that integrate geothermal preheating reduce the electric demand, thereby improving both the cost competitiveness and CI exposure [9].
4.4. Calculation Framework
- ▪
- Case-A (EGS + electric): The geothermal duty covers the preheat/isothermal hold, while the trim duty provide the final temperature rise and manages transients.
- ▪
- Case-B (Solar–thermal + electric): Replace with ; storage increases CAPEX but reduces the exposure to electricity.
- ▪
- Case-C (Electric-only): Nearly all of the duty is covered by ≈ , leading to the highest electricity consumption but simpler CAPEX.
4.5. Sensitivities and Expected Findings
- ▪
- Case-A (EGS + electric): This configuration achieves the lowest LCOH when the EGS capacity factor is high and geothermal heat (purchased or owned) is cost-effective. It also provides strong resilience against electricity prices and CI fluctuations.
- ▪
- Case-B (Solar–thermal + electric): This case improves the carbon intensity and reduces the electricity demand compared with the electric-only case. However, CAPEX increases due to the solar field and storage system, and the overall economics are highly dependent on the solar capacity factor and storage sizing.
- ▪
- Case-C (Electric-only): This option features the simplest CAPEX but exhibits the highest variance in LCOH due to the electricity price and CI. It serves as a useful baseline for comparing hybrid options A and B.
- ▪
- Fix nameplate → annual H2 via capacity factor → compute CH4 → calculate C via stoichiometry and yields.
- ▪
- Break CAPEX into blocks → apply CRF → add OPEX components.
- ▪
- Calculate the geothermal/solar duty and trim duty from the heat-integration model (Figure 2) → convert to electricity.
- ▪
- Add revenue streams from the H2 off-take, carbon grade mix, and policy credits.
- ▪
- Run scenarios for Case A, B, and C with the full sensitivity set, and then report the results as tornado diagrams for LCOH and identify the breakeven thresholds (e.g., carbon price versus electricity price).
5. Methods (What to Report So Reviewers Can Reproduce the Results)
5.1. Process Basis and Heat-Integration Data
- ▪
- Basis and boundary: nameplate H2, capacity factor, overall yield after recycling, and site ambient conditions.
- ▪
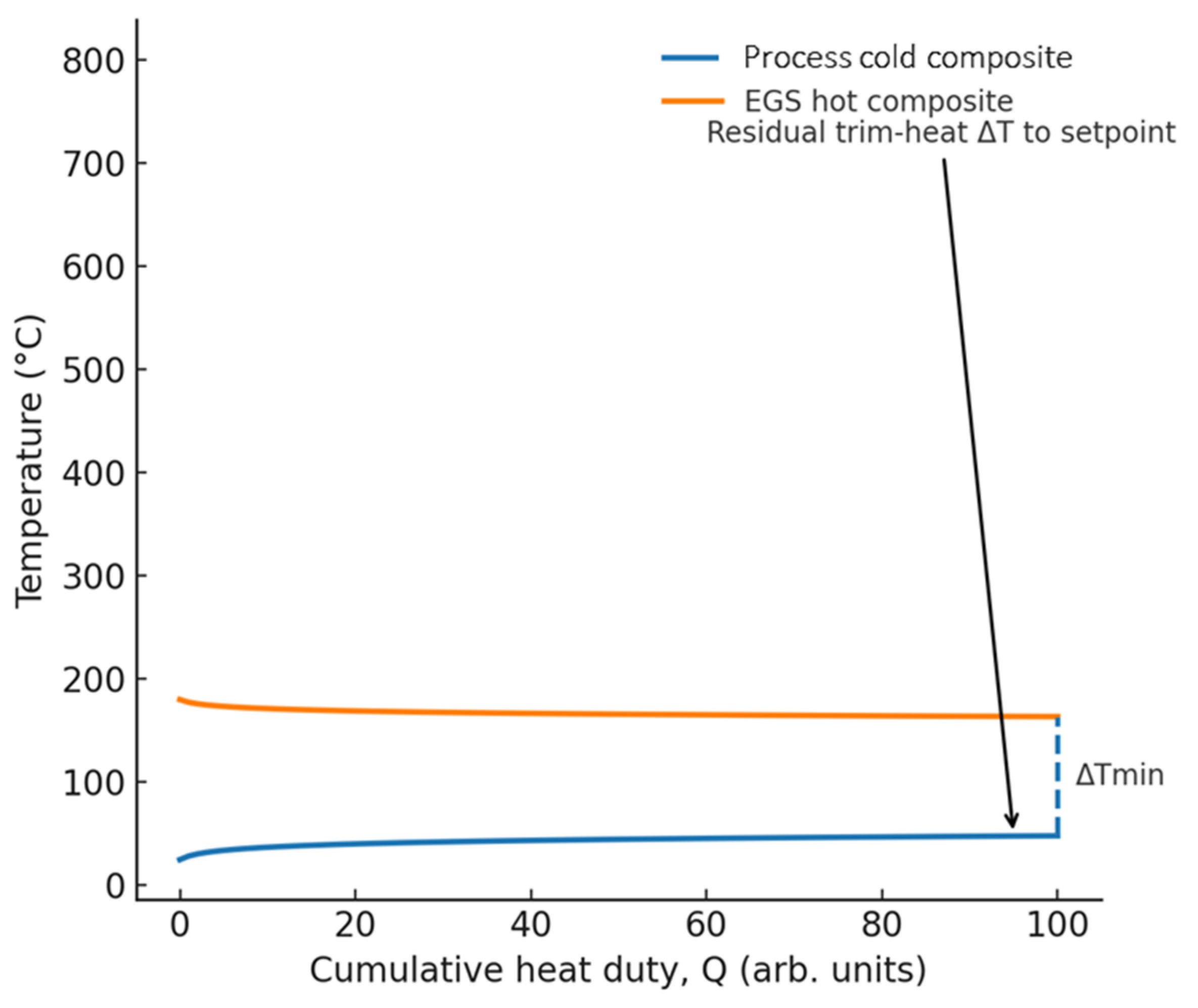
- EGS loop: Key geothermal parameters should include production temperature and flow rate, reinjection temperature, and variability in supply/return (expressed as ±σ or operating bands). If thermal storage is applied, the configuration must be described.
- ▪
- Process cold streams: Identification of mass flow , mean correlation, inlet/outlet temperature, and minimum allowable approach .
- ▪
- Process hot streams (each): For each internal hot utility, provide stream identification along with mass flow rate , , and inlet/outlet temperatures.
- ▪
- Pinch reconstruction: Composite curves (temperature vs. cumulative heat duty) should be published for the EGS supply and the process demand. These curves must be annotated to show the pinch point and the residual trim-heat requirement relative to the target setpoint.
- ▪
5.2. Reactor Details (Geometry, HP/HT Envelope, and Internals)
5.3. Carbon QA/QC (Methods That Tie to Economics)
- ▪
- Particle Size Distribution (PSD): Report by laser diffraction, including details of dispersant, sonication power and time, and refractive index model.
- ▪
- Surface Area: Measure by BET analysis, with reporting of degassing temperature/time and the model fit domain.
- ▪
- Volatiles and Ash Content: Analyze by thermo-gravimetery or muffle furnace procedure, specifying temperatures and hold times; include residual metal content if relevant.
- ▪
- Oil Absorption (DBP): Report DBP number or an alternative structure metric.
- ▪
- Moisture and Surface Chemistry: If linked to pricing, measure elemental O/H ratios and functional groups using methods such as Boehm titration or XPS.
- ▪
- ▪
5.4. TEA Inputs (So the Numbers Are Reproducible)
- ▪
- Documentation Requirement: All parameters and models used for cost estimation and financial analysis must be documented clearly, with references to raw data sources or date-stamped indices, to ensure reproducibility.
- ▪
- ▪
- Indices and Currencies: Identify the cost index employed (e.g., CEPCI or equivalent), the base year, the reference currency, and the method of escalating to current values.
- ▪
- WACC and Finance Assumptions: Provide the nominal and/or real weighted average cost of capital (WACC), the tax rate, and the depreciation method (e.g., MACRS or straight-line). State the assumed plant lifetime in years and the discount rate (i), and present the capital recovery factor (CRF) explicitly (see Figure 3).
- ▪
- ▪
- ▪
- Model Transparency: Upload the full calculation workbook with clearly labeled tabs (Assumptions, Heat Split, CAPEX, OPEX, Revenues, LCOH, and Sensitivity).
5.5. Data and Code Availability
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EGS | Enhanced geothermal system |
| TEA | Techno-Economic Analysis |
| CAPEX | Capital expenditure |
| OPEX | Operational expenditure |
| SMR | Steam methane reforming |
| CCS | Carbon capture and storage |
| LCOH | Levelized cost of hydrogen |
| PSA | Pressure swing adsorption |
| CB | Carbon black |
| PSD | Particle size distribution |
| FEED | Front end engineering design |
| BET | Brunauer–Emmett–Teller |
| DBP | Dibutyl Phthalate absorption number |
| Overall Yield | |
| CI | Carbon Intensity |
| WACC | Weighted average cost of capital |
| CRF | Capital recovery factor |
| Geothermal duty | |
| Residual trim duty |
References
- Patlolla, S.R.; Katsu, K.; Sharafian, A.; Wei, K.; Herrera, O.E.; Mérida, W. A review of methane pyrolysis technologies for hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113323. [Google Scholar] [CrossRef]
- Lang, Z.; Zhu, Y.; Xu, S.; Cao, G.; Duan, H. A mini-review on hydrogen and carbon production from methane pyrolysis by molten media. Energy Fuels 2024, 38, 23175–23191. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Navas-Anguita, Z.; García-Gusano, D.; Dufour, J.; Iribarren, D. Revisiting the role of steam methane reforming with CO2 capture and storage for long-term hydrogen production. Sci. Total Environ. 2021, 771, 145432. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, B.; Matthews, J.W.; McConnaughy, T.B.; Upham, D.C.; McFarland, E.W. Techno-economic analysis of methane pyrolysis in molten metals: Decarbonizing natural gas. Chem. Eng. Technol. 2017, 40, 1022–1030. [Google Scholar] [CrossRef]
- Daghagheleh, O.; Schenk, J.; Zarl, M.A.; Lehner, M.; Farkas, M.; Zheng, H. Feasibility of a plasma furnace for methane pyrolysis. Energies 2023, 17, 167. [Google Scholar] [CrossRef]
- Uehara, T.; Asahara, M.; Miyasaka, T. CO2-free hydrogen production by methane pyrolysis using hydrogen combustion heat. Energies 2024, 17, 367. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Hejazi, S.; Fattahi, M.; Kibria, G.; Thomson, M.J.; AlEisa, R.; Khan, M.A. Methane pyrolysis for hydrogen production: Navigating the path to a net zero future. Energy Environ. Sci. 2025, 18, 1034–1056. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Experimental comparison of solar methane pyrolysis in gas-phase and molten-tin bubbling tubular reactors. Energy 2022, 260, 124943. [Google Scholar] [CrossRef]
- DiPippo, R. Geothermal Power Plants: Principles, Applications, Case Studies and Environmental Impact, 4th ed.; Butterworth–Heinemann (Elsevier): Oxford, UK, 2015. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw–Hill: New York, NY, USA, 2003. [Google Scholar]
- McConnachie, M.; Konarova, M.; Smart, S. Literature review of the catalytic pyrolysis of methane for hydrogen and carbon production. Int. J. Hydrogen Energy 2023, 48, 25660–25682. [Google Scholar] [CrossRef]
- Korányi, T.I.; Németh, M.; Beck, A.; Horváth, A. Recent advances in methane pyrolysis: Turquoise hydrogen with solid carbon production. Energies 2022, 15, 6342. [Google Scholar] [CrossRef]
- Patzschke, C.F.; Parkinson, B.; Willis, J.J.; Nandi, P.; Love, A.M.; Raman, S.; Hellgardt, K. Co-Mn catalysts for H2 production via methane pyrolysis in molten salts. Chem. Eng. J. 2021, 414, 128730. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Wang, B.; Su, X.; Wang, F. The secondary flows in a cyclone separator: A review. Processes 2023, 11, 2935. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Bansal, R.C.; Wang, M.-J. Carbon Black: Science and Technology, 2nd ed.; Marcel Dekker: New York, NY, USA, 1993. [Google Scholar]
- Plevan, M.; Geißler, T.; Abánades, A.; Mehravaran, K.; Rathnam, R.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; Wetzel, T. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis. Int. J. Hydrogen Energy 2015, 40, 8020–8033. [Google Scholar] [CrossRef]
- Karayel, G.K.; Javani, N.; Dincer, I. Green hydrogen production potential in Turkey with wind power. Int. J. Green Energy 2022, 20, 129–138. [Google Scholar] [CrossRef]
- Kang, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic methane pyrolysis in molten MnCl2-KCl. Appl. Catal. B Environ. 2019, 254, 659–666. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design, 3rd ed.; Elsevier: Oxford, UK, 2022. [Google Scholar]
- Green, D.W.; Southard, M.Z. (Eds.) Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw–Hill: New York, NY, USA, 2019. [Google Scholar]
- Aljubran, M.J.; Gandomi, A.G.; Al-Aali, A.; Al-Khalidi, H.A. Power supply characterization of baseload and flexible enhanced geothermal systems. Sci. Rep. 2024, 14, 17619. [Google Scholar] [CrossRef]
- Sorcar, S.; Rosen, B.A. Methane pyrolysis using a multiphase molten metal reactor. ACS Catal. 2023, 13, 10161–10166. [Google Scholar] [CrossRef]
- Palmer, C.; Tarazkar, M.; Kristoffersen, H.H.; Gelinas, J.; Gordon, M.J.; McFarland, E.W.; Metiu, H. Methane pyrolysis with a molten Cu–Bi alloy catalyst. ACS Catal. 2019, 9, 8337–8345. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane pyrolysis for CO2-free hydrogen production: A green process to overcome renewable energies unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane pyrolysis for zero-emission hydrogen production. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kim, S.; Lee, S.; Kim, J. Hydrogen production in methane decomposition reactor using solar thermal energy. Appl. Sci. 2021, 11, 10333. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A short review on Ni-based catalysts and related engineering issues for methane steam reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Punia, A.; Tatum, J.; Kostiuk, L.; Olfert, J.; Secanell, M. Analysis of methane pyrolysis experiments at high pressure: Goal-oriented estimations of kinetics. Chem. Eng. J. 2023, 471, 144183. [Google Scholar] [CrossRef]
- Pototschnig, U.; Matas, M.; Scheiblehner, D.; Neuschitzer, D.; Obenaus-Emler, R.; Antrekowitsch, H.; Holec, D. A predictive model for catalytic methane pyrolysis. J. Phys. Chem. C 2024, 128, 9034–9040. [Google Scholar] [CrossRef]
- Pangestu, M.R.G.; Zahid, U. Techno-economic analysis of integrating methane pyrolysis and reforming for low-carbon ammonia production. Energy Convers. Manag. 2024, 322, 119125. [Google Scholar] [CrossRef]
- Grant, M.; Bixley, P. Geothermal Reservoir Engineering, 2nd ed.; Academic Press: Burlington, MA, USA, 2011. [Google Scholar]
- Rosner, F.; Bhagde, T.; Slaughter, D.S.; Zorba, V.; Stokes-Draut, J. Techno-economic and carbon dioxide emission assessment of carbon black production. J. Clean. Prod. 2023, 436, 140224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiam, A.; Watson, M.; Gamadi, T. Towards Carbon-Neutral Hydrogen: Integrating Methane Pyrolysis with Geothermal Energy. Processes 2025, 13, 3195. https://doi.org/10.3390/pr13103195
Tiam A, Watson M, Gamadi T. Towards Carbon-Neutral Hydrogen: Integrating Methane Pyrolysis with Geothermal Energy. Processes. 2025; 13(10):3195. https://doi.org/10.3390/pr13103195
Chicago/Turabian StyleTiam, Ayann, Marshall Watson, and Talal Gamadi. 2025. "Towards Carbon-Neutral Hydrogen: Integrating Methane Pyrolysis with Geothermal Energy" Processes 13, no. 10: 3195. https://doi.org/10.3390/pr13103195
APA StyleTiam, A., Watson, M., & Gamadi, T. (2025). Towards Carbon-Neutral Hydrogen: Integrating Methane Pyrolysis with Geothermal Energy. Processes, 13(10), 3195. https://doi.org/10.3390/pr13103195





