Solubility Measurement and Correlation of Cis-1,1,1,4,4,4-Hexafluoro-2-butene in Dipentaerythritol Hexaheptanoate and Dipentaerythritol Isononanoate from 293.15 K to 343.15 K
Abstract
1. Introduction
2. Experimental Method
2.1. Experimental Samples
2.2. Experimental Setup
2.3. NRTL Model
3. Experimental Results and Discussion
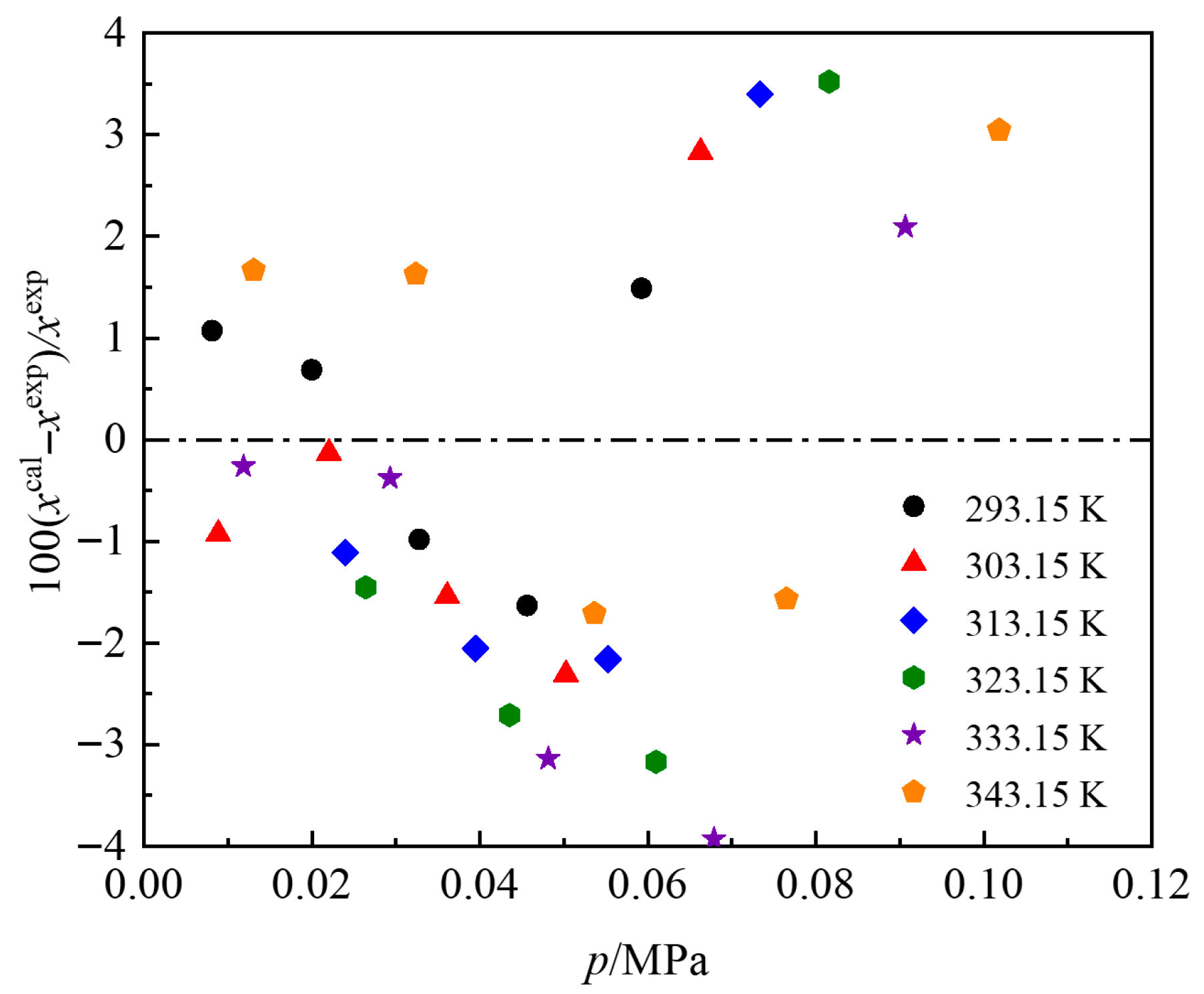
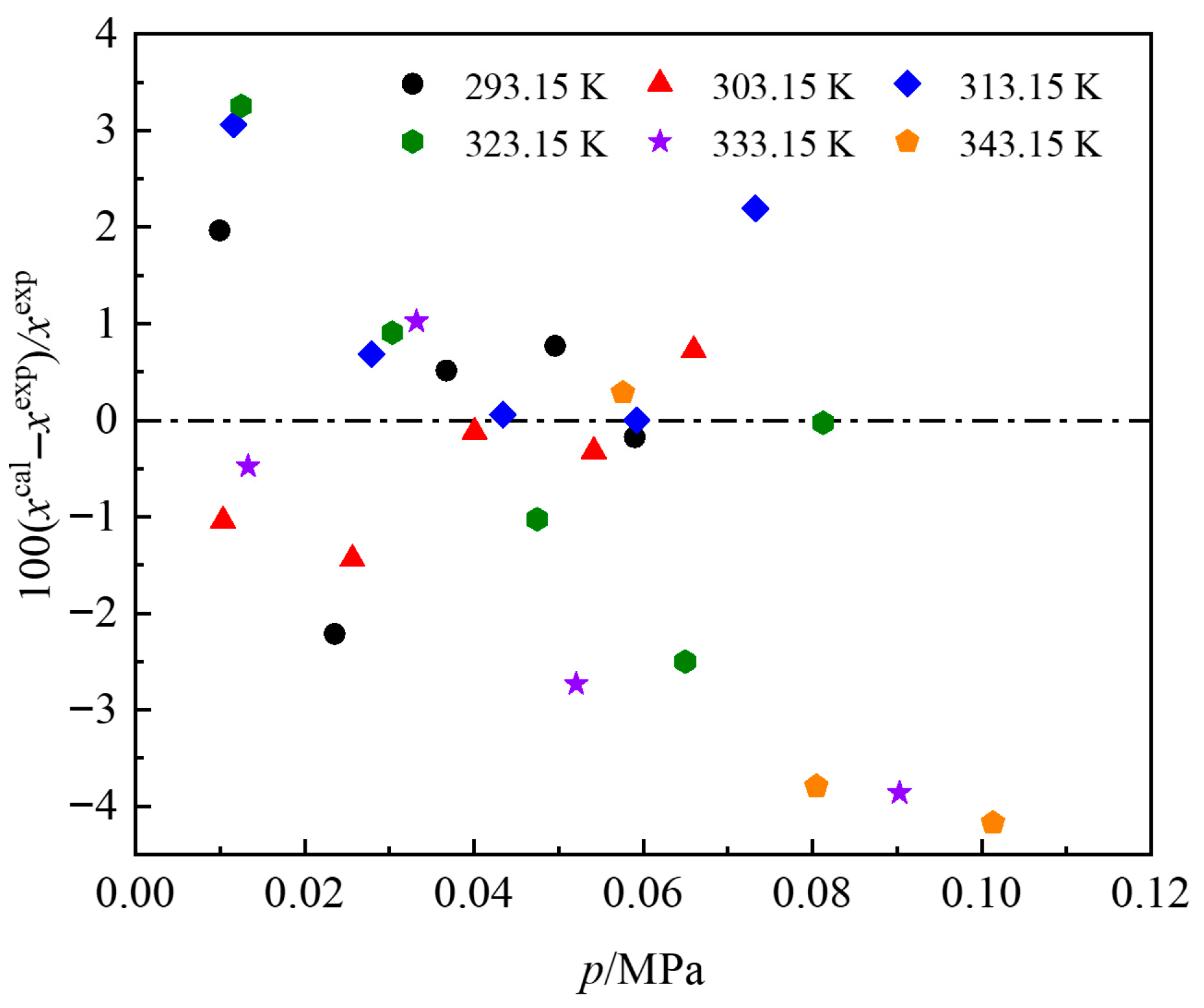
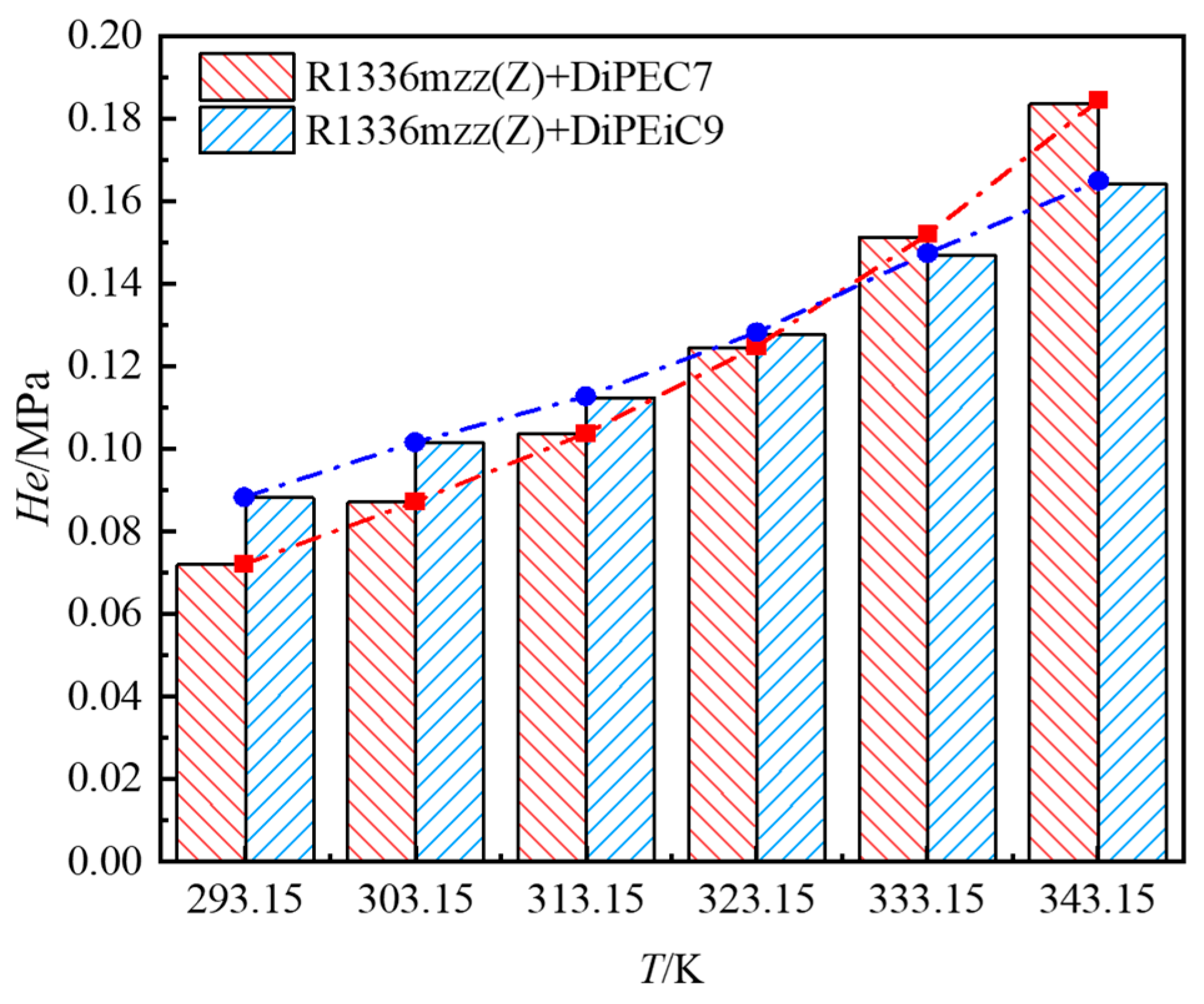
3.1. Experimental Data and Correlation
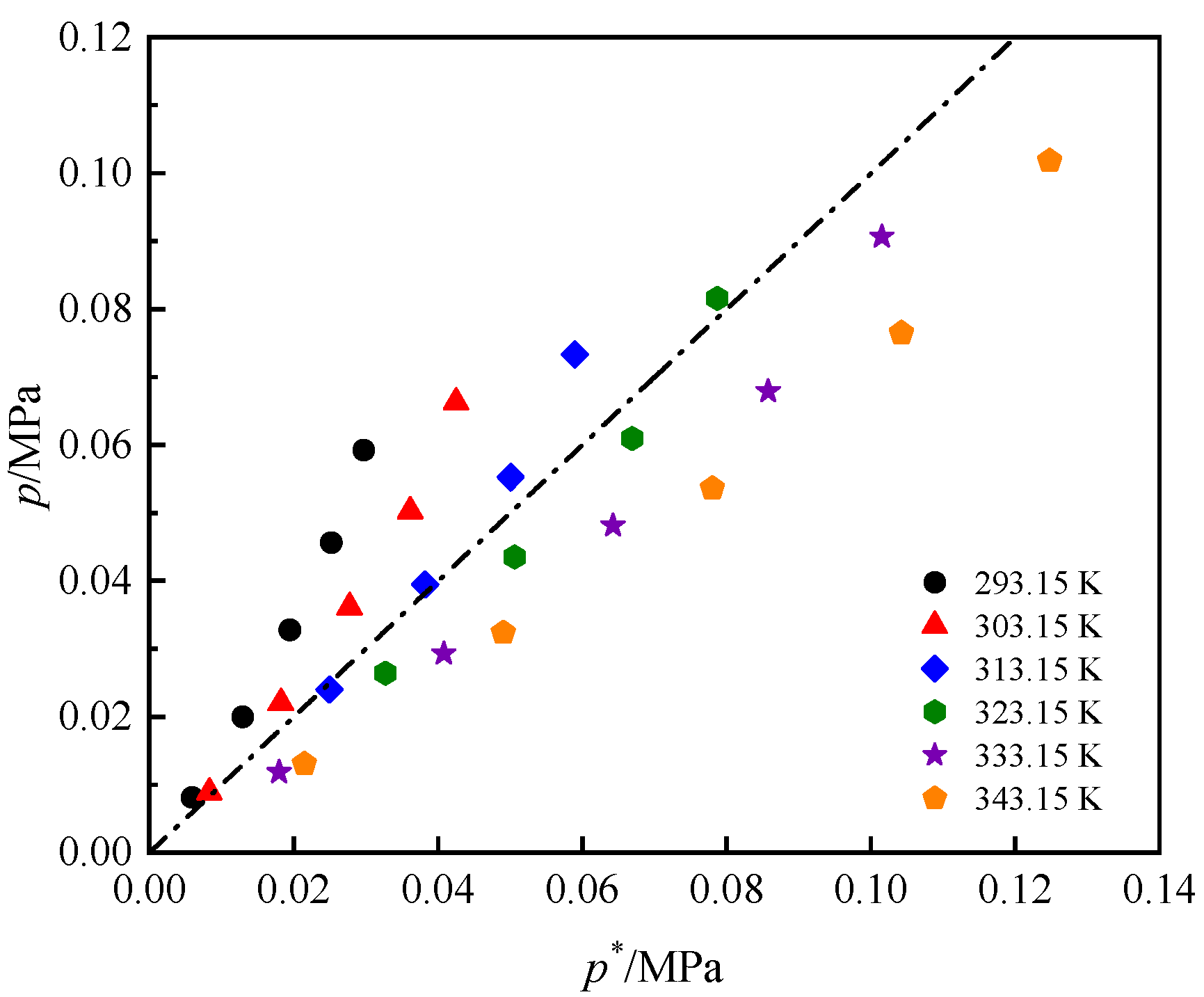
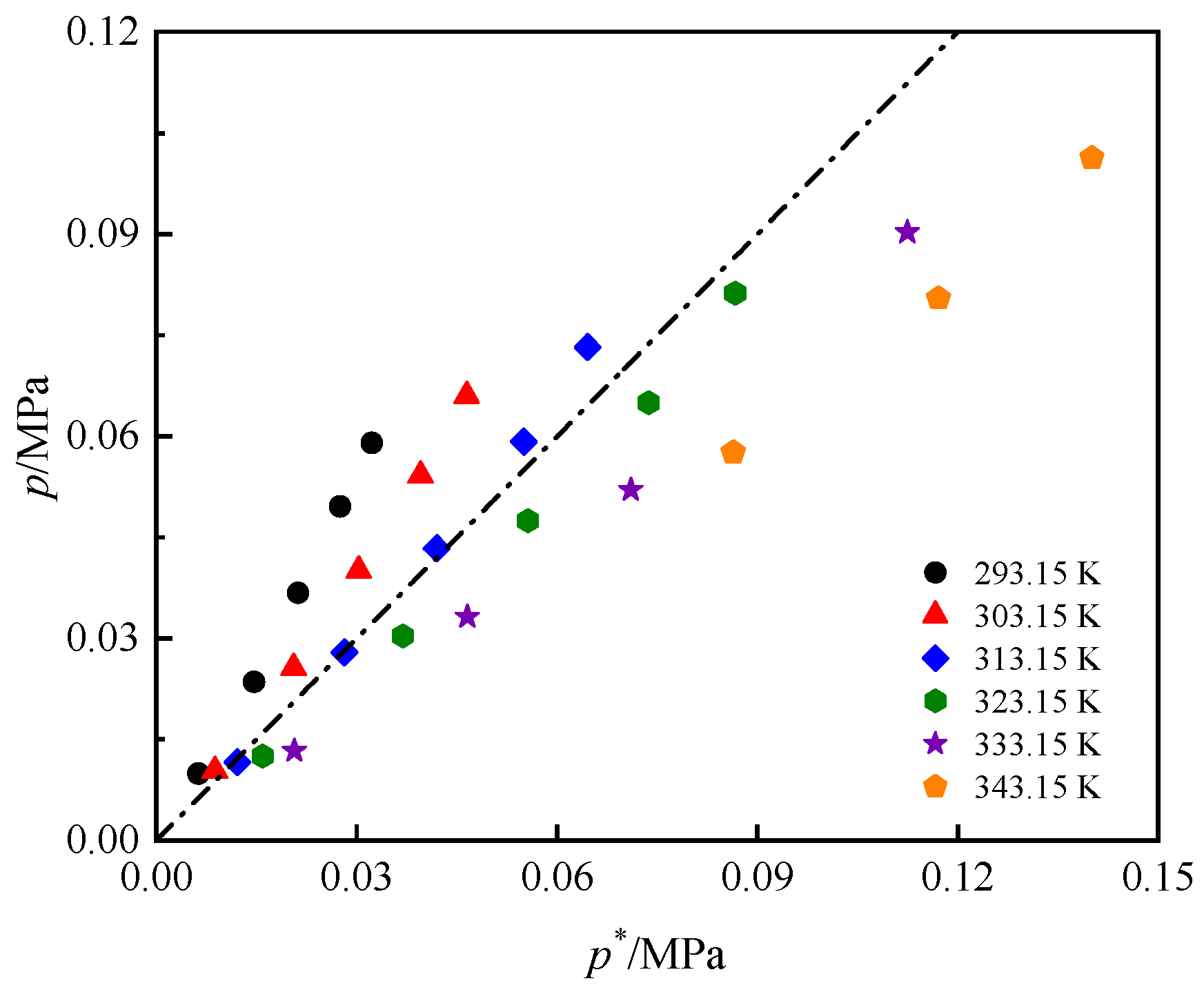
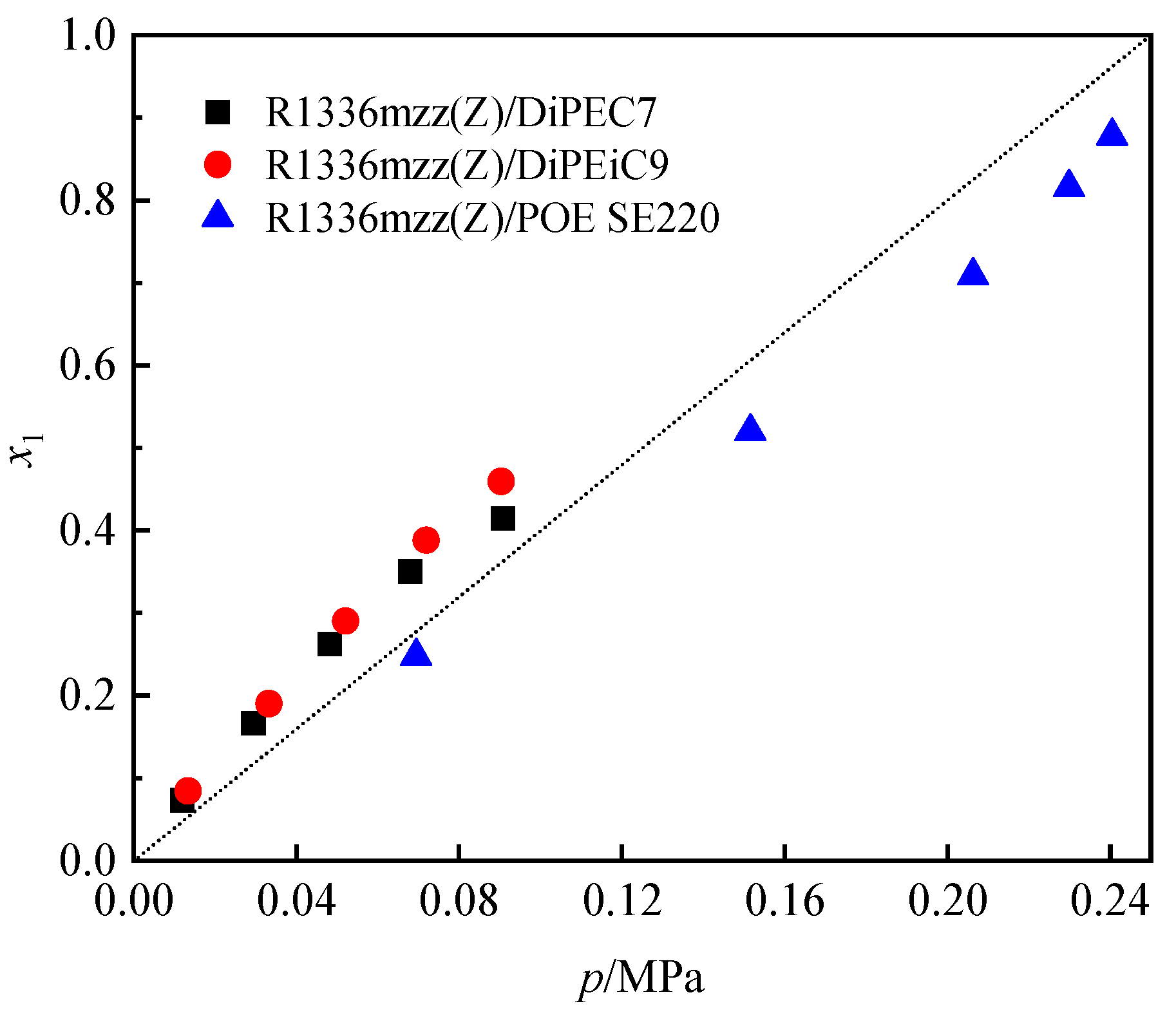
3.2. Solubility Comparison
3.3. Mixing Thermodynamic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vuppaladadiyam, A.K.; Antunes, E.; Vuppaladadiyam, S.S.V.; Baig, Z.T.; Subiantoro, A.; Lei, G.Y.; Leu, S.Y.; Sarmah, A.K.; Duan, H.B. Progress in the development and use of refrigerants and unintended environmental consequences. Sci. Total Environ. 2022, 823, 153670. [Google Scholar] [CrossRef]
- Perry, C.; Nickson, T.; Starr, C.; Grabiel, T.; Geoghegan, S.; Porter, B.; Mahapatra, A.; Walravens, F. More to offer from the Montreal protocol: How the ozone treaty can secure further significant greenhouse gas emission reductions in the future. J. Integr. Environ. Sci. 2024, 21, 2362124. [Google Scholar] [CrossRef]
- Liu, H.P.; Duan, H.B.; Zhang, N.; Ma, Y.; Liu, G.; Miller, T.R.; Mao, R.C.; Xu, M.; Li, J.H.; Yang, J.K. Rethinking time-lagged emissions and abatement potential of fluorocarbons in the post-Kigali Amendment era. Nat. Commun. 2024, 15, 6687. [Google Scholar] [CrossRef]
- Heath, E.A. Amendment to the Montreal Protocol on substances that deplete the ozone layer (Kigali Amendment). Int. Leg. Mater. 2017, 56, 193–205. [Google Scholar] [CrossRef]
- Nair, V. HFO refrigerants: A review of present status and future prospects. Int. J. Refrig. 2021, 122, 156–170. [Google Scholar] [CrossRef]
- Molés, F.; Navarro-Esbrí, J.; Peris, B.; Mota-Babiloni, A.; Barragán-Cervera, Á.; Kontomaris, K. Low GWP alternatives to HFC-245fa in Organic Rankine Cycles for low temperature heat recovery: HCFO-1233zd-E and HFO-1336mzz-Z. Appl. Therm. Eng. 2014, 71, 204–212. [Google Scholar] [CrossRef]
- Tanaka, K.; Akasaka, R.; Sakaue, E.; Ishikawa, J.; Kontomaris, K.K. Measurements of the critical parameters for cis-1,1,1,4,4,4-Hexafluoro-2-butene. J. Chem. Eng. Data 2017, 62, 1135–1138. [Google Scholar] [CrossRef]
- Arpagaus, C.; Bless, F.; Uhlmann, M.; Schiffmann, J.; Bertsch, S.S. High temperature heat pumps: Market overview, state of the art, research status, refrigerants, and application potentials. Energy 2018, 152, 985–1010. [Google Scholar] [CrossRef]
- Sakoda, N.; Higashi, Y.; Akasaka, R. Measurements of vapor pressures for trans-1-Chloro-3,3,3-trifluoropropene (R1233zd(E)) and cis-1,1,1,4,4,4-hexafluoro-2-butene (R1336mzz(Z)). J. Chem. Eng. Data 2020, 65, 4285–4289. [Google Scholar] [CrossRef]
- Kano, Y. Thermophysical properties evaluation for a polar fluid on the basis of the experimentally determined heat capacity and dipole moment in the ideal gas states. J. Therm. Anal. Calorim. 2023, 148, 5573–5587. [Google Scholar] [CrossRef]
- Alam, M.J.; Miyara, A.; Kariya, K.; Kontomaris, K.K. Measurement of viscosity of cis-1,1,1,4,4,4-Hexafluoro-2-butene (R-1336mzz(Z)) by Tandem capillary tubes method. J. Chem. Eng. Data 2018, 63, 1706–1712. [Google Scholar] [CrossRef]
- Alam, M.J.; Islam, M.A.; Kariya, K.; Miyara, A. Measurement of thermal conductivity of cis-1,1,1,4,4,4-hexafluoro-2-butene (R-1336mzz(Z)) by the transient hot-wire method. Int. J. Refrig. 2017, 84, 220–227. [Google Scholar] [CrossRef]
- Perkins, R.A.; Huber, M.L. Measurement and correlation of the thermal conductivity of cis-1,1,1,4,4,4-hexafluoro-2-butene. Int. J. Thermophys. 2020, 41, 103. [Google Scholar] [CrossRef]
- Tijani, M.E.H.; Otero Rodriguez, G.J.; Ramirez, M.; Poulsen, J.L.; Spoelstra, S. Selection of lubricant oils for high temperature heat pumps: A review and selection methodology guidelines. Appl. Therm. Eng. 2025, 273, 126483. [Google Scholar] [CrossRef]
- García, J.; Abou Naccoul, R.; Fernández, J.; Razzouk, A.; Mokbel, I. Vapor-pressure measurements and modeling of dipentaerythritol ester lubricants. Ind. Eng. Chem. Res. 2011, 50, 4231–4237. [Google Scholar] [CrossRef]
- Emel’ianov, V.V.; Krasnykh, E.L.; Sokolov, A.B. Synthetic oils based on pentaerythritol esters. Kinematic viscosity. Fluid Phase Equilib. 2024, 581, 114074. [Google Scholar] [CrossRef]
- Paredes, X.; Pensado, A.S.; Comuñas, M.J.P.; Fernández, J. Experimental dynamic viscosities of dipentaerythritol ester lubricants at high pressure. J. Chem. Eng. Data 2010, 55, 3216–3223. [Google Scholar] [CrossRef]
- Harris, K.R. Temperature and pressure dependence of the viscosities of Krytox GPL102 Oil and di(pentaerythritol) hexa(isononanoate). J. Chem. Eng. Data 2015, 60, 1510–1519. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.J.; Wang, X.P. Density, viscosity, and R1234yf solubility in dipentaerythritol esters. J. Chem. Eng. Data 2025, 70, 2057–2067. [Google Scholar] [CrossRef]
- Morales-Espejel, G.E.; Wallin, H.H.; Hauleitner, R.; Arvidsson, M. Progress in rolling bearing technology for refrigerant compressors. Proc. Inst. Mech. Eng. Part C-J. Mech. Eng. Sci. 2017, 232, 2948–2961. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wang, X.D.; Wang, S.B.; Meng, X.Z. Viscosity measurement and correlation of saturated solutions of R-290 with three lubricants. Int. J. Refrig. 2023, 155, 1–6. [Google Scholar] [CrossRef]
- Wang, C.C.; Hafner, A.; Kuo, C.S.; Hsieh, W.D. An overview of the effect of lubricant on the heat transfer performance on conventional refrigerants and natural refrigerant R-744. Renew. Sustain. Energy Rev. 2012, 16, 5071–5086. [Google Scholar] [CrossRef]
- Brocus, J.; Valtz, A.; Coquelet, C.; De Carlan, F. Solubility measurements of refrigerants in polyolesters lubricants at temperature from 323 K to 383 K. Int. J. Refrig. 2022, 134, 278–292. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Z.; Lu, C.; Wang, X.P. Vapor-liquid equilibrium measurements of 3, 3, 3-trifluoropropene with pentaerythritol tetraheptanoate and pentaerythritol tetranonanoate. J. Chem. Thermodyn. 2022, 174, 106874. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.J.; Wang, X.P. Solubility measurements of R1234ze(E), R1243zf, and R1234yf in POE46 at temperatures from 283.15 to 343.15 K. J. Chem. Eng. Data 2024, 69, 1677–1684. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, X.J.; Meng, X.Y.; Wu, J.T. Measurement and correlation of the liquid density and viscosity of HFO-1336mzz(Z) (cis-1,1,1,4,4,4-hexafluoro-2-butene) at high pressure. J. Chem. Eng. Data 2019, 64, 395–403. [Google Scholar] [CrossRef]
- Bich, W.; Cox, M.G.; Dybkaer, R.; Elster, C.; Estler, W.T.; Hibbert, B.; Imai, H.; Kool, W.; Michotte, C.; Nielsen, L.; et al. Revision of the ‘Guide to the Expression of Uncertainty in Measurement’. Metrologia 2012, 49, 702. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Moosavi, M.; Abareshi, M. Calculation of thermodynamic properties of lubricant + refrigerant mixtures using GMA equation of state. Int. J. Therm. Sci. 2007, 46, 944–952. [Google Scholar] [CrossRef]
- Comuñas, M.J.P.; Baylaucq, A.; Boned, C.; Canet, X.; Fernández, J. High-pressure volumetric behavior of x 1,1,1,2-tetrafluoroethane + (1 − x) 2,5,8,11,14-pentaoxapentadecane (TEGDME) mixtures. J. Chem. Eng. Data 2002, 47, 233–238. [Google Scholar] [CrossRef]
- Saggion, A.; Faraldo, R.; Pierno, M. Thermodynamics: Fundamental Principles and Applications; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Beattie, J.A.; Stockmayer, W.H. The Second Virial Coefficient for Gas Mixtures. J. Chem. Phys. 1942, 10, 473–476. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermo-Dynamic and Transport Properties-REFPROP, Version 10.0.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 2004, 14, 135–144. [Google Scholar] [CrossRef]
- Masuku, C.M.; Ma, W.; Hildebrandt, D.; Glasser, D.; Davis, B.H. A vapor–liquid equilibrium thermodynamic model for a Fischer–Tropsch reactor. Fluid Phase Equilibr. 2012, 314, 38–45. [Google Scholar] [CrossRef]
- Gunn, R.D.; Yamada, T.; Whitman, D. Corresponding states. III. Henry’constants for nonpolar binary mixtures. AIChE J. 1974, 20, 906–915. [Google Scholar] [CrossRef]
- Sun, Y.J.; Di, G.L.; Wang, J.; Wang, X.P.; He, M.G. Phase behavior of R1234yf and R600a in pentaerythritol tetranonanoate. Int. J. Refrig. 2020, 109, 135–142. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; de Azevedo, E.G. Molecular Thermodynamics of Fluid Phase Equilibria, 3rd ed.; Prentice Hall PTR: Hoboken, NJ, USA, 1999. [Google Scholar]
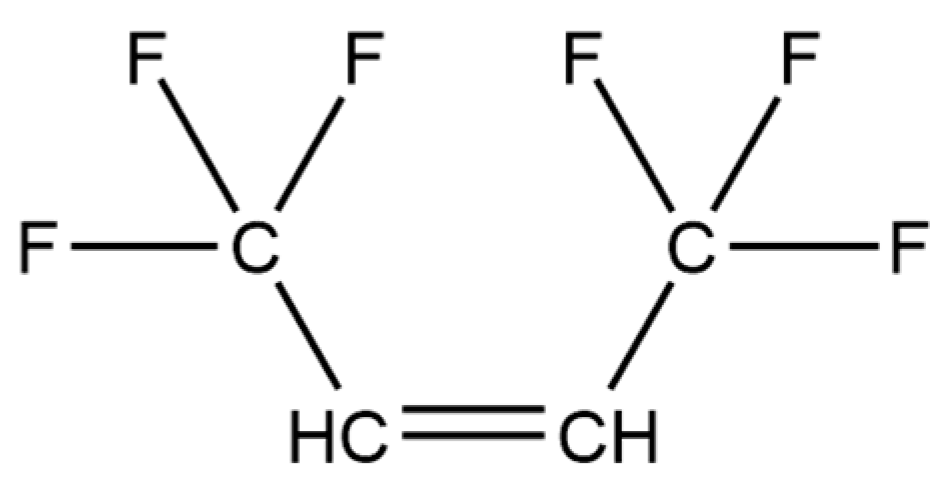
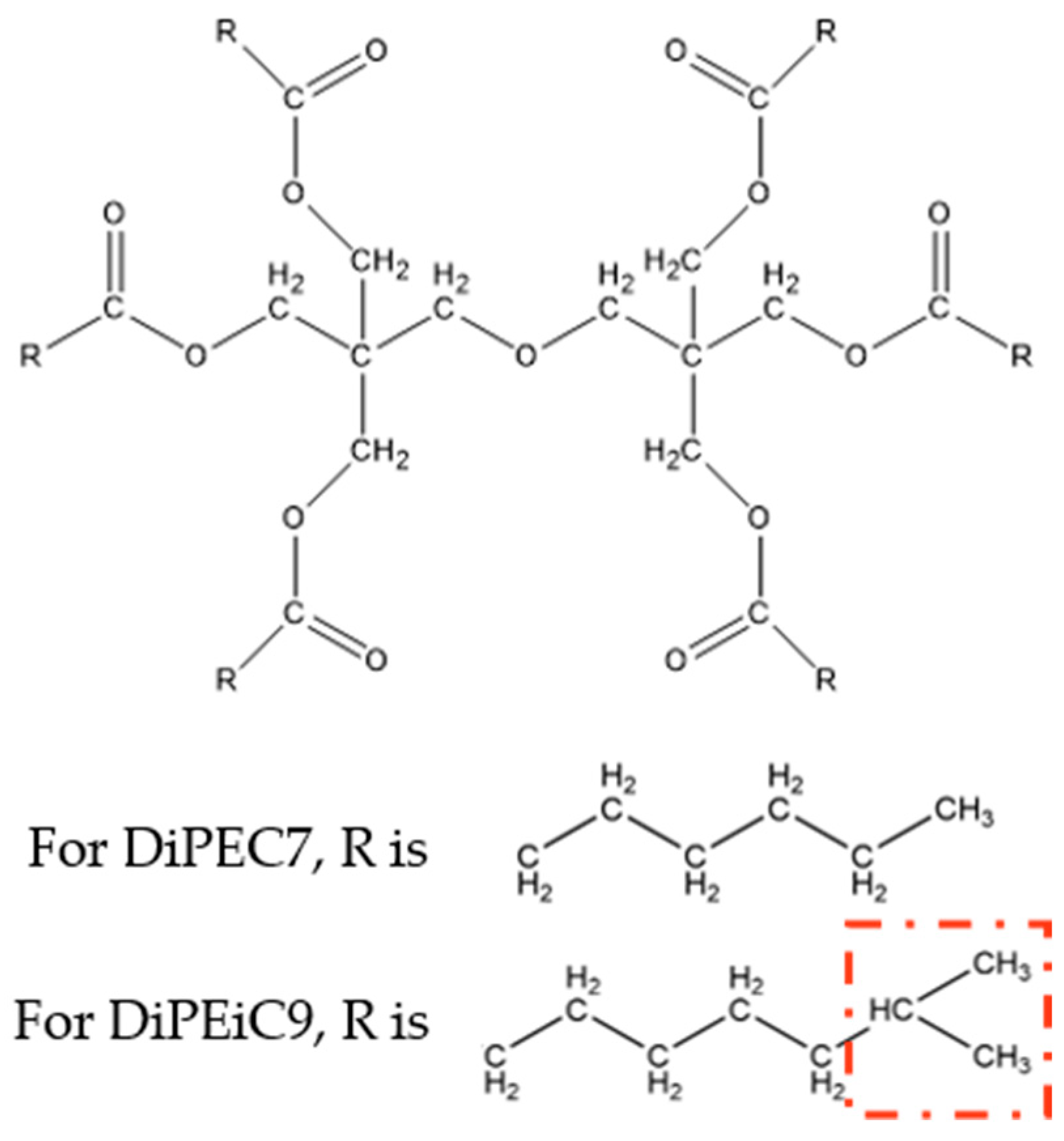
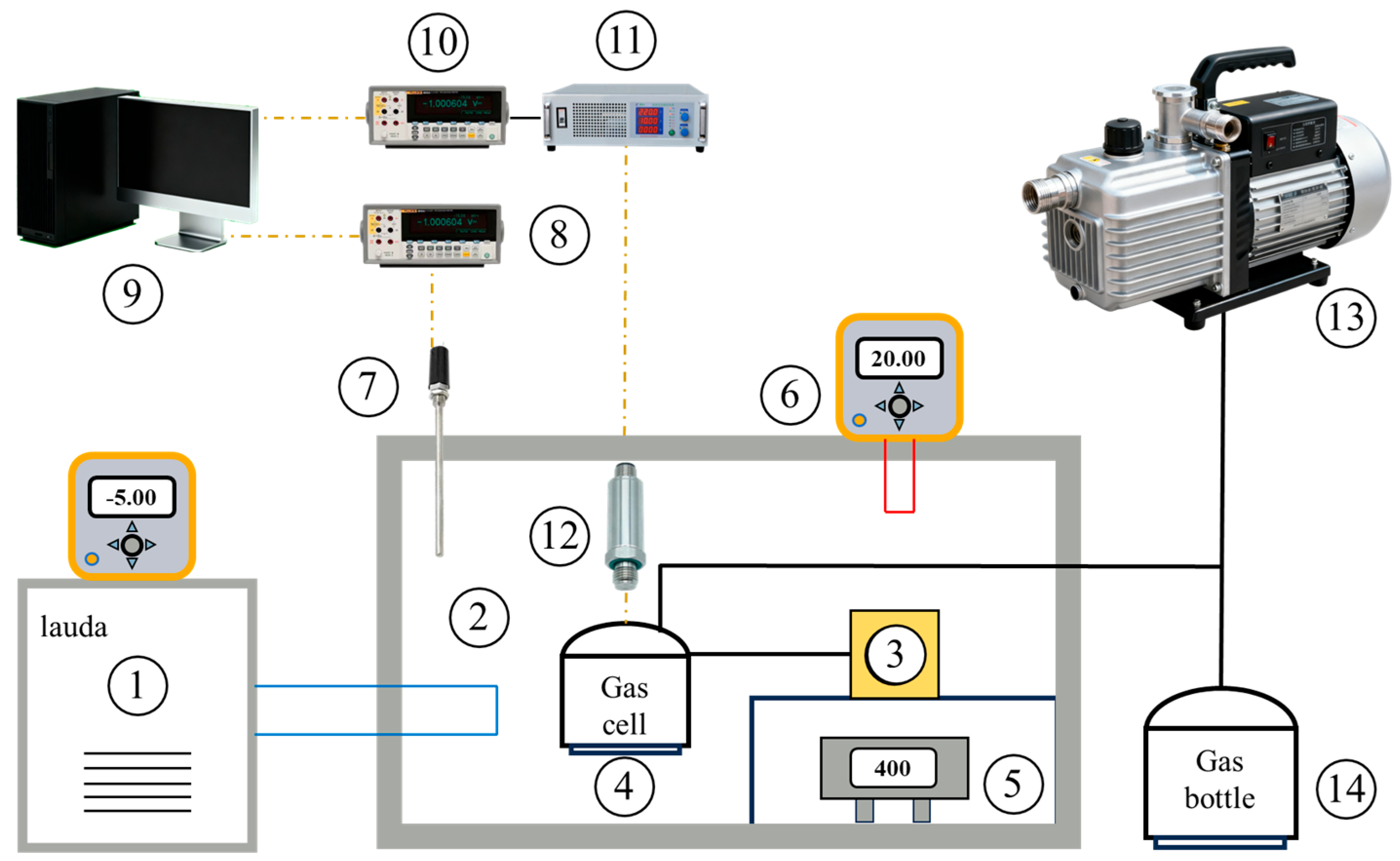

| Formula | CAS No. | Supplier | Mass Fraction Purity | |
|---|---|---|---|---|
| R1336mzz(Z) a | C4H2F6 | 692-49-9 | Honeywell | 0.999 |
| DiPEC7 b | C52H94O13 | 76939-66-7 | MingKT Corporation | 0.99 |
| DiPEiC9 c | C64H118O13 | 127304-08-9 | MingKT Corporation | 0.99 |
| Source of Uncertainty | Uncertainty | Source of Uncertainty | Uncertainty |
|---|---|---|---|
| u(Vgc) | 0.02 cm3 | u() | 0.005 |
| u(Vec) | 0.02 cm3 | u() | 0.005 |
| u(ρ2) | 0.0008 g·cm−3 | u() | 0.005 |
| u(m2) | 0.0002 g |
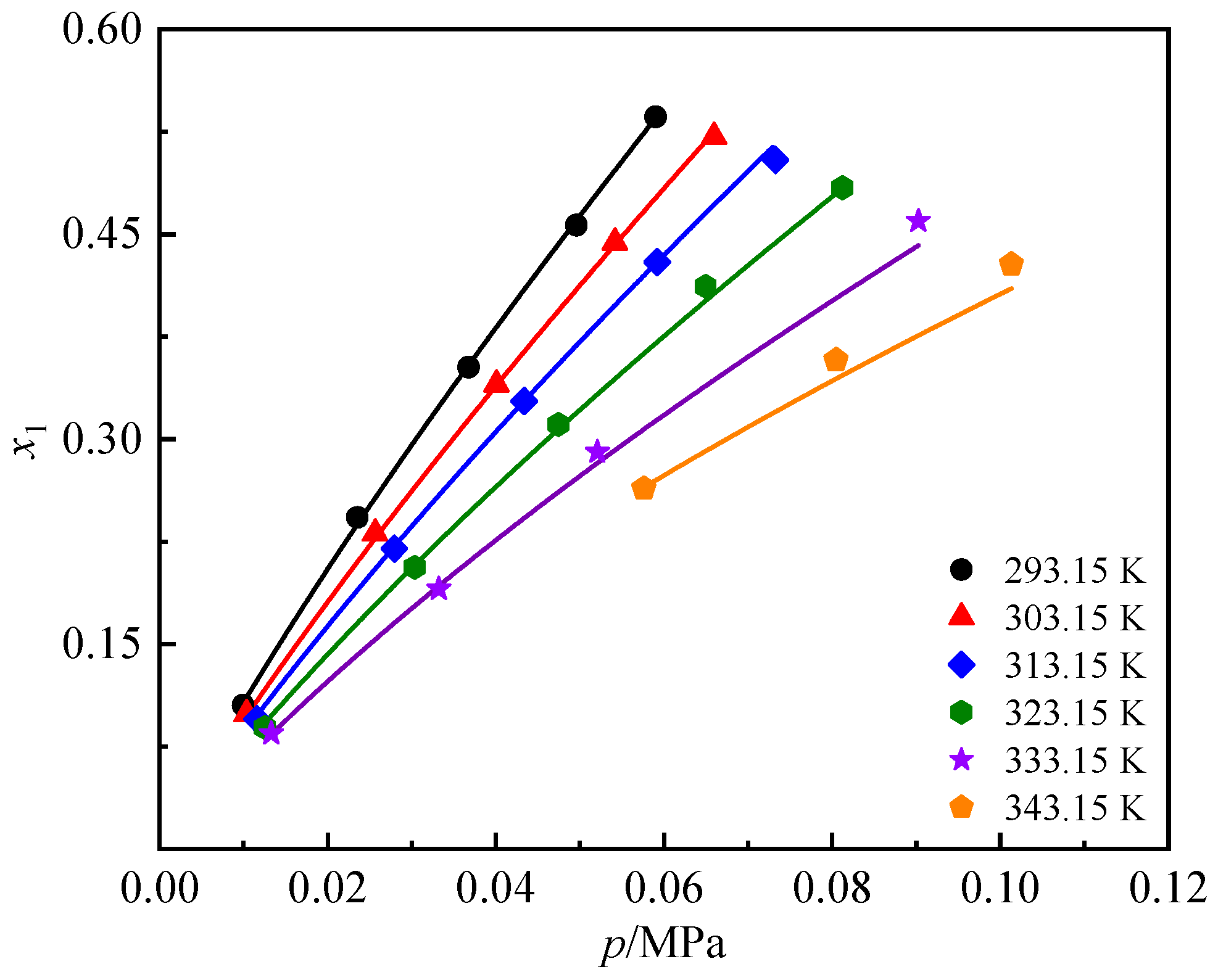
| T (K) | p (MPa) | x1 | w1 | T (K) | p (MPa) | x1 | w1 |
|---|---|---|---|---|---|---|---|
| 293.15 | 0.008 | 0.099 | 0.019 | 323.15 | 0.026 | 0.183 | 0.038 |
| 293.15 | 0.020 | 0.215 | 0.046 | 323.15 | 0.044 | 0.283 | 0.065 |
| 293.15 | 0.033 | 0.324 | 0.078 | 323.15 | 0.061 | 0.374 | 0.095 |
| 293.15 | 0.046 | 0.419 | 0.113 | 323.15 | 0.082 | 0.440 | 0.122 |
| 293.15 | 0.059 | 0.494 | 0.147 | 333.15 | 0.012 | 0.074 | 0.014 |
| 303.15 | 0.009 | 0.093 | 0.018 | 333.15 | 0.029 | 0.167 | 0.034 |
| 303.15 | 0.022 | 0.205 | 0.044 | 333.15 | 0.048 | 0.263 | 0.059 |
| 303.15 | 0.036 | 0.311 | 0.074 | 333.15 | 0.068 | 0.351 | 0.087 |
| 303.15 | 0.050 | 0.406 | 0.108 | 333.15 | 0.091 | 0.415 | 0.112 |
| 303.15 | 0.066 | 0.477 | 0.139 | 343.15 | 0.013 | 0.066 | 0.012 |
| 313.15 | 0.024 | 0.195 | 0.041 | 343.15 | 0.032 | 0.150 | 0.030 |
| 313.15 | 0.039 | 0.299 | 0.070 | 343.15 | 0.054 | 0.238 | 0.052 |
| 313.15 | 0.055 | 0.391 | 0.102 | 343.15 | 0.076 | 0.318 | 0.076 |
| 313.15 | 0.073 | 0.461 | 0.131 | 343.15 | 0.102 | 0.381 | 0.098 |
| T (K) | p (MPa) | x1 | w1 | T (K) | p (MPa) | x1 | w1 |
|---|---|---|---|---|---|---|---|
| 293.15 | 0.010 | 0.105 | 0.017 | 313.15 | 0.073 | 0.504 | 0.132 |
| 293.15 | 0.024 | 0.243 | 0.046 | 323.15 | 0.013 | 0.089 | 0.014 |
| 293.15 | 0.037 | 0.353 | 0.075 | 323.15 | 0.030 | 0.206 | 0.037 |
| 293.15 | 0.050 | 0.457 | 0.112 | 323.15 | 0.047 | 0.311 | 0.063 |
| 293.15 | 0.059 | 0.536 | 0.147 | 323.15 | 0.065 | 0.412 | 0.095 |
| 303.15 | 0.010 | 0.099 | 0.016 | 323.15 | 0.081 | 0.484 | 0.123 |
| 303.15 | 0.026 | 0.231 | 0.043 | 333.15 | 0.013 | 0.085 | 0.014 |
| 303.15 | 0.040 | 0.340 | 0.072 | 333.15 | 0.033 | 0.190 | 0.034 |
| 303.15 | 0.054 | 0.444 | 0.107 | 333.15 | 0.052 | 0.290 | 0.058 |
| 303.15 | 0.066 | 0.521 | 0.140 | 333.15 | 0.090 | 0.460 | 0.113 |
| 313.15 | 0.012 | 0.095 | 0.015 | 343.15 | 0.058 | 0.264 | 0.051 |
| 313.15 | 0.028 | 0.220 | 0.040 | 343.15 | 0.080 | 0.358 | 0.077 |
| 313.15 | 0.043 | 0.328 | 0.068 | 343.15 | 0.101 | 0.428 | 0.101 |
| 313.15 | 0.059 | 0.430 | 0.101 |
| R1336mzz(Z)/DiPEC7 | R1336mzz(Z)/DiPEiC9 | |
|---|---|---|
| τ12,0 | 659.1357 | 4000.9680 |
| τ12,1 | −4.6958 | −27.4281 |
| τ12,2 | 0.0090 | 0.0490 |
| τ21,0 | −15.6776 | −18.0989 |
| τ21,1 | 0.0924 | 0.1185 |
| τ21,2 | −0.0001 | −0.0002 |
| AARD/% a | 1.88 | 1.46 |
| MARD/% b | 3.93 | 4.17 |
| T (K) | x1 | ΔHmix (J·mol−1) | ΔSmix (J·mol−1·K−1) | ΔGmix (J·mol−1) |
|---|---|---|---|---|
| 293.15 | 0.099 | 1351.05 | 7.05 | −714.70 |
| 293.15 | 0.215 | 3090.70 | 14.23 | −1079.48 |
| 293.15 | 0.324 | 4854.82 | 20.65 | −1198.80 |
| 293.15 | 0.419 | 6494.43 | 26.13 | −1164.56 |
| 293.15 | 0.494 | 7836.38 | 30.33 | −1054.97 |
| 303.15 | 0.093 | 1387.28 | 7.08 | −760.49 |
| 303.15 | 0.205 | 3152.40 | 14.36 | −1200.81 |
| 303.15 | 0.311 | 4945.46 | 20.94 | −1401.67 |
| 303.15 | 0.406 | 6623.76 | 26.60 | −1440.40 |
| 303.15 | 0.477 | 7927.58 | 30.74 | −1391.36 |
| 313.15 | 0.195 | 2615.67 | 12.52 | −1305.82 |
| 313.15 | 0.299 | 4103.34 | 18.15 | −1580.41 |
| 313.15 | 0.391 | 5491.69 | 22.93 | −1688.02 |
| 313.15 | 0.461 | 6570.35 | 26.39 | −1692.17 |
| 323.15 | 0.183 | 782.05 | 4.85 | −1369.67 |
| 323.15 | 0.283 | 2920.31 | 14.32 | −1707.72 |
| 323.15 | 0.374 | 3907.61 | 17.90 | −1876.84 |
| 323.15 | 0.440 | 4636.07 | 20.31 | −1926.24 |
| 333.15 | 0.074 | 565.17 | 4.08 | −795.70 |
| 333.15 | 0.167 | 1268.71 | 7.96 | −1384.44 |
| 333.15 | 0.263 | 1973.29 | 11.26 | −1776.79 |
| 333.15 | 0.351 | 2604.05 | 13.81 | −1998.38 |
| 333.15 | 0.415 | 3056.67 | 15.44 | −2087.74 |
| 343.15 | 0.066 | 457.35 | 3.57 | −768.60 |
| 343.15 | 0.150 | 999.06 | 6.89 | −1365.10 |
| 343.15 | 0.238 | 1513.76 | 9.64 | −1792.98 |
| 343.15 | 0.318 | 1929.18 | 11.60 | −2052.46 |
| 343.15 | 0.381 | 2221.33 | 12.84 | −2183.77 |
| T (K) | x1 | ΔHmix (J·mol−1) | ΔSmix (J·mol−1·K−1) | ΔGmix (J·mol−1) |
|---|---|---|---|---|
| 293.15 | 0.105 | 1564.06 | 7.73 | −701.76 |
| 293.15 | 0.243 | 3677.08 | 16.15 | −1057.84 |
| 293.15 | 0.353 | 5418.21 | 22.35 | −1132.62 |
| 293.15 | 0.457 | 7105.54 | 27.89 | −1069.34 |
| 293.15 | 0.536 | 8410.79 | 31.90 | −940.54 |
| 303.15 | 0.099 | 2036.09 | 9.23 | −760.53 |
| 303.15 | 0.231 | 4743.18 | 19.68 | −1222.57 |
| 303.15 | 0.340 | 6955.06 | 27.52 | −1387.48 |
| 303.15 | 0.444 | 9043.68 | 34.47 | −1404.86 |
| 303.15 | 0.521 | 10582.22 | 39.32 | −1336.88 |
| 313.15 | 0.095 | 1731.47 | 8.17 | −827.96 |
| 313.15 | 0.220 | 3904.71 | 16.87 | −1377.41 |
| 313.15 | 0.328 | 5692.07 | 23.39 | −1631.97 |
| 313.15 | 0.430 | 7291.74 | 28.80 | −1727.94 |
| 313.15 | 0.504 | 8412.21 | 32.35 | −1718.08 |
| 323.15 | 0.089 | 1262.21 | 6.58 | −863.68 |
| 323.15 | 0.206 | 2746.49 | 13.07 | −1477.49 |
| 323.15 | 0.311 | 3899.37 | 17.64 | −1801.77 |
| 323.15 | 0.412 | 4858.54 | 21.11 | −1963.76 |
| 323.15 | 0.484 | 5450.15 | 23.05 | −1997.78 |
| 333.15 | 0.085 | 1111.99 | 6.02 | −893.60 |
| 333.15 | 0.190 | 2277.23 | 11.43 | −1529.48 |
| 333.15 | 0.290 | 3142.57 | 15.16 | −1909.42 |
| 333.15 | 0.460 | 4080.98 | 18.84 | −2195.77 |
| 343.15 | 0.264 | 3323.86 | 15.43 | −1972.47 |
| 343.15 | 0.358 | 3973.34 | 18.13 | −2246.47 |
| 343.15 | 0.428 | 4272.83 | 19.33 | −2359.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Dang, W.; Wang, X. Solubility Measurement and Correlation of Cis-1,1,1,4,4,4-Hexafluoro-2-butene in Dipentaerythritol Hexaheptanoate and Dipentaerythritol Isononanoate from 293.15 K to 343.15 K. Processes 2025, 13, 3704. https://doi.org/10.3390/pr13113704
Lu C, Dang W, Wang X. Solubility Measurement and Correlation of Cis-1,1,1,4,4,4-Hexafluoro-2-butene in Dipentaerythritol Hexaheptanoate and Dipentaerythritol Isononanoate from 293.15 K to 343.15 K. Processes. 2025; 13(11):3704. https://doi.org/10.3390/pr13113704
Chicago/Turabian StyleLu, Cheng, Wenzhe Dang, and Xiaopo Wang. 2025. "Solubility Measurement and Correlation of Cis-1,1,1,4,4,4-Hexafluoro-2-butene in Dipentaerythritol Hexaheptanoate and Dipentaerythritol Isononanoate from 293.15 K to 343.15 K" Processes 13, no. 11: 3704. https://doi.org/10.3390/pr13113704
APA StyleLu, C., Dang, W., & Wang, X. (2025). Solubility Measurement and Correlation of Cis-1,1,1,4,4,4-Hexafluoro-2-butene in Dipentaerythritol Hexaheptanoate and Dipentaerythritol Isononanoate from 293.15 K to 343.15 K. Processes, 13(11), 3704. https://doi.org/10.3390/pr13113704






