Transglutaminase Effects on Texture and Flow Behaviour of Fermented Milk During Storage Using Concentrated Kombucha Inoculum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacture of Samples
2.3. Methods
2.3.1. Monitoring of the Fermentation Process
2.3.2. Textural Characteristics
2.3.3. Rheological Analyses
2.4. Production Cost Analysis
2.5. Statistical Analysis
3. Results
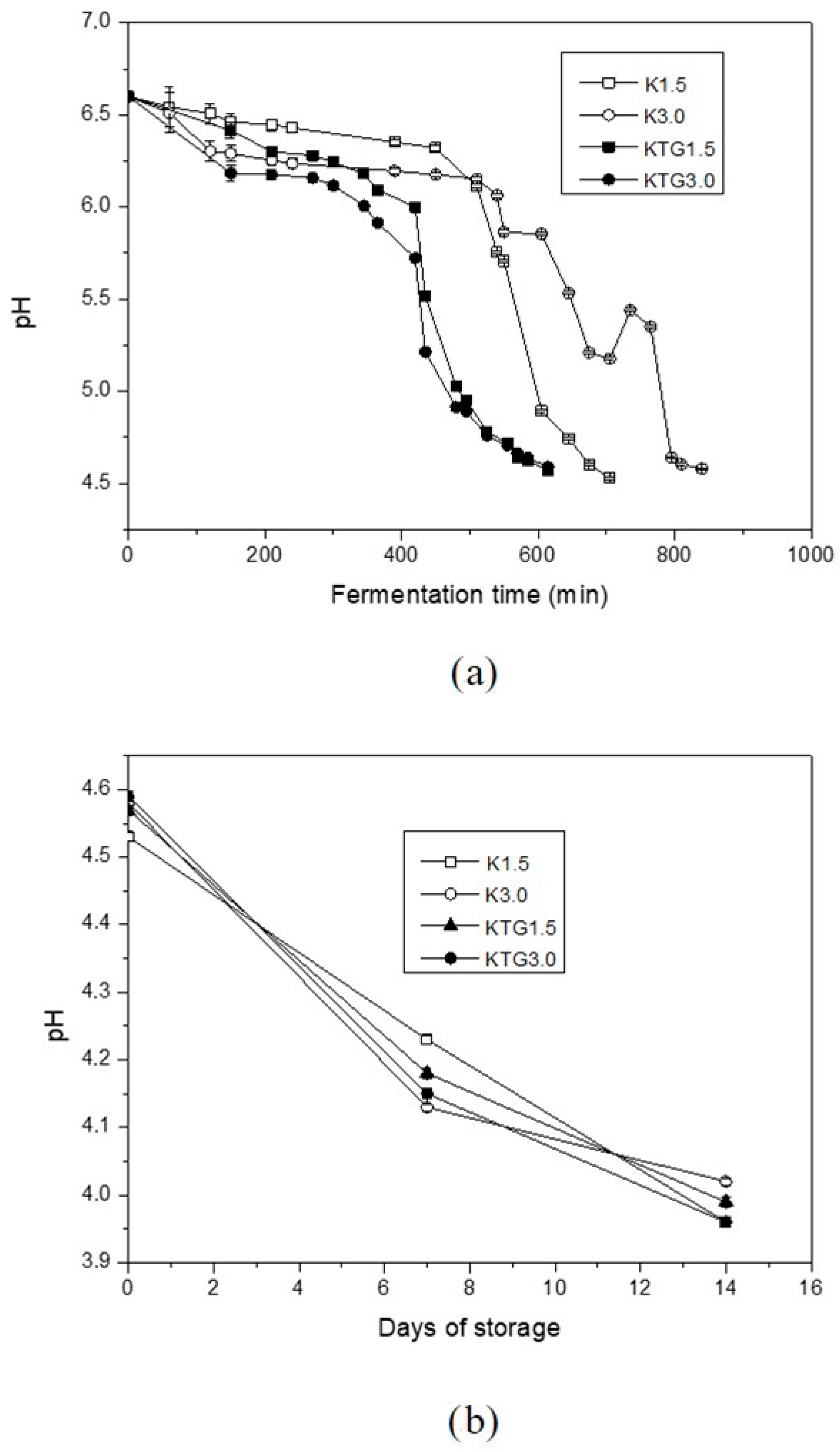
3.1. Fermentation Development and pH Evolution
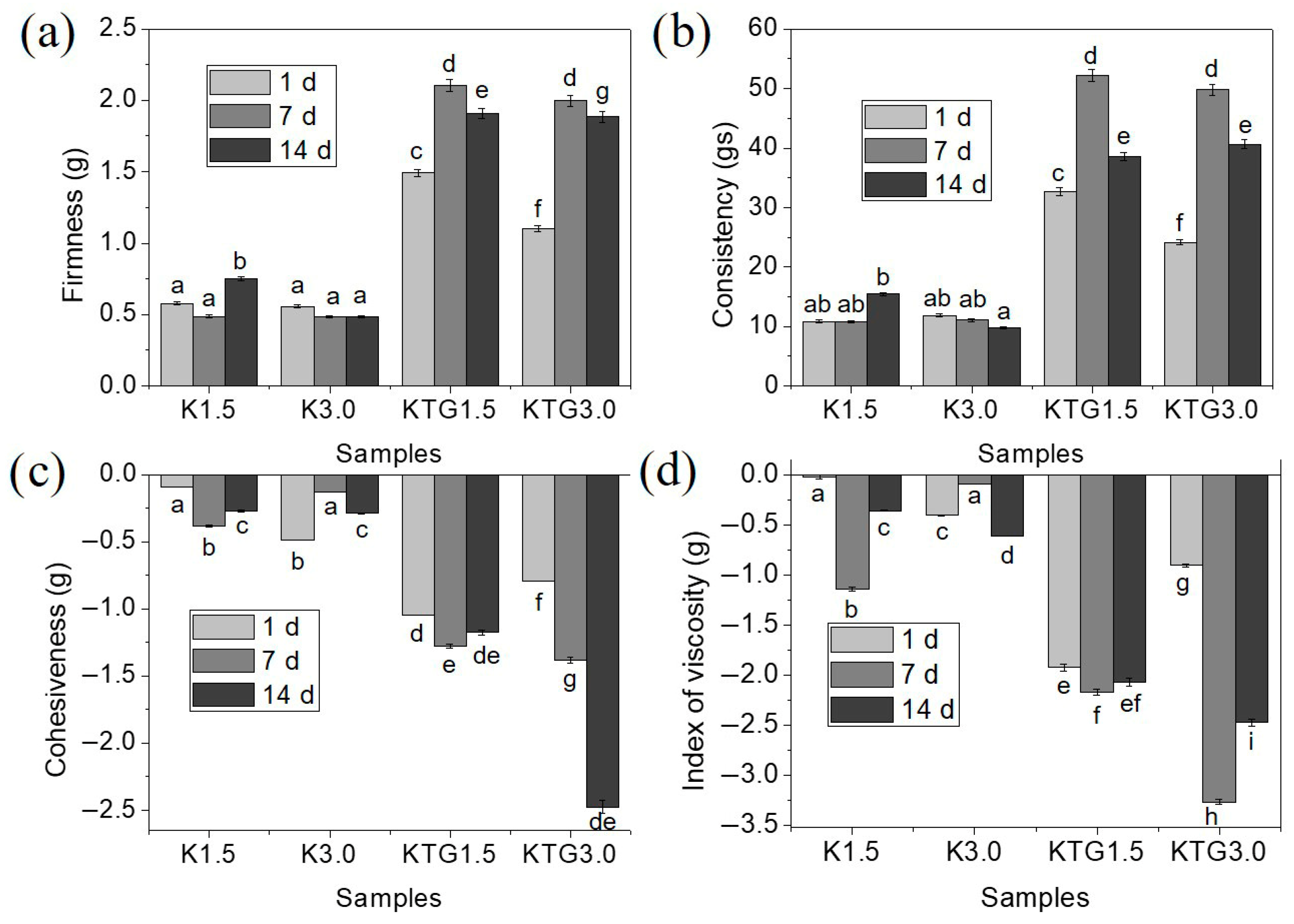
3.2. Textural Properties
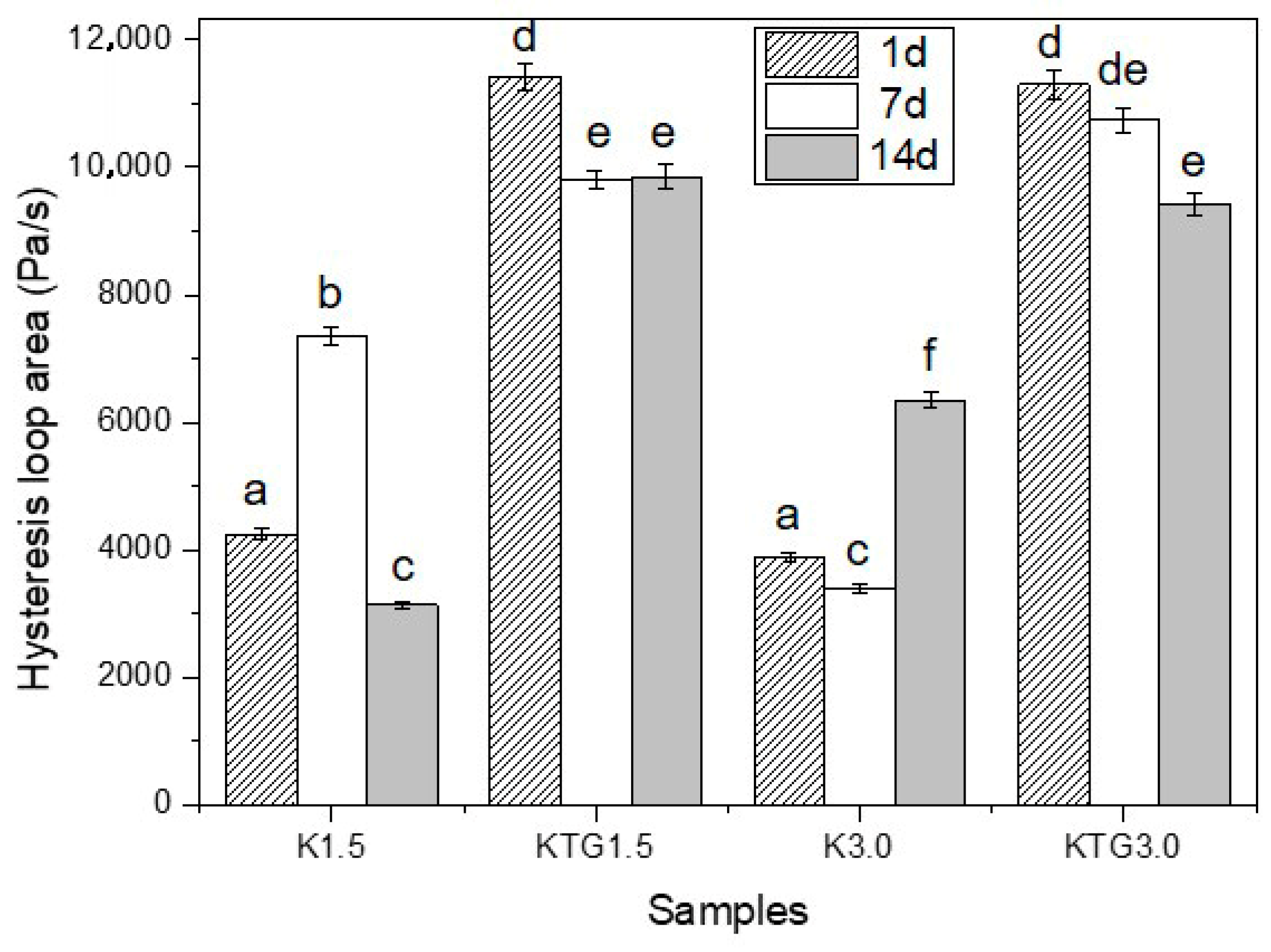
3.3. Rheological Properties
3.4. Cost Structure of Kombucha-Fermented Milk Products
3.5. Principal Component Analysis
4. Discussion
4.1. Fermentation Development and pH Evolution
4.2. Textural Properties
4.3. Rheological Properties
4.4. Cost Structure of Kombucha-Fermented Milk Products
4.5. Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TG | Transglutaminase |
| K1.5 | Fermented milk sample with 1.5% kombucha concentrate |
| K3.0 | Fermented milk sample with 3.0% kombucha concentrate |
| KTG1.5 | Fermented milk sample with 1.5% kombucha concentrate and added transglutaminase |
| KTG3.0 | Fermented milk sample with 3.0% kombucha concentrate and added transglutaminase |
References
- Vukić, V.; Iličić, M.; Hrnjez, D.; Kocić-Tanackov, S.; Pavlić, B.; Bjekić, M.; Kanurić, K.; Degenek, J.; Zeković, Z. The application of kombucha inoculum as an innovative starter culture in fresh cheese production. LWT Food Sci. Technol. 2021, 151, 112142. [Google Scholar] [CrossRef]
- Degenek, J.; Kanurić, K.; Iličić, M.; Vukić, D.; Mrkonjić, Ž.; Pavlić, B.; Zeković, Z.; Vukić, V. Fortification of fresh kombucha cheese with wild thyme (Thymus serpyllum L.) herbal dust and its influence on antioxidant activity. Food Biosci. 2023, 51, 103161. [Google Scholar] [CrossRef]
- Ningtiyas, W.D.; Irfan, M.; Mukhlisah, A.N.; Syah, S.P.; Rab, S.A.; Mutmainna, A. The Effectiveness of Kombucha as a Starter in the Production of Fermented Milk Beverages. J. Agripet 2025, 25, 1–6. Available online: https://jurnal.usk.ac.id/agripet (accessed on 14 July 2025). [CrossRef]
- Marsh, A.J.; Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha tea fungus samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.R.; Neto, R.O.; Dos Santos D’Almeida, C.T.; Pimenta di Nascimento, T.; Girotto Pressete, C.; Azevedo, L.; Duarte Martino, H.C.; Cameron, L.C.; Larraz Ferreira, M.S.; Ribeiro de Barros, F.A. Kombucha from green and black teas have different phenolic profile, which impact their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Malbaša, R.; Jevrić, L.; Lončar, E.; Vitas, J.; Podunavac-Kuzmanović, S.; Milanović, S.; Kovačević, S. Chemometric approach to texture profile analysis of kombucha fermented milk products. J. Food Sci. Technol. 2015, 52, 5968–5974. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Razavi, S.H.; Kieliszek, M. Recent advances in microbial transglutaminase biosynthesis and its application in the food industry. Trends Food Sci. Technol. 2021, 110, 458–469. [Google Scholar] [CrossRef]
- Di Renzo, T.; Reale, A. Process Optimization and Quality Improvement of Fermented Foods and Beverages. Foods 2025, 14, 1238. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Liang, H. Food Hydrocolloids: Structure, Properties, and Applications. Foods 2024, 13, 1077. [Google Scholar] [CrossRef]
- Mahmood, W.A.; Sebo, N.H. Improvement of yoghurt properties by microbial transglutaminase. Jordan J. Agric. Sci. 2012, 173, 1–21. [Google Scholar]
- García-Gómez, B.; Romero-Rodríguez, Á.; Vázquez-Odériz, L.; Muñoz-Ferreiro, N.; Vázquez, M. Physicochemical evaluation of low-fat yoghurt produced with microbial transglutaminase. J. Sci. Food Agric. 2018, 98, 5479–5485. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Yang, Y.; Zhang, R.; Jiao, A.; Jin, Z. Construction of transglutaminase covalently cross-linked hydrogel and high internal phase emulsion gel from pea protein modified by high-intensity ultrasound. J. Sci. Food Agric. 2023, 103, 1874–1884. [Google Scholar] [CrossRef]
- Iličić, M.; Milanović, S.; Carić, M.; Dokić, L.P.; Kanurić, K. Effect of transglutaminase on texture and flow properties of stirred probiotic yoghurt during storage. J. Texture Stud. 2014, 45, 13–19. [Google Scholar] [CrossRef]
- Ozturkoglu-Budak, S.; Akal, H.C.; Buran, İ.; Yetişemiyen, A. Effect of inulin polymerization degree on various properties of synbiotic fermented milk including Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12. J. Dairy Sci. 2019, 102, 6901–6913. [Google Scholar] [CrossRef] [PubMed]
- Iličić, M.; Milanović, S.; Kanurić, K.; Vukić, V.; Vukić, D.; Stojanović, B.J. Poboljšanje teksture i reoloških svojstava čvrstih i tekućih kombucha fermentiranih mliječnih napitaka s dodatkom transglutaminaze. Mljekarstvo 2021, 71, 155–164. [Google Scholar] [CrossRef]
- Vukić, D.V.; Vukić, V.R.; Milanović, S.D.; Iličić, M.D.; Kanurić, K.G. Modeling of rheological characteristics of the fermented dairy products obtained by novel and traditional starter cultures. J. Food Sci. Technol. 2018, 55, 2180–2188. [Google Scholar] [CrossRef]
- Masiá, C.; Ong, L.; Logan, A.; Stockmann, R.; Gambetta, J.; Jensen, P.E.; Yazdi, S.R.; Gras, S. Enhancing the textural and rheological properties of fermentation-induced pea protein emulsion gels with transglutaminase. Soft Matter 2024, 20, 133–143. [Google Scholar] [CrossRef]
- Malbaša, R.; Milanović, S.; Lončar, E.; Djurić, M.; Carić, M.; Iličić, M.; Kolarov, L. Milk-based beverages obtained by kombucha application. Food Chem. 2009, 112, 178–184. [Google Scholar] [CrossRef]
- Hassan, A.N.; Ipsen, R.; Janzen, T.; Qvist, K.B. Microstructure and Rheology of Yogurt Made with Cultures Differing Only in Their Ability to Produce Exopolysaccharides. J. Dairy Sci. 2003, 86, 1632–1638. [Google Scholar] [CrossRef]
- Dokić, P.; Sovilj, V.; Šefer, I.; Rašulić, G. Thixotropy evaluation by parameters of flow equation. Acta Period. Technol. 1999, 29–30, 67–79. [Google Scholar]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Vitas, J.S.; Malbaša, R.V.; Grahovac, J.A.; Lončar, E.S. The antioxidant activity of kombucha fermented milk products with stinging nettle and winter savory. Chem. Ind. Chem. Eng. Q. 2013, 19, 129–139. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Z.; Zhang, J.; Mu, G.; Jiang, S. Characteristics of the mixed yogurt fermented from cow–soy milk in the presence of transglutaminase. Foods 2024, 13, 2120. [Google Scholar] [CrossRef]
- Sarkaya, P.; Akan, E.; Kinik, O. Use of kombucha culture in the production of fermented dairy beverages. LWT Food Sci. Technol. 2021, 137, 110326. [Google Scholar] [CrossRef]
- Beirami-Serizkani, F.; Hojjati, M.; Jooyandeh, H. The effect of microbial transglutaminase enzyme and Persian gum on the characteristics of traditional kefir drink. Int. Dairy J. 2021, 112, 104843. [Google Scholar] [CrossRef]
- Taşkoparan, Ş.; Altınay, C.; Özer, H.B. Recent updates of probiotic dairy-based beverages. Food Funct. 2025, 16, 1656–1669. [Google Scholar] [CrossRef]
- Velázquez-Domínguez, A.; Hiolle, M.; Abdallah, M.; Delaplace, G.; Peixoto, P.P.S. Transglutaminase cross-linking on dairy proteins: Functionalities, patents, and commercial uses. Int. Dairy J. 2023, 143, 105688. [Google Scholar] [CrossRef]
- Hovjecki, M. Influence of Selected Factors on the Acid and Rennet Coagulation of Goat’s Milk and the Quality of Yoghurts and Cheeses. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2021. Available online: https://nardus.mpn.gov.rs/handle/123456789/18511 (accessed on 9 October 2025).
- Lorenzen, P.C.; Neve, H.; Mautner, A.; Schlimme, E. Effect of enzymatic cross-linking of milk proteins on functional properties of set-style yoghurt. Int. J. Dairy Technol. 2002, 55, 152–157. [Google Scholar] [CrossRef]
- Neve, V.H.; Lorenzen, P.C.; Mautner, A.; Schlimme, E.; Heller, K.J. Effects of transglutaminase treatment on the production of set skim milk yoghurt: Microbiological aspects. Kieler Milchwirtsch. Forschber. 2001, 53, 347–361. [Google Scholar]
- Ziarno, M.; Zaręba, D. The effect of the addition of microbial transglutaminase before the fermentation process on the quality characteristics of three types of yogurt. Food Sci. Biotechnol. 2020, 29, 109–119. [Google Scholar] [CrossRef]
- Domagała, J.; Wszołek, M.; Tamime, A.Y.; Kupiec-Teahan, B. The effect of transglutaminase concentration on the texture, syneresis and microstructure of set-type goat’s milk yoghurt during the storage period. Small Rumin. Res. 2013, 112, 154–161. [Google Scholar] [CrossRef]
- Temiz, H.; Çakmak, E. The effect of microbial transglutaminase on probiotic fermented milk produced using a mixture of bovine milk and soy drink. Int. J. Dairy Technol. 2018, 71, 906–920. [Google Scholar] [CrossRef]
- Darnay, L.; Csehi, B.; Barna, N.; Pasztorné-Huszar, K.; Horvath, M. The effect of microbial transglutaminase on the viscosity and protein network of kefir made from cow, goat or donkey milk. Fermentation 2021, 7, 214. [Google Scholar] [CrossRef]
- Bönisch, M.P.; Huss, M.; Lauber, S.; Kulozik, U. Yoghurt gel formation by means of enzymatic protein cross-linking during microbial fermentation. Food Hydrocoll. 2007, 21, 585–595. [Google Scholar] [CrossRef]
- Kuraishi, C.; Sakamoto, J.; Soeda, T. The usefulness of transglutaminase for food processing. In American Chemical Society Symposium Series; American Chemical Society: Washington, DC, USA, 1996; Volume 637, pp. 1–14. [Google Scholar]
- Færgemand, M.; Sørensen, M.; Jørgensen, U.; Budolsfen, G.; Qvist, K.B. Transglutaminase: Effect on instrumental and sensory texture of set-style yoghurt. Milchwissenschaft 1999, 54, 563–566. [Google Scholar]
- Niu, Q.; Liu, E.; Huo, R.; Zhang, F.; He, R.; Yang, J.; Zhao, Z. Effects of transglutaminase and heat treatment on the structure and gelation properties of camel casein protein. Foods 2025, 14, 1644. [Google Scholar] [CrossRef] [PubMed]
- Tang, I.; Roos, Y.H.; Miao, S. Effect of microbial transglutaminase on functional, rheological, and structural properties of lentil protein-casein binary gels. Food Hydrocoll. 2023, 143, 108838. [Google Scholar] [CrossRef]
- Popović, R.; Milanović, S.; Iličić, M.; Ranogajec, M.; Kanurić, K.; Vukić, V.; Hrnjez, D. Nutritive characteristics and market prospects of kombucha fermented milk beverages. Agro FOOD Ind. Hi Tech. 2016, 27, 48–52. [Google Scholar]
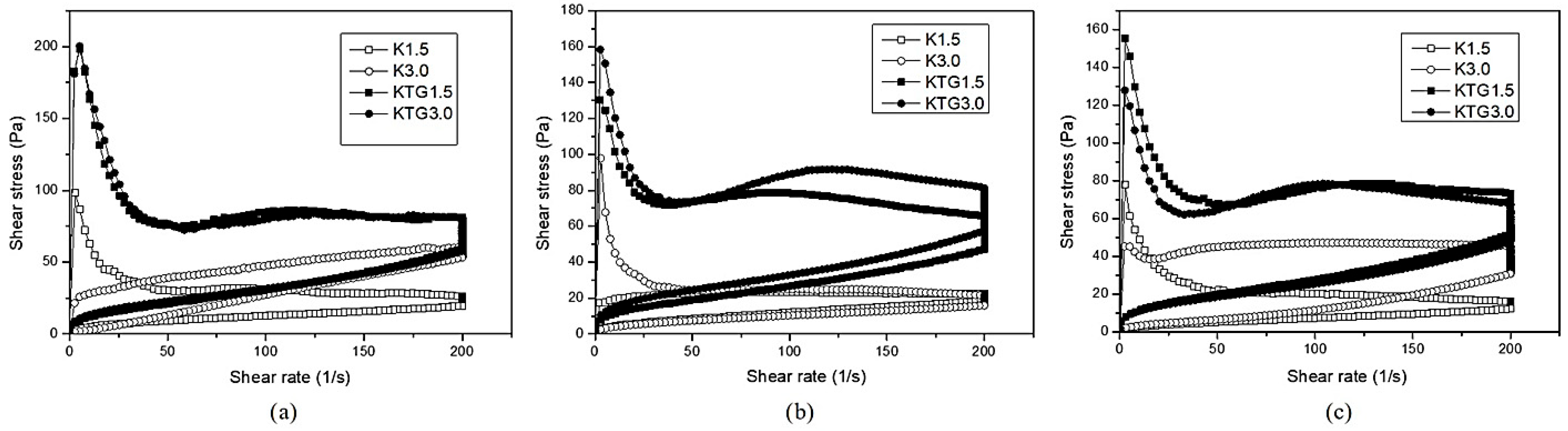

| Samples | Day | τ0 a (H.B.) (Pa) | Km b (Pas n) | nm c | R2 d | τ0 e (Pa) | η f (Pas) | Kd g |
|---|---|---|---|---|---|---|---|---|
| K1.5 | 1 | 2.68 ± 0.05 c | 0.474 ± 0.009 f | 0.665 ± 0.013 a | 0.996 | 98.43 ± 1.9 e | 33.95 ± 0.67 e | 0.633 ± 0.012 |
| 7 | 1.92 ± 0.03 ab | 0.001 ± 0.000 a | 2.103 ± 0.000 bd | 0.996 | 38.65 ± 0.8 b | 15.16 ± 0.30 b | 0.734 ± 0.014 | |
| 14 | 1.79 ± 0.03 ab | 0.133 ± 0.002 b | 0.813 ± 0.001 ac | 0.998 | 77.93 ± 1.6 d | 28.01 ± 0.56 d | 0.684 ± 0.013 | |
| K3.0 | 1 | - | 0.297 ± 0.005 d | 0.381 ± 0.006 c | 0.996 | 21.58 ± 0.5 a | 8.37 ± 0.15 a | 0.422 ± 0.007 |
| 7 | 2.02 ± 0.04 a | 0.392 ± 0.007 e | 0.606 ± 0.012 cef | 0.996 | 97.87 ± 1.9 e | 33.58 ± 0.67 e | 0.629 ± 0.012 | |
| 14 | 3.65 ± 0.07 b | 0.002 ± 0.000 a | 1.736 ± 0.035 df | 0.942 | 45.27 ± 0.9 c | 17.51 ± 0.35 c | 0.705 ± 0.014 | |
| KTG1.5 | 1 | 15.48 ± 0.31 h | 0.001 ± 0.000 a | 2.005 ± 0.040 bd | 0.992 | 183.0 ± 3.7 h | 72.0 ± 1.44 h | 0.665 ± 0.013 |
| 7 | 9.64 ± 0.19 d | 0.151 ± 0.003 b | 1.030 ± 0.020 ae | 0.990 | 130.4 ± 2.6 f | 49.71 ± 0.99 f | 0.644 ± 0.011 | |
| 14 | 10.68 ± 0.21 e | 0.091 ± 0.001 c | 1.142 ± 0.022 a | 0.988 | 155.4 ± 3.1 g | 58.08 ± 1.16 g | 0.632 ± 0.012 | |
| KTG3.0 | 1 | 11.8 ± 0.23 f | 0.148 ± 0.002 b | 1.069 ± 0.021 aef | 0.988 | 181.1 ± 3.6 h | 74.64 ± 1.49 h | 0.636 ± 0.013 |
| 7 | 13.38 ± 0.26 g | 0.169 ± 0.003 b | 1.036 ± 0.020 aef | 0.988 | 158.4 ± 3.2 g | 59.61 ± 1.19 g | 0.616 ± 0.012 | |
| 14 | 10.60 ± 0.21 e | 0.063 ± 0.001 a | 1.189 ± 0.023 bef | 0.996 | 127.8 ± 2.6 f | 48.57 ± 0.97 f | 0.641 ± 0.013 |
| Unit | Quantity | Price EUR/Unit | Amount | |
|---|---|---|---|---|
| Black Tea | kg | 0.0015 | 8.3759 | 0.0126 |
| Sucrose | kg | 0.07 | 0.6122 | 0.0429 |
| Depreciation | 1 | 0.0298 | 0.0298 | |
| Overhead cost | 1 | 0.2296 | 0.2296 | |
| TOTAL: | 0.3148 |
| Kombucha Milk Fermented Beverage | Plain Yoghurt with 2.8% Milk Fat | ||||
|---|---|---|---|---|---|
| K1.5 | K3.0 | KTG1.5 | KTG3.0 | ||
| Raw milk | 0.2679 | 0.2679 | 0.2679 | 0.2679 | 0.2679 |
| Inoculum kombucha/ starter culture | 0.0236 | 0.0472 | 0.0236 | 0.0472 | 0.0060 |
| TG | 0.0000 | 0.0000 | 0.0170 | 0.0170 | 0.0000 |
| Packaging | 0.0995 | 0.0995 | 0.0995 | 0.0995 | 0.0995 |
| Depreciation | 0.0204 | 0.0204 | 0.0204 | 0.0204 | 0.0204 |
| Overhead cost | 0.0765 | 0.0765 | 0.0765 | 0.0765 | 0.0765 |
| TOTAL: | 0.4879 | 0.5115 | 0.5049 | 0.5285 | 0.4702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iličić, M.; Degenek, J.; Vukić, V.; Dokić, L.; Kanurić, K.; Popović, R.; Vukić, D. Transglutaminase Effects on Texture and Flow Behaviour of Fermented Milk During Storage Using Concentrated Kombucha Inoculum. Processes 2025, 13, 3598. https://doi.org/10.3390/pr13113598
Iličić M, Degenek J, Vukić V, Dokić L, Kanurić K, Popović R, Vukić D. Transglutaminase Effects on Texture and Flow Behaviour of Fermented Milk During Storage Using Concentrated Kombucha Inoculum. Processes. 2025; 13(11):3598. https://doi.org/10.3390/pr13113598
Chicago/Turabian StyleIličić, Mirela, Jovana Degenek, Vladimir Vukić, Ljubica Dokić, Katarina Kanurić, Rade Popović, and Dajana Vukić. 2025. "Transglutaminase Effects on Texture and Flow Behaviour of Fermented Milk During Storage Using Concentrated Kombucha Inoculum" Processes 13, no. 11: 3598. https://doi.org/10.3390/pr13113598
APA StyleIličić, M., Degenek, J., Vukić, V., Dokić, L., Kanurić, K., Popović, R., & Vukić, D. (2025). Transglutaminase Effects on Texture and Flow Behaviour of Fermented Milk During Storage Using Concentrated Kombucha Inoculum. Processes, 13(11), 3598. https://doi.org/10.3390/pr13113598








