Structure, Synthesis and Properties of Antimony Oxychlorides: A Brief Review
Abstract
1. Introduction
2. Antimony: Elemental Properties, Historical Use, and Strategic Significance
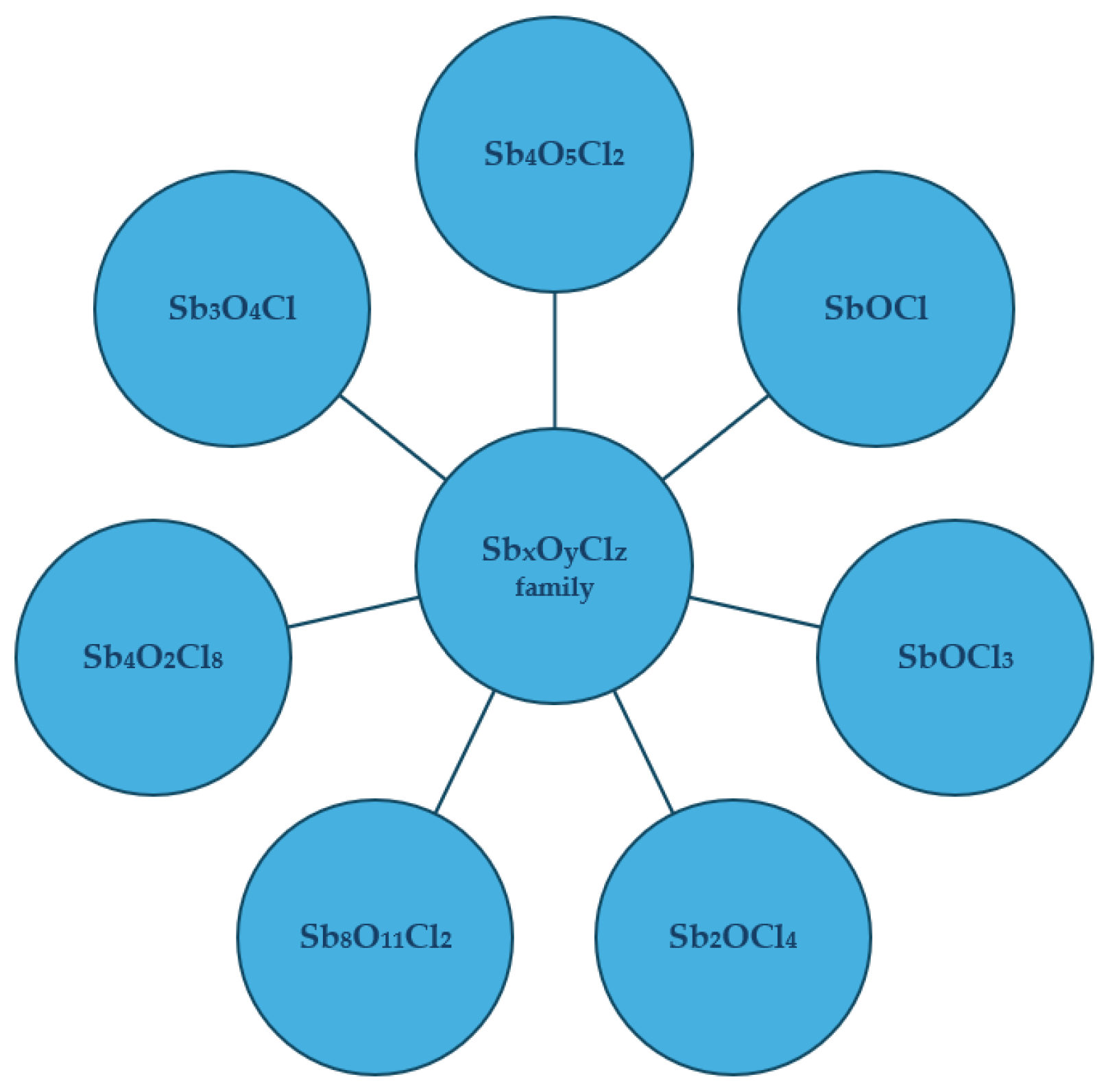
3. Structural Foundations of Antimony Oxychlorides
4. Fabrication Methods of Antimony Oxychlorides
5. Challenges and Future Prospects of Antimony Oxychlorides Compounds
5.1. Prospects for the Development of Antimony Oxychloride-Based Materials
5.1.1. Molecular Engineering and Doping
5.1.2. Hybrid Structures and Composites
5.1.3. Application in Flame-Retardant Systems
5.1.4. Development of Vapor-Phase Transport Methods
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weston, K.; Taylor, R.A.; Kisslinger, K.; Mantripragada, S. Effect of Growth Dynamics on the Structural, Photophysical and Pseudocapacitance Properties of Famatinite Copper Antimony Sulphide Colloidal Nanostructures (Including Nanosheets). Dalton Trans. 2025, 54, 3174–3187. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Wu, Y.; Liu, Y.; Xu, J.; Guo, X.; Chen, J.; Ouyang, X. Source, Distribution and Potential Risk of Antimony in Water and Sediments of Danjiangkou Reservoir: Impact from Dam. Int. J. Environ. Res. Public Health 2022, 19, 12367. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Chen, D.; Tachibana, Y.; Suzuki, H.; Abe, R.; Caruso, R.A. Developing Sustainable, High-Performance Perovskites in Photocatalysis: Design Strategies and Applications. Chem. Soc. Rev. 2021, 50, 13692–13729. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Lee, K.H.; Soni, H.L.; Mangi, K.H.; Channa, A.S.; Khan, R.; Lee, K.G. Coagulation Behavior of Antimony Oxyanions in Water: Influence of pH, Inorganic and Organic Matter on the Physicochemical Characteristics of Iron Precipitates. Molecules 2022, 27, 1663. [Google Scholar] [CrossRef]
- Amutha, E.; Rajaduraipandian, S.; Sivakavinesan, M.; Annadurai, G. Hydrothermal Synthesis and Characterization of the Antimony–Tin Oxide Nanomaterial and Its Application as a High-Performance Asymmetric Supercapacitor, Photocatalyst, and Antibacterial Agent. Nanoscale Adv. 2023, 5, 255–267. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Jeong, W. Study on the Flame Retardancy and Hazard Evaluation of Poly(Acrylonitrile-co-Vinylidene Chloride) Fibers by the Addition of Antimony-Based Flame Retardants. Polymers 2021, 14, 42. [Google Scholar] [CrossRef]
- Aqeel, T.; Galstyan, V.; Comini, E.; Bumajdad, A. Efficient One-Pot Synthesis of Antimony-Containing Mesoporous Tin Dioxide Nanostructures for Gas-Sensing Applications. Arab. J. Chem. 2023, 16, 104797. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Wong, T.K.S.; Kundu, S.; Chakraborty, A.K.; Zhuk, S. Emerging Trends in Sulfide and Selenide Based Materials for Emerging Applications: Sustainable Energy Harvesting and Storage Technology. In Sulfide and Selenide Based Materials for Emerging Applications; Elsevier: Amsterdam, The Netherlands, 2022; p. 195. [Google Scholar]
- Li, T.; He, X.; Yang, J.; Nazarov, R.S.; Deribina, E.I.; Kapitonov, Y.; Zhai, T. Recent Research Progress of Antimony-Based Two-Dimensional Materials for Electronics and Optoelectronics. Adv. Mater. 2025, 37, 2503509. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Han, M.F.; Zhao, X.J.; Ma, Y.Y.; Jing, C.Q.; Pan, H.M.; Lei, X.W. Structural Dimensionality Modulation toward Enhanced Photoluminescence Efficiencies of Hybrid Lead-Free Antimony Halides. Adv. Opt. Mater. 2021, 9, 2100556. [Google Scholar] [CrossRef]
- Komal, N.; Malik, Z.; Ali, N.Z.; Chaudhary, A.J. Synthesis, Characterization and Properties of Hierarchically Assembled Antimony Oxyhalides Nanonetworks. Mater. Res. Express 2019, 6, 065035. [Google Scholar] [CrossRef]
- Al-Hamdi, A.; Sillanpää, M. Photocatalytic Activities of Antimony, Iodide, and Rare Earth Metals on the Photodegradation of Phenol over SnO2 under UV, Solar, and Visible Light Irradiations. In Advanced Water Treatment: Advanced Oxidation Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–288. [Google Scholar] [CrossRef]
- Petrovic, C.; Mamantov, G.; Soerlie, M.; Lietzke, M.H.; Smith, G.P. Electrical Conductivity of Molten Mixtures of Antimony(III) Chloride–Aluminum Chloride and Antimony(III) Chloride–Aluminum Chloride–Sodium Chloride. J. Phys. Chem. 1982, 86, 4598–4605. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, J.; Lee, Y.W. Adsorption Removal Characteristics of Hazardous Metalloids (Antimony and Arsenic) According to Their Ionic Properties. Water 2024, 16, 767. [Google Scholar] [CrossRef]
- Biver, M. Some Kinetic Aspects of the Mobilization of Antimony from Natural Sources. Ph.D. Thesis, Ruprecht-Karls-Universität, Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Meradji, H.; Drablia, S.; Ghemid, S.; Belkhir, H.; Bouhafs, B.; Tadjer, A. First-Principles Elastic Constants and Electronic Structure of BP, BAs, and BSb. Phys. Status Solidi B 2004, 241, 2881–2885. [Google Scholar] [CrossRef]
- Norman, N.C. (Ed.) Chemistry of Arsenic, Antimony and Bismuth; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Weast, R.C. The Elements (Continued). In Handbook of Chemistry and Physics, 69th ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. B-8,B-73–B-74. [Google Scholar]
- Ruetschi, P. Review on the Lead–Acid Battery Science and Technology. J. Power Sources 1977, 2, 3–120. [Google Scholar] [CrossRef]
- Schwarz-Schampera, U. Antimony. In Critical Metals Handbook; Wiley: Hoboken, NJ, USA, 2014; pp. 70–98. [Google Scholar] [CrossRef]
- Jochum, K.P.; Hofmann, A.W. Constraints on Earth Evolution from Antimony in Mantle-Derived Rocks. Chem. Geol. 1997, 139, 39–49. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of Metals and Metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef]
- Köhler, A.R. Material Scarcity: A Reason for Responsibility in Technology Development and Product Design. Sci. Eng. Ethics 2013, 19, 1165–1179. [Google Scholar] [CrossRef]
- Jawadand, S.; Randive, K. A Sustainable Approach to Transforming Mining Waste into Value-Added Products. In Innovations in Sustainable Mining: Balancing Environment, Ecology and Economy; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Hatayama, H. The Metals Industry and the Sustainable Development Goals: The Relationship Explored Based on SDG Reporting. Resour. Conserv. Recycl. 2022, 178, 106081. [Google Scholar] [CrossRef]
- Parra, C.; Lewis, B.; Ali, S.H. (Eds.) . Mining, Materials, and the Sustainable Development Goals (SDGs): 2030 and Beyond; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- U.S. Department of the Interior; U.S. Geological Survey. Mineral Commodity Summaries 2023; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2024. [Google Scholar]
- U.S. Department of the Interior; U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2025. [Google Scholar]
- Anderson, C.G. The Metallurgy of Antimony. Chem. Erde 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Claude, F. Non-Ferrous Metal: From Ag to Zn. Lannoo: Tielt, Belgium, 2002. [Google Scholar]
- Jones, J.S.; Gabbai, F.P. Coordination- and Redox-Noninnocent Behavior of Ambiphilic Ligands Containing Antimony. Acc. Chem. Res. 2016, 49, 857–867. [Google Scholar] [CrossRef]
- Ling, M.; Zhang, S.-G.; Pan, D.-A. Antimony Recovery from SbCl5 Acid Solution by Hydrolysis and Aging. Rare Met. 2015, 34, 436–439. [Google Scholar] [CrossRef]
- Zheng, G.-Q.; Zhi, B.; Chen, J.-Z. Hydrolysis of Antimony Pentachloride. Chin. J. Nonferrous Met. 2006, 16, 1628–1633. [Google Scholar]
- Freedman, L.D.; Doak, G.O.; Long, G.G.; Mahmood, T.; Lindhal, C.B. Antimony Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Li, Y. Theoretical Simulation and Experimental Study of Hydrolysis Separation of SbCl3 in Complexation–Precipitation System. Trans. Nonferrous Met. Soc. China 2016, 26, 2746–2753. [Google Scholar] [CrossRef]
- Collins, H. Halide Transfer Reactions of Arsenic, Antimony and Bismuth. Ph.D. Thesis, University of Warwick, Coventry, UK, 1991. [Google Scholar]
- Cowell, G.W.; Ledwith, A.; White, A.C.; Woods, H.J. Electron-Transfer Oxidation of Organic Compounds with Hexachloroantimonate [SbCl6]− Ion. J. Chem. Soc. B Phys. Org. 1970, 227–231. [Google Scholar] [CrossRef]
- Kisenyi, J.M. Studies of the Coordination Chemistry of As(III), Sb(III), Bi(III), Sn(IV) and Ti(IV) Species. Ph.D. Thesis, University of Warwick, Coventry, UK, 1983. [Google Scholar]
- Hu, C.; Chu, D.; Hou, X.; Zhang, F.; Han, J. Exploration of Antimony(III) Oxyhalides via Single-Site Substitution in Quest of Large Birefringence. Inorg. Chem. Front. 2024, 11, 3367–3376. [Google Scholar] [CrossRef]
- Hao, R.; Duan, C.K. Unraveling the Photoluminescent Properties of Sb-Doped Cd-Based Inorganic Halides: A First-Principles Study. Inorg. Chem. 2024, 63, 3152–3164. [Google Scholar] [CrossRef]
- Becker, G.; Mundt, O.; Standelmann, H. Element-Element-Bindungen. VI. Kristallines 2-Chlor-1,3,2-Benzoxathiastibol—Ein Mehrfach Verknüpftes Koordinationspolymeres. Z. Anorg. Allg. Chem. 1990, 580, 139–150. [Google Scholar] [CrossRef]
- Edstrand, M.; Brodersen, R.; Sillén, L.G.; Linnasalmi, A.; Laukkanen, P. On the Crystal Structure of the Antimony Oxychloride Sb4O5Cl2 and Isomorphous Oxybromide. Acta Chem. Scand. 1947, 1, 178–203. [Google Scholar] [CrossRef]
- Schwarz, W.; Hausen, H.D. Kristall- und Molekülstruktur von Tetramethyl(dimethylthiophosphinato)stiboran (CH3)4SbOP(S)(CH3)2. Z. Anorg. Allg. Chem. 1978, 441, 175–180. [Google Scholar] [CrossRef]
- Menchetti, S.; Sabelli, C.; Trosti-Ferroni, R. The Structures of Onoratoite, Sb8O11Cl2 and Sb8O11Cl2·6H2O. Cryst. Struct. Commun. 1984, 40, 1506–1510. [Google Scholar] [CrossRef]
- Peng, X.; Li, X.; Zhang, Y.; Dong, X.; Huang, L.; Cao, L.; Zou, G. Harnessing the dual role of DMSO in the synthesis of SbOCl·DMSO: An excellent nonlinear optical crystal with unique 1D spiral chain. Inorg. Chem. Front. 2025, 12, 5054–5062. [Google Scholar] [CrossRef]
- Płotek, J.; Kulka, A.; Maximenko, A.; Kondracki, Ł.; Trabesinger, S.; Moździerz, M.; Molenda, J. Sb/Sb4O5Cl2/C composite as a stable anode for sodium-ion batteries. Energy Storage Mater. 2024, 72, 103780. [Google Scholar] [CrossRef]
- Jiang, Q.; Yuan, X.; Wang, H.; Chen, X.; Gu, S.; Liu, Y.; Zeng, G. A facile hydrothermal method to synthesize Sb2S3/Sb4O5Cl2 composites with three-dimensional spherical structures. RSC Adv. 2015, 5, 53019–53024. [Google Scholar] [CrossRef]
- Xie, J.; Pei, Y.; Liu, L.; Guo, S.; Xia, J.; Li, M.; Wang, X. Hydrothermal synthesis of antimony oxychloride submicron rods as anode materials for lithium-ion and sodium-ion batteries. Electrochim. Acta 2017, 254, 246–254. [Google Scholar] [CrossRef]
- Shen, K.; Lu, X.; Shen, S.; Xu, P.; Zeng, Y.; Li, L.; Wang, H. Effect of cobalt on lifetime of Sb4O5Cl2–graphene anode in chloride-ion batteries. ChemSusChem 2024, 17, e202301392. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Yu, W.; Zeng, J.; Shi, Y.; Lin, S.; Ji, Z. Photocatalytic Study of a Novel Crystal-Facets-Sensitive Heterojunction between Sb8O11Cl2 and Anatase TiO2 with Different Exposed Facets. Dyes Pigm. 2019, 160, 530–539. [Google Scholar] [CrossRef]
- Lin, C.Q.; Li, J.; Shi, J.; Wang, J.R.; Bo, R.L.; Gong, L.K.; Pan, C.-Y.; Lei, X.-W.; Gong, Z. Zero-dimensional antimony oxychloride scintillators with high photoluminescent efficiency. Inorg. Chem. 2025, 64, 18490–18498. [Google Scholar] [CrossRef]
- Rabbani, F.; Shaikh, A.J.; Khan, J.; Ajaz, H.; Rafique, M.; Khan, Z.U.H.; Shah, G.M. Removal of Organic Colorants Using Nano Copper Antimony Oxychloride Synthesized by Non-Solvated System. J. Inorg. Organomet. Polym. Mater. 2019, 29, 893–900. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, W.; Li, Z.; Xu, T.; Fei, H. Facile Synthesis of Phase-Pure Sb8O11Cl2 Microrods as Anode Materials for Sodium-Ion Batteries with High Capacity. Ionics 2017, 23, 3197–3202. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Liu, C.; Li, Y.; Gao, J. A Simple Preparation of antimony metal by carbothermal reduction of antimony oxide powder in a microwave field: Mechanism and process. J. Sust. Metall. 2024, 10, 603–624. [Google Scholar] [CrossRef]
- Weng, S.; Wang, X.; Zhou, H.; He, K.; Chen, C.; Lu, X. Nickel-Doped Sb4O5Cl2 Enables Bifunctional Electrochemical Systems for Efficient Energy Storage and Saline Water Treatment. Nanotechnology 2025, 36, 295703. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Zhou, D.; Wu, T.; Xiao, Z. A Flower-Like Sb4O5Cl2 Cluster-Based Material as Anode for Potassium-Ion Batteries. Appl. Surf. Sci. 2022, 583, 152509. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.; Wang, H.; Chen, X.; Gu, S.; Jiang, Q.; Zeng, G. Novel Visible-Light-Induced g-C3N4–Sb2S3/Sb4O5Cl2 Composite Photocatalysts for Efficient Degradation of Methyl Orange. Catal. Commun. 2015, 70, 17–20. [Google Scholar] [CrossRef]
- Makhloufi, R.; Hachani, S.E.; Fettah, A.; Tair, W.; Zekri, Z. Synthesis of Sb2S3–Sb4O5Cl2 Composite Used as a Photocatalyst for Crystal Violet Cationic Dye Degradation. Chem. Data Collect. 2022, 39, 100867. [Google Scholar] [CrossRef]
- Zeng, Y.; Liao, C.; Xu, Z.; Liu, F. The Mechanism of Selective Separation of Antimony from Chloride Leachate of Copper Anode Slime. Rev. Chim. 2022, 73, 99–113. [Google Scholar] [CrossRef]
- Mayerová, Z.; Johnsson, M.; Lidin, S. The Structure of Onoratoite, Sb8O11X2 (X = Cl, Br) Revisited. Solid State Sci. 2006, 8, 849–854. [Google Scholar] [CrossRef]
- Allaf, A.W.; Ajji, Z. The Gas-Phase On-Line Formation of Antimony Oxide Trihalides, SbOX3 (X = F, Cl), and Their Identification by Infrared Spectroscopy. Spectrochim. Acta Part A 2000, 56, 1971–1977. [Google Scholar] [CrossRef]
- Ullah, I.; Chang, S.; Hou, W.; Valle-Perez, A.D.; Du, X.; Katiyar, S.; Wu, X. Concentrated Chloride Electrolytes Enable High-Efficiency, Long-Cycling, and Dendrite-Free Aqueous Trivalent Antimony Batteries. Angew. Chem. Int. Ed. 2025, 64, e202502279. [Google Scholar] [CrossRef]
- Bogdanova, V.V.; Fedeev, S.S.; Lesnikovich, A.I.; Klimovtsova, I.A.; Sviridov, V.V. The Formation of Antimony Oxychloride in Flame-Retardant Mixtures and Its Influence on Flame-Retardant Efficiency. Polym. Degrad. Stab. 1985, 11, 205–210. [Google Scholar] [CrossRef]
- Buchholcz, B.; Haspel, H.; Boldizsár, T.; Kukovecz, Á.; Kónya, Z. pH-Regulated Antimony Oxychloride Nanoparticle Formation on Titanium Oxide Nanostructures: A Photocatalytically Active Heterojunction. CrystEngComm 2017, 19, 1408–1416. [Google Scholar] [CrossRef]
- Burgard, M.; Brunette, J.P.; Leroy, M.J. Vibrational Spectroscopic Study of Some Oxo Adducts of Antimony Pentachloride. Inorg. Chem. 1976, 15, 1225–1227. [Google Scholar] [CrossRef]
- Chen, X.Y.; Huh, H.S.; Lee, S.W. Hydrothermal Synthesis of Antimony Oxychloride and Oxide Nanocrystals: Sb4O5Cl2, Sb8O11Cl2, and Sb2O3. J. Solid State Chem. 2008, 181, 2127–2132. [Google Scholar] [CrossRef]
- Costa, L.; Goberti, P.; Paganetto, G.; Camino, G.; Sgarzi, P. Thermal Behaviour of Chlorine–Antimony Fire-Retardant Systems. Polym. Degrad. Stab. 1990, 30, 13–28. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, H.; Li, L.; Guo, L. Shape Evolution of Antimony Oxychloride from Sheaf-Like to Quasi-Wafer Structures. Chin. Sci. Bull. 2011, 56, 3817–3822. [Google Scholar] [CrossRef]
- Lakshmi, K.P.; Janas, K.J.; Shaijumon, M.M. Antimony Oxychloride Embedded Graphene Nanocomposite as Efficient Cathode Material for Chloride Ion Batteries. J. Power Sources 2019, 433, 126685. [Google Scholar] [CrossRef]
- Wlaźlak, E.; Blachecki, A.; Bisztyga-Szklarz, M.; Klejna, S.; Mazur, T.; Mech, K.; Szaciłowski, K. Heavy Pnictogen Chalcohalides: The Synthesis, Structure and Properties of These Rediscovered Semiconductors. Chem. Commun. 2018, 54, 12133–12162. [Google Scholar] [CrossRef]



| Property | Significance | Ref |
|---|---|---|
| Density | 6.697 g/cm3 (at 20 °C) | [18] |
| Melting point | 630.6 °C (903.8 K) | |
| Specific heat capacity | ≈ 0.207 J/(g·K) | |
| Gas thermal conductivity | ≈ 18 W/(m·K) | |
| Standard enthalpy of formation | 0 kJ/mol (elemental Sb, standard state) | |
| Melting heat | 19.79 kJ/mol |
| Country | Mine Production | Reserves | |
|---|---|---|---|
| 2023 | 2024 | ||
| China | 62,300 | 60,000 | 670,000 |
| Russia | 13,000 | 13,000 | 350,000 |
| Tajikistan | 17,000 | 17,000 | 50,000 |
| Australia | 1860 | 2000 | 140,000 |
| United States | - | - | 860,000 |
| Canada | - | - | 78,000 |
| Bolivia | 3700 | 3700 | 310,000 |
| Turkey | 1600 | 1600 | 99,000 |
| Vietnam | 300 | 300 | 54,000 |
| Mexico | 800 | 800 | 18,000 |
| 106,000 | 100,000 | >2,000,000 | |
| Compound | Space Group Name | Band Gap (eV) | Ref |
|---|---|---|---|
| SbOCl·DMSO | Pca21 | 3.74 | [45] |
| Sb4O5Cl2 | P21/a | Between 2.45 and 2.83 | [46,47] |
| Sb4O5Cl2 | P21/c | 2.583 | [48,49] |
| Sb8O11Cl2 | C2/m | 3.59 | [48,50] |
| Sb2Cl7O | P21/c | 3.38, 3.05 (DFT) | [51] |
| Sb3O4Cl | P2/c | 2.737 | [52] |
| Synthesis Conditions | Reagents | Crystal Structure/Space Group | Morphology | Material Type | Application | Features | Ref |
|---|---|---|---|---|---|---|---|
| Hydrothermal, 100 °C, 12 h | SbCl3, ethylenediamine, ethanol | Monoclinic, P21/c | 3D flower-like nanosheets | Pure Sb4O5Cl2 | Anode material for potassium-ion batteries (PIBs) | 3D flower-like morphology, high reversible capacity (≈530 mAh g−1 @ 50 mA g−1), stable structure after cycling | [57], 2022 |
| Hydrothermal, unspecified | SbCl3, thiourea | Monoclinic, P21/a | Mixed-phase composite (Sb2S3, Sb4O5Cl2) | Composite (Sb2S3 Sb4O5Cl2) | Photocatalyst for crystal violet dye degradation | Sb2S3, Sb4O5Cl2 composite with enhanced charge carrier separation | [59], 2022 |
| Hydrolysis/alcoholysis 25 °C, pH ≈ 2 | SbCl3+ H2O/EtOH/EG | SbOCl: P21/a Sb4O5Cl2: P21/c Sb3O4Cl: P2/c | Fine precipitates after hydrolysis | Mixed products SbOCl, Sb3O4Cl, Sb4O5Cl2 | Selective extraction of Sb from anode slime | Formation of stable SbOCl, Sb3O4Cl, Sb4O5Cl2 depending on pH, confirmed by DFT | [52], 2022 |
| Solid-state reaction, 120 °C, 5 days | CuO, CuCl2, SbCl3 | Crystalline Cu-Sb-O-Cl phase (nanocrystalline, 2–8 nm) | Rough and uneven surface; small spherical/cylindrical aggregates (5–50 µm) | Ternary oxide halide (Cu Sb O Cl) | Removal of organic dyes | Cu-Sb oxychloride nanomaterial, high stability | [60], 2019 |
| Sealed evacuated silica tubes, 500 °C, 96 h | Sb2O3 + SbCl3, (11:2 molar ratio) | Triclinic/P-1 | Rod-like colorless crystals | Sb8O11Cl2 | Mineral form (Onoratoite), | Tubular Sb–O structures with [SbO4E] and [SbO3E]; halide ions between tubes | [61], 2006 |
| Hydrothermal + variation | SbCl3, ethanol, ammonia, PVP, etc. | Monoclinic, P21/c | Nanorods (2 μm), Nanosheets (50–150 nm) | Nanostructured Sb4O5Cl2 | Photocatalysis, optics, dielectric materials | Sb4O5Cl2 nanostructures (nanorods, nanosheets), varied morphologies, and high dielectric constants | [11], 2019 |
| On-line gas-phase formation at 230–550 °C, under 0.2–0.5 torr | SbCl3, Ag2O, NaF | C3v symmetry (molecular) | Gas phase species (SbOX3) | SbOCl3 | Spectroscopy, IR characterization of molecules | SbOX3 (X=F, Cl), first-time IR band assignment for O-Sb bond | [62], 2000 |
| Electrochemical deposition in concentrated electrolyte, room temperature | SbCl3 + LiCl aqueous solution | XRD-confirmed formation of SbOCl | Uniform spherical deposits (500 nm), dendrite-free | SbOCl (condensed phase) | Aqueous trivalent Sb batteries | High Coulombic efficiency (99.7–99.8%), long lifespan (7300 h), reversible Sb↔SbOCl transformation, stable morphology | [63], 2025 |
| Gas–solid reaction, 523–773 K | Sb2O3 + HCl vapor; | Monoclinic, orthorhombic | Polycrystalline SbOCl (h) and SbOCl (l) | SbOCl (h), SbOCl | Flame retardants in polymers (PE, PP) | Phase transformation affects flame-retardant efficiency; | [64], 1985 |
| Solvothermal, 120 °C, 12 h | SbCl3 + H2O/ethylene glycol (1:1) + TiONT | Sb4O5Cl2 (monoclinic, Sb8O11Cl2 (orthorhombic) | 8–11 nm nanoparticles on titanate nanotubes | SbxOyClz/TiONT heterostructures | Photocatalytic degradation of methyl orange under UV/Vis | Band gap ≈3.05 eV; enhanced charge separation; activity strongly dependent on pH; stable up to 300 °C | [65], 2017 |
| Direct addition at 0 °C or in CH2Cl2 solution | SbCl5 + oxygen-donor ligands | Molecular adducts, local C4v symmetry around Sb | Crystalline solids; no defined morphology | SbCl5·L (L = O-donor ligand) | Vibrational spectroscopy studies | C4v-like symmetry, distinct Sb-O and Sb-Cl modes, donor strength affects stretching frequencies | [66], 1976 |
| Hydrothermal, 120 °C, 12 h, pH 1–2, solvent: EG–H2O or EtOH–H2O | SbCl3 | Monoclinic (Sb4O5Cl2) | Nanoparticles | Sb4O5Cl2 | Flame retardants, optical properties | Synthesized in acidic conditions, first report on nanostructured form | [67], 2008 |
| Hydrothermal, 120 °C, 12 h, solvent: EG–H2O | SbCl3 + NaOH | Monoclinic | Nanobelts and nanowires | Sb8O11Cl2 | Rare antimony oxychloride phase, nanowire | [67], 2008 | |
| Hydrothermal, 120 °C, 12 h, pH 8–9, solvent: EG–H2O or EtOH–H2O | SbCl3 + NaOH | Cubic (senarmontite) or orthorhombic (valentinite) | Nanocrystals | Sb2O3 | Catalysis, flame retardants, optical | Selective phase control via solvent and pH; strong photoluminescence | [67], 2008 |
| Heating mixture of Sb2O3 and organochlorine compounds | Sb2O3 + chlorinated organics | Main phase: Sb4O5Cl2 (monoclinic P21/c) | Fine solid products; XRD-identified Sb4O5Cl2 | Antimony oxychloride (mainly Sb4O5Cl2) | Flame-retardant additives for polymers | Sb4O5Cl2 is thermally stable at 400–600 °C; reacts to form volatile SbCl3; | [68], 1990 |
| Preparation Method | Influence on Morphology and Structure | Advantages | Limitations |
|---|---|---|---|
| Hydrothermal | Nanoplates and microrods of Sb4O5Cl2 are formed with a well-defined orthorhombic structure; crystallites are oriented along the (001) direction. | High crystallinity; single-phase products; controlled morphology (plates, rods); improved photocatalytic and electrochemical properties. | Requires long heating time (up to 24 h); partial formation of Sb2O3 impurities; pressure control is required. multistage washing and vacuum drying. |
| Sonochemical | Needle-like microcrystals of Sb8O11Cl2 (1–3 μm), weakly ordered. | Rapid process (≤1 h); no heating or pressure required; easily scalable. | Moderate phase purity; relatively large particles (microscale); difficult morphology control. |
| CVD | Dense thin films of SbxOyClz and mixed phases are formed; the structure is layered, with crystallites oriented along the (001) direction. | Enables deposition of uniform films with strong adhesion and good compositional reproducibility; allows doping and fabrication of multilayer structures | Requires volatile Sb precursors; high temperatures (350–500 °C) may induce phase transformation to Sb2O3; difficult to control the Cl/O ratio. |
| Gas-phase method | SbOCl(h) and Sb4O5Cl2 are formed with ordered layered structures; the structure depends on temperature. | The stability of SbOCl at high temperatures is confirmed, along with the formation of stable phases. | High processing temperatures lead to partial sublimation of SbCl3 and the formation of metallic Sb, resulting in an uneven product surface. |
| Wet–Chemical | Micron-sized Sb4O5Cl2 microplates are formed, assembling into “sand-rose” structures; the crystals are orthorhombic and anisotropic. | Simple and reproducible synthesis procedure; high photocatalytic activity (up to 94% degradation of methylene blue). | Large particle size (microscale); non-uniform thickness. |
| Sol–gel method | Porous xerogels of Sb4O5Cl2 and Sb8O11Cl2 are formed with nanoplate-like morphology; the porous structure is preserved during drying below 200 °C, while densification occurs at 400 °C. | Low synthesis temperature; possibility of obtaining nanoporous coatings and monolithic structures. | Long gelation stage (12–48 h); difficult to control Cl− content during prolonged drying; possible dehydration leading to Sb2O3 formation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shongalova, A.; Kemelbekova, A.; Umirzakov, A.; Tashmukhanbetova, I.; Dmitriyeva, E. Structure, Synthesis and Properties of Antimony Oxychlorides: A Brief Review. Processes 2025, 13, 3560. https://doi.org/10.3390/pr13113560
Shongalova A, Kemelbekova A, Umirzakov A, Tashmukhanbetova I, Dmitriyeva E. Structure, Synthesis and Properties of Antimony Oxychlorides: A Brief Review. Processes. 2025; 13(11):3560. https://doi.org/10.3390/pr13113560
Chicago/Turabian StyleShongalova, Aigul, Ainagul Kemelbekova, Arman Umirzakov, Indira Tashmukhanbetova, and Elena Dmitriyeva. 2025. "Structure, Synthesis and Properties of Antimony Oxychlorides: A Brief Review" Processes 13, no. 11: 3560. https://doi.org/10.3390/pr13113560
APA StyleShongalova, A., Kemelbekova, A., Umirzakov, A., Tashmukhanbetova, I., & Dmitriyeva, E. (2025). Structure, Synthesis and Properties of Antimony Oxychlorides: A Brief Review. Processes, 13(11), 3560. https://doi.org/10.3390/pr13113560






