Graphene-Stabilized δ-MnO2 Cathode for High-Capacity Aqueous Aluminum-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Cathode Materials
2.2. Battery Assembly
2.3. Electrochemical Measurements
2.4. Material Characterization
3. Results and Discussion
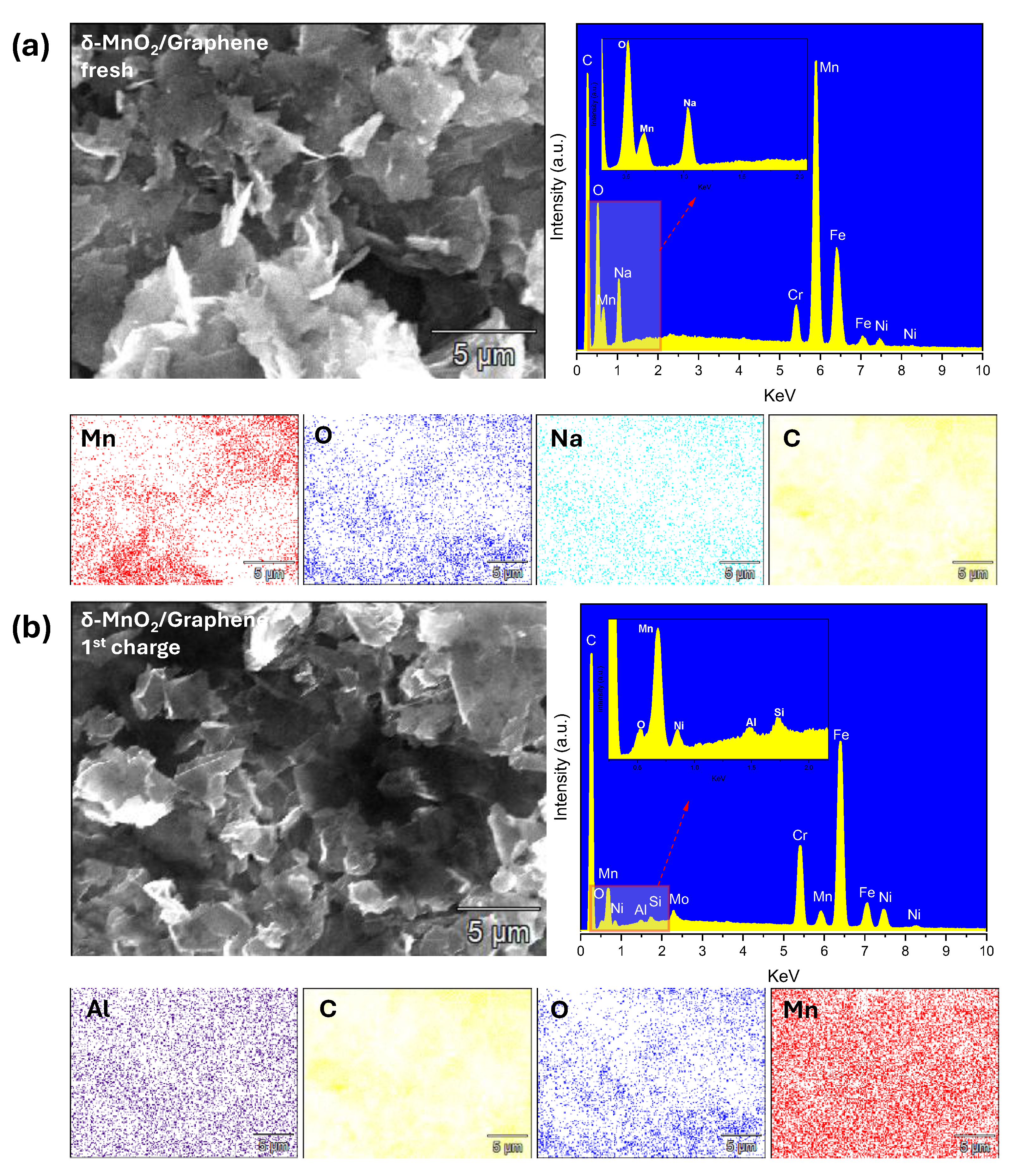
3.1. Synthesized Cathode Materials Characterization
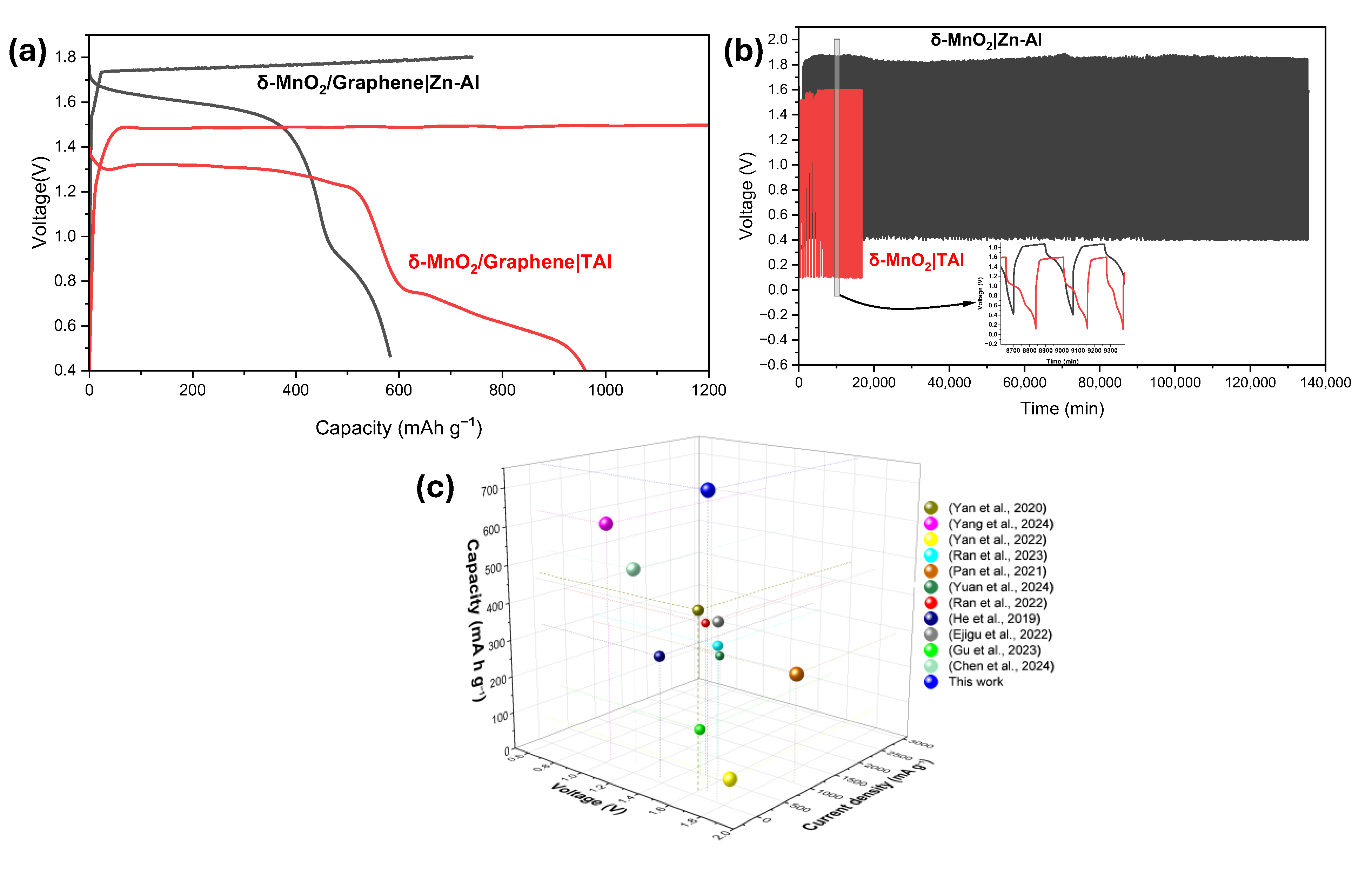
3.2. Study of the Battery System Utilizing TAl Anode
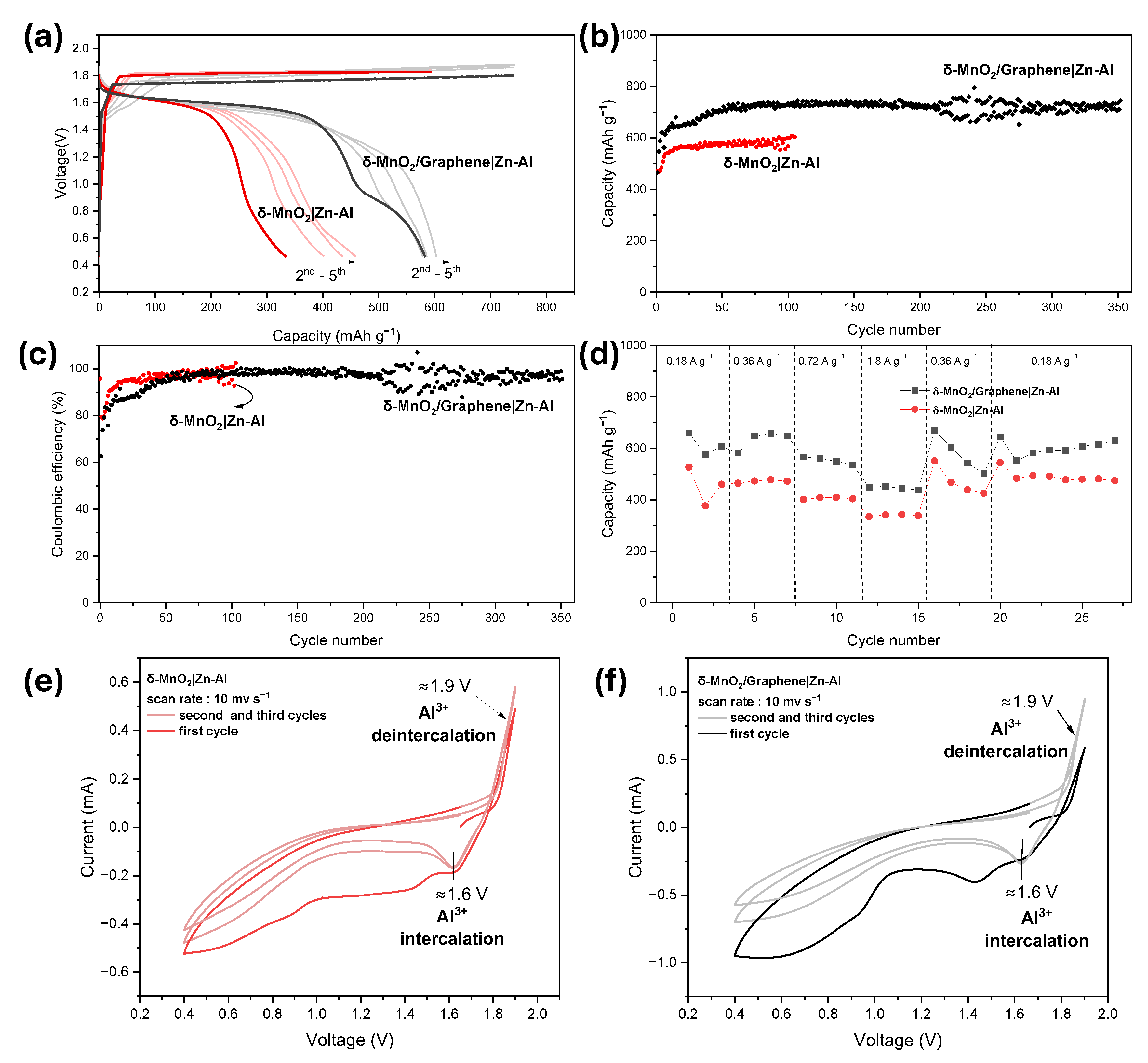
3.3. Study of the Battery System Utilizing Zn–Al Anode

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ejigu, A.; Le Fevre, L.W.; Elgendy, A.; Spencer, B.F.; Bawn, C.; Dryfe, R.A.W. Optimization of Electrolytes for High-Performance Aqueous Aluminum-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 25232–25245. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.L.; Lei, H.; Yu, Z.; Chen, L.L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Selvamani, T.; Tak, Y.; Lee, G. Multi-Ionic Capacity of Zn-Al/V6O13 Systems Enable Fast-Charging and Ultra-Stable Aqueous Aluminium-Ion Batteries. ChemElectroChem 2022, 9, e202200964. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Y.; Deng, T.; Zhu, J.; Sun, B.; Su, Y.; Ti, R.; Yang, J.; Wu, W.; Cheng, N.; et al. Recent progress in aqueous aluminum-ion batteries. Nanotechnology 2024, 35, 362004. [Google Scholar] [CrossRef]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Zhang, X.; Chen, J.; Wang, Z.; Yang, T.; Liu, Z.; Liang, Y.; Wang, B.; Liu, S.; et al. A High-Energy Aqueous Aluminum-Manganese Battery. Adv. Funct. Mater. 2019, 29, 1905228. [Google Scholar] [CrossRef]
- Lv, H.; Yang, S.; Li, C.; Han, C.; Tang, Y.; Li, X.; Wang, W.; Li, H.; Zhi, C. Suppressing passivation layer of Al anode in aqueous electrolytes by complexation of H2PO4− to Al3⁺ and an electrochromic Al ion battery. Energy Storage Mater. 2021, 36, 350–357. [Google Scholar] [CrossRef]
- Yan, C.; Lv, C.; Wang, L.; Cui, W.; Zhang, L.; Dinh, K.N.; Tan, H.; Wu, C.; Wu, T.; Ren, Y.; et al. Architecting a Stable High-Energy Aqueous Al-Ion Battery. J. Am. Chem. Soc. 2020, 142, 15295–15304. [Google Scholar] [CrossRef]
- Yan, C.; Lv, C.; Jia, B.E.; Zhong, L.; Cao, X.; Guo, X.; Liu, H.; Xu, W.; Liu, D.; Yang, L.; et al. Reversible Al Metal Anodes Enabled by Amorphization for Aqueous Aluminum Batteries. J. Am. Chem. Soc. 2022, 144, 11444–11455. [Google Scholar] [CrossRef]
- Ran, Q.; Zeng, S.P.; Zhu, M.H.; Wan, W.B.; Meng, H.; Shi, H.; Wen, Z.; Lang, X.Y.; Jiang, Q. Uniformly MXene-Grafted Eutectic Aluminum-Cerium Alloys as Flexible and Reversible Anode Materials for Rechargeable Aluminum-Ion Battery. Adv. Funct. Mater. 2023, 33, 2211271. [Google Scholar] [CrossRef]
- Chen, S.; Kong, Y.; Tang, C.; Gadelhak, N.A.; Nanjundan, A.K.; Du, A.; Yu, C.; Huang, X. Doping Regulation Stabilizing δ-MnO2 Cathode for High-Performance Aqueous Aluminium-ion Batteries. Small 2024, 20, 2312229. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Shi, H.; Meng, H.; Zeng, S.P.; Wan, W.B.; Zhang, W.; Wen, Z.; Lang, X.Y.; Jiang, Q. Aluminum-copper alloy anode materials for high-energy aqueous aluminum batteries. Nat. Commun. 2022, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Nacimiento, F.; Cabello, M.; Alcántara, R.; Lavela, P.; Tirado, J.L. Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries. RSC Adv. 2016, 6, 62157–62164. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Yin, J.; Zheng, J.; Zhang, D.; Chen, J.U.; Archer, L.A.; Zhao, Q.; Yin, J.; Frederick, L.A.A.R.; et al. Proton Intercalation/De-Intercalation Dynamics in Vanadium Oxides for Aqueous Aluminum Electrochemical Cells. Angew. Chem. Int. Ed. 2020, 59, 3048–3052. [Google Scholar] [CrossRef]
- Kumar, S.; Satish, R.; Verma, V.; Ren, H.; Kidkhunthod, P.; Manalastas, W.; Srinivasan, M. Investigating FeVO4 as a cathode material for aqueous aluminum-ion battery. J. Power Sources 2019, 426, 151–161. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, S.; Yu, X.; He, W.; Zhang, S.; Tian, Y.; Xing, M.; Fu, Y.; Luo, X. Realizing reversible storage of trivalent aluminum ions using VOPO4·2H2O nanosheets as cathode material in aqueous aluminum metal batteries. J. Alloys Compd. 2021, 885, 161008. [Google Scholar] [CrossRef]
- Nandi, S.; Das, S.K. An electrochemical study on bismuth oxide (Bi2O3) as an electrode material for rechargeable aqueous aluminum-ion battery. Solid. State Ion. 2020, 347, 115228. [Google Scholar] [CrossRef]
- Lahan, H.; Das, S.K. Reversible Al3+ ion insertion into tungsten trioxide (WO3) for aqueous aluminum-ion batteries. Dalton Trans. 2019, 48, 6337–6340. [Google Scholar] [CrossRef]
- Joseph, J.; Nerkar, J.; Tang, C.; Du, A.; O’Mullane, A.P.; Ostrikov, K.K. Reversible Intercalation of Multivalent Al3+ Ions into Potassium-Rich Cryptomelane Nanowires for Aqueous Rechargeable Al-Ion Batteries. ChemSusChem 2019, 12, 3753–3760. [Google Scholar] [CrossRef]
- Wu, C.; Gu, S.; Zhang, Q.; Bai, Y.; Li, M.; Yuan, Y.; Wang, H.; Liu, X.; Yuan, Y.; Zhu, N.; et al. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat. Commun. 2019, 10, 73. [Google Scholar] [CrossRef]
- Melzack, N.; Wills, R.G.A. A Review of Energy Storage Mechanisms in Aqueous Aluminium Technology. Front. Chem. Eng. 2022, 4, 778265. [Google Scholar] [CrossRef]
- Gu, H.; Yang, X.; Chen, S.; Zhang, W.; Yang, H.Y.; Li, Z. Oxygen Vacancies Boosted Proton Intercalation Kinetics for Aqueous Aluminum-Manganese Batteries. Nano Lett. 2023, 23, 11842–11849. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Mao, J.; Wang, Y.; Zhao, X.; Leong, K.W.; Luo, S.; Chen, Y.; Leung, D.Y.C. High-Performance MnO2/Al Battery with In Situ Electrochemically Reformed AlxMnO2 Nanosphere Cathode. Small Methods 2021, 5, 2100491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, J.; Lin, L.; Liu, Y.; Li, S.; Li, Q.; Liu, X.X.; Sun, X. A polydopamine coating enabling the stable cycling of MnO2 cathode materials in aqueous zinc batteries. Chem. Sci. 2024, 15, 3545–3551. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jia, Z.; Wu, W.; Shi, H.Y.; Lin, Z.; Li, C.; Liu, X.X.; Sun, X. The back-deposition of dissolved Mn2+ to MnO2 cathodes for stable cycling in aqueous zinc batteries. Chem. Commun. 2022, 58, 4845–4848. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Han, Y.; Yang, C.; Gao, J. Constructing accommodational space in MnO2 cathode for Mn2+ transport and electrodeposition for aqueous zinc-ion batteries. Ionics 2022, 28, 4295–4301. [Google Scholar] [CrossRef]
- Ren, Y.; Li, H.; Rao, Y.; Zhou, H.; Guo, S. Aqueous MnO2/Mn2+ electrochemistry in batteries: Progress, challenges, and perspectives. Energy Environ. Sci. 2024, 17, 425–441. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhang, W.; Shi, Z.; Feng, Y.; Liu, C.; Fu, H.; Yong, Z.; Li, Q. Hydrogen-bond chemistry inhibits Jahn–Teller distortion caused by Mn 3d orbitals for long-lifespan aqueous Zn//MnO2 batteries. J. Mater. Chem. A Mater. 2024, 12, 25491–25503. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, W.; Chen, N.; Chen, S.; Xu, W.; Cai, R.; Brown, C.L.; Yang, D.; Yao, X. Generating oxygen vacancies in MnO hexagonal sheets for ultralong life lithium storage with high capacity. ACS Nano 2019, 13, 2062–2071. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Q.; Chai, L.; Chen, S.; Zhang, W.; Yang, H.Y.; Li, Z. α-MnO2 Cathode with Oxygen Vacancies Accelerated Affinity Electrolyte for Dual-Ion Co-Encapsulated Aqueous Aluminum Ion Batteries. Small 2024, 20, 2400335. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Xiao, X.; Wu, S.; Zhong, G.; Xu, K.; Wei, Z.; Su, W.; Lu, X. γ-MnO2 nanorods/graphene composite as efficient cathode for advanced rechargeable aqueous zinc-ion battery. J. Energy Chem. 2020, 43, 182–187. [Google Scholar] [CrossRef]
- Li, J.; Kang, L.; Luo, K.; Huang, Y.; Zhong, S.; Yan, D. Encapsulating Cu2Se into 3D porous carbon as high-voltage electrode materials for aluminum-ion batteries. Ceram. Int. 2023, 49, 2613–2618. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, H.; Wang, X.; Xu, T.; Zhang, M.; Wang, Y.; Zhu, L.; Tong, S.; Zhou, X.; Li, J.; et al. Highly reversible and rapid charge transfer Zn-MnO2 battery by MnO2 nanosheet arrays anchored nanocellulose-based carbon aerogel. Adv. Compos. Hybrid. Mater. 2024, 7, 90. [Google Scholar] [CrossRef]
- Wang, X.; Yin, M.; Xue, H.; Su, Y.; Tian, S. Simple microwave synthesis and improved electrochemical performance of Nb-doped MnO2/reduced graphene oxide composite as anode material for lithium-ion batteries. Ionics 2018, 24, 2583–2590. [Google Scholar] [CrossRef]
- Saadi-motaallegh, S.; Javanbakht, M.; Omidvar, H.; Habibzadeh, S.; Gao, B. Nanostructure Design of MnO2/Partially Oxidized Graphene Nanocomposite Cathode via One-Step Pulse Potential Strategy: Toward Ultralong-Life Zinc-Ion Batteries. ACS Appl. Eng. Mater. 2023, 1, 2941–2953. [Google Scholar] [CrossRef]
- Rosaiah, P.; Divya, P.; Sambasivam, S.; Tighezza, A.M.; Kalaivani, V.; Muthukrishnaraj, A.; Ayyar, M.; Niyitanga, T.; Kim, H. Carbon based manganese oxide (MnO2, MnO2/MWCNT and MnO2/rGO) composite electrodes for high-stability Li-ion batteries. Carbon. Lett. 2024, 34, 215–225. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, H.; Song, T.; Wang, X.; Li, C.; Li, W. MnO2-graphene based composites for supercapacitors: Synthesis, performance and prospects. J. Alloys Compd. 2022, 914, 165343. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, M.; Qin, R.; Fang, J.; Ren, H.; Yi, H.; Liu, L.; Zhao, W.; Li, Y.; Yao, L.; et al. Oxygen-Deficient β-MnO2@Graphene Oxide Cathode for High-Rate and Long-Life Aqueous Zinc Ion Batteries. Nanomicro Lett. 2021, 13, 173. [Google Scholar] [CrossRef]
- Shi, J.; Yu, C.; Zou, Z.; Zheng, S.; Zhang, X.; Wang, B. Reasonable Designing of Free-standing MnO2/Graphene Composite Membrane for Lithium-ion Storage. Chem. Res. Chin. Univ. 2024, 40, 508–512. [Google Scholar] [CrossRef]
- Liu, X.; Liang, B.; Hong, X.; Long, J. Electrochemical Performance of MnO2/Graphene Flower-like Microspheres Prepared by Thermally-Exfoliated Graphite. Front. Chem. 2022, 10, 870541. [Google Scholar] [CrossRef]
- Zhong, X.; Yang, C.; Zhao, Y.; Qiu, J.; Zang, L. Rational Design of Layered MnO2@Graphene with Hierarchical Structure for Flexible Quasisolid-State Aqueous Zinc-Ion Battery via Laser Activation. Adv. Mater. Technol. 2023, 8, 2201430. [Google Scholar] [CrossRef]
- Abdi, A.; Sarraf-Mamoory, R.; Stich, M.; Baumer, C.; Ullmann, F.; Krischok, S.; Bund, A. Optimization of in situ hydrothermal synthesis of birnessite MnO2/graphene composite: Thermodynamic insights and enhanced electrochemical performance for Li-ion battery anodes. J. Mater. Sci. Mater. Electron. 2025, 36, 1750. [Google Scholar] [CrossRef]
- Liu, Z.H.; Ooi, K.; Kanoh, H.; Tang, W.P.; Tomida, T. Swelling and delamination behaviors of birnessite-type manganese oxide by intercalation of tetraalkylammonium ions. Langmuir 2000, 16, 4154–4164. [Google Scholar] [CrossRef]
- Qiu, S.; Li, R.; Huang, Z.; Huang, Z.; Tsui, C.P.; He, C.; Han, X.; Yang, Y. Scalable sonochemical synthesis of petal-like MnO2/graphene hierarchical composites for high-performance supercapacitors. Compos. B Eng. 2019, 161, 37–43. [Google Scholar] [CrossRef]
- Zahoor, A.; Faizan, R.; Elsaid, K.; Hashmi, S.; Butt, F.A.; Ghouri, Z.K. Synthesis and experimental investigation of δ-MnO2/N-rGO nanocomposite for Li-O2 batteries applications. Chem. Eng. J. Adv. 2021, 7, 100115. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Tavares, A.C. Innovative approach for the synthesis of graphene/MnO2 nanocomposites and their electrochemical behavior. Electrochem. Sci. Adv. 2022, 2, 2100029. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, H.; Du, S.; Wang, Y. A facile hydrothermal synthesis of MnO2 nanorod–reduced graphene oxide nanocomposites possessing excellent microwave absorption properties. RSC Adv. 2015, 5, 88979–88988. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Nie, F.; Zhang, R.; Fang, X.; Wang, Y. The role of MnO2 crystal morphological scale and crystal structure in selective catalytic degradation of azo dye. Environ. Sci. Pollut. Res. 2023, 30, 15377–15391. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Yang, Z.; Zhou, X.; Qiu, D.; Qiu, H.; Huang, X.; Yu, Y. Unraveling the Role of Nitrogen-Doped Carbon Nanowires Incorporated with MnO2 Nanosheets as High Performance Cathode for Zinc-Ion Batteries. Energy Environ. Mater. 2023, 6, e12378. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Huang, Z.H.; Lei, L.; Duan, X.; Li, H.; Ma, T. Shielding Mn3+ Disproportionation with Graphitic Carbon-Interlayered Manganese Oxide Cathodes for Enhanced Aqueous Energy Storage System. Small 2024, 20, 2401849. [Google Scholar] [CrossRef]
- Huang, L.; Luo, X.; Chen, C.; Jiang, Q. A high specific capacity aqueous zinc-manganese battery with a ε-MnO2 cathode. Ionics 2021, 27, 3933–3941. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Liang, S.; Shan, L.; Xie, X.; Yi, Z.; Wang, Y.; Guo, S.; Zhou, J. Oxygen Defects in β-MnO2 Enabling High-Performance Rechargeable Aqueous Zinc/Manganese Dioxide Battery. IScience 2020, 23, 100797. [Google Scholar] [CrossRef]
- Zhong, W.; Zhao, R.; Zhu, Y.; Xu, Y.; Chen, W.; Peng, C. Vacancy Engineering on MnSe Cathode Enables High-Rate and Stable Zinc-Ion Storage. Adv. Funct. Mater. 2024, 35, 2419720. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Camargo, P.H.C.; Wang, J. Investigating the effect of MnO2 band gap in hybrid MnO2-Au materials over the SPR-mediated activities under visible light. J. Mater. Chem. A 2019, 7, 925–931. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Deng, S.; Tie, Z.; Yue, F.; Cao, H.; Yao, M.; Niu, Z. Rational Design of ZnMn2O4 Quantum Dots in a Carbon Framework for Durable Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202115877. [Google Scholar] [CrossRef] [PubMed]
- Awang, H.; Talalah, N.I. Synthesis of Reduced Graphene Oxide-Titanium (rGO-TiO2) Composite Using a Solvothermal and Hydrothermal Methods and Characterized via XRD and UV-Vis. Nat. Resour. 2019, 10, 17–28. [Google Scholar] [CrossRef]
- Susee, S.K.; Sandhya, S.; Kumar, M.S.; Chidhambararajan, B. Reduced graphene oxide–MnO2 nanocomposites by hydrothermal method for histamine sensor and photocatalytic activity. J. Mater. Sci. Mater. Electron. 2024, 35, 1948. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W.D. Heterostructural Graphene Quantum Dot/MnO2 Nanosheets toward High-Potential Window Electrodes for High-Performance Supercapacitors. Adv. Sci. 2018, 5, 1700887. [Google Scholar] [CrossRef]
- Zhang, Y.; Bian, Y.; Lv, Z.; Han, Y.; Lin, M.C. Aqueous Aluminum Cells: Mechanisms of Aluminum Anode Reactions and Role of the Artificial Solid Electrolyte Interphase. ACS Appl. Mater. Interfaces 2021, 13, 37091–37101. [Google Scholar] [CrossRef]
- Zeng, K.; Zhu, J. Surface morphology, elastic modulus and hardness of thin film cathodes for Li-ion rechargeable batteries. Mech. Mater. 2015, 91, 323–332. [Google Scholar] [CrossRef]
- Hamade, F.; Radich, E.; Davis, V.A. Microstructure and electrochemical properties of high performance graphene/manganese oxide hybrid electrodes. RSC Adv. 2021, 11, 31608–31620. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Nie, Y.; Wu, J.; Yu, L.; Liu, L.; Xi, J. An aqueous alkaline zinc–sulfur flow battery. Chem. Commun. 2024, 60, 2946–2949. [Google Scholar] [CrossRef] [PubMed]
- Ette, P.M.; Bosubabu, D.; Ramesha, K. Graphene anchored mesoporous MnO2 nanostructures as stable and high-performance anode materials for Li-ion batteries. Electrochim. Acta 2022, 414, 140164. [Google Scholar] [CrossRef]
- Wang, X.; Xi, Z.; Zhao, Q. Progress on aqueous rechargeable aluminium metal batteries. Ind. Chem. Mater. 2025, 3, 7–30. [Google Scholar] [CrossRef]
- Zheng, X.; Han, C.; Lee, C.S.; Yao, W.; Zhi, C.; Tang, Y. Materials challenges for aluminum ion based aqueous energy storage devices: Progress and prospects. Prog. Mater. Sci. 2024, 143, 101253. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Shi, Y.; Wang, C.; Qiu, H.; Ji, Y.; Cao, M.; Zhang, D. Recent progress in aluminum anodes for high-performance rechargeable aqueous Al-ion batteries. Inorg. Chem. Front. 2024, 11, 2246–2259. [Google Scholar] [CrossRef]
- Meng, H.; Ran, Q.; Zhu, M.H.; Zhao, Q.Z.; Han, G.F.; Wang, T.H.; Wen, Z.; Lang, X.Y.; Jiang, Q. Benzoquinone-Lubricated Intercalation in Manganese Oxide for High-Capacity and High-Rate Aqueous Aluminum-Ion Battery. Small 2024, 20, 2310722. [Google Scholar] [CrossRef]
- Siamionau, U.V.; Aniskevich, Y.M.; Ragoisha, G.A.; Streltsov, E.A. MnO2 electrodeposition at the positive electrode of zinc-ion aqueous battery containing Zn2+ and Mn2+ cations. J. Solid. State Electrochem. 2023, 27, 1911–1918. [Google Scholar] [CrossRef]
- Hu, E.; Jia, B.E.; Zhu, Q.; Xu, J.; Loh, X.J.; Chen, J.; Pan, H.; Yan, Q. Engineering High Voltage Aqueous Aluminum-Ion Batteries. Small 2024, 2309252. [Google Scholar] [CrossRef]
- Yang, X.; Gu, H.; Chai, L.; Chen, S.; Zhang, W.; Yang, H.Y.; Li, Z. Construction of V2O5@MXene Cathodes toward a High Specific Capacity Aqueous Aluminum-Ion Battery. Nano Lett. 2024, 24, 8542–8549. [Google Scholar] [CrossRef]
- Yuan, X.; Yuan, X.; Zhang, S.; Chen, P.; Wu, X.; Ye, J.; Liu, L.; Fu, L.; Wang, T.; Ozoemena, K.I.; et al. An Aqueous Rechargeable Al-Ion Battery Based on Cobalt Hexacyanoferrate and Al Metal. Adv. Energy Mater. 2024, 14, 2302712. [Google Scholar] [CrossRef]
- Mohammadi, E.; Taheri-Nassaj, E.; Shahalizade, T.; Shahroudi, H. Synthesis and electrochemical performance of SnSe/PANI composite as a long-cycle-life lithium-ion battery anode. J. Electroanal. Chem. 2025, 979, 118930. [Google Scholar] [CrossRef]
- Jin, D.; Dong, X.; Xin, S.; Yang, L.; Liu, J.; Pang, Q. Effect of MnSO4 concentration on the electrochemical performance of β-MnO2/3D graphene-carbon nanotube hybrids cathode for aqueous zinc-ion batteries. Ionics 2024, 30, 3329–3338. [Google Scholar] [CrossRef]






| Cathode | R1 (Ω) | R2 (Ω) | R3 (Ω) | Q1 (μF.s1−n) | Q2 (μF.s1−n) |
|---|---|---|---|---|---|
| δ-MnO2 | 2.60 ± 0.05 | 36.9 ± 2.6 | 1333 ± 30 | 150 ± 5 | 14.5 ± 3.1 |
| δ-MnO2/Graphene | 1.78 ± 0.04 | 5.90 ± 0.48 | 1110 ± 20 | 530 ± 1 | 280 ± 4 |
| No. | Cathode | Anode | Electrolyte | Voltage (V) | Capacity (mAh g−1), Cycle Number | Ref. |
|---|---|---|---|---|---|---|
| 1 | AlxMnO2 | Zn-Al | 2 M Al(OTF)3 | 1.6 | 460, 80 | [8] |
| 2 | V2O5@MXene | TAl | 5 M Al(OTF)3 | 1 | 626, 100 | [72] |
| 3 | MnO2 | Zn-Al | 3 M Al[TFSI]3 | 1.75 | 450, 400 | [1] |
| 4 | AlxMnO2 | a-Al | 0.5 M Al2(SO4)3 | 1.8 | <60, 200 | [9] |
| 5 | AlxMn(1−x)O2 | TAl | 2 M Al(OTF)3, 0.5 M MnSO4 | 1.35 | 320, 65 | [6] |
| 6 | AlxMnO2/C | MXene/ EAl97Ce3 | 2 M Al(OTF)3,0.2 M Mn(OTF)2 | 1.4 | 306, 500 | [10] |
| 7 | MnAlxO2 | Al | 16.57 M AlCl3, 3.86 M MnSO4 | 1.9 | 285, 500 | [23] |
| 8 | V6O13 | Al | 3 M Al(OTF)3 | 0.7 | 100, 1400 | [73] |
| 9 | AlxMnO2 | EAl82Cu18 | 2 M Al(OTF)3 | 1.5 | 400, 400 | [12] |
| 10 | V-δ-MnO2 | Al alloy | 2 M Al(OTF)3 | 1.14 | 518, 400 | [11] |
| 11 | Ov-MnO2 | TAl | 5 M Al(OTF)3 | 1.5 | 128, 200 | [22] |
| 12 | AlxMn(1−x)O2/Graphene | Zn-Al | 0.5 M Al2(SO4)3, 0.4 M MnSO4 | 1.63 | 746, 352 | This work |
| Cathode | R1 (Ω) | R2 (Ω) | R3 (Ω) | Q1 (μF.s1−n) | Q2 (μF.s1−n) |
|---|---|---|---|---|---|
| δ-MnO2 After 3rd cycle | 4.12 ± 0.32 | 7.1 ± 0.4 | 3891 ± 100 | 87.7 ± 5.1 | 42.1 ± 3.4 |
| δ-MnO2/Graphene After 3rd cycle | 3.9 ± 0.21 | 4.4 ± 0.1 | 3055 ± 63 | 3.1 ± 0.4 | 69.5 ± 1.2 |
| δ-MnO2 After 100th cycle | 7.40 ± 0.05 | 47.2 ± 0.6 | 276 ± 3 | 26.7 ± 1.7 | 28.6 ± 0.4 |
| δ-MnO2/Graphene After 100th cycle | 6.70 ± 0.04 | 12.1 ± 0.1 | 136 ± 1 | 7.0 ± 0.1 | 141 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, A.; Sarraf-Mamoory, R.; Stich, M.; Baumer, C.; Kurniawan, M.; Bund, A. Graphene-Stabilized δ-MnO2 Cathode for High-Capacity Aqueous Aluminum-Ion Batteries. Processes 2025, 13, 3551. https://doi.org/10.3390/pr13113551
Abdi A, Sarraf-Mamoory R, Stich M, Baumer C, Kurniawan M, Bund A. Graphene-Stabilized δ-MnO2 Cathode for High-Capacity Aqueous Aluminum-Ion Batteries. Processes. 2025; 13(11):3551. https://doi.org/10.3390/pr13113551
Chicago/Turabian StyleAbdi, Azadeh, Rasoul Sarraf-Mamoory, Michael Stich, Christoph Baumer, Mario Kurniawan, and Andreas Bund. 2025. "Graphene-Stabilized δ-MnO2 Cathode for High-Capacity Aqueous Aluminum-Ion Batteries" Processes 13, no. 11: 3551. https://doi.org/10.3390/pr13113551
APA StyleAbdi, A., Sarraf-Mamoory, R., Stich, M., Baumer, C., Kurniawan, M., & Bund, A. (2025). Graphene-Stabilized δ-MnO2 Cathode for High-Capacity Aqueous Aluminum-Ion Batteries. Processes, 13(11), 3551. https://doi.org/10.3390/pr13113551






