Abstract
Non-road mobile machinery (NRMM) emits a significant amount of NOx and particulate matter (PM), yet there is still a lack of economically feasible purification technologies up to now. In response to this issue, and taking into account the relatively flexible installation conditions for exhaust purification systems in NRMM, this study proposes a novel technical approach for the simultaneous removal of NOx and PM based on gas-phase O3 oxidation combined with Venturi scrubbing. The effects of O3 oxidation on the PM physicochemical properties, O3 concentration, liquid-to-gas (L/G) ratio, and surfactant addition in the scrubbing liquid on the PM removal and simultaneous removal of PM and NOx were investigated. The results showed that O3 oxidation significantly promoted PM removal in the absence of NO, which was attributed to the increase in the hydrophilicity of PM resulting from O3 oxidation. However, O3 preferentially reacted with NO, thereby reducing the removal efficiency of PM under the conditions when PM and NO coexisted. Adding surfactants to the scrubbing liquid improved PM removal and increasing the liquid-to-gas ratio improved the removal of both PM and NOx. When both the O3/NO molar ratio and liquid-to-gas ratio were 1.5, the removal efficiencies of NOx and PM reached 87% and 92%, respectively, and O3 escape was also effectively controlled. These findings demonstrated that the combination of gas-phase O3 oxidation and Venturi scrubbing is a promising purification technology for NRMM exhausts.
1. Introduction
NRMM typically uses diesel and heavy oil as fuel and emits various air pollutants, especially NOx and particulate matter (PM). According to the “2024 China Mobile Source Environmental Management Annual Report” released by the Ministry of Ecology and Environment, China [1], NRMM emitted 4.532 million tons of NOx and 224,000 tons of PM in 2023. NOx not only poses a direct health threat but also acts as one of the precursors of atmospheric haze and ozone (O3) pollution. Although PM emissions from NRMM are not large relative to national total emissions, these emissions occur near the breathing zone and mainly consist of nanoscale soot, posing significant health and environmental risks. With the strengthening of atmospheric pollution control requirements, controlling NOx and PM emissions from NRMM has become one of the key tasks in China’s air pollution control efforts [2].
Although significant progress in controlling pollutant formation has been achieved through fuel optimization and in-cylinder combustion technology, these measures still remain insufficient to meet stringent emission standards. Therefore, exhaust post-treatment has become a rigid requirement. Diesel Oxidation Catalyst (DOC) + Diesel Particulate Filter (DPF) + Selective Catalytic Reduction (SCR) + Ammonia Slip Catalyst (ASC) has been widely applied as a mature exhaust gas purification technology for road diesel vehicles [3,4]. However, the fuel quality and engine technology of NRMM are generally inferior to those of road diesel vehicles. Moreover, NRMM operation modes change more frequently and significantly, with especially more extended periods of operation at low temperatures, which results in a wider fluctuation in NOx emissions and higher PM concentrations.
To adapt the NH3-SCR de-NOx technology to the wide exhaust temperature range of NRMM, extensive studies have been conducted on optimizing catalyst formulations [5,6,7,8,9]. However, tests have shown that the performance of NH3-SCR de-NOx systems for NRMM is still unsatisfactory, especially during cold starts and idle periods [10,11,12]. Although the filtration efficiency of DPF for PM from road diesel vehicle exhaust is generally above 90% [13], the frequent combustion regeneration of DPF leads to the decrease in both fuel economy and PM filtration efficiency during regeneration periods when using DPF to filter the high-concentration PM from NRMM exhausts [14,15,16]. For this reason, many studies have been conducted on particle catalytic oxidation (POC) for PM purification; the results show that the efficiency of POC in PM purification is generally less than 70% due to the limited solid–solid contact conditions between PM and catalyst [17,18,19,20]. Additionally, studies using conventional water scrubbing technology to purify PM from diesel engine exhaust have shown that the purification efficiency is only around 40% [21,22,23]. Recently, Kawakami et al. conducted an exploratory study on the removal of NOx and PM from diesel engine exhaust using non-thermal plasma. Their results showed that the removal efficiencies for PM and NOx could reach up to 98% and 70%, respectively, with NOx being converted into HNO3. However, this process required a high energy input of 2400 J/L [24].
Considering the relatively flexible installation conditions for an NRMM exhaust purification system, this study puts forward a technical approach based on the combination of gas-phase O3 oxidation and Venturi scrubbing for purifying both NOx and PM. The reliability of the efficient purification of NOx from industrial flue gas by the combination of O3 oxidation and absorption has been verified through both experimental research and engineering practice. As long as the molar ratio of O3/NO is larger than 1.5, NOx can be oxidized into higher-valence nitric oxides such as N2O5 and NO3, which have good hydrophilicity and can be effectively removed by absorption [25,26,27,28,29,30,31]. Besides O3 oxidation, the synergistic oxidation of vacuum ultraviolet (VUV)/Oxone/hydrogen peroxide (H2O2) coupled with absorption has also been studied and achieved a relatively good denitrification efficiency. However, overall, its comprehensive performance is inferior to that of ozone oxidation [32]. Because NOx concentration level and other characteristics of NRMM exhaust are similar to those of the industrial flue gas, it can be deduced that this technology is also feasible for the purification of NOx from NRMM exhaust. On the other hand, some studies have been conducted on the effect of O3 oxidation on the physicochemical and toxicological properties of soot (or black carbon). The results show that the physical properties such as the particle size and soot morphology will change to varying extents after O3 treatment [33,34]. Theoretically, O3 oxidation can be utilized to modify the PM of NRMM exhaust, especially to enhance the hydrophilicity and thus promote the growth of PM particle size during Venturi scrubbing, thereby improving the separation effect.
However, the removal of both NOx and PM based on the combination of gas-phase O3 oxidation and Venturi scrubbing remains unexplored up to now. The stringent regulations demanding deep reductions in NRMM emissions of NOx and PM pose a significant challenge, which makes the existing research gap particularly pressing. This study is designed precisely to address this shortcoming by examining the impact of O3 oxidation on the physicochemical properties of PM and the efficacy of oxidation-coordinated scrubbing in achieving simultaneous NOx and PM removal.
2. Experimental Methodologies
2.1. Experimental System
A schematic diagram of the experimental setup is shown in Figure 1. The system comprises four units: the simulated NRMM exhaust gas unit, the O3 generation and oxidation unit, the Venturi scrubbing and gas–liquid separation unit, and the detection unit. NRMM exhaust was simulated by mixing air with NO from a gas cylinder and flue gas containing a high concentration of black smoke (PM), produced from methane combustion under oxygen-deficient conditions. The ozone oxidation combined with a Venturi scrubbing system was positioned downstream of the Diesel Oxidation Catalyst (DOC). Therefore, it was not necessary to consider HC and CO in the simulated gas. Furthermore, it has been extensively reported that SO2 is not preferentially oxidized by ozone over NOx and PM. Additionally, since diesel engines typically operate under excess air conditions, the use of air containing NOx and PM to simulate diesel exhaust is acceptable. The O3 generator produced O3 by high-voltage discharge in the O2 atmosphere. The oxidation reaction tank is a stainless-steel cylinder with a diameter of 50 mm and a length of 80 mm. The Venturi scrubber, with a total length of 117 mm, a throat diameter of 3 mm, and a throat length of 10 mm, and a cyclonic liquid–gas separator were serially connected and fixed on an iron stand.
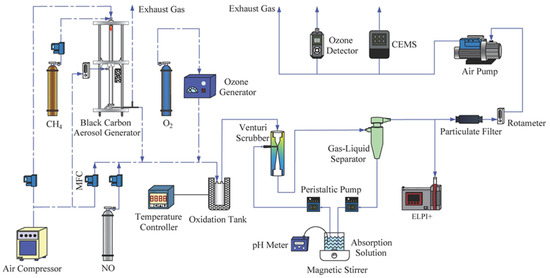
Figure 1.
Schematic diagram of the experimental setup.
2.2. Experimental Methods
O3 and the simulated exhaust gas were thoroughly mixed and then reacted in the oxidation tank. The gas after oxidation entered the Venturi scrubber, where the scrubbing liquid was atomized and accelerated by a high-speed gas flow at the throat. Theoretically, higher-valence NOx such as N2O5 and NO3 are effectively absorbed by aqueous solution. Simultaneously, moisture condenses and adheres to the surface of the oxidized PM, which makes PM separated easier in the liquid–gas separator. A peristaltic pump was employed to circulate the scrubbing liquid. The purified exhaust gas entered the detection unit, where the O3 concentration was analyzed using an ozone detector (Model 106-M, 2B Technologies, Inc., Broomfield, CO, USA), the NO and NO2 concentrations were analyzed by a UV flue gas analyzer (MODEL 3080UV, Beijing SDL Technology Co., Ltd., Beijing, China), and the PM number concentration and number size distribution were detected by an ELPI+TMparticle analyzer (Dekati, Kangasala, Finland). To ensure measurement accuracy, some necessary calibration of the relevant instruments was performed before the experiments commenced. All subsequent detection and measurement processes were carried out in strict compliance with the standardized operating procedures. Unless otherwise specified, the investigation was conducted under the conditions of an oxidation time of 0.3 s, a reaction temperature of 25 °C, an O3 concentration of 300 ppm, a NO concentration of 200 ppm, a PM concentration of 3.65 (±0.25) × 105/cm3, a liquid–gas ratio of 1.0 L/m3, and a total gas flow rate of 30 L/min.
NOx removal efficiency is as follows:
is the NOx removal efficiency, %; and are the NO concentrations before and after the oxidation–scrubbing–separation, ppm; and is the NO2 concentration after the oxidation–scrubbing–separation, ppm.
PM removal efficiency is as follows:
is the PM removal efficiency, %; NPM,in and NPM,out are the PM number concentrations before and after the oxidation–scrubbing–separation, particles/cm3, respectively.
In order to investigate the effects of O3 oxidation on the physicochemical properties of PM, PM produced from methane combustion under oxygen-deficient conditions was collected and dried at 70 °C for 6 h. Then, PM oxidation was conducted under the conditions of O3 concentration of 450 ppm. Subsequently, the number concentration and number size distribution, functional groups, electronegativity, and water contact angle of PM before and after oxidation were analyzed utilizing an ELPI+TM particle analyzer (Dekati, Finlant), a FT-IR spectrometer (IR Tracer 100, Shimadzu, Kyoto, Japan), a Zeta potential meter (Zetasizer Nano, Malvern Panalytical, UK), and a contact angle meter (LSA100, Lauda, Germany).
3. Results and Discussion
3.1. Effects of O3 Oxidation on Physicochemical Properties of PM
Figure 2 shows the number size distribution of PM before and after O3 oxidation for 30 min. It can be seen that the O3 oxidation resulted in a decrease in PM percentage with a large diameter (≥0.05 µm) and an increase in PM percentage with a small diameter (≤0.04 µm), which is similar to Liu’s research results [33]. This is because PM is not composed of dense, spherical particles. Instead, it consists of aggregates formed from thousands of nano-sized primary soot particles (approximately 20–50 nm in diameter) bound together by van der Waals forces and covalent bonds, creating complex, fluffy chain-like or cluster-like structures. The aerodynamic diameter of these aggregates can range up to several hundred nanometers or even micrometers. Ozone attacks the bridging sites that connect these primary particles. These junctions are typically defects or active sites within the carbon network, which are more vulnerable to oxidation than the crystalline structure itself. When these critical bridging points are oxidized and broken, the large aggregates disintegrate and fragment into several smaller aggregate fragments, or even individual primary particles. Consequently, the particle size distribution shifts, showing a decrease in the concentration of particles in the larger size ranges and a significant increase in the number of smaller particles.
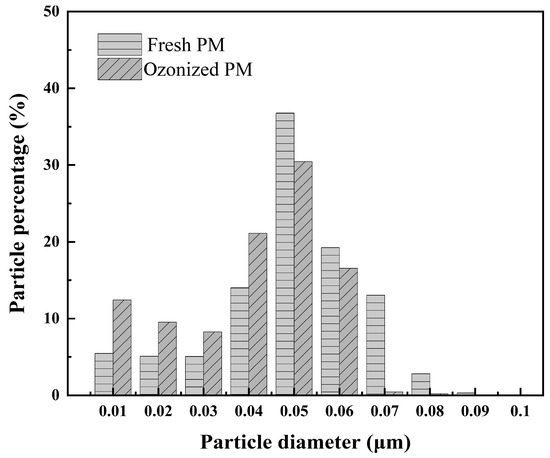
Figure 2.
Number size distribution of fresh PM and PM oxidized for 30 min.
The surface charge characterization indicated that after PM was oxidized for 30 min, the Zeta potential increased from −8.1 mV to −14.7 mV, which suggested that following O3 oxidation, the acidic oxygen-containing functional groups formed on the PM surface and underwent weak acidic dissociation in suspension solution, thereby augmenting the negative charge density of the suspension solution.
Furthermore, the PM undergoing oxidation for 30 min was characterized by FTIR. As shown in Figure 3, the FTIR spectra of fresh and oxidized PM exhibited identical peak positions. However, the disparities existed in peak intensities. After oxidation, the absorption peaks corresponding to C=C stretching vibrations in aromatic rings or condensed rings at 1578 cm−1, alkenes and alkynes at 890–1092 cm−1, trisubstituted C-H aryl at 755 cm−1, and tetrasubstituted C-H aryl at 843 cm−1 weakened, whereas the absorption peak corresponding to C=O stretching vibrations in aldehydes, ketones, and carboxylic acids at 1629–1704 cm−1 intensified. It was speculated that after O3 oxidation, the condensation degree of the aromatic structure diminished. The aromatic rings and/or side chains underwent restructuring during oxidation, with a remarkable increase in highly active oxygen-containing functional groups. Unsaturated bonds were oxidized to aldehydes, ketones, and carboxylic acids. These outcomes indicated that ozone oxidation reduced the aromatic structure on the PM surface and significantly augmented the surface oxygen-containing functional groups, which corroborated the results of Li et al. [34].
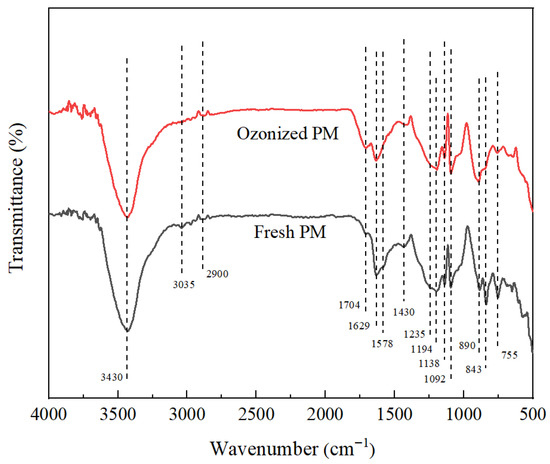
Figure 3.
FTIR spectra of fresh PM and PM oxidized for 30 min.
The water contact angle (WCA) of PM before and after O3 oxidation at different times was detected. As shown in Figure 4, the WCA of fresh PM was 132°, manifesting its strong hydrophobicity. After O3 oxidation for 1 min, 5 min, and 15 min, the WCA decreased to 121°, 116°, and 64°, respectively, signifying that the PM transformed from hydrophobic to hydrophilic, which is consistent with the results reported by Liu et al. [33].
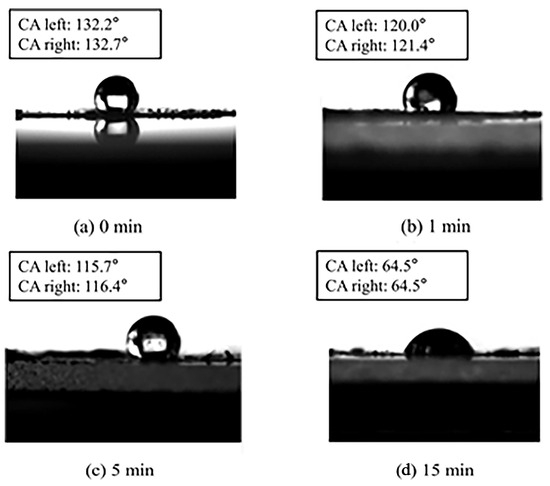
Figure 4.
Water contact angle of PM oxidized at different times.
3.2. Removal of PM by the Combination of Ozone Oxidation and Scrubbing
3.2.1. The Influence of O3 Concentration on PM Removal
Figure 5 shows the effect of O3 concentration on PM removal by the combination of O3 oxidation and scrubbing using water as the scrubbing liquid. It can be seen from Figure 5a that the total removal efficiency of PM gradually rose with the increase of O3 concentration. When the O3 concentration increased from 0 to 450 ppm, the PM removal efficiency increased from 54% to 80%. Figure 5b shows that the removal efficiency of PM in each particle size segment also increased with the increase of O3 concentration and the removal efficiency of PM with a small size was higher than that of PM with a large size. The maximum and minimum PM removal efficiencies were obtained for 0.02 μm and 0.32 μm, respectively. The former was attributed to the most substantial diffusion effect, the latter was due to the relatively weak inertial collision, interception, and diffusion effects. On the other hand, the smaller the particle size, the higher the surface energy, and thus the stronger the hygroscopicity. Therefore, it was deduced that the increases in PM percentage with a small particle size (Figure 2), oxygen-containing functional groups (Figure 3), and hydrophilicity (Figure 4) after O3 oxidation facilitated the wetting, water adhesion, and growth of PM in the scrubber, and thus promoted its removal.
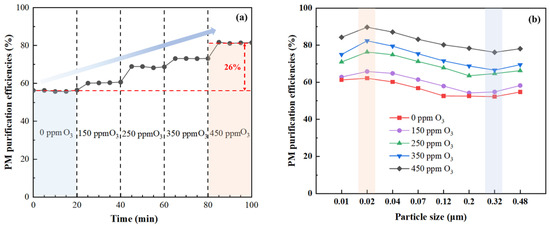
Figure 5.
Effects of O3 concentration on PM removal. (a) Total removal efficiency, (b) fractional removal efficiency.
3.2.2. The Influence of Scrubbing Conditions on PM Removal
- (i)
- The influence of liquid-to-gas ratio
The liquid-to-gas ratio (L/G ratio) is one of the key parameters affecting the purification performance of the Venturi scrubber. In this study, water was used as the scrubbing liquid, and the influence of the L/G ratio on PM removal was investigated. As shown in Figure 6a, with the increase in the L/G ratio, the total removal efficiency of PM significantly enhanced whether O3 was added or not. This was attributed to the fact that when more liquid was supplied, more mist particles could be formed, thereby increasing the gas–liquid contact area and contact probability, and subsequently promoting the growth of particles. It can also be seen in Figure 6a that the promotion effect of O3 oxidation weakened at higher L/G ratios, suggesting the dominating role of liquid supply. As shown in Figure 6b, the removal efficiencies of PM with different sizes all conspicuously increased with the increase in the L/G ratio.
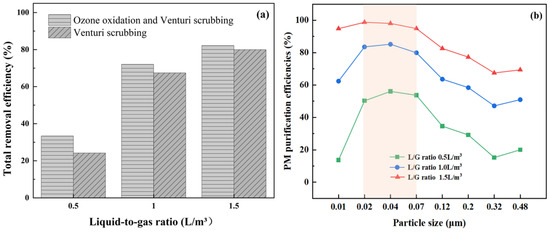
Figure 6.
Effects of L/G ratio on PM removal. (a) Total removal efficiency, (b) fractional removal efficiency.
- (ii)
- The influence of adding surfactants
Theoretically, surfactants can reduce the surface tension of scrubbing liquid, thereby enhancing the wetting properties and subsequently improving PM removal performance. In this study, the influence of surfactant addition on PM removal was investigated by adding three representative surfactants, namely LAS, CTAB, and AEO-9 (mass concentration of 0.25%). The results demonstrated that the addition of surfactants increased the PM removal efficiency. In particular, adding LAS made the PM removal efficiency increase from 56.3% to 73.3%. This improvement may be attributed to a large π bond contained in the LAS molecular structure, which exhibits a strong affinity with the hydrophobic groups of PM. Moreover, the electrostatic repulsion between PM particles reduced and the wettability of mist particles enhanced, and the formed micelles encapsulated PM particles, thereby facilitating PM growth.
3.3. Simultaneous Removal of NOx and PM by the Combination of O3 Oxidation and Scrubbing
3.3.1. The Influence of O3 Concentration on Simultaneous Removal of NOx and PM
The effects of O3 concentration on the simultaneous removal of NOx and PM were investigated using a 0.1% (w/w) Na2CO3 aqueous solution as the scrubbing liquid. As shown in Figure 7a, the NOx removal efficiency initially increased linearly with the increase in the O3 concentration. When the O3 concentration reached 360 ppm, corresponding to an O3/NO molar ratio of 1.8, NOx removal efficiency reached 100%. Clearly, NO was oxidized to higher-valence nitric oxides with good water solubility, promoting the absorption removal of NOx. On the other hand, with the increase of O3 concentration, the increase in the PM removal efficiency was not as significant as that observed without NO in the simulated exhaust gas (Figure 5a). Cleary, a competitive relationship exists between O3 reactions with NO and PM. O3 preferentially oxidizes NO to form highly soluble, higher-valent nitrogen oxides. While this reaction significantly enhances NOx removal in the subsequent scrubbing process, it also reduces the amount of O3 available for direct PM oxidation, leading to a lower PM removal efficiency compared with conditions without NO in the simulated gas. Nevertheless, at higher O3 concentrations, the residual O3 and the generated higher-valent nitrogen oxides can still modify the PM surface, enhancing its hydrophilicity and thereby improving its wettability and removal during washing to a certain extent.
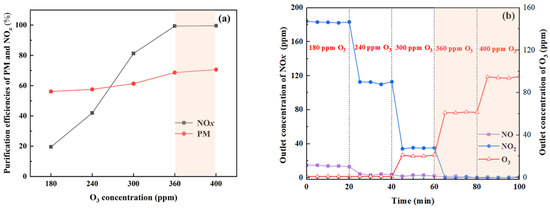
Figure 7.
Effects of O3 concentration on simultaneous removal of NOx and PM. (a) Removal efficiencies of NOx and PM; (b) NOx and O3 concentrations at the outlet.
It can be seen from Figure 7b that the NO2 concentration gradually decreased when the O3 concentration increased from 180 ppm to 400 ppm, which indicated that NO2 was the main oxidation product at the low O3 concentration (O3/NO ratio ≤ 1), the absorption of which was limited. At higher O3 concentrations, NO2 was further oxidized to nitric oxides such as N2O5 and NO3, which can be easily absorbed. Figure 7b also showed that the O3 concentration at the outlet of the reaction system increased with the concentration of O3 at the inlet when it reached 300 ppm and above (O3/NO ratio ≥ 1.5). Therefore, further research on optimizing the technical pathway and process parameters to prevent ozone escape is necessary.
3.3.2. The Influence of Scrubbing Conditions on Simultaneous Removal of NOx and PM
- (i)
- The influence of scrubbing liquid compositions
Figure 8 shows the effects of the scrubbing liquid compositions on the removal of NOx and PM. It can be seen that the removal efficiency of NOx was relatively high (around 87%) when 0.1% (w/w) Na2CO3 was added into the scrubbing liquid, no matter whether 0.25% (w/w) LAS was added or not. On the other hand, the removal efficiency of PM was relatively high when 0.25% (w/w) LAS was added into the scrubbing liquid, especially when 0.1% (w/w) Na2CO3 was also added. These results suggest that the basicity of Na2CO3 is important for the absorption of NOx while LAS, as a typical surfactant, mainly enhances the removal of PM. As expected, the highest removal efficiencies of NOx and PM were obtained when both 0.1% (w/w) Na2CO3 and 0.25% (w/w) LAS were added into the scrubbing liquid (Figure 8).
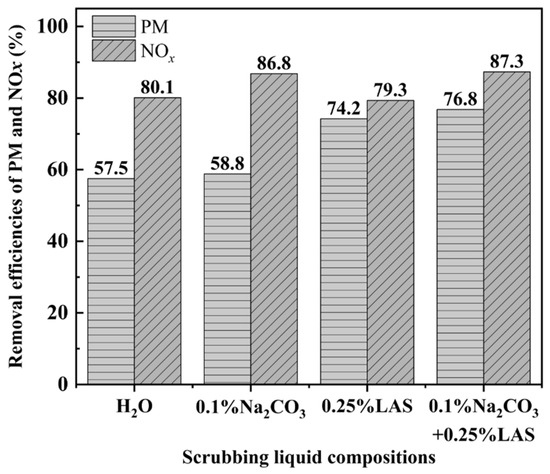
Figure 8.
Effects of scrubbing liquid compositions on removal efficiencies of NOx and PM.
- (ii)
- The influence of liquid-to-gas ratio
The effects of the L/G ratio on the removal efficiencies of NOx and PM, as well as the escape concentration of O3 were investigated using an aqueous solution containing 0.1% Na2CO3 and 0.25% LAS as the scrubbing liquid. As shown in Figure 9, the L/G ratio exerted a negligible influence on NOx removal. However, the PM removal efficiency significantly increased as the L/G ratio rose. When the L/G ratio was 1.5 L/min, the removal efficiencies of PM and NOx reached 92% and 87%, respectively. Notably, as the L/G ratio increased, the O3 concentration at the outlet gradually diminished, indicating that the presence of more mist particles also alleviated O3 escape.
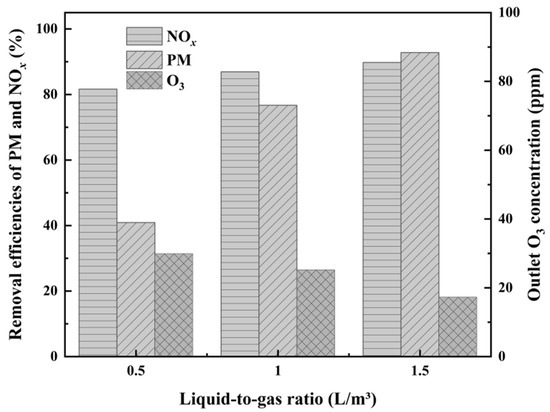
Figure 9.
Effects of liquid-to-gas ratio on removal efficiencies of NOx and PM.
3.4. Practical Challenges and Future Perspectives
While laboratory-scale results demonstrate the promising potential of O3 oxidation-coordinated scrubbing technology for the efficient synergistic removal of NOx and PM, several challenges must be addressed for its practical engineering application. Firstly, the on-site generation of ozone involves certain energy consumption and equipment costs. However, the energy efficiency of ozone generators continues to improve with advancements in technology. More importantly, as this technology enables the synergistic control of multiple pollutants within a single system, it holds the potential to replace or simplify traditional multi-stage treatment devices, thereby offering cost competitiveness at the system level.
Secondly, safety concerns regarding ozone and system maintenance require careful consideration. The deployment of ozone leakage monitoring and interlock protection systems can effectively mitigate safety risks. Issues such as reactor clogging or corrosion can be addressed through modular design and regular predictive maintenance.
Future research will focus on developing intelligent ozone dosing control algorithms linked to engine operating conditions, aiming to optimize the balance between energy consumption and removal efficiency. Additionally, pilot-scale durability testing will be conducted to validate the long-term operational stability and economic feasibility of this technology under real-world conditions.
4. Conclusions
This study demonstrates that an integrated O3 oxidation and Venturi scrubbing system achieves the efficient synergistic removal of NOx (87%) and PM (92%) under optimized conditions (O3/NO = 1.5, L/G = 1.5), with effective control of O3 escape. It was observed that an increased O3 concentration enhances PM removal by elevating PM surface polarity and hydrophilicity; however, the competing reaction—where O3 preferentially oxidizes NO over PM—resulted in a discernible decline in PM removal efficiency in the presence of NOx. Furthermore, both elevating the L/G ratio and incorporating surfactants into the scrubbing liquid were shown to improve PM capture. Raising the L/G ratio increases the number of mist droplets, thereby enhancing the probability of contact with PM, whereas surfactants reduce liquid surface tension and improve water dispersion—both mechanisms promote the adhesion of water to PM surfaces and facilitate particle growth. However, this research is limited to simulated exhaust under steady-state conditions and does not assess the energy consumption or potential secondary by-products of continuous O3 generation. Future work should focus on dynamic engine testing, system durability, and techno-economic analyses to evaluate the technology’s feasibility for real-world NRMM applications.
Author Contributions
Conceptualization, T.Z.; Methodology, X.L.; Investigation, Y.S. and M.L.; Data Curation, Y.G. and J.F.; Writing—Original Draft, Y.S. and M.L.; Writing—Review and Editing, T.Z.; Supervision, T.Z.; Funding Acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (52370106).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministry of Ecology and Environment of the People’s Republic of China. 2024 China Mobile Source Environment Management Annual Report. Available online: https://www.mee.gov.cn/hjzl/sthjzk/ydyhjgl/202503/W020250326518388591055.pdf (accessed on 26 May 2025).
- Bie, P.; Ji, L.; Cui, H.; Li, G.; Liu, S.; Yuan, Y.; He, K.; Liu, H. A review and evaluation of nonroad diesel mobile machinery emission control in China. J. Environ. Sci. 2023, 123, 30–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Shan, Y.; Wang, F.; Liu, J.; Wang, M.; Liu, Z.; Yan, Y.; Xu, G.; He, G.; et al. Toward synergetic reduction of pollutant and greenhouse gas emissions from vehicles: A catalysis perspective. Chem. Soc. Rev. 2025, 54, 1151–1215. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Shan, W.; Yu, Y.; Shan, Y.; Wu, X.; Wu, Y.; Zhang, S.; He, L.; Shuai, S.; Pang, H.; et al. Advances in emission control of diesel vehicles in China. J. Environ. Sci. 2023, 123, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, Z.; Shen, B.; Yuan, P.; Ma, J.; Wang, Z.; Kong, W. An overview of the deactivation mechanism and modification methods of the SCR catalysts for denitration from marine engine exhaust. J. Environ. Manag. 2022, 317, 115457. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shan, W.; Lian, Z.; Liu, J.; He, H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 230, 165–176. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn-Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, O.; Cho, G.; Kim, H. Improvement of low-temperature NH3-SCR performance of Cu-zeolite and vanadium catalysts through ozone injection. Energy 2025, 322, 135510. [Google Scholar] [CrossRef]
- Nie, W.; Song, X.; Hua, Y.; Liu, C.; Lian, J.; Wu, H.; Wang, C. Effect of cerium-zirconium oxide-loaded red mud on the selective catalytic reduction of NO in downhole diesel vehicle exhaust. Environ. Pollut. 2025, 368, 125690. [Google Scholar] [CrossRef]
- Desouza, C.D.; Marsh, D.J.; Beevers, S.D.; Molden, N.; Green, D.C. Real-world emissions from non-road mobile machinery in London. Atmos. Environ. 2020, 223, 117301. [Google Scholar] [CrossRef]
- Wardana, M.K.A.; Lim, O. Review of improving the NOx conversion efficiency in various diesel engines fitted with SCR System technology. Catalysts 2023, 13, 67. [Google Scholar] [CrossRef]
- Pietikäinen, M.; Väliheikki, A.; Oravisjärvi, K.; Kolli, T.; Huuhtanen, M.; Niemi, S.; Virtanen, S.; Karhu, T.; Keiski, R.L. Particle and NOx emissions of a non-road diesel engine with an SCR unit: The effect of fuel. Renew. Energy 2015, 77, 377–385. [Google Scholar] [CrossRef]
- Kong, X.; Li, Z.; Shen, B.; Wu, Y.; Zhang, Y.; Dong, C. Simulation of flow and soot particle distribution in wall-flow DPF based on lattice Boltzmann method. Chem. Eng. Sci. 2019, 202, 169–185. [Google Scholar] [CrossRef]
- Chen, C.; Yao, A.; Yao, C.; Qu, G. Experimental study of the active and passive regeneration procedures of a diesel particulate filter in a diesel methanol dual fuel engine. Fuel 2020, 264, 116801. [Google Scholar] [CrossRef]
- Feng, R.; Sun, Z.; Hu, X.; Li, G.; Deng, B. Role of particle oxidation catalyst on emission reduction of a non-road diesel engine: A multi case study. Chem. Eng. Sci. 2022, 260, 117914. [Google Scholar] [CrossRef]
- Landi, G.; Di Sarli, V.; Lisi, L. A Numerical investigation of the combined effects of initial temperature and catalyst activity on the dynamics of soot combustion in a catalytic diesel particulate filter. Top. Catal. 2021, 64, 270–287. [Google Scholar] [CrossRef]
- Guan, C.; Li, X.; Liao, B.; Huang, Z. Effects of fuel injection strategies on emissions characteristics of a diesel engine equipped with a particle oxidation catalyst (POC). J. Environ. Chem. Eng. 2016, 4 Pt B, 4822–4829. [Google Scholar] [CrossRef]
- Castoldi, L.; Aneggi, E.; Matarrese, R.; Bonzi, R.; Trovarelli, A.; Lietti, L. Simultaneous removal of soot and NOx Over silver and ruthenium-based catalysts. Top. Catal. 2017, 60, 209–213. [Google Scholar] [CrossRef]
- Feng, R.; Hu, X.; Li, G.; Sun, Z.; Deng, B. A comparative investigation between particle oxidation catalyst (POC) and diesel particulate filter (DPF) coupling aftertreatment system on emission reduction of a non-road diesel engine. Ecotoxicol. Environ. Saf. 2022, 238, 113576. [Google Scholar] [CrossRef]
- Hu, J.; Liao, J.; Hu, Y.; Lei, J.; Zhang, M.; Zhong, J.; Yan, F.; Cai, Z. Experimental investigation on emission characteristics of non-road diesel engine equipped with integrated DOC + CDPF + SCR aftertreatment. Fuel 2021, 305, 121586. [Google Scholar] [CrossRef]
- Lack, D.A.; Cappa, C.D.; Langridge, J.; Bahreini, R.; Buffaloe, G.; Brock, C.; Cerully, K.; Coffman, D.; Hayden, K.; Holloway, J.; et al. Impact of fuel quality regulation and speed reductions on shipping emissions: Implications for climate and air quality. Environ. Sci. Technol. 2011, 45, 9052–9060. [Google Scholar] [CrossRef]
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Khobragade, R.; Singh, S.K.; Shukla, P.C.; Gupta, T.; Al-Fatesh, A.S.; Agarwal, A.K.; Labhasetwar, N.K. Chemical composition of diesel particulate matter and its control. Catal. Rev.-Sci. Eng. 2019, 61, 447–515. [Google Scholar] [CrossRef]
- Kawakami, K.; Watatani, K.; Yamasaki, H.; Kuroki, T.; Okubo, M. Performance evaluation of PM, NOx, and hydrocarbon removal in diesel engine exhaust by surface discharge -induce plasma. J. Hazardou Mater. 2024, 462, 132685–132696. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhuang, Z.; Sun, C.; Zhao, N.; Liu, Y.; Wu, Z. Numerical evaluation of the effectiveness of NO2 and N2O5 generation during the NO ozonation process. J. Environ. Sci. 2016, 41, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, N.; Zhuang, Z.; Wang, H.; Liu, Y.; Weng, X.; Wu, Z. Mechanisms and reaction pathways for simultaneous oxidation of NOx and SO2 by ozone determined by in situ IR measurements. J. Hazard. Mater. 2014, 274, 376–383. [Google Scholar] [CrossRef]
- Ji, R.; Wang, J.; Xu, W.; Liu, X.; Zhu, T.; Yan, C.; Song, J. Study on the key factors of NO oxidation using O3: The oxidation product composition and oxidation selectivity. Ind. Eng. Chem. Res. 2018, 57, 14440–14447. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Sun, C.L.; Zhao, N.; Wang, H.; Wu, Z. Simultaneous absorption of NOx and SO2 using magnesia slurry combined with ozone oxidation. Energy Fuels 2015, 29, 3276–3283. [Google Scholar] [CrossRef]
- Shao, J.; Yang, Y.; Whiddon, R.; Wang, Z.; Lin, F.; He, Y.; Kumar, S.; Cen, K. Investigation of NO removal with ozone deep oxidation in Na2CO3 solution. Energy Fuels 2019, 33, 4454–4461. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Xiao, X.; Jia, Y.; Fang, P.; Huang, J.-H.; Wu, H.-W.; Tang, Z.-J.; Chen, D.-Y. Simultaneous removal of SO2 and NO by O3 oxidation combined with wet absorption. Acs Omega 2020, 5, 5844–5853. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Gao, X.; Pan, J.; Liu, Y. Remvoal of SO2 and NO in flue gas using a vacuum ultraviolet light/oxone/H2O2 ocidation system. Fuel 2025, 390, 134539–134551. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Ma, J.; Ma, Q.; He, H. Structural and hygroscopic changes of soot during heterogeneous reaction with O3. Phys. Chem. Chem. Phys. 2010, 12, 10896–10903. [Google Scholar] [CrossRef]
- Li, Q.; Shang, J.; Zhu, T. Physicochemical characteristics and toxic effects of ozone-oxidized black carbon particles. Atmos. Environ. 2013, 81, 68–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).