TLC in the Analysis of Plant Material
Abstract
1. Introduction
2. TLC in Qualitative and Quantitative Analysis of Plant Materials and Herbal Formulations
2.1. TLC in Qualitative Analysis
2.1.1. Profiling Plant Material Using TLC
2.1.2. Application in Profiling HPTLC Plates and Other Modern Methods Improving the Separation of Components
2.1.3. Application of TLC for Profiling Plant Formulations and Detecting Adulterations and Contaminants in Them
2.1.4. TLC Coupled with Other Methods
TLC Coupled with Chemometric Methods and Image Analysis
The Selected Methods for Identifying Components of Plant Extracts Separated by TLC
2.2. TLC in Quantitative Analysis
| Matrix/Plant Materials | Chromatographic Conditions | Chromatographic and Statistical Parameters | Refs. |
|---|---|---|---|
| Plants | |||
| Medicine mulberry (Morus nigra L.) Cyanidin-3-O-glicoside (C3G) Cyanidin-3-O-rutinoside (C3R) | HPTLC–densitometry λ = 550 nm Silica gel 60F254 formic acid–water–n-butanol (9.1:8.4:32.5, v/v) | Linearity (μg/spot): 0.2–1.0 (for C3G, C3R) LOD (µg/spot): 0.048 (for C3G); 0.036 (for C3R) LOQ (µg/spot): 0.15 (for C3G); 0.11 (for C3R) Intraday precision: 1.32% (for C3G); 3.62% (for C3R) Interday precision: 2.67% (for C3G); 3.04% (for C3R) Recovery: 98.09% and RSD = 3.08% (for C3G); 98.66% and RSD = 2.93% (for C3R) | [136] |
| Solanum xanthocarpum Schrad. & Wendl. Solasonine (SN) Solamargine (SM) Khasianine (K) Solasodine (SD) Diosgenin (D) | HPTLC–densitometry λ = 640 nm for SN, 440 nm for K and SM; 610 nm for SD, and 430 nm for D Silica gel 60 GF254 n-propanol–ethyl acetate –10% glacial acetic acid in water (4:8:3, v/v) for SN, SM, K toluene–ethyl acetate– diethylamine (6:2:0.3, v/v) for SD, D | RF = 0.15 ± 0.01, 0.22 ± 0.02, and 0.31 ± 0.02 for SN, SM,K, respectively; and 0.38 ± 0.04 and 0.50 ± 0.03 for SD and D Linearity (µg/spot): 0.2–1.0 (for SN, SM, K, SD, D) LOD (µg/spot): 0.031 (for SN), 0.009 (for SM), 0.033 (for K), 0.045 (for SD), 0.053 (for D) LOQ (µg/spot): 0.094 (for SN), 0.029 (for SM), 0.10(for K), 0.136 (for SD), 0.16 (for D) Intraday precision: 2.08–2.87% (for SN, SM, K, SD), 3.25 (for D) Interday precision: 1.58% (for SN); 2.44–2.95 (for SM, K, SD), 3.14 (for D) Recovery (%): 98.41, 98.58, 98.82, 100.34, 99.89 (for SN, SM, K, SD, D, respectively) | [137] |
| Hypericum species Hyperforin (HyF) Hypericin (HyP) Hyperoside (HyS) | HPTLC–densitometry λ = 366 nm Silica gel 60F254 n-hexane–ethyl acetate (8:2, v/v) (for HyF) toluene–chloroform–ethyl acetate–formic acid (8:5:35:0.6, v/v) (for HyP) ethyl acetate–formic acid–acetic acid -water (15:2:2:1, v/v) (for HyS) | Linearity (ng/band): 400–1400 (for HyF) 20–100 (for HyP) 10–100 (for HyS) LOD (ng/band): 120 (for HyF); 6 (for HyP); 3 (for HyS) LOQ (ng/band): 400 (for HyF); 20 (for HyP); 10 (for HyS) Intraday precision: <2% (for HyF, HyP, HyS) Interday precision: <2% (for HyF, HyS), <3% (for HyP) Accuracy (Recovery): RSD (%) 1.35–1.93 (for HyF), 1.14–1.61 (for HyP), 0.76–1.81 (for HyS) | [138] |
| Alisma orientale (Sam.) Alisol B 23-acetate (ABA) Alisol A (AA) Alisol B (AB) Alisol C 23-acetate (ACA) | HPTLC–densitometry λ = 254 nm (ACA) and 208 nm (ABA, AA, AB) Silica gel 60F254 cyclohexane–ethyl acetate (1:1, v/v) | Linearity (µg/zone): 0.125–2.0 (for ABA); 0.0834–2.0 (for AA, AB, ACA) Intraday precision: <1% (for ABA, AA, AB, ACA) Interday precision: <1% (for ABA, AA, AB, ACA) Repeability: RSD < 1% (for ABA, AB); <3% (for AA, ACA) Stability: RSD < 1% (for ABA, AB); <3% (for AA, ACA) Accuracy (Recovery): RSD (%) 3.27 (for ABA), 4.05 (for ACA), 2.07 (for AB), 2.78 (for AA) | [126] |
| Millettia pinnata (L.) Pierre (stem, bark) Karanjin (KR) | HPTLC–densitometry λ = 260 nm HPTLC–MS/MS for identification of isolated compound Silica gel 60F254 toluene–ethyl acetate–formic acid (7:3:0.3, v/v) | Linearity range (ng/band): 200–1200 LOD (ng/band): 21.5 LOQ (ng/band): 65.3 Repeatability (%SD): 1.88 Intraday precision (%RSD): 1.88–1.95 Interday precision (%RSD): 1.87–1.88 % Recovery: 94–104 Robustness (n = 6) (%RSD): <4% | [135] |
| Gum samples of Sterculia urens Roxb. Glucuronic acid (GlcUA) | HPTLC–densitometry λ = 580 nm Silica gel 60F254 1-propanol–water (7:3, v/v) | RF = 0.43 Linearity range (ng/band): 300–700 LOD (ng/band): 201.54 LOQ (ng/band): 610.74 Intraday precision (%RSD): 1.77 Interday precision (%RSD): 1.27 Accuracy: % Recovery 101.56; %RSD = 1.00 Specificity: Specific | [123] |
| Ziziphus mauritiana Lam. and Ziziphus nummularia (Burm.f.) Wight & Arn. Betulinic acid (BAC) | HPTLC–densitometry λ = 580 nm Silica gel 60F254 petroleum ether–ethyl acetate– toluene (7:2:1, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | Linearity (µg/spot): 2–10 LOD (µg/spot): 0.379 LOQ (µg/spot): 1.149 Intraday precision: 0.85% Interday precision: 1.22% Accuracy: 99.54% | [139] |
| fruits, leaves, root bark and stem bark of Dillenia indica Linn Betulinic acid (BAC) β-Sitosterol (BS) Lupeol (LU) | TLC–densitometry λ = 525 nm Silica gel 60F254 toluene–methanol–chloroform (8:1:1, v/v) | RF = 0.38 ± 0.01 for BAC, 0.54 ± 0.01 for BS and 0.65 ± 0.02 for LU Linearity (ng/band): 2000–6000; 200–1000; and 200–600 for BAC, BS, LU, respectively LOD (ng/band): 2.98, 95.36, 118.51 for BAC, BS, LU, respectively LOQ (ng/band): 9.02, 288.97, 359.12 for BAC, BS, LU, respectively Intraday precision (%RSD): 1.11, 1.73, 1.40 for BAC, BS, LU, respectively Interday precision (%RSD): 0.87, 1.81, 1.47 for BAC, BS, LU, respectively Accuracy (% Recovery) 99.19, 99.69, 100.95% for BAC, BS, LU, respectively Robustness (%RSD): <2 | [99] |
| Flowers, fruits, root, stem bark and leaves of Cassia fistula L. β-Sitosterol (BS) Lupeol (LU) | HPTLC–densitometry λ = 525 nm Silica gel 60F254 toluene–methanol–chloroform (8:1:1, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.25 ± 0.01 for BS and 0.37 ± 0.01 for LU Linearity (ng/band): 40–120 for BS, LU LOD (ng/band): 13.86, 13.01 for BS, LU, respectively LOQ (ng/band): 41.99, 39.42 for BS, LU, respectively Intraday precision (%RSD): 1.33 for RT, 1.82–1.97 for Q Interday precision (%RSD): 1.02 for RT, 0.68 for Q Accuracy (% Recovery) 99.81% for RT, 100.97% for Q Robustness (%RSD): <2 | [100] |
| Different plant parts of Uraria picta (Jacq.) Desv. β-Sitosterol (BS) Lupeol (LU) | HPTLC–densitometry λ = 525 nm Silica gel 60F254 toluene–methanol–chloroform (8:1:1, v/v) post-chromatographic derivatization using anisaldehyde-sulphuric reagent | RF = 0.53 ± 0.01 and 0.63 ± 0.01 (for BS and LU, respectively) Linearity range (ng/band): 200–600 for BS and LU LOD (ng/band): 129.455 for BS and 88.687 for LU LOQ (ng/band): 392.287 for BS and 268.749 for LU Intraday precision (%RSD): 1.43 for BS and 1.21 for LU Interday precision (%RSD): 1.27 for BS and 0.92 for LU Accuracy: % Recovery 99.86 for BS and 101.07 for LU %RSD 1.97 for BS and 0.64 for LU Specificity: Specific | [101] |
| Leaf extracts of Bauhinia vahlii Fern.-Vill. β-Sitosterol (BS) Lupeol (LU) | HPTLC–densitometry λ = 514 nm toluene–ethyl acetate–formic acid (8:2:0.2, v/v post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent for detection of BS | RF = 0.53 and 0.68 for BS and LU, respectively Linearity range (ng/band): 200–1400 for LU and 600–1300 for BS LOD (ng/band): 0.92 for LU and 1.69 for BS LOQ (ng/band): 3.07 for LU and 5.63 for BS Intraday precision (%RSD): <2 Interday precision (%RSD): <2 Accuracy (% Recovery) 106.41% for LU and 109.52% for BS %RSD of recovery: 1.038 for LU and 1.168 for BS Specificity: Specific | [102] |
| Tuber extract of Amorphophallus paeoniifolius (Dennst.) Nicolson Resveratrol (RV) β-Sitosterol (BS) | TLC–densitometry λ = 305 nm for RV (before derivatization) and 662 nm for BS (after derivatization) Silica gel G60 F254 toluene–ethyl acetate (7:3, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent for detection of BS | RF = 0.24 and 0.60 for RV and BS, respectively Linearity range (ng/band): 100–1000 LOD (ng/band): 10.6 for RV and 11.8 for BS LOQ (ng/band): 32.2 for RV and 35.9 for BS Intraday precision (%RSD): <2 Interday precision (%RSD): <2 Accuracy (% Recovery) 96.99–98.32% for RV and 98.31–99.27% for BS Repeatability (%RSD): 0.15 for RV and 1.01 for BS Specificity: Specific Robustness: Robust | [103] |
| Phyllanthus niruri L. Phyllanthin (PL) | HPTLC–densitometry λ = 279 nm Silica gel 60F254 toluene–ethyl acetate–formic acid (15:10.5:1.5, v/v) | RF = 0.67 Linearity (µg/band): 2.36–11.8 LOD (µg/band): 0.532 LOQ (µg/band): 1.612 Intraday precision (%RSD) = 8.87–9.43 Interday precision (%RSD) = 6.94 Specificity: Specific | [140] |
| Eight rhizomes from plants of the Zingiberaceae family Curcumin (C) | HPTLC–densitometry λ = 422 nm Silica gel 60F254 chloroform–methanol (40:1, v/v) | RF = 0.38 Linearity (ng/band): 200–1400 LOD (ng/band): 199.35 LOQ (ng/band): 604.08 Intraday precision (%RSD) = 2.94–5.03 Interday precision (%RSD) = 6.71 Accuracy (% Recovery): 91.62% and 102.42% Specificity: Specific | [124] |
| Leaves of Clerodendrum philippinum Schauer Hispidulin (H) | HPTLC–densitometry λ = 267 nm Silica gel 60F254 chloroform–methanol–formic acid (9:1:0.1, v/v) post-chromatographic derivatization using sulfuric acid–methanol reagent (5%) | RF = 0.53 Linearity (ng/spot): 100–500 LOD (ng/spot): 17 LOQ (ng/spot): 50 Intraday precision (%RSD): <2 Interday precision (%RSD) = <2 Accuracy (% Recovery): 97.73 Robustness: Robust | [133] |
| Leaf of Murraya koenigii L. Mahanimbine (MB) | HPTLC–densitometry λ = 285 nm Silica gel 60F254 hexane–ethyl acetate (7:3, v/v) | RF = 0.60 Linearity (µg/mL): 100–400 LOD (ng/spot): 45.50 LOQ (ng/spot): 77.92 Reproducibility: Reproducible | [130] |
| Citrus aurantium peel. Neohesperidin (NP) | TLC–densitometry λ = 254 nm Silica gel 60F254 ethyl acetate–methanol–water– formic acid (7.1:1.4:1:0.5, v/v) | RF = 0.54 ± 0.02 Linearity (ng/spot): 1000–3000 LOD (ng/spot): 290.05 LOQ (ng/spot): 878.96 Intraday precision (%RSD): <2 Interday precision (%RSD) = <2 Accuracy (% Recovery): 99.6–101.81, Robustness: %RSD < 2 Robust | [134] |
| Different parts of Capparis zeylanica Linn. Rutin (RT) | HPTLC–densitometry λ = 264 nm Silica gel 60F254 ethyl acetate-glacial acetic acid- formic acid–water (10:1.1:1.1:2.6, v/v) | RF = 0.418 ± 0.004 Linearity range (ng/spot): 400–1400 LOD (ng/spot): 14.10 LOQ (ng/spot): 42.73 Intraday precision (%RSD): <1 Interday precision (%RSD): <2 % Recovery 97.73–98.12 %RSD ≤ 0.01 Specificity: Specific | [116] |
| Herbal plants, including Ocimum basilicum L. Rutin (RT) Quercetin (Q) | HPTLC–densitometry λ = 254 nm Silica gel 60F254 toluene–ethyl acetate–methanol–formic acid (6:4:3:1, v/v) | RF = 0.25 ± 2.01 for RT and 0.80 ± 0.64 for Q Linearity (ng/band): 300–1300 for RT, Q LOD (ng/spot): 46.52, 81.79 for RT, Q, respectively LOQ (ng/spot): 140.96, 247.84 for RT, Q, respectively Intraday precision (%RSD): 1.54–1.79 for RT, 1.24–1.97 for Q Interday precision (%RSD): 1.82–2.41 for RT, 1.96–2.17 for Q Accuracy (% Recovery) 22.84–25.19% for RT, 54.00–55.29% for Q Robustness (%RSD): <2 | [106] |
| Leaf of Annona reticulata L. Galic acid (GA) Quercetin (Q) | HPTLC–densitometry λ = 254 nm Silica gel GF254 toluene–ethyl acetate–formic acid (9:10:1.6, v/v) | Linearity (ng/spot): 200–1000 for Q and 200–1200 for GA LOD (ng/spot): 21.31 (for Q); 14.86 (for GA) LOQ (ng/spot): 64.57 (for Q); 55.04 (for GA) Intraday precision: <1% (for GA and Q) Interday precision: <2% (for GA and Q) Robustness (%, RSD): <1 (for AA, Q, C) Repeatability of measurement (%RSD): <1 (for GA and Q) Repeatability of application (%RSD): <1 (for GA and Q) Accuracy (%): 98.02–99.09 for Q and 99.28–100.26 (for GA) Specificity: Specific | [107] |
| Cyperus rotundus L. Quercetin (Q) | HPTLC–densitometry λ = 257 nm toluene–ethyl acetate–formic acid (3:4:2.5, v/v) | RF = 0.80 Linearity (ng/band): 100–700 LOD (ng/band): 30.08 LOQ (ng/band): 91.17 Instrument precision (n = 5): RSD = 0.94% Repeatability (n = 5): RSD = 1.05% Recovery 98–99% Specific—Q separated from rutin and catechin | [108] |
| Leaf extract of Manilkara hexandra Dubard Myricetin (M) Quercetin (Q) | HPTLC–densitometry λ = 254 nm Silica gel 60F254 toluene–ethyl acetate–formic acid (6:6:2.4, v/v) | RF = 0.6 for M, 0.7 for Q Linearity (µg/band): 0.5–3, 0.4–1.4 for M and Q respectively LOD (µg/band): 0.13, 0.072 for M and Q, respectively LOQ (µg/band): 0.40, 0.21 for M and Q, respectively Intraday precision (%RSD): 0.21–0.69, 0.29–0.78 for M and Q, respectively Interday precision (%RSD): 0.50–1.66, 0.58–1.44 for M and Q, respectively Accuracy (% Recovery) 99.85–100.12, 99.23–100.83 for M and Q, respectively Robustness (%RSD): <2 | [109] |
| Desmodium oojeinensis (Roxb.) Hochr. bark and roots Betulin (BT) Stigmasterol (ST) Lupeol (LU) | TLC–densitometry λ = 520 nm Silica gel 60F254 hexane–ethyl acetate (8.5:1.5, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.42 ± 0.01, 0.27 ± 0.01, and 0.19 ± 0.01 for LU, ST and BT, respectively Linearity (ng/band): 200–600 for LU, ST and BT LOD (ng/band): 12.75, 18.02, 13.35 for LU, ST, BT, respectively LOQ (ng/band): 38.64, 54,59, 40.46 for LU, ST, BT, respectively Intraday precision (%RSD): <2 Interday precision (%RSD): <2 Accuracy (% Recovery): 97.43–97.69 for LU, 97.02–97.89 for ST, 97.68–98.18 for BT Robustness (%RSD): <2 | [105] |
| Leaves, flowers, stems, seeds, and roots of Hygrophila schulli (Schumach.) Heine Stigmasterol (ST) | HPTLC–densitometry λ = 520 nm Silica gel 60F254 toluene—methanol (9:1, v/v) post-chromatographic derivatization using anisaldehyde—sulphuric acid reagent | RF = 0.47 ± 0.02 Linearity (ng/band): 100–500 LOD (ng/band): 6.87 LOQ (ng/band): 20.82 Precision (%RSD): <2 Accuracy (% Recovery): 98.86–99.22, %RSD < 1 Robustness: Robust | [132] |
| Parkia roxburghii (DC.) Merr. seed Catechin (CT) | HPTLC–densitometry λ = 302 nm Silica gel 60F254 ethyl acetate–acetic acid–formic acid–water (10:1:0.75:1, v/v) | RF = 0.61 Range of calibration curve (µg): 2–10 LOD (ng/spot): 12.32 LOQ (ng/spot): 37.23 Intraday precision (%RSD): <1 Interday precision (%RSD): <1 Accuracy (% Recovery): 99.54 Robustness: robust Specificity: specific | [128] |
| Gynura cusimbua S.Moore leaves Chlorogenic acid (CGA) | HPTLC–densitometry λ = 366 nm Silica gel 60F254 ethyl acetate–formic acid–acetic acid–water (100:11:11:2.6, v/v) | RF = 0.43 ± 0.01 Range of calibration curve (ng/spot): 50–250 LOD (ng/spot): 14.36 LOQ (ng/spot): 43.12 Intraday precision (%RSD): 0.63 Interday precision (%RSD): 1.78 Accuracy (% Recovery): 97.79–98.30 Specificity: specific | [127] |
| Different parts of Carica papaya L. Syringic acid (SA) Gallic acid (GA) p-Coumarin (PC) Caffeic acid (CFA) | TLC–densitometry λ = 302 nm for SA, 256 nm for PC, 200 nm for GA, 296 nm for CFA Silica gel 60F254 toluene–ethyl acetate–glacial acetic acid (8.5:1.5:0.1, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.51 ± 0.002, 0.62 ± 0.001, 0.29 ± 0.05, and 0.38 ± 0.01 for SA, PC, GA, and CFA, respectively Linearity (ng): 100–600 for SA, GA, PC, CFA LOD (ng): 60, 30, 40, 40 for SA, PC, GA, and CFA, respectively LOQ (µg): 200, 100, 100, 100 for SA, PC, GA, and CFA, respectively Intraday precision (%RSD): <1% Interday precision (%RSD): <2% Accuracy (% Recovery): 97.23–98.35, 97.72–98.36, 98.12–99.71, 98.26–99.13 for SA, PC, GA, and CFA, respectively Specificity: specific | [125] |
| Caesalpinia bonduc leaf extract β-Caesalpin (βCLP) α-Caesalpin (αCLP) | TLC–densitometry TLC-MS λ = 580 nm Silica gel 60F254 n-hexane–ethyl acetate (6:4, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.64 (for βCLP) 0.78 (for αCLP) Linearity (ng/band): 200–1200 for βCLP and αCLP LOD (µg/band): 43.34 and 22.74 for βCLP and αCLP, respectively LOQ (µg/spot): 131.36 and 68.91 for βCLP and αCLP, respectively Intraday precision: 1.52–1.78 for βCLP and 0.52–1.30 for αCLP Interday precision: 1.59–1.75 for βCLP and 0.89–1.65 for αCLP Accuracy: 95.16–99.75% for βCLP and 97.49–99.86% for αCLP Injection repeatability: 1.62 and 1.75 for βCLP and αCLP, respectively Scanning repeatability: 0.47 and 0.58 for βCLP and αCLP, respectively Robustness: Robust Specificity: Spectific | [87] |
| Herbal formulations | |||
| Majun Nisyan (α + β) Boswelics acids (BA) β-Asarone (A) Isoeugenol (IS) 6-Gingerol (G) Piperine (P) | HPTLC–densitometry Silica gel 60F254 toluene–ethyl acetate -chloroform–acetic acid (8:2:5:0.1, v/v) | RF = 0.102 ± 0.01 (for BA 0.982 ± 0.04 (for A), 0.850 ± 0.03 (for IS), 0.698 ± 0.03 (for G) 0.355 ± 0.01(for P) Linearity (µg/spot): 1–12 (for BA, A, IS, G, P) LOD (µg/spot): 1.060 (for BA); 1.405. (for A); 1.973 (for IS); 1.691 (for G); 2.090 (for P) LOQ (µg/spot): 3.214 (for BA); 4.258. (for A); 5.979 (for IS); 5.125 (for G); 6.334 (for P) Intraday precision: <2% (for BA, A, IS, G, P) Interday precision: <2% (for BA, A, IS, G, P) Accuracy: 86.42–105.75% | [111] |
| Polyherbal formulation Galic acid (GA) Eugenol (E) | HPTLC–densitometry Silica gel 60F254 Isopropyl alcohol–n-hexane–ethyl Acetate–glacial acetic acid (10:6:6:0.1, v/v) | RF = 0.608 ± 0.041 (for GA); 0.752 ± 0.035 (for E) Linearity (ng/mL): 1–10 (for GA, E) LOD (ng/mL): 7.85 (for GA); 8.78 (for E) LOQ ((ng/mL): 23.80 (for GA); 26.60 (for E) Intraday precision: <1% (for GA); <2% (for E) Interday precision: <2% (for GA); <1% (for E) Accuracy: 98.05–99.41% | [112] |
| Polyherbal formulations containing Terminalia species Galic acid (GA) Quercetin (Q) | HPTLC–densitometry λ = 271 nm (for GA) and 366 nm (for Q) Silica gel 60F254 toluene-isopropyl alcohol-acetic acid (7:2.5:0.5, v/v) post-chromatographic derivatization using anisaldehyde reagent for phenolic compounds; 2, 2-diphenyl-1 picrylhydrazyl reagent (DPPH) for antioxidant activity; vanilin reagent for terpenoids and phenolic compounds | Linearity (μg/mL): 5–10 (for GA); 1–6 (for Q) LOD (ppm): 800 (for GA); 5 (for Q) LOQ (ppm): 2400 (for GA); 8 (for Q) Reproducibility, RSD = 0.44–9.71% | [110] |
| Kapacurak Kuṭinīr Cūraṇam Andrographolide (AG) Columbin (CL) Gallic acid (GA) p-Coumaric acid (CA) Piperine (P) Oleanolic acid (OA) | HPTLC–densitometry λ = 254 nm for GA, CA, P (before derivatization) and 520 nm for AG, CL and OA (after derivatization) Silica gel 60F254 toluene: ethyl acetate: formic acid (7:3:0.5, v/v) post-chromatographic derivatization using vanillin–sulphuric acid reagent for AG, CL and OA | RF = 0.19 (for AG), 0.23 (GA), 0.28 (CL), 0.57 (CA), 0.64 (PP) and 0.66 (OA) Linearity (µg/band): 1–5 (for each compounds) LOD (ng/band): 0.0069, 0.0044, 0.0063, 0.0042, 0.0022, 0.00037 (for AG, GA, CL, CA, PP, OA, respectively) LOQ (ng/band): 0.0209, 0.0133, 0.0189, 0.0127, 0.0067, 0.00113 (for AG, GA, CL, CA, PP, OA, respectively) Intraday precision (%RSD): <3 Interday precision (%RSD): <3 Accuracy (% Recovery): 97.73 Standard stability (% RSD) <5 | [113] |
| Herbal formulations Quercetin (Q) Curcumin (C) Ascorbic acid (ASA) | HPTLC–densitometry λ = 265 nm Silica gel 60F254 chloroform–ethyl acetate–formic acid (6:6:2.5, v/v) | Linearity (ng/spot): 500–1000 (for AA, Q, C); LOD (ng/spot): 12 (for AA); 6 (for Q); 4 (for C) LOQ (ng/spot): 36 (for AA); 18 (for Q); 13 (for C) Intraday precision: <2% (for AA); <1% (for Q, C) Interday precision: <2% (for AA); <1% (for Q, C) Robustness (%, RSD): <1 (for AA, Q, C) Repeatability (%RSD): <1 (for AA, Q, C) | [114] |
| Sitopaladi churna— Ayurvedic multi-herbal preparation Piperine (P) Cinnamaldehyde (CD) 1,8-Cineole (CN) | HPTLC–densitometry λ = 307 nm for PP, CD (before derivatization) and 599 for CN (after derivatization) Silica gel 60F254 toluene–methanol (9:1, v/v) post-chromatographic derivatization using vanillin– sulphuric acid reagent | RF = 0.22 ± 0.01 for PP, 0.54 ± 0.01 for CD, and 0.65 ± 0.01 for CN Linearity (ng/band): 100–500 ng/spot for PP, CD, and 600–3000 for CN LOD (ng/spot): 18, 24, 27 for PP, CD, CN, respectively LOQ (ng/spot): 54, 73, 483 for PP, CD, CN, respectively Intraday precision (%RSD): ≤2 for PP, CD and 2.4–4.1 for CN Interday precision (%RSD): <1 for PP, ≤2 for CD and 2.4–3.5 for CN Accuracy (% Recovery) 99.1–101.6 for PP, 98.8–100.7 for CD, 98.3–102.7 for CN Robustness (%RSD): <2 for PP, CD, and <5 for CN | [115] |
| Ayurvedic formulations Alizarin (AL) | TLC–densitometry λ = 259 nm Silica gel 60F254 toluene–ethyl acetate–formic acid (9:1.5:1, v/v) | RF = 0.50 ± 0.02 Linearity (ng/spot): 100–1000 LOD (ng/spot): 30.45 LOQ (ng/band): 92.28 Intraday precision (%RSD): <1 Interday precision (%RSD): <1 Accuracy (% Recovery) 96.75–100.43 Specificity: specific | [129] |
| Herbal hepatoprotective formulation Andrographolide (AD) | HPTLC–densitometry λ = 254 nm Silica gel 60F254 dichloromethane–toluene–ethyl acetate–formic acid (6:4:1:0.5, v/v) | RF = 0.69 Linearity (ng/spot): 500–3000 LOD (ng/spot): 31.50 LOQ (ng/spot): 95.48 Intraday precision (%RSD): <3 Interday precision (%RSD): <2 Accuracy (% Recovery) 99.74–99.84 Robustness (%RSD): <1 Ruggedness (%RSD): <1 | [117] |
| Marketed herbal formulations Mahanimbine (MB) Koenimbine (KB) | HPTLC–densitometry λ = 285 nm for MB and 291 nm for KB Silica gel 60F254 hexane–ethyl acetate (7:3, v/v) | RF =0.48 and 0.60 for KB and MB, respectively Linearity range (ng/spot): 100–400 for MB and 50–450 for KB LOD (ng/spot): 32.81 for MB and 18.44 for KB LOQ (ng/spot): 72.81 for MB and 31.57 for KB Intraday precision (%RSD): <3 Interday precision (%RSD): <3 Accuracy (% Recovery) 95.1–98.4 for MB Reproducibility: Reproducible | [118] |
| Mansyadi Kwatha Atropine (AT) Rutin (R) Vanillin (V) | TLC–densitometry λ = 206 nm Silica gel 60F254 tetrahydrofuran–toluene– methanol–formic acid (5:3.5:2:0.5, v/v) | RF = 0.090 ± 0.0039, 0.290 ± 0.0099 and 0.679 ± 0.0056 for AT, R, and V, respectively Linearity (ng/band): 500–50000 for AT, and 500–5000 for R, and V LOD (ng/band): 1427.070, 119.559, 109.974, for AT, R, and V, respectively LOQ (µg/band): 4324.454, 362.300, 333.254 for AT, R, and V, respectively Intraday precision (%RSD): <2% Interday precision (%RSD): <2% Accuracy (% Recovery): 65.26–103.00, 98.27–104.70, 95.40–104.28 for AT, R, and V, respectively Robustness (%RSD): <2 Specificity: specific | [119] |
| Other samples containing the biological active substances occurring in plant | |||
| Rasam/a South Indian spice soup Piperine (P) Capsaicin (CP) | TLC–densitometry λ = 527 nm Silica gel 60F254 toluene–ethyl acetate (7:3, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.51 for PP and 0.40 for CP Linearity (µg/spot): 1–5 LOD (ng): 23.57 for PP and 7.67 for CP LOQ (ng): 76.84 for PP and 25 for CP Intraday precision (%RSD): 2% Interday precision (%RSD): <2% Accuracy (% Recovery): 97.78–99.18 for PP and 96.15–102.13 for CP | [121] |
| Rasam, a polyherbal soup Curcumin (CC) Piperine (P) Capsaicin (CP) | TLC–densitometry λ = 254 nm Silica gel 60F254 toluene–ethyl acetate (7:3, v/v) post-chromatographic derivatization using anisaldehyde–sulphuric acid reagent | RF = 0.26, 0.40 and 0.47 for CC, PP, and CP, respectively Linearity (µg/spot): 2–7 LOD (µg): 3.98, 3.75, and 3.13 for CC, PP, and CP, respectively LOQ (µg): 12.05, 11.36, and 9.49 for CC, PP, and CP, respectively Instrumental precision (%RSD): 2 Intraday precision (%RSD): 1% Interday precision (%RSD): <2% Accuracy (% Recovery): 99.96–101.48 for CC, 99.93–101.48 for PP and 92.25–100.62 for CP Specificity: specific | [120] |
| Coffee bean infusions/ green, light and dark roasted coffee bean infusions Trigonelline (TG) Caffeine (CF) Chlorogenic acid (CGA) | TLC–densitometry λ = 270, 275 and 330 nm for TG, CF, and CGA, respectively Silica gel 60F254 with concentrating zone chloroform–ethyl acetate– methanol–formic acid, (10:6:3:1, v/v) | RF = 0.06, 0.18, 0.73 for TG, CGA, and CF, respectively Linearity (µg/band): 1.00–3.00 for TG, 1.50–5.00 for CGA and 1.00–3.00 for CF LOD (µg/band): 0.28, 0,27, 0,04 for TG, CGA, and CF, respectively LOQ (µg/band): 0.84, 0.82, 0.12 for TG, CGA, and CF, respectively Intraday precision (%CV): <3% Interday precision (%CV): <3% Accuracy (% Recovery): Coffee from an espresso machine: 95.4–101.8 for TG, 96.3–102.4 for CLA, 98.1–102.1 for CF Brewed coffee: 96.2–103.9 for TG, 98.7–104.1 for CLA, and 97.2–101.7 for CF Accuracy (% CV) Coffee from an espresso machine: 2.12, 2.33, and 1.75 for TG, CGA, and CF, respectively Brewed coffee: 2.54, 2.19, and 1.78 for TG, CGA, and CF, respectively Specificity: specific | [98] |
| Counterfeit herbal antidiabetic products Metformin HCl (MET) Pioglitazone HCl (PIO) Glipizide (GLP) Glimepiride (GLM) | HPTLC–densitometry and HPTLC-MS λ = 232 nm Silica gel 60F254 cyclohexane–dichloromethane– 1-propanol–saturated solution of ammonium acetate in acetic acid (7:5:2:2, v/v) | RF = 0.255, 0.461, 0.551, 0.791 (for MET, PIO, GLP, GLM, respectively) Linearity (ng/spot): 200–1200 (for MET, PIO, GLP, GLM) LOD (ng/spot): 186.39 (for MET), 191.66 (for PIO), 153.47 (for GLP), 222.34 (for GLM) LOQ (ng/spot): 564.84 (for MET), 580.77 (for PIO), 465.07 (for GLP), 673.77 (for GLM) Intraday precision: <9% Interday precision: <9% Accuracy: 97.40–105.43, 98.19–105.41, 100.25–103.13, and 98.82–104.38% (for MET, PIO, GLP, GLM, respectively) Robustness (%): <16% | [77] |
| Pesticide residues in thyme and guava leaves Imidacloprid (IMD) Deltamethrin (DLM) Dibutyl phthalate (internal standard—IS) | HPTLC–densitometry λ = 270.0 nm for IMD and 230.0 nm for DLM Silica gel 60F254 impregnated in chitosan nanoparticles (ChTNPs) 0.5% isopropyl alcohol for IMD and IS n–hexane–toluene–ethyl acetate (7:3:1, v/v) for DLM and IS | RF = 0.51 for of IMD and 0.89 for IS RF = 0.80 for of DLM and 0.61 for IS Linearity (µg/spot): 0.2–2.2 for IMD and 0.2–2.4 for DLM Accuracy [mean% ± SD]: 100.49 ±1.62 for IMD and 100.57 ± 0.39 DLM Intraday precision (%RSD): 1.92 for IMD, 1.39 for DLM Interday precision (%RSD): 1.92 for IMD, 1.92 for DLM LOD (µg/spot): 0.002 for IMD and 0.00116 for DLM LOQ (µg/spot): 0.0054 for IMD and 0.0035 for DLM Robustness (%RSD): <3% for IMD and DLM | [122] |
3. Thin Layer Chromatography with Effect-Oriented Analysis
3.1. Detection of Antimicrobial Substances
3.2. Detection of Substances with Antioxidant Activity
3.3. Detection of Enzyme-Inhibiting Substances
3.4. Detecting Substances Affecting Endocrine Management
3.5. Detection of Genotoxic Substances
3.6. Detection of Cytotoxic Substances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TLC | Thin Layer Chromatography |
| HPTLC | High Performance Thin Layer Chromatography |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| IA | Image Analysis |
| PCA | Principal Component Analysis |
| HCA | Hierarchical Cluster Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| HPLC | High Performance Liquid Chromatography |
| HPLC-MS | High Performance Liquid Chromatography—Mass Spectrometry |
| LC-MS | Liquid Chromatography—Mass Spectrometry |
| GC-MS | Gas Chromatography—Mass Spectrometry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GDM | Multiple Gradient Method |
| UV-Vis | Ultraviolet-Visible Spectroscopy |
| SRD | Sum of Ranking Differences |
| TLC-IA | Thin Layer Chromatography—Image Analysis |
| SERS | Surface-Enhanced Raman Spectroscopy |
| AChE | Acetylcholinesterase |
| HPTLC-MSn | High Performance Thin Layer Chromatography—Multistage Mass Spectroscopy |
| ChTNPs | Chitosan Nanoparticles |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| COX | Cyclooxygenase |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| INT | 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride |
| ROS | Reactive Oxygen Species |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| FRAP | Ferric Reducing Antioxidant Power |
| DBF | Dibenzylfluorescein |
| PGH2 | Prostaglandin H2 |
| PGG2 | Prostaglandin G2 |
| FDG | Fluorescein di-β-D-galactopyranoside |
| pYAS | Planar Yeast Androgen Screen |
| pYES | Planar Yeast Estrogen Screen |
| HPLC-DAD | High-Performance Liquid Chromatography-Diode Array Detection |
| LC-ESI-QTOF-MS | Liquid Chromatography-Electrospray Ionization-Quadrupole-Time-of-Flight-Mass Spectrometry |
| DESI | Desorption Electrospray Ionization |
| PCACI | Principal Component Artificial Coloring of Images |
| CCD | Charge-Coupled Device |
| DART | Direct Analysis in Real Time |
References
- Durazzo, A.; Lucarini, M.; Heinrich, M. Editorial: Dietary supplements, botanicals and herbs at the interface of food and medicine. Front. Pharmacol. 2022, 13, 899499. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in European herbs. Phytochem. Rev. 2022, 21, 1549–1575. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, L.; Zhou, S.; Hong, Y.; Zhang, X.; Chen, H. Analysis of differences in the accumulation of tea compounds under various processing techniques, geographical origins, and harvesting seasons. Food Chem. 2024, 430, 137000. [Google Scholar] [CrossRef]
- Gafner, S.; Blumenthal, M.; Foster, S.; Cardellina, J.H.; Khan, I.A.; Upton, R. Botanical ingredient forensics: Detection of attempts to deceive commonly used analytical methods for authenticating herbal dietary and food ingredients and supplements. J. Nat. Prod. 2023, 86, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Alyas, A.A.; Aldewachi, H.; Aladul, M.I. Adulteration of herbal medicine and its detection methods. Pharmacogn. J. 2024, 16, 248–254. [Google Scholar] [CrossRef]
- Luo, P.; Feng, X.; Liu, S.; Jiang, Y. Traditional uses, phytochemistry, pharmacology and toxicology of Ruta graveolens L.: A critical review and future perspectives. Drug Des. Devel. Ther. 2024, 18, 6459–6485. [Google Scholar] [CrossRef]
- Sheng, Y.-H.; Wang, J.; Jiang, Y.-P. Comparison of metabolomics peak-picking parameter optimization algorithms based on chromatographic peak shape. Chin. J. Anal. Chem. 2024, 52, 130–137. [Google Scholar] [CrossRef]
- Gong, H.; Tan, X.; Hou, J.; Gong, Z.; Qin, X.; Nie, J.; Zhu, H.; Zhong, S. Separation, purification, structure characterization, and immune activity of a polysaccharide from Alocasia cucullata obtained by freeze-thaw treatment. Int. J. Biol. Macromol. 2024, 282, 137232. [Google Scholar] [CrossRef]
- Vagare, R.D.; Mane, S.R.; Bais, S.K. Review on phytochemical analysis of finished product by chromatographic techniques. Int. J. Pharm. Herb. Technol. 2025, 3, 3399–3418. [Google Scholar]
- Pawar, K.N.; Kadam, S.P.; Redasani, V.K. A systematic review on high performance thin layer chromatography (HPTLC). Int. J. Pharm. Res. Appl. 2025, 10, 402–416. [Google Scholar] [CrossRef]
- Akabari, A.H.; Gajiwala, H.; Patel, S.K.; Surati, J.; Solanki, D.; Shah, K.V.; Patel, T.J.; Patel, S.P. Stability-indicating TLC-densitometric and HPLC methods for simultaneous determination of teneligliptin and pioglitazone in pharmaceutical dosage forms with eco-friendly assessment. J. Chromatogr. Sci. 2025, 63, bmae038. [Google Scholar] [CrossRef]
- Wilson, I.D.; Poole, C.F. Planar chromatography—Current practice and future prospects. J. Chromatogr. B 2023, 1214, 123553. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Indrayanto, G.; Rohman, A. Advances in fingerprint analysis for standardization and quality control of herbal medicines. Front. Pharmacol. 2022, 13, 853023. [Google Scholar] [CrossRef]
- Long, H.; Yao, S.; Tian, W.; Hou, J.; Lei, M.; Zhang, Z.; Guo, D.; Wu, W. A simple and effective method for identification of Fraxini cortex from different sources by multi-mode fingerprint combined with chemometrics. J. Sep. Sci. 2022, 45, 788–803. [Google Scholar] [CrossRef]
- Kartini, K.; Sabatini, S.S.; Haridsa, N.M.; Jayani, N.I.E.; Setiawan, F.; Hadiyat, M.A. TLC-fingerprinting and chemometrics for identification of Curcuma xanthorrhiza from different geographical origins in Indonesia. Biodiversitas 2023, 24, 6557–6566. [Google Scholar] [CrossRef]
- Choma, I.M.; Nikolaichuk, H. TLC bioprofiling—A tool for quality evaluation of medicinal plants. In Evidence-Based Validation of Herbal Medicine: Translational Research on Botanicals; Elsevier: Amsterdam, The Netherland, 2022; pp. 407–422. ISBN 9780323855426. [Google Scholar]
- Morlock, G.E. Planar chromatographic super-hyphenations for rapid dereplication. Phytochem. Rev. 2025, 24, 1–12. [Google Scholar] [CrossRef]
- Sharma, B.; Islam, A.; Sharma, A. HPTLC-MS: An advance approach in herbal drugs using fingerprint spectra and mass spectroscopy. Tradit. Med. Res. 2023, 8, 10. [Google Scholar] [CrossRef]
- Chaitanya, K.; Sri, K.B.; Sumakanth, M. A review: High performance thin layer chromatography coupled with mass spectroscopy. Int. J. Pharm. Sci. Rev. Res. 2025, 85, 180–184. [Google Scholar] [CrossRef]
- Aulia, D.A.P.; Supandi. Identification of paracetamol compound in traditional herbal medicine as muscle reliever using thin layer chromatography-densitometry. J. Pharm. Nat. Sci. 2025, 2, 76–85. [Google Scholar] [CrossRef]
- Octaria, R.; Diana, D.; Setiawan, H.K. Analytical method validation of sildenafil citrate and caffeine in herbal medicine for increasing stamina using thin layer chromatography—Densitometry. Proceeding Int. Conf. Innov. Sci. Technol. Educ. Child. Health 2025, 5, 158–164. [Google Scholar] [CrossRef]
- Spangenberg, B.; Poole, C.F.; Weins, C. Quantitative Thin Layer Chromatography: A Practical Survery; Springer: Berlin, Germany, 2011; ISBN 978-3-642-10727-6. [Google Scholar]
- Hahn-Deinstrop, E. Appiled Thin-Layer Chromatography: Best Practice And Avoidance Of Mistakes; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; ISBN 9783527315536. [Google Scholar]
- Hameed, K.; Khan, M.S.; Fatima, A.; Shah, S.M.; Abdullah, M.A. Exploring the word of thin-layer chromatography: A review. Asian J. Appl. Chem. Res. 2023, 14, 23–38. [Google Scholar] [CrossRef]
- Coman, M.V.; Herghelegiu, M.C. Thin-layer chromatography in forensic analysis. J. Planar Chromatogr. Mod. TLC 2025, 38, 247–333. [Google Scholar] [CrossRef]
- García-Zavala, A.; Jiménez, C.C.; Martínez-Bourget, D.; Romero-Ávila, M. Exploring thin-layer and column chromatography fundamentals via experiential learning with simple and affordable materials. J. Chem. Educ. 2025, 102, 2181–2189. [Google Scholar] [CrossRef]
- Silver, J. Let us teach proper thin layer chromatography technique! J. Chem. Educ. 2020, 97, 4217–4219. [Google Scholar] [CrossRef]
- Bitwell, C.; Sen Indra, S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, V.; et al. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Darwin, R.; Valmon, R.; Chithanna, S.; Galla, S.H.; Syed, S.H.; Mohathasim Billah, A.A.; Kumar Reddy, K.T.; Naga Venkata Arjun, U.V. Sustainable extraction and purification of phytochemicals: A review of green solvents and techniques. Chem. Methodol. 2025, 9, 356–385. [Google Scholar] [CrossRef]
- Faboro, E.O.; Adekunle, D.O.; Obisesan, I.A.; Oyinlola, T.A. Optimization of extraction conditions for phytochemicals from Senna fistula using cheminformatics. SN Appl. Sci. 2023, 5, 209. [Google Scholar] [CrossRef]
- Bārzdiņa, A.; Paulausks, A.; Bandere, D.; Brangule, A. The potential use of herbal fingerprints by means of HPLC and TLC for characterization and identification of herbal extracts and the distinction of Latvian native medicinal plants. Molecules 2022, 27, 2555. [Google Scholar] [CrossRef]
- Jović, M.; Ristivojević, P.; Živković-Radovanović, V.; Andrić, F.; Dimkić, I.; Milojković-Opsenica, D.; Trifković, J. Statistical analysis-based green planar chromatographic methodology for the quality assessment of food supplements: A case study on Origanum vulgare L. commercial products. J. Planar Chromatogr. Mod. TLC 2023, 36, 493–502. [Google Scholar] [CrossRef]
- Pratiwi, E.D.; Dewi, N.P. Screening of phytochemical secondary metabolites of Muntingia calabura: A potential as hepatoprotector. J. Fundam. Appl. Pharm. Sci. 2022, 2, 59–65. [Google Scholar] [CrossRef]
- Gadad, D.; Holeyache, D.; Hiremath, D. Comparative physico chemical and phyto chemical study of commercial samples of Trivrut (Operculina turpethum Silva. Manso.) from herbal drug markets of India. Ann. Ayurvedic Med. 2022, 11, 221–230. [Google Scholar] [CrossRef]
- Kumar, P.; Bhushan, A.; Gupta, P.; Gairola, S. Comparative morpho-anatomical standardization and chemical profiling of root drugs for distinction of fourteen species of family Apocynaceae. Bot. Stud. 2022, 63, 12. [Google Scholar] [CrossRef] [PubMed]
- Alamsjah, F.; Agustien, A.; Sinurat, A.Y.; Muqarramah, M. Antibacterial activity and compound identification of Eurya acuminata leaf fractions against bacteria-causing skin infections. Biodiversitas 2024, 25, 3441–3448. [Google Scholar] [CrossRef]
- Hidayatullah, M.; Yuwono, M.; Primaharinastiti, R. Optimization method and stability test to determinate luteolin, quercetin, apigenin, and sinensetin levels in herbal medicines using TLC-densitometry. J. Farm. Ilmu Kefarmasian Indones. 2022, 9, 235–241. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Saputri, F.C.; Yanuar, A.; Ningrum, R.A.; Mun’im, A.; Hayati, H. Microscopical evaluation and TLC analysis of Pluchea indica (L.) less: Leaf, stem, and root. HAYATI J. Biosci. 2024, 31, 71–81. [Google Scholar] [CrossRef]
- Bationo, R.K.; Kaboré, D.S.; Abdoulaye, Y.; Arrounan, N.; Toe, M.; Dabiré, C.M.; Pale, E.; Nébié, R.H.C. Phytochemical constituent and cumulative or antagonistic effects of crops plant organ combination on free radical scavenging capacity and antioxidant compound content. Asian J. Chem. Sci. 2024, 14, 10–28. [Google Scholar] [CrossRef]
- Guimarães, S.F.; Amorim, J.M.; Silva, T.F.; de Melo Lima, I.; Shim, J.H.; Castilho, R.O.; Modolo, L.V. Flavone-rich Passiflora edulis fruit shells as urease inhibitors for sustainable agricultural solutions. Theor. Exp. Plant Physiol. 2024, 36, 313–324. [Google Scholar] [CrossRef]
- Dirgantara, S.; Insanu, M.; Fidrianny, I. Evaluation of xanthine oxidase inhibitory, antioxidative activity of five selected Papua medicinal plants and correlation with phytochemical content. Pharmacia 2022, 69, 965–972. [Google Scholar] [CrossRef]
- Machaba, T.C.; Mahlo, S.; Eloff, J. Antifungal and antioxidant properties of medicinal plants used against fungal infections. J. Med. Plants Econ. Dev. 2024, 8, a214. [Google Scholar] [CrossRef]
- Sapkota, S.; Maharjan, A.; Tiwari, S.; Rajbhandari, M. Phytochemical analysis, antioxidant potential and antibacterial activities of different anatomical parts of Hypericum cordifolium Choisy. Sci. World J. 2024, 2024, 8128813. [Google Scholar] [CrossRef]
- Manyawi, M.; Mozirandi, W.Y.; Tagwireyi, D.; Mukanganyama, S. Fractionation and antibacterial evaluation of the surface compounds from the leaves of Combretum zeyheri on selected pathogenic bacteria. Sci. World J. 2023, 2023, 2322068. [Google Scholar] [CrossRef]
- Ho, Y.L.; Au, T.T.D.; Wu, H.Y.; Wu, K.C.; Chang, Y.S. Comparative study of Scleromitrion diffusum and Oldenlandia corymbosa: Microscopy, TLC, HPLC, and antioxidant activity. Microsc. Res. Tech. 2024, 87, 2371–2384. [Google Scholar] [CrossRef]
- Mubinov, A.R.; Kurkin, V.A.; Smirnova, E.A. Chemical composition and standardization of Nigella sativa L. herb. Pharm. Chem. J. 2023, 57, 842–846. [Google Scholar] [CrossRef]
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.; Nikolova, M.; Gavrilov, G. Phytochemical screening of Satureja kitaibelii Wierzb. ex Heuff. extracts by GC/MS and TLC. Farmacia 2023, 71, 91–96. [Google Scholar] [CrossRef]
- Santhose, B.I.; Adhikary, P.; Bharathi, S.S.; Kayali, A.; Sathishkumar, K.; Almutairi, B.O.; Gaurav, G.K.; Thanigaivel, S. In vitro screening and characterization of phytochemical products from Alstonia scholaris (Linn) and its bioactive potential for sustainable application. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Castillo-Mendoza, E.; Zamilpa, A.; González-Cortazar, M.; Ble-González, E.A.; Tovar-Sánchez, E. Chemical constituents and their production in Mexican oaks (Q. rugosa, Q. glabrescens and Q. obtusata). Plants 2022, 11, 2610. [Google Scholar] [CrossRef]
- Ismail, H.; Khalid, D.; Waseem, D.; Ijaz, M.U.; Dilshad, E.; Haq, I.U.; Bhatti, M.Z.; Anwaar, S.; Ahmed, M.; Saleem, S. Bioassays guided isolation of berberine from Berberis lycium and its neuroprotective role in aluminium chloride induced rat model of Alzheimer’s disease combined with in silico molecular docking. PLoS ONE 2023, 18, e0286349. [Google Scholar] [CrossRef]
- Momina, S.S.; Gandla, K. Flavonoid-rich Trianthema decandra ameliorates cognitive dysfunction in the hyperglycemic rats. Biochem. Genet. 2025, 63, 1400–1435. [Google Scholar] [CrossRef]
- Zhou, R.; Dzomba, P.; Gwatidzo, L. Chemical profiling of antifungal Dicerocaryum senecioides and Diospyros mespiliformis extracts using TLC-p-iodonitrotetrazolium violet assay and GC–MS/MS. Future J. Pharm. Sci. 2023, 9, 112. [Google Scholar] [CrossRef]
- Velmurugan, Y.; Natarajan, S.R.; Chakkarapani, N.; Jayaraman, S.; Madhukar, H.; Venkatachalam, R. In silico and in vitro studies for the identification of small molecular inhibitors from Euphorbia hirta Linn for rheumatoid arthritis: Targeting TNF-α-mediated inflammation. Mol. Divers. 2025, 29, 1189–1206. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I. Anticholinesterase activity of selected medicinal plants from Navarra region of Spain and a detailed phytochemical investigation of Origanum vulgare L. ssp. vulgare. Molecules 2022, 27, 7100. [Google Scholar] [CrossRef]
- Hechaichi, F.Z.; Bendif, H.; Bensouici, C.; Alsalamah, S.A.; Zaidi, B.; Bouhenna, M.M.; Souilah, N.; Alghonaim, M.I.; Benslama, A.; Medjekal, S.; et al. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of Centaurea parviflora Desf. extracts. Molecules 2023, 28, 2263. [Google Scholar] [CrossRef]
- Prajapati, P.; Maitreya, B.B.; Rawal, R.M. Qualitative and quantitative phytochemical screening and chemical fingerprint analysis of Conocarpus lancifolius plant using HPTLC. Vegetos 2024, 38, 1506–1514. [Google Scholar] [CrossRef]
- Solanki, P.; Abdul, A.P.J. Determination of berberine and quercetin in Tinospora cordifolia with the help of HPLC and TLC methods. NeuroQuantology 2022, 20, 5623–5629. [Google Scholar]
- Ganesan, R.; Mahesh, F.; Sneha, R.; Yuvaraj, K.; Aathithya, J.; Shakila, R.; Satheesh, D. In-vitro antidiabetic, hepatoprotective activities and HPTLC finger print profile of Azadirachta indica flower. Int. J. Ayurvedic Med. 2025, 16, 311–317. [Google Scholar] [CrossRef]
- Sameemabegum, S.; Prabha, T.; Sribhuvaneswari, S.; Sivakumar, T. Morphoanatomical, pharmacotaxonomical, physiochemical and phytochemical profiles, including TLC and HPTLC analysis of Ipomoea pes-tigridis L. Ann. Phytomed. 2023, 12, 882–891. [Google Scholar] [CrossRef]
- Reguigui, A.; Ott, P.G.; Darcsi, A.; Bakonyi, J.; Romdhane, M.; Móricz, Á.M. Nine-dimensional bioprofiles of Tunisian sages (Salvia officinalis, S. aegyptiaca and S. verbenaca) by high-performance thin-layer chromatography—Effect-directed analyses. J. Chromatogr. A 2023, 1688, 463704. [Google Scholar] [CrossRef]
- Darina, V.; Gegechkori, V.; Morton, D.W.; Agatonovic-Kustrin, S. The impact of spontaneous fermentation on phenolic and antioxidant profiles of selected aromatic plant extracts. J. Planar Chromatogr. Mod. TLC 2025, 38, 391–399. [Google Scholar] [CrossRef]
- Spangenberg, B.; Seigel, A.; Brämer, R. Screening of orange peel waste on valuable compounds by gradient multiple development diode-array high-performance thin-layer chromatography. J. Planar Chromatogr. Mod. TLC 2022, 35, 313–330. [Google Scholar] [CrossRef]
- Punitha, D.; Elansekaran, D.; Sudha Revathy, S.; Ramamurthy, M.; Srinivasan, V.; Gayatri, R.; Christian, G. Qualitative and quantitative analysis of siddha herbal Formulation Kabasura kudineer in various concentrations. Int. J. Ayurvedic Med. 2023, 14, 976–981. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, V.B.; Tiwari, R.C.; Gupta, O.P. Physico-chemical analysis of a herbal classical formulation- Shleshmatakadhya Agada Ghanavati. Ayushdhara 2022, 9, 55–63. [Google Scholar] [CrossRef]
- Mandal, A.K.; Ramachandran, S. Pharmacopoeial Standards for Venpucani Ilakam—A classical Siddha medicine. Int. J. Ayurvedic Med. 2023, 13, 939–943. [Google Scholar] [CrossRef]
- Paul, C.; Mariappan, A. Physiochemical and phytochemical analysis of Karanthai legium—Siddha herbomineral formulation. Int. J. Ayurvedic Med. 2024, 15, 564–569. [Google Scholar] [CrossRef]
- Deepa, P.; Nataraj, H.R.; Prajwal, H.N.; Anushree, C.G. Standardization of Dooshivishahari Agada through HPTLC. Int. J. Ayurvedic Med. 2022, 13, 479–482. [Google Scholar] [CrossRef]
- Swaminath, M.; Hiremath, R.S.; Mannur, V.S. Development and evaluation of lavangadi vati in the form of suspension—A polyherbal novel liquid dosage form. Int. J. Ayurvedic Med. 2024, 14, 1026–1032. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, Y.T.; Verma, N.; Mishra, A.K.; Mani, M.; Shukla, D.; Ahmad, S. Establishment of quality and safety markers for the identification of Amomum seed and Cinnamon leaf. Int. J. Ayurvedic Med. 2024, 15, 546–555. [Google Scholar] [CrossRef]
- Pratyusha, G.; Hiremath, R.S. Chemical profiling of Mandak—A novel polyherbal combination. Int. J. Ayurvedic Med. 2025, 15, 1012–1020. [Google Scholar] [CrossRef]
- Owolabi, T.; Amodu, E. Bioactive composition and TLC profile data on PAX herbal health tea and PAX herbal diatea. Int. J. Adv. Chem. 2022, 10, 46–49. [Google Scholar] [CrossRef]
- Owolabi, T.; Osaretin, D.; Eyinayan, B. Bioactive composition and TLC profile data on Pax Herbal Malatreat tea. Drug Anal. Res. 2022, 6, 35–39. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Liu, J.; Wang, Y.; Li, Z.; Jiang, B.; Lu, J.; Yang, S. An herbal formulation “Shenshuaifu Granule” alleviates cisplatin-induced nephrotoxicity by suppressing inflammation and apoptosis through inhibition of the TLR4/MyD88/NF-κB pathway. J. Ethnopharmacol. 2023, 306, 116168. [Google Scholar] [CrossRef]
- ul Haq, I.; Taj, R.; Nafees, M.; Hussain, A. Mycotoxin detection in selected medicinal plants using chromatographic techniques. Biomed. Chromatogr. 2024, 38, e5831. [Google Scholar] [CrossRef]
- Purohit, D.C.; Vadalia, J.; Joshi, H.; Vegad, U.G. Rapid screening of undeclared hypoglycemics in counterfeit herbal antidiabetic products using HPTLC-MS. J. Liq. Chromatogr. Relat. Technol. 2022, 45, 100–106. [Google Scholar] [CrossRef]
- Minh, D.T.C.; Tram, L.T.B.; Phong, N.H.; Huong, H.T.L.; Van Vu, L.; Thi, L.A.; Anh, N.T.K.; Ha, P.T.T. Single versus double coffee-ring effect patterns in thin-layer chromatography coupled with surface-enhanced Raman spectroscopic analysis of anti-diabetic drugs adulterated in herbal products. Molecules 2023, 28, 5492. [Google Scholar] [CrossRef]
- Mwankuna, C.J.; Mariki, E.E.; Mabiki, F.P.; Malebo, H.M.; Styrishave, B.; Mdegela, R.H. Thin layer chromatographic method for detection of conventional drug adulterants in herbal products. Separations 2023, 10, 23. [Google Scholar] [CrossRef]
- Dahiya, J.; Mangal, A.K.; Bolleddu, R.; Kumar, D.; Abdullah, S.; Prasad, S.B.; Dutta, S.; Mall, S.; Hazra, K.; Babu, G. HPTLC based marker and fingerprint analysis coupled with multivariate analysis of different parts of Cyanthillium cinereum from different geographical locations. Chromatographia 2025, 88, 95–106. [Google Scholar] [CrossRef]
- Pei, W.; Huang, Y.; Qu, Y.; Cui, X.; Zhou, L.; Yang, H.; Zhao, M.; Zhang, Z.; He, F.; Zhou, H. A strategy for quality evaluation of complex herbal preparations based on multi-color scale and efficacy-oriented high-performance thin-layer chromatography characteristic fingerprint combined with chemometric method: Sanwujiao Pills as an example. Heliyon 2023, 9, e22098. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Liang, Y.; Li, F.; Lin, N.; Jiang, L.; Lin, Q.; Chen, Q. Quality evaluation of kidney tea granules from different origins based on TLC, HPLC fingerprinting, and quantitative analysis combined with chemical pattern recognition. Phytochem. Anal. 2025, 36, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Kartika Dewi, B.A.A.S.; Kartini, K. System optimization and validation to improvethin-layer chromatography of roselle calyces (Hibiscus sabdariffa L.)required by the Indonesian Herbal Pharmacopoeia Edition II. J. Pharm. Pharmacogn. Res. 2023, 11, 243–254. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Wei, W.; Li, Z.; Zhang, J.; Yao, C.; Li, J.; Bi, Q.; Qu, H.; Pan, H.; et al. Species discrimination of multiple botanical origins of Fritillaria species based on infrared spectroscopy, thin layer chromatography-image analysis and untargeted metabolomics. Phytomedicine 2024, 123, 155228. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Szkolak, J.; Cwener, A.; Komsta, Ł. Novel hyperspectral analysis of thin-layer chromatographic plates—An application to fingerprinting of 70 Polish grasses. Molecules 2023, 28, 3745. [Google Scholar] [CrossRef]
- Gadowski, S.; Tomiczak, K.; Komsta, Ł. High dynamic range in videodensitometry—A comparative study to classic videoscanning on Gentiana extracts. J. Planar Chromatogr. Mod. TLC 2023, 36, 3–8. [Google Scholar] [CrossRef]
- Tandel, J.N.; Chhalotiya, U.; Kachhiya, H.; Tandel, D. Advanced thin-layer chromatography–mass spectrometry validation and comprehensive analysis of bioactive phytochemicals in Caesalpinia bonduc leaf extract. J. Planar Chromatogr. Mod. TLC 2025, 38, 83–93. [Google Scholar] [CrossRef]
- Vasquez-Delgado, J.S.; Vivas-Moncayo, J.E.; Lopez-Cortes, J.V.; Combariza, M.Y.; Montoya, G. Pharmacokinetic assessment and phytochemical triterpene control from Cecropia angustifolia using plant biotechnology. Phytochem. Anal. 2023, 34, 641–651. [Google Scholar] [CrossRef]
- Samal, M.; Siddiqui, A.; Srivastava, V.; Dar, M.I.; Khan, M.; Insaf, A.; Ansari, S.H.; Ahmad, S. Identification of acetylcholinesterase inhibitory metabolites from hydroalcoholic extract of Itrifal Muqawwi Dimagh using thin-layer chromatography–bioautography–mass spectroscopy and its validation using in silico molecular approach. J. Planar Chromatogr. Mod. TLC 2024, 37, 271–282. [Google Scholar] [CrossRef]
- Kumari, S.; Pattnaik, A.K. Unraveling the anti-obesity potential of Haldina cordifolia bioactive fractions in 3T3-L1 adipocytes differentiation: In vitro, high-performance thin-layer chromatography–multistage mass spectrometry and in silico studies. J. Planar Chromatogr. Mod. TLC 2024, 37, 283–297. [Google Scholar] [CrossRef]
- Glavnik, V.; Bensa, M.; Vovk, I.; Guzelmeric, E. High-performance thin-layer chromatography–multi-stage mass spectrometry methods for analyses of bee pollen botanically originating from sweet chestnut (Castanea sativa Mill.). J. Planar Chromatogr. Mod. TLC 2023, 36, 471–482. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Chen, W.; van Vuuren, S.; Combrinck, S.; Viljoen, A.M. Bioautography-guided HPTLC–MS as a rapid hyphenated technique for the identification of antimicrobial compounds from selected South African Combretaceae species. Phytochem. Anal. 2022, 33, 1177–1189. [Google Scholar] [CrossRef]
- Tandon, D.; Gupta, A.K. Bioautography, synergistic effect and HPTLC-MS and SEM analysis of antimicrobial and antioxidant compounds of inflorescence extract of Sphaeranthus indicus. Future J. Pharm. Sci. 2023, 9, 72. [Google Scholar] [CrossRef]
- Sanguansermsri, D.; Sanguansermsri, P.; Buaban, K.; Choommongkol, V.; Akekawatchai, C.; Charoensri, N.; Fraser, I.; Wongkattiya, N. Antibacterial activity of Dioscorea bulbifera Linn. extract and its active component flavanthrinin against skin-associated bacteria. BMC Complement. Med. Ther. 2024, 24, 180. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, X.; Yuan, G.; Zhang, T.; Deng, B.; Feng, X.; Wang, Q. Stachydrine, a bioactive equilibrist for synephrine, identified from four Citrus Chinese herbs. Molecules 2023, 28, 3813. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Wong, S.; Dolzhenko, A.V.; Gegechkori, V.; Ku, H.; Tucci, J.; Morton, D.W. Evaluation of bioactive compounds from Ficus carica L. leaf extracts via high-performance thin-layer chromatography combined with effect-directed analysis. J. Chromatogr. A 2023, 1706, 464241. [Google Scholar] [CrossRef]
- Nikolaichuk, H.; Studziński, M.; Stankevič, M.; Choma, I.M. Qualitative and quantitative evaluation of rosavin, salidroside, and p-tyrosol in artic root products via TLC-screening, HPLC-DAD, and NMR spectroscopy. Molecules 2022, 27, 8299. [Google Scholar] [CrossRef]
- Zych, M.; Leopold, K.; Pyka-Pająk, A. Determination of caffeine, trigonelline and chlorogenic acid by high-performance thin-layer chromatography in coffee infusions and study of the effect of these infusions on the α-amylase activity. Farm. Pol. 2023, 79, 651–663. [Google Scholar] [CrossRef]
- Parihar, S.; Saxena, H.O.; Pawar, G.; Ginwal, H.S. Validated high performance thin layer chromatographic method for simultaneous quantification of betulinic acid, β-sitosterol and lupeol in fruits, leaves, root bark and stem bark of Dillenia indica Linn. Acta Chromatogr. 2025, 37, 183–193. [Google Scholar] [CrossRef]
- Parihar, S.; Saxena, H.O.; Pawar, G.; Ginwal, H.S.; Singh, N. A validated thin-layer chromatography method for the concurrent determination of β-sitosterol and lupeol in Cassia fistula L.—An important species of Ayurveda. J. Planar Chromatogr. Mod. TLC 2025, 38, 25–36. [Google Scholar] [CrossRef]
- Saxena, H.O.; Parihar, S.; Pawar, G.; Rao, G.R. Simultaneous densitometric determination of β-sitosterol and lupeol through validated HPTLC method in different plant parts of Uraria picta (Jacq.) Desv. ex DC.—A dashmool species. Acta Chromatogr. 2023, 35, 99–105. [Google Scholar] [CrossRef]
- Sharma, H.; Mishra, S.K.; Khan, R.; Prasad, S.B.; Narasimhaji, C.V.; Srikanth, N.; Acharya, R. A validated phytochemical marker based HPTLC method for the segregation of Bauhinia vahlii Wight and Arn. from geologically different samples of Indian zones. J. Planar Chromatogr. Mod. TLC 2024, 37, 331–343. [Google Scholar] [CrossRef]
- Patel, H.; Chhalotiya, U.; Tandel, J. Simultaneous estimation of biomarkers in hydroalcoholic tuber extract of Amorphophallus paeoniifolius by a validated instrumental thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2024, 37, 521–531. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Sharma, V. Simultaneous high-performance thin-layer chromatography analysis of phytoconstituents and antioxidant potential of Inula grandiflora Willd. from India. J. Planar Chromatogr. Mod. TLC 2022, 35, 609–616. [Google Scholar] [CrossRef]
- Ingole, S.; Ghule, B.; Patil, K.; Takale, N. Simultaneous estimation of lupeol, stigmasterol and betulin in Desmodium oojeinensis bark and roots by a validated instrumental thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2024, 37, 207–218. [Google Scholar] [CrossRef]
- Ravindrakumar, P.; Vyas, N.; Sandip, P. Urolithiasis: HPTLC method for quantitative detection of rutin and quercetin in an herbal plant. J. Nat. Remedies 2022, 22, 371–379. [Google Scholar] [CrossRef]
- Pathak, K.; Das, R.J.; Gogoi, N.; Saikia, R.; Sarma, H.; Das, A. A validated high-performance thin-layer chromatography method for the simultaneous determination of quercetin and gallic acid in Annona reticulata L. J. Planar Chromatogr. Mod. TLC 2022, 35, 35–41. [Google Scholar] [CrossRef]
- Jain, D.; Upadhyay, R.; Jain, S.; Prakash, A.; Janmeda, P. TLC and HPTLC finger printing analysis of Cyperus rotundus (Linn.). Lett. Appl. NanoBioSci. 2022, 11, 3861–3870. [Google Scholar] [CrossRef]
- Sharma, S.; Modi, K.; Shah, M. Development and validation of high-performance thin-layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC) methods for the simultaneous determination of myricetin and quercetin in Manilkara hexandra. J. Planar Chromatogr. Mod. TLC 2024, 37, 511–519. [Google Scholar] [CrossRef]
- Bidikar, C.M.; Hurkadale, P.J.; Nandanwadkar, S.M.; Hegde, H.V. A validated spectro densitometric regulatory compliant USP-HP-TLC protocol for quantification of polyphenols and antioxidants from polyherbal formulations containing Terminalia species. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1207, 123379. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.P.; Vora, A.; Kaur, G.; Akhtar, J.; Kumar, P. Simultaneous estimation of (α + β) boswellic acids, β-asarone, isoeugenol, 6-gingerol, and piperine in Majun Nisyan by high-performance thin-layer chromatography. J. Planar Chromatogr. Mod. TLC 2024, 37, 129–136. [Google Scholar] [CrossRef]
- Balekundri, A.R.; Mannur, V.K.S.; Chouhan, M.K. A Simple and validated HP-TLC method for simultaneous analysis of ethno-medicine gallic acid and eugenol. Indian Drugs 2022, 59, 82–87. [Google Scholar] [CrossRef]
- Shanmugam, M.; Subramanian, S.; Ramachandran, S. Method development and validation for quantification of six bioactive compounds (andrographolide, columbin, piperine, gallic, paracoumaric and oleanolic acids) by HPTLC. J. Complement. Integr. Med. 2023, 20, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kagathara, C.; Odedra, K.; Vadia, N. Development of HPTLC method for the simultaneous estimation of quercetin, curcumin, and ascorbic acid in herbal formulations. J. Iran. Chem. Soc. 2022, 19, 4129–4138. [Google Scholar] [CrossRef]
- Narigara, P.; Thummar, K.; Vegad, U.; Chauhan, S.; Vadalia, J. Quantification of the main constituents of “sitopaladi churna—Ayurvedic multi-herbal preparation” using a validated high-performance thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2024, 37, 119–127. [Google Scholar] [CrossRef]
- Pawar, H.; Ghule, B.; Sahu, A.; Takale, N.; Kotagale, N. High-performance thin-layer chromatography method development and validation for quantification of rutin in different parts of Capparis zeylanica Linn. plant. J. Planar Chromatogr. Mod. TLC 2024, 37, 137–149. [Google Scholar] [CrossRef]
- Ahmad, S.; Mujawar, T.; Batewal, B.; More, P.; Gaikwad, A.; Chumbhale, D.; Tare, H. RP-UHPLC and HPTLC method development and validation for analysis of andrographolide from herbal hepatoprotective formulation. Int. J. Pharm. Qual. Assur. 2023, 14, 96–104. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Ahmed, R.; Mishra, K.; Sandbhor, R.; Sharma, R.J.; Kaki, V.R. A validated, precise TLC-densitometry method for simultaneous quantification of mahanimbine and koenimbine in marketed herbal formulations. Future J. Pharm. Sci. 2024, 10, 23. [Google Scholar] [CrossRef]
- Varma, V.R.; Gupta, A.A.; Dhande, S.R. A novel validated instrumental thin-layer chromatographic method and marker-based standardization of liquid herbal formulation using atropine, rutin and vanillin as biomarkers. J. Planar Chromatogr. Mod. TLC 2024, 37, 379–385. [Google Scholar] [CrossRef]
- Panseriya, N.; Mohan Maruga Raja, M.K. Simultaneous quantification of curcumin, piperine and capsaicin by HPTLC in Rasam, a polyherbal soup. Int. J. Ayurvedic Med. 2022, 13, 647–650. [Google Scholar] [CrossRef]
- Sharma, A.; Mohan Maruga Raja, M.K. A HPTLC method for the quantitative determination of piperine and capsaicin in Rasam, A South Indian spice soup. Int. J. Ayurvedic Med. 2022, 13, 483–486. [Google Scholar] [CrossRef]
- Elbaz, G.A.; Zaazaa, H.E.; Monir, H.H.; Abd El Halim, L.M. Chitosan nanoparticles modified TLC-densitometry for determination of imidacloprid and deltamethrin residues in plants: Greenness assessment. BMC Chem. 2023, 17, 29. [Google Scholar] [CrossRef]
- Saxena, H.O.; Parihar, S.; Pawar, G.; Sahu, V.R. High-performance thin-layer chromatography method development and validation for quantification of glucuronic acid in gum samples of Sterculia urens Roxb. J. Planar Chromatogr. Mod. TLC 2022, 35, 153–159. [Google Scholar] [CrossRef]
- Kartini, K.; Ariyani, V.M.; Ang, W.; Aini, Q.; Jayani, N.I.E.; Oktaviyanti, N.D.; Setiawan, F.; Azminah, A. A validated TLC-densitometric analysis of curcumin in eight important Zingiberaceae rhizomes and their ATR-FTIR fingerprint profiles. Food Anal. Methods 2025, 18, 717–731. [Google Scholar] [CrossRef]
- Jadaun, V.; Prateeksha, P.; Nailwal, T.; Singh, B.N. Antioxidant activity and simultaneous estimation of four polyphenolics in different parts of Carica papaya L. by a validated high-performance thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2023, 36, 211–221. [Google Scholar] [CrossRef]
- Yang, F.; Kim, M.; Gu, L.; Li, L.; Yang, L.; Wang, Z. Stimulation quantification of four natural lipase inhibitors from Alismatis Rhizoma by high-performance thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2022, 35, 3–12. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Kar, A.; Bhardwaj, P.K.; Sharma, N.; Devi, S.I.; Mukherjee, P.K. A validated high-performance thin-layer chromatography method for the quantification of chlorogenic acid in the hydroalcoholic extract of Gynura cusimbua leaves. J. Planar Chromatogr. Mod. TLC 2023, 36, 45–53. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Lalvenhimi, S.; Biswas, S.; Chanda, J.; Kar, A.; Bhardwaj, P.K.; Sharma, N.; Mukherjee, P.K. High-performance thin-layer chromatography (HPTLC) method development and validation for the quantification of catechin in the hydroalcoholic extract of Parkia roxburghii seed. J. Planar Chromatogr. Mod. TLC 2022, 35, 161–167. [Google Scholar] [CrossRef]
- Pawar, S.C.; Metkari, D.D.; Jadhav, A.P.; Jagdale, D.; Khanvilkar, V.V.; Gavali, R.D. Validated stability-indicating instrumental thin-layer chromatography method for the quantification of alizarin from Ayurvedic formulations. J. Planar Chromatogr. Mod. TLC 2024, 37, 463–470. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Mishra, K.; Ahmed, R.; Sandbhor, R.; Sharma, R.J.; Kaki, V.R. Determination of mahanimbine from Murraya koenigii, collected from different geographical regions of India, by TLC-densitometry. J. Anal. Chem. 2024, 79, 1121–1131. [Google Scholar] [CrossRef]
- Nagy-Turák, A.; Végh, Z.; Ferenczi-Fodor, K.V. Validation of the quantitative planar chromatographic analysis of drug substances.III. Robustness testing in OPLC. J. Planar Chromatogr. Mod. TLC 1995, 8, 188–193. [Google Scholar]
- Takale, N.; Kothawale, T.; Ghule, B.; Kotagale, N. Isolation, identification, and quantification of stigmasterol in Hygrophila schulli plant by a validated high-performance thin-layer chromatography–densitometric method. J. Planar Chromatogr. Mod. TLC 2023, 36, 223–235. [Google Scholar] [CrossRef]
- Rout, K.K.; Kar, M.K.; Agarwal, P.C.; Dash, S.K. Analysis of bioactive hispidulin: An anticancer flavone of Clerodendrum philippinum. J. Planar Chromatogr. Mod. TLC 2024, 37, 49–56. [Google Scholar] [CrossRef]
- Tatkare, P.C.; Jadhav, A.P. Development and validation of a novel high-performance thin-layer chromatography method for the quantitative estimation of neohesperidin from Citrus aurantium peel extract. J. Planar Chromatogr. Mod. TLC 2022, 35, 579–584. [Google Scholar] [CrossRef]
- Ravat, F.; Prajapati, D.; Goswami, J.; Dudhatra, B.; Vadalia, J.; Chauhan, S.; Thummar, K. Phytochemical analysis, isolation and quantitative estimation of karanjin in the stem bark of Millettia pinnata by a validated high-performance thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2024, 37, 11–20. [Google Scholar] [CrossRef]
- Zhang, C.; Mamattursun, A.; Ma, X.; Pang, T.; Wu, Y.; Ma, X. High-performance thin-layer chromatography and high-performance liquid chromatography determination of two anthocyanins in medicine mulberry. J. Planar Chromatogr. Mod. TLC 2024, 37, 345–355. [Google Scholar] [CrossRef]
- Tripathi, D.; Chaudhary, M.K.; Misra, A.; Srivastava, M.; Srivastava, S. High-performance thin-layer chromatography-guided chemotaxonomic studies of pharmacologically active steroidal alkaloids in Solanum xanthocarpum Schrad. & Wendl. collected from Central India. J. Planar Chromatogr. Mod. TLC 2025, 38, 95–103. [Google Scholar] [CrossRef]
- Saçıcı, E.; Yesilada, E. Development of new and validated HPTLC methods for the qualitative and quantitative analysis of hyperforin, hypericin and hyperoside contents in Hypericum species. Phytochem. Anal. 2022, 33, 355–364. [Google Scholar] [CrossRef]
- Sareen, A.; Mawal, P.; Gupta, R.C.; Bansal, G. Estimation of betulinic acid from wild fruit extracts of Ziziphus mauritiana and Ziziphus nummularia from different regions of North India by a validated high-performance thin-layer chromatography method. J. Planar Chromatogr. Mod. TLC 2022, 35, 585–591. [Google Scholar] [CrossRef]
- Kartini, K.; Wijayati, A.S.; Jayani, N.I.E.; Setiawan, F.; Budiono, R. Straightforward thin-layer chromatography–densitometric method for the determination of phyllanthin in Phyllanthus niruri from different phytogeographical zones. J. Planar Chromatogr. Mod. TLC 2024, 37, 1–10. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, G.; Banaszek, K.; Gnat, S.; Waksmundzka-Hajnos, M. Planar chromatography of bactericidal active fractions of extracts obtained from selected varieties of hops. J. Planar Chromatogr. Mod. TLC 2022, 35, 331–337. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Bhujbal, S.S.; Chawale, B.G.; Kale, M.A. Application based studies of HPTLC-bioautography in evaluation of botanicals: A review. J. Anal. Chem. 2022, 77, 473–483. [Google Scholar] [CrossRef]
- He, C.K.; Hung, M.C.; Hxu, C.H.; Hsieh, Y.H.; Lin, Y.S. Pitfalls in measuring solution toxicity using the level of bioluminescence inhibition in Aliivibrio fischeri. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 287, 110067. [Google Scholar] [CrossRef] [PubMed]
- Nikolaichuk, H.; Choma, I.M.; Morlock, G.E. Bioactivity profiles on 15 different effect mechanisms for 15 golden root products via high-performance thin-layer chromatography, planar assays, and high-resolution mass spectrometry. Molecules 2023, 28, 1535. [Google Scholar] [CrossRef]
- Adegun, A.A.; Adesegun, S.A.; Usman, A.R.; Odukoya, O.A. Thin layer chromatography bio-autography guided identification of antibacterial constituents of leaf extract of Stereospermum kunthianum Cham. (Bignoniaceae). Niger. J. Pharm. 2023, 57, 582–591. [Google Scholar] [CrossRef]
- Ambarwati, N.; Elya, B.; Malik, A.; Omar, H.; Hanafi, M.; Ahmad, I. New robustaflavone from Garcinia latissima Miq. leave and its antibacterial activity. J. Adv. Pharm. Technol. Res. 2022, 13, 50–55. [Google Scholar] [CrossRef]
- Jankov, M.S.; Milojković Opsenica, D.M.; Trifković, J.; Janaćković, P.T.; Ristivojević, P.M. Antibacterial profiling of Sempervivum tectorum L. (common houseleek) leaves extracts using high-performance thin-layer chromatography coupled with chemometrics. J. Planar Chromatogr. Mod. TLC 2023, 36, 521–528. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Lv, L.; Qiao, S.; Chen, M.; Song, H. Guided strategy for the detection of phthalides with antimicrobial and antioxidant activities from Ligusticum chuanxiong essential oil. Phytochem. Anal. 2025, 36, 1130–1140. [Google Scholar] [CrossRef]
- Wen, W.; Xiang, H.; Qiu, H.; Chen, J.; Ye, X.; Wu, L.; Chen, Z.; Tong, S. Screening and identification of antibacterial components in Artemisia argyi essential oil by TLC–direct bioautography combined with comprehensive 2D GC × GC-TOFMS. J. Chromatogr. B 2024, 1234, 124026. [Google Scholar] [CrossRef]
- Bakó, C.; Balázs, V.L.; Kerekes, E.; Kocsis, B.; Nagy, D.U.; Szabó, P.; Micalizzi, G.; Mondello, L.; Krisch, J.; Pethő, D.; et al. Flowering phenophases influence the antibacterial and anti-biofilm effects of Thymus vulgaris L. essential oil. BMC Complement. Med. Ther. 2023, 23, 168. [Google Scholar] [CrossRef] [PubMed]
- Jović, M.D.; Agatonovic-Kustrin, S.; Ristivojević, P.M.; Trifković, J.D.; Morton, D.W. Bioassay-guided assessment of antioxidative, anti-inflammatory and antimicrobial activities of extracts from medicinal plants via high-performance thin-layer chromatography. Molecules 2023, 28, 7346. [Google Scholar] [CrossRef]
- Nikolaichuk, H.; Choma, I.M.; Morlock, G.E. Effect-directed profiling of Akebia quinata and Clitoria ternatea via high-performance thin-layer chromatography, planar assays and high-resolution mass spectrometry. Molecules 2023, 28, 2893. [Google Scholar] [CrossRef]
- Sobstyl, E.; Szopa, A.; Dziurka, M.; Ekiert, H.; Nikolaichuk, H.; Choma, I.M. Schisandra rubriflora fruit and leaves as promising new materials of high biological potential: Lignan profiling and effect-directed analysis. Molecules 2022, 27, 2116. [Google Scholar] [CrossRef]
- Sobstyl, E.; Szopa, A.; Olszowy-Tomczyk, M.; Gnat, S.; Jafernik, K.; Choma, I.M. Chromatographic and biological screening of chosen species of Schisandraceae Family: Schisandra chinensis, S. rubriflora, S. sphenanthera, S. henryi and Kadsura japonica. Chem. Biodivers. 2023, 20, e202300741. [Google Scholar] [CrossRef]
- Oresanya, I.O.; Orhan, I.E.; Heil, J.; Morlock, G.E. African under-utilized medicinal leafy vegetables studied by microtiter plate assays and high-performance thin-layer chromatography-planar assays. Molecules 2024, 29, 733. [Google Scholar] [CrossRef]
- Inarejos-Garcia, A.M.; Heil, J.; Martorell, P.; Álvarez, B.; Llopis, S.; Helbig, I.; Liu, J.; Quebbeman, B.; Nemeth, T.; Holmgren, D.; et al. Effect-directed, chemical and taxonomic profiling of peppermint proprietary varieties and corresponding leaf extracts. Antioxidants 2023, 12, 476. [Google Scholar] [CrossRef]
- Hilaire, V.; Michel, G.; Majoor, A.; Hadji-Minaglou, F.; Landreau, A.; Fernandez, X. New method for screening anti-Leishmania compounds in plants extracts by HPTLC-bioautography. J. Chromatogr. B 2022, 1188, 123061. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, Y.; Han, Y.; Yang, L.; Wang, Z. A TLC-direct bioassay method for detection of anti-lipid peroxidation constituents from fruits of Perilla frutescens. LWT Food Sci. Technol. 2023, 182, 114779. [Google Scholar] [CrossRef]
- Kowalska, T.; Sajewicz, M. Thin-layer chromatography (TLC) in the screening of botanicals–its versatile potential and selected applications. Molecules 2022, 27, 6607. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.I.; Dabhi, R.C.; Trivedi, P.G.; Thakar, M.S.; Maru, J.J.; Sindhav, G.M. Isolation and characterization of undescribed flavonoid from Abrus precatorius L. based on HPTLC-DPPH bioautography and its cytotoxicity evaluation. Future J. Pharm. Sci. 2023, 9, 119. [Google Scholar] [CrossRef]
- Gahtori, R.; Tripathi, A.H.; Chand, G.; Pande, A.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Phytochemical screening of Nyctanthes arbor-tristis plant extracts and their antioxidant and antibacterial activity analysis. Appl. Biochem. Biotechnol. 2024, 196, 436–456. [Google Scholar] [CrossRef]
- Irfan Dar, M.; Qureshi, M.I.; Zahiruddin, S.; Abass, S.; Jan, B.; Sultan, A.; Ahmad, S. In silico analysis of PTP1B inhibitors and TLC-MS bioautography-based identification of free radical scavenging and α-amylase inhibitory compounds from heartwood extract of Pterocarpus marsupium. ACS Omega 2022, 7, 46156–46173. [Google Scholar] [CrossRef]
- Jajo, H.; Baishya, T.; Das, P.; Ashraf, G.J.; Dua, T.K.; Paul, P.; Nandi, G.; Sahu, R. GC-MS and HPTLC bioautography-based phytochemical profiling and evaluation of biological activity Neptunia prostrata Linn whole plant and leaves. Pharmacol. Res. Nat. Prod. 2024, 2, 100013. [Google Scholar] [CrossRef]
- Jan, B.; Zahiruddin, S.; Basist, P.; Irfan, M.; Abass, S.; Ahmad, S. Metabolomic profiling and identification of antioxidant and antidiabetic compounds from leaves of different varieties of Morus alba Linn grown in Kashmir. ACS Omega 2022, 7, 24317–24328. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Isidorov, V.A.; Krauze-Baranowska, M. Characterization of secondary metabolites of leaf buds from some species and hybrids of Populus by gas chromatography coupled with mass detection and two-dimensional high-performance thin-layer chromatography methods with assessment of their antioxidant. Int. J. Mol. Sci. 2024, 25, 3971. [Google Scholar] [CrossRef]
- Sen, N.B.; Guzelmeric, E.; Vovk, I.; Glavnik, V.; Kırmızıbekmez, H.; Yesilada, E. Phytochemical and bioactivity studies on Hedera helix L. (Ivy) flower pollen and ivy bee pollen. Antioxidants 2023, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, S.; Sarmamy, A.O.I.; Mirzajani, F. Inhibitory effects of Marrubium vulgare L. Extract on the female hormones based on bioautography -HPTLC-MS. Iran. J. Pharm. Res. 2024, 23, e148259. [Google Scholar] [CrossRef] [PubMed]
- Baumli, J.; Mărincean, A.I.; Cimpoiu, C. Scanning of chicoric acid in different parts of Cichorium intybus by high-performance thin-layer chromatography with quantitation by image analysis. J. Planar Chromatogr. Mod. TLC 2024, 37, 491–497. [Google Scholar] [CrossRef]
- Karavuş, Ş.N.; Çaşkurlu, A.; Karadağ, A.E.; Demirci, F. Bioautography for evaluation of several Lavandula L. and Origanum species antimicrobial and antioxidant activity. Acta Pharm. Sci. 2023, 61, 141–151. [Google Scholar] [CrossRef]
- Urbain, A.; Trabelssi, N.; Bardot, V. Development of an enzyme-based thin-layer chromatographic assay for the detection of cyclooxygenase-2 inhibitors. Separations 2022, 9, 238. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. The TLC-bioautography as a tool for rapid enzyme inhibitors detection—A review. Crit. Rev. Anal. Chem. 2022, 52, 275–293. [Google Scholar] [CrossRef]
- Cabezudo, I.; Salazar, M.O.; Ramallo, I.A.; Furlan, R.L.E. Effect-directed analysis in food by thin-layer chromatography assays. Food Chem. 2022, 390, 132937. [Google Scholar] [CrossRef] [PubMed]
- Galarce-Bustos, O.; Obregon, C.; Vallejos-Almirall, A.; Folch, C.; Acevedo, F. Application of effect-directed analysis using TLC—Bioautography for rapid isolation and identification of antidiabetic compounds from the leaves of Annona cherimola Mill. Phytochem. Anal. 2023, 34, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Anwar, N.; Zahiruddin, S.; Ahmad, S. TLC-bioautography-MS-based Identification of Antioxidant, α-Amylase and α-Glucosidase Inhibitory Compounds in a Polyherbal Formulation “Sugreen-120”. Pharmacogn. Mag. 2023, 19, 254–268. [Google Scholar] [CrossRef]
- Hua, X.; Hong, H.J.; Zhang, D.Y.; Liu, Q.; Leong, F.; Yang, Q.; Hu, Y.J.; Chen, X.J. Rapid screening of lipase inhibitors from Ophiopogonis radix using high-performance thin layer chromatography by two step gradient elution combined with bioautographic method. Molecules 2022, 27, 1155. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.; Fernandes, D.C.; Danuello, A.; Regasini, L.O.; Cardoso-Lopes, E.M.; Young, M.C.M.; Brandão Torres, L.M.; Campos, V.P.; Silva, D.H.S.; da Silva Bolzani, V.; et al. Nematostatic activity of isoprenylated guanidine alkaloids from Pterogyne nitens and their interaction with acetylcholinesterase. Exp. Parasitol. 2023, 250, 108542. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Zgórka, G. In-depth phytochemical and biological studies on potential AChE inhibitors in red and zigzag clover dry extracts using reversed–phase liquid chromatography (RP-LC) coupled with photodiode array (PDA) and electron spray ionization-quadrupole/time of flight. Food Chem. 2022, 375, 131846. [Google Scholar] [CrossRef]
- Nagar, S.; Pigott, M.; Kukula-Koch, W.; Sheridan, H. Unravelling novel phytochemicals and anticholinesterase activity in Irish Cladonia portentosa. Molecules 2023, 28, 4145. [Google Scholar] [CrossRef]
- Harahap, A.; Triamarta, S.; Kharisma, D.; Hanifah, W.; Iqbal, M.; Arifa, N.; Ismed, F. Evaluation of the anti-tyrosinase-anti-aging potential and metabolite profiling from the bioactive fraction of corn cob (Zea mays L.). Int. J. Appl. Pharm. 2024, 16, 71–76. [Google Scholar] [CrossRef]
- Insaf, A.; Parveen, R.; Srivastava, V.; Samal, M.; Khan, M.; Ahmad, S. TLC—MS-bioautographic identification of antityrosinase compounds and preparation of a topical gel formulation from a bioactive fraction of an RSM-optimized alcoholic extract of Rubia cordifolia L. stem. J. AOAC Int. 2023, 106, 1598–1607. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and evaluation of dermo-cosmetic activities of the invasive plant species Polygonum cuspidatum. Plants 2023, 12, 83. [Google Scholar] [CrossRef]
- Gąsowska-Bajger, B.; Wojtasek, H. Oxidation of baicalein by tyrosinase and by o-quinones. Int. J. Biol. Macromol. 2023, 231, 123317. [Google Scholar] [CrossRef]
- Dawood, H.M.; Shawky, E.; Hammoda, H.M.; Metwally, A.M.; Ibrahim, R.S. Development of a validated HPTLC-bioautographic method for evaluation of aromatase inhibitory activity of plant extracts and their constituents. Phytochem. Anal. 2022, 33, 115–126. [Google Scholar] [CrossRef]
- Oyarzún, P.; Carrasco, J.; Peterssen, D.; Tereucan, G.; Aranda, M.; Henríquez-Aedo, K. A high throughput method for detection of cyclooxygenase-2 enzyme inhibitors by effect-directed analysis applying high performance thin layer chromatography-bioassay-mass spectrometry. J. Chromatogr. A 2023, 1711, 464426. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Ronzheimer, A.; Friz, M.; Morlock, G.E. Multiplex planar bioassay with reduced diffusion on normal phase, identifying androgens, verified antiandrogens and synergists in botanicals via 12D hyphenation. Food Chem. 2022, 395, 133610. [Google Scholar] [CrossRef] [PubMed]
- Ronzheimer, A.; Schreiner, T.; Morlock, G.E. Multiplex planar bioassay detecting estrogens, antiestrogens, false-positives and synergists as sharp zones on normal phase. Phytomedicine 2022, 103, 154230. [Google Scholar] [CrossRef]
- Morlock, G.E.; Meyer, D. Designed genotoxicity profiling detects genotoxic compounds in staple food such as healthy oils. Food Chem. 2023, 408, 135253. [Google Scholar] [CrossRef] [PubMed]
- Windisch, M.; Kittinger, C.; Heil, J.; Morlock, G.E. Simple performance of the planar SOS-Umu-C–FLD genotoxicity bioassay shown for perfume and packaging material analysis. J. Planar Chromatogr. Mod. TLC 2023, 36, 513–520. [Google Scholar] [CrossRef]
- Schmidtmann, K.; Lemme, J.; Morlock, G.E. Ames assay transferred from the microtiter plate to the planar assay format. J. Xenobiotics 2025, 15, 67. [Google Scholar] [CrossRef]
- Mügge, F.L.B.; Morlock, G.E. Planar bioluminescent cytotoxicity assay via genetically modified adherent human reporter cell lines, applied to authenticity screening of Saussurea costus root. J. Chromatogr. A 2022, 1683, 463522. [Google Scholar] [CrossRef]
- Mügge, F.L.B.; Morlock, G.E. Chemical and cytotoxicity profiles of 11 pink pepper (Schinus spp.) samples via non-targeted hyphenated high-performance thin-layer chromatography. Metabolomics 2023, 19, 48. [Google Scholar] [CrossRef] [PubMed]
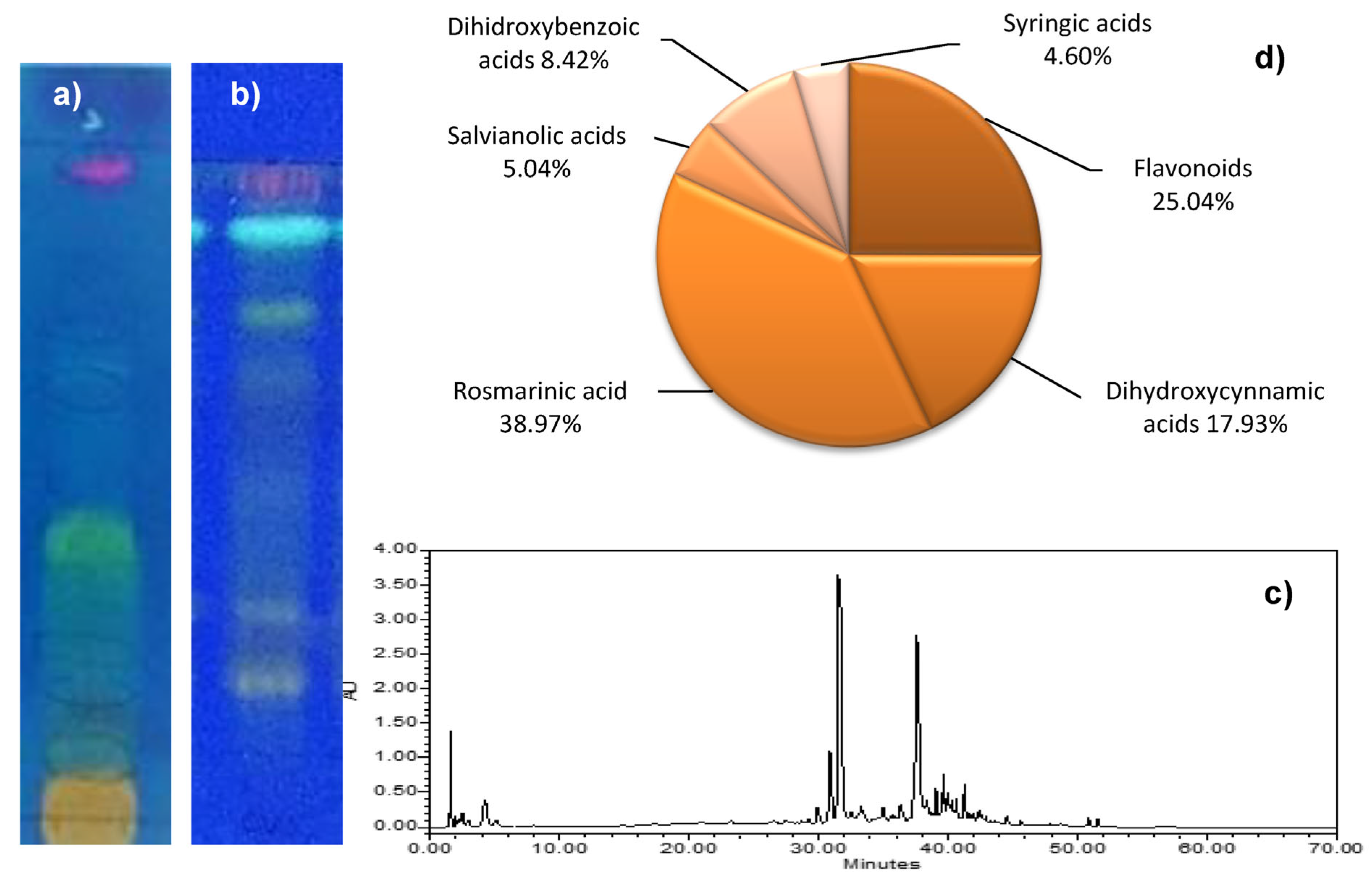

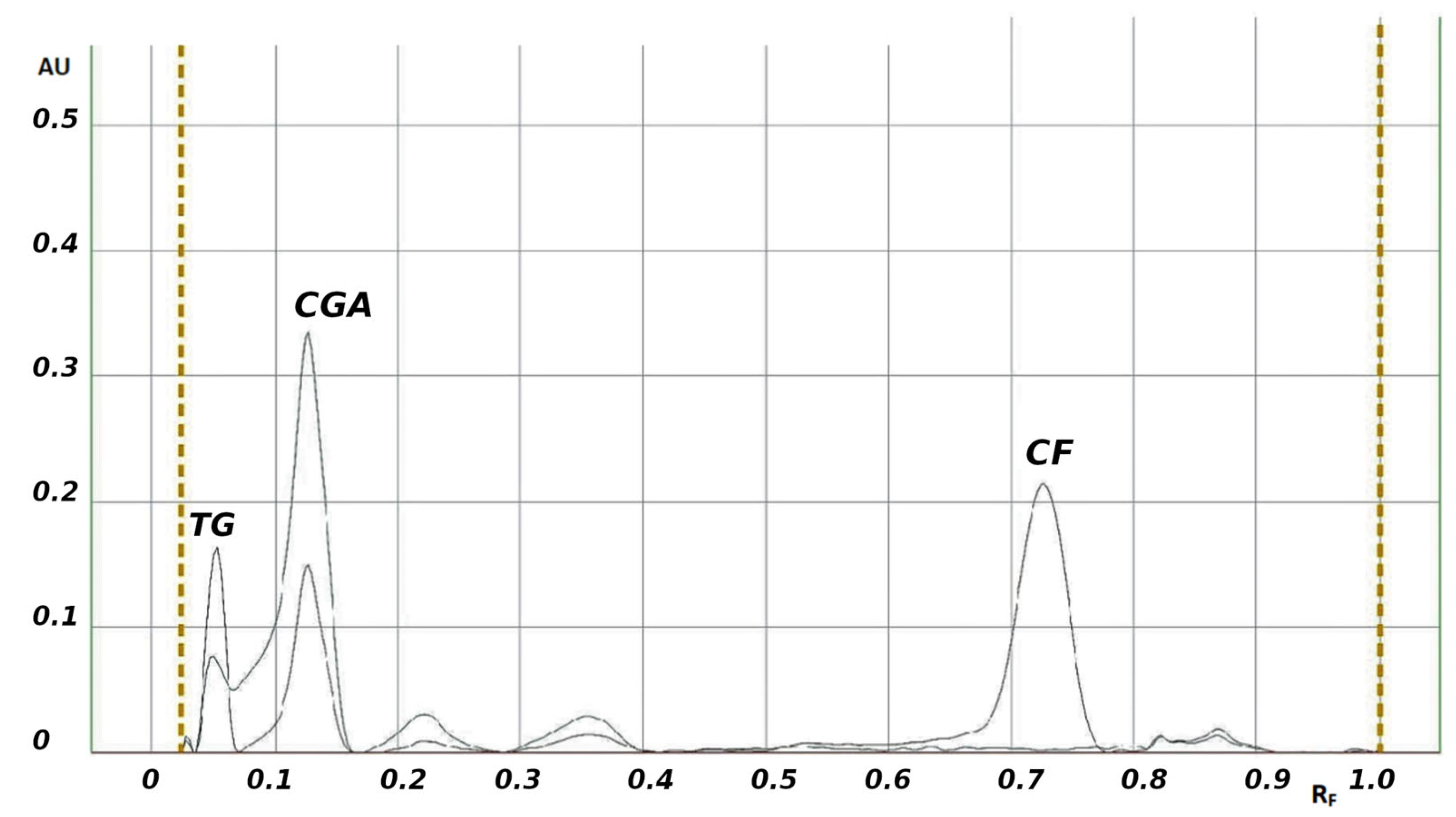
| Matrix/Plant Materials | Microorganism/ Microorganisms | Bioautography Method/Way of Visualization | Refs. |
|---|---|---|---|
| Leaf of Stereospermum kunthianum Cham. | Bacillus subtilis *, Staphylococcus aureus *, Salmonella typhi *, Pseudomonas aeruginosa *, Aspergillus niger **, Candida albicans ** | Overlay bioautography/ MTT | [147] |
| Leaf of Garcinia latissima Miq. | Bacillus subtilis * | Contact bioautography | [148] |
| Origanum vulgare L. | Bacillus subtilis *, Micrococcus lysodeikticus *, Escherichia coli * | Direct bioautography/ MTT | [33] |
| Inflorescence of Sphaeranthus indicus L. | Staphylococcus aureus *, Klebsiella pneumonia * | Overlay bioautography/ TTC | [93] |
| Various hop varieties | Bacillus subtilis * | Direct bioautography/ MTT | [142] |
| Dioscorea bulbifera L. | Staphylococcus aureus *, methicillin-resistant Staphylococcus aureus *, Staphylococcus epidermidis *, Pseudomonas aeruginosa * | Overlay bioautography/ MTT | [94] |
| Leaves and stems of Dicerocaryum senecioides (Klotzsch) Byng & Christenh. and fresh fruits of Diospyros mespiliformis Hochst. ex A.DC. | Candida albicans **, Trichophyton rubrum **, Epidermophyton floccosum ** | Overlay bioautography/ INT | [54] |
| 15 selected plant species from four genera: Combretum, Pteleopsis, Quisqualis, Terminalia | Staphylococcus aureus *, Bacillus cereus *, Escherichia coli *, Salmonella typhimurium * | Overlay bioautography/ INT | [92] |
| Leaf of Sempervivum tectorum L. | Bacillus subtilis *, Micrococcus lysodeikticus *, methicillin-resistant Staphylococcus aureus *, Staphylococcus aureus *, Escherichia coli *, Klebsiella pneumoniae * | Direct bioautography/ MTT | [149] |
| Aerial parts of Salvia aegyptiaca L., S. verbenaca L. and the leaves of S. officinalis L. | Aliivibrio fischeri *, Bacillus subtilis *, Rhodococcus fascians *, Bipolaris sorokiniana **, Fusarium avenaceum ** | Direct bioautography/MTT, luminescence (for Aliivibrio fischeri) | [62] |
| Ligusticum chuanxiong Hort. rhizome essential oil | Candida albicans ** | Overlay bioautography/ MTT | [150] |
| Artemisia argyi H.Lév. & Vaniot essential oil | Staphylococcus aureus *, Escherichia coli * | Direct bioautography/ MTT | [151] |
| Thymus vulgaris L. essential oil | Haemophilus spp. * (Haemophilus influenzae and H. parainfluenzae), Pseudomonas aeruginosa * | Direct bioautography/ MTT | [152] |
| Nineteen medicinal plants purchased as herbal teas in Belgrade | Escherichia coli *, Staphylococcus aureus * | Direct bioautography/ MTT | [153] |
| Fifteen golden root (Rhodiola rosea L.) products | Bacillus subtilis * Aliivibrio fischeri * | Direct bioautography/ MTT, luminescence (for Aliivibrio fischeri) | [146] |
| Akebia quinata D. leaves or fruits, and Clitoria ternatea L. flowers | Bacillus subtilis * Aliivibrio fischeri * | Direct bioautography/ MTT, luminescence (for Aliivibrio fischeri) | [154] |
| Schisandra rubriflora (Franch.) Rehd. et Wils fruit and leaf | Bacillus subtilis * | Direct bioautography/ MTT | [155] |
| Leaves of Schisandra chinensis (Turcz.) Baill., S. rubriflora Rehder & E.H.Wilson, S. sphenanthera Rehder & E.H.Wilson, S. henryi C.B.Clarke and Kadsura japonica (L.) Dunal | Bacillus subtilis * | Direct bioautography/ MTT | [156] |
| African leafy vegetables | Bacillus subtilis * Aliivibrio fischeri * | Direct bioautography/ MTT, luminescence (for Aliivibrio fischeri) | [157] |
| Leaves of Ficus carica L. | Enterococcus faecalis * | Direct bioautography/ MTT | [96] |
| Different peppermint products | Bacillus subtilis * Aliivibrio fischeri * | Direct bioautography/ MTT, luminescence (for Aliivibrio fischeri) | [158] |
| Matrix/Plant Materials | Type of Stationary Phase | DPPH Solution Concentration and Solvent Type | Time from Spraying the Plate to Analysis | Refs. |
|---|---|---|---|---|
| Leaves of Abrus precatorius L. | Silica gel 60F254 TLC | 0.2%, methanol | 30 min | [163] |
| Leaves and flowers of Nyctanthes arbor-tristis L. | No information available | 0.02%, methanol | overnight | [164] |
| Heartwood of Pterocarpus marsupium Roxb. | Silica gel 60F254 TLC | 5 mM, methanol | No information available | [165] |
| Neptunia prostrata Baill. | Silica gel 60F254 TLC | 2%, unknown solvent | 30 min | [166] |
| Leaves of Morus alba L. | Silica gel 60F254 TLC | 5 mM, methanol | No information available | [167] |
| Origanum vulgare L. | Silica gel 60 HPTLC | 0.1%, methanol | 30 min | [33] |
| Leaf buds of two species and two hybrids of the genus Populus | Silica gel 60F254 TLC | 0.05%, methanol | 30 min | [168] |
| Aerial parts of Salvia aegyptiaca and Salvia verbenaca and the leaves of Salvia officinalis | Silica gel 60F254 HPTLC | 0.02%, methanol | No information available | [62] |
| Hedera helix L. flower pollen | Silica gel 60F254 HPTLC | 0.1%, unknown solvent | 30 min | [169] |
| Inflorescence of Sphaeranthus indicus L. | Silica gel 60F254 TLC | 50 µM, methanol | 10 min | [93] |
| Ligusticum chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu essential oil | Silica gel 60F254 HPTLC | 0.092 mg in mL methanol | 40 min | [150] |
| Schisandra chinensis (Turcz.) Baill., S. rubriflora Rehder & E.H.Wilson, S. sphenanthera Rehder & E.H.Wilson, S. henryi C.B.Clarke and Kadsura japonica (L.) Dunal | Silica gel 60F254 TLC | 0.2%, methanol | immediately after spraying | [156] |
| Fruits of Terminalia bellirica (Gaertn.) Roxb. and T. chebula Retz. | Silica gel 60F254 TLC | 8 mg in 200 mL ethanol | 10–15 min | [110] |
| Herb of Marrubium vulgare L. | Silica gel 60F254 HPTLC | 6 × 10−5 M, methanol | 10 min | [170] |
| Nineteen medicinal plants purchased as herbal teas in Belgrade | Silica gel 60F254 HPTLC | 0.25%, methanol | 30 min | [153] |
| Flowers, leaves, roots and aerial of Cichorium intybus L. | Silica gel 60F254 HPTLC | 0.03%, ethanol | 30 min | [171] |
| The essential oil of Lavandula sp. and Origanum sp. | Silica gel 60F254 TLC | 0.2%, unknown solvent | 30 min | [172] |
| Syzygium aromaticum (L.) Merr. & L.M.Perry, Fomitopsis pinicola and Hypholoma fasciculare | Silica gel 60F254 HPTLC | 0.1% (m/v), methanol | No information available | [173] |
| Leaves of Rosmarinus officinalis, Ficus carica, Backhousia citriodora, Salvia officinalis, Salvia apiana, leaves and flowers of Olea europaea L. | Silica gel 60F254 HPTLC | 0.2%, methanol | 30 min | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zych, M.; Pyka-Pająk, A. TLC in the Analysis of Plant Material. Processes 2025, 13, 3497. https://doi.org/10.3390/pr13113497
Zych M, Pyka-Pająk A. TLC in the Analysis of Plant Material. Processes. 2025; 13(11):3497. https://doi.org/10.3390/pr13113497
Chicago/Turabian StyleZych, Maria, and Alina Pyka-Pająk. 2025. "TLC in the Analysis of Plant Material" Processes 13, no. 11: 3497. https://doi.org/10.3390/pr13113497
APA StyleZych, M., & Pyka-Pająk, A. (2025). TLC in the Analysis of Plant Material. Processes, 13(11), 3497. https://doi.org/10.3390/pr13113497








