Abstract
Sake is a traditional Japanese alcoholic beverage that contains ethyl caproate (EC), a compound that enhances its economic value due to consumer preference. Procyanidins are polyphenolic compounds found in fruits such as apples, with procyanidin B2 (PB2) commonly used as a standard compound. Detecting EC and PB2 directly in intact or simply prepared beverages (e.g., dilution without organic solvent) requires liposomes as a model system. In this study, we focused on liquid-ordered/liquid-disordered liposomes, particularly reverse-domain liposomes, which were identified by fluorescence microscopy. EC and PB2 were found to increase and decrease the ratio of reverse-domain liposomes, respectively. The observation of cell-sized liposomes, combined with calibration curves, enabled a simplified analysis of EC and PB2. This approach has potential applications in the development, optimization, and practical implementation of food analytical methods for routine monitoring of beverage quality. These findings also provide a foundation for further studies on the biophysical and physiological properties of EC and PB2.
1. Introduction
Ethyl caproate (EC) imparts a pleasant characteristic flavor to beverages and is found in a wide range of products, including apple juice [1] and sake [2]. The detection and quantification of EC in beverages have traditionally been performed using gas chromatography [2,3]. In addition, Kuribayashi et al. reported a simple method for EC measurement based on enzyme reactions [3]. Monitoring the concentration of EC in sake is particularly crucial for quality control, as EC serves as a key indicator of sake’s aroma and flavor, which is directly linked to consumer preferences. Therefore, developing simple and reliable methods for EC quantification is essential for maintaining beverage quality and enhancing consumer satisfaction.
Initial research on procyanidins primarily focused on their antioxidant effects, with particular attention given to epicatechin and procyanidin B2 (PB2) due to their high abundance and well-documented health benefits [4]. PB2, a polyphenol with both antioxidant and lipid-lowering properties, is also commonly used as a marker in functional food labeling. However, other procyanidins, such as procyanidin B1 (PB1) and procyanidin C (PC), have also been shown to possess significant antioxidant activities and should be considered for a more comprehensive evaluation of procyanidin content.
Procyanidins are widely found in various fruits and beverages, including apple juice [5,6], where their presence enhances the health-promoting properties of these products. The measurement of procyanidins in foods is thus an important indicator of functional quality. Traditionally, procyanidin contents, including PB2, are quantified using high-performance liquid chromatography (HPLC) [5,6], which requires an extraction step involving organic solvents.
Developing simple, solvent-free analytical methods for procyanidin quantification could significantly advance quality control practices in the functional food industry.
In previous studies, a group to which Yoda previously belonged directly observed dynamic changes in the shape and phase separation of cell-sized liposomes made from phospholipids [7,8,9,10,11,12]. Cell-sized liposomes are composed of lipid bilayer membranes, similar to those found in biological cell membranes. Unlike conventional liposomes, which are typically nanoscale and cannot be observed using optical microscopy, cell-sized liposomes are in the micrometer range. This allows for direct observation under an optical microscope at a scale comparable to that of actual cells. Therefore, cell-sized liposomes serve as convenient and effective models for studying membrane behaviors at the cellular level.
In these studies, Yoda found that biological compound-containing liposomes changed the physicochemical properties, which corresponded to adding compounds [12]. Recently, EC was reported to decrease both the diameter of liposomes [2] and their phase separation [13]. Other studies have reported on PB2 detection using standard reagents for PB2 extracted from apple juice based on diameter size [14] and the phase separation of liposomes [15]. These phenomena may be caused by changes in fluidity, resulting from the physicochemical interactions between lipids and EC or PB2 [2,13,14,15]. In the present study, EC or PB2 was used in the preparation of the liposomes, which could not be used in intact drinks because organic solvents, such as acetone, are required for the extraction processes.
This study was initiated with the aim of efficiently analyzing EC and PB2 in beverages. The primary objective is to estimate the content of EC and PB2 in beverages through the observation of the phase-separated structure of cell-sized liposomes. Addressing the gaps and limitations identified in previous literature, particularly the time-consuming and solvent-intensive nature of EC and PB2 analysis, the author aims to contribute to the advancement of a method for rapid estimation of EC and PB2 content in beverages. This contribution is geared towards minimizing the use of organic solvents by leveraging the observation of the phase-separated structure of cell-sized liposomes.
In the present study, EC and PB2 detection in liposomes were performed using either the intact state of beverages or simple prepared beverages (such as simple dilution without organic solvent). To this end, a hydration step was added for the preparation of the liposomes. This study marks the inaugural observation of liposomes hydrated using sake and apple juice. The investigation then delved into the influence of EC and PB2 contents in the beverages. This study primarily focused on the phase separation behavior of liquid-ordered (Lo) and liquid-disordered (Ld) phases in liposomes, with particular attention to the formation of reverse-domain structures, as observed through fluorescence microscopy. Reverse-domain formation is thought to arise from differences in line tension and lipid packing between Lo and Ld phases, particularly under asymmetric molecular interactions [16]. To the best of our knowledge, reverse-domain formation has been less explored in previous studies, which have mainly focused on traditional Lo/Ld phase separation. Our findings suggest that such liposome systems can be applied for detecting and estimating the concentrations of functional compounds in beverages, offering a simple, cost-effective, and efficient alternative to conventional analytical methods. These findings provide insights into the use of liposomes for the detection and estimation of the concentration of functional compounds in beverages, which represents an economical, efficient, and low-cost alternative to current methods.
2. Materials and Methods
2.1. Materials
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and cholesterol (Chol) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (Rhodamine DHPE), was purchased from Invitrogen (Waltham, MA, USA). Ultrapure water generated using an RFD240NC purification system (ADVANTEC, Tokyo, Japan) was employed for reagent preparation and cleaning glassware. Acetone and methanol were obtained from Wako Pure Chemical (Osaka, Japan), while chloroform and EC were sourced from Kanto Chemical (Tokyo, Japan). PB2 was supplied by Fuji Film Wako Pure Chemical (Osaka, Japan). To maintain purity and stability, PB2 and EC were stored at −20 °C following supplier recommendations. Commercial apple juice (1 L bottles) was procured from the Farm Village Industry Federation of Aomori Prefectural Agricultural (Hirosaki, Japan), and commercial sake (350 mL packs) from Kiku-Masamune Sake Brewing Co., Ltd. (Kobe, Japan). All beverage samples were stored at −20 °C in small aliquots and thawed under running water immediately prior to use to minimize degradation.
2.2. Preparation of Liposomes
Glass test tubes were first cleaned with acetone and air-dried. Various types of liposomes, including giant unilamellar vesicles, were prepared using a slightly modified natural swelling method from dry lipid films, as reported previously [2,7,8,9,10,11,12,13,14,15,17,18]. Lipids and Rhodamine DHPE were dissolved in chloroform under argon in the cleaned test tubes, followed by vacuum drying for 3 h to generate thin lipid films.
Hydration was carried out at 50 °C for 3 h using ultrapure water, apple juice, or sake in a Block Heater (Fisherbrand™ 88860022, Fisher Scientific, Pittsburgh, PA, USA), reaching a final concentration of 200 µM total lipid and 0.1 µM Rhodamine DHPE. To evaluate phase separation, DOPC/DPPC/Chol mixtures at ratios of 40:40:20 and 45:45:10 were used, which are commonly applied to reproduce biologically relevant domain structures [9,13,15,18].
Apple juice was diluted to various extents to achieve visible cell-sized liposomes under the microscope, whereas sake was used without dilution. PB2 (1.4 µM) was added to apple juice for selected experiments. EC was added to sake at 10 v/v%. The final concentrations were calculated based on literature values: PB2 in apple juice (100 mg/L) [19] and EC in sake (7.2 mg/L) [20]. Dilution during liposome preparation resulted in PB2 at 1–2 mg/L and EC at 1–93.6 mg/L, depending on the sample.
2.3. Microscopic Observation
Liposome samples (6 µL) were placed within a silicone-bordered (0.2 mm) enclosed area on a glass slide and covered with a cover glass. Fluorescence microscopy (BX51, Olympus, Tokyo, Japan) was used to monitor liposome diameters and phase-separated domains at room temperature. The microscope was equipped with an oil immersion lens (Uplan S-Apo, Olympus), LED excitation (U-HGLGPS), and dichroic mirrors at 410, 505, and 570 nm (all from Olympus, Tokyo, Japan). Ld phases were labeled with Rhodamine DHPE and imaged under green excitation. Photographs were captured using a WRAYCAM-VEX830 camera (Wraymer, Osaka, Japan).
For each experimental condition, a minimum of 60 liposomes were examined. Bias was reduced by observing multiple fields and performing repeated measurements. Because cell-sized liposomes were produced via natural swelling, uniform diameters could not be achieved; thus, liposomes approximately >5 µm were randomly selected. Preparations were repeated at least three times, and liposome size distributions were reproducible across batches, with a standard deviation of ~5 µm.
2.4. Calibration Curve Method for Estimating Concentrations of EC and PB2
An EC solution made from ultrapure water was prepared with concentrations of 0 mg/L, 5 mg/L, 10 mg/L, and 15 mg/L to create the calibration curve. Similarly, the PB2 concentration in apple juice was estimated, when estimating the concentration of PB2 in juice using the calibration curve, the displayed concentration represented the original concentration without dilution. The concentrations were 0 mg/L, 100 mg/L, 200 mg/L, and 300 mg/L. To construct the calibration curve, a PB2 solution was prepared using ultrapure water, referencing the actual measurements of PB2 from a previous report.
2.5. Characterization for PB2 Concentration Using HPLC
For the juice sample, 2 mL of the thawed product was accurately measured using a volumetric pipette and transferred in-to a 10 mL measuring flask. Subsequently, 7 mL of acetone was added, and the final volume was adjusted with 5% acetic acid containing 0.5% ascorbic acid. The resulting mixture was filtered using a 0.45 mm membrane filter. The concentration of PB2 was determined using HPLC. Instrument settings, columns, and eluent conditions followed previously reported studies [5,6,21]. In brief, an Inertstil WP300 diol column (GL Sciences, Tokyo, Japan) was used. Two mobile phases were utilized as follows: mobile phase A, consisting of acetonitrile and acetic acid (98:2, v/v), and mobile phase B, a mixture of methanol, ultrapure water, and acetic acid (95:3:2, v/v/v). The elution method involved isocratic elution with 7% B for 0 to 1.5 min, followed by isocratic elution with 98% B for 8.5 min. The mobile phase was returned to its initial condition (7% B) to re equilibrate over 10 min. The sample injection volume was 5 or 10 μL, with a flow rate of 1.0 mL/min. PB2 was detected using fluorescence excitation and emission wave-lengths set at 230 and 321 nm, respectively.
2.6. Statistical Analysis
Data summarization and figure preparation were performed using Microsoft Excel and the R statistical software (https://www.r-project.org/, accessed on 11 July 2025). Statistical comparisons were performed using Student’s t-test, with n > 60 for each group, and significant differences were indicated by an asterisk (*) for p < 0.05.
3. Results and Discussion
First, the impact of PB2 and EC content in apple juice and sake, respectively, on the sizes of cell-sized liposomes was investigated. Second, the effects of apple juice and sake with PB2 and EC, respectively, on the phase-separation of the membranes of cell-sized liposomes were assessed. Experiments were performed to reveal both the effect of apple juice and sake and the effects of PB2 and EC on the membranes of cell-sized liposomes were, as well as the potential use of these liposomes in concentration detection.
3.1. Diameter of Liposomes
First, the formation of cell-sized liposomes was investigated. Because cell-sized liposomes may not form using intact bever-ages, the experimental beverages were diluted using pure water. The conditions for liposome formation are shown in Table 1. When apple juice was used as a hydration medium for liposomes, a 100-fold dilution was needed. In contrast, unmodified sake could be used as a hydration medium for liposomes. Based on these findings, apple juice with a 100-fold dilution was used in subsequent experiments.

Table 1.
Liposome formation using apple juice and sake.
Next, the effect of the PB2 and EC content in apple juice and sake, respectively, on the size of the membranes of cell-sized liposomes was investigated (Figure 1). To this end, the diameter of the liposomes was measured using fluorescent observation. This method was used as beverages may have color, for which phase contrast observation is not suitable. However, as a result, the size of the control data set was slightly smaller than in previous studies [2,14] which may be due to differences in the observation conditions, since phase contrast observation was used in the literature, while fluorescent observation was used herein. We have shown the observed liposome sizes in Figure 1. However, in this study, we did not perform comprehensive size measurements using a particle size analyzer or similar methods. This study aimed to apply the microscopic observation results directly to concentration estimation, so detailed particle size distributions were not required.
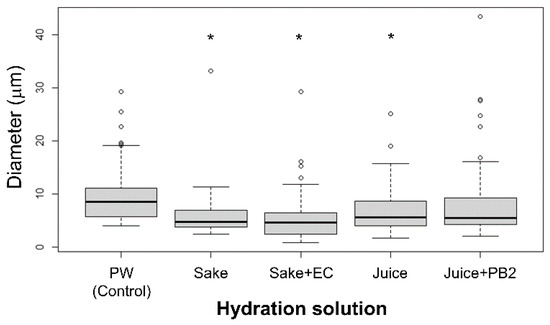
Figure 1.
Liposome diameter as a function of the hydration solution. All liposomes were composed solely of DOPC, and diameters were measured for 60 liposomes under each condition. The actual concentrations of EC and PB2 used in the experiments were as follows: Pure water (PW, control; EC: 0 mg/L, PB2: 0 mg/L), Sake (EC: 7.2 mg/L), Sake + EC (EC: 93.6 mg/L), Juice (PB2: 1 mg/L), and Juice + PB2 (PB2: 2 mg/L). Asterisks (*) indicate a statistically significant difference from the PW (control (p < 0.05)). Small circles represent outliers. Whiskers denote the minimum and maximum data points, excluding outliers. The lower and upper quartiles are represented by the edges of the box, while the median diameter for each condition is indicated by a horizontal bar within the box.
Despite using fewer values, the addition of EC was found to decrease the liposome size, while the addition of PB2 increased their size, in agreement with the literature [2,16,18]. Furthermore, while previous studies have added membranes in prepared liposomes, in the present study, the experimental beverages and compounds were found to affect membranes via hydration. Herein, the respective compounds found in the experimental beverages, namely EC or PB2, were found to affect the fluidity of the lipid molecules, with changes observed in their corresponding size, which induced changes in the size of the liposomes [2,14,17,22]. This method may be used to evaluate the content of EC or PB2 in beverages.
The transfer condition determines the difference between the concentration of the inner droplet solution and that of the outer solution. In a previous study, the concentration of both the inner and outer solution was found to regulate the size of the resulting liposomes [23]. Another study also reported that the size of liposomes was dependent on the droplet size [24]. Using the hydration method, liposome size depends on the concentration of the compounds in the hydration solution, such as calcium chloride or potassium sulfate [25,26]. As a result, the study of the mechanisms underlying liposome size is de pendent on the electrostatic repulsion of the lipid head group [26,27], as this is contains negatively charged phosphatidylglycerol. On the other hand, in nano-size scale liposomes made from even neutral lipids, the type of hydrated organic solvent used has an effect of the size of the liposomes [27]. In present study, micro-scale liposomes were prepared based on the hydration method using neutral lipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), which was not charged. Therefore, in the present study, it may be hypothesized that the size of the liposomes was dependent on changes in the fluidity caused by interactions with the molecules found in the experimental beverages.
3.2. Phase Separation of Liposomes
The observed phase separation states of cell-sized liposomes in this study are summarized in Table 2.

Table 2.
Summary of observed phase separation states and representative conditions.
In previous studies, our experimental group found that phase separation decreases were caused by compounds found in the hydration media [13,15]. To evaluate the effects of the experimental beverages (e.g., sake and apple juice, and their corresponding EC and PB2 content), phase separation was evaluated under each hydration condition. As a result, the same condition (DOPC:1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC): Chol (45:45:10)) was found to produce three different phase-separated liposomes (Figure 2 (sake) and Figure 3 (juice)): homogeneous phase (Figure 2A and Figure 3A), liquid ordered (Lo)/Ld phase separation (Figure 2B and Figure 3B), and solid ordered (So)/liquid disordered (Ld) phase separation (Figure 2C and Figure 3C). Herein, two kinds of Lo/Ld phase-separation were observed, notably so called “reverse” liposomes (Figure 3B), which have been previously observed [28]. Over 60 of these vesicles were observed under each condition (Figure 2D and Figure 3D; the ratios of reverse liposomes are shown as an average of three observations). Although previous studies have reported occurrences near the region where all three phases coexist in a single liposome on a phase diagram for DOPC/DPPC/Chol liposomes [28,29,30], in the present study, only homogenous, Lo/Ld, and Ld/So phase separations were considered. The effects of both sake with EC and apple juice with PB2 on the So/Ld membranes were initially investigated under hydration conditions. Since conventional studies have reported So/Ld phase-separation in liposomes made from DOPC/DPPC/Chol (45:45:10) hydrated using pure water [13,15], these liposomes were used as a control. Herein, 89% So/Ld phase separation was observed at DOPC/DPPC/Chol (45/45/10) (Figure 2D and Figure 3D). The corresponding results for the control sample were in agreement with previous studies [14,17]. The total effect of apple juice with PB2 on the So/Ld domains was larger than that for sake with EC. In the present study, EC and PB2, this represents the first instances that phase-separated liposomes hydrated using sake and apple juice are observed. The So/Ld ratio de creased (89 (±5)%), while homogeneity increased (8 ((±5)%) with the concentration of DOPC/DPPC/Chol (45/45/10) hydrated using either sake (44 (±8)% So/Ld ratio and 54 (±10)% homogeneity) or apple juice (41 (±7)% So/Ld ratio and 33 (±12)% homogeneity) (Figure 2D and Figure 3D). Using sake as the hydration medium, EC led to weaker effects (Figure 2D). In contrast, using apple juice for hydration, PB2 led to stronger effects (Figure 3D).
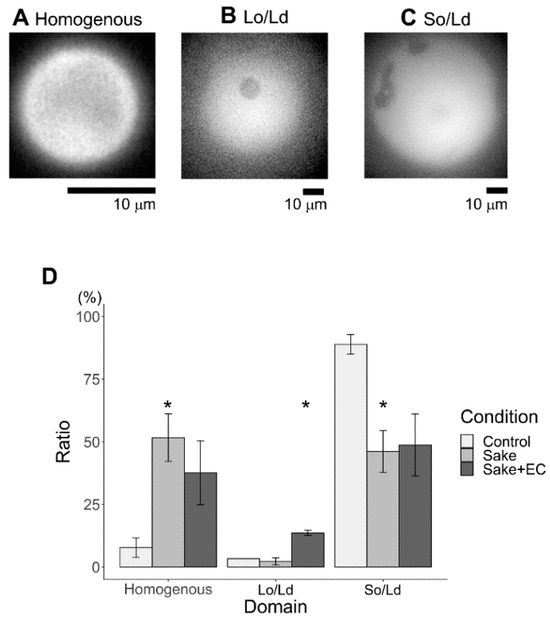
Figure 2.
Major phase separation of control liposomes via solid ordered (So)/liquid disordered (Ld) phase separation with sake. (A) Homogenous, (B) Lo/Ld, (C) So/Ld, and percentages of phase-separated liposomes are calculated using the total number of observed liposomes (D, (* p < 0.05)). Asterisks (*) indicate statistically significant differences compared with the respective control (p < 0.05). All liposomes were made from DOPC:DPPC:Chol (45:45:10) using sake as the hydration medium. All experiments were performed at 20 °C.
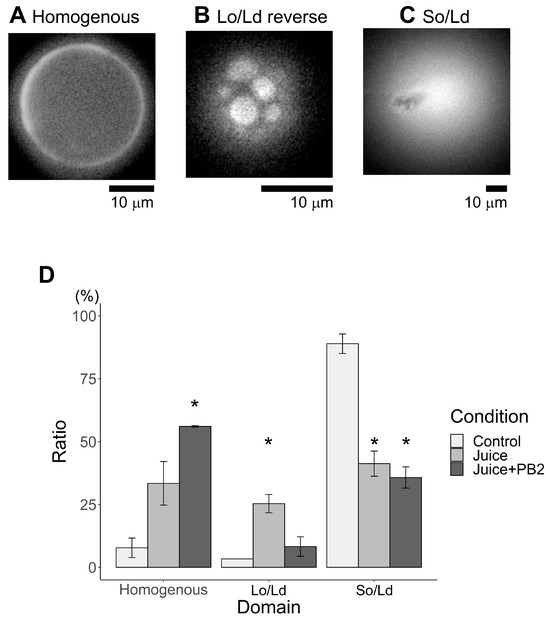
Figure 3.
Major phase separation of control liposomes via solid ordered (So)/liquid disordered (Ld) phase separation using apple juice. (A) Homogenous, (B) Lo/Ld reverse, (C) So/Ld, and percentages of phase-separated liposomes are calculated using the total number of observed liposomes ((D), (* p < 0.05)). Asterisks (*) indicate statistically significant differences compared with the respective control (p < 0.05). All liposomes were made from DOPC:DPPC:Chol (45:45:10) hydrated using apple juice. All experiments were performed at 20 °C.
Next, the effects of both sake with EC and apple juice with PB2 on the Lo/Ld membranes were investigated. Herein, DOPC/DPPC/Chol (40/40/20) liposomes were used as control for Lo/Ld phase separation, since previous studies have re-ported that mainly Lo/Ld phase-separation is observed when liposomes made from DOPC/DPPC/Chol 40:40:20 are hydrated using pure water [13,15,18,31,32,33,34,35,36,37]. These liposomes were used as a control. In the current investigation, it was found that 71% of Lo/Ld phase separation liposomes were observed in the case of DOPC/DPPC/Chol (40/40/20) (Figure 4 and Figure 5). The total effect of apple juice with PB2 on So/Ld domains was larger than that for sake with EC. Interestingly, the same trend was observed for So/Ld phase separation. The Lo/Ld ratio de-creased (71 (±6)%), while homogeneity increased (20 ((±7)%) with the concentration of DOPC/DPPC/Chol (40/40/20) hydrated using either sake (36 (±11)% Lo/Ld ratio and 54 (±2)% homogeneity) or apple juice (11 (±4)% Lo/Ld ratio and 88 (±10)% homogeneity) (Figure 4 and Figure 5). The concentration of neither EC nor PB2 affected the ratio of homogenous and Lo/Ld (Figure 4 and Figure 5).
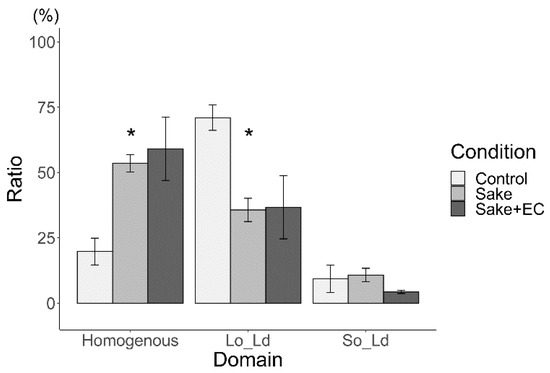
Figure 4.
Major phase separation of control liposomes via liquid ordered (Lo)/liquid disordered (Ld) phase separation with sake (* p < 0.05). Asterisks (*) indicate statistically significant differences compared with the respective control (p < 0.05). All liposomes were made from DOPC:DPPC:Chol (40:40:20) in hydrated using sake.
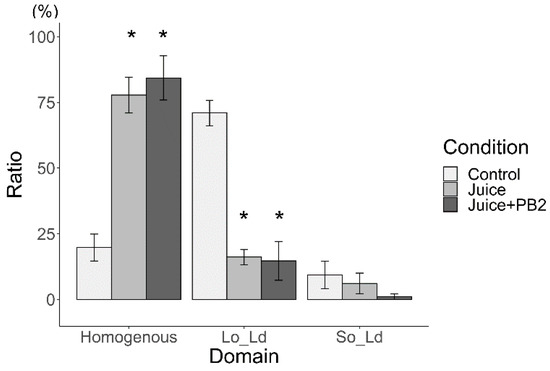
Figure 5.
Major phase separation of control liposomes via liquid ordered (Lo)/liquid disordered (Ld) phase separation with apple juice (* p < 0.05). Asterisks (*) indicate statistically significant differences compared with the respective control (p < 0.05). All liposomes were made from DOPC:DPPC:Chol (40:40:20) hydrated using apple juice.
To investigate phase separation further, two types of Lo/Ld phase separation investigated, namely Lo/Ld (Figure 2B) and Lo/Ld reverse (Figure 3B). Here, Lo/Ld (typical cell-sized liposomes are shown in Figure 2B) and Lo/Ld reverse (typical cell-sized liposomes are shown in Figure 3B). The characteristics shared and differentiated between Lo/Ld phase-separated liposomes and reverse phase-separated liposomes are elucidated as follows: Normal Lo/Ld phase-separated liposomes demonstrate Lo domains within the Ld phase, whereas reverse phase-separated liposomes exhibit Ld phase domains within the Lo phase. Despite both types exhibiting Lo/Ld phase separation, they possess distinct structural variations, as previously described. Reverse liposomes were not observed in either of the control liposomes (DOPC/DPPC/Chol (45/45/10 and 40/40/20) hydrated using pure water). The reverse liposomes hydrated using apple juice were larger than those hydrated using sake.
The ratios of reverse liposomes in total Lo/Ld are shown in Figure 6 (using sake as the hydration medium) and Figure 7 (using apple juice as the hydration medium). Because reverse liposomes could not be observed in either of the control liposomes (DOPC/DPPC/Chol (45/45/10 and 40/40/20) hydrated using pure water), the ratio was not described in those Figure 6 and Figure 7. Reverse liposomes were observed upon hydration with sake (containing EC) and apple juice (containing PB2) in DOPC/DPPC/Chol liposomes (40/40/20) and upon hydration with sake (with EC) and apple juice (with PB2) in DOPC/DPPC/Chol liposomes (45/45/10). The presence of EC increased the ratio of reverse liposomes, while PB2 decreased this ratio in liposomes made from DOPC/DPPC/Chol (40/40/20 and 45/45/10). In the future, the ratio of phase separation liposomes should focus on compound detection. Changes in the Lo/Ld ratio may not apply when two types of state, such as Lo/Ld and reverse, are not distinguished. Care should be paid to these characteristics when applied in detection systems, particularly autodetection systems using machine learning Lo/Ld and reverse separation [18].
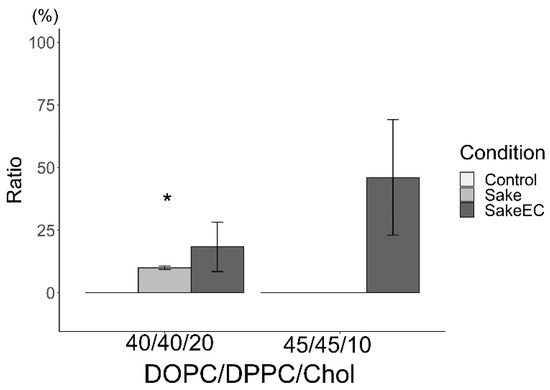
Figure 6.
Ratio of reverse domain for liposomes hydrated using sake (* p < 0.05). Asterisks (*) indicate statistically significant differences compared with the respective control (p < 0.05). Liposomes were made from DOPC:DPPC:Chol (40:40:20 and 45:45:10).
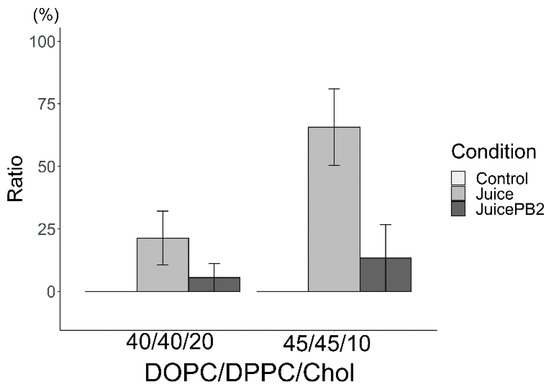
Figure 7.
Ratio of reverse domain for liposomes hydrated using apple juice. Liposomes were made from DOPC:DPPC:Chol (40:40:20 and 45:45:10).
Based on the experimental results, it is discussed the effects of EC and PB2 on the formation of phase-separated domains. First, the impact of EC on these domains was considered.
In previous observations, it was examined that cell-sized liposomes containing EC or Sake with EC were incorporated into the membrane [13,36]. The results indicated a trend where the proportion of cell-sized liposomes with phase-separated domains decreased as the EC concentration increased. Similarly, in this study, where EC was added during hydration, the percentage of cell-sized liposomes with phase-separated domains also tended to decrease with increasing EC concentration. Additionally, this study reports a trend of growing reverse domains with higher EC concentrations. Building on the discussion from the previous research, the molecular mechanism by which EC impedes the formation of phase-separated domains and promotes the formation of reverse domains is examined below from the perspectives of energy balance and lipid packing in lipid membranes. These hypotheses are based on established models of lipid phase separation, where the state is influenced by lipid packing and aggregation in aqueous environments, as reported in previous studies [37].
Observable domain types were considered to reflect the following energy balance. When homogeneity is present, mixing is favorable due to the low mixing energy required. In a homogeneous situation, relatively small fluidic differences within each domain or region seemed possible. In the case of So/Ld phase separation, the mixing energy is high because the fluidic difference between the two regions is significant. In control cell-sized liposomes, mainly So/Ld phase separation was observed with DOPC/DPPC/Chol 45/45/10 (Figure 2D). In the presence of sake or sake with added EC, the So domain was observed, suggesting that So domains may be formed by DPPC, Chol, and EC. As EC concentration increases, domain fluidity in-creases because EC has a short carbon chain, causing some domains to change from So to Lo (Figure 2D). EC may also integrate into the Ld domain, decreasing the fluidity difference between So and Ld or Lo and Ld. In such a situation, in-creased homogeneity is advantageous due to the lower mixing energy required.
In control cell-sized liposomes, mainly Lo/Ld phase separation was observed with DOPC/DPPC/Chol 40/40/20 (Figure 4). Similarly, in the presence of sake or sake with added EC, Lo domains were observed, suggesting that Lo domains may be formed by DPPC, Chol, and EC. Here, also EC may integrate into the Ld domain, reducing the fluidity difference between Lo and Ld, thus favoring increased homogeneity due to the lower mixing energy required.
Moreover, prior research and recent findings have indicated that membrane tension, induced by factors such as osmotic pressure, promotes the formation of phase-separated domains [32,38]. In this study, the author analyzed the influence of EC on the development of membrane phase-separated domains, including both So/Ld and Lo/Ld phases, from the standpoint of energy balance and membrane lipid packing, particularly how EC impedes and complicates their formation. Given that tension enhances lipid membrane packing and fosters the formation of phase-separated structures, it is hypothesized that EC reduces membrane tension compared to a state where it is absent, thus hindering the formation of these domains.
The increase in reverse domains is thought to be due to EC acting like DPPC (Figure 6). As a result, the area of Lo increases, leading to reverse domains with Ld in Lo, as opposed to Lo in Ld, and thereby increasing the percentage of reverse domains. However, previous research has shown that EC behaves like an unsaturated lipid [13]. This difference is likely because EC was added as sake or sake with EC rather than as pure EC during hydration, resulting in the hydrophobic components of EC and sake being present and associating with the DPPC region.
Next, the effect of PB2 on the formation of phase-separated domains was considered. Previously, cell-sized liposomes containing PB2 or apple juice containing PB2 in the membrane were observed [15]. As a result, the proportion of cell-sized liposomes with domains formed by phase separation structures tended to decrease with increasing PB2 concentration. Similarly, in this study, where apple juice and apple juice with PB2 were added during hydration, the percentage of cell-sized liposomes with phase-separated domains also tended to decrease with increasing PB2 concentration. Additionally, this study reports a trend of decreasing reverse domains with higher PB2 concentrations. Building on the discussion from the previous research, the molecular mechanism by which PB2 impedes the formation of phase-separated domains and the formation of reverse domains is examined below from the perspectives of energy balance and lipid packing in lipid membranes.
As procyanidin contains a carbon ring and a hydroxyl group, it appears to have a higher affinity for So or Lo domains. The position of procyanidin should be at the interface of the hydrophilic and hydrophobic regions in lipids on the So or Lo domain of the membranes [38]. In control cell-sized liposomes, mainly So/Ld phase separation was observed with DOPC/DPPC/Chol 45/45/10 (Figure 3), or mainly Lo/Ld phase separation was observed with DOPC/DPPC/Chol 4/40/20 (Figure 5). Similarly, in the presence of apple juice or apple juice with PB2, So or Lo domains were observed, suggesting that So or Lo domains may be formed by DPPC, Chol, and PB2. Here, PB2 may also integrate into the Ld domain, reducing the fluidity difference between So or Lo and Ld, thus favoring increased homogeneity due to the lower mixing energy required.
Similarly, the author considered the impact of PB2 on lipid membrane tension. Based on the findings of this study, the author evaluated the effects of PB2 on the formation of membrane phase-separated domains, both So/Ld and Lo/Ld, using a similar approach to that used for EC, focusing on energy balance and lipid packing. Since membrane tension is known to increase lipid packing and promote phase separation [32,39], the author proposes that PB2 also hinders the formation of phase-separated domains, thereby reducing membrane tension relative to a state where PB2 is not present.
The decrease in reverse domains is thought to be due to PB2 acting like DOPC (Figure 7). As a result, the area of Ld in-creases, leading to Lo domains in Ld, as opposed to Ld in Lo, and thereby decreasing the percentage of reverse domains.
On the other hand, previous research has shown that PB2 affected the decrease only for Lo/Ld phase separation, not for So/Ld [15]. This difference is likely because PB2 was added as apple juice or apple juice with PB2 rather than as pure PB2 during hydration, resulting in the hydrophobic components of PB2 and apple juice being present and associating with both the So (DPPC) and Lo (DPPC and Chol) regions. In previous research, PB2 was used as a standard reagent and added during film formation [15], allowing it to integrate only into the Lo region but not the inflexible So region. In contrast, adding PB2 during hydration, where it existed in apple juice along with other hydrophobic compounds, allowed PB2 to integrate into both the Lo and So regions. Therefore, in the present study, PB2 could affect So/Ld phase separation.
The utilization of cell-sized liposome observations for the quantification of EC and PB2 is discussed as follows.
Experiments were conducted to utilize the calibration curve method for quantifying the concentrations of both EC and PB2, as previous suggestions indicated the potential utility of this method for such measurements [40,41]. Standard reagents and ultrapure water were employed to establish the calibration curves. This study marks the first observation of cell-sized liposomes made from DOPC/DPPC/Chol (with ratios of 45/45/10 and 40/40/20) lipid compositions in the presence of EC and PB2 solutions.
Previous discussions focused on phase-separated domains, such as So/Ld, Lo/Ld, and Lo/Lo. Based on the results from sake and sake with EC (Figure 4), it was observed that the ratio of homogeneous liposomes could serve as a simple indicator, as it increased linearly. Cell-sized liposomes derived from DOPC/DPPC/Chol (both ratios 45/45/10 and 40/40/20) various EC concentrations exhibited a linear increase in the ratio of homogeneous liposomes with increasing EC concentration (Figure 8). The calibration equations for EC were obtained as follows: For DOPC/DPPC/Chol (45/45/10): [EC] = ([Homogenous liposomes/total liposomes] − 0.15)/0.030, R2 = 0.99, For DOPC/DPPC/Chol (40/40/20): [EC] = ([Homogenous liposomes/total liposomes] − 0.025)/0.013, R2 = 0.98. These equations indicate a strong correlation between liposome response and analyte concentration. The calibration plots are shown in Figure 8. To create both calibration curves and equations, cell-sized liposomes were made at least three times to ensure reproducibility, with no significant bias observed across different preparations. Data are shown as the mean ± standard error from three independent observations, consistent for both EC and PB2 analyses. The EC experiments revealed that when EC was added during lipid film preparation to liposomes with a lipid composition that facilitated phase separation [13], the ratio of phase-separated cell-sized liposomes decreased in a concentration-dependent manner. This suggests that the effect of EC is similar whether it is present in the lipid film or hydrated ultrapure water.
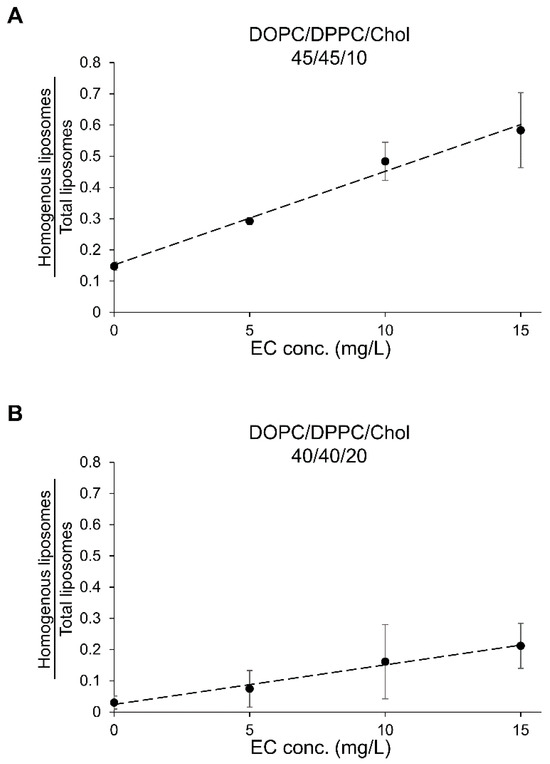
Figure 8.
Calibration curves for ethyl caproate (EC) using the liposome observation method. The calibration equations were determined as follows: For DOPC/DPPC/Chol (45/45/10): [EC] = ([Homogenous liposomes/total liposomes] − 0.15)/0.030, R2 = 0.99, For DOPC/DPPC/Chol (40/40/20): [EC] = ([Homogenous liposomes/total liposomes] − 0.025)/0.013, R2 = 0.98. Panels (A,B) depict the calibration curves for EC in DOPC/DPPC/Chol (45/45/10) and DOPC/DPPC/Chol (40/40/20), respectively.
In previous studies, the ratio of So/Ld phase-separation in cell-sized liposomes did not correspond to PB2 concentration in the lipid film, whereas the Lo/Ld phase-separation [15]. Notably, in previous studies, PB2 was added during lipid film production, while in this study, PB2 was added during hydration. Unlike earlier findings, the current results show a linear increase in the ratio of homogeneous liposomes with increasing PB2 concentration, even in So/Ld main composition DOPC/DPPC/Chol 45/45/10 (Figure 9). The calibration equations for PB2 were obtained as follows: For DOPC/DPPC/Chol (45/45/10): [PB2] = ([Homogenous liposomes/total liposomes] − 0.16)/0.0012, R2 = 0.97, For DOPC/DPPC/Chol (40/40/20): [PB2] = ([Homogenous liposomes/total liposomes] − 0.11)/0.021, R2 = 0.99. These equations indicate a strong correlation between liposome response and analyte concentration. The calibration plots are shown in Figure 9. This suggests that PB2, when dissolved in pure water during hydration, can affect the self-assembly of lipid molecules to form membrane structures, potentially making it difficult for liposomes to undergo So/Ld phase separation, influenced by the PB2 concentration in pure water.
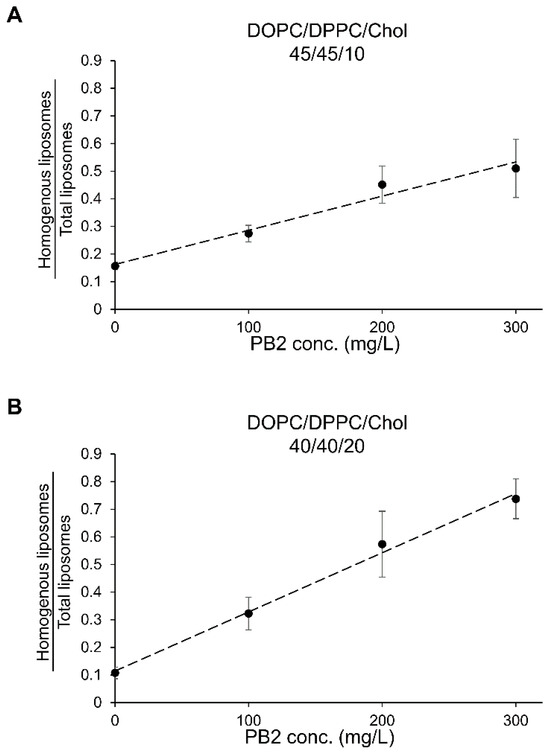
Figure 9.
Calibration curves for procyanidin B2 (PB2) using the liposome observation method. The calibration equations were determined as follows: For DOPC/DPPC/Chol (45/45/10): [PB2] = ([Homogenous liposomes/total liposomes] − 0.16)/0.0012, R2 = 0.97, For DOPC/DPPC/Chol (40/40/20): [PB2] = ([Homogenous liposomes/total liposomes] − 0.11)/0.021, R2 = 0.99. Panels (A,B) depict the calibration curves for PB2 in DOPC/DPPC/Chol (45/45/10) and DOPC/DPPC/Chol (40/40/20), respectively.
For estimating EC concentration in sake using the calibration curve, the ultrapure water (control), sake, and EC-added sake were found to have concentrations of −0.1 mg/L, 6.1 mg/L, and 13.7 mg/L, respectively, using DOPC/DPPC/Chol 45/45/10 liposomes, and 1.8 mg/L, 40.0 mg/L, and 56.7 mg/L, respectively, using DOPC/DPPC/Chol 40/40/20 liposomes. The actual concentrations were 0 mg/L, 7.2 mg/L, and 93.6 mg/L, respectively. The estimated concentrations for ultrapure water and sake were similar to those with DOPC/DPPC/Chol 45/45/10 liposomes, though the EC-added sake concentration was underestimated. For DOPC/DPPC/Chol 40/40/20 liposomes, the ultrapure water estimate matched the actual concentration, but the sake estimate was higher, and the EC-added sake was underestimated.
When estimating PB2 concentration using the calibration curve, the ultrapure water (control), apple juice, and PB2-added apple juice concentrations were −10 mg/L, 81 mg/L, and 327 mg/L, respectively, using DOPC/DPPC/Chol 45/45/10 liposomes, and 2 mg/L, 250 mg/L, and 390 mg/L, respectively, using DOPC/DPPC/Chol 40/40/20 liposomes. The actual concentrations were 0.0 mg/L, 56.4 (±0.2) mg/L, and 156.4 (±0.2) mg/L, respectively. The measured values were shown as the average of three measurements and standard error. The estimated concentrations for ultrapure water and juice were close to the actual values, though PB2-added apple juice concentrations were higher than actual with DOPC/DPPC/Chol 45/45/10 liposomes. With DOPC/DPPC/Chol 40/40/20 liposomes, the ultrapure water estimate matched the precise concentration, but the estimated values for both apple juice and PB2-added apple juice were higher. Applied the calibration curve method, especially with DOPC/DPPC/Chol 45/45/10 liposomes, successfully estimated PB2 concentration within the 0–100 mg/L range. It is well known that concentration deviations are significantly changed when samples are stored, particularly when subjected to repeated freezing and thawing. For example, we have observed that the PB2 concentration decreased to 26.6 (±4.3) mg/L, approximately half of that in the plain juice used in the HPLC experiment. In the cell-sized liposome experiment, fresh samples were used. We also measurements were conducted promptly. However, it is important to acknowledge that concentration deviations may still occur depending on factors such as lot variations, storage conditions, and preservation methods, even when using the same juice.
Although precise EC or PB2 concentrations were limitedly achieved, the data effectively represented approximate relative concentrations, as the order was correct. The calibration curve method can identify differences between fermentation tanks and be used for prototype product creation and process optimization. Given that EC content in sake varies with plant and microbial composition, and PB2 content in apple juice varies due to individual differences between apples, regular monitoring is crucial. This method, especially counting phase-separated liposomes, confirms alignment with existing lots, providing valuable consumer information. While the accuracy of quantification using the liposome observation method is limited compared to traditional chromatographic techniques (e.g., GC, HPLC), the approach provides a rapid and solvent-free assessment of EC and PB2 interactions in beverage matrices. While GC and HPLC provide higher sensitivity and specificity, they require expensive instrumentation and longer processing times. Our liposome-based method offers a simpler and lower-cost alternative, albeit with lower sensitivity. This method could serve as a screening tool for preliminary evaluation in food quality control. Although the accuracy is lower than that of conventional methods, this approach is low-cost and rapid. It can be useful for selecting product candidates containing high amounts of EC or PB2 from numerous prototypes. This method is also suitable for companies that produce a wide variety of products in small quantities, especially small companies without access to their own GC or HPLC equipment. Using only a fluorescence microscope, this method can estimate concentrations and reduce the initial cost of introducing conventional analytical equipment by approximately 90%.
While we used calibration curves prepared with pure compounds, matrix effects from sake and apple juice could influence fluorescence intensity. We therefore discussed these potential effects and clarified this limitation. Further, we did not perform separate experiments to isolate the effects of other sake or juice ingredients. Based on these findings, we further evaluated the limitations and future perspectives of our method, focusing on the complexity of beverage matrices such as sake and apple juice. This study acknowledges several limitations in the quantitative estimation of EC and PB2 concentrations using the current liposome-based method. First, the measurable concentration range is constrained by the calibration curves and the complexity of the sample matrices. In sake, for example, other components such as isoamyl acetate (IA), isovaleraldehyde (IVA), caproic acid (CA), and isoamyl alcohol (IAA), along with sugars, can influence membrane fluidity and liposome formation, making it challenging to precisely quantify EC alone. Future work will incorporate ethanol-based calibration systems, as ethanol can influence membrane properties, to improve the accuracy of EC estimation. Despite these limitations, our results suggest that this method can serve as a useful screening tool for detecting EC across a range of sake samples with varying characteristics, particularly when applied under similar conditions where the major components remain consistent.
Similarly, apple juice contains various polyphenols besides procyanidins, such as catechins, and previous studies have shown that other polyphenols, including theaflavin, can affect membrane phase separation. Although procyanidins are the predominant polyphenols in apple juice, it remains necessary to consider the potential influence of other polyphenols and variations in sugar and acid contents among different apple varieties and production processes. Sugars and acids have been reported to affect liposome size and membrane phase separation, suggesting that these components could indirectly impact procyanidin estimation.
Given these factors, our ongoing research aims to refine the concentration estimation system by accounting for the effects of sugars, acids, and other polyphenols on membrane phase separation. We are currently conducting further studies using apple juice samples from different varieties and processing conditions to evaluate the robustness and applicability of this method for complex food matrices.
By considering these variables, this method has the potential to evolve into a practical tool for comprehensive analysis of functional components in beverages, allowing for simple and effective screening of flavors and health-related compounds. These considerations highlight the necessity for continued refinement of the method to improve its robustness for broader practical applications in food analysis.
Although this report presents preliminary results, it was planned to continue our research to develop a reliable and simple evaluation system for EC and PB2.
Synthesized molecules inserted into the membranes of liposomes have been reported to induce changes in the liposome characteristics, including their photoresponsivity [42,43,44,45] and osmotic responsiveness [46]. It has also been reported that liposomes made from natural lipids induce changes in the mechanical properties, such as endocytosis, caused by physicochemical, stress such as osmotic stress [9], oxidative stress [7] or thermo-stress [8,17]. Previously, we reported that EC changed the membrane properties by decreasing liposomes size [2] and Lo/Ld phase separation [13]. It has also been previously reported that PB2 also changes the membrane properties, resulting in an increased liposome size [14] and decreasing Lo/Ld phase separation [15]. Previous studies have found that the natural product capsaicin and synthesized local anesthetics lidocaine and tetracaine [34,35] decrease phase separation. In almost all cases, the addition of compounds, whether natural or synthesized, was found to reduce the phase separation of micro-scale liposomes from ternary lipids [10,13,15,34,35]. This may be due to the fact that the lipid composition was shifted from the central region, facilitating the phase separation of lipids to the outer region of the phase diagram, enhancing homogeneity resulting from the addition of compounds. In this context, the use of ternary lipids, such as cholesterol to ergosterol, increased the phase separation [18], with similar findings reported in nanoscale liposomes [47,48,49]. In nanoscale liposomes containing an oxidized cholesterol, the interaction between the lipid chain and the hydrophobic region of the phospholipid was enhanced, stabilizing the Lo domain, compared with liposomes containing cholesterol. In other studies, these compounds were added to the membranes of liposomes under organic solvent conditions [10,13,15,34,35]. Previous studies examined the impact of phase-separated structures introduced into liposome membranes during the preparation of lipid solutions under organic solvent conditions. In contrast, this study unveiled a novel finding that the components of the aqueous solution utilized for hydration influenced the phase-separated structure of liposomes. Furthermore, similar studies have prepared liposomes only from lipids, either from biological or inorganic compounds, to investigate the effect of compounds on lipid membranes [11,12,31,50,51,52,53,54].
In this study, both EC and PB2 were found to induce changes in the membrane structure and phase separation states of liposomes. Previous reports have shown that membrane receptors can respond to specific molecules [55,56]. Furthermore, the function of potassium ion channels is known to be influenced by lipid phase behavior and membrane phase transitions [57,58,59]. These effects may be mediated by changes in the conformation or spatial organization of membrane receptors or channels in response to EC.
On the other hand, procyanidins have been reported to modulate lipid metabolic pathways, potentially through interactions with membrane receptors and/or ion channels [18,38]. For instance, procyanidins have been shown to inhibit the regulation of potassium transport through potassium channels [60].
Thus, while this study primarily investigated interactions between lipid molecules and EC or PB2, the findings also suggest broader significance as a “cell membrane model.” Specifically, this liposome-based system may provide insights into the interactions between lipid molecules and other membrane-associated components, such as membrane proteins, within biological membranes. This highlights the potential of liposome systems as simplified models for studying molecular interactions in complex biological membranes.
The author has taken into consideration the biological implications of this research. However, the emphasis is placed on its contribution to beverage evaluation. In this context, the author believes that cell-sized liposomes offer an advantage over cells, which demand culture and quality control, as they can be stored as reagents and prepared as needed. Herein, the ratio of reverse-domain liposomes was found to increase by EC and decrease by PB2. With regard to the mechanisms underlying these findings, the author hypothesized that acyl chain compounds, such as EC, for materials of the Lo domain and PB2 for materials of the Ld domain were responsible for these results. Materials of the Lo domain compounds increased the ratio of reverse-domain liposomes. Based on the hypothesis, sake (EC) and apple juice (PB2) increased and decreased the ratio of reverse domains, respectively (Figure 6 and Figure 7). Sake and apple juice should contain compounds which has acyl chain. Previously, many fatty acids compounds with the acyl chains have been reported in sake [3]. Similarly, research group of the author also previously observed many fatty acids with acyl chains in apple juice using gas chromatography–mass spectrometry (GC/MS) [1]. According to these results, it is these fatty acids compounds in the experimental beverages that give rise to reverse-domain liposomes (Figure 6 and Figure 7). However, while EC was found to increase the ratio of these reverse-domain liposomes (Figure 6), PB2 also has some hydroxyl group, which increases the Ld domain region and thereby decreases the ratio of reverse-domain liposomes. To the best of my knowledge, reverse-domain formation has not been research at length, with the literature focusing mainly on Lo/Ld phase separation [10].
Other membrane systems for the detection of organic solvent and flavor have also been reported [61,62]. Previous studies have reported on the measurement of membrane response via the transduction of mechanical stresses using different solvents, including alcohols (e.g., methanol, ethanol, and 2-propanol), acetone, ethyl acetate, alkanes (e.g., n-hexane and n-heptane), and aromatic compounds (e.g., benzene and toluene) [61,62]. Research has proposed that membranes are used to detect molecular mass through molecular mass analysis [62]. The response patterns of membranes have the potential to be used as a sensor. Since membrane patterns, such as phase separation, have been used as a microscopic picture to determine the quality of membranes, these could be similarly used to detect the quality of beverages without the need for complex methods, such as chromatography (e.g., GC/MS or high-performance liquid chromatography), which tend be costly. Using the method proposed herein would only require a microscope and a personal computer with artificial intelligence (AI) or machine learning systems to improve reproducibility and reduce bias (or for use in the analysis of microscopic images) [63]. Any ongoing costs would only be for liposome solution and glassware, both of which are reasonably inexpensive. Furthermore, machine learning for the identification of the phase separation of liposomes using microscopic images has already been performed successfully [18]. The characterization of compound concentration faces challenges due to factors like liposome type and preparation methods, particularly in complex systems like fruit drinks. Our approach may not be suitable for precise quantification but may have potential in other fields, such as nutraceutical research or liposomal drug delivery [64], because edible compounds PB2 and EC could change the physicochemical properties of lipids membrane. Thus, the methodology proposed in this study remains at an early stage. To achieve minimal acceptance, this approach should integrate official analysis techniques to enable result comparisons and validation. Considering the utilization of a calibration curve could be instrumental in this regard.
4. Conclusions
This study demonstrated a simple analytical method for detecting ethyl caproate (EC) in sake and procyanidin B2 (PB2) in apple juice using phase separation of liposomes. The observation of liquid-ordered/liquid-disordered liposomes via fluorescence microscopy provided insight into the interactions of EC and PB2 with lipid membranes, revealing that EC increased and PB2 decreased the ratio of phase separation on liposomes. The use of calibration curves further enhanced the potential for quantitative analysis. This approach offers a simplified and practical methodology for food quality monitoring and routine analysis in food laboratories. The findings also provide a basis for future investigations into the biophysical and physiological properties of EC, PB2, and other bioactive food compounds.
Author Contributions
Conceptualization, T.Y.; methodology, T.Y. and N.N.; formal analysis and investigation, T.Y. and N.N.; experiments, T.Y. and N.N.; data curation, and writing—original draft preparation, T.Y. writing—review and editing, T.Y., K.Y. and N.N.; supervision, T.Y., K.Y. and N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Konica Minolta Imaging Science Encouragement Award, the Shorai Foundation for Science and Technology, the Japan Society for the Promotion of Science (JSPS) KAKENHI, grant number JP20K19699, and grants from the Aomori Prefectural Industrial Technology Research Center for a research challenge, third term research project and research for which the executives have set a special framework.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to thank Hiroshi Miyaki for kind suggestions regarding the research plan. Thank to Shoji Sasaki for providing a comfortable environment for our research. The authors also extend their appreciation to Hirosaki industrial institute, Aomori Prefectural Industrial Technology Research Center for providing access to their instruments.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| EC | ethyl caproate |
| PB2 | procyanidin B2 |
| PB1 | procyanidin B1 |
| PC | procyanidin C |
| HPLC | high-performance liquid chromatography |
| GC/MS | Gas chromatography–mass spectrometry |
| Lo | liquid-ordered |
| Ld | liquid-disordered |
| So | Solid-ordered |
| DOPC | 1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| Chol | cholesterol |
| Rhodamine SHPE | Lissamine™ rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine and triethylammonium salt |
| IA | isoamyl acetate |
| IVA | isovaleraldehyde |
| IAA | isoamyl alcohol |
| AI | artificial intelligence |
References
- Yoda, T.; Miyaki, H.; Saito, T. Freeze concentrated apple juice maintains its flavor. Sci. Rep. 2021, 11, 12679. [Google Scholar] [CrossRef]
- Yoda, T.; Saito, T. Size of Cells and Physicochemical Properties of Membranes are Related to Flavor Production during Sake Brewing in the Yeast Saccharomyces cerevisiae. Membranes 2020, 10, 440. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Kaneoke, M.; Hirata, D.; Watanabe, K.I. Analysis of free fatty acids in sake by an enzymatic method and its application for estimating ethyl caproate and selecting yeast with high productivity of the ester. Biosci. Biotechnol. Biochem. 2012, 76, 391–394. [Google Scholar] [CrossRef]
- Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Gu, L.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption1,2. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Kanda, T.; Ohtake, Y. Apple (Malus pumila) procyanidins fractionated according to the degree of polymerization using normal-phase chromatography and characterized by HPLC-ESI/MS and MALDI-TOF/MS. J. Chromatogr. A 2008, 1102, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T. Methods of Detection for Procyanidins on Apples. Available online: http://fmric.or.jp/ffd/ffmanual/manual40107.pdf (accessed on 31 January 2025). (English Title Translated by the Author, Written in Japanese).
- Yoda, T.; Vestergaard, M.C.; Akazawa-Ogawa, Y.; Yoshida, Y.; Hamada, T.; Takagi, M. Dynamic Response of a Cholesterol-containing Model Membrane to Oxidative Stress. Chem. Lett. 2010, 39, 1273–1274. [Google Scholar] [CrossRef]
- Yoda, T.; Vestergaard, M.C.; Hamada, T.; Le, P.T.M.; Takagi, M. Thermo-induced Vesicular Dynamics of Membranes Containing Cholesterol Derivatives. Lipids 2012, 47, 813–820. [Google Scholar] [CrossRef]
- Dhingra, S.; Morita, M.; Yoda, T.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Dynamic Morphological Changes Induced by GM1 and Protein Interactions on the Surface of Cell-Sized Liposomes. Materials 2013, 6, 2522–2533. [Google Scholar] [CrossRef]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Hata, T.; Morita, M.; Yoda, T.; Hamada, T.; Vestergaard, M.C.; Takagi, M. The effect of oxysterols on the interaction of Alzheimer’s amyloid beta with model membranes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2487–2495. [Google Scholar] [CrossRef]
- Yoda, T. Quality Evaluation of Drinks Based on Liposome Shape Changes Induced by Flavor Molecules. ACS Omega 2022, 7, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. Phase—Separated Structures of Sake Flavors—Containing Cell Model Membranes. Chem. Biodivers. 2023, 20, e202200750. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. Direct Observation of Cell—Sized Liposomes Containing a Functional Polyphenol Procyanidin B2 from Apple. ChemistrySelect 2022, 7, e202201808. [Google Scholar] [CrossRef]
- Yoda, T. The Flavonoid Molecule Procyanidin Reduces Phase Separation in Model Membranes. Membranes 2022, 12, 943. [Google Scholar] [CrossRef]
- Blosser, M.C.; Cornell, C.E.; Rayermann, S.P.; Keller, S.L. Phase diagrams and tie lines in giant unilamellar vesicles. In The Giant Vesicle Book; Dimova, R., Marques, C.M., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2022; Chapter 18; pp. 401–416. [Google Scholar]
- Yoda, T.; Yamada, Y.; Chounan, Y. Effects of isovaleraldehyde on cell-sized lipid bilayer vesicles. Biophys. Chem. 2021, 279, 106698. [Google Scholar] [CrossRef]
- Yoda, T. Phase Separation in Liposomes Determined by Ergosterol and Classified Using Machine Learning. Microsc. Microanal. 2022, 28, 2130–2137. [Google Scholar] [CrossRef]
- Yamazaki, S.; Nakada, M.; Osawa, K. Study on Functional Ingredients Contained in Apples and Leeks Produced in Nagano Prefecture. Available online: https://www.gitc.pref.nagano.lg.jp/reports/pdf/H29/H29F19.pdf (accessed on 11 July 2025). (In Japanese).
- Takahashi, T.; Ohara, Y.; Sueno, K. Breeding of a sake yeast mutant with enhanced ethyl caproate productivity in sake brewing using rice milled at a high polishing ratio. J. Biosci. Bioeng. 2017, 123, 707–713. [Google Scholar] [CrossRef]
- JAS 0024:2022; Determination of the Procyanidins in Apple Juice―High Performance Liquid Chromatographic Method. Singapore Food Agency: Singapore, 2022. Available online: https://www.maff.go.jp/j/jas/jas_kikaku/attach/pdf/kokujikaisei-226.pdf (accessed on 11 July 2025). (In Japanese)
- Arisawa, K.; Mitsudome, H.; Yoshida, K.; Sugimoto, S.; Ishikawa, T.; Fujiwara, Y.; Ichi, I. Saturated fatty acid in the phospholipid monolayer contributes to the formation of large lipid droplets. Biochem. Biophys. Res. Commun. 2016, 480, 641–647. [Google Scholar] [CrossRef]
- Hamada, T.; Miura, Y.; Komatsu, Y.; Kishimoto, Y.; Vestergaard, M.C.; Takagi, M. Construction of Asymmetric Cell-Sized Lipid Vesicles from Lipid-Coated Water-in-Oil Microdroplets. J. Phys. Chem. B 2008, 112, 14678–14681. [Google Scholar] [CrossRef]
- Morita, M.; Onoe, H.; Yanagisawa, M.; Ito, H.; Ichikawa, M.; Fujiwara, K.; Saito, H.; Takinoue, M. Droplet-Shooting and Size-Filtration (DSSF) Method for Synthesis of Cell-Sized Liposomes with Controlled Lipid Compositions. ChemBioChem 2015, 16, 2029–2035. [Google Scholar] [CrossRef]
- Akashi, K.; Miyata, H.; Itoh, H.; Kinosita, K., Jr. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 1996, 71, 3242–3250. [Google Scholar] [CrossRef]
- Akashi, K.; Miyata, H.; Itoh, H.; Kinosita, K., Jr. Formation of giant liposomes promoted by divalent cations: Critical role of electrostatic repulsion. Biophys. J. 1998, 74, 2973–2982. [Google Scholar] [CrossRef]
- Webb, C.; Khadke, S.; Schmidt, S.T.; Roces, C.B.; Forbes, N.; Berrie, G.; Perrie, Y. The Impact of Solvent Selection: Strategies to Guide the Manufacturing of Liposomes Using Microfluidics. Pharmaceutics 2019, 11, 653. [Google Scholar] [CrossRef]
- Himeno, H.; Shimokawa, N.; Komura, S.; Andelman, D.; Hamada, T.; Takagi, M. Charge-induced phase separation in lipid membranes. Soft Matter 2014, 10, 7959–7967. [Google Scholar] [CrossRef]
- Davis, J.H.; Clair, J.J.; Juhasz, J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009, 96, 521–539. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.F.M.; Borst, J.; Fedorov, A.; Prieto, M.; Visser, A.J.W.G. Complexity of Lipid Domains and Rafts in Giant Unilamellar Vesicles Revealed by Combining Imaging and Microscopic and Macroscopic Time-Resolved Fluorescence. Biophys. J. 2007, 93, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Morita, M.; Miyakawa, M.; Sugimoto, R.; Hatanaka, A.; Vestergaard, M.C.; Takagi, M. Size-Dependent Partitioning of Nano/Microparticles Mediated by Membrane Lateral Heterogeneity. J. Am. Chem. Soc. 2012, 134, 13990–13996. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Kishimoto, Y.; Nagasaki, T.; Takagi, M. Lateral phase separation in tense membranes. Soft Matter 2011, 7, 9061–9068. [Google Scholar] [CrossRef]
- Hamada, T.; Mizuno, S.; Kitahata, H. Domain dynamics of phase-separated lipid membranes under shear flow. Soft Matter 2022, 18, 9069–9075. [Google Scholar] [CrossRef]
- Sugahara, K.; Shimokawa, N.; Takagi, M. Destabilization of Phase-separated Structures in Local Anesthetic-containing Model Biomembranes. Chem. Lett. 2015, 44, 1604–1606. [Google Scholar] [CrossRef]
- Sugahara, K.; Shimokawa, N.; Takagi, M. Thermal Stability of Phase-Separated Domains in Multicomponent Lipid Membranes with Local Anesthetics. Membranes 2017, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. Micro-Scale Phase-Separation Liposome Detection System of Flavor Concentrations in Sake, a Traditional Alcoholic Drink in Japan. In Proceedings of the 2022 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 27–30 November 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Nair, P.; Christian, D.; Discher, D.E. Polymersomes. In The Giant Vesicle Book; Dimova, R., Marques, C.M., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2022; Chapter 26; pp. 537–550. [Google Scholar]
- Wang, R.; Dang, M.; Zhu, W.; Li, C. Galloyl Group in B-type Proanthocyanidin Dimers Was Responsible for Its Differential Inhibitory Activity on 3T3-L1 Preadipocytes due to the Strong Lipid Raft-Perturbing Potency. J. Agric. Food Chem. 2021, 69, 5216–5225. [Google Scholar] [CrossRef] [PubMed]
- Sambre, P.D.; Ho, J.C.S.; Parikh, A.N. Intravesicular Solute Delivery and Surface Area Regulation in Giant Unilamellar Vesicles Driven by Cycles of Osmotic Stresses. J. Am. Chem. Soc. 2024, 146, 3250–3261. [Google Scholar] [CrossRef] [PubMed]
- Yoda, T. Materials evaluation using cell-sized liposomes. Anal. Methods 2024, 16, 5509–5518. [Google Scholar] [CrossRef]
- Yoda, T. Assessment of beverage quality for ethyl caproate and procyanidin B2 utilizing binary liposomes. Anal. Methods 2024, 16, 6845–6855. [Google Scholar] [CrossRef]
- Hamada, T.; Sato, Y.T.; Nagasaki, T.; Yoshikawa, K. Reversible Photoswitching in a Cell-Sized Vesicle. Langmuir 2005, 21, 7626–7628. [Google Scholar] [CrossRef]
- Ishii, K.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible Control of Exo- and Endo-Budding Transitions in a Photosensitive Lipid Membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef]
- Hamada, T.; Sugimoto, R.; Vestergaard, M.; Nagasaki, T.; Takagi, M. Membrane Disk and Sphere: Controllable Mesoscopic Structures for the Capture and Release of a Targeted Object. J. Am. Chem. Soc. 2010, 132, 10528–10532. [Google Scholar] [CrossRef]
- Hamada, T.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Photochemical control of membrane raft organization. Soft Matter 2011, 7, 220–224. [Google Scholar] [CrossRef]
- Muraoka, T.; Shima, T.; Hamada, T.; Morita, M.; Takagi, M.; Tabata, K.V.; Noji, H.; Kinbara, K. Ion Permeation by a Folded Multiblock Amphiphilic Oligomer Achieved by Hierarchical Construction of Self-Assembled Nanopores. J. Am. Chem. Soc. 2012, 134, 19788–19794. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Kuhl, T.L.; Umakoshi, H. Melting-Temperature-Dependent Interactions of Ergosterol with Unsaturated and Saturated Lipids in Model Membranes. Langmuir 2019, 35, 10640–10647. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Ergosterol-Induced Ordered Phase in Ternary Lipid Mixture Systems of Unsaturated and Saturated Phospholipid Membranes. J. Phys. Chem. B 2019, 123, 6161–6168. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Vestergaard, M.; Hamada, T.; Takagi, M. Real-time observation of model membrane dynamics induced by Alzheimer’s amyloid beta. Biophys. Chem. 2010, 147, 81–86. [Google Scholar] [CrossRef]
- Morita, M.; Hamada, T.; Tendo, Y.; Hata, T.; Vestergaard, M.C.; Takagi, M. Selective localization of Alzheimer’s amyloid beta in membrane lateral compartments. Soft Matter 2012, 8, 2816–2819. [Google Scholar] [CrossRef]
- Hamada, T.; Hagihara, H.; Morita, M.; Vestergaard, M.C.; Tsujino, Y.; Takagi, M. Physicochemical Profiling of Surfactant-Induced Mem-brane Dynamics in a Cell-Sized Liposome. J. Phys. Chem. Lett. 2012, 3, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.C.; Morita, M.; Hamada, T.; Takagi, M. Membrane fusion and vesicular transformation induced by Alzheimer’s amyloid beta. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hamada, T.; Vestergaard, M.C.; Takagi, M. Endo- and exocytic budding transformation of slow-diffusing membrane domains induced by Alzheimer’s amyloid beta. Phys. Chem. Chem. Phys. 2014, 16, 8773–8777. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Touhara, K. Myr-Ric-8A Enhances Gα15-Mediated Ca2+ Response of Vertebrate Olfactory Receptors. Chem. Senses 2009, 34, 15–23. [Google Scholar] [CrossRef]
- Katada, S.; Hirokawa, T.; Oka, Y.; Suwa, M.; Touhara, K. Structural Basis for a Broad but Selective Ligand Spectrum of a Mouse Olfactory Receptor: Mapping the Odorant-Binding Site. J. Neurosci. 2005, 25, 1806–1815. [Google Scholar] [CrossRef]
- Kelkar, D.A.; Chattopadhyay, A. The gramicidin ion channel: A model membrane protein. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2011–2025. [Google Scholar] [CrossRef]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The crystal structure of a voltage-gated sodium channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Ma, B.; Shi, S.; Chen, Y.; Zhang, H.; Zhan, Y.; An, H. Procyanidin B1, a novel and specific inhibitor of Kv10.1 channel, suppresses the evolution of hepatoma. Biochem. Pharmacol. 2020, 178, 114089. [Google Scholar] [CrossRef]
- Osica, I.; Melo, A.F.A.A.; Lima, F.C.D.A.; Shiba, K.; Imamura, G.; Crespilho, F.N.; Betlej, J.; Kurzydowski, K.J.; Yoshikawa, G.; Ariga, K. Nanomechanical Recognition and Discrimination of Volatile Molecules by Au Nanocages Deposited on Membrane-Type Surface Stress Sensors. ACS Appl. Nano Mater. 2020, 3, 4061–4068. [Google Scholar] [CrossRef]
- Shiba, K.; Yoshikawa, G. Aero-Thermo-Dynamic Mass Analysis. Sci. Rep. 2016, 6, 28849. [Google Scholar] [CrossRef] [PubMed]
- Genç, İ.Y.; Remzi, G.; Açikgözoğlu, E. Quality Determination of Frozen-Thawed Shrimp Using Machine Learning Algorithms Powered by Explainable Artificial Intelligence. Food Anal. Methods 2025, 18, 935–945. [Google Scholar] [CrossRef]
- Uchida, N.; Ryu, Y.; Takagi, Y.; Yoshizawa, K.; Suzuki, K.; Anraku, Y.; Ajioka, I.; Shimokawa, N.; Takagi, M.; Hoshino, N.; et al. Endocytosis-Like Vesicle Fission Mediated by a Membrane-Expanding Molecular Machine Enables Virus Encapsulation for In Vivo Delivery. J. Am. Chem. Soc. 2023, 145, 6210–6220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).