Facile Microwave Production and Photocatalytic Activity of Bismuth Vanadate Nanoparticles over the Acid Orange 7
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BiVO4
2.3. Characterization
2.4. Evaluation of Photocatalytic Activity
2.5. Antibacterial Properties
3. Results and Discussion
3.1. Structural and Morphological Properties of the Microwave-Synthesized BiVO4
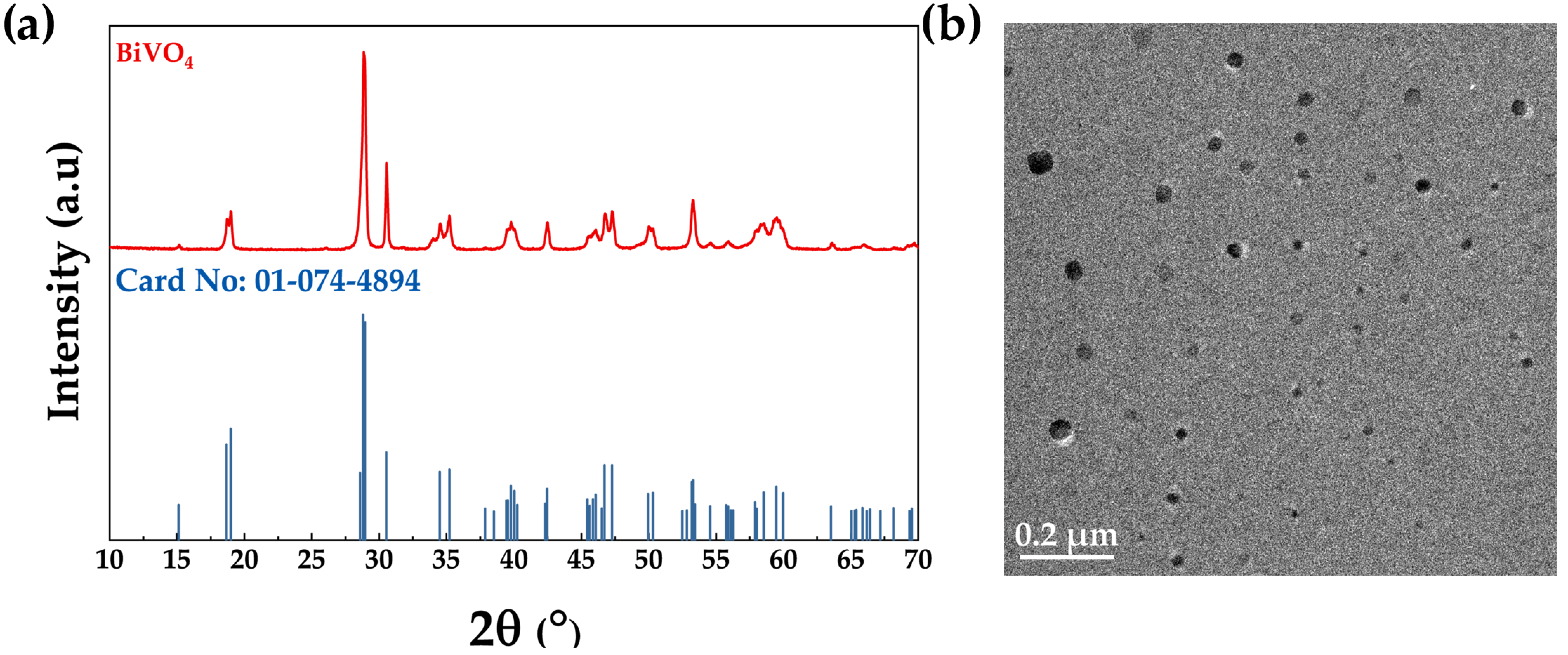
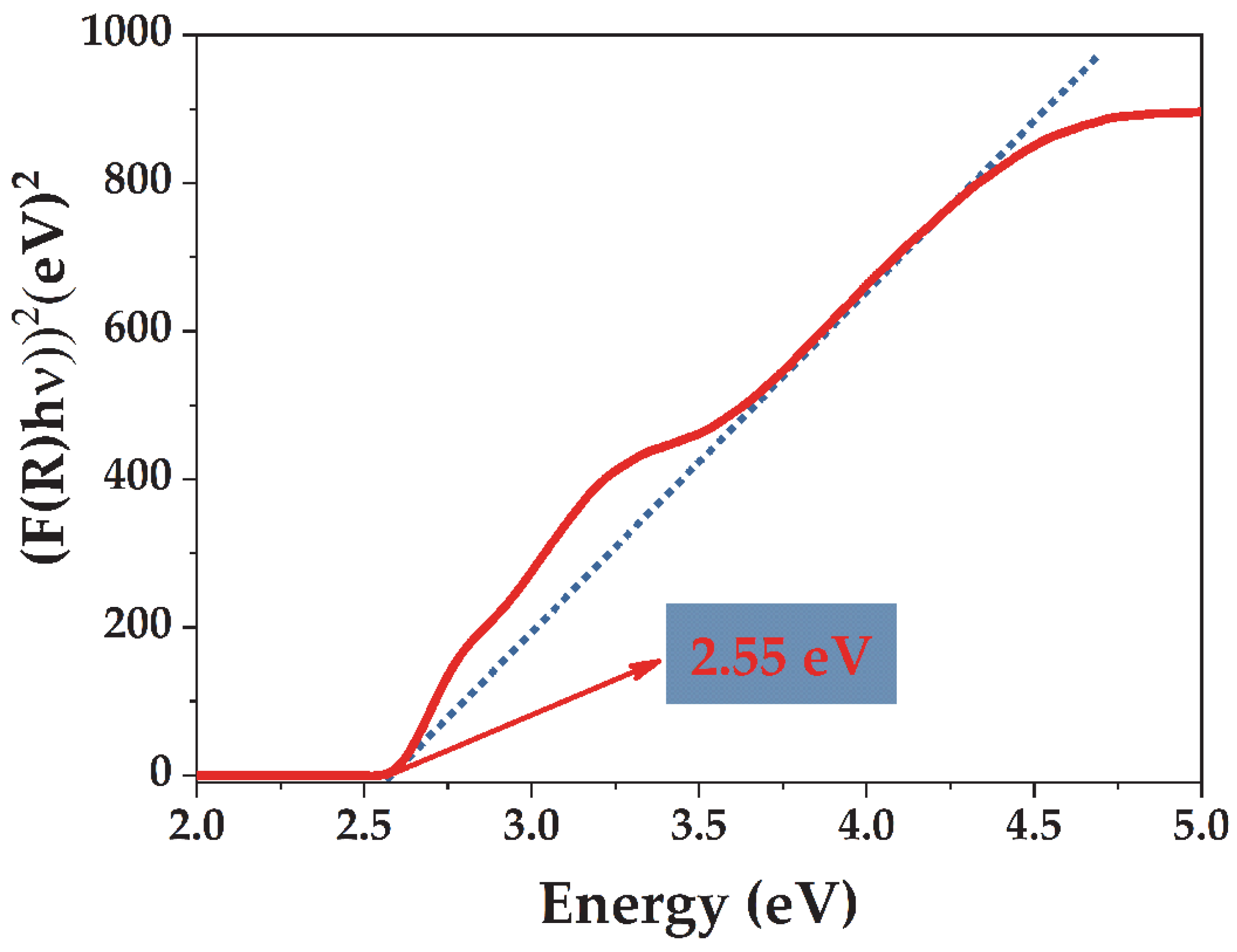
3.2. Textural, Vibrational, and Optical Properties of the Microwave-Synthesized BiVO4
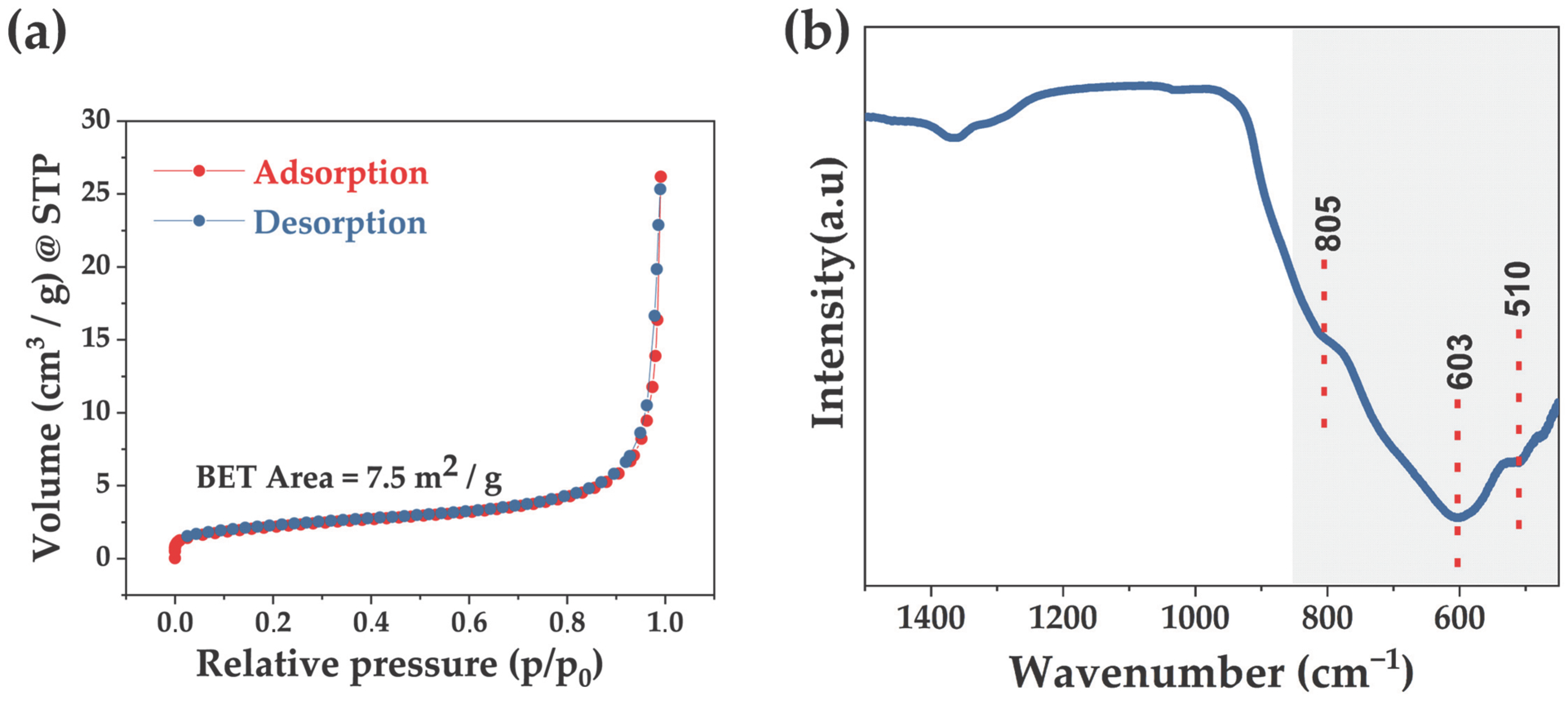
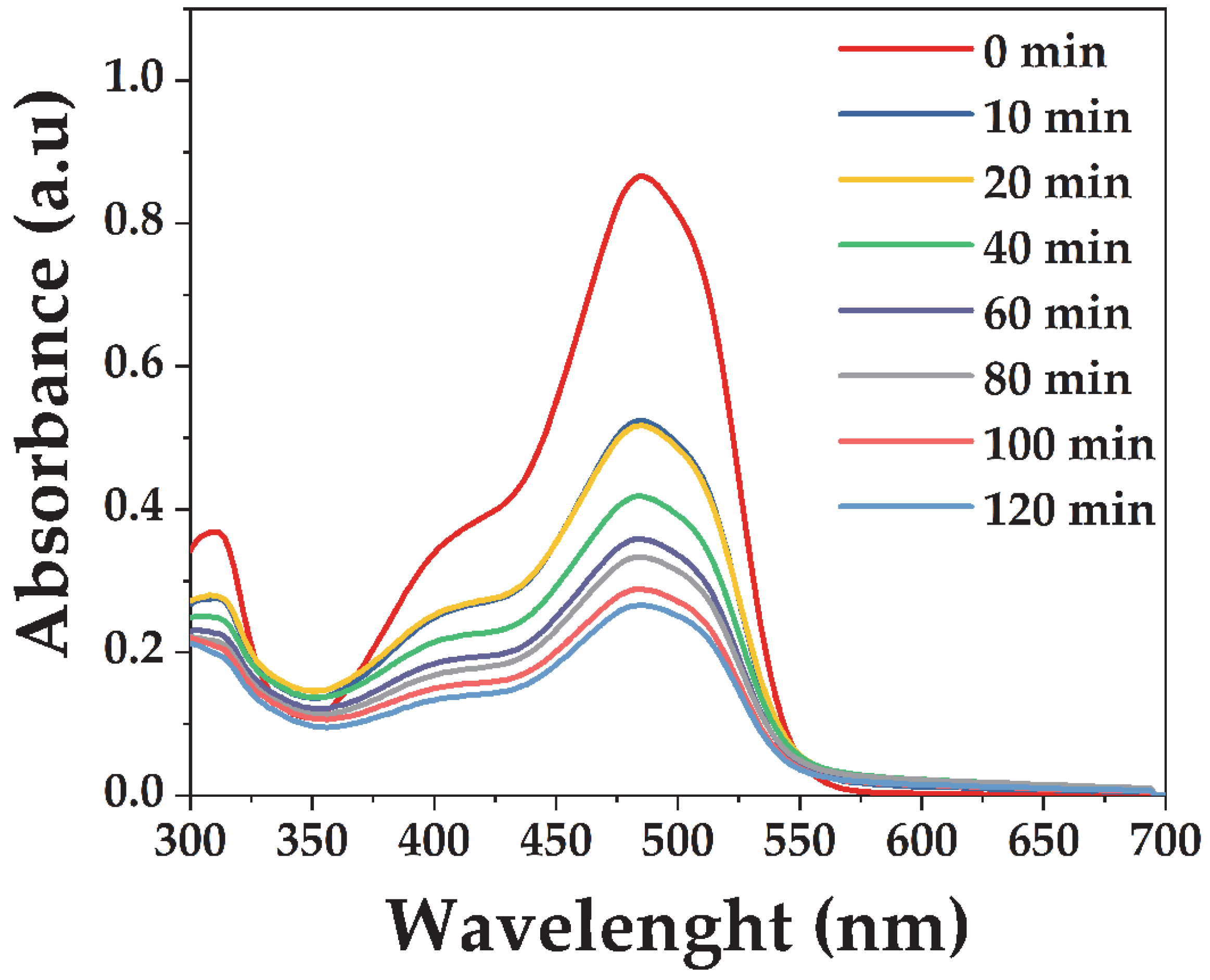
3.3. Photocatalytic Performance of the Microwave-Synthesized BiVO4
3.3.1. The Effect of Catalyst Dosage on the Degradation of Acid Orange 7
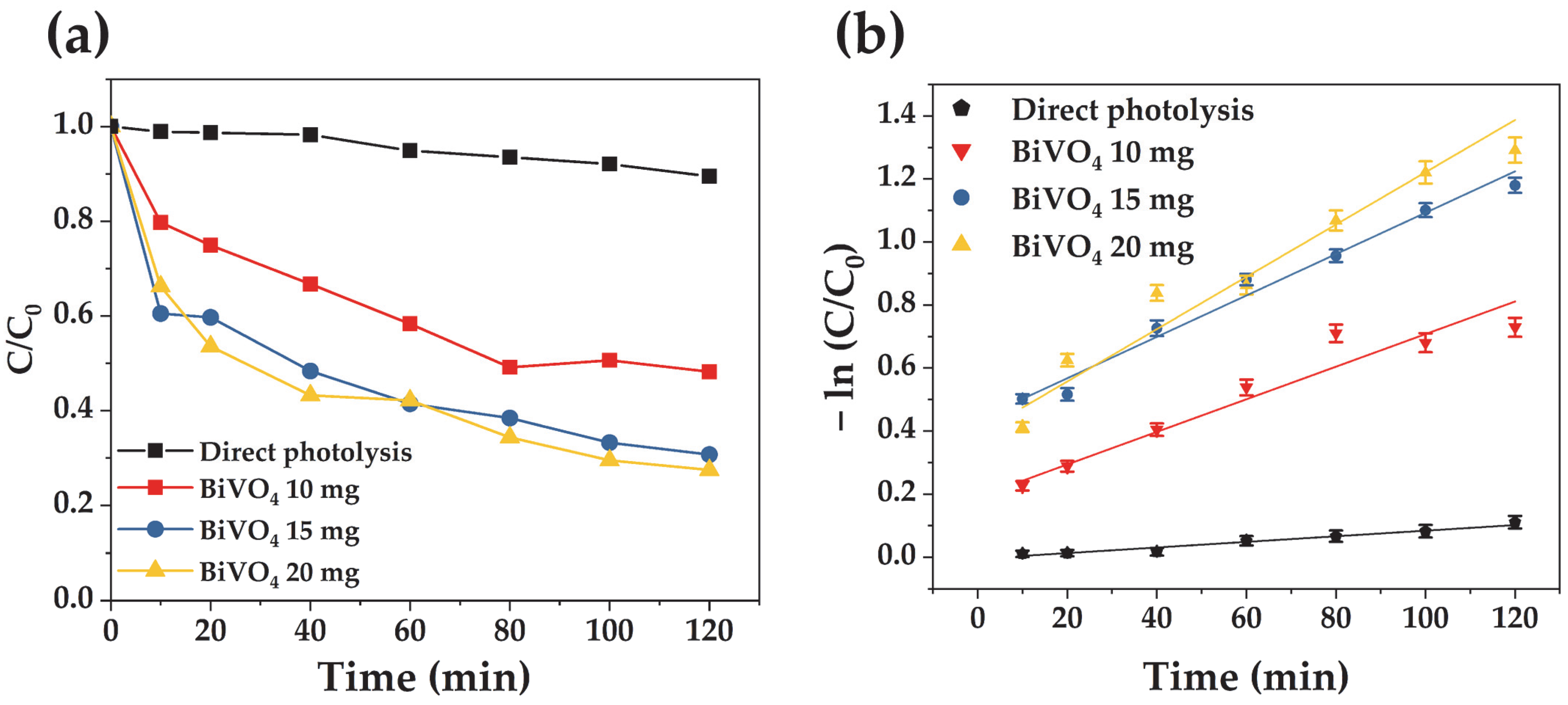
3.3.2. Influence of the Initial Acid Orange 7 Concentration on Visible-Light Photodegradation Performance

3.4. Possible Photocatalytic Pathway for the Degradation of Acid Orange 7
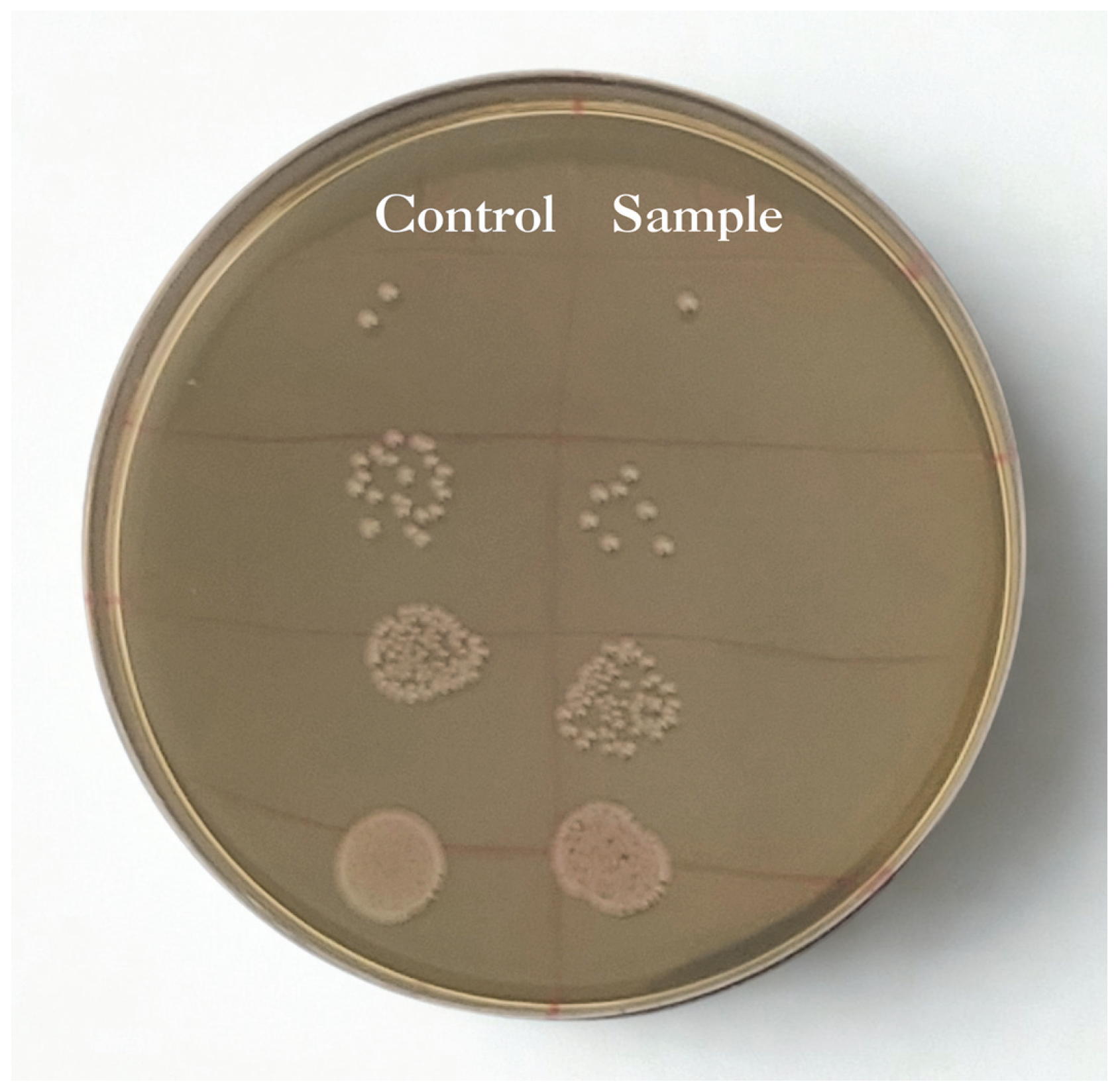
3.5. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghimi Dehkordi, M.; Pournuroz Nodeh, Z.; Soleimani Dehkordi, K.; Salmanvandi, H.; Rasouli Khorjestan, R.; Ghaffarzadeh, M. Soil, Air, and Water Pollution from Mining and Industrial Activities: Sources of Pollution, Environmental Impacts, and Prevention and Control Methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Shetty, S.S.; D, D.; S, H.; Sonkusare, S.; Naik, P.B.; Kumari N, S.; Madhyastha, H. Environmental Pollutants and Their Effects on Human Health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Kusumlata; Ambade, B.; Kumar, A.; Gautam, S. Sustainable Solutions: Reviewing the Future of Textile Dye Contaminant Removal with Emerging Biological Treatments. Limnol. Rev. 2024, 24, 126–149. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of Textile Dyes in Aquatic Environment: Adverse Impacts on Aquatic Ecosystem and Human Health, and Its Management Using Bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Daneshvar, N.; Aber, S.; Hosseinzadeh, F. Study of C.I. Acid Orange 7 Removal in Contaminated Water by Photo Oxidation Processes. Glob. NEST J. 2008, 10, 16–23. [Google Scholar] [CrossRef]
- Kathi, S.; El Din Mahmoud, A. Trends in Effective Removal of Emerging Contaminants from Wastewater: A Comprehensive Review. Desalination Water Treat. 2024, 317, 100258. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]
- Tanos, F.; Razzouk, A.; Lesage, G.; Cretin, M.; Bechelany, M. A Comprehensive Review on Modification of Titanium Dioxide-Based Catalysts in Advanced Oxidation Processes for Water Treatment. ChemSusChem 2024, 17, e202301139. [Google Scholar] [CrossRef]
- Pham, H.-C.; Kim, K.-S. Effect of TiO2 Thin Film Thickness on NO and SO2 Removals by Dielectric Barrier Discharge-Photocatalyst Hybrid Process. Ind. Eng. Chem. Res. 2013, 52, 5296–5301. [Google Scholar] [CrossRef]
- Guo, Q.; Ma, Z.; Zhou, C.; Ren, Z.; Yang, X. Single Molecule Photocatalysis on TiO2 Surfaces: Focus Review. Chem. Rev. 2019, 119, 11020–11041. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Kaiba, A.; Alansi, A.M.; Oubelkacem, A.; Chabri, I.; Hameed, S.T.; Afzal, N.; Rafique, M.; Qahtan, T.F. Sunlight-Driven Synthesis of TiO2/(MA)2SnCl4 Nanocomposite Films for Enhanced Photocatalytic Degradation of Organic Pollutants. Catalysts 2025, 15, 214. [Google Scholar] [CrossRef]
- Ishigaki, T.; Nakada, Y.; Tarutani, N.; Uchikoshi, T.; Tsujimoto, Y.; Isobe, M.; Ogata, H.; Zhang, C.; Hao, D. Enhanced Visible-Light Photocatalytic Activity of Anatase-Rutile Mixed-Phase Nano-Size Powder given by High-Temperature Heat Treatment. R. Soc. Open Sci. 2020, 7, 191539. [Google Scholar] [CrossRef]
- Kamble, G.S.; Natarajan, T.S.; Patil, S.S.; Thomas, M.; Chougale, R.K.; Sanadi, P.D.; Siddharth, U.S.; Ling, Y.-C. BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials 2023, 13, 1528. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Štrbac, D.; Far, L.Đ.; Dramićanin, M.D. Improved Coloristic Properties and High NIR Reflectance of Environment-Friendly Yellow Pigments Based on Bismuth Vanadate. Ceram. Int. 2018, 44, 22731–22737. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Smits, K.; Babić, B.; Marinović-Cincović, M.; Porobić, S.; Dramićanin, M.D. A Comparative Study of Photocatalytically Active Nanocrystalline Tetragonal Zyrcon-Type and Monoclinic Scheelite-Type Bismuth Vanadate. Ceram. Int. 2018, 44, 17953–17961. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Choi, K.S.; Shin, H.-M.; Kim, T.L.; Song, J.; Yoon, S.; Jang, H.W.; Yoon, M.-H.; Jeon, C.; Lee, J.; et al. Enhanced Photocatalytic Performance Depending on Morphology of Bismuth Vanadate Thin Film Synthesized by Pulsed Laser Deposition. ACS Appl. Mater. Interfaces 2017, 9, 505–512. [Google Scholar] [CrossRef]
- Jelić, S.T.; Ćirković, J.; Jovanović, J.; Novaković, T.; Podlogar, M.; Mitrić, J.; Branković, G.; Branković, Z. High Efficiency Solar Light Photocatalytic Degradation of Mordant Blue 9 by Monoclinic BiVO4 Nanopowder. Mater. Chem. Phys. 2025, 333, 130341. [Google Scholar] [CrossRef]
- Regmi, C.; Dhakal, D.; Lee, S.W. Visible-Light-Induced Ag/BiVO4 Semiconductor with Enhanced Photocatalytic and Antibacterial Performance. Nanotechnology 2018, 29, 064001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, J. Effects of Europium Doping on the Photocatalytic Behavior of BiVO4. J. Hazard. Mater. 2010, 173, 265–272. [Google Scholar] [CrossRef]
- Drisya, K.T.; Solís-López, M.; Ríos-Ramírez, J.J.; Durán-Álvarez, J.C.; Rousseau, A.; Velumani, S.; Asomoza, R.; Kassiba, A.; Jantrania, A.; Castaneda, H. Electronic and Optical Competence of TiO2/BiVO4 Nanocomposites in the Photocatalytic Processes. Sci. Rep. 2020, 10, 13507. [Google Scholar] [CrossRef]
- Ran, J.-H.; Fei, X.; Ni, L.; Telegin, F. Enhanced Photocatalytic Degradation of Acid Orange 7 by AgBr/BiVO4 under Visible Light. J. Fiber Bioeng. Inform. 2018, 11, 151–161. [Google Scholar] [CrossRef]
- Bulut, D.T. Exploring the Dual Role of BiVO4 Nanoparticles: Unveiling Enhanced Antimicrobial Efficacy and Photocatalytic Performance. J. Sol-Gel Sci. Technol. 2025, 114, 198–222. [Google Scholar] [CrossRef]
- Pramila, S.; Nagaraju, G.; Mallikarjunaswamy, C.; Latha, K.C.; Chandan, S.; Ramu, R.; Rashmi, V.; Lakshmi Ranganatha, V. Green Synthesis of BiVO4 Nanoparticles by Microwave Method Using Aegle marmelos Juice as a Fuel: Photocatalytic and Antimicrobial Study. Anal. Chem. Lett. 2020, 10, 298–306. [Google Scholar] [CrossRef]
- Marinković, D.; Righini, G.C.; Ferrari, M. Advances in Synthesis and Applications of Bismuth Vanadate-Based Structures. Inorganics 2025, 13, 268. [Google Scholar] [CrossRef]
- Marinković, D.; Righini, G.C.; Ferrari, M. Synthesis, Optical, and Photocatalytic Properties of the BiVO4 Semiconductor Nanoparticles with Tetragonal Zircon-Type Structure. Photonics 2025, 12, 438. [Google Scholar] [CrossRef]
- Li, B.; Gao, X.; Qu, J.; Xiong, F.; Xuan, H.; Jin, Y.; Yuan, H. Visible-Light-Driven Antimicrobial Activity and Mechanism of Polydopamine-Reduced Graphene Oxide/BiVO4 Composite. Int. J. Mol. Sci. 2022, 23, 7712. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Yu, H.-Q.; Li, Q.-R. Radiolytic Degradation of Acid Orange 7: A Mechanistic Study. Chemosphere 2005, 61, 1003–1011. [Google Scholar] [CrossRef]
- Iqbal, N.; Huang, X.; Mohamedali Hamid, K.; Yuan, H.; Batool, I.; Yang, Y. Efficient Visible-Light-Driven Photocatalysis of BiVO4@Diatomite for Degradation of Methoxychlor. Catalysts 2025, 15, 672. [Google Scholar] [CrossRef]
- Maran, M.A.; Zheng, A.L.T.; Tan, H.Y.; Sarbini, S.R.; Tan, K.B.; Boonyuen, S.; Wong, K.K.S.; Chung, E.L.T.; Lease, J.; Andou, Y. Assessing the Photocatalytic Performance of Hydrothermally Synthesized Fe-Doped BiVO4 Under Low-Intensity UV Irradiation. Arab. J. Sci. Eng. 2025, in press. [Google Scholar] [CrossRef]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L. Visible-Light-Driven AO7 Photocatalytic Degradation and Toxicity Removal at Bi-Doped SrTiO3. Materials 2022, 15, 2465. [Google Scholar] [CrossRef] [PubMed]
- Reza, K.M.; Kurny, A.; Gulshan, F. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2: A Review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct Use of the Langmuir–Hinshelwood Equation for Proving the Absence of a Synergy Effect in the Photocatalytic Degradation of Phenol on a Suspended Mixture of Titania and Activated Carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Chansaenpak, K.; Channei, D. Evaluating the Photocatalytic Efficiency of the BiVO4/rGO Photocatalyst. Sci. Rep. 2019, 9, 16091. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Bonelli, B.; Esposito, S.; Freyria, F.S.; Blangetti, N.; Sannino, D. Visible Light-Driven Photocatalytic Activity and Kinetics of Fe-Doped TiO2 Prepared by a Three-Block Copolymer Templating Approach. Materials 2021, 14, 3105. [Google Scholar] [CrossRef]
- Abdullahi, N.; Saion, E.; Shaari, A.H.; Al-Hada, N.M.; Keiteb, A. Optimisation of the Photonic Efficiency of TiO2 Decorated on MWCNTs for Methylene Blue Photodegradation. PLoS ONE 2015, 10, e0125511. [Google Scholar] [CrossRef]
- Viswanathan, B. Photocatalytic Degradation of Dyes: An Overview. Curr. Catal. 2018, 7, 99–121. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-Light-Activated TiO2-Based Photocatalysts for the Inactivation of Pathogenic Bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Narváez, B.; Mendoza-Mendoza, E.; Peralta-Rodríguez, R.; Bach, H.; Barriga-Castro, E.; Segovia-Sandoval, S.; Martinez-Gutierrez, F. Visible-Leds-Induced Enhanced Photocatalytic and Antibacterial Activity of BiVO4 Based Green Photocatalysts Decorated with Silver and Graphene. J. Photochem. Photobiol. A Chem. 2023, 447, 115191. [Google Scholar] [CrossRef]

| Reagent Name | Chemical Formula | Purity | CAS No. |
|---|---|---|---|
| Bismuth nitrate pentahydrate | Bi(NO3)3 × 5H2O | ≥99% (AR) | 10035-06-0 |
| Ammonium metavanadate | NH4VO3 | 99% | 7803-55-6 |
| Nitric acid | HNO3 | ≥99% (AR) | 7697-37-2 |
| Acid Orange 7(AO7) | C16H11N2NaO4S | 95% (dye grade) | 633-96-5 |
| Run | AO7 (ppm) | Catalyst Mass (mg) | k (min−1) | R2 |
|---|---|---|---|---|
| Mass series at AO7 = 20 ppm | ||||
| m1 | 20 | 10 | 0.00635 | 0.967 |
| m2 | 20 | 15 | 0.00963 | 0.996 |
| m3 | 20 | 20 | 0.01036 | 0.989 |
| Concentration series at mass 15 mg | ||||
| C1 | 15 | 15 | 0.00983 | 0.995 |
| C2 | 20 | 15 | 0.00963 | 0.996 |
| C3 | 25 | 15 | 0.00811 | 0.983 |
| Photocatalyst | Pollutant | m (mg) | C0 (ppm) | Light Source | k (min−1) | Ref. |
|---|---|---|---|---|---|---|
| TiO2 P25 | Acid Orange 7 | 100 | 10 | 10 W white LEDs (visible) | 0.0016 | [40] |
| TiO2 P25 | Methylene Blue | 100 | 10 | 500 W halogen | 0.0036 | [41] |
| BiVO4 | Acid Orange 7 | 15 | 20 | 300 W Osram Vitalux lamp | 0.00963 | This work |
| BiVO4 | Mordant Blue 9 | 100 | 10 | 300 W solar simulator | 0.0126 | [21] |
| BiVO4/TiO2 | Acid Blue 113 | 1000 | 40 | 1.6 kW Xe lamp | 0.083 | [24] |
| Eu/BiVO4 | Methyl Orange | 200 | 10 | 500 W Xe lamp | 0.0039 | [23] |
| Ag/BiVO4 | Methylene Blue | 100 | 10 | Visible light (λ ≥ 420 nm) | 0.031 | [22] |
| Ag/BiVO4 | Rhodamine B | 100 | 10 | Visible light (λ ≥ 420 nm) | 0.023 | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tot, N.; Vasiljević, B.; Davidović, S.; Pustak, A.; Marić, I.; Prekodravac Filipović, J.; Marinković, D. Facile Microwave Production and Photocatalytic Activity of Bismuth Vanadate Nanoparticles over the Acid Orange 7. Processes 2025, 13, 3485. https://doi.org/10.3390/pr13113485
Tot N, Vasiljević B, Davidović S, Pustak A, Marić I, Prekodravac Filipović J, Marinković D. Facile Microwave Production and Photocatalytic Activity of Bismuth Vanadate Nanoparticles over the Acid Orange 7. Processes. 2025; 13(11):3485. https://doi.org/10.3390/pr13113485
Chicago/Turabian StyleTot, Nataša, Bojana Vasiljević, Slađana Davidović, Anđela Pustak, Ivan Marić, Jovana Prekodravac Filipović, and Dragana Marinković. 2025. "Facile Microwave Production and Photocatalytic Activity of Bismuth Vanadate Nanoparticles over the Acid Orange 7" Processes 13, no. 11: 3485. https://doi.org/10.3390/pr13113485
APA StyleTot, N., Vasiljević, B., Davidović, S., Pustak, A., Marić, I., Prekodravac Filipović, J., & Marinković, D. (2025). Facile Microwave Production and Photocatalytic Activity of Bismuth Vanadate Nanoparticles over the Acid Orange 7. Processes, 13(11), 3485. https://doi.org/10.3390/pr13113485










