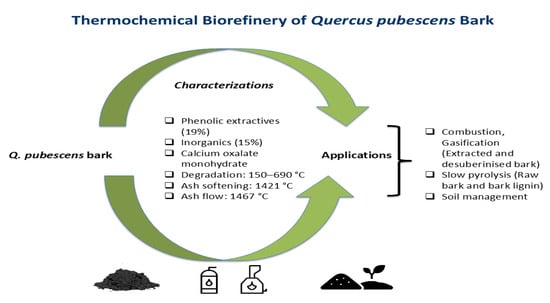
Chemical Composition and Reactivity of Quercus pubescens Bark and Bark Fractions for Thermochemical Biorefinery Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Structural and Chemical Characterization
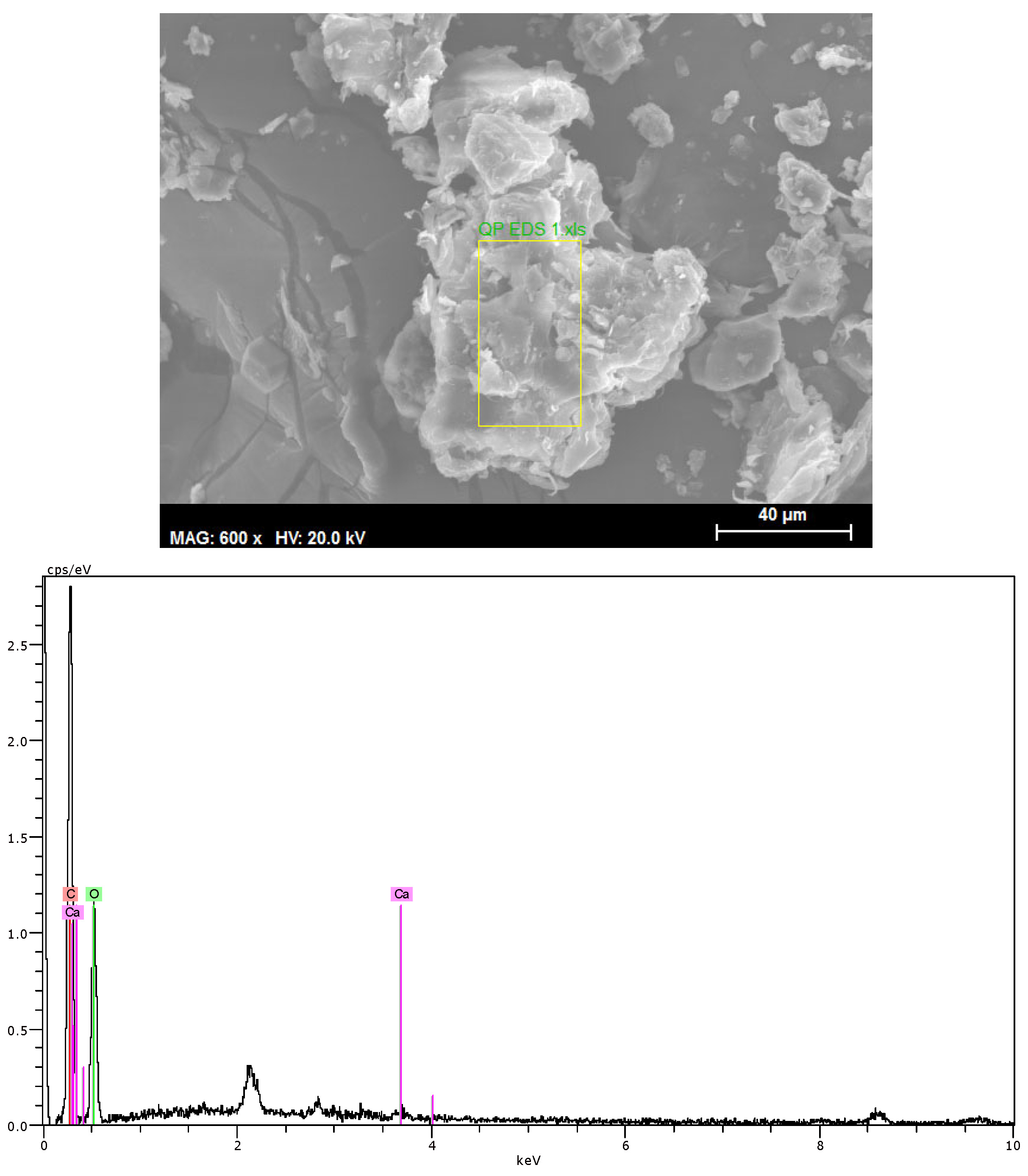
2.2.1. Scanning Electron Microscopy
2.2.2. X-Ray Diffraction Analysis
2.2.3. FT-IR Analysis
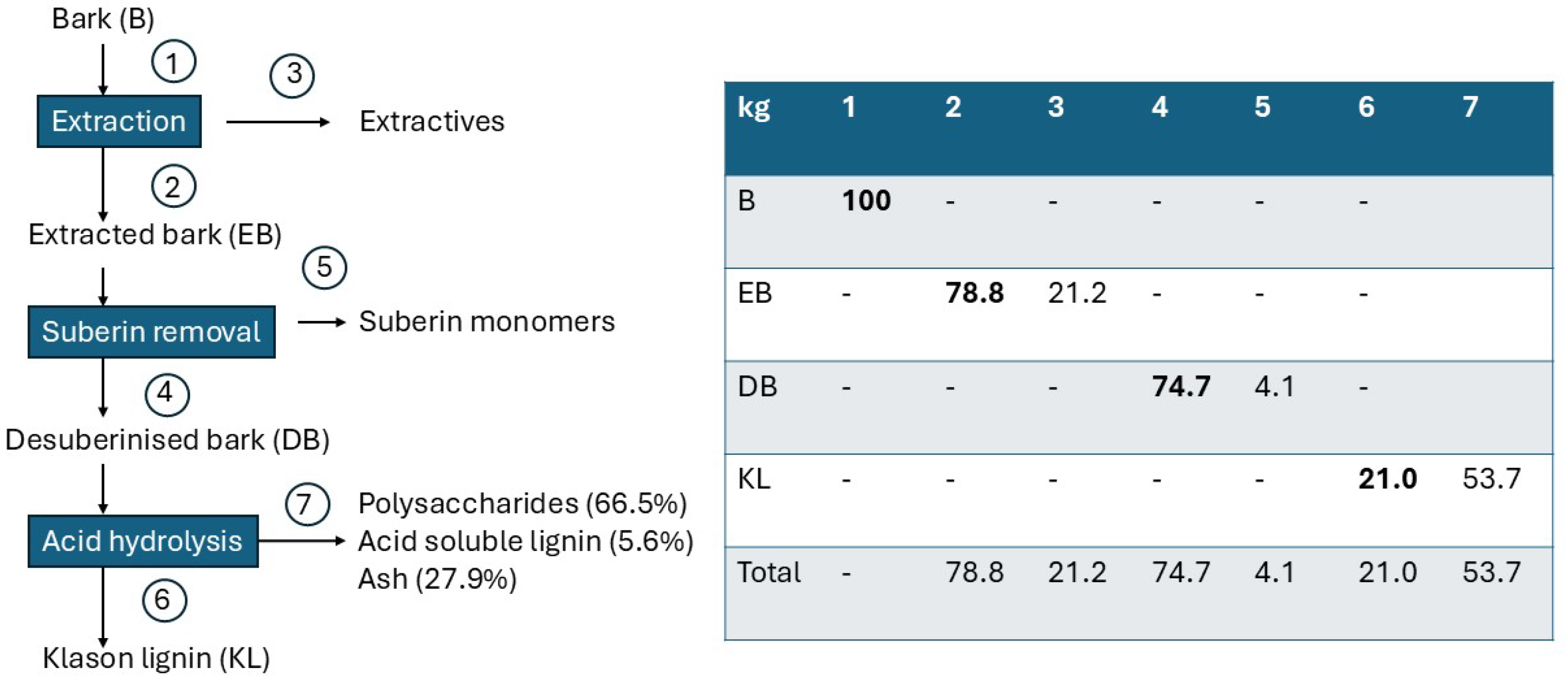
2.2.4. Chemical Analysis and Fractionation
2.2.5. XRF Analysis
2.3. Thermochemical Characteristics
2.3.1. Thermogravimetric Analysis
2.3.2. Pyrolysis Kinetics
2.3.3. Solid Fuel Characterization
2.4. Slagging and Fouling Behavior
3. Results and Discussion
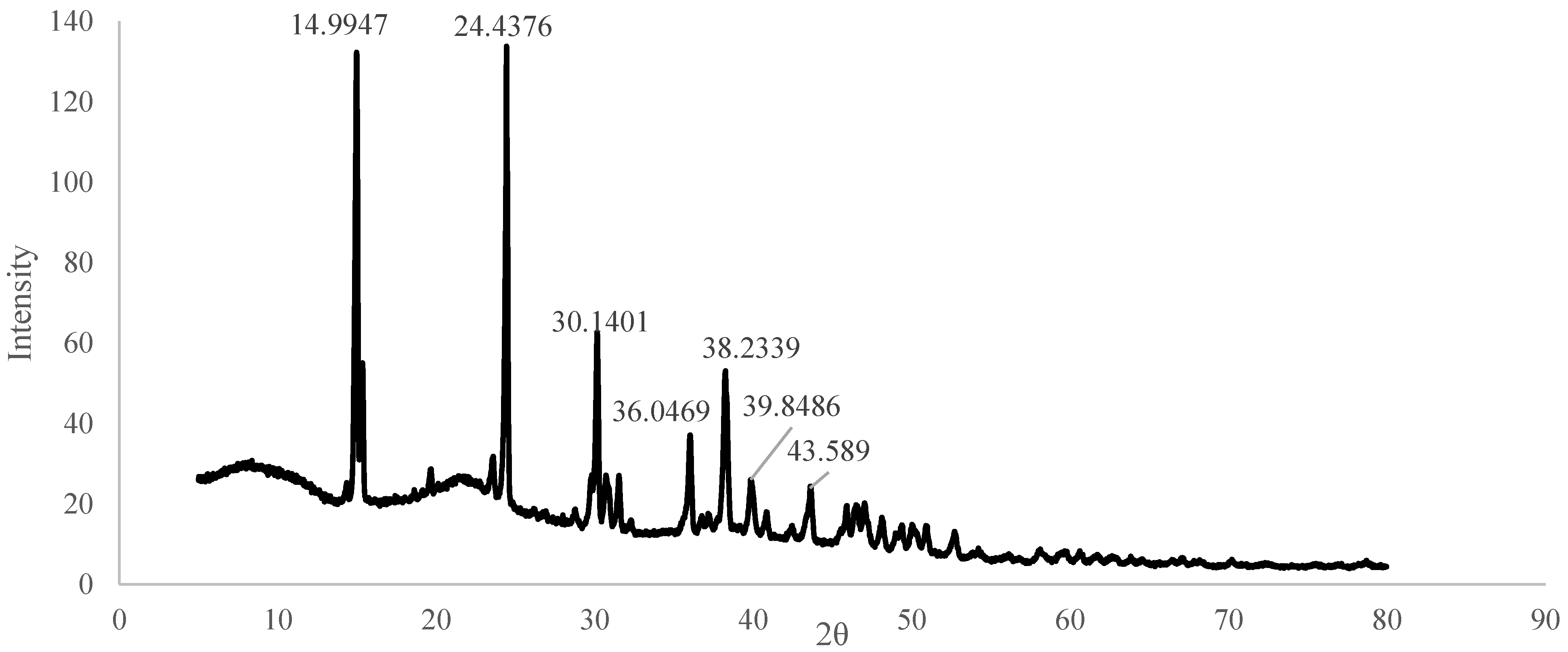
3.1. Structural Characterization
3.2. Chemical Composition
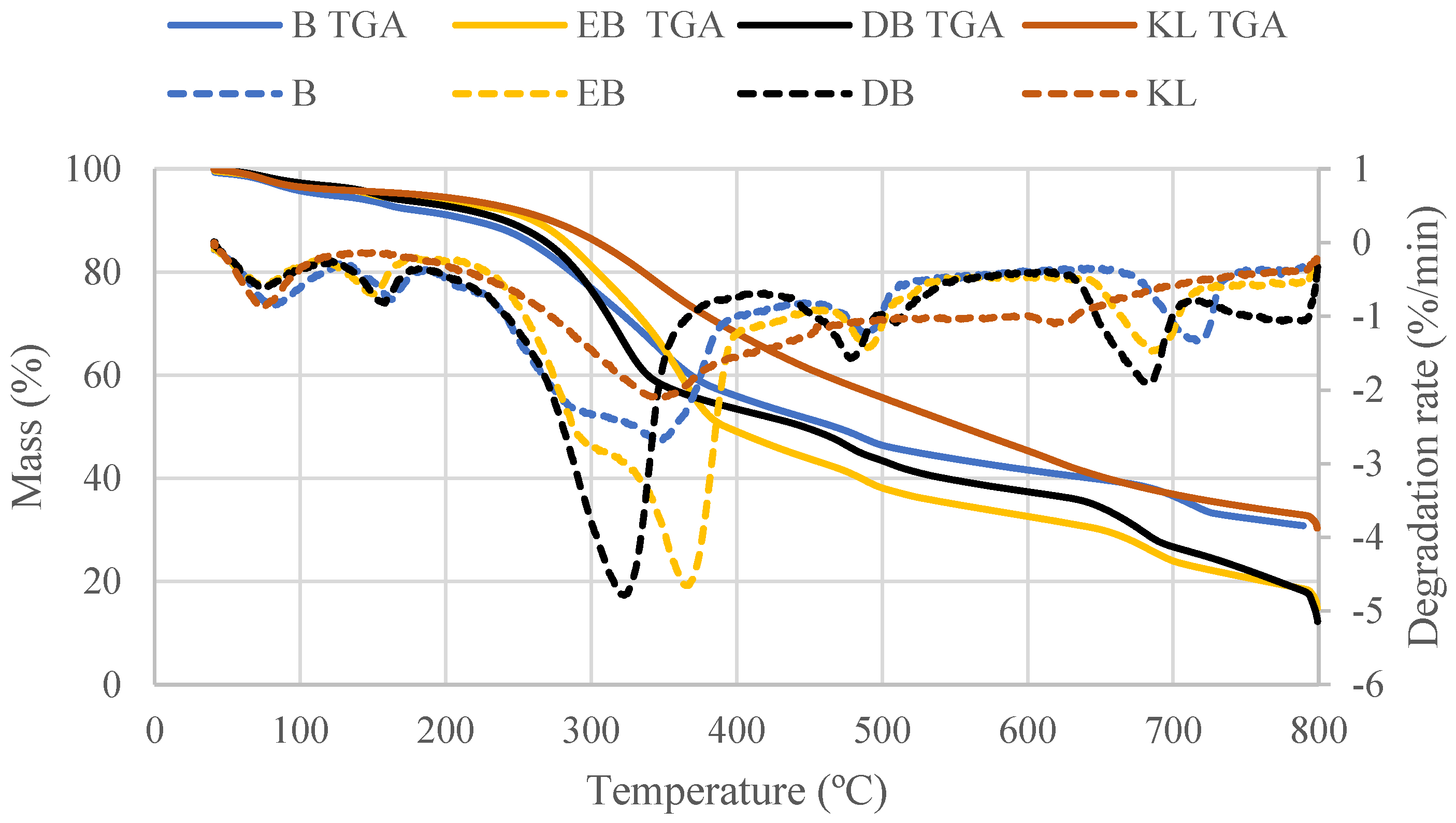
3.3. Thermochemical Behavior
3.3.1. Pyrolysis Behavior
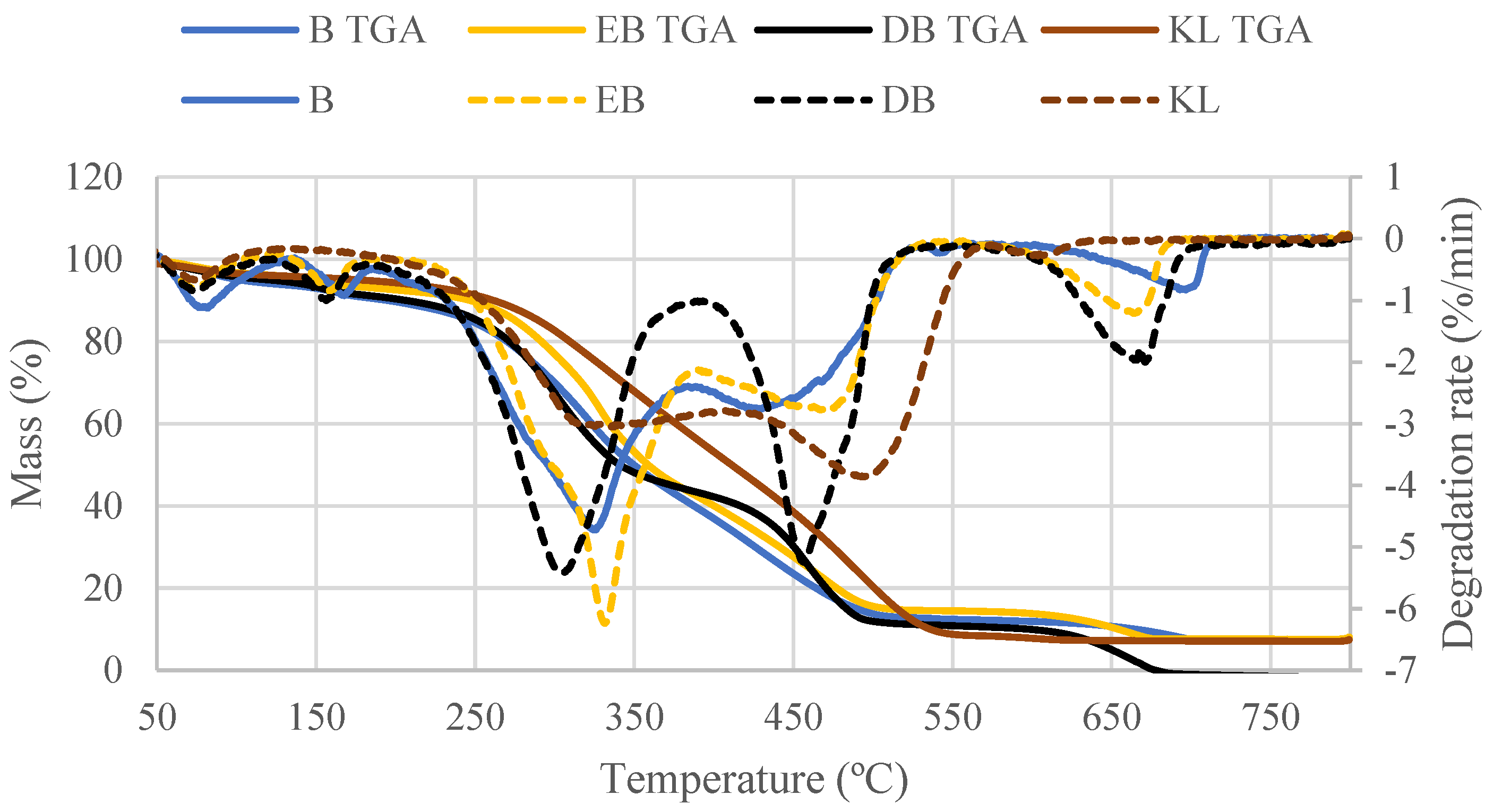
3.3.2. Combustion Behavior and Fuel Properties
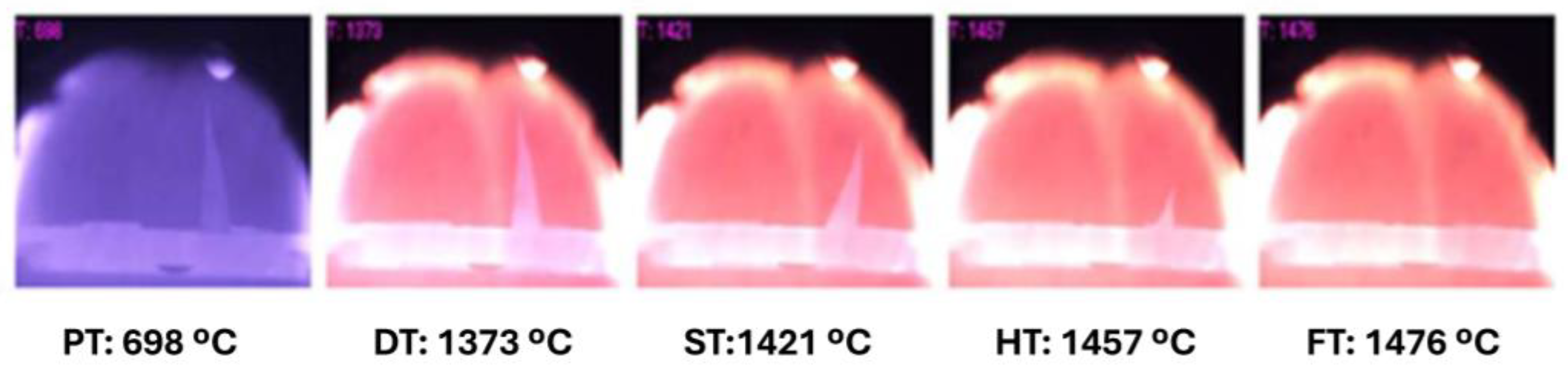
3.4. Slagging and Fouling Risks
3.5. Techno-Ecological Pathways and Process Implications
4. Conclusions
- Q. pubescens bark contains a significant amount of phenolic extractives (21%) and inorganics (15%). The inorganic fraction of the bark is dominated by calcium oxalate, mainly in monohydrate form (COM).
- Thermal degradation of the bark occurs between 150 °C and 690 °C. The ash softening temperature is 1421 °C, and the ash flow temperature is 1467 °C. The raw bark has a very low slagging risk and a moderate fouling risk.
- Extractives enhance the bark thermal degradation by catalytic activity. Suberin decreases the thermal degradation by forming a physical barrier.
- Reactivity analyses of bark fractions favor their different thermochemical biorefinery routing: extracted bark (EB) and desuberinised bark (DB) are highly reactive and well-suited to combustion/gasification, whereas raw bark (RB) and Klason lignin (KL) exhibit higher thermal stability and yield more persistent char
- The proposed extraction and desuberinization steps were designed to demonstrate fraction-specific valorization routes rather than to define an optimized industrial process. Although such pre-processing would entail additional energy input at larger scales, these costs can potentially be offset through co-product valorization and process heat integration in future techno-economic and life-cycle assessments.
- Although the high mineral and extractive content of Q. pubescens bark may limit direct pyrolysis efficiency compared with feedstocks with more polysaccharides and lignin, these same characteristics open opportunities for integrated process optimization. Targeted fractionation can convert these compositional constraints into functional advantages. Therefore, the present dataset provides a foundation for future process-integration and techno-economic studies aimed at overcoming these inherent limitations and enabling efficient utilization of tree barks in circular biorefineries.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFT | Ash fusion temperatures |
| B | Bark |
| Csa | Hot-summer Mediterranean climate |
| DB | Desuberinised bark |
| DSC | Differential scanning calorimetry |
| EB | Extracted bark |
| EDS | Energy dispersive X-ray spectroscopy |
| FT-IR | Fourier-transform infrared spectroscopy |
| HHV | Higher heating value |
| HR | Heating rate |
| KL | Klason lignin |
| MC | Moisture content |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
Appendix A

References
- Yaltırık, F. Türkiye Meşeleri Teşhis Kılavuzu [Guide for Identification of Turkish Oaks]; Tarım Orman ve Köyişleri Genel Müdürlüğü: İstanbul, Turkey, 1984. [Google Scholar]
- Mévy, J.-P.; Loriod, B.; Liu, X.; Corre, E.; Torres, M.; Büttner, M.; Haguenauer, A.; Reiter, I.M.; Fernandez, C.; Gauquelin, T. Response of downy oak (Quercus pubescens Willd.) to climate change: Transcriptome assembly, differential gene analysis and targeted metabolomics. Plants 2020, 9, 1149. [Google Scholar] [CrossRef]
- Pasta, S.; De Rigo, D.; Caudullo, G. Quercus Pubescens in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; pp. 156–157. [Google Scholar]
- Kätzel, R. Die Vorkommen der Flaum-Eiche und ihrer Hybriden nördlich der Alpen. Landbauforsch. Appl. Agric. For. Res. 2014, 64, 73–84. [Google Scholar]
- Todaro, L.; Rita, A.; Negro, F.; Moretti, N.; Saracino, A.; Zanuttini, R. Behavior of pubescent oak (Quercus pubescens Willd.) wood to different thermal treatments. iForest 2015, 8, 748–756. [Google Scholar] [CrossRef]
- Davis, P.H. Flora of Turkey and the East Aegean Islands; University Press: Edinburgh, UK, 1982; Volume 7, pp. 672–673. [Google Scholar]
- Di Pietro, R.; Di Marzio, P.; Antonecchia, G.; Conte, A.L.; Fortini, P. Preliminary characterization of the Quercus pubescens complex in southern Italy using molecular markers. Acta Bot. Croat. 2020, 79, 15–25. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S. Wood anatomy of some trees with diffuse- and ring-porous wood: Some functional and ecological interpretations. Plant Biosyst. 1990, 124, 601–613. [Google Scholar] [CrossRef]
- Humar, M.; Balzano, A.; Grbec, S.; Gričar, J.; Kržišnik, D.; Lesar, B.; Vek, V. Investigation of the material resistance and moisture performance of pubescent oak (Quercus pubescens). Holzforschung 2021, 75, 22–36. [Google Scholar] [CrossRef]
- Moretti, N.; Bufo, S.A.; Fouilloux, C.; Lelario, F.; Capogrossi, A.; Sinisgalli, C.A.; Milella, L.; Scrano, L. Primi risultati sulla utilizzazione del legname di Quercus pubescens Willd. (roverella) per la produzione di botti da invecchiamento. L’Italia For. Mont. 2019, 74, 143–154. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Correia, A.C.; Jordão, A.M. The impact of Quercus pubescens wood chips on chemical and sensory characteristics of a Serbian ‘Kadarka’ red wine during aging: A comparison with other oak species (Q. petraea, Q. alba, and Q. pyrenaica). Vitis 2023, 62, 41–58. [Google Scholar] [CrossRef]
- Nișca, A.; Ștefănescu, R.; Mocan, A.; Babotă, M.; Nicolescu, A.; Mare, A.D.; Ciurea, C.N.; Man, A.; Tănase, C. A comparative analysis of polyphenol content and biological potential of Quercus petraea Matt. and Q. pubescens Willd. bark extracts. Forests 2023, 14, 116. [Google Scholar] [CrossRef]
- Vangeel, T.; Neiva, D.M.; Quilhó, T.; Costa, R.A.; Sousa, V.; Sels, B.F.; Pereira, H. Tree bark characterization envisioning an integrated use in a biorefinery. Biomass Convers. Biorefin. 2021, 11, 2029–2043. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. The bark biorefinery: A side-stream of the forest industry converted into nanocomposites with high oxygen-barrier properties. Cellulose 2014, 21, 4583–4594. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; de Cássia Carneiro, A.; Pereira, H. Potential of Eucalyptus globulus industrial bark as a biorefinery feedstock: Chemical and fuel characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 1984. [Google Scholar]
- McBride, M.B.; Kelch, S.; Schmidt, M.; Zhou, Y.; Aristilde, L.; Martinez, C.E. Lead solubility and mineral structures of coprecipitated lead/calcium oxalates. Environ. Sci. Technol. 2019, 53, 13794–13801. [Google Scholar] [CrossRef] [PubMed]
- TAPPI T204 om-84; Solvent Extractives of Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 1987.
- TAPPI T207 om-93; Water Solubility of Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 1993.
- Graça, J.; Pereira, H. Methanolysis of bark suberins: Analysis of glycerol and acid monomers. Phytochem. Anal. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- TAPPI T222 om-88; Acid Insoluble Lignin in Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 1988.
- TAPPI UM 250; Acid-Soluble Lignin in Wood and Pulp. TAPPI Press: Atlanta, GA, USA, 1991.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass (NREL LAP 1617); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Lu, J.-J.; Chen, W.-H. Investigation on the ignition and burnout temperatures of bamboo and sugarcane bagasse by thermogravimetric analysis. Appl. Energy 2015, 160, 49–57. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, W.; Zhang, X.; Han, D.; Liu, W.; Jia, J. Pyrolysis and combustion behavior study of PMMA waste from micro-scale to bench-scale experiments. Fuel 2022, 319, 123717. [Google Scholar] [CrossRef]
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D1102-84; Test Method for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2008.
- Lavrič, M.; Eler, K.; Ferlan, M.; Vodnik, D.; Gričar, J. Chronological sequence of leaf phenology, xylem and phloem formation and sap flow of Quercus pubescens from abandoned Karst grasslands. Front. Plant Sci. 2017, 8, 314. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Calcium oxalate crystals in the bark of Quercus robur, Ulmus glabra, Populus tremula and Betula pendula. Ann. Bot. 1995, 75, 281–284. [Google Scholar] [CrossRef]
- Şen, A.; Marques, A.V.; Gominho, J.; Pereira, H. Study of thermochemical treatments of cork in the 150–400 °C range using colour analysis and FTIR spectroscopy. Ind. Crops Prod. 2012, 38, 132–138. [Google Scholar] [CrossRef]
- Şen, A.U.; Simões, R.; Yücedağ, C.; Quilhó, T.; Sousa, V.; Miranda, I.; Fernandes, Â.; Pereira, H. Bark-based biorefineries: Anatomical and chemical characterization of the bark of endemic Quercus vulcanica of Turkey. Wood Sci. Technol. 2024, 58, 333–355. [Google Scholar] [CrossRef]
- Maruyama, M.; Sawada, K.P.; Tanaka, Y.; Okada, A.; Momma, K.; Nakamura, M.; Mori, R.; Furukawa, Y.; Sugiura, Y.; Tajiri, R. Quantitative analysis of calcium oxalate monohydrate and dihydrate for elucidating the formation mechanism of calcium oxalate kidney stones. PLoS ONE 2023, 18, e0282743. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y. Pyrolysis characteristics of cellulosic biomass in the presence of alkali and alkaline-earth-metal (AAEM) oxalates. Cellulose 2021, 28, 3473–3483. [Google Scholar] [CrossRef]
- Trubetskaya, A. Reactivity effects of inorganic content in biomass gasification: A review. Energies 2022, 15, 3137. [Google Scholar] [CrossRef]
- Şen, U.; Esteves, B.; Pereira, H. Pyrolysis and extraction of bark in a biorefineries context: A critical review. Energies 2023, 16, 4848. [Google Scholar] [CrossRef]
- Leite, C.; Pereira, H. Cork-containing barks—A review. Front. Mater. 2017, 3, 63. [Google Scholar] [CrossRef]
- Şen, A.; Van den Bulcke, J.; Defoirdt, N.; Van Acker, J.; Pereira, H. Thermal behaviour of cork and cork components. Thermochim. Acta 2014, 582, 94–100. [Google Scholar] [CrossRef]
- Fetisova, O.Y.; Mikova, N.M.; Chudina, A.I.; Kazachenko, A.S. Kinetic study of pyrolysis of coniferous bark wood and modified fir bark wood. Fire 2023, 6, 59. [Google Scholar] [CrossRef]
- Qian, L. Influence of extractives on mechanism of biomass pyrolysis. J. Fuel Chem. Technol. 2010, 38, 42–46. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Qian, J. Effects of extractives on degradation characteristics and VOCs released during wood heat treatment. BioResources 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Ren, X.; Guo, J.; Li, S.; Chang, J. Thermogravimetric analysis–Fourier transform infrared spectroscopy study on the effect of extraction pretreatment on the pyrolysis properties of Eucalyptus wood waste. ACS Omega 2020, 5, 23364–23371. [Google Scholar] [CrossRef] [PubMed]
- Weiland, F.; Lundström, S.; Ögren, Y. Oxygen-blown gasification of pulp mill bark residues for synthetic fuel production. Processes 2021, 9, 163. [Google Scholar] [CrossRef]
- Fang, X.; Jia, L. Experimental study on ash fusion characteristics of biomass. Bioresour. Technol. 2012, 104, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Baláš, M.; Lisý, M.; Lisá, H.; Milčák, P.; Elbl, P. An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Li, Q.H.; Zhang, Y.G.; Meng, A.H.; Li, L.; Li, G.X. Study on ash fusion temperature using original and simulated biomass ashes. Fuel Process. Technol. 2013, 107, 107–112. [Google Scholar] [CrossRef]
- Yang, Z.; Li, F.; Ma, M.; Liu, X.; Fan, H.; Li, Z.; Wang, Y.; Fang, Y. Regulation mechanism of solid waste on ash fusion characteristics of sorghum straw under O2/CO2 atmosphere. Energies 2023, 16, 7052. [Google Scholar] [CrossRef]
- ASTM D1857; Standard Test Method for Fusibility of Coal and Coke Ash. ASTM International: West Conshohocken, PA, USA, 2024.
- Čajová Kantová, N.; Holubčík, M.; Trnka, J.; Čaja, A. Analysis of ash melting temperatures of agricultural pellets detected during different conditions. Fire 2023, 6, 88. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Ning, X.; Zhang, J.; Li, Y.; Jiang, C. Effect of CaO mineral change on coal ash melting characteristics. J. Energy Inst. 2020, 93, 642–648. [Google Scholar] [CrossRef]
- Bgasheva, T.; Falyakhov, T.; Petukhov, S.; Sheindlin, M.; Vasin, A.; Vervikishko, P. Laser-pulse melting of calcium oxide and some peculiarities of its high-temperature behavior. J. Am. Ceram. Soc. 2021, 104, 3461–3477. [Google Scholar] [CrossRef]
- Lawson-Wood, K.; Robertson, I. Study of the Decomposition of Calcium Oxalate Monohydrate Using a Hyphenated Thermogravimetric Analyser–FT-IR System (TG-IR). PerkinElmer Application Note, 2016. Available online: https://www.s4science.at/wordpress/wp-content/uploads/2018/10/TL8000-TG-IR-Decomposition-Calcium-Oxalate-Monohydrate-Application-Note.pdf (accessed on 1 August 2025).
- Wang, J.; Zhao, B.; Zhu, D.; Huang, F.; Zhang, W.; Yang, H.; Chen, L.; Guan, H.; Sun, L.; Yang, S. Mechanism on catalytic cracking tar with CaO-based catalysts for hydrogen-rich gas by DFT and experiments. Int. J. Hydrogen Energy 2021, 46, 6522–6531. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Z.; Tang, A.; Huang, H.; Wei, D.; Yu, E.; Lu, W. Steam-gasification of biomass with CaO as catalyst for hydrogen-rich syngas production. J. Energy Inst. 2019, 92, 1641–1646. [Google Scholar] [CrossRef]
- Carrier, M.; Windt, M.; Ziegler, B.; Appelt, J.; Saake, B.; Meier, D.; Bridgwater, A. Quantitative insights into the fast pyrolysis of extracted cellulose, hemicelluloses, and lignin. ChemSusChem 2017, 10, 3212–3224. [Google Scholar] [CrossRef]


| Chemical Components | Dry Mass (%) | Ash-Free Mass (%) |
|---|---|---|
| Ash | 15.03 ± 1.99 | - |
| DCM extractives | 2.00 ± 0.30 | 2.4 |
| EtOH extractives | 9.96 ± 0.48 | 11.7 |
| H2O Extractives | 9.24 ± 0.25 | 10.9 |
| Total extractives | 21.20 ± 1.23 | 24.9 |
| Suberin | 4.07 ± 0.73 | 4.8 |
| Klason lignin | 21.02 ± 2.04 | 24.7 |
| Acid-soluble lignin | 2.99 ± 0.29 | 3.5 |
| Total lignin | 24.01 ± 2.33 | 28.3 |
| Polysaccharides/lignin ratio | 1.49 | |
| Suberin/lignin ratio | 0.17 | |
| Major Elements | Composition (mg kg−1) | % Major Elements |
|---|---|---|
| Na | 108.9 | 0.2 |
| K | 647.9 | 1.1 |
| Ca | 55,023.9 | 96.1 |
| Mg | 753.5 | 1.3 |
| P | 152.4 | 0.3 |
| S | 294.4 | 0.5 |
| Fe | 96.3 | 0.2 |
| Oligoelements | Composition (mg kg−1) | % Oligoelements |
| Cu | 8.9 | 5.3 |
| Zn | 3.9 | 2.3 |
| Mn | 154.9 | 92.4 |
| Total | 57,244.9 | 100.0 |
| Oxides | Composition (mg kg−1) | % Oxides |
| CaO | 76,987.2 | 95.7 |
| K2O | 780.9 | 1.0 |
| Na2O | 146.9 | 0.2 |
| MgO | 1249.4 | 1.6 |
| P2O5 | 349.4 | 0.4 |
| Fe2O3 | 137.7 | 0.2 |
| SO3 | 734.6 | 0.9 |
| Wavenumber (cm−1) | Assignment | Functional Group and Origin |
|---|---|---|
| 3347 | O–H stretching (broad) | Hydroxyl groups in cellulose, hemicellulose, lignin, and absorbed water |
| 2931 | C–H stretching | Aliphatic chains in lignin side groups and suberin aliphatics |
| 1737 | C=O | Ester bonds in suberin |
| 1622 | C=O stretching/aromatic skeletal vibration | Conjugated carbonyls and aromatic rings in lignin and phenolic extractives; bound water bending |
| 1314 | C–H deformation/O–H bending | Cellulose and lignin structural vibrations; phenolic groups |
| 1037 | C–O–C stretching | Polysaccharide (cellulose and hemicellulose) glycosidic linkages |
| 780 | Aromatic C–H out-of-plane bending | Aromatic rings (lignin/phenolics); C–C/C–O deformation in calcium oxalate monohydrate |
| 516 | M–O stretching | Ca–O (calcium oxalate), possible other inorganic mineral vibrations |
| Water Loss Region (40–120 °C) | Low-Temperature Mass Loss Region (40–210 °C) | Main Mass Loss Region (210–405 °C) | Char Mass Loss Region (405–740 °C) | Residual Char | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mass loss (%) | Tmax (°C) | Mass loss (%) | Tmax (°C) | Onset (°C) | Mass loss (%) | Tmax (°C) | Mass loss (%) | Tmax (°C) | Mass (%) | |
| B | 5.1 | 81.8 | 8.4 | 163.4 | 211.7 | 34.9 | 346.5 | 22.8 | 718.4 | 30.8 |
| EB | 3.4 | 68.7 | 6.2 | 150.8 | 224.0 | 41.6 | 366.9 | 27.1 | 687.6 | 14.8 |
| DB | 3.3 | 74.6 | 6.7 | 157.1 | 234.3 | 34.4 | 323.2 | 29.8 | 684.3 | 12.3 |
| KL | 3.9 | 73.7 | 5.2 | 73.7 | 223.9 | 23.9 | 349.8 | 32.4 | 627.3 | 30.4 |
| Apparent Ea (kJ/mol) | A (1/s) | R2 | Best-Fit Kinetic Model | |
|---|---|---|---|---|
| B | 69.88 | 1.93 × 105 | 0.9926 | Jander |
| EB | 103.25 | 7.86 × 107 | 0.9880 | Jander |
| DB | 87.53 | 3.97 × 106 | 0.9732 | Jander |
| KL | 98.57 | 1.74 × 107 | 0.9873 | Jander |
| Tm (°C) | Tb (°C) | |
|---|---|---|
| B | 325.7 | 691.8 |
| EB | 328.3 | 662.7 |
| DB | 301.3 | 684.5 |
| KL | 493.1 | 568.5 |
| Proximate Composition (%) | ||||
|---|---|---|---|---|
| Basis | MC | Ash | VM | FC |
| As-received | 7.8 | 16.3 | 65.8 | 10.1 |
| Dry | 0.0 | 17.7 | 71.4 | 10.9 |
| Dry-ash-free | 0.0 | 0.0 | 86.7 | 13.3 |
| Calorific value (MJ/kg) | 14.9 | |||
| Stages | Preheat | Deformation | Softening | Hemisphere | Flow |
|---|---|---|---|---|---|
| Temperatures (°C) | 698 | 1373 | 1421 | 1457 | 1476 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, U.; Balcı, B.; Arıcı, Ș.; Şat, B.; Miranda, I.; Pereira, H. Chemical Composition and Reactivity of Quercus pubescens Bark and Bark Fractions for Thermochemical Biorefinery Applications. Processes 2025, 13, 3484. https://doi.org/10.3390/pr13113484
Şen U, Balcı B, Arıcı Ș, Şat B, Miranda I, Pereira H. Chemical Composition and Reactivity of Quercus pubescens Bark and Bark Fractions for Thermochemical Biorefinery Applications. Processes. 2025; 13(11):3484. https://doi.org/10.3390/pr13113484
Chicago/Turabian StyleŞen, Umut, Büşra Balcı, Șefik Arıcı, Beyza Şat, Isabel Miranda, and Helena Pereira. 2025. "Chemical Composition and Reactivity of Quercus pubescens Bark and Bark Fractions for Thermochemical Biorefinery Applications" Processes 13, no. 11: 3484. https://doi.org/10.3390/pr13113484
APA StyleŞen, U., Balcı, B., Arıcı, Ș., Şat, B., Miranda, I., & Pereira, H. (2025). Chemical Composition and Reactivity of Quercus pubescens Bark and Bark Fractions for Thermochemical Biorefinery Applications. Processes, 13(11), 3484. https://doi.org/10.3390/pr13113484