Methane Sorption Behavior in Nanopores of Coal: A Molecular Dynamics Simulation Based on a Reconstructed Macromolecular Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information
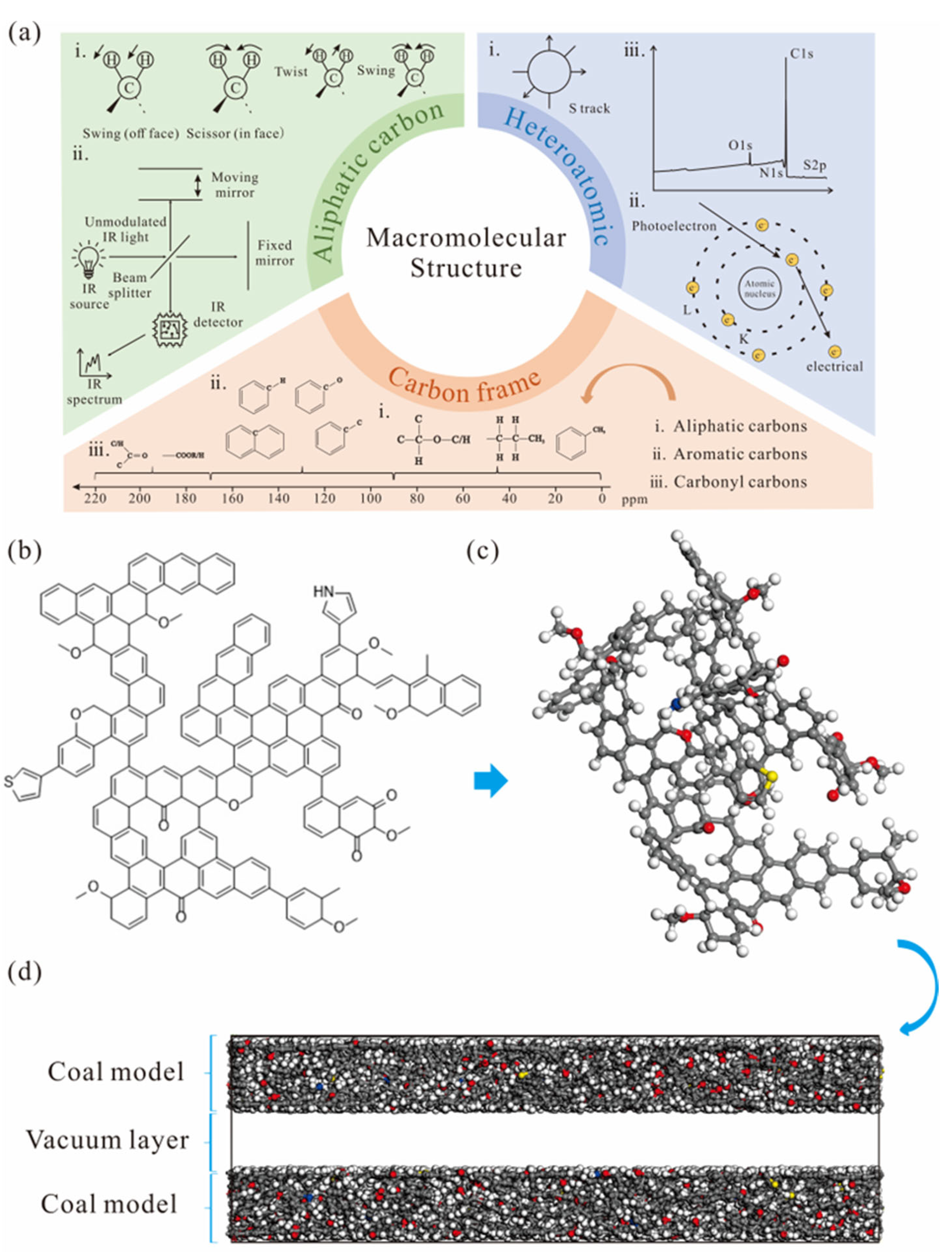
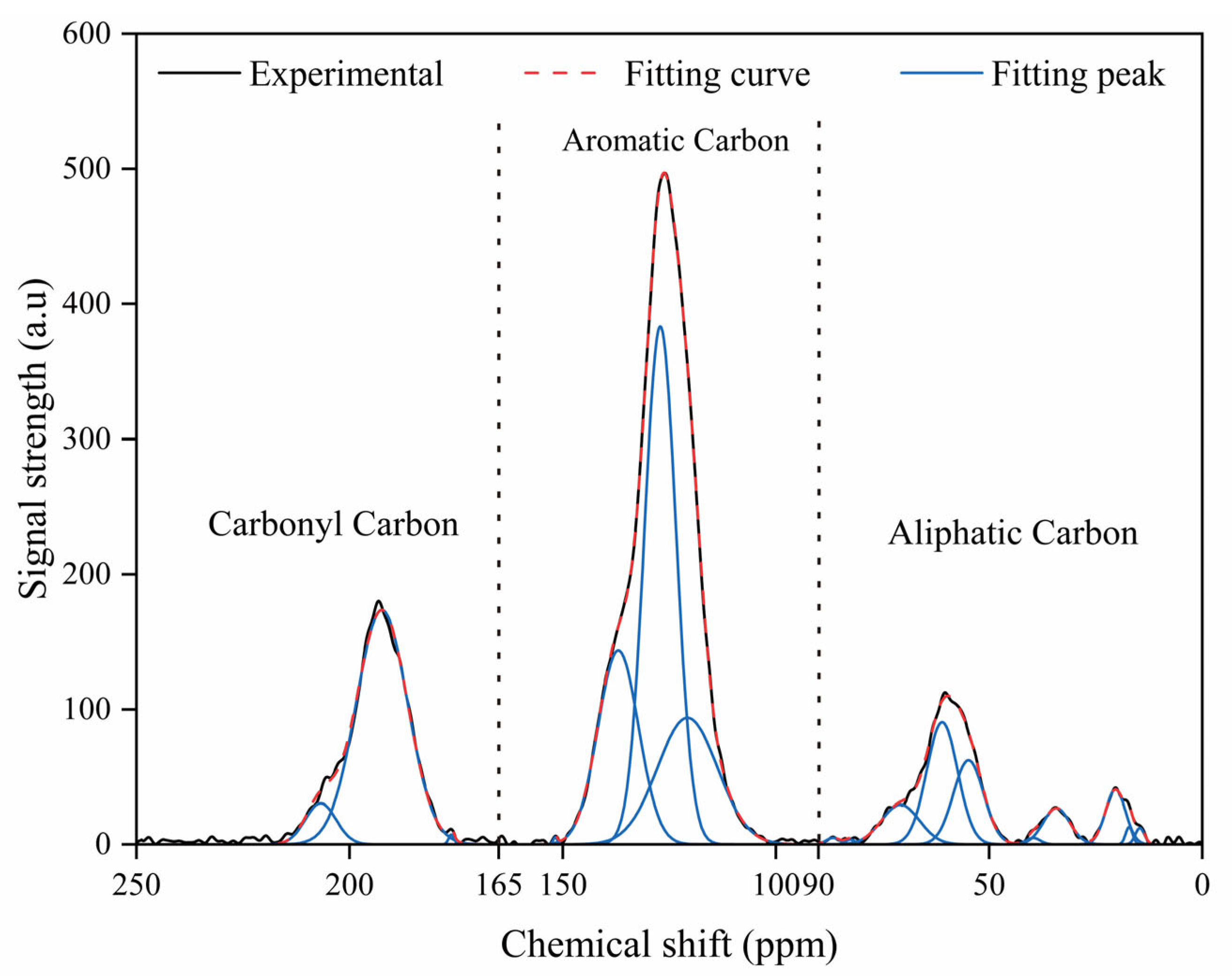
2.2. 13C Nuclear Magnetic Resonance Spectra
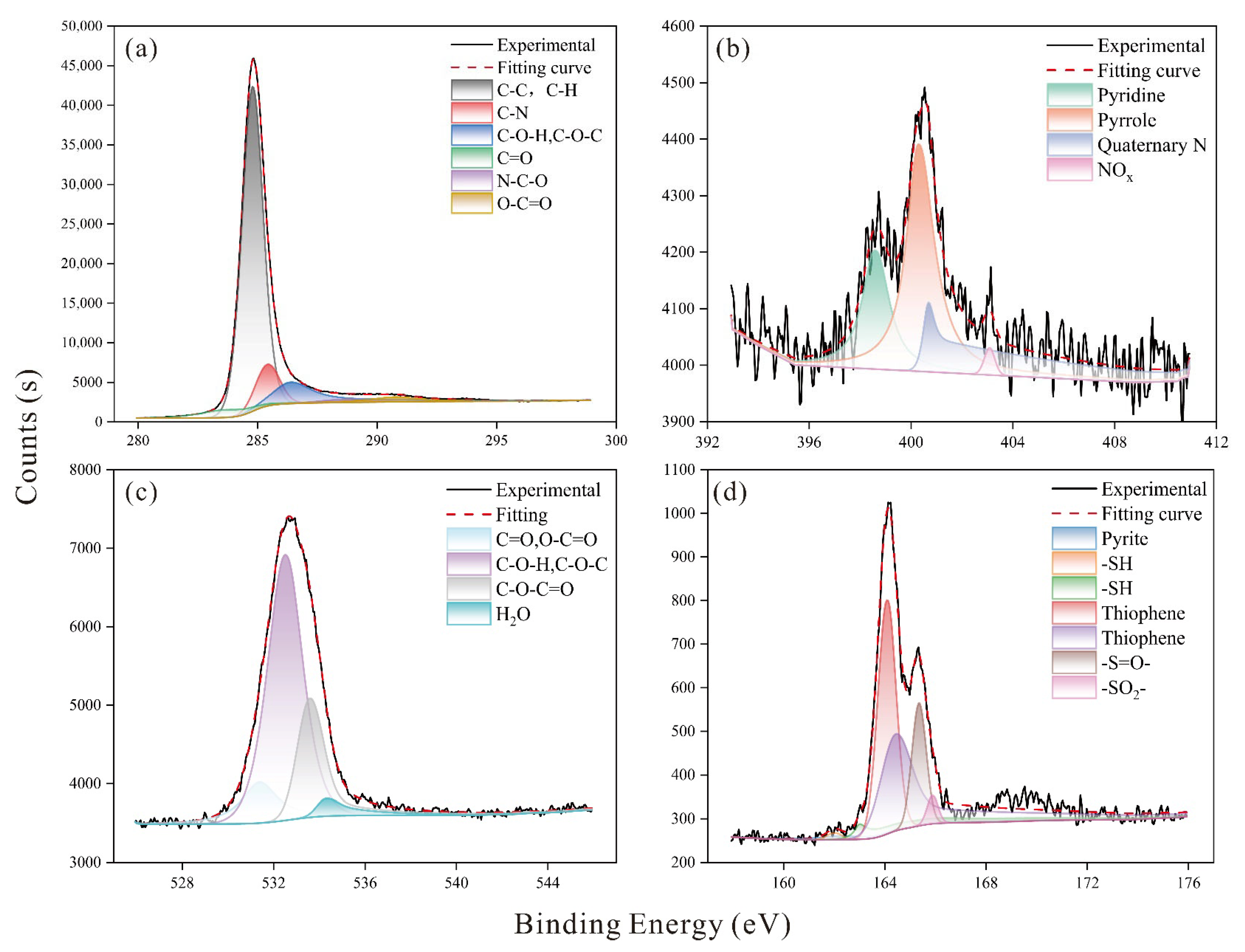
2.3. X-Ray Photoelectron Spectroscopy
2.4. Fourier Transform Infrared Spectroscopy
2.5. Molecular Mechanics and Molecular Dynamics Calculations
3. Results and Discussion
3.1. Construction of the Macromolecular Structure of Coal
3.1.1. Coal Molecular Structure Information
3.1.2. Three-Dimensional Coal Macromolecular Model
3.2. Methane Absolute Adsorption Characteristic Analysis Under Different Pore Sizes
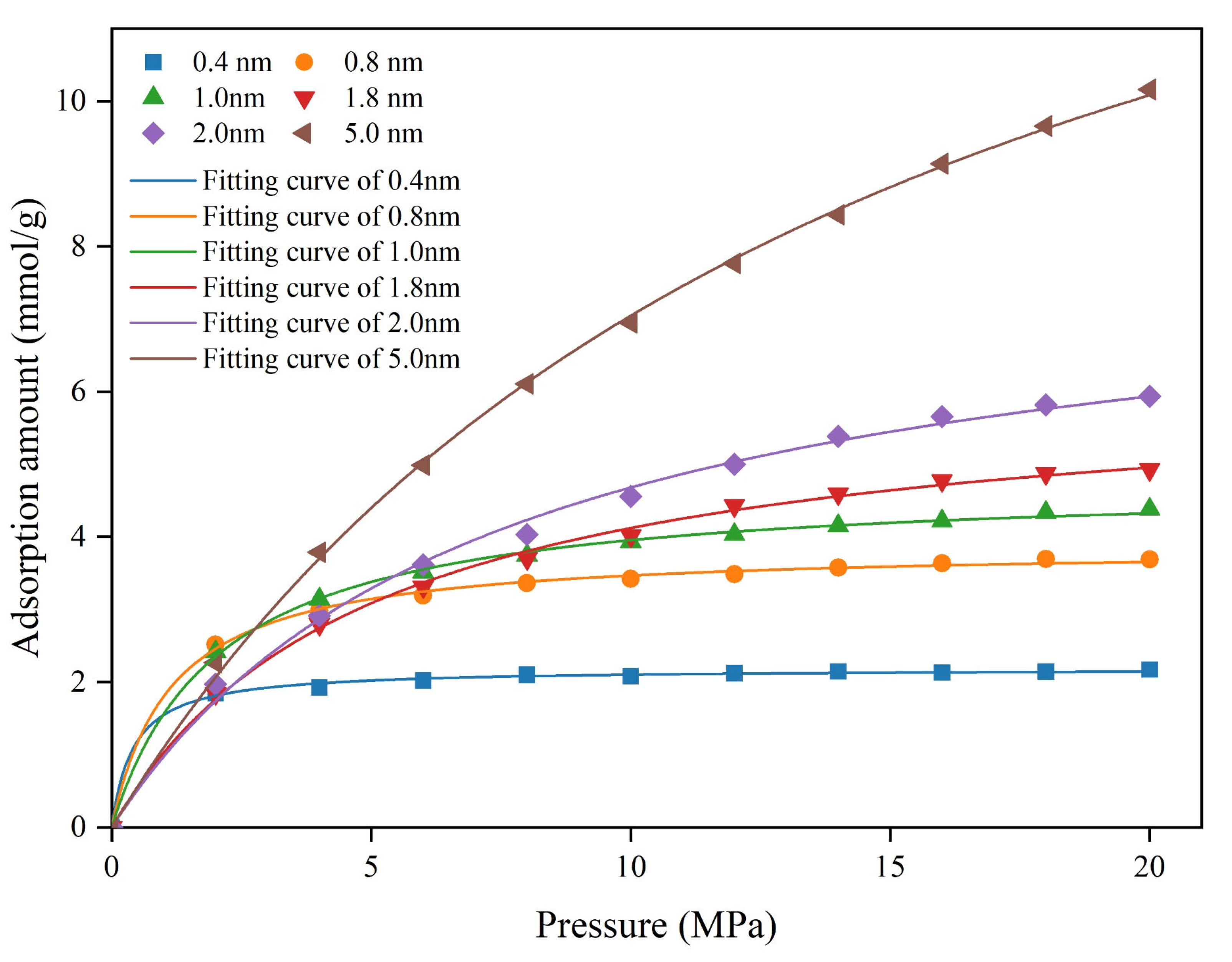
3.2.1. Methane Absolute Adsorption Isotherm Characteristic Analysis
3.2.2. Methane Adsorption Layer Characteristic Analysis
3.3. Methane Excess Adsorption Characteristic Analysis Under Different Pore Sizes
3.3.1. Methane Excess Adsorption Density Characteristic Analysis
3.3.2. Methane Excess Adsorption Capacity Characteristic Analysis
3.4. Methane Adsorption Heat Characteristic Analysis Under Different Pore Sizes
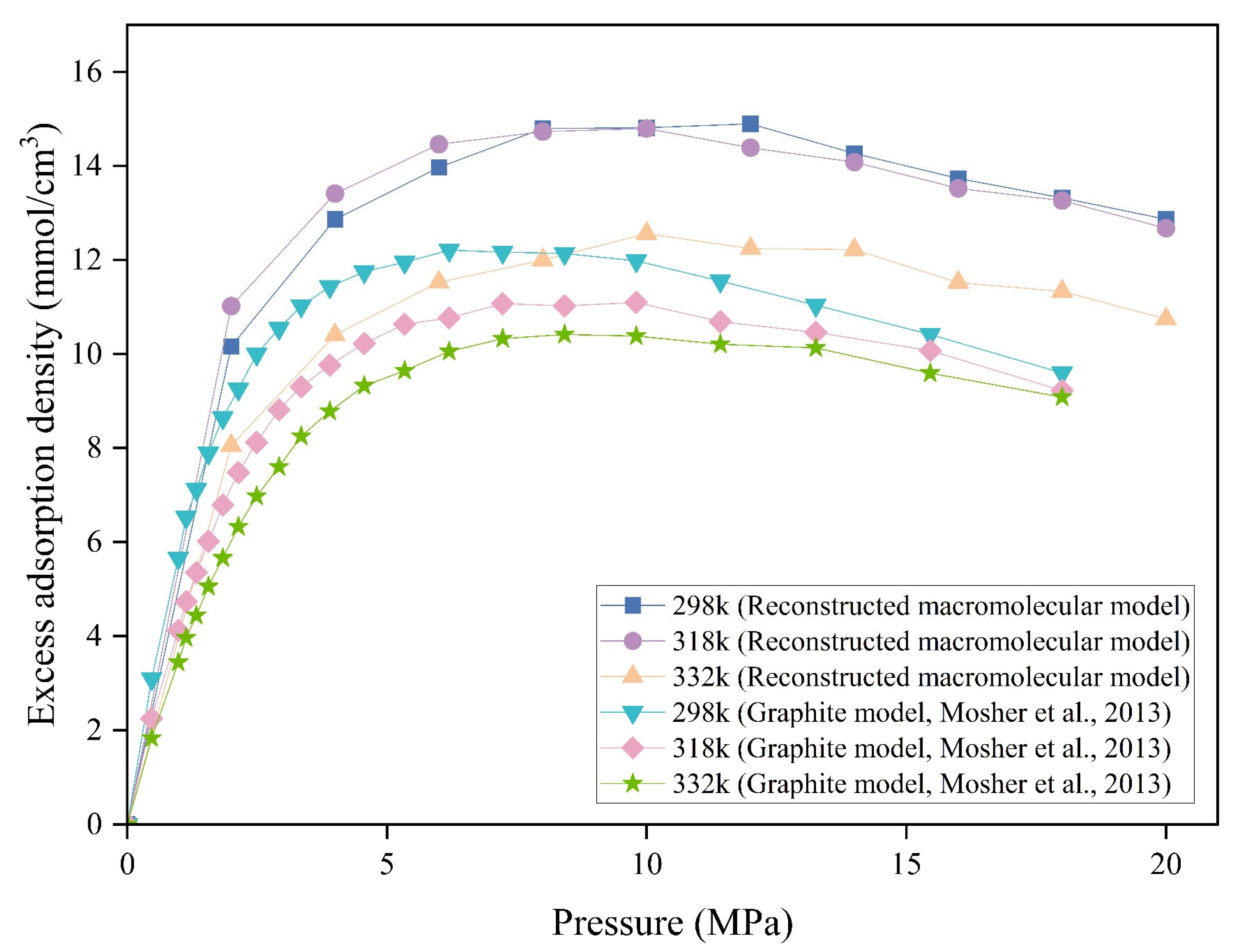
3.5. Comparison of Methane Adsorption Across Different Coal Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Deng, C.; Jin, Z.; Gao, T. Molecular Simulation of the Adsorption Characteristics of Methane in Pores of Coal with Different Metamorphic Degrees. Molecules 2021, 26, 7217. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Fink, R.; Littke, R.; Zieger, L. Methane Sorption Behaviour of Coals Altered by Igneous Intrusion, South Sumatra Basin. Int. J. Coal Geol. 2019, 214, 103250. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, D.; Cai, Y.; Liu, N.; Qiu, Y. Methane Adsorption Interpreting with Adsorption Potential and Its Controlling Factors in Various Rank Coals. Processes 2020, 8, 390. [Google Scholar] [CrossRef]
- Shi, Y.; He, Y.; Wan, J.; Sun, J.; Zeng, J.; Cui, R. The Primary Controlling Factors of the Occurrence State of Deep High-Rank Coalbed Methane in Eastern Ordos Basin. Front. Earth Sci. 2024, 12, 1340523. [Google Scholar] [CrossRef]
- Tan, X.; Ma, X.; Li, X.; Li, Y. An Adsorption Model Considering Fictitious Stress. Fractal Fract. 2025, 9, 17. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, H.; Zhang, X.; Ranjith, P.G.; Perera, M.S.A. Molecular Simulation of Methane Adsorption in Nanoscale Rough Slits. J. Nat. Gas Sci. Eng. 2022, 102, 104608. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z.; Hu, D.; Miao, C.; Lu, B.; Gao, D. Construction of Macromolecular Model and Analysis of Oxygen Absorption Characteristics of Hongyang No. 2 Coal Mine. Arab. J. Chem. 2023, 16, 104662. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, J.; Xu, X.; Wang, B. Molecular Structure Characterization of Middle-High Rank Coal via 13C NMR, XPS, and FTIR Spectroscopy. China Geol. 2024, 7, 702–713. [Google Scholar] [CrossRef]
- Nie, B.; Zhu, X.; Liu, P.; Zhao, D.; Liu, X.; Deng, B.; Lun, J.; Wang, M.; Qin, F. Novel Insights into Gas Sorption Mechanisms in Multiscale Coal Nanopores via Molecular Simulation. Energy Fuels 2025, 39, 9373–9387. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Fang, Z.; Wei, N.; Li, Q. Small-Molecule Gas Sorption and Diffusion in Coal: Molecular Simulation. Energy 2010, 35, 2939–2944. [Google Scholar] [CrossRef]
- Su, X.; Li, N.; Li, L.; Tuerhong, R.; Yu, Y.; Zhang, P.; Su, Q.; Shen, T.; Sun, M.; Ma, X. Structural Parameters and Molecular Model of Shendong Subbituminous Coal. Chin. J. Chem. Eng. 2024, 76, 124–134. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Cai, Y.; Li, X. Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China. Energies 2019, 12, 986. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore Structure Characterization of Different Rank Coals Using Gas Adsorption and Scanning Electron Microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Chen, C.; Sun, J.; Zhang, Y.; Mu, J.; Li, W.; Song, Y. Adsorption Characteristics of CH4 and CO2 in Organic-Inorganic Slit Pores. Fuel 2020, 265, 116969. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Li, J.; Sun, Y.; Lv, B.; Ma, X.; Zhou, M.; Zhang, J. Investigation of Methane Adsorption Mechanism in Low-Rank Bituminous Coal Considering Internal Water Distribution: A Molecular Simulation and Experimental Study. Chem. Eng. J. 2024, 495, 153562. [Google Scholar] [CrossRef]
- Teng, T.; Chen, Y.; Wang, Y.; Qiao, X. In Situ Nuclear Magnetic Resonance Observation of Pore Fractures and Permeability Evolution in Rock and Coal under Triaxial Compression. J. Energy Eng. 2025, 151, 4025036. [Google Scholar] [CrossRef]
- Cao, Z.; Xiong, Y.; Xue, Y.; Du, F.; Li, Z.; Huang, C.; Wang, S.; Yu, Y.; Wang, W.; Zhai, M.; et al. Diffusion Evolution Rules of Grouting Slurry in Mining-Induced Cracks in Overlying Strata. Rock Mech. Rock Eng. 2025, 58, 6493–6512. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Cao, Z.; Liu, J.; Xue, Y.; Wang, P.; Cao, X.; Liu, Y. Thermo-Mechanical Degradation and Fracture Evolution in Low-Permeability Coal Subjected to Cyclic Heating–Cryogenic Cooling. Phys. Fluids 2025, 37, 086617. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, T.; Aziz, N. Influences of Temperature and Moisture on Coal Sorption Characteristics of a Bituminous Coal from the Sydney Basin, Australia. IJOGCT 2014, 8, 62. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, D.; Yang, S.; Mangi, H.N.; Fu, H.; Wang, G.; Yang, X.; Zhang, B.; Li, T.; Liang, W.; et al. Effects of Sequence Stratigraphy on Coal Characteristics and CH4 Adsorption Capacity of the Low-Rank Coal in Santanghu Basin, China. J. Nat. Gas Sci. Eng. 2020, 81, 103467. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, C.; Sun, Y.; Gao, Y.; Qiao, H.; Tai, Z. Impact of Fractal Features on Gas Adsorption and Desorption Capacities and Ad-/Desorption Hysteresis in Coals Based on Synchrotron Radiation SAXS. Front. Earth Sci. 2022, 10, 824348. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Y.; Hu, B.; He, X. Quantitative Analysis of Difference in CH4 and CO2 Adsorption Capacity in Coal Based on Adsorption Model. J. Nat. Gas Sci. Eng. 2022, 102, 104541. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, D.; Qin, Y.; Xu, H.; Liu, Y.; Wu, H. Characteristics of Methane (CH4) Diffusion in Coal and Its Influencing Factors in the Qinshui and Ordos Basins. Energy Fuels 2018, 32, 1196–1205. [Google Scholar] [CrossRef]
- Kumar, H.; Mishra, M.K.; Mishra, S. Sorption Capacity of Indian Coal and Its Variation with Rank Parameters. J. Pet. Explor. Prod. Technol. 2019, 9, 2175–2184. [Google Scholar] [CrossRef]
- Kiani, A.; Sakurovs, R.; Grigore, M.; Sokolova, A. Gas Sorption Capacity, Gas Sorption Rates and Nanoporosity in Coals. Int. J. Coal Geol. 2018, 200, 77–86. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Li, Z.; Liu, B.; Wang, R. Effect of Pore Structure on Competitive Sorption and Diffusion of Mixed Methane and Carbon Dioxide in Anthracite, South Qinshui Basin, China. Int. J. Coal Geol. 2022, 253, 103956. [Google Scholar] [CrossRef]
- Karacan, C.O.; Okandan, E. Adsorption and Gas Transport in Coal Microstructure: Investigation and Evaluation by Quantitative X-Ray CT Imaging. Fuel 2001, 80, 509–520. [Google Scholar] [CrossRef]
- Li, S.-G.; Bai, Y.; Lin, H.-F.; Shu, C.-M.; Yan, M.; Laiwang, B. Molecular Simulation of Adsorption of Gas in Coal Slit Model under the Action of Liquid Nitrogen. Fuel 2019, 255, 115775. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Ju, X.; Zhou, A. Adsorption and Diffusion Behavior of CH4 and CO2 in Closed and Open Pores from Zhaozhuang Coal. Energy Fuels 2022, 36, 2582–2590. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Cai, J.; Xie, H.; Chen, S. Investigation of the Impact of Micro-Nano Pore Geometry on CH4 Adsorption. Chem. Eng. J. 2024, 500, 157162. [Google Scholar] [CrossRef]
- Song, W.; Yao, J.; Ma, J.; Li, A.; Li, Y.; Sun, H.; Zhang, L. Grand Canonical Monte Carlo Simulations of Pore Structure Influence on Methane Adsorption in Micro-Porous Carbons with Applications to Coal and Shale Systems. Fuel 2018, 215, 196–203. [Google Scholar] [CrossRef]
- Mosher, K.; He, J.; Liu, Y.; Rupp, E.; Wilcox, J. Molecular Simulation of Methane Adsorption in Micro- and Mesoporous Carbons with Applications to Coal and Gas Shale Systems. Int. J. Coal Geol. 2013, 109–110, 36–44. [Google Scholar] [CrossRef]
- Cui, C.; Wang, D.; Zhang, L.; Yang, M. Molecular Simulation on the Desorption and Extraction of Methane in the Slits with Varying Surface Activity. Chem. Phys. 2023, 572, 111975. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, F.; Cong, Q.; Pang, X.; Ma, K.; Shi, K.; Pang, B.; Chen, D.; Pang, H.; Yang, X.; et al. Adsorption Characteristics of Shale Gas in Organic–Inorganic Slit Pores. Energy 2023, 278, 127788. [Google Scholar] [CrossRef]
- Lin, K.; Yuan, Q.; Zhao, Y.-P. Using Graphene to Simplify the Adsorption of Methane on Shale in MD Simulations. Comput. Mater. Sci. 2017, 133, 99–107. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Li, W.; Xiang, J.; Wang, Y.; Li, J.; Zeng, F. Molecular Simulation of Methane Adsorption in Shale Based on Grand Canonical Monte Carlo Method and Pore Size Distribution. J. Nat. Gas Sci. Eng. 2016, 30, 119–126. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Wei, C.; Dai, X.; Quan, F.; Hou, C.; Cheng, G. A Review on the Application of Molecular Dynamics to the Study of Coalbed Methane Geology. Front. Earth Sci. 2021, 9, 775497. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Zhu, J.; Wang, Z.; Zhou, J.; Cen, K. Removal of Oxygen Functional Groups in Lignite by Hydrothermal Dewatering: An Experimental and DFT Study. Fuel 2016, 178, 85–92. [Google Scholar] [CrossRef]
- Huang, J.; Zhai, C.; Sun, Y.; Lai, Y.; Xu, H.; Huang, T.; Wang, Y.; Li, Y.; Xu, J. Microscopic Scale Influence of Functional Groups on the Displacement Behavior of Coalbed Methane by Hot Humid Flue Gas: A Molecular Simulation Study Based on the Dynamic Injection Process. Gas Sci. Eng. 2024, 131, 205477. [Google Scholar] [CrossRef]
- Fu, X.; Lun, Z.; Zhao, C.; Zhou, X.; Wang, H.; Zhou, X.; Xu, Y.; Zhang, H.; Zhang, D. Influences of Controlled Microwave Field Irradiation on Physicochemical Property and Methane Adsorption and Desorption Capability of Coals: Implications for Coalbed Methane (CBM) Production. Fuel 2021, 301, 121022. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of Chemical Functional Groups in Macerals across Different Coal Ranks via Micro-FTIR Spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Hao, S.; Wen, J.; Yu, X.; Chu, W. Effect of the Surface Oxygen Groups on Methane Adsorption on Coals. Appl. Surf. Sci. 2013, 264, 433–442. [Google Scholar] [CrossRef]
- Bastos-Neto, M.; Canabrava, D.V.; Torres, A.E.B.; Rodriguez-Castellón, E.; Jiménez-López, A.; Azevedo, D.C.S.; Cavalcante, C.L. Effects of Textural and Surface Characteristics of Microporous Activated Carbons on the Methane Adsorption Capacity at High Pressures. Appl. Surf. Sci. 2007, 253, 5721–5725. [Google Scholar] [CrossRef]
- Li, J.; Ge, S.; Liu, S. Differential Adsorption of H2S/CH4 by Bituminous Coal and Its Competitive Adsorption Properties. Chem. Eng. J. 2024, 500, 157447. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, S.; Tang, S.; Xin, D.; Zhang, S.; Wang, B.; Zhang, Q.; Zhang, K.; Lin, D.; Yang, W. Density and Volume of Adsorbed Methane in Key Applications for In-Situ Gas Content: Insights from Molecular Simulation. Chem. Eng. J. 2024, 490, 151809. [Google Scholar] [CrossRef]
- Yang, Q.; Xue, J.; Lin, H.; Jin, Z. Two-Way Coupling Dynamics of CH4 Adsorption and Coal Matrix Deformation: Insights from Hybrid GCMC/MD Simulations. Chem. Eng. J. 2024, 498, 155321. [Google Scholar] [CrossRef]
- Han, Q.; Deng, C.; Gao, T.; Jin, Z.; Zhang, H. Construction of Different Metamorphic Degree Coal Models and Corresponding Adsorption Characteristics Based on Molecular Simulation. J. Mol. Struct. 2023, 1274, 134517. [Google Scholar] [CrossRef]
- Pan, J.; Jiao, F.; Wang, K.; Li, Y.; Song, D.; Hou, Q. Molecular Simulations of the Effects of CO2 and N2 Injection on CH4 Adsorption, Coal Porosity and Permeability. Adv. Geo-Energy Res. 2024, 12, 205–222. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, M.; Kang, Y.; You, L.; Li, P.; Chen, X.; Zhang, F. Molecular Simulation Insights into Methane-Water Adsorption and Transport Mechanisms in Deep Shale Multiscale Nanoslits under High-Temperature and High-Pressure Conditions. Fuel 2025, 396, 135267. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zhang, X.; Cheng, J.; Lu, W.; Bai, E.; You, Z. Thermodynamic Characteristics of CH4/CO2 Adsorption in Different Rank Coals and Its Molecular Mechanism. Langmuir 2025, 41, 5419–5438. [Google Scholar] [CrossRef]
- Burnaz, L.; Zieger, L.; Schmatz, J.; Botero, A.E.; Amberg, S.; Thüns, N.; Littke, R. Preparation Techniques for Microscopic Observation of Dispersed Organic Matter and Their Effect on Vitrinite Reflectance. Int. J. Coal Geol. 2023, 272, 104249. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Li, H.; Zhang, H.; Bai, H.; Guo, Q. Exploring Molecular Structure Characteristics and Chemical Index of Qinghua Bituminous Coal: A Comprehensive Insight from Single Molecule of Macerals to Particles with Various Sizes. Powder Technol. 2022, 396, 36–49. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, C.; Zhang, M.; Liu, X.; Xue, S. Model Construction and Optimization of Coal Molecular Structure. J. Mol. Struct. 2023, 1290, 135960. [Google Scholar] [CrossRef]
- Zhu, B.; Dong, X.; Fan, Y.; Ma, X.; Yao, S.; Fu, Y.; Chen, R.; Chang, M. Structural Characterization and Molecular Model Construction of High-Ash Coal from Northern China. Molecules 2023, 28, 5593. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiang, J.; Deng, X.; Gao, W. Molecular Simulation of the Adsorption and Diffusion Properties of CH4 and CO2 in the Microporous System of Coal. Fuel 2024, 360, 130566. [Google Scholar] [CrossRef]
- Alam, M.d.S.; Jeong, J.H. MD Simulation Estimation of Saturation Pressure and Vapor-Liquid Equilibrium for Binary Blends of HFO-1234yf and HFO-1123. Int. J. Air-Cond. Refrig. 2022, 30, 9. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Zhang, X.; Wei, J.; Chen, F. Construction of Molecular Structure Model of Tunlan Coal and Its Microscopic Physicochemical Mechanism. Fuel 2022, 308, 121936. [Google Scholar] [CrossRef]
- Takanohashi, T.; Nakamura, K.; Terao, Y.; Iino, M. Computer Simulation of Solvent Swelling of Coal Molecules: Effect of Different Solvents. Energy Fuels 2000, 14, 393–399. [Google Scholar] [CrossRef]
- Hu, F.; Han, F.; Wu, K.; Liu, M.; Niu, G.; Xue, D.; Wang, H. Construction and Optimization of Liuzhuang Coal Molecular Model for Wettability Mechanism Analysis. Fuel 2026, 404, 136187. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Babaei, S.; Tesson, S.; Azamat, J.; Ostadhassan, M. From Excess to Absolute Adsorption Isotherm: The Effect of the Adsorbed Density. Chem. Eng. J. 2021, 425, 131495. [Google Scholar] [CrossRef]
- Qiu, T.; Hu, S.; Zhang, J. Application of the Material Balance Equation Based on the BET Multimolecular Fractal Theory in a Shale Gas Reservoir. Front. Energy Res. 2022, 10, 829800. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Sang, S.; Liu, T.; Zheng, S. Effects of Pore Structure Changes on the CH4 Adsorption Capacity of Coal during CO2-ECBM. Fuel 2022, 330, 125529. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, X.; Zhang, K.; Zeng, F.; Wang, Z. Investigation of Dynamical Properties of Methane in Slit-like Quartz Pores Using Molecular Simulation. RSC Adv. 2018, 8, 33798–33816. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Bao, X. The Effects of Confinement inside Carbon Nanotubes on Catalysis. Acc. Chem. Res. 2011, 44, 553–562. [Google Scholar] [CrossRef]
- Guan, J.; Pan, X.; Liu, X.; Bao, X. Syngas Segregation Induced by Confinement in Carbon Nanotubes: A Combined First-Principles and Monte Carlo Study. J. Phys. Chem. C 2009, 113, 21687–21692. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, Z.; Tan, Z.; Liu, H.; Yang, M. Study on Heat Adsorption Based on the Experiment of Coal Adsorption of Methane by Using the Weight Method. J. Nat. Gas Geosci. 2021, 6, 245–253. [Google Scholar] [CrossRef]
- Xiang, J.; Zeng, F.; Liang, H.; Li, B.; Song, X. Molecular Simulation of the CH4/CO2/H2O Adsorption onto the Molecular Structure of Coal. Sci. China Earth Sci. 2014, 57, 1749–1759. [Google Scholar] [CrossRef]
- Gao, C.; Liu, D.; Vandeginste, V.; Cai, Y.; Sun, F. Thermodynamic Energy Change and Occurrence Mechanism of Multiple Fluids in Coal Reservoirs. Energy 2023, 283, 129089. [Google Scholar] [CrossRef]
- Hao, M.; Wei, C.; Zhang, H. Adsorption and Diffusion of Methane in Coal Slit Pores: Insights into the Molecular Level. Energy Fuels 2022, 36, 880–886. [Google Scholar] [CrossRef]
- Yang, N.; Yang, D.; Zhang, G.; Chen, L.; Liu, D.; Cai, M.; Fan, X. The Effects of Graphene Stacking on the Performance of Methane Sensor: A First-Principles Study on the Adsorption, Band Gap and Doping of Graphene. Sensors 2018, 18, 422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, C.; Wang, Z.; Xie, J.; Zhao, W.; Meng, F.; Han, Y. Micropore Distribution and Methane Adsorption Process and Mechanism in Bituminous Coals: A Molecular Dynamics Simulation Study. J. Environ. Chem. Eng. 2024, 12, 112139. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Yang, T.; Chen, X.; Li, W.; Wang, T. Mechanistic Study of Carbon Dioxide Sequestration Technology Using Molecular Simulations to Assess the Adsorption of Methane and Carbon Dioxide by Different Functional Groups. Appl. Surf. Sci. 2024, 677, 161058. [Google Scholar] [CrossRef]
- Zhao, D.; Ke, X.; Huang, M.; He, W.; Tong, M.; Chen, B.; Du, Q. The Study on the Adsorption Characteristics of Anthracite under Different Temperature and Pressure Conditions. PLoS ONE 2025, 20, e0310863. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Lan, F. Competitive Adsorption of CO2/N2/CH4 onto Coal Vitrinite Macromolecular: Effects of Electrostatic Interactions and Oxygen Functionalities. Fuel 2019, 235, 23–38. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular Simulation of CO2/CH4 Adsorption in Brown Coal: Effect of Oxygen-, Nitrogen-, and Sulfur-Containing Functional Groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Liu, Y.; Wilcox, J. Molecular Simulation of CO2 Adsorption in Micro- and Mesoporous Carbons with Surface Heterogeneity. Int. J. Coal Geol. 2012, 104, 83–95. [Google Scholar] [CrossRef]


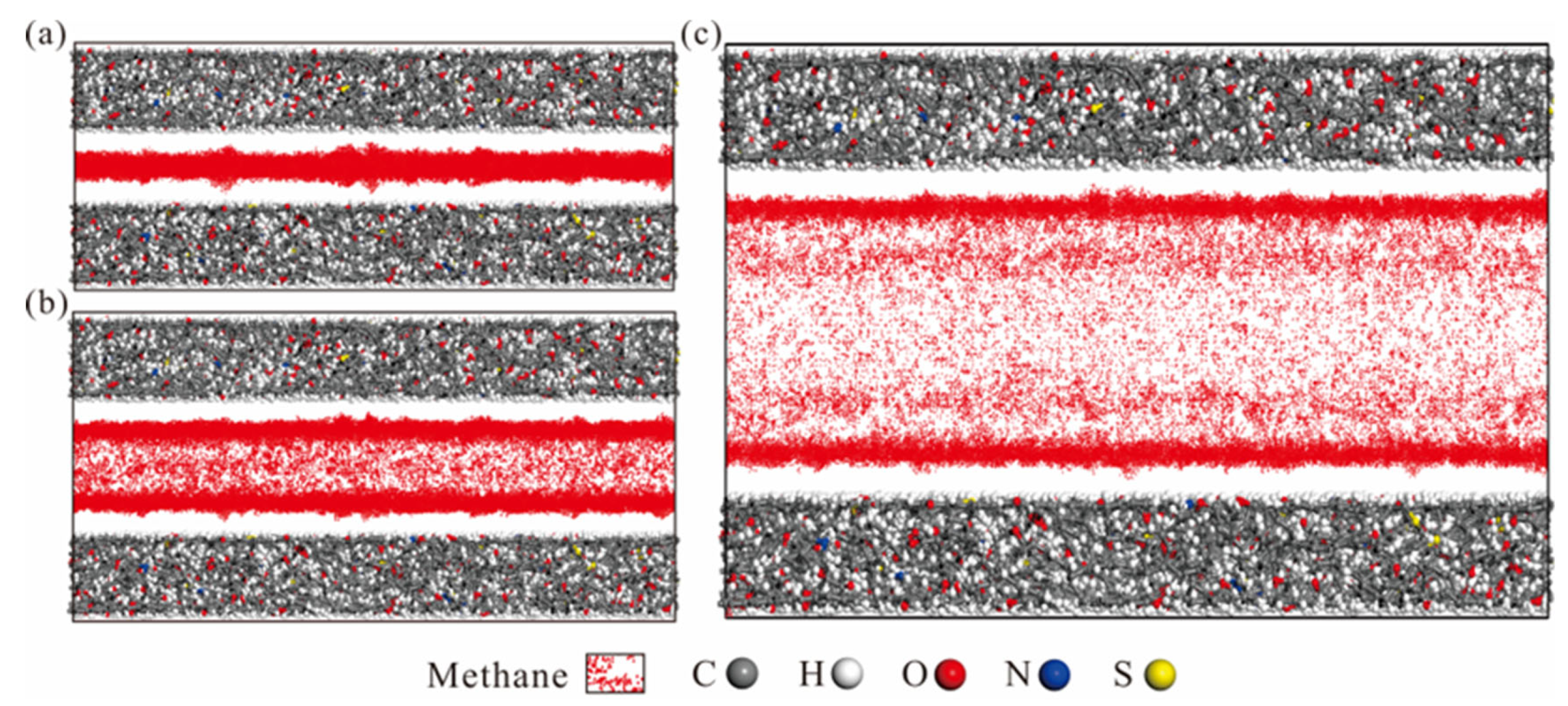
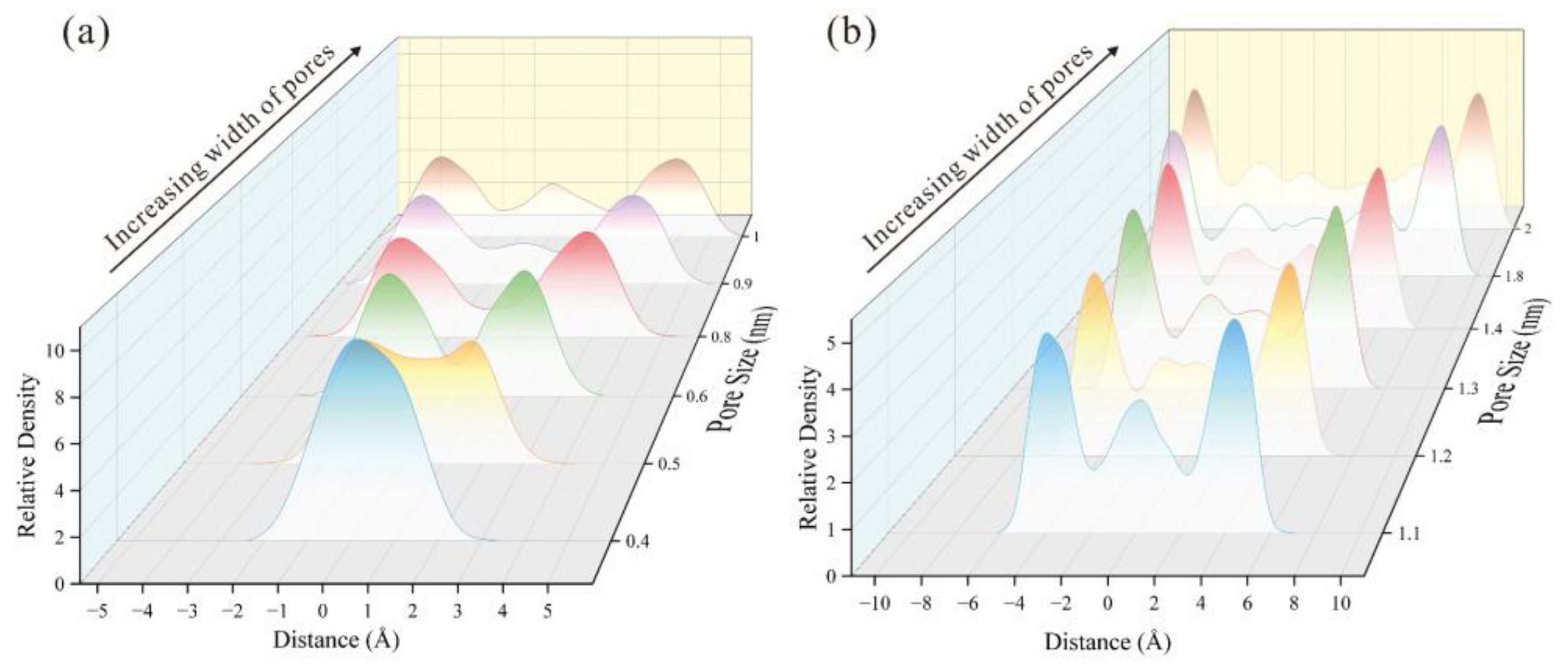
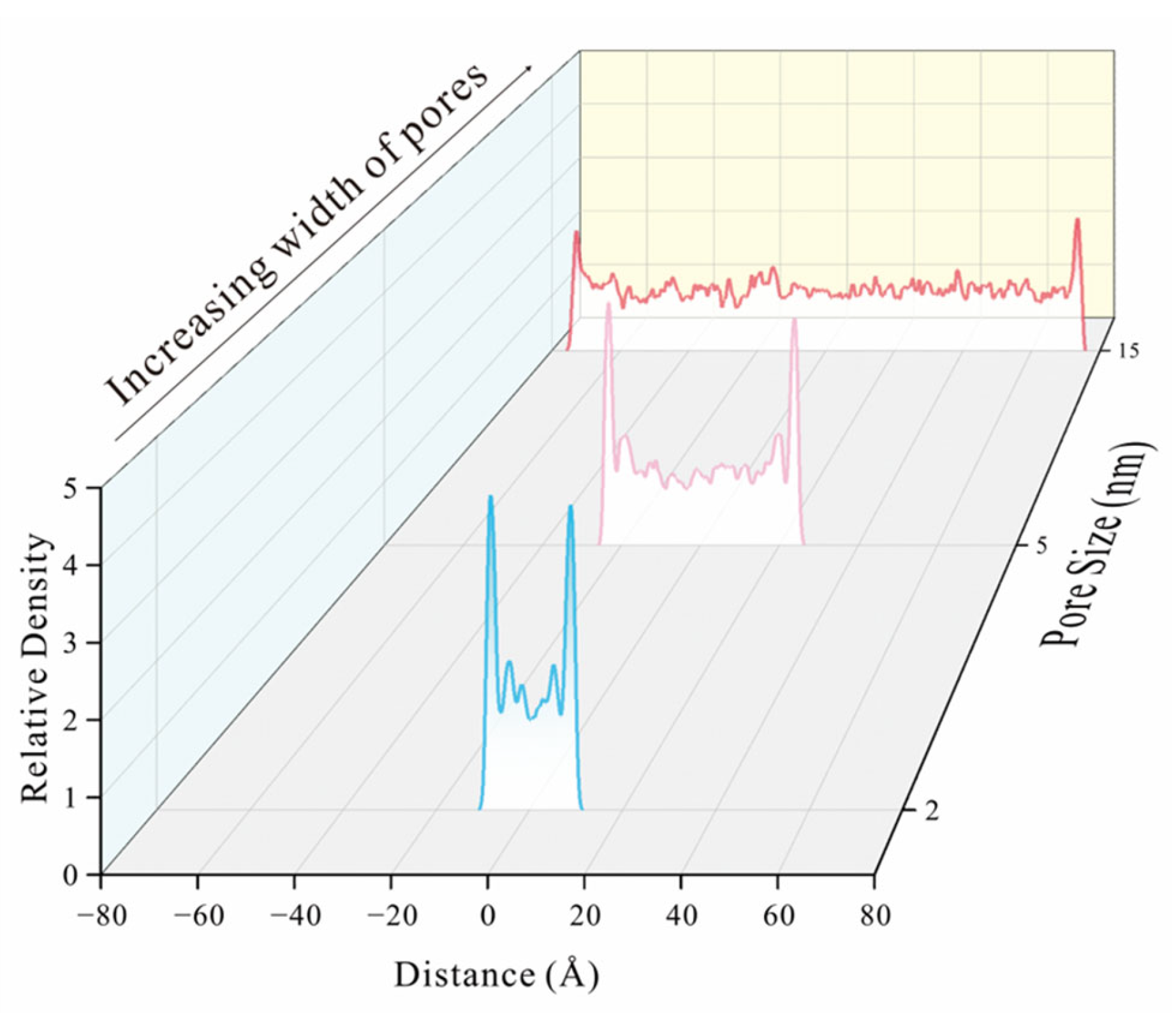


| Type | Organic Petrology | Industrial Analysis | Element Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ro | TOC | St | Mad | Ad | Vdaf | FCdaf | C | H | N | S | O | |
| Content (%) | 2.25 | 32.35 | 2.15 | 0.87 | 6.48 | 9.45 | 90.55 | 86.87 | 3.07 | 1.04 | 2.02 | 6.99 |
| Type | Chemical Shift (ppm) | Proportions (%) | Functional Group |
|---|---|---|---|
| Aliphatic Carbon | 14.63 | 0.24 | Aliphatic methyl carbon |
| 17.15 | 0.20 | Aromatic methyl carbon | |
| 20.35 | 1.77 | Aromatic methyl carbon | |
| 34.19 | 1.54 | Methylene | |
| 39.63 | 0.15 | Submethyl, quaternary carbon | |
| 54.82 | 4.07 | Oxygenated methylene carbon | |
| 61.00 | 6.07 | Oxygenated methylene carbon | |
| 70.78 | 2.42 | Oxygenated methylene carbon | |
| 82.56 | 0.08 | Oxygenated quaternary carbon | |
| 86.58 | 0.14 | Oxygenated quaternary carbon | |
| Aromatic Carbon | 120.75 | 12.24 | Protonated aromatic carbon |
| 120.75 | 10.58 | Protonated aromatic carbon | |
| 127.12 | 26.37 | Protonated aromatic carbon | |
| 136.97 | 12.67 | Bridged aromatic carbon | |
| 151.76 | 0.05 | Oxygenated aromatic carbon | |
| Carbonyl Carbon | 176.13 | 0.09 | Carbonyl carbon |
| 192.42 | 19.30 | Carboxyl carbon | |
| 206.78 | 2.03 | Carboxyl carbon |
| Type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 83.3 | 21.4 | 61.9 | 12.7 | 49.2 | 0.0 | 0.0 | 12.7 | 16.7 | 2.2 | 1.7 | 12.8 |
| Type | Energy (eV) | Functional Group | Proportions (%) |
|---|---|---|---|
| C(1S) | 284.79 | C-C, C-H | 69.82 |
| 285.42 | C-N | 9.87 | |
| 286.38 | C-O-H, C-O-C | 11.66 | |
| 283.66 | C=O | 4.08 | |
| 288.71 | N-C-O | 1.56 | |
| 290.76 | O-C=O | 3.01 | |
| N(1S) | 398.56 | Pyridine | 24.37 |
| 400.28 | Pyrrole | 49.32 | |
| 400.68 | Quaternary N | 24.70 | |
| 403.08 | NOx | 1.61 | |
| O(1S) | 531.39 | C=O, O-C=O | 11.91 |
| 532.50 | C-O-H, C-O-C | 60.65 | |
| 533.59 | C-O-C=O | 23.40 | |
| 534.30 | H2O | 4.04 | |
| S(2P) | 162.20 | Pyrite | 2.51 |
| 161.99 | -SH | 0.81 | |
| 163.01 | -SH | 8.82 | |
| 164.08 | Thiophene | 34.27 | |
| 164.44 | Thiophene | 37.01 | |
| 165.35 | -S=O- | 14.12 | |
| 165.88 | -SO2- | 2.46 |
| Type | Wave Number (cm−1) | Proportions (%) | Functional Group |
|---|---|---|---|
| Aromatic hydrocarbon spectra | 716.09 | 0.49 | aromatic rings |
| 743.59 | 2.45 | aromatic rings | |
| 800.39 | 1.37 | aromatic rings | |
| 831.99 | 0.07 | aromatic rings | |
| 870.90 | 2.46 | aromatic rings | |
| Oxygen-containing functional group | 1027.26 | 0.79 | C-O |
| 1160.58 | 0.56 | C-O | |
| 1264.51 | 3.53 | C-O | |
| 1337.17 | 3.26 | -CH2- | |
| 1378.54 | 1.30 | -CH2- | |
| 1431.71 | 10.16 | -CH2, -CH3 | |
| 1601.41 | 9.02 | C=C | |
| 1653.23 | 1.30 | C=O | |
| Aliphatic hydrocarbon spectra | 2804.44 | 0.01 | -COOH |
| 2817.14 | 0.05 | -COOH | |
| 2828.36 | 0.07 | CH2 | |
| 2851.32 | 0.45 | CH2 | |
| 2886.86 | 0.75 | CH2 | |
| 2908.47 | 0.19 | CH2 | |
| 2922.28 | 0.21 | CH3 | |
| 2935.91 | 0.17 | CH3 | |
| 2957.09 | 0.15 | CH3 | |
| 2969.15 | 0.02 | -OH | |
| 2986.75 | 0.02 | -OH | |
| Hydroxyl hydrogen bond spectra | 3040.81 | 0.98 | hydrogen bond |
| 3273.45 | 10.48 | hydrogen bond | |
| 3412.95 | 33.45 | -OH | |
| 3485.29 | 11.90 | -OH | |
| 3542.30 | 4.37 | -OH |
| Pore Size (nm) | 0.4 | 0.8 | 1 | 1.8 | 2 | 5 |
|---|---|---|---|---|---|---|
| a (mmol/g) | 2.19 | 3.86 | 4.76 | 6.20 | 8.10 | 17.71 |
| b (MPa−1) | 2.44 | 0.88 | 0.49 | 0.20 | 0.14 | 0.07 |
| Pressure (MPa) | Isosteric Heat of Adsorption (kJ/mol) | ||||||
|---|---|---|---|---|---|---|---|
| 0.4 nm | 0.5 nm | 0.6 nm | 1.2 nm | 1.4 nm | 2 nm | 5 nm | |
| 2 | 25.88 | 20.69 | 21.58 | 14.66 | 14.07 | 13.66 | 10.68 |
| 4 | 25.97 | 21.64 | 22.31 | 15.18 | 14.59 | 13.71 | 10.63 |
| 6 | 26.09 | 22.08 | 22.81 | 15.64 | 14.80 | 13.51 | 10.53 |
| 8 | 26.17 | 22.12 | 23.00 | 15.79 | 14.98 | 13.42 | 10.30 |
| 10 | 26.16 | 22.37 | 23.15 | 15.97 | 15.17 | 13.44 | 10.27 |
| 12 | 26.16 | 22.46 | 23.27 | 16.43 | 15.36 | 13.64 | 10.17 |
| 14 | 26.18 | 22.46 | 23.33 | 16.43 | 15.67 | 13.75 | 10.15 |
| 16 | 26.15 | 22.63 | 23.35 | 16.72 | 15.67 | 13.94 | 10.21 |
| 18 | 26.21 | 22.74 | 23.52 | 16.76 | 15.72 | 13.99 | 10.30 |
| 20 | 26.22 | 22.81 | 23.53 | 16.77 | 15.72 | 13.97 | 10.28 |
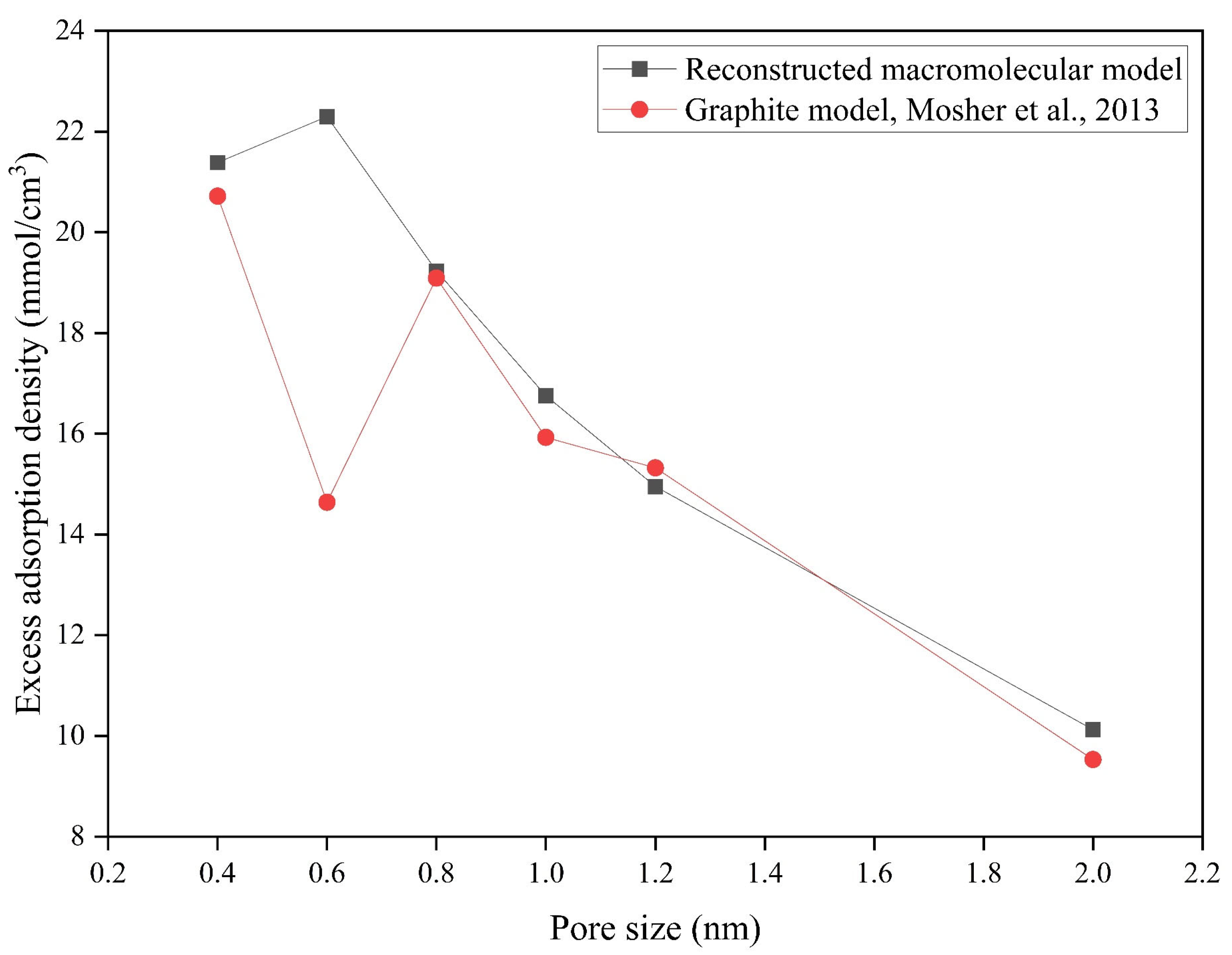
| Pore Size (nm) | Excess Adsorption Density (mmol/cm3) | |||
|---|---|---|---|---|
| RMM | Graphite Model | Δ (RMM and Graphite Model) | Reduction Proportion (%) | |
| 0.4 | 21.39 | 20.72 | 0.67 | 3.13 |
| 0.6 | 22.30 | 14.64 | 7.66 | 34.34 |
| 0.8 | 19.24 | 19.09 | 0.14 | 0.75 |
| 1 | 16.76 | 15.93 | 0.83 | 4.95 |
| 1.2 | 14.96 | 15.33 | −0.37 | −2.48 |
| 2 | 10.12 | 9.53 | 0.59 | 5.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Liu, H.; Yang, X.; Lei, T.; Guo, Q. Methane Sorption Behavior in Nanopores of Coal: A Molecular Dynamics Simulation Based on a Reconstructed Macromolecular Model. Processes 2025, 13, 3478. https://doi.org/10.3390/pr13113478
Cheng J, Liu H, Yang X, Lei T, Guo Q. Methane Sorption Behavior in Nanopores of Coal: A Molecular Dynamics Simulation Based on a Reconstructed Macromolecular Model. Processes. 2025; 13(11):3478. https://doi.org/10.3390/pr13113478
Chicago/Turabian StyleCheng, Junhan, Hanlin Liu, Xin Yang, Tao Lei, and Qiulei Guo. 2025. "Methane Sorption Behavior in Nanopores of Coal: A Molecular Dynamics Simulation Based on a Reconstructed Macromolecular Model" Processes 13, no. 11: 3478. https://doi.org/10.3390/pr13113478
APA StyleCheng, J., Liu, H., Yang, X., Lei, T., & Guo, Q. (2025). Methane Sorption Behavior in Nanopores of Coal: A Molecular Dynamics Simulation Based on a Reconstructed Macromolecular Model. Processes, 13(11), 3478. https://doi.org/10.3390/pr13113478






