Distinct Pollution Profiles and Spatio-Temporal Dynamics in Adjacent Ramsar Lakes (Algeria): An Integrated Assessment and High-Resolution Mapping for Targeted Conservation
Abstract
1. Introduction
- Identify the major pollution sources affecting Lakes Tonga and Oubeira.
- Evaluate the spatial and temporal heterogeneity of pollution both between and within the two lakes, and demonstrate how high-resolution spatial mapping contributes to visualizing these patterns.
- Assess the implications of the observed pollution levels for the conservation of these valuable ecosystems, including their biodiversity and the public health of surrounding communities.
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Protocol
2.3. Laboratory Analyses
2.3.1. Physico-Chemical Analyses
2.3.2. Heavy Metal Analysis
2.3.3. Microbiological Analyses
2.4. Water Quality Index (WQI) Calculation
2.5. Statistical Analysis
3. Results
3.1. Physico-Chemical and Microbiological Characterization of Lakes Tonga and Oubeira
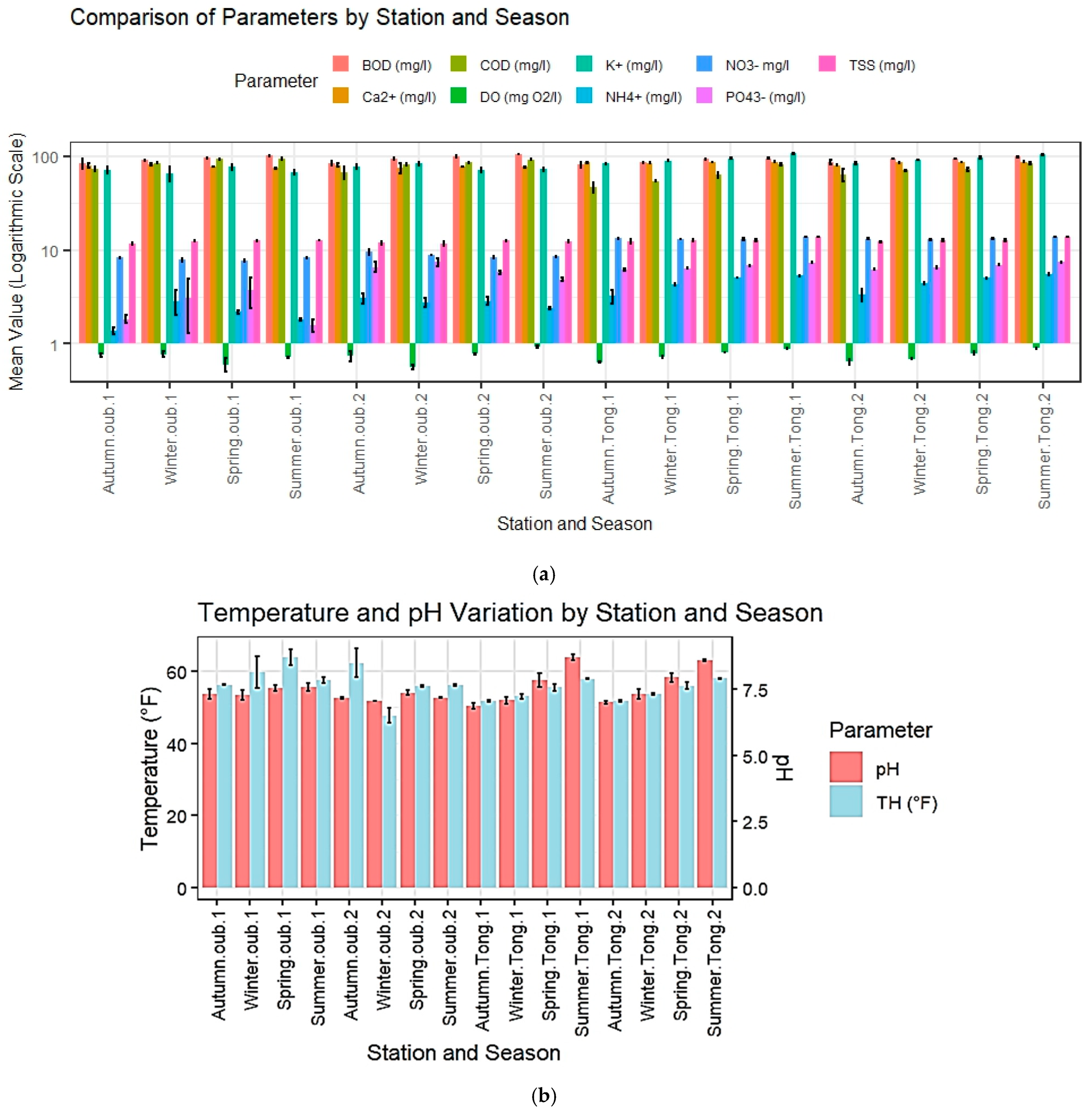
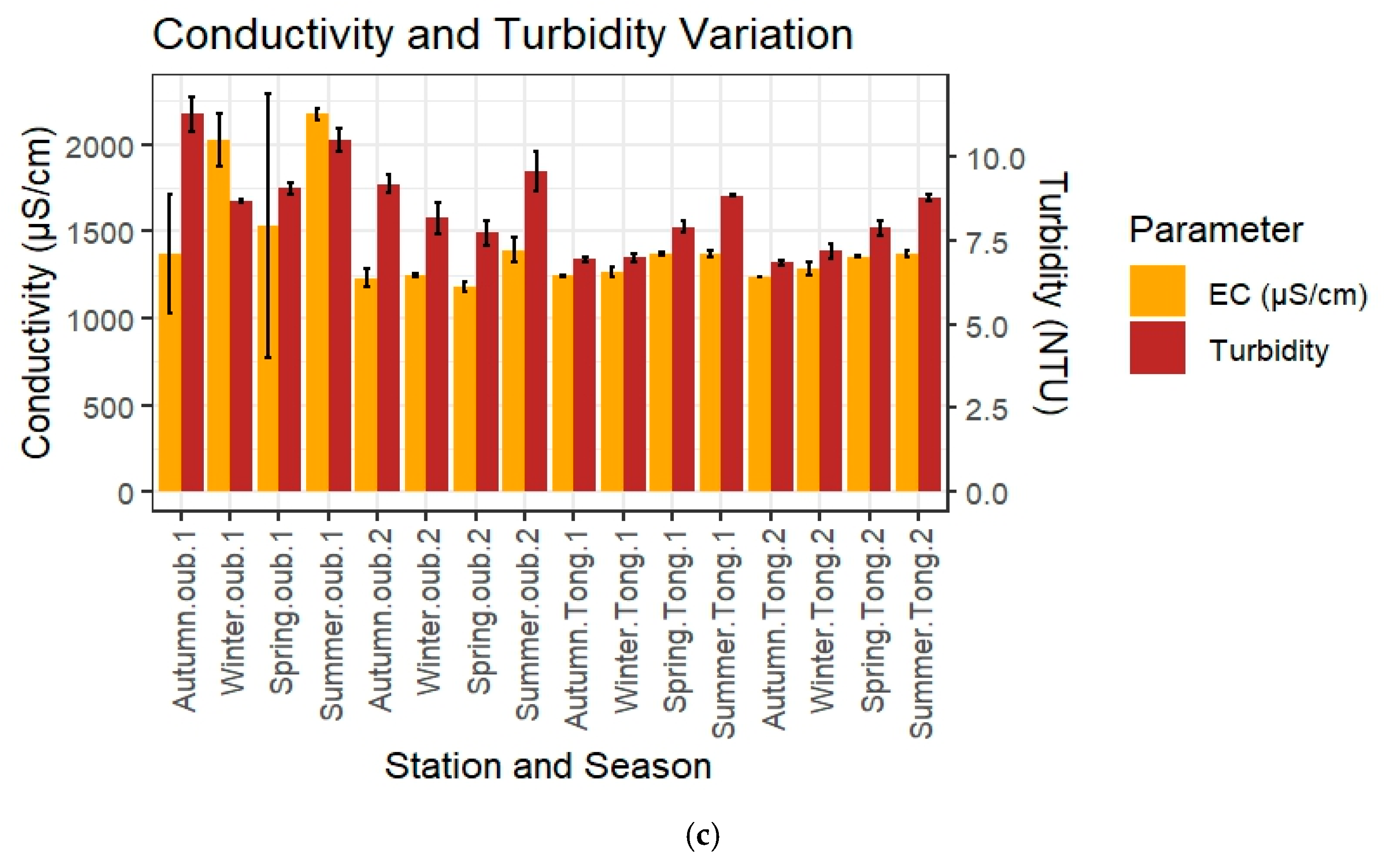
3.1.1. Physico-Chemical Parameters
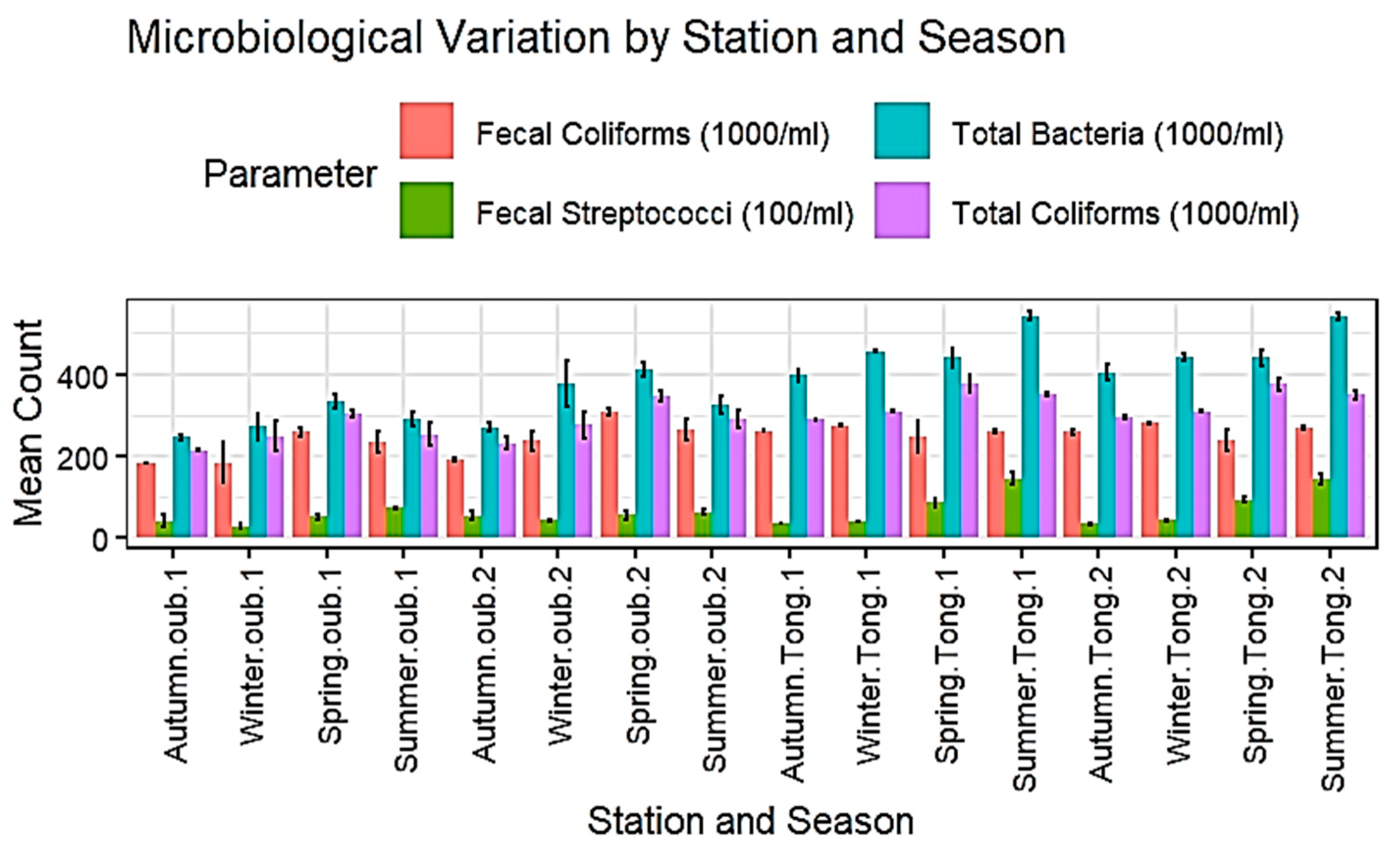
3.1.2. Microbiological Parameters
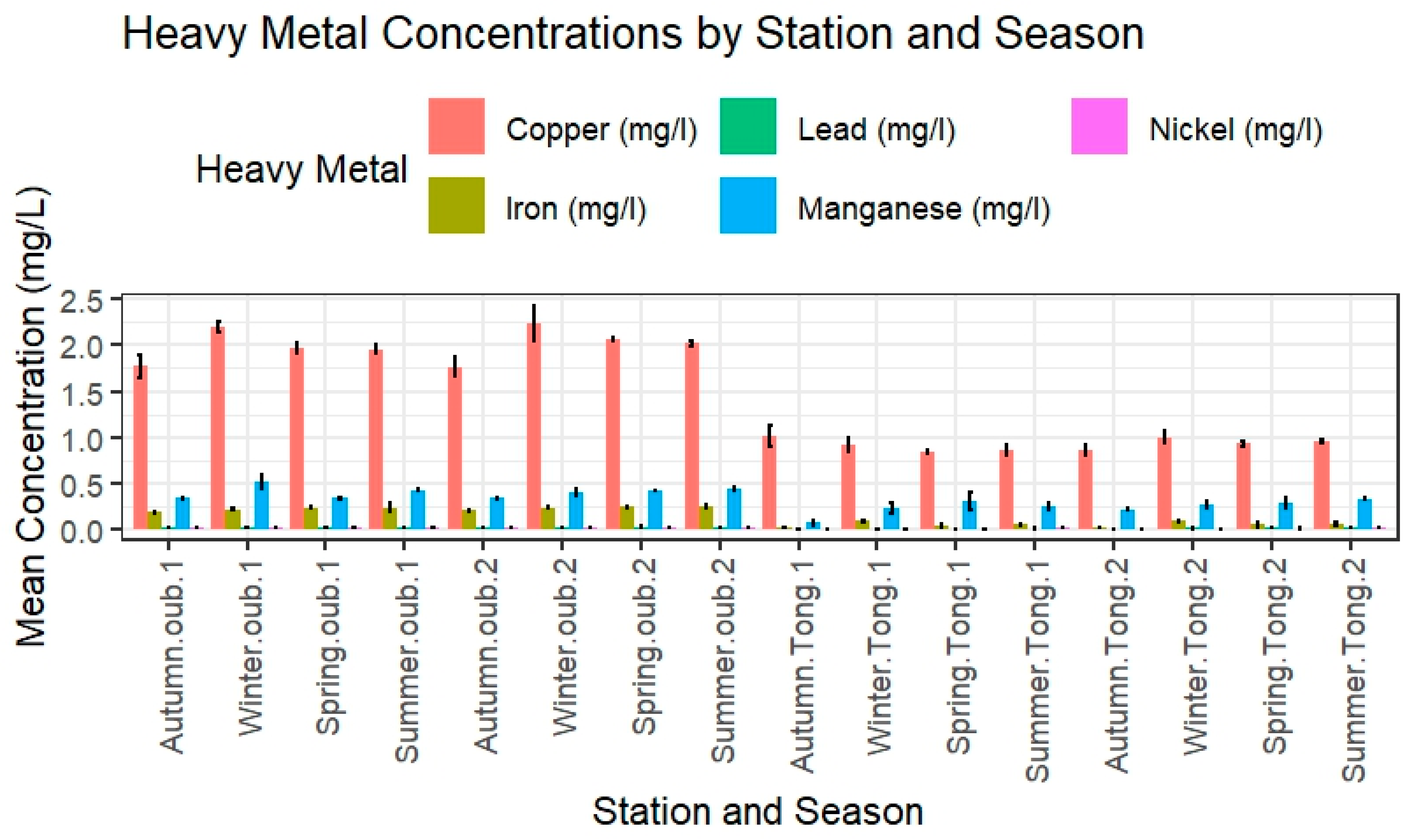
3.1.3. Heavy Metal Concentrations
3.2. Statistical Analyses
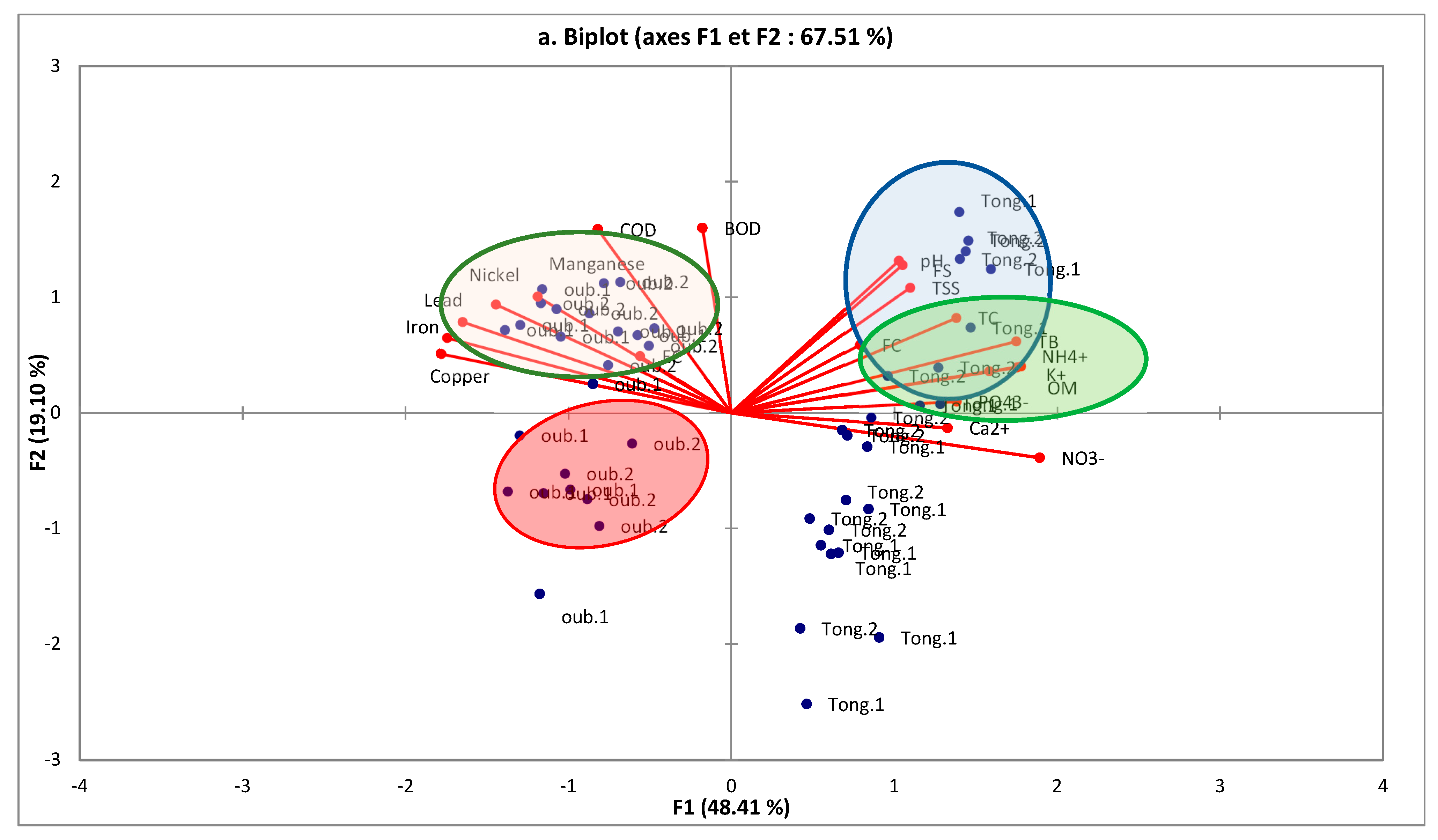
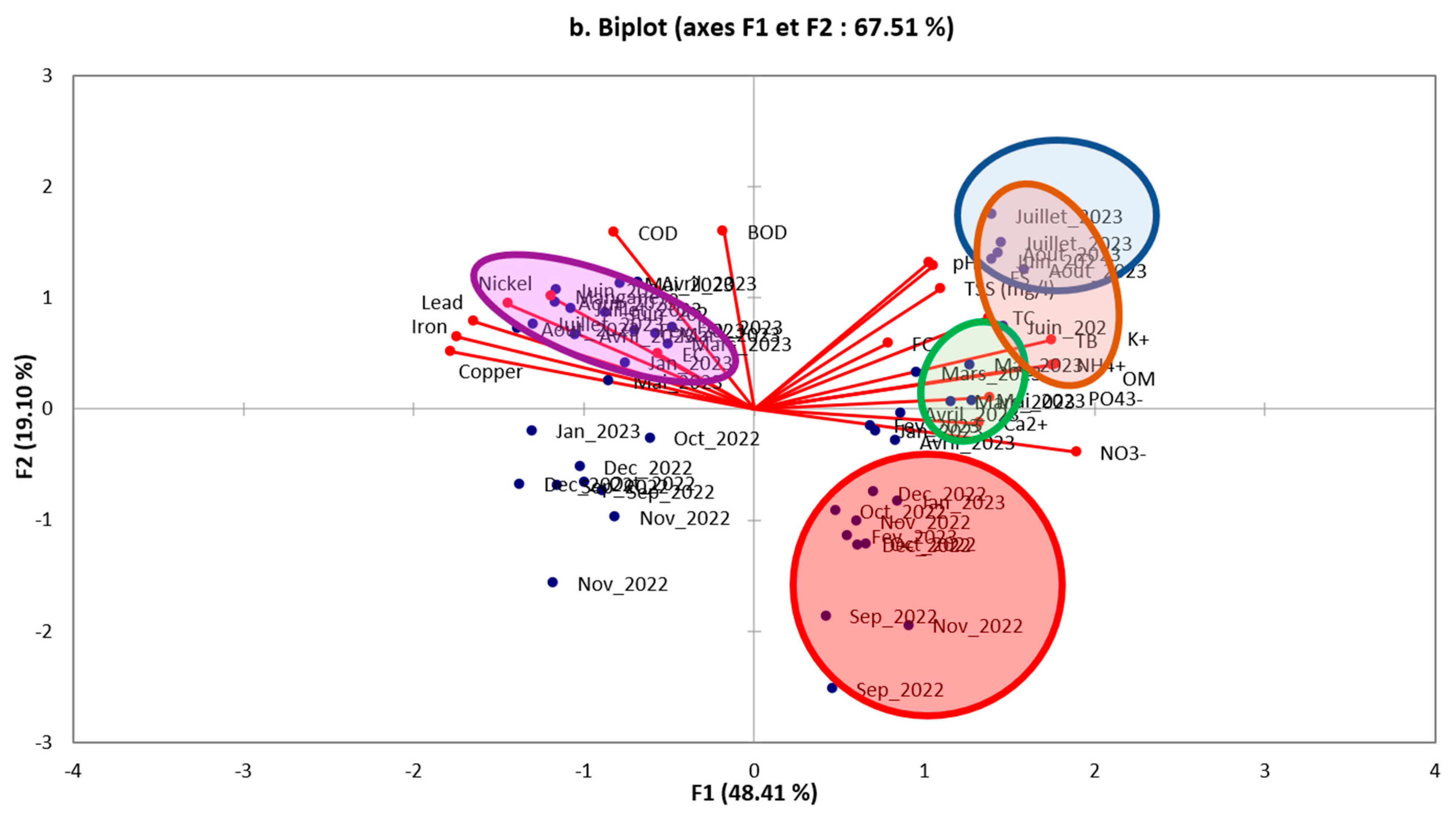
3.2.1. Principal Component Analysis (PCA)
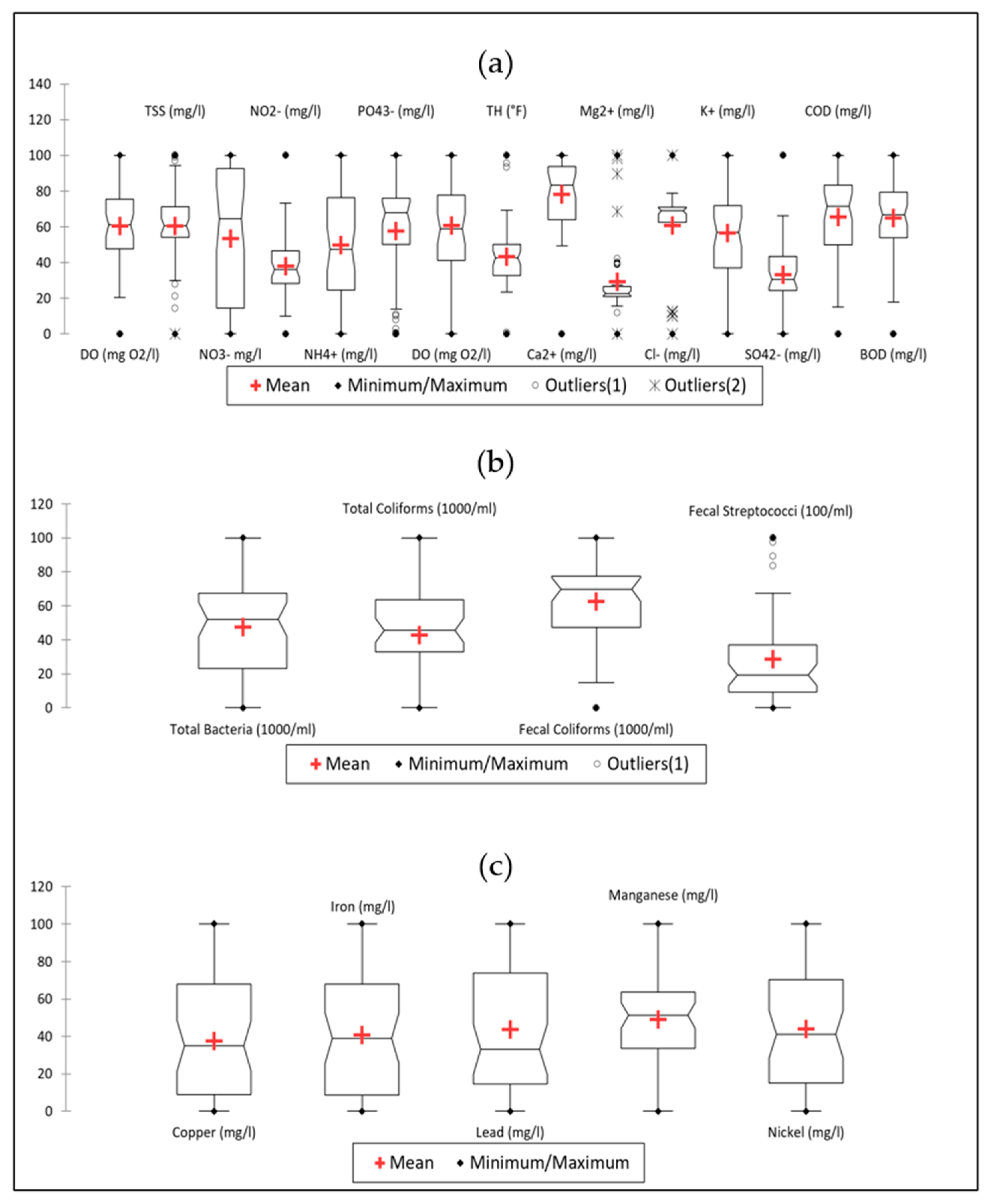
3.2.2. Boxplot Analysis
3.2.3. Water Quality Index (WQI): Spatio-Temporal Assessment
3.2.4. Analysis of Variance (ANOVA)
3.3. Exceedances of Water Quality Standards and Cartographic Representation
3.3.1. Exceedances of Water Quality Standards
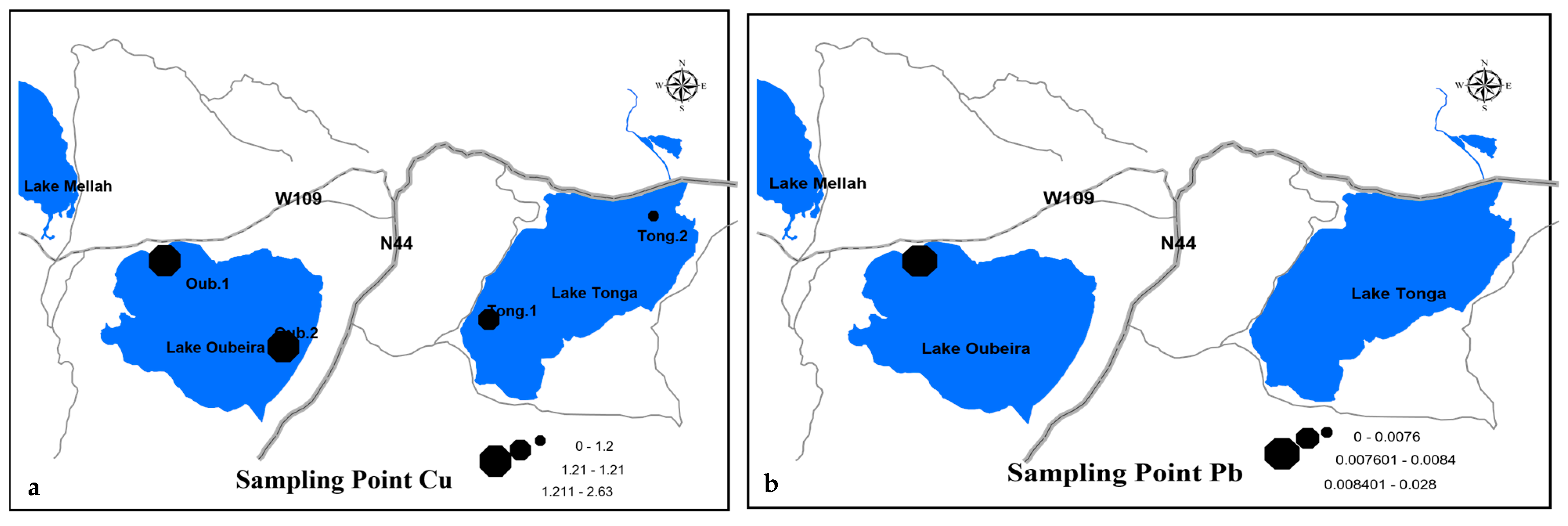
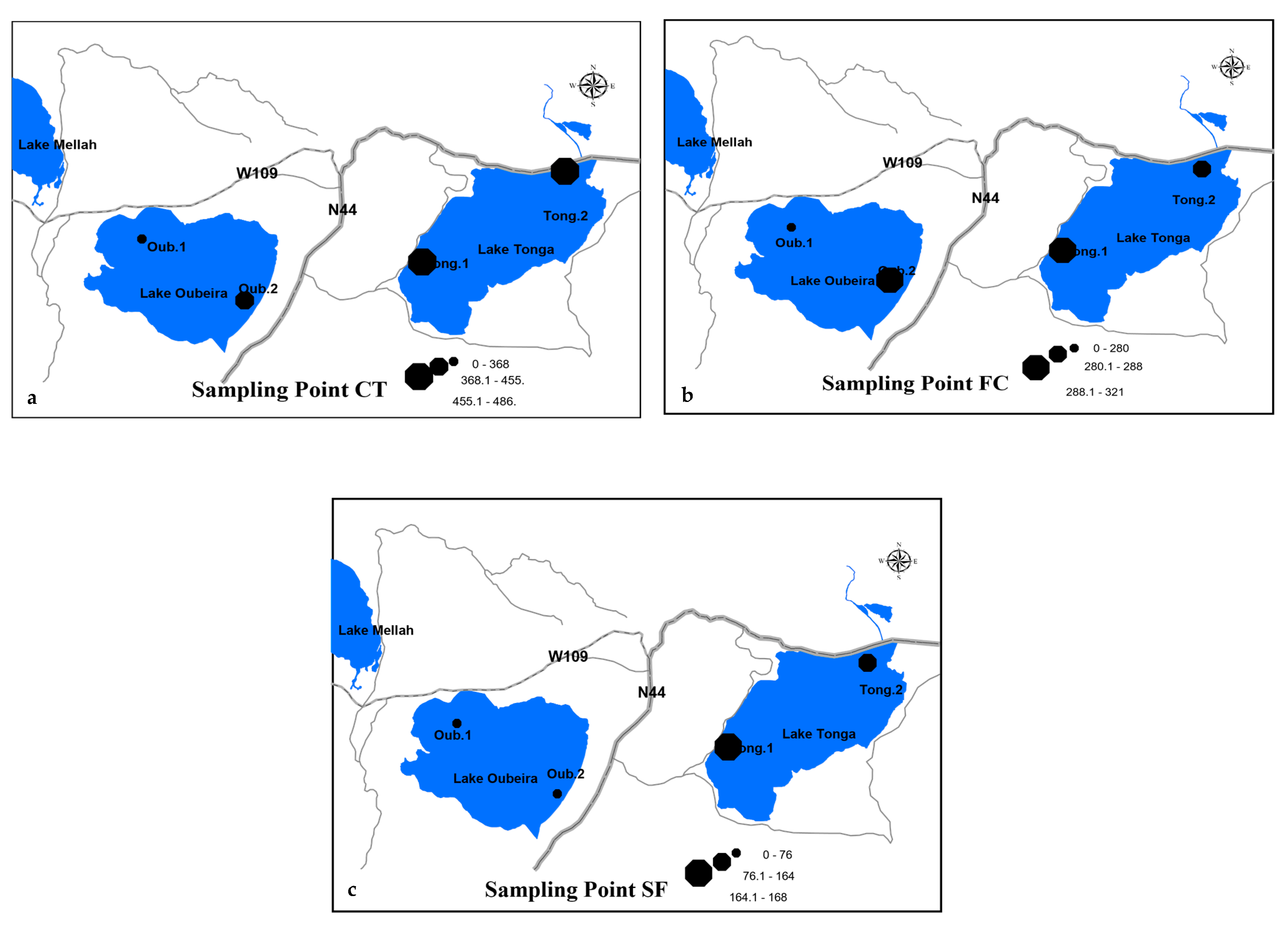
3.3.2. Cartographic Representation of the Spatial Distribution of Pollutants
Nutrients
Heavy Metals
Microbiological Contamination Indicators
4. Discussion
- For Lake Tonga, management efforts must prioritize the reduction in nutrient and microbial inputs from non-point sources. Key actions should include the implementation of agricultural best management practices, such as optimizing fertilizer use and establishing vegetated buffer strips along drainage channels and the lake shore to intercept runoff [59]. Concurrently, improving domestic wastewater collection and investing in effective, low-cost treatment solutions for the surrounding rural settlements is critical to curb fecal pollution [60].
- For Lake Oubeira, the priority is to address the severe metal and organic pollution. An urgent investigation is required to identify the precise sources of copper, differentiating between potential industrial point sources, diffuse agricultural inputs (e.g., fungicides), or legacy contamination from sediments.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Jung, M.; Arnell, A.; de Lamo, X.; García-Rangel, S.; Lewis, M.; Mark, J.; Merow, C.; Miles, L.; Ondo, I.; Pironon, S.; et al. Areas of global importance for conserving terrestrial biodiversity, carbon and water. Nat. Ecol. Evol. 2021, 5, 1499–1509. [Google Scholar] [CrossRef]
- Ramsar Convention Secretariat. The Ramsar Convention Manual: A Guide to the Convention on Wetlands; Ramsar Convention Secretariat: Gland, Switzerland, 2021; Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119692621.ch2 (accessed on 10 December 2024).
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Woolway, R.I.; Sharma, S.; Smol, J.P. Lakes in Hot Water: The Impacts of a Changing Climate on Aquatic Ecosystems. Bioscience 2022, 72, 1050–1061. [Google Scholar] [CrossRef]
- Meybeck, M.; Lestel, L.; Carré, C.; Bouleau, G.; Garnier, J.; Mouchel, J.M. Trajectories of river chemical quality issues over the Longue Durée: The Seine River (1900S–2010). Environ. Sci. Pollut. Res. 2016, 25, 23468–23484. [Google Scholar] [CrossRef]
- Nyenje, P.M.; Foppen, J.W.; Uhlenbrook, S. Eutrophication and nutrient release in urban areas of sub-Saharan Africa—A review. Sci. Total Environ. 2010, 408, 447–455. [Google Scholar] [CrossRef]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef]
- Ayele, G.; Atlabachew, M. Review of characterization, factors, impacts, and solutions of Lake eutrophication: Lesson for lake Tana, Ethiopia. Environ. Sci. Pollut. Res. 2021, 28, 14233–14252. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Salama, E.; Han, H.; Zheng, Y.; Kulshrestha, S.; Jalalah, M.; Harraz, F.; Alsareii, S.; Harraz, F.; Alsareii, S.; et al. World eutrophic pollution of lake and river: Biotreatment potential and future perspectives. Environ. Technol. Innov. 2021, 23, 101604. [Google Scholar] [CrossRef]
- Suresh, K.; Tang, T.; Van Vliet, M.T.; Bierkens, M.F.P.; Strokal, M.; Sorger-Domenigg, F.; Wada, Y. Recent advancement in water quality indicators for eutrophication in global freshwater lakes. Environ. Res. Lett. 2023, 18, 073001. [Google Scholar] [CrossRef]
- Pereira, A.; Mulligan, C.N. Practices for Eutrophic Shallow Lake Water Remediation and Restoration: A Critical Literature Review. Water 2023, 15, 2270. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Belings, I.; Verspagen, P.A.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Downing, J. Limnology and oceanography: Two estranged twins reuniting by global change. Inland Waters 2014, 4, 215–232. [Google Scholar] [CrossRef]
- Gherib, A.; Lazli, A.; Naili, S.; Boucheker, A.; Ikhlef, D.; Mechaka, N. Avifauna diversity and phenology in a Ramsar site: Lake Tonga (Northeastern Algeria). Arx. Miscel·Lània Zool. 2022, 19, 321–344. [Google Scholar] [CrossRef]
- Djamai, S.; Mimeche, F.; Bensaci, E.; Oliva-Paterna, F. Diversity of macro-invertebrates in Lake Tonga (northeast Algeria). Biharean Biol. 2019, 13, 8–11. [Google Scholar]
- Nebbache, M.; Lazli, A.; Boucheker, A. An ecological survey of Laro-limicolae in Northeastern Algeria. Arx. Miscel·Lània Zool. 2023, 21, 261–274. [Google Scholar] [CrossRef]
- Dis, C.; Kara, F.; Bouamama, S.; Sahra, F.; Boucena, M. Eco-biological Study of the Mosquitofish Gambusia affinis from Oubeira lake. J. Aquac. Fish Health 2022, 11, 201–209. [Google Scholar]
- Bensaâd-Bendjedid, L.; Telailia, S.; Alliouche, F.; Touati, H.; Ladjama, I. First record of the occurrence of the Chinese pond mussel Sinanodonta woodiana (Lea, 1834) (Bivalvia: Unionidae: Unionidae) in African freshwaters: Oubeira Lake, Algeria. Turk. J. Zool. 2023, 47, 94–102. [Google Scholar] [CrossRef]
- Nasri, A.; Bouaïcha, N.; Fastner, J. First Report of a Microcystin-Containing Bloom of the Cyanobacteria microcystis spp. in Lake Oubeira, Eastern Algeria. Arch. Environ. Contam. Toxicol. 2004, 46, 197–202. [Google Scholar] [CrossRef]
- Nasri, H.; Herry, E.; Bouaïcha, N. First reported case of turtle deaths during a toxic Microcystis spp. bloom in Lake Oubeira, Algeria. Ecotoxicol. Environ. Saf. 2008, 71, 535–544. [Google Scholar] [CrossRef]
- Hasna, C.; Faiza, M.; Aicha, T. Diatoms diversity in Oubeïra Lake, northeastern Algeria. Biodiversitas 2020, 11, 573–580. [Google Scholar] [CrossRef]
- Djelita, B.; Benadda, L. Spatiotemporal Assessment of the Water Quality of the Hammam Boughrara Dam (North-West Algeria). J. Ecol. Eng. 2021, 22, 92–103. [Google Scholar] [CrossRef]
- Loucif, K.; Neffar, S.; Menasria, T.; Maazi, M.C.; Houhamdi, M.; Chenchouni, H. Physico-chemical and bacteriological quality assessment of surface water at Lake Tonga in Algeria. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100284. [Google Scholar] [CrossRef]
- Loucif, K. Ecology of Avian Settlements in Lake Tonga (Northern Algeria). Zool. Sci. 2020, 54, 275–284. [Google Scholar] [CrossRef]
- Naili, S.; Boucheker, A.; Gherib, A.; Djelloul, R.; Lazli, A. Seasonal variation in physico-chemical characteristics and load contamination of lake Tonga and their effects on waterbird populations. Ukr. J. Ecol. 2021, 11, 103–112. [Google Scholar]
- Derradji, F.; Benmeziane, F.; Benaabidate, L.; Maoui, A.; Bousnoubra, H.; Kherici, N. Heavy metals analysis in the water of Mellah, Oubeira and Tonga Lakes of El kala wetland complex, North east Algeria. Int. J. Innov. Sci. Res. 2015, 13, 107–113. [Google Scholar]
- Belabed, B.-E.; Bendjema, A.; Boudjelida, H.; Djabri, L.; Bensouilah, M. Evaluation of the metal contaminations in the surface sediments of the Oubeira lagoon, National park of El Kala, Algeria. Arch. Appl. Sci. Res. 2011, 3, 51–62. [Google Scholar]
- Loucif, K.; Maazi, M.; Houhamdi, M.; Chenchouni, H. Nest site selection and breeding ecology of the Ferruginous Duck (Aythya nyroca) in Algeria. Glob. Ecol. Conserv. 2021, 26, e01524. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Bendali-Saoudi, F.; Soltani, N. Do water physicochemical parameters explain richness and phenology of aquatic beetles (Coleoptera) in Tonga Lake (Northeast Algeria)? Orient. Insects 2022, 57, 1–24. [Google Scholar] [CrossRef]
- Alayat, H.; Lamouroux, C. Caractérisation physico-chimique des eaux thermo–minérales des monts de la Cheffia (Extrême nord-est Algérien). Presse Therm. Clim. 2007, 144, 191–199. [Google Scholar]
- Alayat, H.; Kherici, N.; Lamouroux, C. Evolution Spatiale Des Caracteristiques Physico-Chimiques Des Eaux Du Lac Oubeira Impose Par Les Conditions Severes De La Secheresse (Extreme Ne Algerien). Eur. Sci. J. 2013, 9, 564–579. [Google Scholar]
- Alayat, H.; Kherici, N.; Lamouroux, C. Space evolution of the silting of Oubeira Lake imposed by erosion (extreme NE Algerian). Rev. Ljee 2014, 15, 28–38. [Google Scholar]
- Bouchaala, L.; Charchar, N.; Mezoug, S.E.; Sellam, F.; Guednouse, A.; Houhamdi, M. Point position of the bacteriological physicochemical quality and wetlands water mapping of Guerbes-Sanhadja eco-complex (Skikda, Northeastern Algeria). Int. J. Innov. Sci. Res. 2019, 46, 80–93. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N. L’Analyse de L’Eau: Eaux Naturelles, Eaux Résiduaires, Eau de Mer, 9th ed.; Dunod: Malakoff, France, 2009; Available online: http://www.sudoc.fr/134445627 (accessed on 1 November 2024).
- ISO 13395:1996; Water Quality—Determination of Nitrate Nitrogen and Nitrite Nitrogen and the Sum of Both by Flow Analysis (CFA and FIA) and Spectrometric Detection. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 6777:1984; Water Quality—Determination of Nitrite—Molecular Absorption Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984.
- NF EN 1189:2007; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. Association Française de Normalisation (AFNOR): Paris, France, 2007.
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- EN 1899-1:1998; Water Quality—Determination of Biochemical Oxygen Demand After n Days (BODn)—Part 1: Dilution and Seeding Method with Allylthiourea Addition. European Committee for Standardization (CEN): Brussels, Belgium, 1998.
- NF EN 872:2005; Water Quality—Determination of Suspended Solids—Method by Filtration Through Glass Fibre Filters. Association Française de Normalisation (AFNOR): Paris, France, 2005.
- ISO 5667-16:2017; Water Quality—Sampling—Part 16: Guidance on Sample Preservation and Handling of Water Samples. International Organization for Standardization: Geneva, Switzerland, 2017.
- U.S. EPA. Method 6010D: Inductively Coupled Plasma-Optical Emission Spectrometry. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; SW-846; U.S. Environmental Protection Agency: Washington, DC, USA, 2018. [Google Scholar]
- ISO 9308-2:2012; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 2: Most Probable Number Method. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 9308-3:1998; Water Quality—Detection and Enumeration of Escherichia coli and Coliform Bacteria in Surface and Waste Water—Part 3: Miniaturized Method (Most Probable Number) for the Detection and Enumeration of E. coli. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 7899-2:2000; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. International Organization for Standardization: Geneva, Switzerland, 2000.
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Messerer, Y.; Retima, A.; Amira, A.B.; Djebar, A.B. Climatic changes, hydrology and trophic status of Lake Oubeira (extreme northeast of Algeria). AACL Bioflux 2019, 12, 4. [Google Scholar]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Bouhezila, F.; Hacène, H.; Aichouni, M. Water quality assessment in Réghaïa (North of Algeria) lake basin by using traditional approach and water quality indices. Kuwait J. Sci. 2020, 47, 57–71. [Google Scholar]
- Tong, Y.; Xu, X.; Qi, M.; Sun, J.; Zhang, Y.; Zhang, W.; Wang, M.; Wang, X.; Zhang, Y. Lake warming intensifies the seasonal pattern of internal nutrient cycling in the eutrophic lake and potential impacts on algal blooms. Water Res. 2020, 188, 116570. [Google Scholar]
- Madene, E.; Boufekane, A.; Busico, G.; Meddi, M.; Maizi, D. Temporal evaluation of surface water quality in semiarid regions: A case study of the Ouled Mellouk dam in north-western Algeria. Water Supply 2023, 23, 2387–2403. [Google Scholar] [CrossRef]
- Boussaha, A.; Bezzalla, A.; Zebsa, R.; Amari, H.; Houhamdi, M.; Chenchouni, H. Monitoring and assessment of spatial and seasonal variability in water quality at Lake of Birds (Algeria) using physicochemical parameters and bacterial quality indicators. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100955. [Google Scholar] [CrossRef]
- Mebarkia, A.; Boufekane, A. Human activity impact on surface water quality in semi-arid regions: A case study of Aïnzeda lake (North-East Algeria). Water Supply 2020, 20, 1726–1744. [Google Scholar] [CrossRef]
- Loucif, K.; Chenchouni, H. Water physicochemical quality as driver of spatial and temporal patterns of microbial community composition in lake ecosystems. Appl. Water Sci. 2024, 14, 115. [Google Scholar] [CrossRef]
- Guemmaz, F.; Neffar, S.; Chenchouni, H. Physicochemical and Bacteriological Quality of Surface Water Resources Receiving Common Wastewater Effluents in Drylands of Algeria. In Water Resources in Algeria: Part II; Negm, A., Bouderbala, A., Chenchouni, H., Barceló, D., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2020; Volume 100. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Senouci, N.; Bemmoussat-Dekkak, S.; Ammouri, R.; Abdellaoui-Hassaine, K. Water Quality Evaluation Using Benthic Macroinvertebrates, Physicochemical Parameters and Heavy Metal Levels in Two Lakes (Northwestern Algeria). Appl. Ecol. Environ. Res. 2023, 21, 5069–5090. [Google Scholar] [CrossRef]
- Tammeorg, O.; Chorus, I.; Spears, B.; Nõges, P.; Nürnberg, G.K.; Tammeorg, P.; Søndergaard, M.; Jeppesen, E.; Paerl, H.; Huser, B.; et al. Sustainable lake restoration: From challenges to solutions. Wiley Interdiscip. Rev. Water 2023, 11, e1689. [Google Scholar] [CrossRef]
- Bouchaala, L.; Charchar, N.; Gherib, A.E. Ressources hydriques: Traitement et réutilisation des eaux usées en Algérie. Alger. J. Arid Environ. 2017, 7, 84–95. [Google Scholar] [CrossRef]
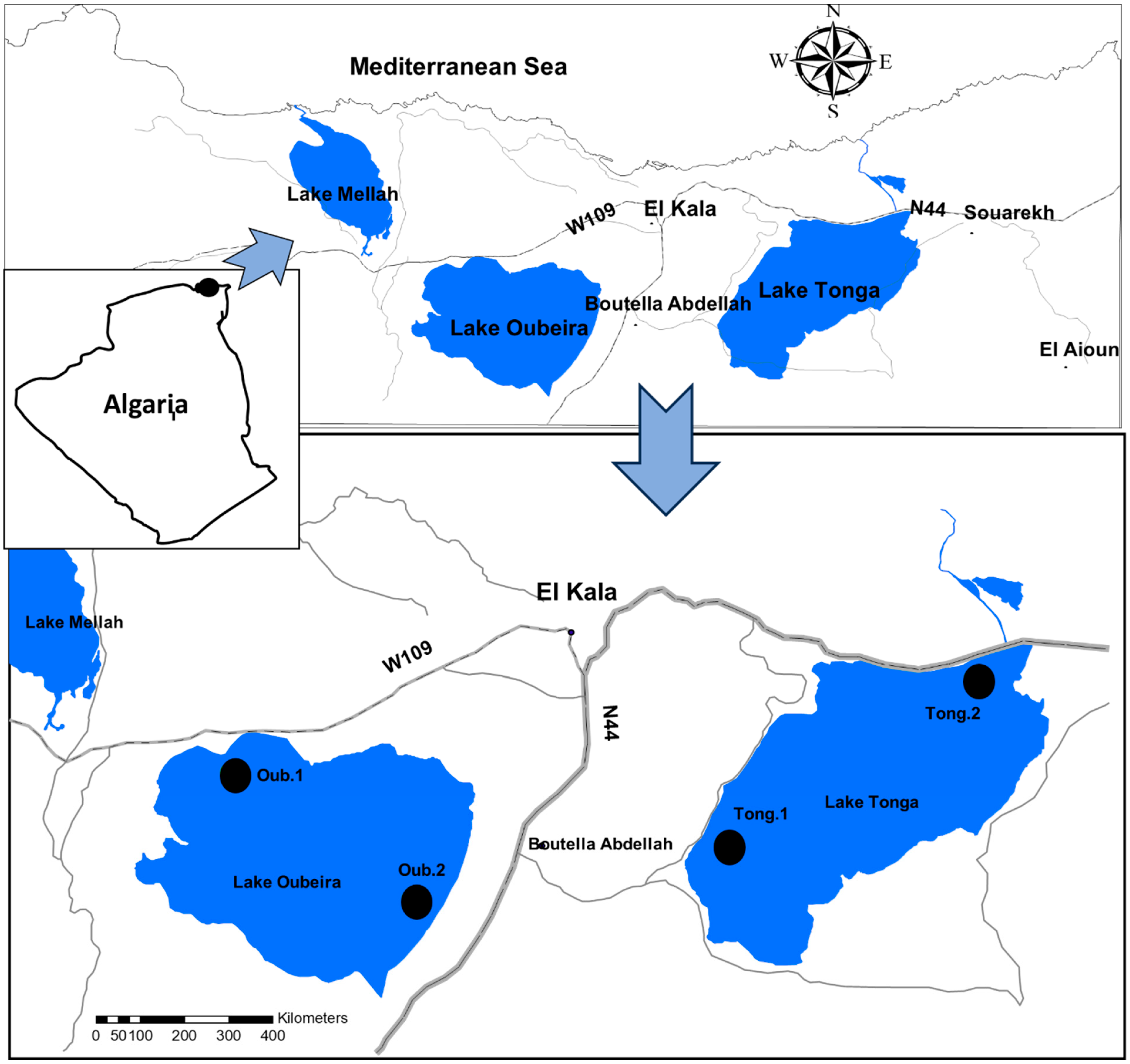

| Station | Latitude | Longitude | Station Justification |
|---|---|---|---|
| Tong.1 | 36°84′44″ N | 8°47′20″ E | Located on the western shore of Lake Tonga, this station is in close proximity to cultivated fields and an agricultural drainage canal, representing the zone potentially most impacted by agricultural runoff. Accessible via an existing agricultural road. |
| Tong.2 | 36°87′98″ N | 8°52′51″ E | Situated in the center of Lake Tonga, this station is considered to be less directly influenced by point sources of pollution and representative of open-water conditions. Accessible by light boat. |
| Oub.1 | 36°86′39″ N | 8°37′32″ E | Located in the northern part of Lake Oubeira, near the primary water input zone (where small chaabates drain rainwater into the lake), allowing for assessment of incoming water quality. Accessible from the bank. |
| Oub.2 | 36°83′50″ N | 8°40′77″ E | Positioned on the southeastern shore of Lake Oubeira, this station is in proximity to rural dwellings and a potential diffuse discharge point for domestic wastewater. Accessible via a walking path. |
| Station | Season | Mean WQI ± SD | WQI Classification (Seasonal) |
|---|---|---|---|
| Tong.1 | Autumn 2022 | 56.0 ± 1.41 | Moderate quality |
| Tong.1 | Winter 2022–2023 | 58.4 ± 0.37 | Moderate quality |
| Tong.1 | Spring 2023 | 60.2 ± 0.27 | Moderate quality |
| Tong.1 | Summer 2023 | 63.1 ± 0.65 | Moderate quality |
| Tong.2 | Autumn 2022 | 56.6 ± 1.73 | Moderate quality |
| Tong.2 | Winter 2022–2023 | 59.5 ± 0.98 | Moderate quality |
| Tong.2 | Spring 2023 | 60.2 ± 1.59 | Moderate quality |
| Tong.2 | Summer 2023 | 63.5 ± 0.30 | Moderate quality |
| Oub.1 | Autumn 2022 | 56.0 ± 2.72 | Moderate quality |
| Oub.1 | Winter 2022–2023 | 60.9 ± 4.07 | Moderate quality |
| Oub.1 | Spring 2023 | 60.6 ± 1.30 | Moderate quality |
| Oub.1 | Summer 2023 | 61.1 ± 0.41 | Moderate quality |
| Oub.2 | Autumn 2022 | 61.4 ± 1.06 | Moderate quality |
| Oub.2 | Winter 2022–2023 | 61.4 ± 1.96 | Moderate quality |
| Oub.2 | Spring 2023 | 63.3 ± 0.09 | Moderate quality |
| Oub.2 | Summer 2023 | 62.6 ± 0.39 | Moderate quality |
| Parameter | F-Value | p-Value | Post Hoc Groupings (Tukey HSD) 1 |
|---|---|---|---|
| Turbidity | 13.782 | <0.001 | Oub.1 (A), oub.2 (B), Tong.2 Tong.1 (C) |
| EC (µS/cm) | 5.273 | 0.003 | Oub.1 (A), oub.2 (B), Tong.2 Tong.1 (C) |
| TSS (mg/L) | 3.660 | 0.019 | Oub.2 (A), Tong.2 (B), oub.1 (BC), Tong.1 (C) |
| NO3− (mg/L) | 369.859 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| NH4+ (mg/L) | 27.233 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| PO43− (mg/L) | 33.835 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| OM (mg O2/L) | 15.653 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| TH (°F) | 3.080 | 0.037 | Tong.1 Tong.2 (A), oub.2 oub.1 (B) |
| Ca2+ (mg/L) | 9.522 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| K+ (mg/L) | 21.458 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| COD (mg/L) | 9.559 | <0.001 | Oub.1 oub.2 (A), Tong.2 (B), Tong.1 (C) |
| Copper (mg/L) | 150.825 | <0.001 | Oub.2 oub.1 (A), Tong.2 Tong.1 (B) |
| Iron (mg/L) | 103.405 | <0.001 | Oub.2 oub.1 (A), Tong.2 Tong.1 (B) |
| Lead (mg/L) | 134.181 | <0.001 | Oub.2 oub.1 (A), Tong.2 Tong.1 (B) |
| Manganese (mg/L) | 13.049 | <0.001 | Oub.1 oub.2 (A), Tong.2 (B), Tong.1 (C) |
| Nickel (mg/L) | 43.402 | <0.001 | Oub.2 oub.1 (A), Tong.2 (B), Tong.1 (C) |
| Total Bacteria | 24.432 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| Total Coliforms | 8.231 | <0.001 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| Fecal Coliforms | 3.200 | 0.032 | Tong.1 Tong.2 (A), oub.2 (B), oub.1 (C) |
| Site | Parameter | Measured Value | Standard (mg/L or CFU) | Date |
|---|---|---|---|---|
| Tong.1 | NO3− (mg/L) | 13.75 | 10 | June 2023 |
| Tong.1 | Total Coliforms (1000/mL) | 560 | 100 | August 2023 |
| Tong.2 | NH4+ (mg/L) | 5.75 | 4 | August 2023 |
| Oub.2 | PO43− (mg/L) | 8.66 | 10 | December 2022 |
| Oub.2 | Copper (Cu) (mg/L) | 2.63 | 2.0 | February 2023 |
| Oub.2 | Lead (Pb) (mg/L) | 0.028 | 0.05 | May 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houhamdi, I.; Bouaguel, L.; Bouchaala, L.; Grara, N.; Bara, M.; Szparaga, A.; Houhamdi, M. Distinct Pollution Profiles and Spatio-Temporal Dynamics in Adjacent Ramsar Lakes (Algeria): An Integrated Assessment and High-Resolution Mapping for Targeted Conservation. Processes 2025, 13, 3466. https://doi.org/10.3390/pr13113466
Houhamdi I, Bouaguel L, Bouchaala L, Grara N, Bara M, Szparaga A, Houhamdi M. Distinct Pollution Profiles and Spatio-Temporal Dynamics in Adjacent Ramsar Lakes (Algeria): An Integrated Assessment and High-Resolution Mapping for Targeted Conservation. Processes. 2025; 13(11):3466. https://doi.org/10.3390/pr13113466
Chicago/Turabian StyleHouhamdi, Ines, Leila Bouaguel, Laid Bouchaala, Nedjoud Grara, Mouslim Bara, Agnieszka Szparaga, and Moussa Houhamdi. 2025. "Distinct Pollution Profiles and Spatio-Temporal Dynamics in Adjacent Ramsar Lakes (Algeria): An Integrated Assessment and High-Resolution Mapping for Targeted Conservation" Processes 13, no. 11: 3466. https://doi.org/10.3390/pr13113466
APA StyleHouhamdi, I., Bouaguel, L., Bouchaala, L., Grara, N., Bara, M., Szparaga, A., & Houhamdi, M. (2025). Distinct Pollution Profiles and Spatio-Temporal Dynamics in Adjacent Ramsar Lakes (Algeria): An Integrated Assessment and High-Resolution Mapping for Targeted Conservation. Processes, 13(11), 3466. https://doi.org/10.3390/pr13113466







