Abstract
Growing concern over nanoplastics as emerging pollutants calls for effective treatment methods, with advanced oxidation processes (AOPs) showing strong potential for their degradation. This study examines the interaction between polyethylene terephthalate nanoplastics (PET-NPs) and magnetite nanoparticles (MNPs) in a heterogeneous Fenton-like system, focusing on colloidal behavior, hydroxyl radicals (●OH) generation, and potential degradation pathways. Zeta potential (ZP) and particle diameter measurements were used to characterize nanoparticle dispersion and aggregation mechanisms over a pH range of 3–9.5. The results revealed a pronounced pH-dependent stability, with MNPs exhibiting larger hydrodynamic diameters (283 nm) and lower stability at pH 3 (ZP: −9.8 mV) compared with neutral or alkaline conditions (189 nm; ZP: −44 to −42 mV). PET-NPs exhibited minimal agglomeration at a pH of 9.5 (ZP: −25.6 mV). Unlike conventional Fenton systems, ●OH production peaked at pH 7–9.5 (0.3–0.35 μM), attributed to preserved Fe2+ sites and reduced particle agglomeration. Although PET-NPs resisted oxidative degradation, their aggregation with MNPs enabled magnetic recovery (46% efficiency at pH 3) through charge screening, Fe3+/Fe2+ bridging, and hydrophobic interactions. These findings highlight MNPs’ potential for sustainable nanoplastic separation and emphasize the need for optimized catalysts to enhance ●OH-driven degradation. Overall, this work advances understanding of nanoplastic–magnetite interactions and offers insights into AOP applications.
1. Introduction
Several emerging pollutants that have gained attention in recent years are nanoplastics, which are defined as plastic particles smaller than 1000 nm. Their ubiquity, resistance to natural and conventional degradation methods, and negative health effects owing to their potential to carry other pollutants on their surface and the physical obstruction they cause in tissues highlight the urgent need for effective treatment methods [1,2]. Nanoplastics come from many types of plastics, such as polystyrene, polyethylene, and polyethylene terephthalate, and are prevalent in water bodies, wastewater, and water treatment plants, whose conventional treatments are not capable of degrading them because of their recalcitrance.
Advanced oxidation processes (AOPs) have been identified as promising technologies for treating wastewater-containing nanoplastics. AOPs encompass a wide variety of treatments that generate highly reactive oxygen species, particularly hydroxyl radicals (●OH), which possess a remarkable oxidation potential of 2.8 eV, exceeding that of chlorine and ozone, which allows the degradation of recalcitrant organic substances present in aqueous solutions [3,4].
Among AOPs, the Fenton reaction has emerged as a popular treatment for microplastic-containing wastewater because of its versatility, rapid organic compound degradation, and straightforward operation [5]. In this process, iron salts (Fe2+) act as catalysts for the decomposition of hydrogen peroxide (H2O2) and generate hydroxyl radicals in an aqueous solution, initiating a cascade of reactions that can effectively target various pollutants present in wastewater [6]; Fe2+ oxidizes to form Fe3+, which can participate in reaction in a lesser extent. However, for simplicity, Equation (1) is often used to represent the overall process.
Fe2+ + H2O2 → Fe3+ + •OH + OH−
Further investigations into the efficiency of this process revealed that variations in parameters such as pH, hydrogen peroxide, catalyst concentration, and reaction time significantly influenced the degradation rates of recalcitrant pollutants, thereby underscoring the need for optimized process conditions [6,7].
However, this technology has some disadvantages, such as the need to continuously feed the reaction with iron salts and control the pH owing to the formation of Fe(OH)3 precipitates and the dissociation of H2O2 into molecular oxygen when the pH of the medium increases [8]. To overcome these drawbacks, magnetite has been incorporated in the Fenton reaction, making it a heterogeneous process. The advantage of using magnetite instead of iron salts lies in its large surface area, which contains active sites with both oxidation states (Fe2+ and Fe3+) and its easy recovery because of its magnetic properties [9]. In addition, there are other advantages, such as the reduced generation of ferric sludge (unlike conventional Fenton), the lesser secondary contamination (as iron does not remain in solution at high concentrations) and the relative stability of magnetite, which can be reused in multiple reaction cycles.
The use of magnetite in heterogeneous Fenton and photo-Fenton processes has been reported for the degradation of contaminants and pharmaceuticals dissolved in water [10,11,12,13], which demonstrates the effectiveness of the degradation of organic compounds in aqueous solutions by ●OH. Therefore, it is reasonable to hypothesize that nanoplastics composed of polyethylene terephthalate molecules will exhibit surface changes and particle diameter reduction as the polymer chains are degraded.
At present, studies on plastic particle pollution are mainly focused on characterization and its abundance in the environment, and on the degradation of films, fragments, and microplastics, mainly polystyrene, polypropylene, and polyethylene by photocatalysis [3], neglecting the study of nanoplastics generated [14]. Thus, the treatment of microplastics does not address the environmental and health risks.
However, it is important to highlight that the properties of plastics (like any other solid material) change at the nanometric scale; therefore, it is important to evaluate their behavior during AOP treatment. Several studies have reported the treatment of nanoplastics using photocatalysis [15,16,17,18], indicating the need to study other types of nanoplastics.
In this study, the dispersion and behavior of polyethylene terephthalate nanoplastics (PET-NPs) with magnetite nanoparticles (MNPs) in a Fenton-like reaction were evaluated as a potential advanced oxidation treatment for the degradation of emerging water pollutants. Heterogeneous Fenton-like reaction is presented as a novel strategy to take advantage of magnetite without adding soluble iron salts, preventing the sludge generation. Nanoplastics were obtained from commercial bottles to bring these experiments closer to real waste, and the generation of ●OH at different pH levels was validated and evaluated to improve the degradation performance of PET nanoplastics.
2. Materials and Methods
2.1. Experimental Strategy
The experimental design was divided into three stages. In the first stage, the dispersion of MNPs and PET-NPs in water at different pH values was evaluated. In the second stage, the generation of hydroxyl radicals (●OH) from a Fenton-like reaction using hydrogen peroxide and MNPs dispersed in water was assessed, and PET-NPs were added to the MNP dispersion. In the third stage, the behavior of the PET-NPs in the Fenton-like reaction using MNPs in an aqueous dispersion was evaluated. The analysis of the results was conducted considering particle diameter (hydrodynamic diameter), zeta potential, and the morphological changes in PET-NPs and the way they could agglomerate with MNPs.
2.2. Materials
The MNPs (Iron II, III oxide nanopowder) used were sourced from Aldrich (Mexico City, Mexico), with a particle diameter of 50–100 nm and a purity of 97%. Hydrogen peroxide 35% purity was purchased from Golden Bell (Mexico City, Mexico) and coumarin 97% purity was purchased from Sigma (China). To adjust the pH of the dispersions, H2SO4 ACS Fermont (Zapopan, Mexico), NaOH ACS Fermont (Monterrey, Mexico), and ultrapure water obtained from a Milli-Q purification system (model Reference, Billerica, USA) were employed.
2.3. Nanoparticles Preparation
A stock solution of MNPs was prepared and maintained under constant stirring for 24 h. Subsequently, a 2.5 mM dilution was prepared in distilled water at pH levels of 3, 7, and 9.5, and agitated for 24 h prior to particle diameter measurement.
PET-NPs were obtained from commercial bottled water containers, cut into fragments, and ground for 5 h using a commercial mill. The resulting PET powder was collected, and 18 mg was suspended in 30 mL of water, sonicated for 20 min, and agitated for 24 h at 320 rpm. The suspension was centrifuged for 5 min at 6000 rpm, and subsequent dilutions were made until a consistent particle or hydrodynamic diameter was achieved. This final concentration was consistent with nanoplastics found in the ocean.
2.4. Characterization of Nanoparticles
The particle diameters of MNPs and PET-NPs, as well as their zeta potential (ZP), were determined using a dynamic light scattering (DLS) particle analyzer, Anton Paar (model Litesizer 500, Graz, Austria), a scanning electron microscope (SEM)—JEOL (model JSM6610LV, Akishima, Japan)—and an energy dispersive spectrometer (EDS—Oxford Instruments, model X-MAX 51-XMX1002, Oxfordshire, UK). Before SEM/EDS analysis, the samples were covered with gold in a vacuum machine.
2.5. Hydroxyl Radical Quantification
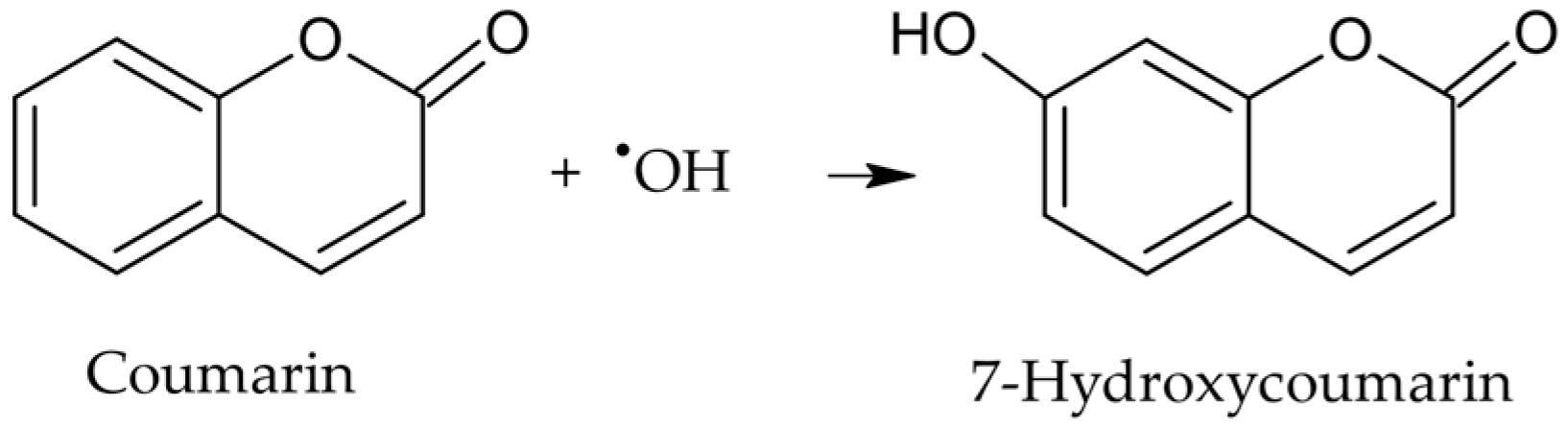
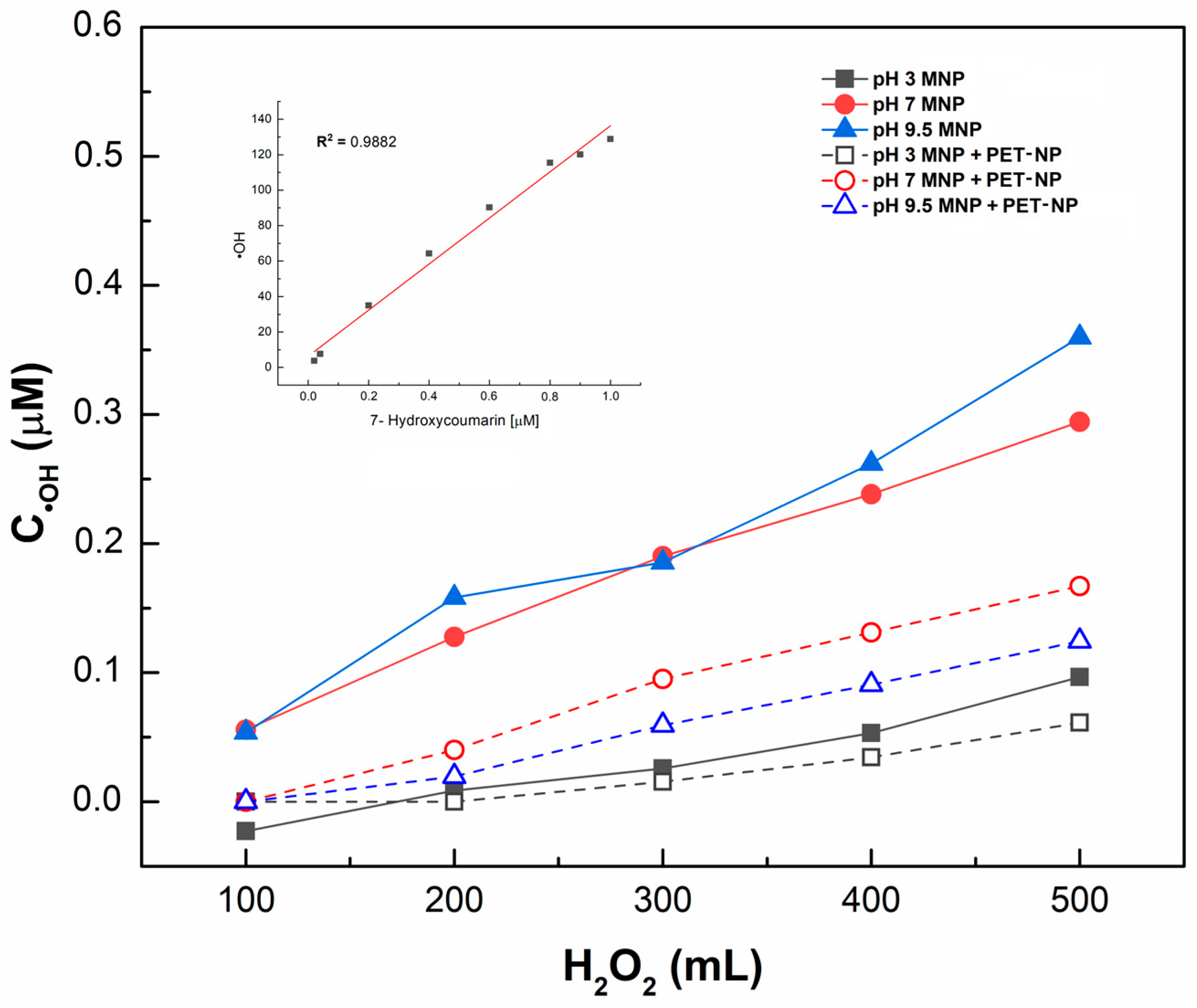
●OH in aqueous solutions was quantified using coumarin, a hydroxyl radical scavenger that oxidizes to form 7-hydroxycoumarin (Figure 1), a highly fluorescent compound [19]. The stoichiometric ratio between the reactants and the reaction product was 1:1:1; therefore, to calculate the concentration of ●OH, a calibration curve of 7-hydroxycoumarin was prepared in the range of 0.021–1.0 µM.
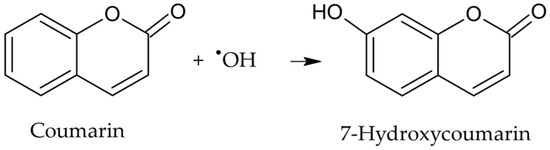
Figure 1.
Oxidation reaction of coumarin by hydroxyl radical to produce 7-hydroxycoumarin.
To determine the concentration of ●OH, solutions with a total volume of 15 mL were prepared, containing 10 mL of distilled water, 5 mL of coumarin solution, and MNPs to reach a concentration of 0.33 mM; three different pH levels were evaluated: 3, 7, and 9.5, and 30% hydrogen peroxide was added incrementally to a total of 500 µL. At the end of the reaction, the samples were centrifuged at 6000 rpm for 5 min, and the fluorescence of the samples was measured as relative fluorescence units (RFUs) at 450 nm, with UV light at 350 nm from a xenon lamp as the excitation source [20,21] using a spectrofluorometer (Jenway, model 6285, USA). A calibration curve within the detection limit of the spectrofluorometer was prepared.
2.6. Evaluation of Nanoplastics Degradation
To figure out whether the nanoplastics underwent degradation, PET-NP samples before and after being subjected to the Fenton-like reaction were analyzed by DLS to measure the hydrodynamic diameter, as well as SEM photographs.
3. Results
3.1. Dispersion and Size of Magnetite Nanoparticles and PET Nanoplastics
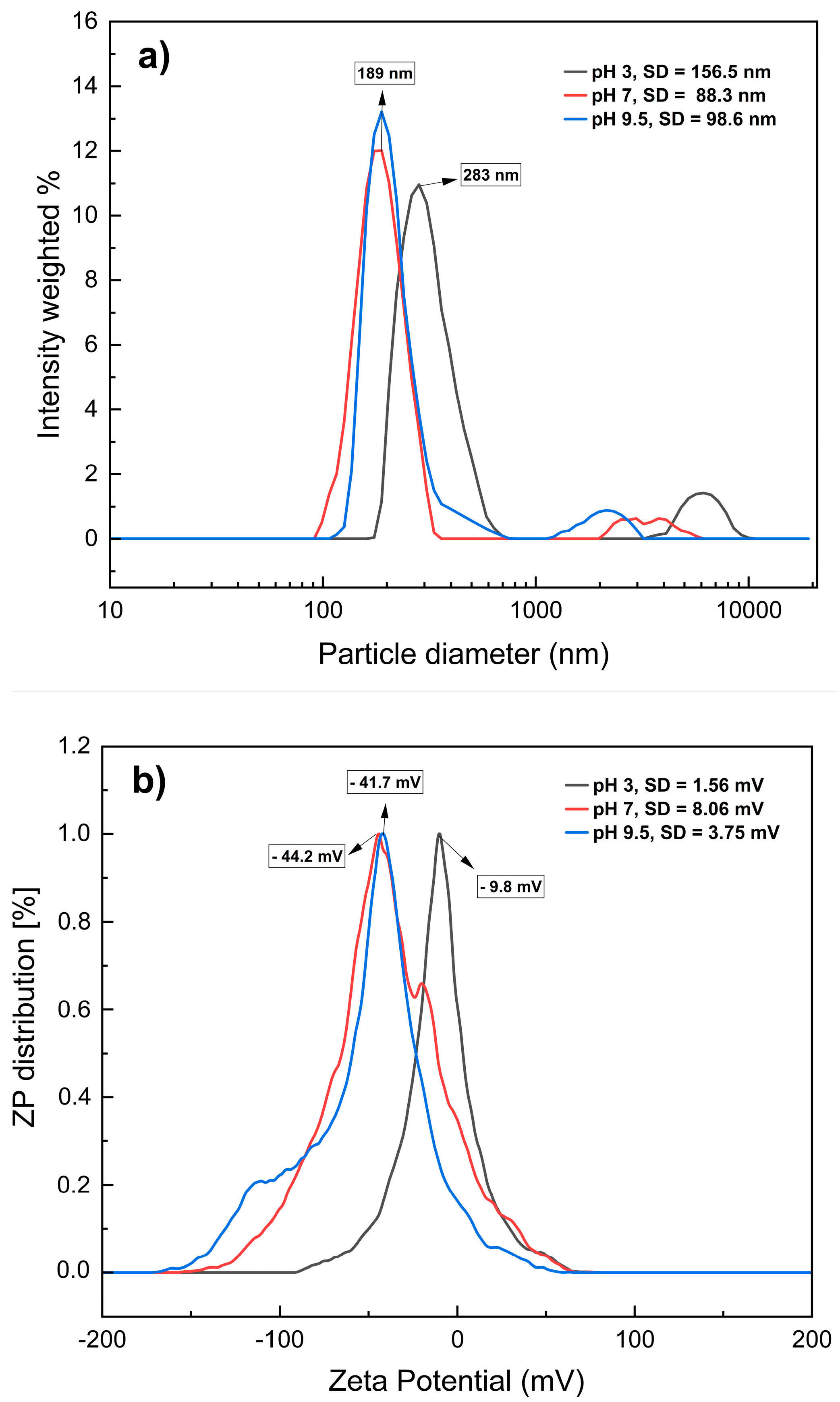
The dispersion and size of the MNPs were vital for validating the nanoparticle diameters within the colloidal systems used in the experiments. Figure 2a shows the hydrodynamic diameters (particle diameters) of the MNPs (x axis) and the number of nanoparticles as light intensity dispersed, measured using the DLS equipment (y axis). At pH 3, the hydrodynamic diameters were 283 nm, whereas for samples dispersed at pH 7 and 9.5, the detected diameters were 189 nm, indicating smaller particle diameters at these two pH values. An ANOVA test (Table 1) was conducted on the hydrodynamic diameters (particle diameters) at each pH and their respective five replicates measured by the DLS instrument, revealing a statistically significant effect of pH (p value (0.0000) < α (0.05)), meaning pH 7 and 9.5 are statistically similar over particle diameter of the magnetite samples. The MNPs used originated from the same stock solution; therefore, their size differences at different pH levels are attributed to heterogeneous dispersion due to agglomeration among the nanoparticles [22]. This can be explained by the electrostatic interactions of MNPs with the medium, which are influenced by the ionic strength of the dispersant, particle diameter, chemical composition, and electrostatic forces [23,24].
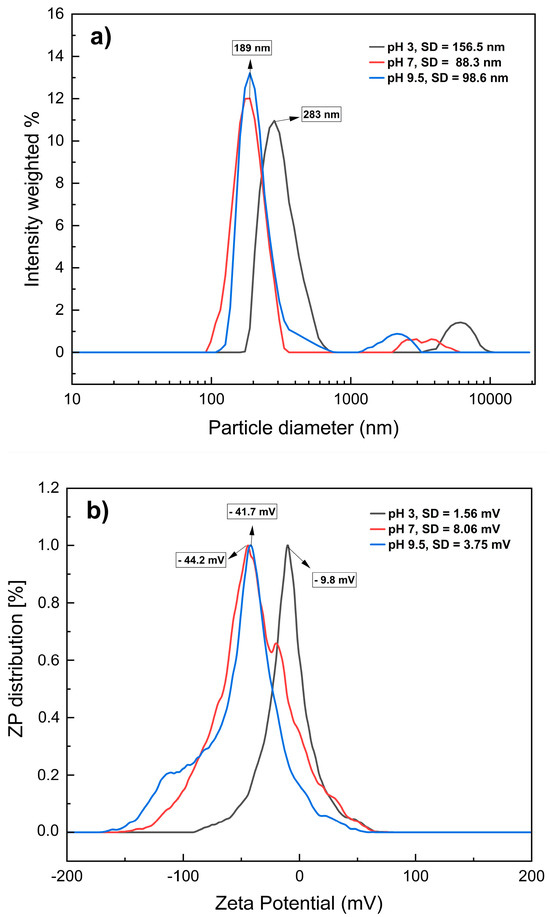
Figure 2.
Hydrodynamic diameters (a) and zeta potential (b) of MNPs in aqueous dispersion at different pH values. SD: Standard deviation.

Table 1.
Analysis of variance for hydrodynamic diameters and ●OH.
To corroborate this hypothesis, the zeta potential (ZP) values were determined for each sample. ZP is a measure of the electrostatic measure of a particle and indicates its stability in a liquid suspension; the ZP results were −9.8, −44.2, and −41.7 mV at pH 3, 7, and 9.5, respectively (Figure 2b). This confirms that at pH 7 and 9.5, there is greater stability in the dispersion of MNPs owing to the magnitude of this parameter [25] and that MNP are strongly anionic under these conditions [26]. These results are consistent with the findings of Yang et al. [27], who observed that MNPs exhibit different ZP values at different pH levels.
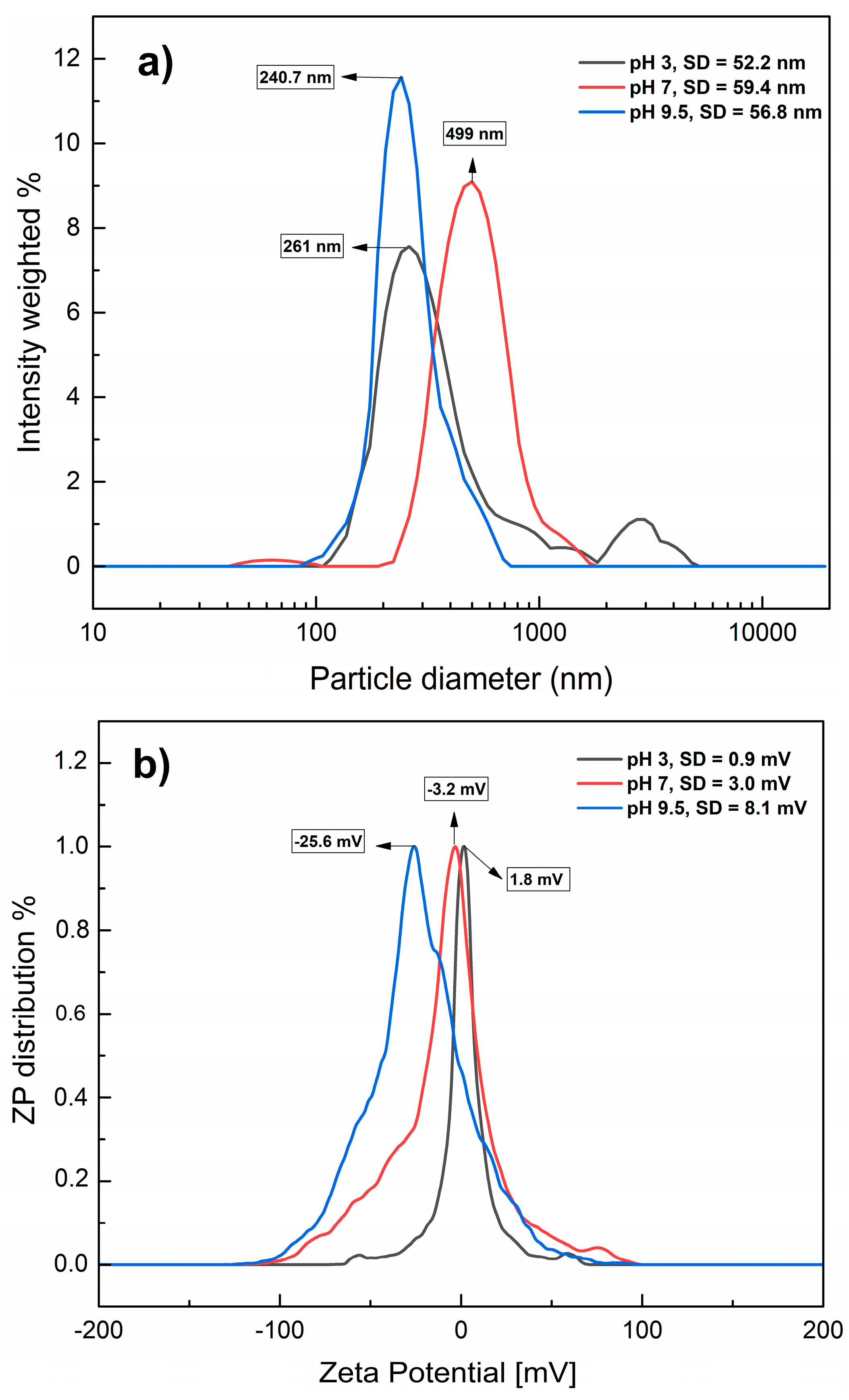
Regarding PET-NPs, Figure 3a shows the hydrodynamic diameters (particle diameters) of the nanoplastic samples at different pH levels. The detected diameters were 261, 499, and 240.7 nm, corresponding to the pH values of 3, 7, and 9.5, respectively. An ANOVA test was also conducted on the hydrodynamic diameters (particle diameters) of PET-NPs at each pH, showing a statistically significant effect of pH (p value (0.0000) < α (0.05)). These results demonstrate the nature of nanoplastics, which agglomerate to a lesser extent under both alkaline and acidic conditions. As with the MNPs, ZP values were determined (Figure 3b), with averages of 1.8, −3.2, and −25.6 mV at pH 3, 7, and 9.5, respectively, suggesting that PET-NPs in water dispersion are more stable at alkaline pH due to the change in charges on their surface, as confirmed by differences in the magnitude of the ZP, in agreement with Patel et al. [28], These values are relatively close to those reported by other authors for PET-NPs; Djapovic et al. [29] reported a ZP of −34.93 mV at a pH close to 6, and Ducoli et al. [30] reported −5.4 mV at pH 7. ZP values have also been reported for other types of nanoplastics, such as polystyrene, ranging from −7 to −35 mV at different pH values [31,32]. These differences in magnitude can be explained by the properties exhibited by nanoplastics with different particle diameters and dispersants; in this study, they ranged from 50 nm to 100 µm and water was the dispersant, while in other studies, the particle diameters were larger and nanoplastics were previously dispersed in different solvents. These results confirmed the presence of PET-NPs and MNPs, and their particle diameters were consistent and homogeneous at an alkaline pH.
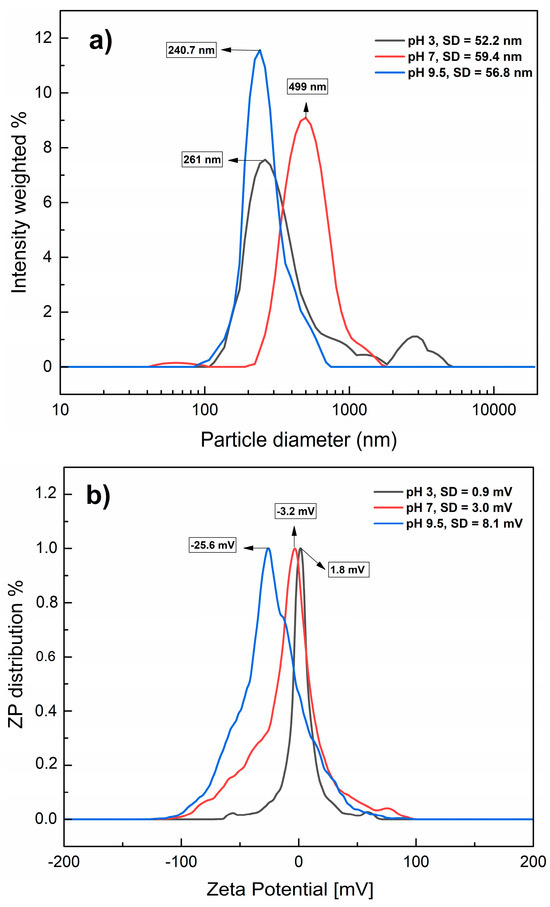
Figure 3.
Hydrodynamic diameters (a) and zeta potential (b) of PET-NPs in aqueous dispersion at different pH values. SD: Standard deviation.
3.2. Hydroxyl Radical Generation During Fenton-like Reaction
●OH generation was determined in the MNP samples dispersed in water at different pH levels using coumarin. A calibration curve of 7-hydroxycoumarin versus RFU, with a determination coefficient (R2) of 0.9892, was used to detect and measure ●OH concentration. Figure 4 illustrates the kinetics of ●OH generation in function of pH and hydrogen peroxide added. As expected, higher concentrations of 7-hydroxycoumarin, and thus, ●OH, were generated with increasing hydrogen peroxide addition. Fenton-like reaction performance at different pH levels was also observed; the lowest ●OH generation occurred at pH 3, whereas higher concentrations were obtained at pH 7 and 9.5. After adding 500 µL of H2O2 and allowing 150 min for reaction, ●OH was generated at a concentration of 0.1 µM at pH 3, while at pH 7 and 9.5, concentrations reached 0.3 and 0.35 µM, respectively. ●OH generation follows first-order kinetics, suggesting a process controlled by a single species (H2O2).
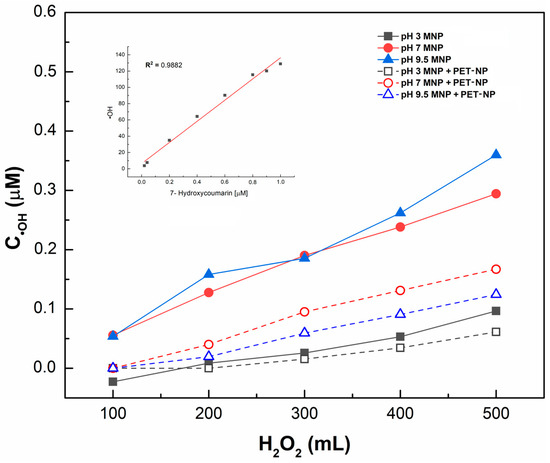
Figure 4.
Hydroxyl radical generation in function of hydrogen peroxide and pH values of medium.
A two-way ANOVA test (Table 1) was conducted considering the doses of H2O2 and pH over the ●OH concentration range. The variance analysis indicated that both the factors and their interactions had a statistically significant effect on ●OH generation. Therefore, to increase ●OH, a pH of 7 or 9.5 is recommended.
The effect of pH on ●OH generation with magnetite and coumarin has not been previously reported, only on the degradation of organic contaminants. However, a similar pH-related result found in this study was reported by Zhou et al. [33] in a heterogeneous Fenton process, in which Fe2+ ions were loaded onto porous magnetic microspheres to degrade methylene blue in wastewater, achieving 90% degradation within 40 min, with the best results at pH 6.18. It can be concluded that the highest concentrations of ●OH were obtained at pH 7 and 9.5, with similar outcomes at both pH levels.
3.3. Interaction Between PET-NPs and MNPs During Fenton-like Reaction
After the hydrodynamic diameters and ZP of MNPs and PET-NPs were measured separately, they were measured in the same suspension at different pH values and then with H2O2. This stage was useful for confirming the conditions under which both nanoparticles disperse before the Fenton-like reaction, and once the reaction begins, the distribution of the hydrodynamic diameters of the nanoparticles could suggest whether degradation or agglomeration of PET-NPs occurs; smaller sizes could indicate degradation of PET-NPs, whereas larger hydrodynamic diameters indicate agglomeration between these two particles. The agglomeration or aggregation of magnetite (or derivatives) with microplastics has been reported previously [34,35].
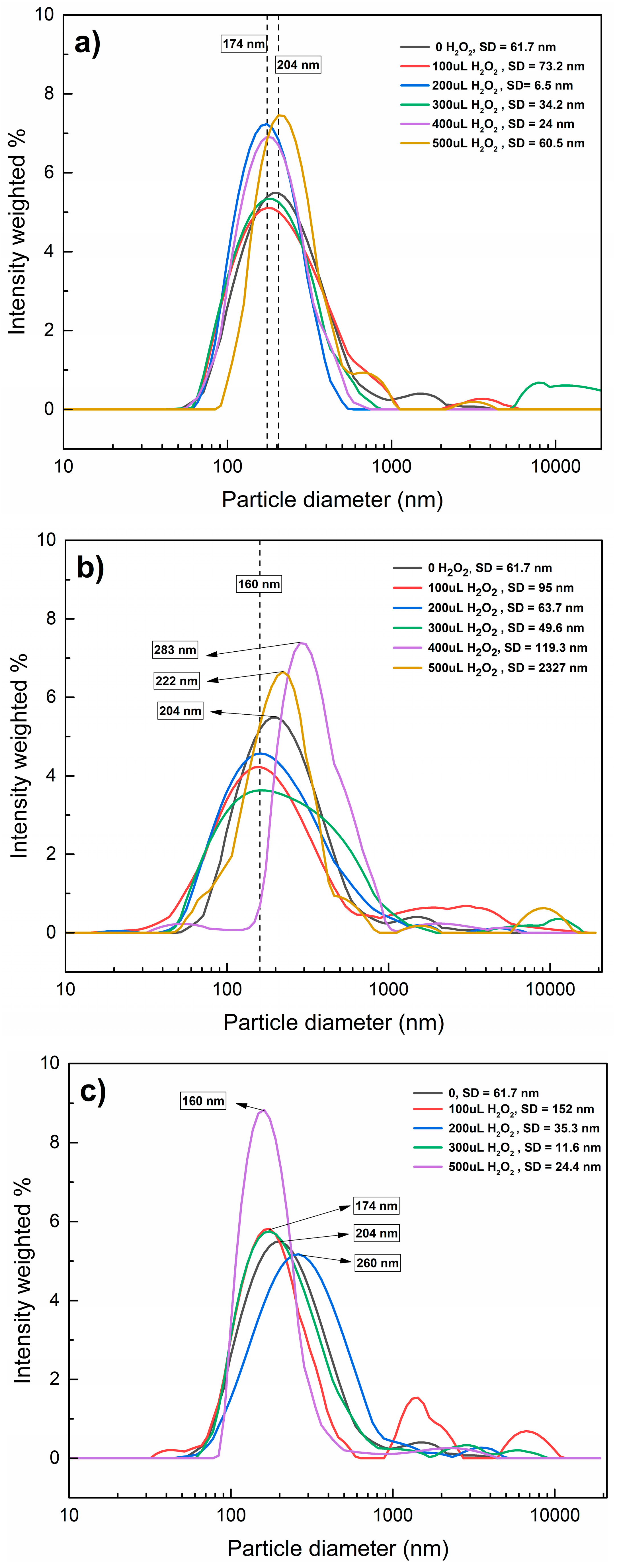
Figure 5 shows the distribution of hydrodynamic diameters through a Fenton-like reaction at different pH values. Under acidic conditions, the particle diameters were similar regardless of the added volume of H2O2, suggesting that the agglomerates persisted. At pH 7 (Figure 5b), the sizes of the agglomerates changed inconsistently; the hydrodynamic diameters decreased with the addition of 100–300 µL of H2O2 but increased with 400 µL of H2O2; at pH 9.5 (Figure 5c), the changes also seem to be inconsistent, decreasing the particle diameter with 100–200 µL of H2O2, which can be explained by the effect of ●OH: At low H2O2 doses, ●OH on the surface of nanoplastics oxides functional groups and changes their charges, enhancing electrostatic repulsion between particles and reducing agglomeration, on the other hand, at high H2O2 doses, Fe3+ ions could promote charge neutralization on the PET-NPs’ surface, reducing electrostatic repulsion and enabling aggregation. These results indicate that the only changes PET-NPs suffer are in their surface charges, possible surface oxidizing, and promoting agglomeration or dispersion, but no significant degradation.
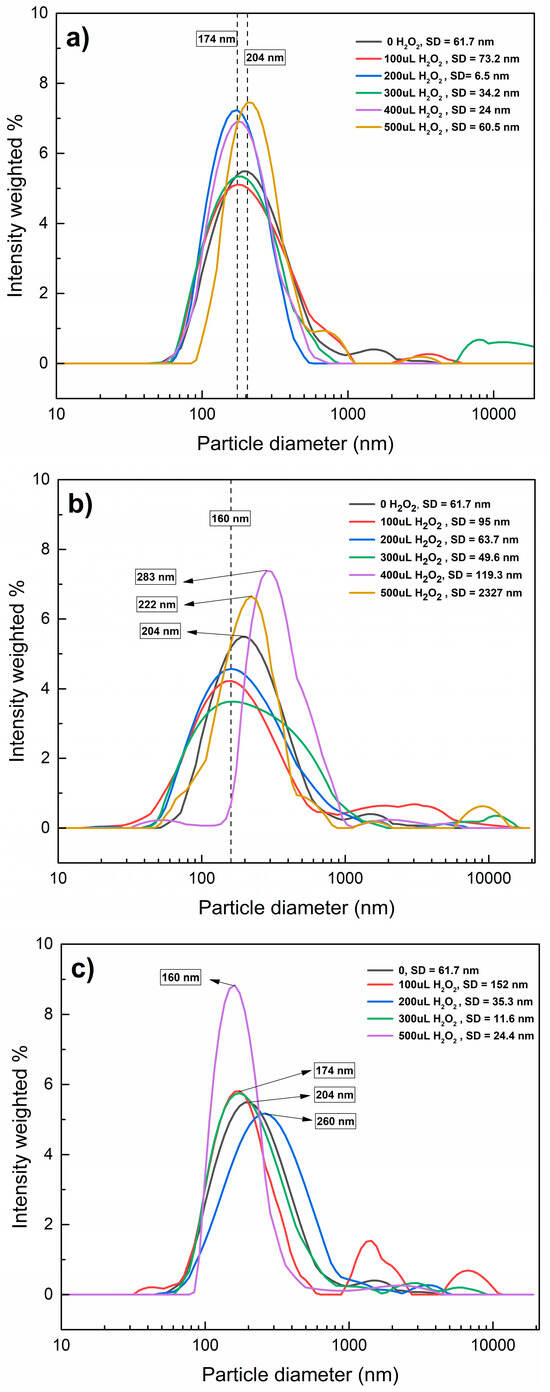
Figure 5.
Distribution of hydrodynamic diameters of MNPs and PET-NPs through Fenton-like reaction at pH 3 (a), 7 (b), and 9.5 (c).
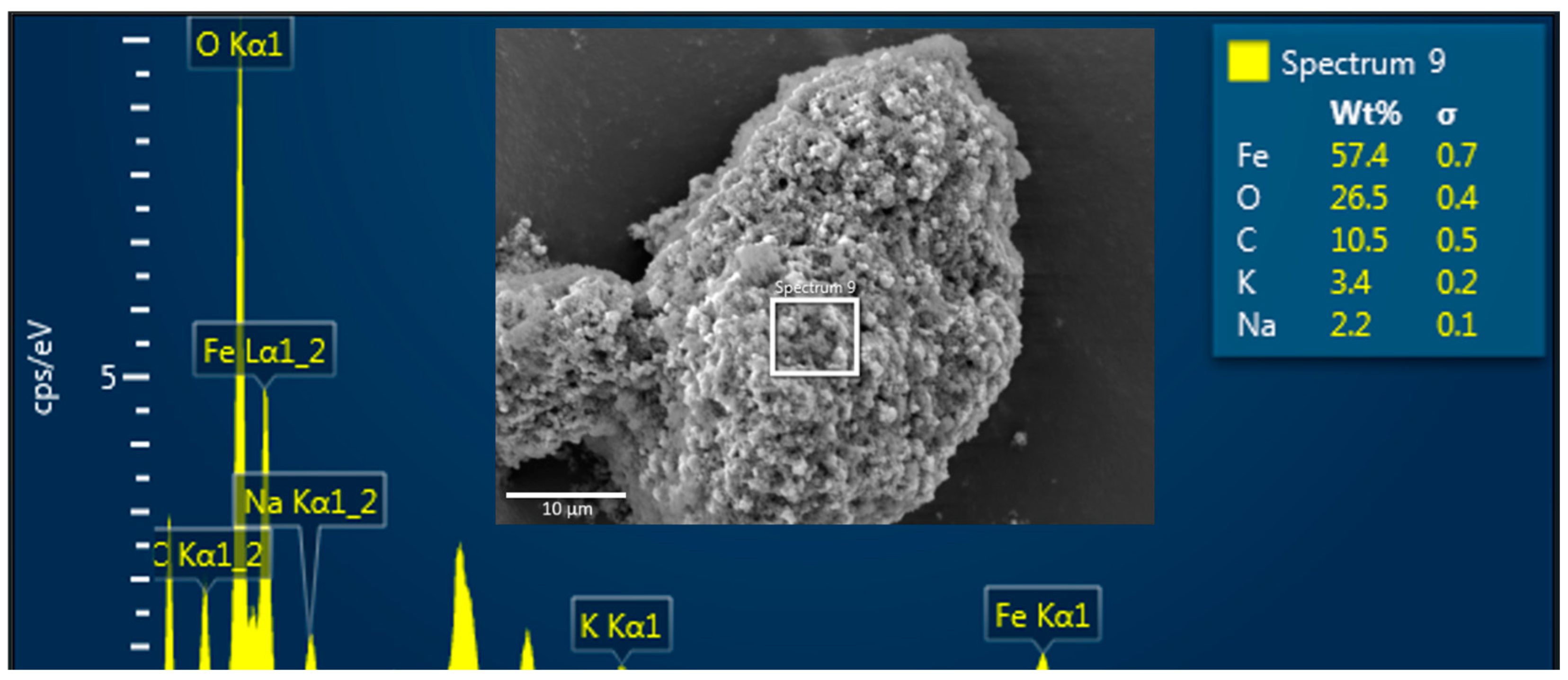
To corroborate the agglomeration between MNPs and PET-NPs, SEM/EDS analysis and ●OH generation were evaluated on the samples during the Fenton-like reaction. Figure 6 shows an SEM image and an EDS spectrum of an agglomerate between both nanoparticles, where the EDS spectrum depicts MNPs as a function of iron (Fe) and oxygen (O) species, while PET-NPs are represented by carbon (C) and oxygen. Potassium (K) and sodium (Na) were detected in the samples because alkaline solutions were used to adjust the pH.
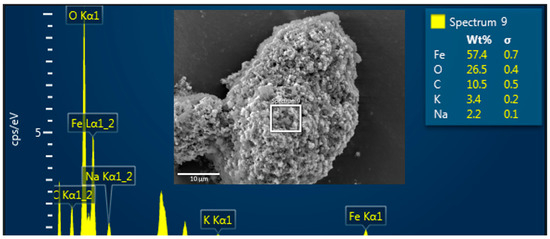
Figure 6.
SEM image and EDS spectrum of MNPs and PET-NPs dispersed in pH 9.5 during Fenton-like reaction.
Concerning ●OH generation in the presence of PET-NPs during the Fenton reaction, a hypothesis was proposed: because of the physical bond between both nanoparticles (agglomeration), the MNPs would have fewer active sites to catalyze the decomposition of H2O2. As shown in Figure 4, in the Fenton-like reaction, 7-hydroxycoumarin continues to be produced because of the generation of ●OH, indicating that the Fenton-like reaction continued to occur, but to a lesser extent, corroborating the proposed hypothesis. Despite the validation of ●OH generation, PET-NPs are resistant to this type of oxidant.
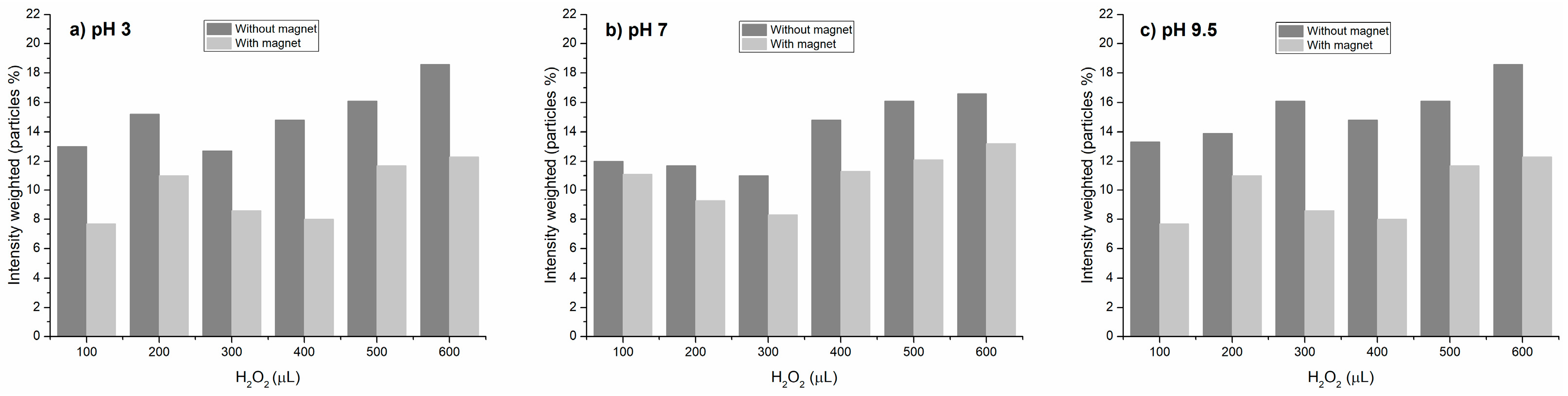
Owing to the agglomeration of both NPs, the resultant advantage of this colloidal system was their recovery by magnetism. Then, the Fenton-like reaction was repeated in the presence of PET-NPs, ultrasonicated, hydrogen peroxide was added to promote the reaction, and a magnet was deployed to separate the MNPs and secondarily drag the PET-NPs. To corroborate the separation of the nanoplastics, the quantity of nanoparticles in the dispersion was determined by DLS before and after using the magnet. Figure 7 shows the quantity of nanoparticles (%) separated from the dispersion by the magnet at different pH values after hydrogen peroxide was added, and each sample was ultrasonicated.
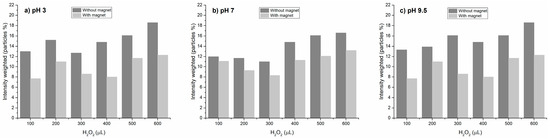
Figure 7.
Effect of magnet on the dragging of PET-NP/MNP agglomerates.
As can be observed, under acidic conditions, NP separation was higher than under neutral and alkaline conditions; at pH 3, the NP dragging ranged from 27 to 46%, while at 7 and 9.5 pH values the dragging was 7.5–25 and 18–38%, respectively. NP separation was statistically significative in function of both H2O2 and pH factors (p value < α). These results confirm that agglomerates formed under acidic conditions facilitate the separation of nanoplastics by the effect of the magnet on MNPs bonded to PET-NPs, which is reported to be caused by van der Waals interactions [25].
Several authors have reported microplastic separation using magnetite-based functionalized nanoparticles. Martin et al. [36] achieved a separation of 90% of polystyrene nanoplastics by employing iron oxide nanoparticles with hydrophobic coating. Sarcletti et al. [37] developed core–shell superparamagnetic iron oxide nanoparticles functionalized with phosphonic acid, achieving up to 90% separation on melamine resin nanoplastics. Bakhteeva et al. [35] synthesized magnetite nanoparticles covered with silica, gelatin, and chitosan, achieving up to 98% PET microplastics. Unfortunately, the preparation of these magnetic nanoparticles requires specific experimental conditions and materials/solvents that must be simplified and economized to make the process sustainable.
4. Discussion
It has been reported that the Fenton reaction should occur under acidic conditions (pH 2.5–3) for the degradation of pollutants and dyes [38,39]; however, interestingly, this study found that neutral and alkaline pH conditions are more favorable for the generation of these radicals. Although these results may seem contradictory, they can be explained by the different reaction conditions of the dissolved iron salts or magnetite (MNPs). In the Fenton reaction with iron salts, acidic pH levels are suggested because Fe3+ precipitates as ferric oxyhydroxides at pH 3, forming sludge that poses a disposal challenge [40]. In the heterogeneous Fenton-like reaction with MNPs at alkaline or neutral pH, the dispersion was stable, minimizing agglomeration and promoting the exposure of more surface-active Fe2+ sites available for catalyzing H2O2 decomposition, thus avoiding the limitation of iron precipitate formation. This was evidenced by the ZP results for MNPs at neutral and alkaline pH levels and their smaller hydrodynamic diameters (Figure 2). The use of a solid catalyst with surface-active Fe2+ sites allows an extended pH range for the reaction [41,42].
The observed aggregation between MNPs and PET-NPs indicated attractive interparticle forces. Potential mechanisms include (1) electrostatic interactions (demonstrated by ZP measurements), which permit the interpretation of colloidal stability, where at acidic pH (near the isoelectric point of magnetite, ~pH 6–7), surface charge neutralization promotes aggregation through van der Waals forces or hydrogen bonding [25]; (2) at alkaline pH (9.5), while the typically negative surfaces of both PET-NPs and MNPs would normally repel each other, the presence of Na+ ions (from NaOH pH adjustment) enables charge screening, facilitating aggregation [43]; and (3) metal ion bridging, where Fe3+/Fe2+ from magnetite forms complexes with surface functional groups (e.g., carboxyl) on nanoplastics, acting as molecular “glue” [44]. Additionally, hydrophobic effects may contribute to aggregation, as hydrophobic PET-NPs and hydrophilic MNPs interact in aqueous media to minimize hydrophobic interfaces [45]. Under alkaline conditions, the size variations suggest pH-dependent surface charge modifications and ●OH effects; however, no significant degradation occurred. This aligns with studies showing the resistance of PET-NPs to oxidative degradation [3,46], as Fenton-generated ●OH radicals fail to break down PET-NPs, as evidenced by aggregate-induced blocking of MNP active sites, reducing ●OH production. This has been reported in several works related to microplastics and AOP, such as the work of Singh et al. [47], who treated microplastics using a combined thermal Fenton process, achieving up to 98% mass loss of these particles within 24 h; Xue et al. [48] demonstrated nearly 100% degradation of polystyrene NP through photocatalytic and photothermal Fenton-like processes in 4 h at 75 °C; Gao et al. [49] applied the electro-Fenton process using magnetite and persulfate, achieving up to 90% degradation of polyethylene microplastics after 20 h of treatment; and Thirunavukkarasu et al. [50] employed a photocatalytic and photo-Fenton process with Fe-modified TiO2 aerogels to degrade polystyrene microplastics, achieving surface-level degradation of the particles. These works report advances in the degradation of micro- and nanoplastics, from surface oxidation to changes in mass loss. Among the aspects they share are the combination of two or more AOPs, which limits their practical application in real-life conditions, in addition to high temperatures and the lack of evaluation of different types of plastic polymers and nanoplastics.
The findings of this work highlight the potential application of magnetite as a sustainable, cost-effective nanoplastic separation process that avoids expensive solvents or complex conditions, while suggesting opportunities for developing enhanced MNP-based catalysts to increase ●OH generation for effective nanoplastic degradation. Finally, this work focuses on simpler, cheaper, and more environmentally relevant systems (neutral pH, mild aqueous conditions), which is a complementary contribution to studies with high degradation but low practical applicability, and it studies specific interaction between magnetite and nanoplastics under heterogeneous Fenton conditions, something little-explored compared to the degradation of polystyrene MPs through more aggressive hybrid processes.
5. Future Perspectives
To enhance the degradation efficiency of nanoplastics in heterogeneous Fenton-like systems, future strategies should focus on optimizing both the catalyst and the reaction environment. Surface modification of magnetite nanoparticles, such as doping with transition metals or applying thin stabilizing coatings, could increase hydroxyl radical yield while minimizing aggregation with nanoplastics. In addition, coupling the system with another AOP, including photo-Fenton under visible/UV-A irradiation or persulfate activation, may provide more reactive species capable of attacking the PET structure. Process intensification approaches, such as controlled H2O2 dosing or the use of mild sonication, could further improve radical availability at the catalyst–nanoplastic interface, where oxidative reactions are most effective. The present findings highlight promising research avenues beyond the direct degradation of PET nanoplastics. The observed pH-dependent aggregation between magnetite and nanoplastics suggests opportunities for magnetic recovery technologies as a complementary remediation strategy. At the same time, the unexpected generation of ●OH at neutral and alkaline conditions opens new pathways for developing heterogeneous Fenton systems with broader environmental applicability. Future work should investigate the stability and recyclability of magnetite under operational conditions, extend the approach to different types of nanoplastics and real water matrices, and explore hybrid processes that combine catalytic oxidation with biological or enzymatic treatments. Such integrative strategies could provide more efficient and sustainable solutions for mitigating nanoplastic pollution.
6. Conclusions
The dispersion of MNPs and PET-NPs as a function of the pH of the medium was evaluated, as well as hydroxyl radical production, to assess the performance of both types of nanoparticles in a heterogeneous Fenton-like reaction. This study revealed that the dispersion of MNPs and PET-NPs varies separately, exhibiting pH-dependent colloidal stability due to intermolecular interaction forces on the nanoparticle surface, with MNPs showing significantly larger hydrodynamic diameters (283 nm) and higher ZP (−9.8 mV) at pH 3 compared to neutral or alkaline conditions (189 nm; −44 to −42 mV), indicating pH-driven agglomeration. PET-NPs similarly demonstrated enhanced stability at alkaline pH (ZP: −25.6 mV), with minimal agglomeration (240.7 nm). The Fenton-like reaction achieves optimal ●OH generation at neutral/alkaline pH (0.3–0.35 µM), challenging conventional acidic pH requirements, due to preserved Fe2+ active sites and reduced MNP agglomeration.
During the Fenton-like reaction, the aggregation between MNPs and PET-NPs is governed by multiple interparticle forces, including electrostatic interactions (charge screening by Na+ at an alkaline pH), Fe3+/Fe2+ bridging, and hydrophobic effects, which collectively promote agglomeration without inducing PET-NP degradation. This was confirmed by the persistent aggregate sizes and SEM/EDS evidence of Fe-O-C interfacial bonds. These findings reveal two key technological pathways. First, pH-tunable aggregation enables the magnetic recovery of PET-NPs (46% efficiency at pH 3) without functionalized nanoparticles, offering a cost-effective alternative to complex nanocomposite systems. Second, the unexpected ●OH generation at neutral or alkaline pH opens new avenues for heterogeneous Fenton catalysis in environmental remediation, although the oxidative stability of PET-NPs necessitates alternative degradation strategies. Future research should explore surface-modified MNPs to simultaneously enhance ●OH yield and nanoplastic degradation while maintaining magnetic separability.
Author Contributions
Conceptualization and methodology, E.D.M.-M. and J.d.R.-O.; Methodology and Formal Analysis, A.M.S.-T. and F.E.E.-P.; investigation and writing—original draft preparation, D.R.O.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study and due to technical limitations. Requests to access the datasets should be directed to daryl.osuna@academicos.udg.mx.
Acknowledgments
The corresponding author thanks SECIHTI for providing a postdoctoral fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MNP | Magnetite Nanoparticles |
| PET-NP | Polyethilene Terephtalate Nanoplastics |
| NP | Nanoparticles |
| SEM | Scanning electron microscope |
| EDS | Energy Dispersive Spectroscopy |
| ZP | Zeta Potential |
References
- WHO Dietary and Inhalation Exposure to Nano- and Microplastic Particles and Potential Implications for Human Health. Available online: https://www.who.int/publications/i/item/9789240054608 (accessed on 23 June 2025).
- Osuna-Laveaga, D.R.; Ojeda-Castillo, V.; Flores-Payán, V.; Gutiérrez-Becerra, A.; Moreno-Medrano, E.D. Micro- and Nanoplastics Current Status: Legislation, Gaps, Limitations and Socio-Economic Prospects for Future. Front. Environ. Sci. 2023, 11, 1241939. [Google Scholar] [CrossRef]
- Kim, S.; Sin, A.; Nam, H.; Park, Y.; Lee, H.; Han, C. Advanced Oxidation Processes for Microplastics Degradation: A Recent Trend. Chem. Eng. J. Adv. 2022, 9, 100213. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Chen, L.; Ma, J.; Li, X.; Zhang, J.; Fang, J.; Guan, Y.; Xie, P. Strong Enhancement on Fenton Oxidation by Addition of Hydroxylamine to Accelerate the Ferric and Ferrous Iron Cycles. Environ. Sci. Technol. 2011, 45, 3925–3930. [Google Scholar] [CrossRef]
- Ghime, D.; Ghosh, P.; Ghime, D.; Ghosh, P. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. In Advanced Oxidation Processes-Applications, Trends, and Prospects; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Pereira, N.; Lima, R.; Faria, J.L.; Gomes, H.T.; Silva, A.M.T. Degradation of Diphenhydramine by Photo-Fenton Using Magnetically Recoverable Iron Oxide Nanoparticles as Catalyst. Chem. Eng. J. 2015, 261, 45–52. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of Magnetite-Based Catalysts and Their Application in Heterogeneous Fenton Oxidation A Review. Appl. Catal. B 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Matta, R.; Hanna, K.; Chiron, S. Fenton-like Oxidation of 2,4,6-Trinitrotoluene Using Different Iron Minerals. Sci. Total Environ. 2007, 385, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hanna, K.; Kone, T.; Medjahdi, G. Synthesis of the Mixed Oxides of Iron and Quartz and Their Catalytic Activities for the Fenton-like Oxidation. Catal. Commun. 2008, 9, 955–959. [Google Scholar] [CrossRef]
- Pérez-Poyatos, L.T.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Magnetite Nanoparticles as Solar Photo-Fenton Catalysts for the Degradation of the 5-Fluorouracil Cytostatic Drug. Nanomaterials 2022, 12, 4438. [Google Scholar] [CrossRef]
- Moreno-Medrano, E.D. Emerging Contaminants Removed by Electro-Fenton Heterogeneous Using Porous Material of Chitosan and Magnetite Nanoparticles. ECS Trans. 2021, 101, 101. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of Nanoplastics during the Degradation of Polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Allé, P.H.; Garcia-Muñoz, P.; Adouby, K.; Keller, N.; Robert, D. Efficient Photocatalytic Mineralization of Polymethylmethacrylate and Polystyrene Nanoplastics by TiO2/β-SiC Alveolar Foams. Environ. Chem. Lett. 2021, 19, 1803–1808. [Google Scholar] [CrossRef]
- Domínguez-Jaimes, L.P.; Cedillo-González, E.I.; Luévano-Hipólito, E.; Acuña-Bedoya, J.D.; Hernández-López, J.M. Degradation of Primary Nanoplastics by Photocatalysis Using Different Anodized TiO2 Structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef] [PubMed]
- Kiendrebeogo, M.; Karimi Estahbanati, M.R.; Ouarda, Y.; Drogui, P.; Tyagi, R.D. Electrochemical Degradation of Nanoplastics in Water: Analysis of the Role of Reactive Oxygen Species. Sci. Total Environ. 2022, 808, 151897. [Google Scholar] [CrossRef]
- Wang, S.; Tan, X.; Wu, Y.; Zhang, J.; Tian, Z.; Ma, J. Isolating Micro/Nanoplastics from Organic-Rich Wastewater: Co/PMS Outweighs Fenton System. J. Hazard. Mater. 2024, 463, 132840. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of Active Oxidative Species in TiO2 Photocatalysis Using the Fluorescence Technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Li, Y.; Zhao, Y.; Pan, X. Photocatalytic Aging Process of Nano-TiO2 Coated Polypropylene Microplastics: Combining Atomic Force Microscopy and Infrared Spectroscopy (AFM-IR) for Nanoscale Chemical Characterization. J. Hazard. Mater. 2021, 404, 124159. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-Based Quantification of Hydroxyl Radicals and Other Reactive Species Generated on Excited Nitrogen-Doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404, 112913. [Google Scholar] [CrossRef]
- Fang, Z.; Sallach, J.B.; Hodson, M.E. Ethanol, Not Water, Should Be Used as the Dispersant When Measuring Microplastic Particle diameter Distribution by Laser Diffraction. Sci. Total Environ. 2023, 902, 166129. [Google Scholar] [CrossRef]
- Reynaud, S.; Aynard, A.; Grassl, B.; Gigault, J. Nanoplastics: From Model Materials to Colloidal Fate. Curr. Opin. Colloid Interface Sci. 2022, 57, 101528. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Yang, S.C.; Paik, S.Y.R.; Ryu, J.; Choi, K.O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic Light Scattering-Based Method to Determine Primary Particle diameter of Iron Oxide Nanoparticles in Simulated Gastrointestinal Fluid. Food Chem. 2014, 161, 185–191. [Google Scholar] [CrossRef]
- Patel, V.; Agrawal, Y. Nanosuspension: An Approach to Enhance Solubility of Drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar] [CrossRef]
- Djapovic, M.; Apostolovic, D.; Postic, V.; Lujic, T.; Jovanovic, V.; Stanic-Vucinic, D.; van Hage, M.; Maslak, V.; Cirkovic Velickovic, T. Characterization of Nanoprecipitated PET Nanoplastics by 1H NMR and Impact of Residual Ionic Surfactant on Viability of Human Primary Mononuclear Cells and Hemolysis of Erythrocytes. Polymers 2023, 15, 4703. [Google Scholar] [CrossRef]
- Ducoli, S.; Federici, S.; Cocca, M.; Gentile, G.; Zendrini, A.; Bergese, P.; Depero, L.E. Characterization of Polyethylene Terephthalate (PET) and Polyamide (PA) True-to-Life Nanoplastics and Their Biological Interactions. Environ. Pollut. 2024, 343, 123150. [Google Scholar] [CrossRef]
- Gaß, H.; Kloos, T.M.; Höfling, A.; Müller, L.; Rockmann, L.; Schubert, D.W.; Halik, M. Magnetic Removal of Micro- and Nanoplastics from Water—From 100 Nm to 100 Μm Debris Size. Small 2024, 20, 2305467. [Google Scholar] [CrossRef] [PubMed]
- Oriekhova, O.; Stoll, S. Heteroaggregation of Nanoplastic Particles in the Presence of Inorganic Colloids and Natural Organic Matter. Environ. Sci Nano 2018, 5, 792–799. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, Y.; Liu, J.; Ye, Z.; Zhang, H.; Ma, J.; Jia, Y.; Gao, W.; Li, Y. Preparation and Characterization of Magnetic Porous Carbon Microspheres for Removal of Methylene Blue by a Heterogeneous Fenton Reaction. ACS Appl. Mater. Interfaces 2014, 6, 7275–7285. [Google Scholar] [CrossRef] [PubMed]
- Filinkova, M.S.; Bakhteeva, Y.A.; Medvedeva, I.V.; Byzov, I.V.; Minin, A.S.; Kurmachev, I.A. Aggregation and Magnetic Separation of Polyethylene Microparticles from Aqueous Solutions. Colloid J. 2024, 86, 967–979. [Google Scholar] [CrossRef]
- Bakhteeva, I.A.; Filinkova, M.S.; Medvedeva, I.V.; Podvalnaya, N.V.; Byzov, I.V.; Zhakov, S.V.; Uimin, M.A.; Kurmachev, I.A. Design and Application of Environmentally Friendly Composite Magnetic Particles for Microplastic Extraction from Water Media. J. Environ. Chem. Eng. 2024, 12, 113287. [Google Scholar] [CrossRef]
- Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef]
- Sarcletti, M.; Park, H.; Wirth, J.; Englisch, S.; Eigen, A.; Drobek, D.; Vivod, D.; Friedrich, B.; Tietze, R.; Alexiou, C.; et al. The Remediation of Nano-/Microplastics from Water. Mater. Today 2021, 48, 38–46. [Google Scholar] [CrossRef]
- Sun, S.P.; Li, C.J.; Sun, J.H.; Shi, S.H.; Fan, M.H.; Zhou, Q. Decolorization of an Azo Dye Orange G in Aqueous Solution by Fenton Oxidation Process: Effect of System Parameters and Kinetic Study. J. Hazard. Mater. 2009, 161, 1052–1057. [Google Scholar] [CrossRef]
- Utset, B.; Garcia, J.; Casado, J.; Domènech, X.; Peral, J. Replacement of H2O2 by O2 in Fenton and Photo-Fenton Reactions. Chemosphere 2000, 41, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit Rev Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A Highly Active Bimetallic Oxides Catalyst Supported on Al-Containing MCM-41 for Fenton Oxidation of Phenol Solution. Appl. Catal. B 2011, 110, 118–125. [Google Scholar] [CrossRef]
- Qian, X.; Fuku, K.; Kuwahara, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Design and Functionalization of Photocatalytic Systems within Mesoporous Silica. ChemSusChem 2014, 7, 1528–1536. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of Dissolved Organic Matter with Natural and Engineered Inorganic Colloids: A Review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Gao, R.; Abdurahman, A.; Dai, J.; Zeng, F. Aggregation Kinetics of Microplastics in Aquatic Environment: Complex Roles of Electrolytes, PH, and Natural Organic Matter. Environ. Pollut. 2018, 237, 126–132. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Zdorovets, M.V.; Taltenov, A.A. Enhancing Hydrophilicity and Water Permeability of PET Track-Etched Membranes by Advanced Oxidation Process. Nucl. Instrum. Methods Phys. Res. B 2015, 365, 651–655. [Google Scholar] [CrossRef]
- Singh, V.; Park, S.Y.; Kim, C.G. Hybrid Oxidation of Microplastics with Fenton and Hydrothermal Reactions. ACS EST Water 2024, 4, 1688–1700. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, X.; Ke, X.; Zhao, J. A novel route for microplastic mineralization: Visible-light-driven heterogeneous photocatalysis and photothermal Fenton-like reaction. Environ. Sci. Nano 2024, 11, 113–122. [Google Scholar] [CrossRef]
- Gao, W.; Tian, T.; Cheng, X.; Zhu, D.; Yuan, L. Sustainable Remediation of Polyethylene Microplastics via a Magnetite-Activated Electro-Fenton System: Enhancing Persulfate Efficiency for Eco-Friendly Pollution Mitigation. Sustainability 2025, 17, 3559. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Motlochová, M.; Bavol, D.; Vykydalová, A.; Kupčík, J.; Navrátil, M.; Kirakci, K.; Pližingrová, E.; Dvoranováb, D.; Šubrt, J. Insights in photocatalytic/Fenton-based degradation of microplastics using iron-modified titanium dioxide aerogel powders. Environ. Sci. Nano. 2025, 12, 1515–1530. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).