Abstract
The LRRK2+ SAA− cohort of Parkinson’s disease (PD), characterized by the absence of hallmark α-synuclein pathology, remains under-explored. This limits opportunities for early detection and targeted intervention. This study analyzes data from this under-characterized subgroup and compares it with the LRRK2+ SAA+ cohort using longitudinal data from the Parkinson’s Progression Markers Initiative (PPMI). The PPMI dataset includes 115 LRRK2+ patients (70 SAA+, 45 SAA−) across 52 features encompassing clinical assessments, cognitive scores, DaTScan SPECT imaging, and motor severity. DaTScan binding ratios were selected as imaging-based indicators of early dopaminergic loss, while NP3TOT (MDS-UPDRS Part III total score) was used as a gold-standard clinical measure of motor symptom severity. Linear mixed-effects models were then applied to evaluate longitudinal predictors of DaTScan decline and NP3TOT progression, and statistical analyses of group comparisons revealed distinct drivers of symptoms differentiating SAA− from SAA+ patients. In SAA− patients, a decline in DaTScan was significantly associated with thermoregulatory impairment (p-value = 0.019), while NP3TOT progression was predicted by constipation (p-value = 0.030), sleep disturbances (p-value = 0.046), and longitudinal time effects (p-value = 0.043). In contrast, SAA+ patients showed significantly lower DaTScan values compared to SAA− (p-value = 0.0004) and stronger coupling with classical motor impairments, including freezing of gait (p-value = 0.016), rising from a chair (p-value = 0.007), and turning in bed (p-value = 0.016), along with cognitive decline (MoCA clock-hands test, p-value = 0.037). These findings support the hypothesis that LRRK2+ SAA− patients follow a distinct pathophysiological course, where progression is influenced more by autonomic and non-motor symptoms than by typical motor dysfunction. This study establishes a robust, multimodal modeling framework for examining heterogeneity in genetic PD and highlights the utility of combining DaTScan, NP3TOT, and symptom-specific features for early subtype differentiation. These findings have direct clinical implications, as stratifying LRRK2 carriers by SAA status may enhance patient monitoring, improve prognostic accuracy, and guide the design of targeted clinical trials for disease-modifying therapies.
1. Introduction
Parkinson’s disease is a neurodegenerative disease caused by the progressive loss of dopaminergic neurons in the substantia nigra, most often affecting individuals over 60 years of age [1,2,3,4]. It is highly prevalent, with ~6 million patients worldwide, and imposes a substantial economic burden of ~$52 billion annually in the United States alone [3,5]. Current therapies, such as levodopa, improve symptoms but show variable responses and do not halt disease progression [6,7,8]. Researchers [6] studying PD examine biomarkers, clinical symptoms, genetic mutations, and environmental exposures to identify causes and predict progression. Non-genetic risk factors for PD include pesticide exposure and prodromal symptoms such as REM sleep behavior disorder (RBD), hyposmia, and constipation [9,10]. RBD is linked to cognitive decline, with MoCA, MMSE, and UPDRS providing standardized measures [9,11]. The MDS-UPDRS is the gold standard for assessing motor and non-motor severity [11]. Imaging biomarkers such as dopamine transporter (DAT) SPECT scans correlate with clinical burden [12,13], and longitudinal PPMI data show that the decline in DaTScan, together with motor changes, olfactory loss, and RBD, predicts conversion [14,15,16]. Biochemical markers, including α-synuclein and NfL, track progression, while higher serum urate is protective [17,18,19]. Urinary BMP [20] and lipid ratios in GBA carriers [21] may serve as trait markers but remain inconsistent. These studies highlight the value of integrating motor, non-motor, imaging, and fluid biomarkers in modeling PD risk and progression.
In addition to clinical and biochemical markers, genetics is also very important in PD. Mutations in LRRK2 and GBA are two of the most studied genetic risk factors, and carriers of these genes often show different patterns of disease [6]. Many LRRK2 carriers remain symptom-free for years, but conversion is often preceded by subtle motor changes, REM sleep disorder, hyposmia, and DaTScan decline [14,16]. Polygenic risk scores (PRSs) help separate patients, as higher values predict earlier onset and greater risk, even in single-mutation carriers [14,22]. Subtype comparisons show that LRRK2 carriers have milder symptoms, slower progression, and more stable cognition, whereas GBA1 carriers often develop early cognitive decline [23,24]. Imaging supports these differences: GBA1-PD shows greater white matter damage, while LRRK2 carriers may display DaTScan abnormalities up to 10 years before onset [25,26]. Sex and PRS can further change how fast disease develops in LRRK2 carriers [22,27]. New methods like “brain age” estimation also show special neurodegeneration patterns, with unusual findings in LRRK2 carriers suggesting their progression is not the same as that of other patients [28].
Biomarkers at the molecular level provide higher levels of separation. The α-synuclein seed amplification assay (SAA) is almost always positive in idiopathic PD and GBA1-PD, but only about two-thirds of LRRK2-PD cases are positive. Those who are SAA+ LRRK2 usually decline faster, with worse DaTScan binding, greater olfactory loss, and more rapid cognitive impairment compared to SAA− carriers [29,30]. Other prodromal features, including apathy [31] and amyloid-β deposition [32], also contribute to risk models. The LRRK2-positive and SAA-negative (LRRK2+ SAA−) subgroup, however, has received far less attention than the SAA+ group, which often shows classical Parkinson’s features such as tremor, rigidity, and Lewy body pathology driven by α-synuclein [33,34]. Evidence suggests that LRRK2+ SAA− patients represent a distinct subtype. Their disease progresses more slowly, motor symptoms are milder, and dopamine levels in movement-related brain regions remain relatively higher [35,36]. Yet, in the early stages, they may exhibit more severe memory and cognitive problems [37]. Demographic differences are also clear: SAA− carriers are more often female, have a later age of onset, and report a stronger family history of Parkinson’s, especially among women [38]. These patterns suggest that tau or other non-α-synuclein mechanisms may drive pathology in LRRK2+ SAA− patients. Studying this group is essential, as their treatment responses may differ from SAA+ patients, and standard trial designs could miss this heterogeneity. Focused research can improve early detection, refine subtyping, and guide new therapies. Moreover, attention to SAA− carriers ensures that precision medicine approaches extend beyond the well-characterized α-synuclein-driven forms, addressing all genetic subtypes of PD [34,35,36,37,38]. There is thus a strong need to thoroughly examine PPMI data for LRRK2+ SAA− patients.
To evaluate this subgroup more systematically, both molecular imaging and clinical motor scales are needed, since they capture different but complementary aspects of disease progression. DaTScan and NP3TOT are two complementary indicators commonly used to evaluate PD. DaTScan is a nuclear imaging technique that records dopamine transporter activity in the brain. In PD, dopamine transporters are reduced even before clear motor symptoms are present, so DaTScan is useful for early detection and distinguishing dopamine deficiency due to Parkinson’s from other forms of Parkinsonism [39]. In contrast, NP3TOT is the total score of the MDS-UPDRS Part III, which evaluates the severity of motor impairment in patients. This scale is widely accepted as a formal clinical indicator of disease progression [11]. Taken together, DaTScan is an imaging biomarker for early dopaminergic loss, while NP3TOT is a clinical measure for motor severity. In this study, both were applied to test their association with SAA status and to identify meaningful variables related to PD progression.
Advanced statistical methods have been broadly applied in analyses of PPMI data. Group comparisons such as ANOVA remain essential for identifying baseline differences in clinical, cognitive, and imaging variables across subtypes, for example, in distinguishing SAA+ from SAA− or comparing LRRK2 with GBA carriers [23,24]. Mixed-effects modeling has been widely adopted to account for repeated measures and to capture longitudinal change. It has been used to characterize longitudinal trajectories of motor progression and assess their role in predicting mild cognitive impairment (MCI) [40]. Using the Statistical Restoration of Fragmented Time course (SReFT), Jin et al. 2024 [27] modeled PD progression and found women and LRRK2 G2019S carriers progress 21–25% more slowly, while other mixed-effects approaches quantified non-motor progression using MDS-UPDRS Part I and SCOPA-AUT [41]. Survival and trajectory models have also refined dementia risk prediction, identifying baseline cognition, age, and non-motor symptom burden as strong predictors [42]. Building on these advances, this study focuses on the understudied LRRK2+ SAA− subgroup and leverages recent multimodal PPMI releases to provide a comprehensive, up-to-date analysis. Complementary methods are applied, including ANOVA for group contrasts, linear mixed-effects for longitudinal change, and dominance analysis for variable importance, to identify both categorical and longitudinal predictors of disease severity. Two complementary outcomes are modeled—DaTScan, which reflects early dopaminergic loss, and NP3TOT, which captures later motor severity—revealing subgroup-specific predictors that differ between SAA− and SAA+ carriers. Using data from 115 LRRK2 mutation carriers (70 SAA+, 45 SAA−) across 10 integrated PPMI datasets with 52 complete clinical, imaging, cognitive, autonomic, and blood features, the study provides an evidence base for stratifying LRRK2 carriers by SAA status to refine early detection, improve patient monitoring, and inform outcome selection in disease-modifying trials.
2. Materials and Methods
2.1. PPMI Data Preparation for LRRK2+ SAA− and SAA+ Analysis
Data were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (https://www.ppmi-info.org) as of 15 June 2025, focusing on Parkinson’s patients carrying the LRRK2 mutation with available SAA information. Nine relevant datasets (Table 1) were selected, covering clinical assessments, imaging, cognition, and blood biomarkers. In particular, these datasets include “MDS-UPDRS Parts I”, “MDS-UPDRS Parts II”, and “MDS-UPDRS Parts III”. They evaluate the progression of PD by testing non-motor symptoms in Part I and motor symptoms in Part II and III. The “REM Sleep Behavior Disorder Questionnaire” dataset records the severity and frequency of abnormal behavior during sleep. In addition, the “MoCA” dataset tests cognitive abilities like memory and attention for early detection of cognitive decline and loss in memory, attention and language skills. The dataset “SCOPA-AUT” is focused on the dysfunction in the gastrointestinal, urinary, cardiovascular, thermoregulatory, and sexual systems. The “Blood Chemistry & Hematology” dataset was also used to find what biomarkers, like liver enzymes, kidney function, blood counts, and inflammation, may be helpful for the diagnosis of PD. The dataset “Age at Visit” was taken to provide demographic information such as race, gender, and age. The “DaTScan SBR Analysis” data analyzes the Striatal Binding Ratio (SBR) to quantify dopamine transporter availability and serve as an objective biomarker of nigrostriatal degeneration. It uses SPECT imaging to assess the degradation of dopamine transporters in the brain and can be used as proof of dopamine-caused Parkinsonism. These datasets were merged by patient ID (PATNO), yielding 52 integrated variables for analysis. Detailed descriptive statistics for all these variables are provided in Appendix A Table A1. After extracting and cleaning data from the 9 datasets, 115 patient samples were retained in the study, with 70 samples of SAA+ and 45 samples of SAA−. Only the samples that contained the information for all 52 feature variables were included in this study.

Table 1.
Summary of Datasets Extracted from the PPMI database.
2.2. Methods
2.2.1. Overview of the Methods
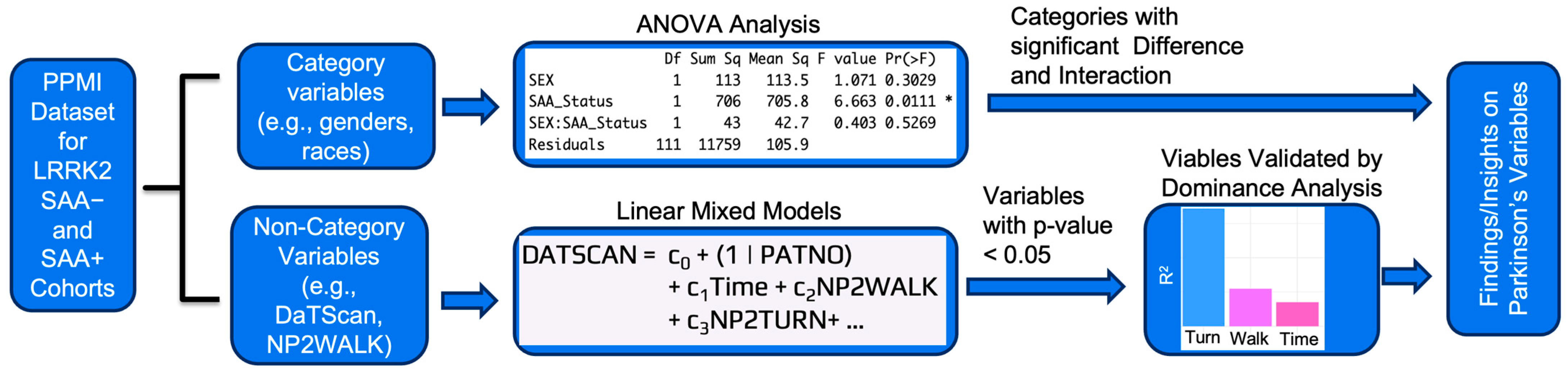
Figure 1 summarizes the overall analysis pipeline, while detailed descriptions of each step are provided in the subsections. The workflow begins with the integration of 52 variables from 10 PPMI datasets for 115 LRRK2 carriers stratified by SAA status. Two outcomes were modeled: DaTScan as an imaging marker of early dopaminergic decline and NP3TOT as a clinical measure of motor severity. Data from the Parkinson’s Progression Markers Initiative (PPMI) were compiled into a CSV file with patients as rows and variables as columns and processed in R (RStudio 2025.05.1). The dataset included both categorical variables (e.g., sex, diagnosis group, medication status, age categories) and non-categorical variables (e.g., motor and non-motor scores, imaging, biomarker levels). For categorical variables (sex, race, ethnicity, handedness, age), two-way ANOVA was applied to test the effects of SAA status on DaTScan and NP3TOT, with interactions (e.g., SAA × sex) considered and Tukey HSD used for post hoc comparisons. For non-categorical variables, linear mixed-effects models (lmer() function, R lme4 package [43]) were constructed for SAA− and SAA+ cohorts separately, with time and clinical, cognitive, autonomic, or biomarker predictors as fixed effects and patient ID as a random intercept. These models identified predictors of longitudinal change in DaTScan (early dopaminergic decline) and NP3TOT (motor severity) while accounting for repeated measures. To complement these models, dominance analysis [44,45] quantified the relative R2 contribution of each predictor across subset models, providing a measure of variable importance even though statistical significance is not directly estimated. Together, this framework integrated categorical comparisons, longitudinal modeling, and predictor ranking to identify robust drivers of progression in LRRK2+ patients stratified by SAA status.

Figure 1.
Overview of the analytical workflow applied to PPMI data from LRRK2 carriers stratified by SAA status (70 SAA+, 45 SAA−). Ten integrated datasets provided 52 variables spanning clinical, cognitive, autonomic, imaging, and blood biomarkers. Two primary outcomes were analyzed: DaTScan (dopaminergic imaging, early disease indicator) and NP3TOT (MDS-UPDRS Part III motor score, later-stage severity measure). Categorical predictors were evaluated with two-way ANOVA and Tukey HSD tests, while non-categorical predictors were modeled using linear mixed-effects approaches with repeated measures. Dominance analysis was then used to validate predictor importance. Examples of results are shown at each step: (i) ANOVA identified different SAA status showing significant differences in NP3TOT, (ii) linear mixed-effects models with DaTScan as the outcome highlighted motor predictors such as walking and turning, and (iii) dominance analysis confirmed turning as an influential contributor to model variance. The asterisk (*) in the figure denotes statistical significance at p-value < 0.05.
2.2.2. ANOVA Analysis
The two-way analysis of variance (ANOVA) allows us to consider interactions between two independent variables, as well as account for the effect of a third variable. In addition to SAA status, the other independent variables examined were sex, race, Hispanic/non-Hispanic, handedness, and age. For each categorical variable (e.g., sex: male vs. female), ANOVA analysis was performed to test whether SAA− and SAA+ phenotypes influenced differences in categorial cohorts (e.g., male vs. female patients) in DaTScan values. This approach also assessed whether the categorical factor (e.g., sex) contributed to variation in DaTScan outcomes for the same SAA phenotype, thereby indicating potential modifiers of early PD detection. A similar analysis was conducted using NP3TOT as the outcome measure to represent the formal clinical detection of PD. ANOVA was performed via the aov() function in R to test group differences, with Tukey HSD applied for pairwise comparisons. The null hypothesis assumed no main effects or interactions between variables. As shown in Figure 2, sex did not significantly affect DaTScan binding (diff = −0.16, p-value = 0.56), whereas SAA status was highly significant, with SAA+ individuals showing lower DaTScan values than SAA− (diff = −1.06, p-value = 0.0004), supporting its role as an early biomarker of nigrostriatal dysfunction. Most sex × SAA interactions were non-significant, though the male SAA+ vs. male SAA− contrast reached significance (diff = −1.10, p-value = 0.031), suggesting a potentially stronger effect of SAA status on DaTScan values in males than in females.

Figure 2.
Example of ANOVA analysis showing the effects of sex and SAA status on DaTScan binding ratios with post hoc comparisons. The “$” symbol is a notation automatically generated by RStudio to indicate a variable or factor in ANOVA.
2.2.3. Linear Mixed-Effects Models
Separate linear mixed-effects models were constructed for the SAA+ and SAA-negative (SAA−) subgroups, with DaTScan binding ratio (DaTScan) representing an early dopaminergic degeneration marker and MDS-UPDRS Part III total score (NP3TOT) representing a formal motor severity outcome, respectively. To ensure interpretability, linear mixed-effects models were constructed separately for each variable category (e.g., MDS-UPDRS Part I, SCOPA-AUT, cognitive measures, imaging, blood biomarkers). The objective was not to select an optimal predictor subset but to evaluate which variables exhibited statistically significant associations with DaTScan or NP3TOT outcomes. Significant predictors were validated through dominance analysis to rank their relative contributions, allowing comparison of progression determinants across SAA+ and SAA− cohorts and identification of domain-specific predictors of early and advanced disease severity.
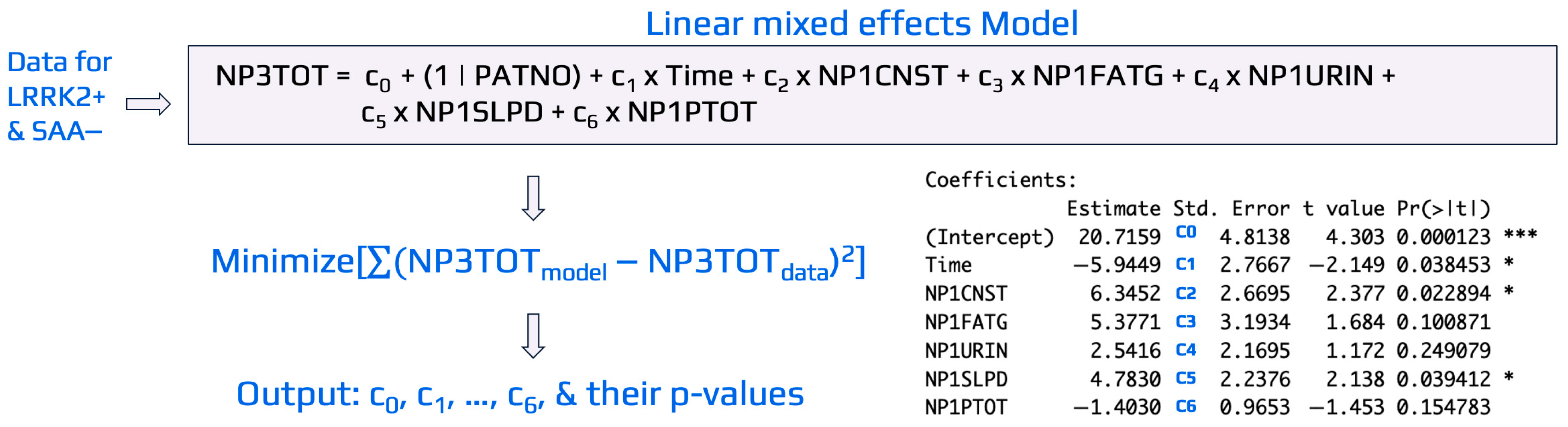
To illustrate the general procedure of linear mixed-effects modeling, Figure 3 presents an example evaluating the influence of domain-specific variables from the MDS-UPDRS_Part_I (e.g., NP1CNST, NP1FATG, NP1URIN, NP1SLPD, NP1PTOT) on the output variable, NP3TOT, for LRRK2+ SAA− cohorts. Similar approaches were implemented to develop linear mixed-effects models for LRRK2+ SAA+ cohorts with NP3TOT or DaTScan as the outputs. In the model, Time was included as a fixed effect to capture the average change between repeated visits for the same patient (i.e., longitudinal measurements per participant). A coefficient ci, i changed from 1 to 8, was assigned to each input variable for the case study shown in Figure 3. As typically implemented in mixed effects models for longitudinal data, a random intercept for patient number (PATNO), specified as c0(1 | PATNO), was included to account for the non-independence of repeated measures within the same individual for the visit record. This random intercept allows each patient to have a unique baseline value [46], while the fixed effects (represented by ci) estimate the average relationship between predictors and the outcome across all patients. Time was modeled as a non-categorical variable (months since baseline), and only random intercepts were used to represent baseline variability across subjects. Random slopes (e.g., time × predictor) were not included to maintain model stability and interpretability, given the limited number of repeated measurements per participant. In this specification, all patients share the same slope for time, reflecting a common average rate of change, but differ in their intercepts.
Figure 3.
An illustrative example of linear mixed-effects modeling used to identify key MDS-UPDRS_Part_I variables associated with NP3TOT in the LRRK2+ SAA− cohort. Data were obtained from PPMI, and the model was implemented in R using the lmer package. Coefficients (ci) were estimated by minimizing the difference between model-predicted and observed NP3TOT values. Variables with p-value < 0.05 were considered significant indicators of NP3TOT for the LRRK2+ SAA− cohort. Symbols * and *** in the figure denotes statistical significance at p-value < 0.05 and p-value < 0.001, respectively.
The linear mixed-effects models were developed using the lmer() function from the lme4 package in R [43] to estimate coefficients and p-values for each predictor, with significance set at p-value < 0.05. Variables with 0.05 ≤ p-value < 0.10 were noted as potentially influential. As shown in Figure 3, Time (p-value = 0.034), NP1CNST (constipation, p-value = 0.023), and NP1SLPD (sleep problems, p-value = 0.040) were significant predictors of NP3TOT, while NP1FATG (fatigue, p-value = 0.100) showed a trend toward significance. The same modeling framework was applied across other variable domains and cohorts to identify predictors of early (DaTScan) and advanced (NP3TOT) disease severity.
2.2.4. Dominance Analysis
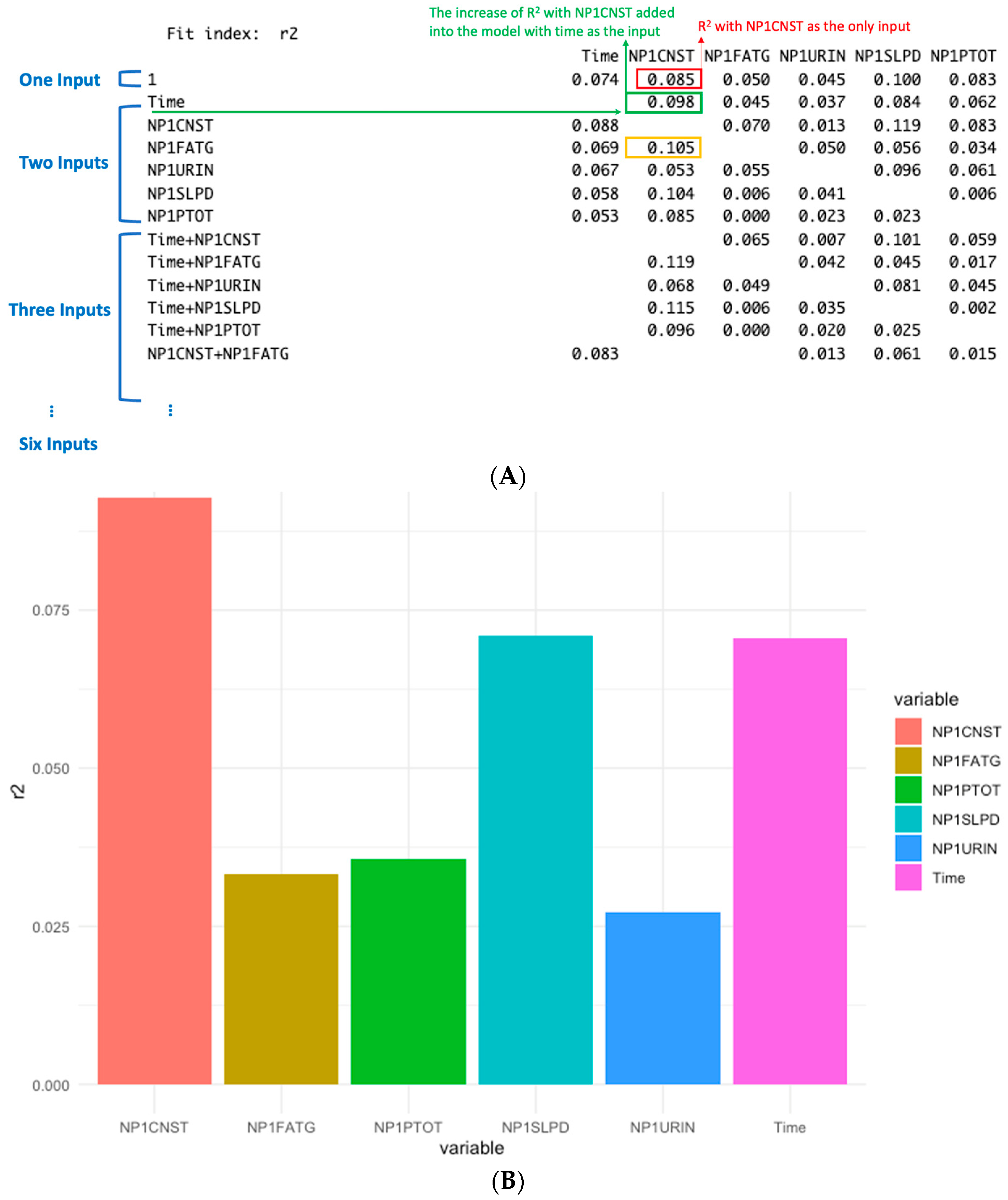
Dominance analysis evaluates the relative importance of predictors by examining how much each variable contributes to the model’s explanatory power, as measured by the change in R2. The basic idea is to add predictors sequentially and quantify the increase in R2 that results from including each new variable. For the example shown in Figure 3, dominance analysis was applied and illustrated in Figure 4A. When NP1CNST (constipation) is the only input for the model, its contribution to R2 is 0.085 (marked in RED in Figure 4A). When it is added to a model already containing Time, the R2 is increased by 0.098 (marked in GREEN in Figure 4A). This improvement reflects the additional explanatory contribution of NP1CNST beyond what is explained by Time alone. Similarly, the increase of R2 is 0.105 if NP1CNST is added into the model with NP1FATG (marked in YELLOW). Dominance analysis then extends this logic by considering all possible combinations of predictors, i.e., three to six inputs. Each variable’s contribution is assessed across single-input models, two-input models, three-input models, and so forth. By averaging the incremental R2 contributions across all possible model combinations, a robust measure of each predictor’s overall importance can be obtained. Figure 4B summarizes these averaged contributions, showing the mean explanatory power of each variable across all combinations rather than from individual models. For example, the average contribution of NP1CNST across all model combinations can be read from the NP1CNST column in Figure 4A. This averaging process yields the final dominance values, which are visualized in Figure 4B. Here, NP1CNST, NP1SLPD (sleep problems), and Time emerge as the strongest contributors to explaining variance in NP3TOT. The most dominant factors identified by dominance analysis are consistent with those highlighted by the linear mixed-effects models in Figure 3. While the mixed-effects models evaluate all variables simultaneously, dominance analysis assesses the contribution of each predictor across models with different numbers and combinations of variables, providing an additional perspective on their relative impact. However, dominance analysis does not quantify statistical significance. In this study, it is therefore used as a complementary tool to validate and reinforce the importance of the factors identified by the linear mixed-effects models.
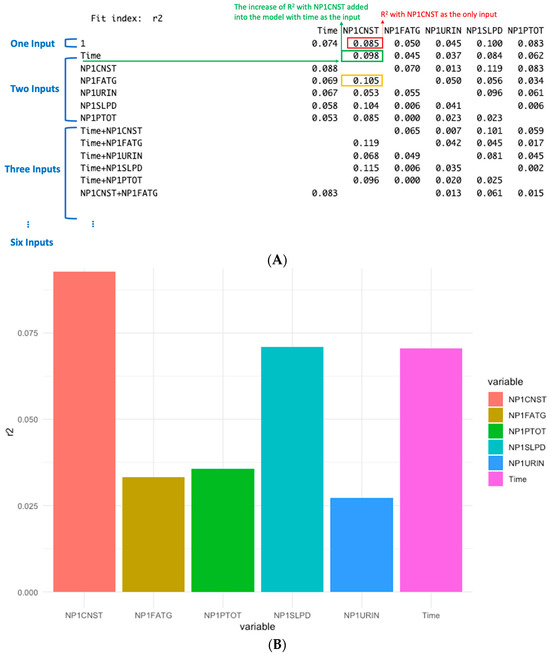
Figure 4.
Illustrative example of dominance analysis. (A) Incremental R2 contributions of individual input variables across all possible model combinations. (B) Average R2 contributions for each variable, calculated by averaging its explanatory power across all model combinations in which it appears. These averaged dominance values reflect the overall relative importance of predictors in explaining NP3TOT variance.
3. Results
3.1. ANOVA Analysis of Category Variables
Across all categorical variables (sex, race, age, Hispanic status, and handedness), SAA− patients showed significantly higher DaTScan values than SAA+ patients (p-value < 0.05), while SAA+ patients exhibited significantly higher NP3TOT motor scores than SAA− patients (p-value < 0.05). Because lower DaTScan binding reflects greater dopaminergic neuron loss, these findings confirm that SAA+ individuals experience more advanced dopaminergic degeneration, consistent with α-synuclein pathology. In contrast, higher DaTScan values in SAA− carriers suggest relatively preserved dopaminergic function, often associated with early-stage or alternative, non-α-synuclein mechanisms of Parkinsonism. Together, these DaTScan and NP3TOT differences indicate that SAA status is a strong biological discriminator between α-synuclein–positive and –negative LRRK2 subgroups. This data indicates that SAA+ patients generally have lower DaTScan than SAA− patients, that is, they have lower dopaminergic neurons and a higher chance of being diagnosed with PD. This is actually reflected in ANOVA tests that SAA− patients also have significantly lower NP3TOT than SAA+ patients. SAA status remained the dominant factor distinguishing patient subgroups across all categorical variables. However, subtle sex-related interactions emerged—female SAA+ patients had lower DaTScan binding than male SAA− patients, while male SAA+ patients exhibited higher NP3TOT scores. These findings suggest that disease expression may differ by sex, with females showing greater dopaminergic loss but males expressing more severe motor symptoms, consistent with known sex-related modulation of PD risk and phenotype.
In addition to the comparison of SAA− vs. SAA+ cases above, Table 2 shows the ANOVA analysis for SAA+ and SAA− patients across the five categories of variables. ANOVA analysis revealed that SAA status remained the most consistent factor distinguishing patient groups across categorical variables. As for sex, ANOVA results revealed clear sex-related interactions between SAA status and PD indicators. For DaTScan, female SAA+ patients showed lower binding ratios than male SAA− patients, suggesting that dopaminergic loss may be more pronounced in females carrying α-synuclein–positive pathology. In contrast, for NP3TOT, male SAA+ patients exhibited higher motor scores than female SAA− patients, indicating that motor impairment progresses more severely among males once dopaminergic loss becomes established. Together, these findings imply that biological sex modifies how α-synuclein–linked neurodegeneration manifests—females may experience greater presynaptic dopamine loss, whereas males display greater downstream motor symptom expression. As for race, no significant racial differences were observed in DaTScan binding across White, Black, and Asian participants. However, NP3TOT scores were higher in Asian participants than in White participants, suggesting possible differences in symptom perception, disease reporting, or genetic modifiers that influence clinical presentation even when dopaminergic loss appears similar. Although the sample size for non-White groups was limited, these results underscore the need to validate racial variability in PD symptom expression using larger, more diverse datasets. Age analysis showed that SAA+ patients aged 60–69 years had lower DaTScan binding ratios than SAA− patients aged 70 years or older, indicating earlier or faster dopaminergic decline among α-synuclein–positive individuals despite being younger on average. No significant age-related differences were detected for NP3TOT, suggesting that once clinical motor symptoms are present, disease severity is less dependent on chronological age. This pattern supports the hypothesis that α-synuclein pathology accelerates presymptomatic neuronal loss before overt motor deterioration occurs. For ethnicity, non-Hispanic SAA+ patients showed lower DaTScan values compared to Hispanic SAA− patients, but NP3TOT outcomes were not significantly different. Finally, handedness did not produce significant differences in either DaTScan or NP3TOT. Overall, these results (Table 2) emphasize that while SAA status is the dominant driver of group differences, additional modifiers such as sex, race, age, and ethnicity contribute to heterogeneity in PD expression.

Table 2.
ANOVA analysis for category variables and LRRK2+ SAA types on early PD indicator (presented by DaTScan) and formal PD indicator (represented by NP3TOT).
3.2. Identification of Important non-Categorical Variables on PD
Linear mixed effects models were developed for both LRR2+ SAA+ and LRR2+ SAA− cohorts from the longitudinal data, with DaTScan and NP3TOT as the output, respectively. The factors identified with significant impacts from mixed models were further validated by dominance analysis. Table 3 summarizes p-values from the non-category variables listed in Appendix A Table A1.

Table 3.
The p-values for MDS-UPDRS Part I Variables Across SAA+ and SAA− Groups by DaTScan and NP3TOP.
For the LRRK2+ SAA− group with DaTScan as the outcome, the strongest signal came from the autonomic system. As reported in Table 3, thermoregulation had a significant association (p-value = 0.019). In addition, several other variables showed p-values close to the significant level, such as NP2SPCH (speech, p-value = 0.086), NP2DRES (dressing, p-value = 0.083), MCAVF (verbal fluency, p-value = 0.061), pupillomotor (p-value = 0.062), and cardio function (p-value = 0.096). These results indicate that in SAA− patients, DaTScan is mainly influenced by thermoregulation, but it may also be impacted by multiple autonomic or motor functions that are weaker.
For the LRRK2+ SAA− group with NP3TOT as the outcome, three variables are significant according to Table 3. These are Time (p-value = 0.043), NP1CNST (constipation, p-value = 0.030), and NP1SLPD (sleep problems, p-value = 0.046). Pupillomotor also shows near-significance (p-value = 0.079). This tells us that in SAA− patients, the motor severity NP3TOT is not only from motor features but is very much connected with long-term progression (time effect) and with patient-reported non-motor problems such as constipation and sleep. The combination of time and non-motor symptoms means that NP3TOT may capture later clinical changes that DaTScan does not.
For the LRRK2+ SAA+ group with DaTScan as the outcome, motor problems were strongly reflected. As shown in Table 3, NP2FREZ (freezing, p-value = 0.016), NP2RISE (rising from chair, p-value = 0.007), and NP2TOT (Part II motoring total score, p-value = 0.030) all had significant effects. Thermoregulation (p-value = 0.013) was also significant, showing that the autonomic system is also important in SAA+. NP2SPCH (speech, p-value = 0.062) was close to significant. In addition, Time had a very strong significance for DaTScan in the SAA+ group (p-value = 0.0024). This means dopaminergic decline in SAA+ is progressing clearly with time, and DaTScan captures this change very well.
For the LRRK2+ SAA+ group with NP3TOT as the outcome, both motor and cognitive domains were important. As indicated in Table 3, NP2TURN (turning in bed, p-value = 0.016) and MCACLCKH (clock-hands test, p-value = 0.037) were significant variables. Anxiety NP1ANXS (p-value = 0.079) was near-significant. This shows that in SAA+ patients, NP3TOT severity is influenced by both axial motor function (turning) and cognition (clock hands), with some contribution from anxiety symptoms.
In summary, Table 3 shows that LRRK2+ SAA− patients are more related to non-motor symptoms and with the time effect when NP3TOT is used as the outcome. Constipation, sleep problems, and time are important for NP3TOT in this group, and pupillomotor and other autonomic measures are close to significant. For DaTScan in SAA− patients, thermoregulation is the main significant factor. On the other hand, LRRK2+ SAA+ patients are more strongly connected with classical motor symptoms and autonomic function when DaTScan is the outcome. In this group, freezing, rising from a chair, Part II motoring total score, and thermoregulation are all significant, and speech is near-significant. Time is also highly significant for DaTScan in SAA+ patients, showing strong longitudinal progression in dopaminergic imaging. For NP3TOT in the SAA+ group, turning in bed and the MoCA clock-hands test are significant, and anxiety is near-significant. These findings together indicate that SAA− and SAA+ are two different subtypes of LRRK2 PD, with different important factors driving disease progression: SAA− is linked to non-motor and time progression, and SAA+ is linked to motor, autonomic, and cognitive impairments.
4. Discussion
4.1. Major Findings from ANOVA Analysis and Linear Mixed Effects Models
This study used both ANOVA analysis and linear mixed-effects modeling to study LRRK2 PD divided by SAA status. From the ANOVA results, the main effect of SAA status was very strong across different demographic categories (sex, race, age, Hispanic, and handedness) when looking at DaTScan and NP3TOT (Table 2). SAA+ patients had significantly lower DaTScan values than SAA− patients, which means stronger dopaminergic neuron loss. At the same time, SAA+ patients also had higher NP3TOT motor scores than SAA− patients, meaning worse motor severity. These findings support that SAA status is an important biological marker that separates two groups of LRRK2 carriers with different disease burden [47].
As shown in the linear mixed models for SAA− patients, NP3TOT was significantly influenced by non-motor symptoms such as constipation and sleep problems, and also by Time, which reflects progression during follow-up [41]. For DaTScan in SAA−, thermoregulation was significant, while other factors like pupillomotor, speech, dressing, and verbal fluency were close to significant (Table 3) [48,49]. In SAA+ patients, DaTScan was strongly associated with motor symptoms such as freezing, rising from a chair, Part II motor total score, and also with thermoregulation [50,51]. For NP3TOT in SAA+, turning in bed and cognitive performance (MoCA clock-hands task) were significant, with anxiety near-significant. These results show different patterns for SAA− and SAA+.
4.2. Comparison of LRRK2+ SAA− and LRRK2+ SAA+
The comparison of SAA− versus SAA+ in LRRK2 carriers is one of the unique parts of our study. LRRK2+ SAA− carriers show more association of NP3TOT with non-motor symptoms and with longitudinal Time. This means their clinical motor severity is not only from dopaminergic loss but is also shaped by non-motor complaints and slow progression. This is consistent with previous reports that some LRRK2 Parkinson’s cases progress more slowly and show different phenotypes [34]. On the other hand, LRRK2+ SAA+ carriers show a more classical Parkinson’s picture: DaTScan is strongly linked with motor deficits, autonomic dysfunction, and also shows a strong decline over time (p-value = 0.0024). This is supported by studies that describe α-synuclein pathology as driving the classic motor–autonomic coupling in PD [49].
Other studies also reported that LRRK2+ patients are heterogeneous. It was reported that cognitive changes in LRRK2 mutation carriers, which matches our result that the MoCA clock-hands task is linked with NP3TOT in SAA+ patients [24]. Hinkle et al., 2018 [48], showed that autonomic dysfunction, especially gastrointestinal, is associated with lower DAT binding, which supports our finding that thermoregulation and pupillomotor domains correlate with DaTScan. Visser et al., 2004 [52], validated SCOPA-AUT and confirmed its domain structure, giving reliability to our autonomic results.
4.3. DaTScan Versus NP3TOT in SAA− Patients
Another important and unique finding of this work is the separation between DaTScan and NP3TOT in LRRK2+ SAA− patients. DaTScan in LRRK2+ SAA− was linked mainly to autonomic function, while NP3TOT was linked to non-motor symptoms (constipation, sleep) and Time. This suggests that in LRRK2+ SAA−, DaTScan may be useful as a biological marker of dopaminergic loss related to autonomic changes, while NP3TOT captures the clinical burden and longitudinal worsening. It was reported that DAT imaging is very useful for early diagnosis [50,51], while clinical scales like UPDRS capture later disability [53]. Our study shows the same pattern in the LRRK2+ SAA− group. Seibyl et al., 2022 [49], also showed that DaTScan SPECT is reliable for progression tracking in longitudinal cohorts, but our data add new information that in LRRK2+ SAA− patients, the coupling of DaTScan with motor severity is weak, and instead NP3TOT and Time are more sensitive for progression.
4.4. Clinical Implication and Significance
The clinical implication of our study is that LRRK2+ SAA− and LRRK2+ SAA+ patients should not be treated as one uniform group in research or clinical trials. For LRR2+ SAA− patients, NP3TOT and non-motor symptoms (constipation, sleep problems) together with longitudinal Time are important for understanding progression, while DaTScan mainly reflects autonomic functions. For LRRK2+ SAA+ patients, DaTScan is closely tied with motor symptoms and autonomic dysfunction, and NP3TOT is influenced by turning in bed and cognitive impairment. This means that outcome measures in clinical trials should be different depending on SAA status: motor and imaging outcomes for SAA+, but mixed non-motor and longitudinal measures for SAA−.
This work is significant because it shows that α-synuclein SAA status is not just a biomarker but also a clinical classifier that reveals two biologically distinct forms of LRRK2 PD. By showing that thermoregulation is consistently significant for DaTScan and that Time is important in different ways across subgroups, our study provides new evidence for precision medicine approaches in Parkinson’s. These findings agree with the new biomarker strategies described in PPMI [38,49] and show the importance of separating LRRK2 carriers by SAA status.
4.5. Limitations and Future Work
Our analyses were restricted to LRRK2+ patients within the PPMI dataset, stratified by SAA status. We deliberately focused on this subgroup because LRRK2+ SAA− is relatively understudied and requires more biomarker discovery. The overall sample size was relatively small (n = 115) due to the limited availability of LRRK2+ cases with complete data across multiple PPMI domains. While the observed trends provide valuable exploratory insights into an underrepresented genetic subgroup, the generalizability of these findings is further limited by the demographic composition of the PPMI cohort, which is predominantly White. Future studies should validate these results in larger and more diverse populations to enhance representativeness and clinical relevance. While the findings cannot be generalized to other genetic cohorts, such as GBA1 or SNCA mutation carriers, or to idiopathic PD, which also display distinct biomarker and progression patterns [54], the data analysis and modeling approaches presented in this study can be applied to the data for other genetic cohorts.
Only participants with complete data for all 52 variables listed in Table 2 were included. We acknowledge that this complete-case approach reduced the sample size and may have introduced selection bias toward participants with more complete clinical assessments, potentially skewing the cohort toward healthier or better-documented individuals. This decision was made to ensure consistency across integrated datasets and to avoid the additional uncertainty that may arise from imputing heterogeneous clinical, imaging and biomarker variables. In future work, we plan to implement multiple imputation or expectation–maximization methods to manage missing data more effectively while retaining a larger, more representative sample. Additional measurements—such as neuroimaging [55,56], fatigue monitoring [57,58], beta oscillations [59], and memory impairment [60]—may help identify new candidate biomarkers for inclusion in future analyses.
The dominance analysis used in this study has certain methodological limitations. Although it provides an intuitive measure of each variable’s relative contribution to model variance, it does not adjust for potential multicollinearity among predictors and does not provide formal statistical significance testing. As such, dominance results should be interpreted descriptively rather than inferentially. In this analysis, dominance analysis served as a complementary validation approach to confirm the consistency of predictors identified from the mixed-effects models. Future work will incorporate additional resampling or regularization techniques to evaluate variable stability and account for correlated predictors.
Although Table 2 included many established predictors from the literature, such as motor and non-motor MDS-UPDRS subscores, sleep-related features reflecting REM sleep behavior disorder, MoCA cognitive variables, depression scales, and SCOPA-AUT autonomic domains, some important biomarkers were not available to us in this analysis. Specifically, we did not include cerebrospinal fluid biomarkers such as tau, amyloid-β, and CSF α-synuclein, which are increasingly recognized as important for predicting cognitive decline and PD progression [61,62,63]. In addition, serum urate, which has been linked to slower disease progression and protective effects in LRRK2 carriers, was not available for inclusion [17,18]. Another missing marker was neurofilament light chain, which is increasingly recognized as a dynamic biomarker of axonal damage and disease progression. Genetic modifiers such as polygenic risk scores were also not included, despite their strong influence on penetrance and phenotypic variability in LRRK2 carriers [22]. Similarly, advanced neuroimaging measures, including diffusion MRI connectometry and brain-age modeling, which have shown value in capturing subtle structural changes and genotype-specific differences, were not incorporated into our models [25]. Finally, novel biomarkers such as urinary bis (monoacylglycerol) phosphate, which may serve as a trait marker in LRRK2/GBA mutation carriers, were unavailable [20].
Although dimensionality reduction techniques such as PCA or penalized regression can mitigate overfitting, they were not applied in this study because the primary objective was to evaluate the statistical significance and biological relevance of individual predictors rather than to optimize predictive performance. While these approaches are powerful, they reduce interpretability by combining correlated variables into latent components, obscuring the contribution of specific clinical or biomarker measures. To balance interpretability and model stability, predictors were analyzed within domain-specific models rather than pooled into a single multivariable framework. As the dataset expands, future analyses will incorporate feature selection and penalized regression to validate model robustness and improve generalizability. Furthermore, expanding the LRRK2+ SAA− cohort with additional fluid biomarkers (e.g., CSF tau, amyloid-β, α-synuclein, serum urate, and NfL), genetic risk scores, and advanced imaging features—alongside the existing clinical, autonomic, and cognitive data available in PPMI—will strengthen predictive modeling for precision medicine and facilitate identification of multimodal biomarkers for improved patient subtyping.
5. Conclusions
This study provides the first systematic characterization of the LRRK2+ SAA− subgroup, revealing that its disease trajectory differs fundamentally from that of LRRK2+ SAA+ carriers. The key discovery is that non-motor and autonomic domains—not classical motor symptoms—drive progression in SAA− patients, while SAA+ carriers show stronger coupling between dopaminergic decline and motor–autonomic impairment. Specifically, thermoregulation emerged as a consistent autonomic correlation of dopaminergic loss, constipation, sleep problems, and time were dominant predictors of worsening motor severity in the SAA− cohort. These findings refine current models of Parkinson’s disease heterogeneity by demonstrating that α-synuclein SAA status defines distinct mechanistic pathways within genetically linked PD. This differentiation supports a precision-medicine framework in which early detection, outcome tracking, and therapeutic targets are tailored by SAA subtype. For example, DaTScan imaging and motor outcomes may better capture disease progression in SAA+ carriers, whereas non-motor and longitudinal measures may serve as more sensitive markers in SAA− carriers. Beyond its immediate results, this work establishes a multimodal analytic framework integrating ANOVA, mixed-effects modeling, and dominance analysis—methods that can be extended to other genetic PD subgroups such as GBA1 and SNCA. Future efforts will expand the dataset by collecting additional longitudinal and diverse patient samples to overcome current data limitations and incorporate fluid biomarkers (e.g., CSF tau, amyloid-β, urate, NfL) and polygenic risk scores to further strengthen our understanding of biologically distinct PD subtypes and enhance biomarker-driven clinical trial design.
Author Contributions
Conceptualization, Z.H.; methodology, V.J., C.K.H., G.G., K.H., L.Y., C.C., A.L. and Z.H.; formal analysis, V.J., C.K.H., G.G., K.H., L.Y., C.C., A.L. and Z.H.; data curation, Z.H.; writing—original draft preparation, V.J., C.K.H., G.G., K.H., L.Y., C.C. and A.L.; writing—review and editing, Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be available upon request.
Acknowledgments
Data used in the preparation of this article were obtained on [15 June 2025] from the Parkinson’s Progression Markers Initiative (PPMI) database (https://www.ppmi-info.org/access-data-specimens/download-data), RRID:SCR_006431. For up-to-date information on the study, visit http://www.ppmi-info.org Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s, AskBio, Avid Radiopharmaceuticals, BIAL, BioArctic, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Capsida Biotherapeutics, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Jazz Pharmaceuticals, Johnson & Johnson Innovative Medicine, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Neuron23, Neuropore, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation and Yumanity Therapeutics.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
The study used samples that contained complete information across 52 variables (Table 2). These variables cover non-motor symptoms, motor impairment, cognition, autonomic function, demographics, imaging, blood markers, and molecular measures. Non-motor symptoms are important because they often appear before the main motor symptoms and can support early diagnosis of PD [6]. In this dataset, non-motor variables from MDS-UPDRS Part I include constipation (NP1CNST, mean 0.62 ± 0.84), fatigue (NP1FATG, 1.06 ± 0.95), urinary problems (NP1URIN, 0.74 ± 0.94), and sleep problems (NP1SLPD, 1.05 ± 0.95). The overall non-motor total score (NP1RTOT) averaged 2.08 ± 2.03, showing mild but variable symptom burden across participants. Marafioti et al., 2023 [9] also emphasized the predictive role of REM sleep behavior disorder and related non-motor variables in cognitive impairment risk. Motor symptoms were measured with MDS-UPDRS Part II [11]. For example, difficulties with walking and balance (NP2WALK) had a mean of 0.94 ± 0.87, tremor (NP2TRMR) averaged 0.98 ± 0.86, and speech problems (NP2SPCH) averaged 0.55 ± 0.83. The Part II total score (NP2PTOT) was 8.43 ± 6.06, reflecting generally mild impairment but wide variation. Motor subtyping (tremor-dominant vs. PIGD) has also been shown to have prognostic value in PPMI data [23].
Sleep-related behaviors also showed measurable presence, such as acting out dreams (DRMFIGHT, 0.22 ± 0.42) and limb movement during sleep (SLPLMBMV, 0.20 ± 0.41). The geriatric depression scale (DEPRS) averaged 0.24 ± 0.43, highlighting the neuropsychiatric dimension of PD. Cognitive performance was assessed with the Montreal Cognitive Assessment (MoCA). Examples include alternating trail making (MCAALTTM, 0.91 ± 0.29) and cube copy (MCACUBE, 0.73 ± 0.44). Verbal fluency averaged 0.88 ± 0.32, while attention measured by serial 7s had a mean of 2.76 ± 0.60. Cognitive impairment in PD has been linked to both RBD [9] and amyloid deposition [32]. Autonomic function was measured with the SCOPA-AUT domains. Urinary function showed a higher burden (mean 4.18 ± 5.18), while cardiovascular (1.43 ± 0.93) and thermoregulation (2.53 ± 2.72) were generally lower. Sexual function averaged 7.13 ± 6.02, indicating high variability among participants.
Other biomarkers included DaTScan striatal-binding ratio (DaTScan, 4.54 ± 1.53), which measures dopamine transporter availability [13,14], and the MDS-UPDRS Part III total motor score (NP3TOT, 20.40 ± 10.32), reflecting the severity of motor impairment [11]. At the molecular level, the α-synuclein seed amplification assay (SAA_Status) was used to stratify patients into SAA+ or SAA− groups [29,30]. Blood markers included LSIRES (28.17 ± 43.78), providing information about systemic inflammation, metabolism and LUSRES (20.72 ± 18.38), which reflects urine-based biochemical markers potentially tied to metabolism or systemic function. Biomarkers like urate [17,18] and NfL have been studied as potential progression indicators in genetic subgroups. Demographic measures included age at visit (mean 64.42 ± 8.90), sex, race, ethnicity, and handedness. Demographic and social determinants have been highlighted as important covariates for equitable modeling.
Overall, these statistical values illustrate that the PPMI dataset captures a wide spectrum of PD features, ranging from mild non-motor symptoms to more variable motor, cognitive, and autonomic changes, supported by imaging and molecular biomarkers. Prior work [15,16,27] further confirms that integrating such multimodal variables can improve predictive modeling of PD onset and progression.

Table A1.
The names, meaning, and values of variables in the dataset from PPMI.
Table A1.
The names, meaning, and values of variables in the dataset from PPMI.
| Abbreviation | Meanings | Values |
|---|---|---|
| EVENT_ID | Visit identifier | V04, V06, V10 |
| SAA_Status | Alpha-synuclein seed amplification assay (SAA) result | SAA+ or SAA− |
| SEX | Sex | 0 = Male, 1 = Female |
| RAASIAN | Race: Asian | 0 = No, 1 = Yes |
| RABLACK | Race: Black or African American | 0 = No, 1 = Yes |
| RAWHITE | Race: White | 0 = No, 1 = Yes |
| HISPLAT | Ethnicity: Hispanic/Latino | 0 = No, 1 = Yes |
| HANDED | Handedness | 1 = Right, 2 = Left, 3 = Ambidextrous |
| AGE_AT_VISIT | Age at the time of visit | 40<, 40–50, 50–60, 60–70, >70 |
| DaTScan | DaTScan binding ratio | 4.54 ± 1.52 |
| NP3TOT | MDS-UPDRS Part III (Motor Examination) total score | 20.40 ± 10.32 |
| SLPINJUR | Sleep-related injury | 0.094 ± 0.29 |
| DRMFIGHT | Acting out dreams (fighting) | 0.22 ± 0.42 |
| DRMUMV | Dream enactment (movement) | 0.11 ± 0.31 |
| SLPLMBMV | Limb movement during sleep | 0.20 ± 0.41 |
| DEPRS | Geriatric Depression Scale (GDS) score | 0.24 ± 0.43 |
| NP1CNST | MDS-UPDRS Part Ia: Constipation | 0.62 ± 0.84 |
| NP1FATG | MDS-UPDRS Part Ia: Fatigue | 1.06 ± 0.95 |
| NP1URIN | MDS-UPDRS Part Ia: Urinary problems | 0.74 ± 0.94 |
| NP1SLPD | MDS-UPDRS Part Ia: Sleep problems | 1.05 ± 0.95 |
| NP1PTOT | MDS-UPDRS Part Ia: total score | 6.65 ± 4.22 |
| NP1COG | MDS-UPDRS Part Ib: Cognitive impairment | 0.46 ± 0.59 |
| NP1APAT | MDS-UPDRS Part Ib: Apathy | 0.43 ± 0.69 |
| NP1DPRS | MDS-UPDRS Part Ib: Depressed mood | 0.46 ± 0.71 |
| NP1ANXS | MDS-UPDRS Part Ib: Anxious mood | 0.52 ± 0.77 |
| NP1DDS | MDS-UPDRS Part Ib: Daytime sleepiness | 0.11 ± 0.34 |
| NP1RTOT | MDS-UPDRS Part Ib (Reported symptoms) total score | 2.08 ± 2.03 |
| NP2WALK | MDS-UPDRS Part II: Walking and balance | 0.94 ± 0.87 |
| NP2FREZ | MDS-UPDRS Part II: Freezing | 0.33 ± 0.72 |
| NP2TRMR | MDS-UPDRS Part II: Tremor | 0.98 ± 0.86 |
| NP2TURN | MDS-UPDRS Part II: Turning in bed | 0.70 ± 0.68 |
| NP2RISE | MDS-UPDRS Part II: Getting up from chair | 0.88 ± 0.79 |
| NP2SPCH | MDS-UPDRS Part II: Speech difficulties | 0.55 ± 0.83 |
| NP2DRES | MDS-UPDRS Part II: Dressing | 0.65 ± 0.71 |
| NP2PTOT | MDS-UPDRS Part II total score | 8.43 ± 6.06 |
| MCAALTTM | MoCA: Alternating trail making | 0.91 ± 0.29 |
| MCACUBE | MoCA: Cube copy | 0.73 ± 0.44 |
| MCACLCKC | MoCA: Clock contour | 0.99 ± 0.09 |
| MCACLCKN | MoCA: Clock numbers | 0.89 ± 0.31 |
| MCACLCKH | MoCA: Clock hands | 0.84 ± 0.37 |
| MCAVFNUM | MoCA: Verbal fluency—number of words | 15.24 ± 5.56 |
| MCAVF | MoCA: Verbal fluency | 0.88 ± 0.32 |
| MCASER7 | MoCA: Serial 7s (attention test) | 2.76 ± 0.60 |
| MCARHINO | MoCA: Naming (rhinoceros) | 0.96 ± 0.20 |
| Urinary | SCOPA-AUT: Urinary domain | 4.18 ± 5.18 |
| Cardio | SCOPA-AUT: Cardiovascular domain | 1.43 ± 0.93 |
| Thermoreg | SCOPA-AUT: Thermoregulation domain | 2.53 ± 2.72 |
| Pupillomotor | SCOPA-AUT: Pupillomotor domain | 0.97 ± 0.68 |
| Sexual functions | SCOPA-AUT: Sexual function domain | 7.13 ± 6.02 |
| LSIRES | Blood test: LSI result | 28.17 ± 43.78 |
| LUSRES | Blood test: LUS result | 20.72 ± 18.38 |
References
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Uitti, R.J. Rasagiline, Parkinson neuroprotection, and delayed-start trials: Still no satisfaction? Neurology 2010, 74, 1143–1148. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Azulay, J.-P.; Odin, P.; Lindvall, S.; Domingos, J.; Alobaidi, A.; Kandukuri, P.L.; Chaudhari, V.S.; Parra, J.C.; Yamazaki, T.; et al. Economic Burden of Parkinson’s Disease: A Multinational, Real-World, Cost-of-Illness Study. Drugs-Real World Outcomes 2024, 11, 1–11. [Google Scholar] [CrossRef]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of dopamine in Parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Dorsey, E.R.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. Npj Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Deal, L.S.; Flood, E.; Myers, D.E.; Devine, J.; Gray, D.L. The Parkinson’s Disease Activities of Daily Living, Interference, and Dependence Instrument. Mov. Disord. Clin. Pract. 2019, 6, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.; Marsh, L. Parkinson’s Disease: Cognitive Impairment. Focus (Madison) 2017, 15, 42–54. [Google Scholar] [CrossRef]
- Marafioti, G.; Corallo, F.; Cardile, D.; Di Lorenzo, G.; Quartarone, A.; Buono, V.L. REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review. J. Clin. Med. 2023, 12, 7397. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Keller, M.F.; Hernandez, D.G.; Chen, L.; Stone, D.J.; Singleton, A.B.; Parkinson’s Progression Marker Initiative (PPMI) investigators. Baseline genetic associations in the Parkinson’s Progression Markers Initiative (PPMI). Mov. Disord. 2016, 31, 79–85. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Surasi, D.S.; Peller, P.J.; Szabo, Z.; Mercier, G.; Subramaniam, R.M. Dopamine transporter SPECT imaging in parkinson disease and dementia. PET Clin. 2013, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Simuni, T.; Uribe, L.; Cho, H.R.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; Singleton, A.; et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): A cross-sectional study. Lancet Neurol. 2020, 19, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Simuni, T.; Brumm, M.C.; Uribe, L.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Alcalay, R.N.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Leucine Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s Disease Participants in the Parkinson’s Progression Markers Initiative: A Cross-Sectional Study. Mov. Disord. 2020, 35, 833–844. [Google Scholar] [CrossRef]
- Sun, X.; Dou, K.; Xue, L.; Xie, Y.; Yang, Y.; Xie, A. Comprehensive analysis of clinical and biological features in Parkinson’s disease associated with the LRRK2 G2019S mutation: Data from the PPMI study. Clin. Transl. Sci. 2024, 17, e13720. [Google Scholar] [CrossRef]
- Simuni, T.; Merchant, K.; Brumm, M.C.; Cho, H.; Caspell-Garcia, C.; Coffey, C.S.; Chahine, L.M.; Alcalay, R.N.; Nudelman, K.; Foroud, T.; et al. Longitudinal clinical and biomarker characteristics of non-manifesting LRRK2 G2019S carriers in the PPMI cohort. Npj Park. Dis. 2022, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Macklin, E.A.; Logan, R.; Zorlu, M.M.; Xia, N.; Crotty, G.F.; Zhang, E.; Chen, X.; Ascherio, A.; Schwarzschild, M.A. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann. Neurol. 2019, 85, 593–599. [Google Scholar] [CrossRef]
- Bougea, A.; Koros, C.; Papagiannakis, N.; Simitsi, A.-M.; Prentakis, A.; Papadimitriou, D.; Pachi, I.; Antonelou, R.; Angelopoulou, E.; Beratis, I.; et al. Serum Uric Acid in LRRK2 Related Parkinson’s Disease: Longitudinal Data from the PPMI Study. J. Park. Dis. 2021, 11, 633–640. [Google Scholar] [CrossRef]
- Chang, B.; Jiang, Y.; Feng, C.; Li, B.; Mei, J.; Niu, C. Effect of purine diet on prognosis of deep brain stimulation for Parkinson’s disease. Food Sci. Hum. Wellness 2025, 14, 9250066. [Google Scholar] [CrossRef]
- Merchant, K.M.; Simuni, T.; Fedler, J.; Caspell-Garcia, C.; Brumm, M.; Nudelman, K.N.H.; Tengstrandt, E.; Hsieh, F.; Alcalay, R.N.; Coffey, C.; et al. LRRK2 and GBA1 variant carriers have higher urinary bis(monacylglycerol) phosphate concentrations in PPMI cohorts. Npj Park. Dis. 2023, 9, 30. [Google Scholar] [CrossRef]
- Huh, Y.E.; Park, H.; Chiang, M.S.R.; Tuncali, I.; Liu, G.; Locascio, J.J.; Shirvan, J.; Hutten, S.J.; Rotunno, M.S.; Viel, C.; et al. Glucosylceramide in cerebrospinal fluid of patients with GBA-associated and idiopathic Parkinson’s disease enrolled in PPMI. Npj Park. Dis. 2021, 7, 102. [Google Scholar] [CrossRef]
- Kmiecik, M.J.; Micheletti, S.; Coker, D.; Heilbron, K.; Shi, J.; Stagaman, K.; Sonmez, T.F.; Fontanillas, P.; Shringarpure, S.; Wetzel, M.; et al. Genetic analysis and natural history of Parkinson’s disease due to the LRRK2 G2019S variant. Brain 2024, 147, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.H.; Lee, J.Y.; Han, S.-K.; Song, Y.S. Motor subtypes and clinical characteristics in sporadic and genetic Parkinson’s disease groups: Analysis of the PPMI cohort. Front. Neurol. 2023, 14, 1276251. [Google Scholar] [CrossRef] [PubMed]
- Thaler, A.; Livne, V.; Rubinstein, E.; Omer, N.; Faust-Socher, A.; Cohen, B.; Giladi, N.; Shirvan, J.C.; Cedarbaum, J.M.; Gana-Weisz, M.; et al. Mild cognitive impairment among LRRK2 and GBA1 patients with Parkinson’s disease. Park. Relat. Disord. 2024, 123, 106970. [Google Scholar] [CrossRef]
- Yu, C.H.; Rodriguez-Porcel, F.; Wilson, S.; Lench, D.H.; Cooper, C.A. Genetic influence on microstructure integrity and motor progression in Parkinson’s disease. Park. Relat. Disord. 2024, 127, 107082. [Google Scholar] [CrossRef]
- Lee, M.J.; Pak, K.; Kim, H.-K.; Nudelman, K.N.; Kim, J.H.; Kim, Y.H.; Kang, J.; Baek, M.S.; Lyoo, C.H. Genetic factors affecting dopaminergic deterioration during the premotor stage of Parkinson disease. Npj Park. Dis. 2021, 7, 104. [Google Scholar] [CrossRef]
- Jin, R.; Yoshioka, H.; Sato, H.; Hisaka, A. Data-driven disease progression model of Parkinson’s disease and effect of sex and genetic variants. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 649–659. [Google Scholar] [CrossRef]
- Teipel, S.J.; Hoffmann, H.; Storch, A.; Hermann, A.; Dyrba, M.; Schumacher, J. Brain age in genetic and idiopathic Parkinson’s disease. Brain Commun. 2024, 6, fcae382. [Google Scholar] [CrossRef]
- Schumacher, J.G.; Zhang, X.; Macklin, E.A.; Wang, J.; Bayati, A.; Dijkstra, J.M.; Watanabe, H.; Schwarzschild, M.A.; Cortese, M.; Zhang, X.; et al. Baseline α-synuclein seeding activity and disease progression in sporadic and genetic Parkinson’s disease in the PPMI cohort. EBioMedicine 2024, 119, 105866. [Google Scholar] [CrossRef]
- Chahine, L.M.; Lafontant, D.E.; Choi, S.H.; Iwaki, H.; Blauwendraat, C.; Singleton, A.B.; Brumm, M.C.; Alcalay, R.N.; Merchant, K.; Nudelman, K.N.H.; et al. LRRK2-associated parkinsonism with and without in vivo evidence of alpha-synuclein aggregates: Longi-tudinal clinical and biomarker characterization. Brain Commun. 2025, 7, fcaf103. [Google Scholar] [CrossRef]
- Pachi, I.; Koros, C.; Simitsi, A.M.; Papadimitriou, D.; Bougea, A.; Prentakis, A.; Papagiannakis, N.; Bozi, M.; Antonelou, R.; Angelopoulou, E.; et al. Apathy: An underestimated feature in GBA and LRRK2 non-manifesting mutation carriers. Park. Relat. Disord. 2021, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mihaescu, A.S.; Valli, M.; Uribe, C.; Diez-Cirarda, M.; Masellis, M.; Graff-Guerrero, A.; Strafella, A.P. Beta amyloid deposition and cognitive decline in Parkinson’s disease: A study of the PPMI cohort. Mol. Brain 2022, 15, 79. [Google Scholar] [CrossRef]
- Srivatsal, S.; Cholerton, B.; Leverenz, J.B.; Wszolek, Z.K.; Uitti, R.J.; Dickson, D.W.; Weintraub, D.; Trojanowski, J.Q.; Van Deerlin, V.M.; Quinn, J.F.; et al. Cognitive profile of LRRK2-related Parkinson’s disease. Mov. Disord. 2015, 30, 728–733. [Google Scholar] [CrossRef]
- Saunders-Pullman, R.; Mirelman, A.; Alcalay, R.N.; Wang, C.; Ortega, R.A.; Raymond, D.; Mejia-Santana, H.; Orbe-Reilly, M.; Johannes, B.A.; Thaler, A.; et al. Progression in the LRRK2-Asssociated Parkinson disease population. JAMA Neurol. 2018, 75, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Melrose, H. Update on the functional biology of Lrrk2. Futur. Neurol. 2008, 3, 669–681. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E.; Hazrati, L.-N.; Fujioka, S.; Wszolek, Z.K.; Dickson, D.W.; Ross, O.A.; Van Deerlin, V.M.; Trojanowski, J.Q.; Hurtig, H.I.; et al. Clinical correlations with lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 2015, 72, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.R.; Lee, B.D. Pathological functions of lrrk2 in parkinson’s disease. Cells 2020, 9, 2565. [Google Scholar] [CrossRef]
- Siderowf, A.; Concha-Marambio, L.; Lafontant, D.-E.; Farris, C.M.; Ma, Y.; AUrenia, P.; Nguyen, H.; Alcalay, R.N.; Chahine, L.M.; Foroud, T.; et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: A cross-sectional study. Lancet Neurol. 2023, 22, 407–417. [Google Scholar] [CrossRef]
- Bega, D.; Kuo, P.H.; Chalkidou, A.; Grzeda, M.T.; Macmillan, T.; Brand, C.; Sheikh, Z.H.; Antonini, A. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: A systematic review and meta-analysis. Npj Park. Dis. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, C.; Ma, J.; Yang, R.; Qi, Q.; Gao, Z.; Du, T.; Zhang, P.; Li, Y.; Cai, M.; et al. Motor progression trajectories and risk of mild cognitive impairment in Parkinson’s disease: A latent class tra-jectory model from PPMI cohort. CNS Neurosci. Ther. 2024, 30, e14918. [Google Scholar] [CrossRef]
- Ahamadi, M.; Mehrotra, N.; Hanan, N.; Lai Yee, K.; Gheyas, F.; Anton, J.; Bani, M.; Boroojerdi, B.; Smit, H.; Weidemann, J.; et al. A Disease Progression Model to Quantify the Nonmotor Symptoms of Parkinson’s Disease in Participants With Leucine-Rich Repeat Kinase 2 Mutation. Clin. Pharmacol. Ther. 2021, 110, 508–518. [Google Scholar] [CrossRef]
- Gallagher, J.; Gochanour, C.; Caspell-Garcia, C.; Dobkin, R.D.; Aarsland, D.; Alcalay, R.N.; Barrett, M.J.; Chahine, L.; Chen-Plotkin, A.S.; Coffey, C.S.; et al. Long-Term Dementia Risk in Parkinson Disease. Neurology 2024, 103, e209699. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Budescu, D.V. Dominance analysis: A new approach to the problem of relative importance of predictors in multiple regression. Psychol. Bull. 1993, 114, 542. [Google Scholar] [CrossRef]
- Azen, R.; Budescu, D.V. The Dominance Analysis Approach for Comparing Predictors in Multiple Regression. Psychol. Methods 2003, 8, 129–148. [Google Scholar] [CrossRef]
- Laird, N.M.; Ware, J.H. Random-Effects Models for Longitudinal Data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, J.T.; Perepezko, K.; Mills, K.A.; Mari, Z.; Butala, A.; Dawson, T.M.; Pantelyat, A.; Rosenthal, L.S.; Pontone, G.M. Dopamine transporter availability reflects gastrointestinal dysautonomia in early Parkinson disease. Park. Relat. Disord. 2018, 55, 8–14. [Google Scholar] [CrossRef]
- Seibyl, J.P.; Kuo, P. What Is the Role of Dopamine Transporter Imaging in Parkinson Prevention Clinical Trials? Neurology 2022, 99, 61–67. [Google Scholar] [CrossRef]
- Brooks, D.J. Imaging approaches to Parkinson disease. J. Nucl. Med. 2010, 51, 596–609. [Google Scholar] [CrossRef]
- Barber, T.R.; Klein, J.C.; Mackay, C.E.; Hu, M.T. Neuroimaging in pre-motor Parkinson’s disease. NeuroImage Clin. 2017, 15, 215–227. [Google Scholar] [CrossRef]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; van Hilten, J.J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord. 2004, 19, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef]
- Sidransky, E.; Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012, 11, 986–998. [Google Scholar] [CrossRef]
- Zhang, H.; He, K.; Zhao, Y.; Peng, Y.; Feng, D.; Wang, J.; Gao, Q. fNIRS Biomarkers for Stratifying Poststroke Cognitive Impairment: Evidence From Frontal and Temporal Cortex Activation. Stroke 2025. [Google Scholar] [CrossRef]
- Wan, L.; Pei, P.; Zhang, Q.; Gao, W. Specificity in the commonalities of inhibition control: Using meta-analysis and regression analysis to identify the key brain regions in psychiatric disorders. Eur. Psychiatry 2024, 67, e69. [Google Scholar] [CrossRef]
- Ou, J.; Li, N.; He, H.; He, J.; Zhang, L.; Jiang, N. Detecting muscle fatigue among community-dwelling senior adults with shape features of the probability density function of sEMG. J. Neuroeng. Rehabil. 2024, 21, 196. [Google Scholar] [CrossRef]
- Li, N.; Ou, J.; He, H.; He, J.; Zhang, L.; Peng, Z.; Zhong, J.; Jiang, N. Exploration of a machine learning approach for diagnosing sarcopenia among Chinese community-dwelling older adults using sEMG-based data. J. Neuroeng. Rehabil. 2024, 21, 69. [Google Scholar] [CrossRef]
- Hu, B.; Wang, X.; Lu, S.; Ying, X. A study of bidirectional control of Parkinson’s beta oscillations by basal ganglia. Chaos Solitons Fractals 2025, 195, 116267. [Google Scholar] [CrossRef]
- Zhu, C. Computational intelligence-based classification system for the diagnosis of memory impairment in psychoactive substance users. J. Cloud Comput. 2024, 13, 119. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Singleton, A.; Shaw, L.M.; Trojanowski, J.Q.; Frasier, M.; Simuni, T.; Iranzo, A.; et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 2019, 34, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Surova, Y.; Öhrfelt, A.; Zetterberg, H.; Lindqvist, D.; Hansson, O. CSF biomarkers and clinical progression of Parkinson disease. Neurology 2015, 84, 57–63. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).