Performance Evaluation of Hybrid and Conventional Coagulants for the Removal of Sunset Yellow and Methylene Violet Dyes from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Coagulants
2.2.1. Preparation of AlCl3 Solution
2.2.2. Preparation of CS Solution
2.2.3. Preparation of Polysilicate Acid Solution
2.2.4. Preparation of Hybrid CS@Al Coagulants
2.2.5. Preparation of Hybrid Al/pSi Coagulants
2.2.6. Preparation of Hybrid CS@Al/pSi Coagulants
2.3. Coagulation Performance
2.3.1. Jar-Tests
2.3.2. Analytical Determinations
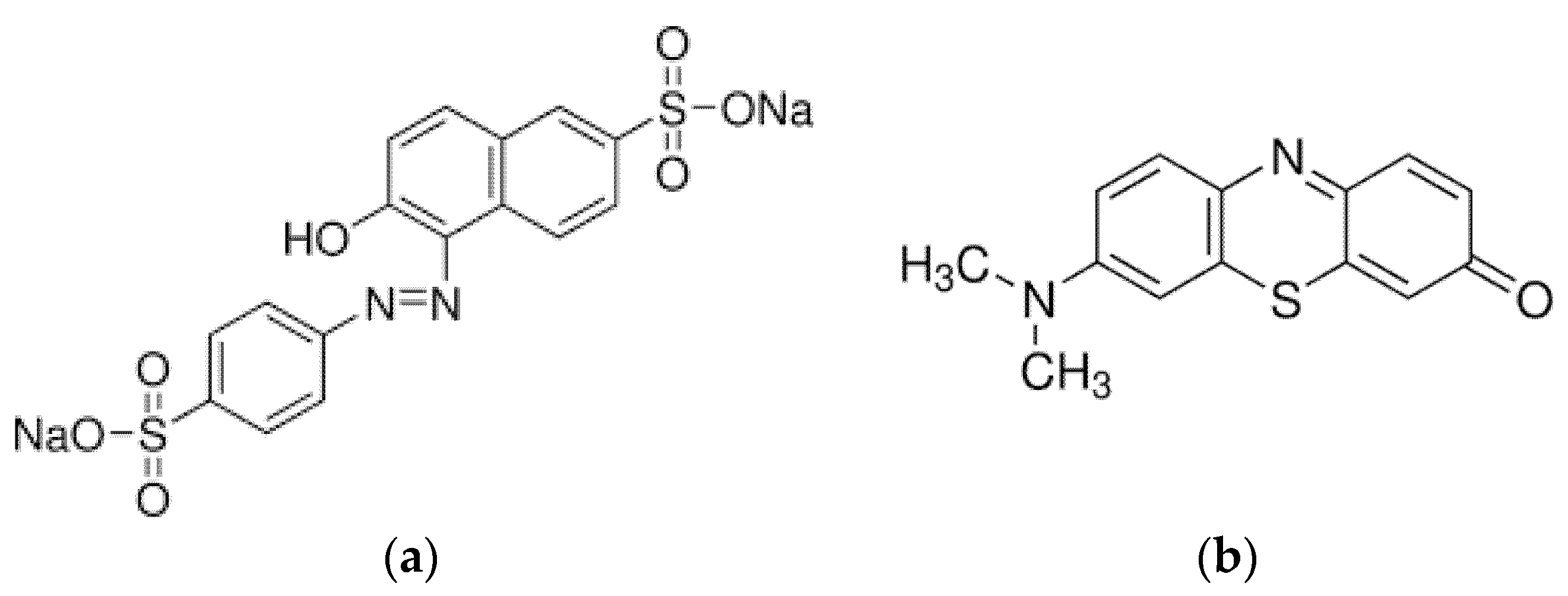
2.3.3. Dye Removal Evaluation
2.4. Characterization Techniques
3. Results and Discussion
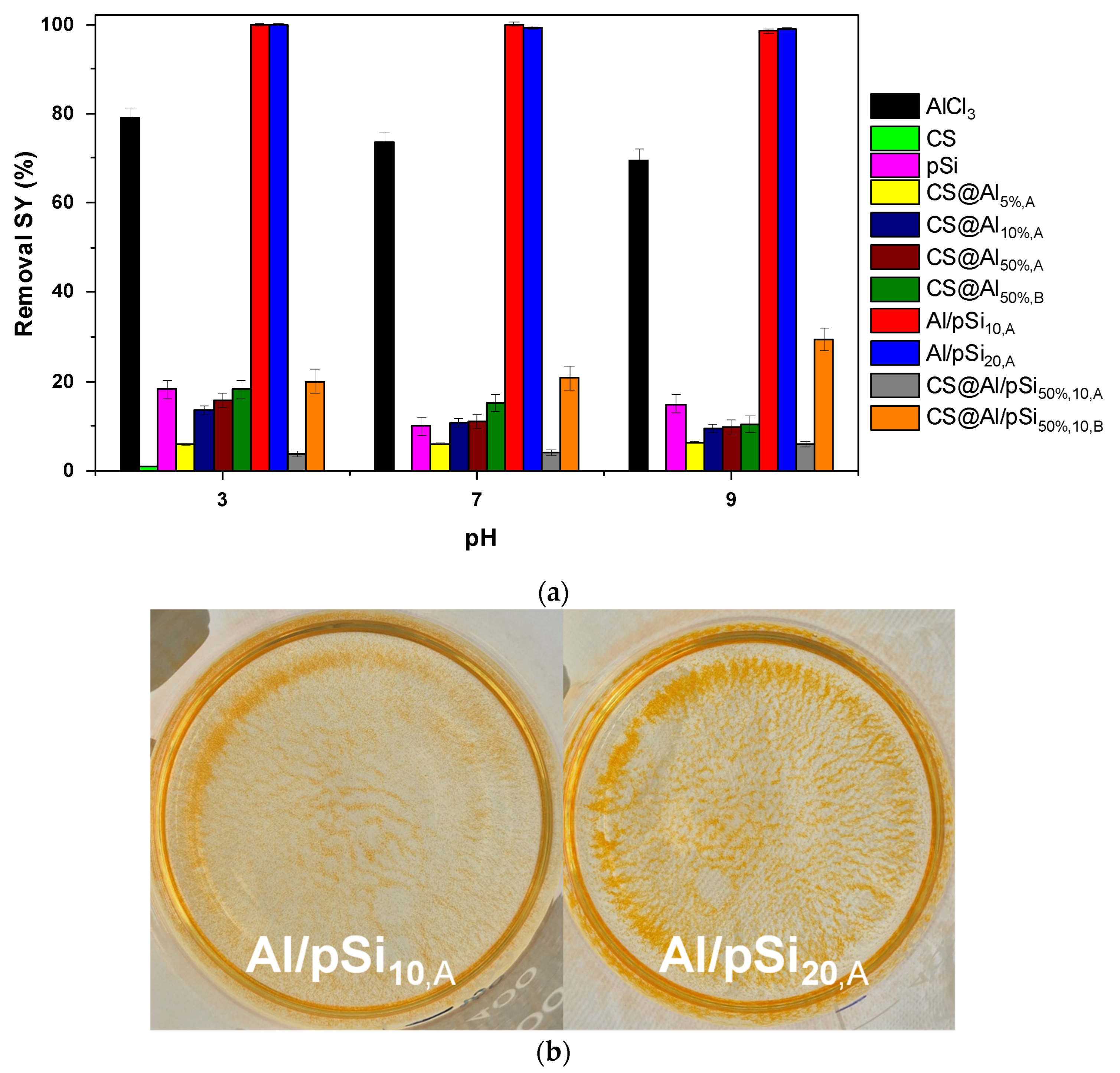
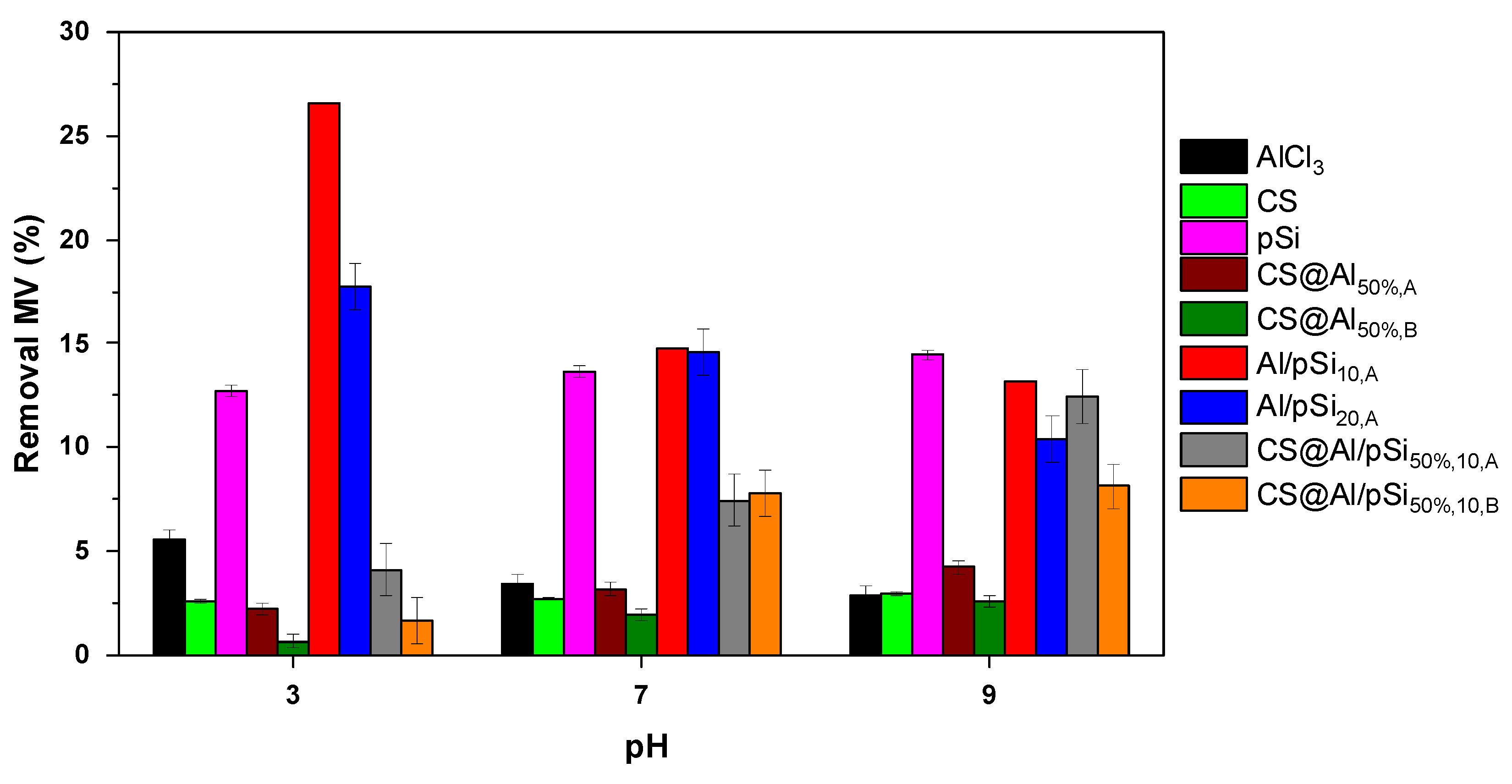
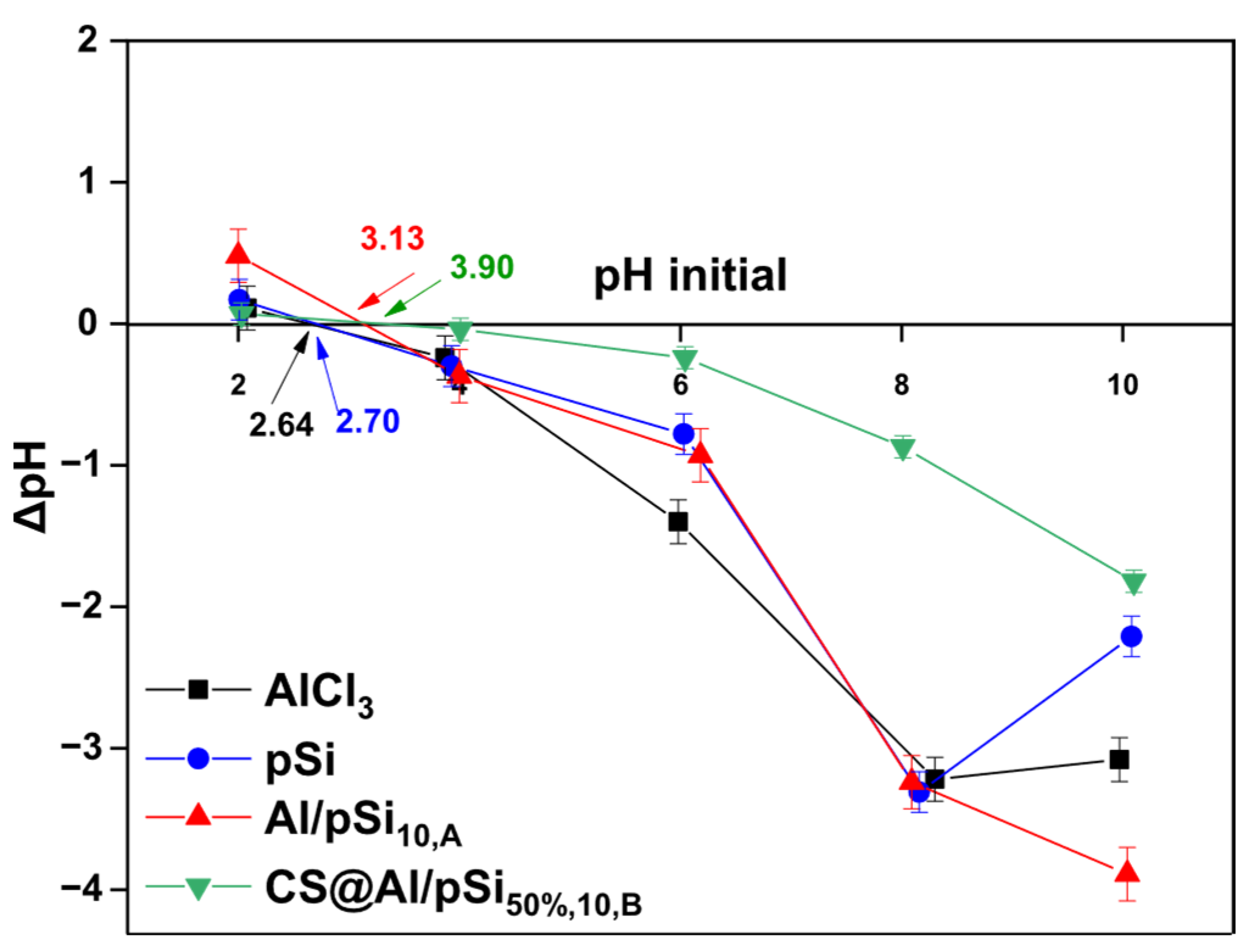
3.1. Effect of pH and Comparison of Coagulants
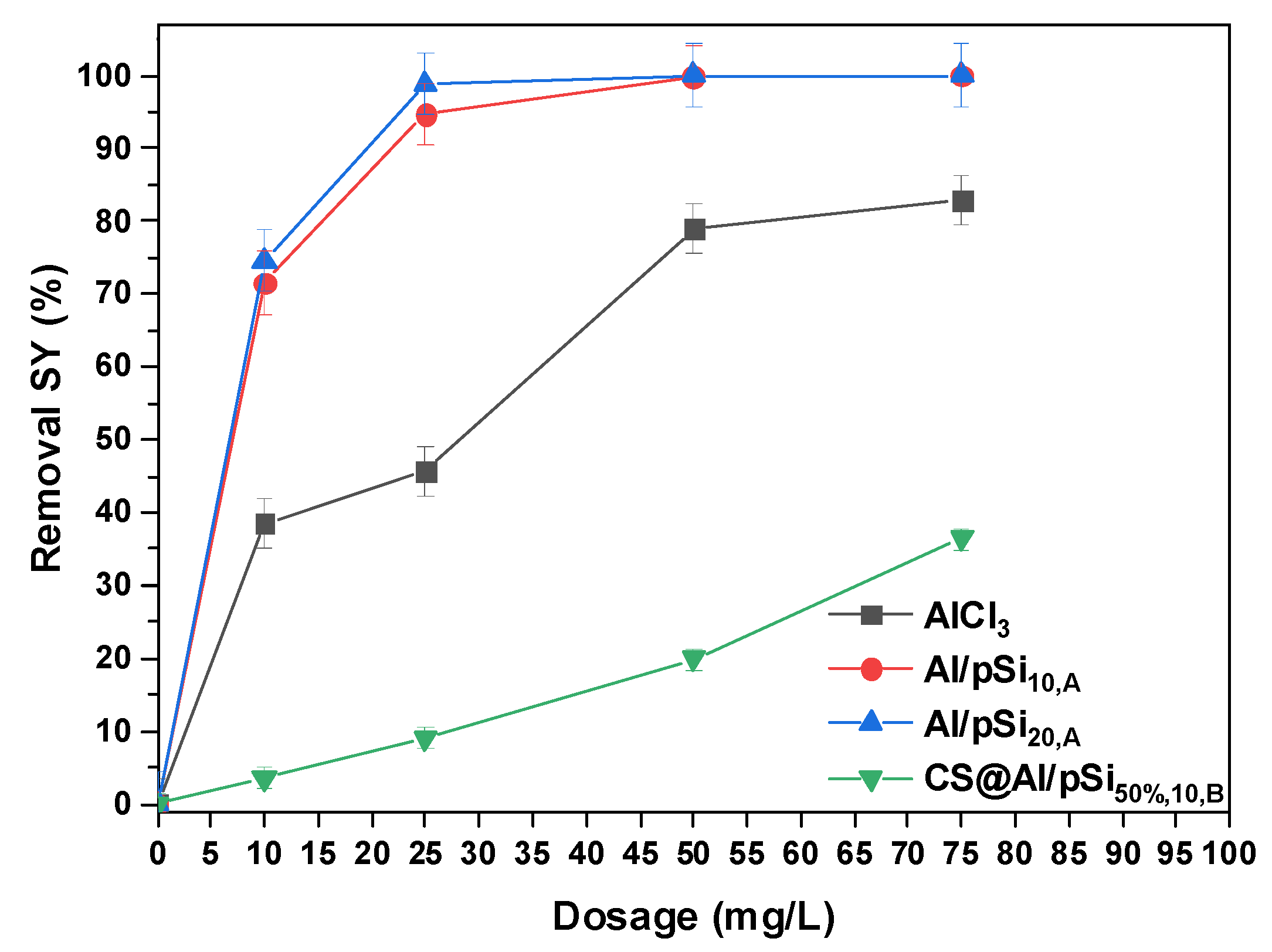
3.2. Effect of Dosage
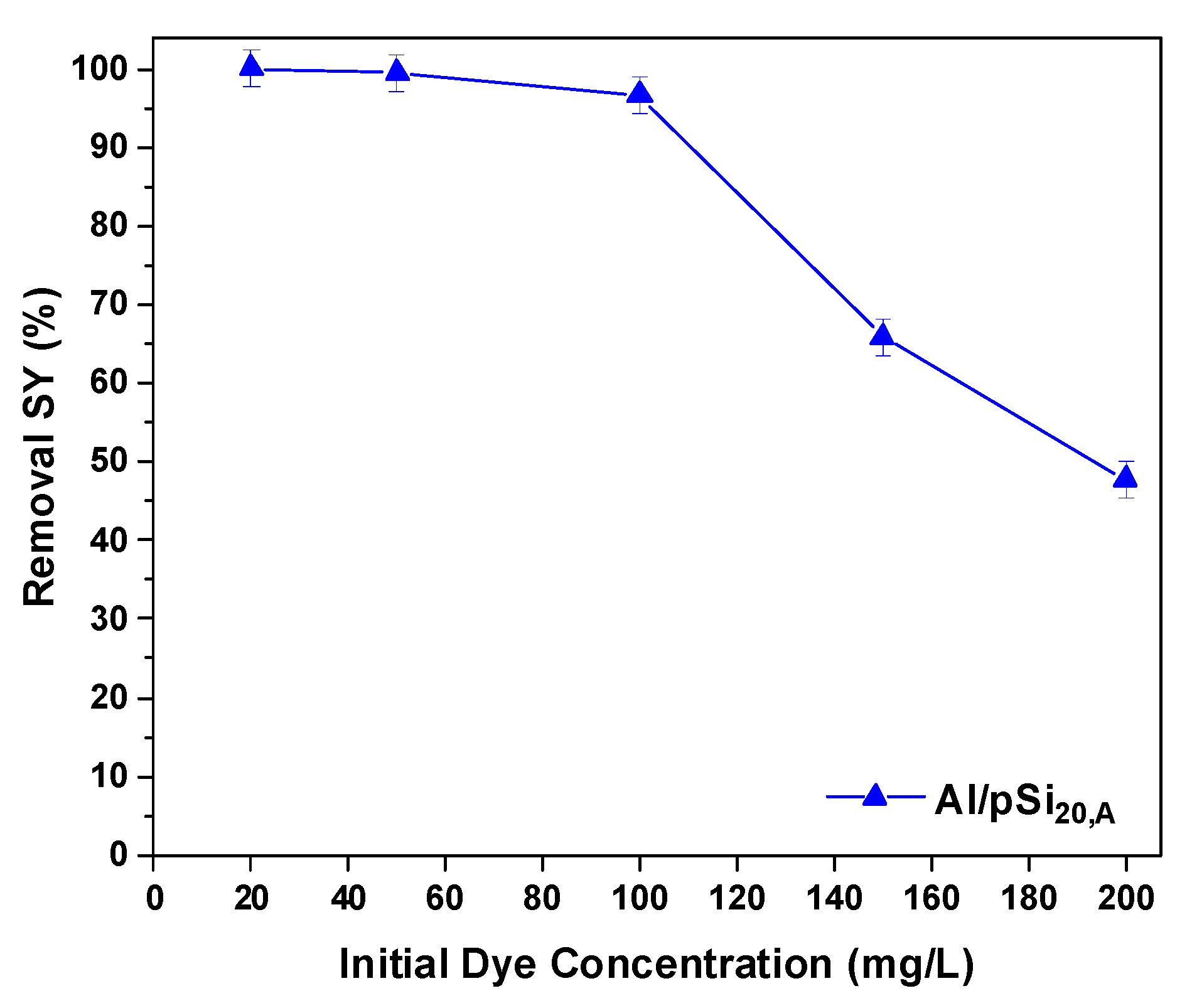
3.3. Effect of Initial Dye Concentration
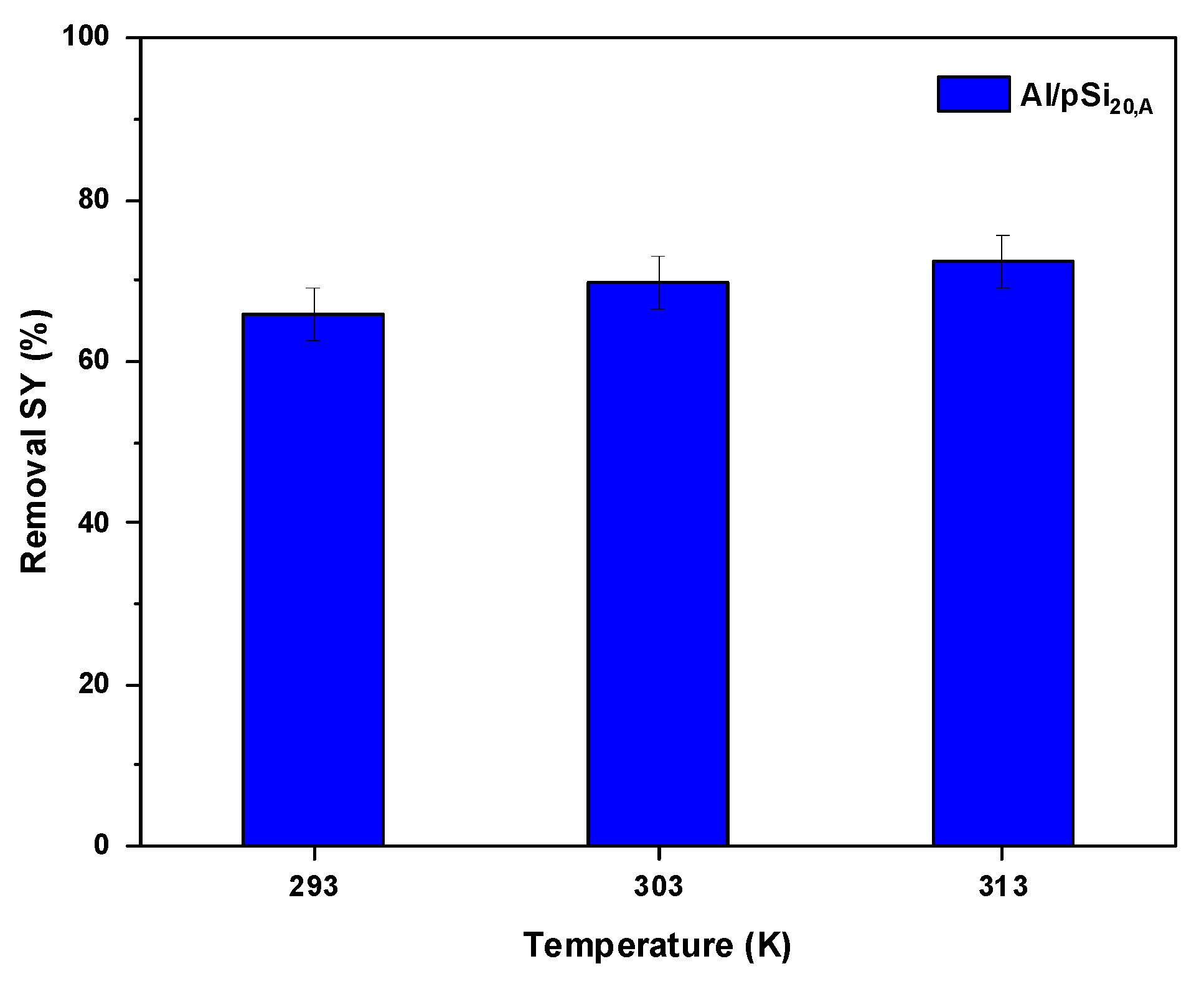
3.4. Effect of Temperature
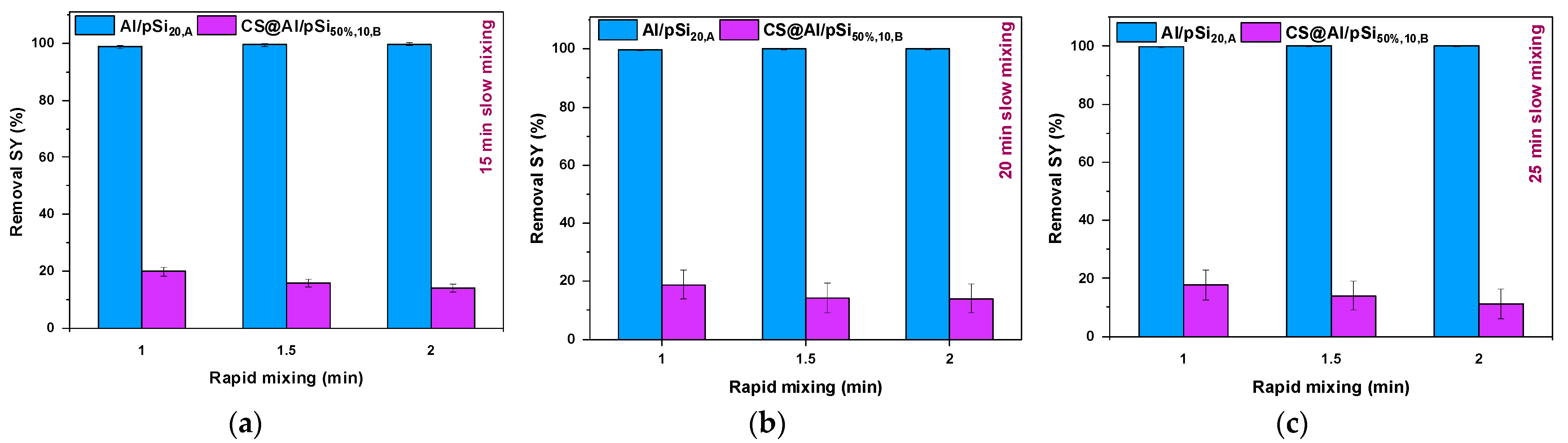
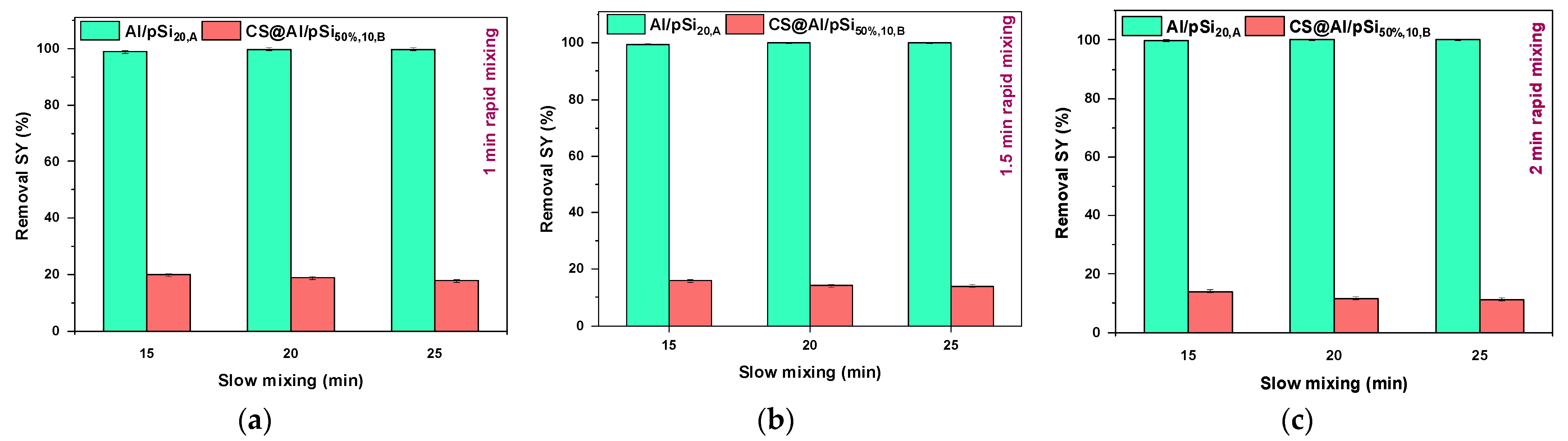
3.5. Effect of Mixing Time
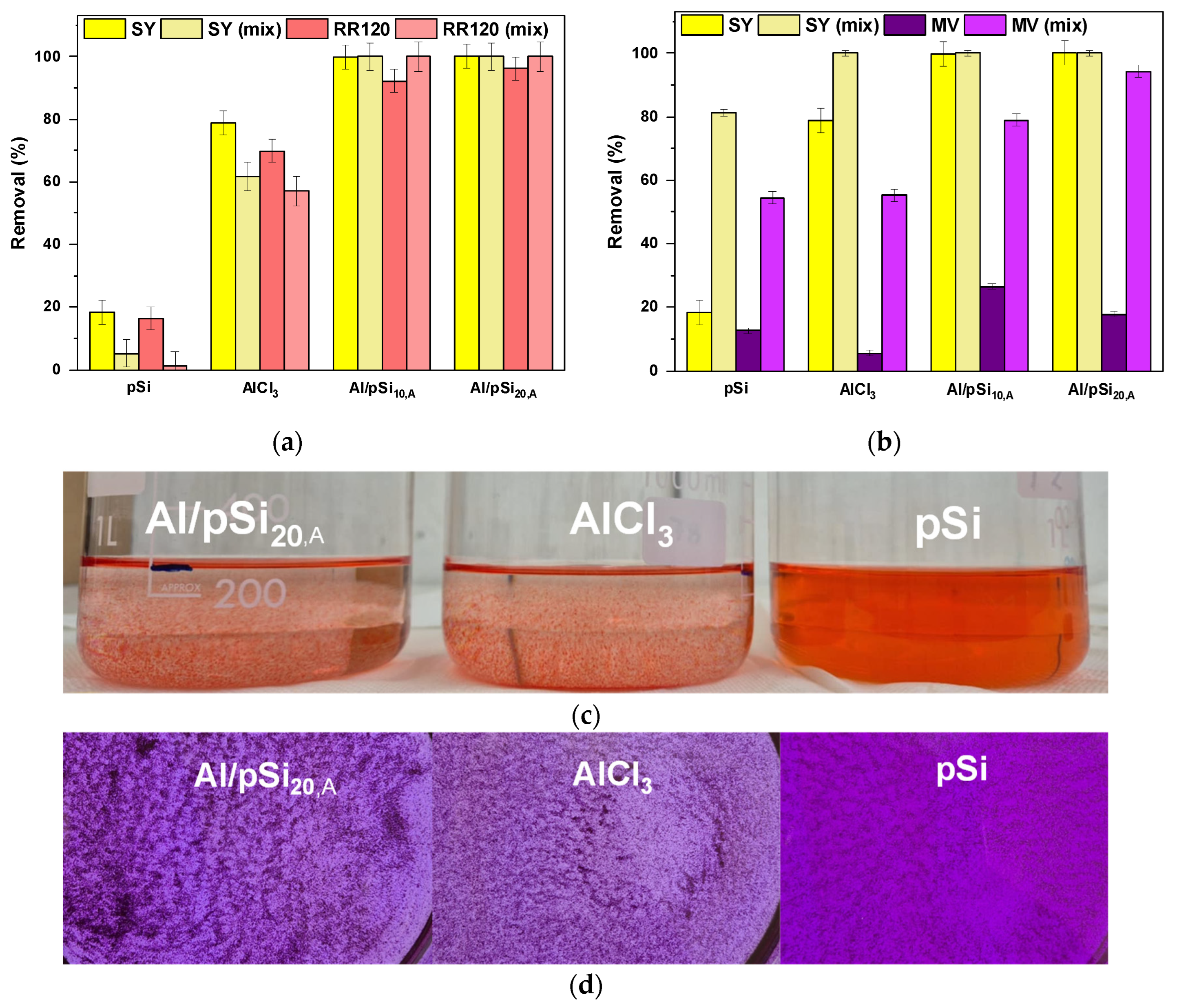
3.6. Mixed Dye Solutions
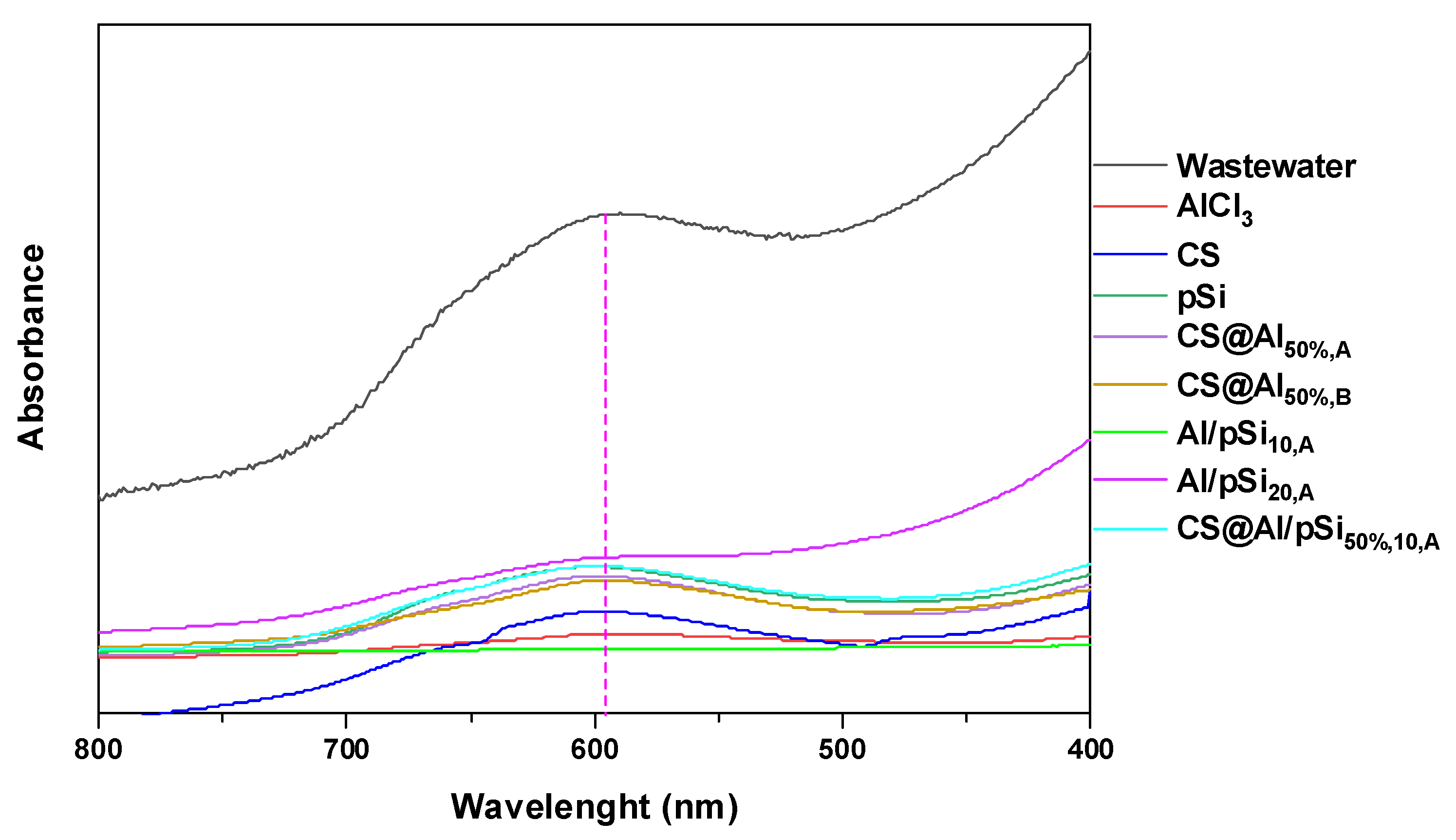
3.7. Real Wastewater Samples
3.8. Comparison with the Literature
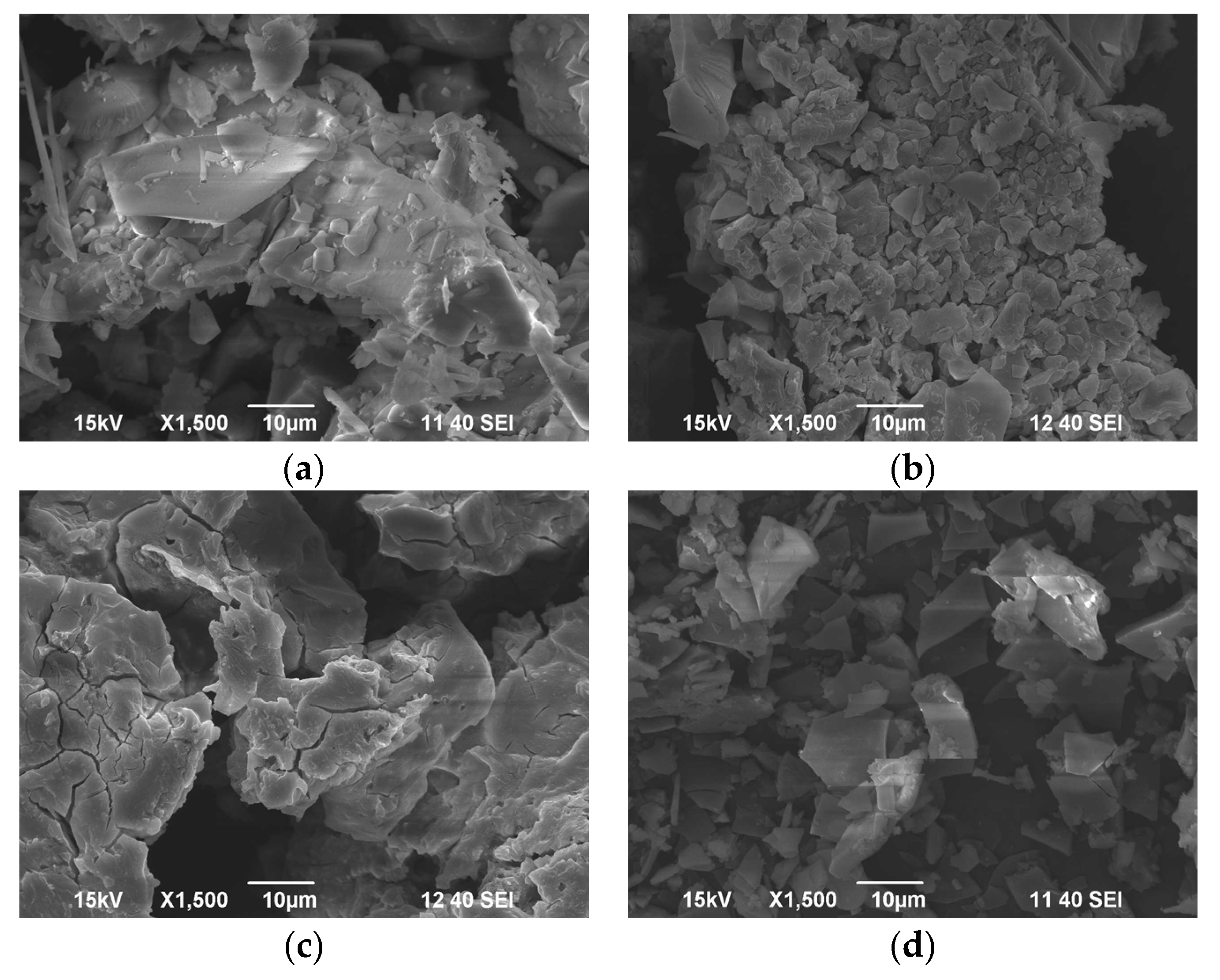
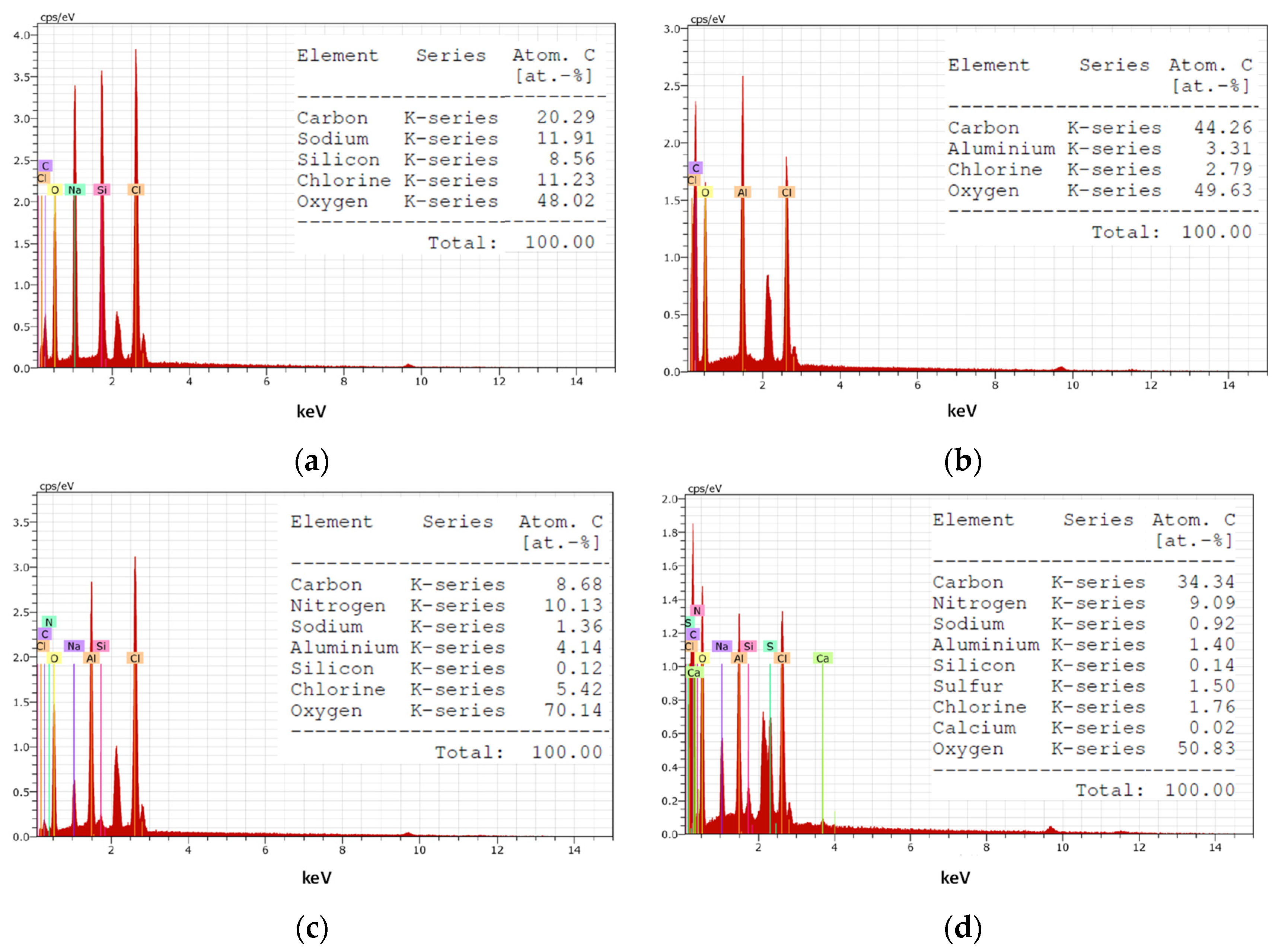
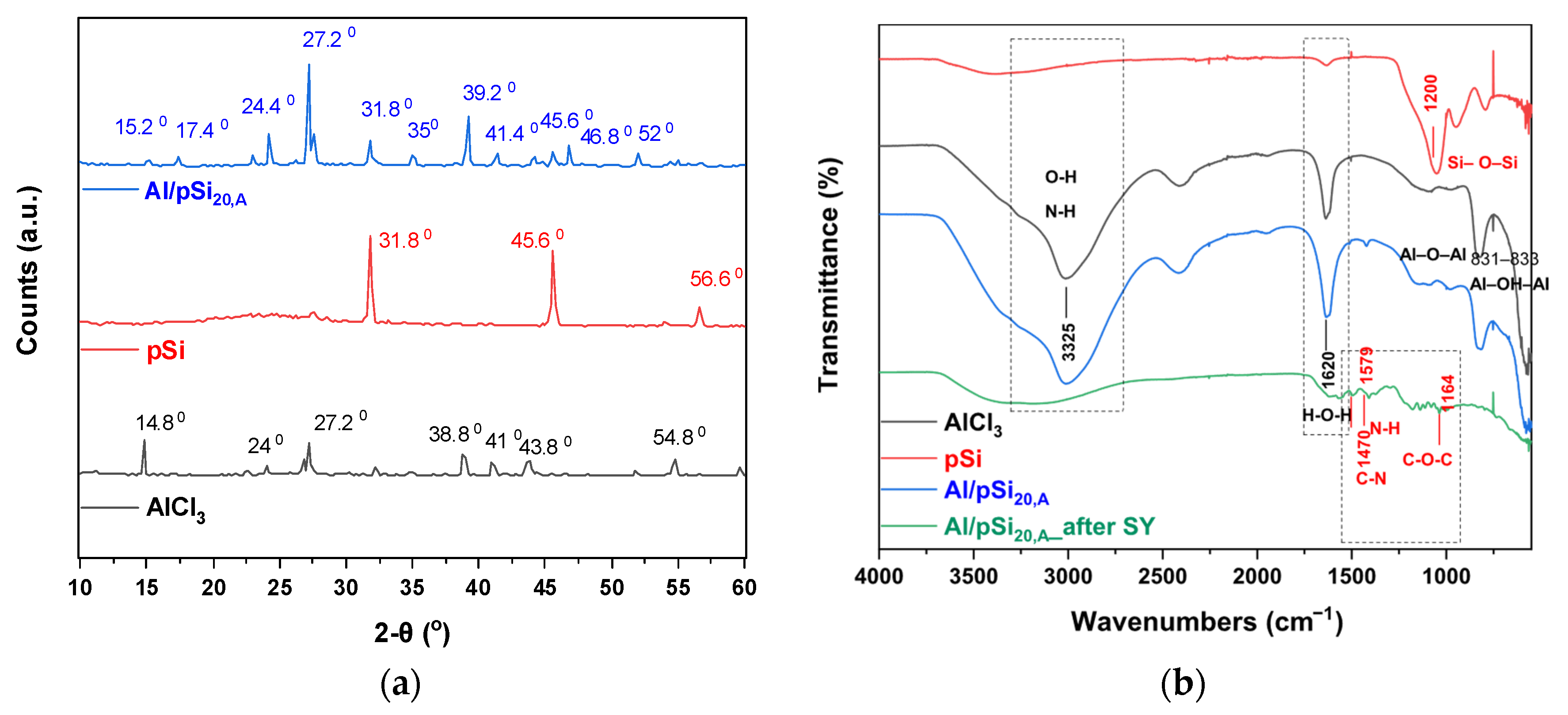
3.9. Characterization of the Coagulants
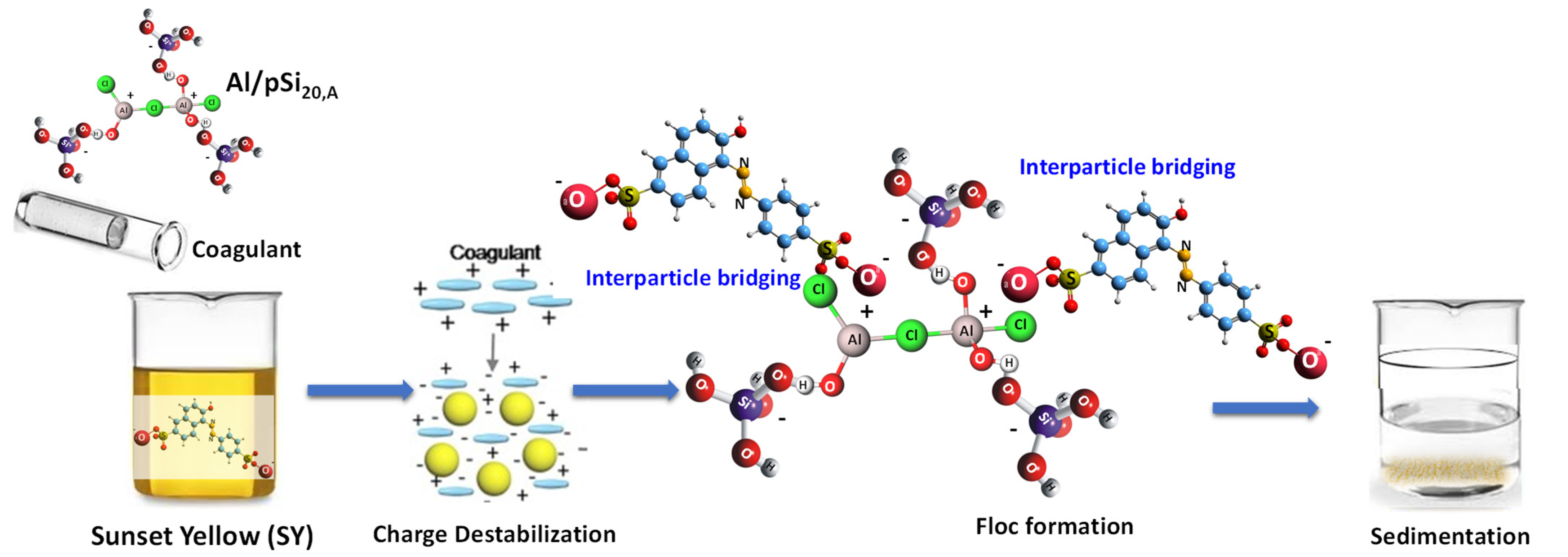
3.10. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Sadiku, M.; Selimi, T.; Berisha, A.; Maloku, A.; Mehmeti, V.; Thaçi, V.; Hasani, N. Removal of Methyl Violet from Aqueous Solution by Adsorption onto Halloysite Nanoclay: Experiment and Theory. Toxics 2022, 10, 445. [Google Scholar] [CrossRef]
- Micheletti, D.H.; da Silva Andrade, J.G.; Porto, C.E.; Alves, B.H.M.; de Carvalho, F.R.; Sakai, O.A.; Batistela, V.R. A Review of Adsorbents for Removal of Yellow Tartrazine Dye from Water and Wastewater. Bioresour. Technol. Rep. 2023, 24, 101598. [Google Scholar] [CrossRef]
- Abbey, J.; Fields, B.; O’Mullane, M.; Tomaska, L.D. Food Additives: Colorants. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 459–465. ISBN 978-0-12-378613-5. [Google Scholar]
- Alsuhybani, M.; Aleid, M.; Alzidan, R.; Bin Bander, K.; Alrehaili, A. High Removal of Methylene Blue and Methyl Violet Dyes from Aqueous Solutions Using Efficient Biomaterial Byproduct. Heliyon 2024, 10, e36731. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Role of Fly Ash in the Removal of Organic Pollutants from Wastewater. Energy Fuels 2009, 23, 1494–1511. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Khan, M.R.; Ng, K.H.; Wongsakulphasatch, S.; Cheng, C.K. Harnessing Renewable Hydrogen-Rich Syngas from Valorization of Palm Oil Mill Effluent (POME) Using Steam Reforming Technique. Renew. Energy 2019, 138, 1114–1126. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, S.; Zhu, Y.; Jin, J. Alkaline-Induced Superhydrophilic/Underwater Superoleophobic Polyacrylonitrile Membranes with Ultralow Oil-Adhesion for High-Efficient Oil/Water Separation. J. Memb. Sci. 2016, 513, 67–73. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Ultrasonication Pre-Treatment of Combined Effluents from Palm Oil, Pulp and Paper Mills for Improving Photofermentative Biohydrogen Production. Energy Convers. Manag. 2016, 119, 142–150. [Google Scholar] [CrossRef]
- Ariffin, N.; Mustafa, M.; Bakri, A.; Remy, M.; Mohd, R.; Zaino, A.; Murshed, M.F.; Faris, M.A. Review on Adsorption of Heavy Metal in Wastewater by Using Geopolymer. MATEC Web Conf. 2017, 97, 01023. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the Art and Sustainability of Natural Coagulants in Water and Wastewater Treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Zhao, J.; Feng, N. Removal of Reactive Dyes from Wastewater Assisted with Kaolin Clay by Magnesium Hydroxide Coagulation Process. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 222–227. [Google Scholar] [CrossRef]
- Mcyotto, F.; Wei, Q.; Macharia, D.K.; Huang, M.; Shen, C.; Chow, C.W.K. Effect of Dye Structure on Color Removal Efficiency by Coagulation. Chem. Eng. J. 2021, 405, 126674. [Google Scholar] [CrossRef]
- Albahnasawi, A. Removal of Reactive Red 141 and Disperse Red 13 Dyes from Aqueous Solutions Using Different Coagulants: An Optimization and Comparison Study. Düzce Üniversitesi Bilim. Teknol. Derg. 2023, 11, 1269–1281. [Google Scholar] [CrossRef]
- Jalal, G.; Abbas, N.; Deeba, F.; Butt, T.; Jilal, S.; Sarfraz, S. Efficient Removal of Dyes in Textile Effluents Using Aluminum-Based Coagulant. Chem. Int. 2021, 7, 197–207. [Google Scholar]
- Wei, Q.; Zhang, Y.; Zhang, K.; Mwasiagi, J.I.; Zhao, X.; Chow, C.W.K.; Tang, R. Removal of Direct Dyes by Coagulation: Adaptability and Mechanism Related to the Molecular Structure. Korean J. Chem. Eng. 2022, 39, 1850–1862. [Google Scholar] [CrossRef]
- Bouyakoub, A.Z.; Lartiges, B.S.; Ouhib, R.; Kacha, S.; El Samrani, A.G.; Ghanbaja, J.; Barres, O. MnCl2 and MgCl2 for the Removal of Reactive Dye Levafix Brilliant Blue EBRA from Synthetic Textile Wastewaters: An Adsorption/Aggregation Mechanism. J. Hazard. Mater. 2011, 187, 264–273. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Giannoulaki, A.; Chalkidi, P.; Arvaniti, E.; Fykari, S.; Kritaki, S.; Kyzas, G.Z. Removal of Anionic and Cationic Dyes from Wastewater by Tetravalent Tin-Based Novel Coagulants. Processes 2025, 13, 2103. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Tzoupanos, N. Alternative Cost-Effective Preparation Method of Polyaluminium Chloride (PAC) Coagulant Agent: Characterization and Comparative Application for Water/Wastewater Treatment. Desalination 2010, 250, 339–344. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Tzollas, N.M.; Tolkou, A.K.; Mitrakas, M.; Ernst, M.; Zouboulis, A.I. Use of Novel Composite Coagulants for Arsenic Removal from Waters-Experimental Insight for the Application of Polyferric Sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Mitrakas, M.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Fluoride Removal from Water by Composite Al/Fe/Si/Mg Pre-Polymerized Coagulants: Characterization and Application. Chemosphere 2019, 231, 528–537. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Tzoupanos, N.D. Polyaluminium Silicate Chloride-A Systematic Study for the Preparation and Application of an Efficient Coagulant for Water or Wastewater Treatment. J. Hazard. Mater. 2009, 162, 1379–1389. [Google Scholar] [CrossRef]
- Gao, B.Y.; Hahn, H.H.; Hoffmann, E. Evaluation of Aluminum-Silicate Polymer Composite as a Coagulant for Water Treatment. Water Res. 2002, 36, 3573–3581. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N. Chitosan as Natural Coagulant in Hybrid Coagulation-Nanofiltration Membrane Process for Water Treatment. J. Environ. Chem. Eng. 2016, 4, 4857–4862. [Google Scholar] [CrossRef]
- Guibal, E.; Roussy, J. Coagulation and Flocculation of Dye-Containing Solutions Using a Biopolymer (Chitosan). React. Funct. Polym. 2007, 67, 33–42. [Google Scholar] [CrossRef]
- Rajabhoj, R.; Rathi, T.; Saravanan, D.; Jugade, R. A Novel Approach towards Chitosan Application Aiming Unprecedented Adsorption Efficacy in Abatement of Dyes. J. Indian Chem. Soc. 2025, 102, 101982. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Shi, F.; Cui, Y.; Wang, J.; Zhang, S.; Zhu, J.; Liu, Q. Chitosan Aided Polyaluminium Ferric Silicate (PAFS) Coagulant for Treatment of Wool Scouring Wastewater. J. Environ. Chem. Eng. 2024, 12, 113662. [Google Scholar] [CrossRef]
- Cen, X.; Li, J.; Jiang, G.; Zheng, M. A Critical Review of Chemical Uses in Urban Sewer Systems. Water Res. 2023, 240, 120108. [Google Scholar] [CrossRef] [PubMed]
- Dago-Serry, Y.; Maroulas, K.N.; Tolkou, A.K.; AbdelAll, N.; Alodhayb, A.N.; Khouqeer, G.A.; Kyzas, G.Z. Composite Super-Adsorbents of Chitosan/Activated Carbon for the Removal of Nonsteroidal Anti-Inflammatory Drug from Wastewaters. J. Mol. Struct. 2024, 1298, 137044. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Synthesis and Coagulation Performance of Composite Poly-Aluminum-Ferric-Silicate-Chloride Coagulants in Water and Wastewater. Desalin. Water Treat. 2015, 53, 3309–3318. [Google Scholar] [CrossRef]
- Tzoupanos, N.D.; Zouboulis, A.I.; Tsoleridis, C.A. A Systematic Study for the Characterization of a Novel Coagulant (Polyaluminium Silicate Chloride). Colloids Surf. A: Physicochem. Eng. Asp. 2009, 342, 30–39. [Google Scholar] [CrossRef]
- Kusterbeck, S. Lululemon Closes the Loop. Apparel 2006, 47, 31–50. [Google Scholar]
- Wang, X.; Du, Y.; Liu, H. Preparation, Characterization and Antimicrobial Activity of Chitosan-Zn Complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Malouchi, N.; Tolkou, A.K.; Maroulas, K.N.; Kosheleva, R.I.; Kostoglou, M.; Katsoyiannis, I.A.; Kyzas, G.Z. Polyphenolic Derivatives of Chitosan as Adsorbents for the Removal of Ibuprofen from Pharmaceutical Solutions. Colloids Surf. A: Physicochem. Eng. Asp. 2025, 715, 136647. [Google Scholar] [CrossRef]
- Kouzoutzoglou-Efremidou, A.A.; Tolkou, A.K.; Maroulas, K.N.; Kosheleva, R.I.; Katsoyiannis, I.A.; Kyzas, G.Z. Interfacial Adsorption Interactions of Dyes and Chitosan/Activated Carbon@Curcumin Derivatives in Single-Component and Binary Solutions. Langmuir 2025, 41, 3603–3622. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Coagulation/Flocculation Process for Dye Removal Using Water Treatment Residuals: Modelling through Artificial Neural Networks. Desalin. Water Treat. 2016, 57, 26392–26400. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review; Springer: Berlin/Heidelberg, Germany, 2019; Volume 16, ISBN 0123456789. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1981. [Google Scholar]
- El Foulani, A.A.; Jamal-eddine, J.; Lekhlif, B. Study of Aluminium Speciation in the Coagulant Composite of Polyaluminium Chloride-Chitosan for the Optimization of Drinking Water Treatment. Process Saf. Environ. Prot. 2022, 158, 400–408. [Google Scholar] [CrossRef]
- Gregory, J.; Duan, J. Hydrolyzing Metal Salts as Coagulants. Pure Appl. Chem. 2001, 73, 2017–2026. [Google Scholar] [CrossRef]
- Kosmulski, M. The PH Dependent Surface Charging and Points of Zero Charge. X. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rutten, S.B.; de Vries-Onclin, B.; Wagterveld, R.M.; de Vos, W.; Lindhoud, S. Evaluating the Efficiency of Enhanced Coagulation for Nanoplastics Removal Using Flow Cytometry. ACS ES&T Water 2025, 5, 3908–3919. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016. [Google Scholar]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Removal of Congo Red Dye from Its Aqueous Solution Using Natural Coagulants. J. Saudi Chem. Soc. 2012, 16, 131–136. [Google Scholar] [CrossRef]
- Islam, M.R.; Golam Mostafa, M. Removal of a reactive dye from synthetic wastewater using pac and FeCl3 coagulants. Life Earth Sci. 2021, 13, 39–44. [Google Scholar]
- Zhang, Z.; Liu, D.; Hu, D.; Li, D.; Ren, X.; Cheng, Y.; Luan, Z. Effects of Slow-Mixing on the Coagulation Performance of Polyaluminum Chloride (PACI). Chin. J. Chem. Eng. 2013, 21, 318–323. [Google Scholar] [CrossRef]
- Pal, P.; Corpuz, A.G.; Hasan, S.W.; Sillanpää, M.; Banat, F. Simultaneous Removal of Single and Mixed Cationic/Anionic Dyes from Aqueous Solutions Using Flotation by Colloidal Gas Aphrons. Sep. Purif. Technol. 2021, 255, 117684. [Google Scholar] [CrossRef]
- Yu, J.X.; Cai, X.L.; Feng, L.Y.; Xiong, W.L.; Zhu, J.; Xu, Y.L.; Zhang, Y.F.; Chi, R.A. Synergistic and Competitive Adsorption of Cationic and Anionic Dyes on Polymer Modified Yeast Prepared at Room Temperature. J. Taiwan Inst. Chem. Eng. 2015, 57, 98–103. [Google Scholar] [CrossRef]
- Shirazi, E.K.; Metzger, J.W.; Fischer, K.; Hassani, A.H. Simultaneous Removal of a Cationic and an Anionic Textile Dye from Water by a Mixed Sorbent of Vermicompost and Persian Charred Dolomite. Chemosphere 2019, 234, 618–629. [Google Scholar] [CrossRef]
- Kumari, B.; Chauhan, S.; Kumar, K.; Singh, S.; Chauhan, G.S. Simultaneous Removal of Cationic and Anionic Dyes from a Complex Mixture Using a Novel Composite Hydrogel Based on Pine Needles, Chitosan, and Gelatin. Int. J. Biol. Macromol. 2025, 307, 141447. [Google Scholar] [CrossRef]
- Tajat, N.; El Hayaoui, W.; El Mouhri, W.; Bougdour, N.; Idlahcen, A.; Radaa, C.; Bakas, I.; Tamimi, M.; Badreddine, M.; Assabbane, A.; et al. Simultaneous Removal of Anionic and Cationic Dyes from Aqueous Solutions Using Nickel–Iron Layered Double Hydroxide Nanosheets. Int. J. Environ. Sci. Technol. 2024, 21, 2843–2862. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Katsoyiannis, I.A.; Kyzas, G.Z. Simultaneous Removal of Anionic and Cationic Dyes on Quaternary Mixtures by Adsorption onto Banana, Orange and Pomegranate Peels. Colloids Surf. A: Physicochem. Eng. Asp. 2024, 685, 133176. [Google Scholar] [CrossRef]
- Zourif, A.; Benbiyi, A.; Kouniba, S.; El Guendouzi, M. Avocado Seed as a Natural Coagulant for Removing Dyes and Turbidity from Wastewater: Behnken Box Design, Sustainable Reuse, and Economic Evaluation. Sustain. Chem. Pharm. 2024, 39, 101621. [Google Scholar] [CrossRef]
- Zourif, A.; Kouniba, S.; El Guendouzi, M. Valorization of Palm Petiole Waste as Natural Biocoagulants: Optimizing Coagulation-Flocculation for Sustainable Wastewater Treatment and Advancing Circular Economy in Agriculture. Biocatal. Agric. Biotechnol. 2025, 63, 103473. [Google Scholar] [CrossRef]
- Goudjil, S.; Guergazi, S.; Ghernaout, D.; Temim, D.; Masmoudi, T. Brilliant Green and Methyl Violet 2B Dyes Removal Using Aluminium Sulfate (AS) in Single and Binary Systems. Desalin. Water Treat. 2024, 319, 100539. [Google Scholar] [CrossRef]
- Rudra Paul, S.; Singh, N.H.; Debnath, A. Quick and Enhanced Separation of Eosin Yellow Dye from Aqueous Solution by FeCl3 Interaction: Thermodynamic Study and Treatment Cost Analysis. Int. J. Environ. Anal. Chem. 2024, 104, 2874–2894. [Google Scholar] [CrossRef]
- Korkmaz, M. Factorial Experimental Design for Removal of Indigo Carmine and Brilliant Yellow Dyes from Solutions by Coagulation. Environ. Res. Technol. 2024, 7, 223–232. [Google Scholar] [CrossRef]
- Lin, T.Y.; Xie, K.H.; Wu, C.Y.; Wang, L.Y.; Liu, C.H.; Shu, C.C. Removal of Anionic Dyes from an Aqueous Solution by a Polyaniline-Modified Bioflocculant. J. Taiwan Inst. Chem. Eng. 2025, 34, 106302. [Google Scholar] [CrossRef]
- Carmo, J.V.C.; Nogueira, J.; Bertoldo, G.M.; Clemente, F.E.; Oliveira, A.C.; Campos, A.F.; Duarte, G.C.S.; Tehuacanero-Cuapa, S.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E. Porous Nanostructured Catalysts Based on Silicates and Their Surface Functionality: Effects of Silica Source and Metal Added in Glycerol Valorization. Catalysts 2024, 14, 526. [Google Scholar] [CrossRef]
- Mane, P.V.; Rego, R.M.; Yap, P.L.; Losic, D.; Kurkuri, M.D. Unveiling Cutting-Edge Advances in High Surface Area Porous Materials for the Efficient Removal of Toxic Metal Ions from Water; Elsevier Ltd.: Amsterdam, The Netherlands, 2024; Volume 146, ISBN 0000000302. [Google Scholar]
- Álvaro-Muñoz, T.; Márquez-Álvarez, C.; Sastre, E. Aluminium Chloride: A New Aluminium Source to Prepare SAPO-34 Catalysts with Enhanced Stability in the MTO Process. Appl. Catal. A Gen. 2014, 472, 72–79. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Trautwein, G.; Pérez-Cadenas, M.; Román-Martínez, M.C. Effects of Compression on the Textural Properties of Porous Solids. Microporous Mesoporous Mater. 2009, 126, 291–301. [Google Scholar] [CrossRef]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared Spectrum Characteristics and Quantification of OH Groups in Coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef] [PubMed]
- Borrajo, J.P.; Liste, S.; Serra, J.; González, P.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. Influence of the Network Modifier Content on the Bioactivity of Silicate Glasses. Key Eng. Mater. 2004, 254–256, 23–26. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Yang, G.; Wang, Q.; Li, R.; Lucia, L.A. Preparation and Characterization of Activated Carbon from Hydrochar by Phosphoric Acid Activation and Its Adsorption Performance in Prehydrolysis Liquor. BioResources 2017, 12, 5928–5941. [Google Scholar] [CrossRef]
- Oh, K.-I.; Baiz, C.R. Empirical S=O Stretch Vibrational Frequency Map. J. Chem. Phys. 2019, 151, 234107. [Google Scholar] [CrossRef]
- Nabilah, M.R.N.; Alwi, M.A.; Su’ait, M.S.; Imperiyka, M.; Hanifah, S.A.; Ahmad, A.; Hassan, N.H.; Rahman, M.Y.A. Effect of Ionic Liquid 1-Butyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide on the Properties of Poly(Glycidyl Methacrylate) Based Solid Polymer Electrolytes. Russ. J. Electrochem. 2016, 52, 362–373. [Google Scholar] [CrossRef]
- Lucas, M.S.; Teixeira, A.R.; Jorge, N.; Peres, J.A. Industrial Wastewater Treatment by Coagulation–Flocculation and Advanced Oxidation Processes: A Review. Water 2025, 17, 1934. [Google Scholar] [CrossRef]
- Khettaf, S.; Khouni, I.; Louhichi, G.; Ghrabi, A.; Bousselmi, L.; Bouhidel, K.-E.; Bouhelassa, M. Optimization of Coagulation–Flocculation Process in the Treatment of Surface Water for a Maximum Dissolved Organic Matter Removal Using RSM Approach. Water Supply 2021, 21, 3042–3056. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N. ‘Izzati; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Xu, M.; Tremblay, P.L.; Joya, M.B.; Kaka, A.Z.H.; Kollah, E.S.; Mwansa, B.K.; Wang, W.; Liu, Y.; Xing, X.; Qiu, F.; et al. An Efficient Bi2MoO6 Adsorbent with a Positive Surface and Abundant Oxygen Vacancies for the Removal of Humic Acid Contaminants. J. Environ. Chem. Eng. 2024, 12, 113296. [Google Scholar] [CrossRef]
| Coagulant Type | Comments | pH | ||
|---|---|---|---|---|
| AlCl3 | 0.1 M with respect to Al | 3.22 | ||
| CS | CS in 2% v/v acetic acid | 4.09 | ||
| pSi | SiO2 in HCl 1 N → ageing → 0.38 M SiO2 respect to Si | 2.01 | ||
| CS@Al5%,A | = = 5% | = = A | 3.67 | |
| CS@Al10%,A | = = A | 3.63 | ||
| CS@Al50%,A | = 50% | = = A | 3.90 | |
| CS@Al50%,B | = 50% | = = B | 4.13 | |
| Al/pSi10,A | = = A | 4.05 | ||
| Al/pSi20,A | = = A | 3.94 | ||
| CS@Al/pSi50%,10,A | = 50% | = | 3.81 | |
| CS@Al/pSi50%,10,B | = 50% | = | 4.11 | |
| Coagulant | Dye | C0 (mg/L) | pH | Dosage (mg/L) | Removal % | Dye Mixture 1 | Real Wastewater 2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Violet cationic dyes | ||||||||
| Avocado seed powder (ASP) | Crystal Violet | 50 | 7.0 | 950 | 95.3 | NO | NO | [56] |
| Palm petiole waste (PPW) | Crystal Violet | 50 | 9.0 | 950 | 98.2 | NO | NO | [57] |
| Alu- minum sulfate (AS) in | Methyl Violet 2B | 30 | 10 | 60 | 45.5 | YES | NO | [58] |
| Al/pSi10,A | Methylene Violet | 20 | 3.0 | 50 | 26.6 | YES | YES | This study |
| Yellow anionic dyes | ||||||||
| FeCl3 | Eosin Yellow (EY) | 100 | 2.0 | 100 | 98.0 | NO | YES | [59] |
| FeCl3 | Brilliant Yellow | 200 | 4.0 | 200 | 90.5 | NO | NO | [60] |
| Polyaniline-modified bioflocculant (PANi) | Direct Yellow 86 (DY86) | 100 | 2.0 | 2500 | 90.0 | NO | NO | [61] |
| Al/pSi20,A | Sunset Yellow | 20 | 3.0 | 25 | 98.8 | YES | YES | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalli, E.; Maroulas, K.N.; Thysiadou, A.A.; Kyzas, G.Z.; Tolkou, A.K. Performance Evaluation of Hybrid and Conventional Coagulants for the Removal of Sunset Yellow and Methylene Violet Dyes from Wastewater. Processes 2025, 13, 3430. https://doi.org/10.3390/pr13113430
Kalli E, Maroulas KN, Thysiadou AA, Kyzas GZ, Tolkou AK. Performance Evaluation of Hybrid and Conventional Coagulants for the Removal of Sunset Yellow and Methylene Violet Dyes from Wastewater. Processes. 2025; 13(11):3430. https://doi.org/10.3390/pr13113430
Chicago/Turabian StyleKalli, Eftychia, Konstantinos N. Maroulas, Anna A. Thysiadou, George Z. Kyzas, and Athanasia K. Tolkou. 2025. "Performance Evaluation of Hybrid and Conventional Coagulants for the Removal of Sunset Yellow and Methylene Violet Dyes from Wastewater" Processes 13, no. 11: 3430. https://doi.org/10.3390/pr13113430
APA StyleKalli, E., Maroulas, K. N., Thysiadou, A. A., Kyzas, G. Z., & Tolkou, A. K. (2025). Performance Evaluation of Hybrid and Conventional Coagulants for the Removal of Sunset Yellow and Methylene Violet Dyes from Wastewater. Processes, 13(11), 3430. https://doi.org/10.3390/pr13113430








