Corn Residue-Based Activated Carbon for Heavy Metal Removal: A Review of Adsorptive Performance and Properties
Abstract
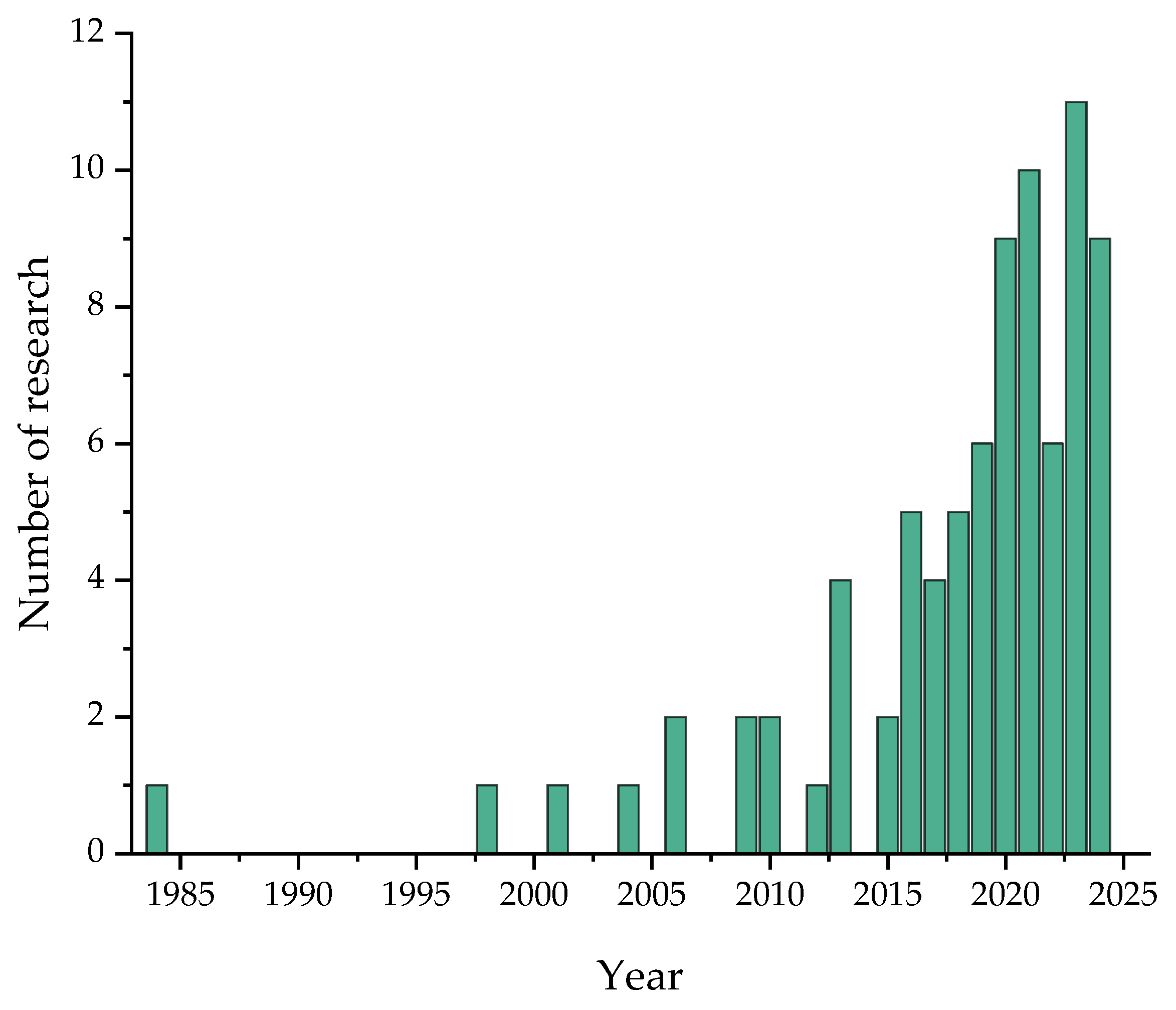
1. Introduction
2. Corn Waste Biomass as a Raw Material for Activated Carbon Production
3. Method of Production and Activation of Activated Carbon
3.1. Pre-Treatment of Biomass
3.2. Carbonization and Activation Methods
3.3. Functionalization and Modification of Activated Carbon
| Biomass (Part of Corn) | Biomass Particle Size | Type of Activation, Activation Agents | Carbonization Parameters (T, Duration, Atmosphere) | Activation Parameters (T, Duration, Atmosphere, Mass Ratio) | Functionalization, Agents, Soaking Time | Reference |
|---|---|---|---|---|---|---|
| Maize tassel | 45–212 μm | Physical, CO2 | 300–700 °C, 1 h, 10 °C/min, N2 | 300–700 °C, | / | [51] |
| Maize tassel | 45–212 μm | Chemical, H3PO4 (85%) | / | 500 °C, /, N2, 1:1; 1:2; 1:3; 1:4 | / | [51] |
| Corn cobs | ≤180 µm | Chemical, KOH | 500 °C, 1 h, 10 °C/min, N2 | 500–900 °C, N2, 0.5–2.5 h, 1:1–1:5; (statistical approach) | KMnO4, 0.02–0.18 mol/L, 12 h | [47] |
| Corn stalk | 0.5–1.0 mm | Chemical, KOH (2–5%) | 600 °C, 1.5 h, /, N2 | 80 °C, 4 h, reflux,—(2.0 g biomass/100 mL KOH sol.) | / | [48] |
| Corn husks | 0.5–3.0 mm | Chemical, H3PO4 (ACP) Chemical, H3PO4 + ZnCl2 (ACP-Zn) Chemical, H3PO4 + ZnCl2 + FeCl3·6H2O (ACP-Zn-Fe) | 700 °C, 2 h (heat rate: 500 °C in 1 h), N2 | Soaked in 50% H3PO4, dried at 80 °C (ACP), ACP + ZnCl2 (sample/salt = 5:1), dried at 80 °C (ACP-Zn) ACP-Zn + FeCl3·6H2O treatment, dried at 80 °C (ACP-Zn-Fe) | / | [57] |
| Corn husk | / | Chemical, H3PO4 | 300 °C for 2 h, 500 °C for 1 h, 5 °C/min, N2 | Impregnation ratio 1:1 (w:v) H3PO4, 60 °C for 12 h, in stainless steel reactor | / | [58] |
| Corn cobs | / | Chemical, H3PO4 | 500 °C, 2 h | Impregnation ratios: 0.25, 0.5, 1, 1.5, 2 (w:w) stirring for 24 h; drying at 80 °C for 24 h | / | [21] |
| Corn cobs | / | Chemical, H3PO4 | 500 °C, 1 h, muffle furnace atmosphere 10 K/min | H3PO4 (m:v = 5:3), 113 °C, 16 h | / | [59] |
| Corn cobs | / | Chemical, H2SO4 pretreatment + KOH activation | 400 °C, 30 min, 800 °C, 1 h; 10 °C/min; under Ar gas | 10% H2SO4, 170 °C for 48 h, mixed with KOH (1:2 mass ratio) | / | [17] |
| Corn cobs | 4–8 mm | Chemical, H3BO3 | 700 °C, 1 h, 5 °C/min, N2 | 60 g CCs + 60 g H3BO3 in 700 mL H2O, 70 °C for 6 h | / | [60] |
| Corn straw | / | Chemical, KOH | 450 (30 min), 650 (30 min), 800 °C (60 min), 5 °C/min, N2 | KOH-to-biomass mass ratio 1:1 | / | [15] |
| Corn cob | 0.7–0.9 mm | Chemical, H3PO4, H3BO3 | 500 °C, 1 h | Pre-carbonized CC + activating agents, then HCl (37%) at 120 °C, 45 min | / | [12] |
| Corn cobs | ≈150 µm | Chemical, H3PO4 + H3BO3 | 400, 450, 500 °C (varied), 1 h, muffle furnace | Immersed at 120 °C for 15, 30, or 45 min in acid mixture | / | [61] |
| Corn straw | ≈150 µm | Chemical, H3PO4 + H3BO3 | 500 °C, 1 h, muffle furnace | Immersed at 120 °C for 45 min in acid mixture | / | [61] |
| Maize cobs | 300 µm | Chemical, NaOH (1.0 M) | 240 °C, 5 min | 24 h, 300 g maize cobs: 1000 cm3 NaOH | / | [20] |
| Maize plant biomass (tassels, cobs, stalks) | / | Chemical, H3PO4 | 500 °C, N2 | 800 °C, N2, H3PO4 | / | [11] |
| Corncob (CC) | 1–2 mm | Physicochemical, KOH + CO2 | 700 °C, 2 h, N2 atmosphere, 10 °C/min | 797 °C, 3 h, CO2 atmosphere, impregnation ratio = 3.5 | / | [53] |
| Corncob | / | Physical, CO2 | 500 °C, 2 h, air (muffle furnace), 20 °C/min | 700 °C, 1.5 h, CO2, pre-N2 atmosphere, 20 °C/min | FeSO4·7H2O + NaOH (precipitation of Fe oxides), 100 °C, 1 h | [14] |
| Corncob | 75–100 µm | Chemical, H3PO4 (85%) | 500 °C, 2 h | Room temp, 12 h, solid–liquid ratio 1:5 (g/mL) | / | [18] |
| Corncob | 75–100 µm | Chemical, AlCl3 | 500 °C, 2 h | AlCl3 (0.01 mol/L), 80 °C, 10 h | / | [18] |
| Corncob | / | Chemical, KOH | 600 °C, 2 h, N2 (100 cm3/min), 5 °C/min | KOH:CCW = 4:1 (impregnated overnight), 600 °C, 2 h, N2, 5 °C/min | / | [7] |
| Corncob | / | Chemical, HNO3 | 600 °C, 2 h, N2 (100 cm3/min), 5 °C/min | 65% HNO3, 24 h soaking, | / | [7] |
| Corn straw | <1 mm | Chemical, H3PO4 (85%) | 600 °C, 1.5 h, N2 | Impregnation in 85% H3PO4 for 24 h, 600 °C, 1.5 h, N2 | / | [9] |
| Corn straw | <1 mm | Chemical, H3PO4 (85%) | 600 °C, 1.5 h, N2 | Impregnation in 85% H3PO4 for 24 h, 600 °C, 1.5 h, N2 | Post-treatment with FeCl3·6H2O: 5 g AC in 500 mL FeCl3 solution (40 g/500 mL) for 24 h, dried 12 h at 105 °C | [9] |
| Maize shells | / | Chemical, H2SO4 | 400 °C, 3 h, muffle furnace (air) | Impregnation ratio 1:1.8 (biomass: H2SO4) | [56] | |
| Maize shells | / | Chemical, H2SO4 | 400 °C, 3 h | Impregnation ratio 1:1.8 (biomass: H2SO4), 105 °C drying | Fe3O4 nanoparticles (20 wt%), soaking for 1 h at 200 rpm, heating at 550 °C, 3 h | [56] |
| Corn husk | / | Chemical, H3PO4 | 400 °C, 30 min, muffle furnace (sealed ceramic container) | Impregnation ratio 1:2 (corn husk: H3PO4), 24 h | 1.0 M citric acid (4 g CC to 25 mL) for 30 min, dried overnight at 50 °C | [62] |
| Maize cob | ≤0.09 mm | Chemical, H3PO4 (75%) | 500 °C, 20 min | Weight ratio H3PO4:char = 0.1, 60 °C for 24 h | / | [63] |
| Maize cob | 2 mm | Chemical, 50% H2SO4 (AC-MC) | 400 °C, 3 h, 10 °C/min, air atmosphere | Soaking ratio H2SO4:precursor = 2:1 (86 mL/60 g) | / | [16] |
| Maize cob | 2 mm | Chemical, 50% H2SO4 | 400 °C, 3 h, 10 °C/min, air atmosphere | Soaking ratio H2SO4:precursor = 2:1 (86 mL/60 g) | Combined with Ag NPs and SiO2 NPs in colloidal form, heated at 70 °C, dried at 80 °C, calcined at 300 °C for 2 h (AC-Ag-SiO2 nanocomposite) | [16] |
| Corn husk | 250 μm | Chemical, 2% HNO3 (v/v) (CHAC) | 300 °C, 2 h 20 °C/min, under N2 | 30 g corn husk + 200 mL HNO3, stirred 2 h | [50] | |
| Corn husk | 250 μm | Chemical, 2% HNO3 (v/v) | 300 °C, 2 h, 20 °C/min, N2 | 30 g corn husk + 200 mL HNO3, stirred 2 h | 20 mL epichlorohydrin + 25 mL DMF + 11 mL ethylenediamine (80 °C, 1 h) + 25 mL trimethylamine (1 h) + 20 mL pyridine + 20 g CHAC (90 °C, 2 h), dried at 75 °C for 6 h (AF-CHAC) | [50] |
| Corn cob | ≈355 nm | Chemical, KOH | 700 °C, 2 h, 5 °C/min, N2 | KOH (1:0.3 wt) | / | [22] |
| Corn cob | ≈355 nm | Chemical, KOH | 700 °C, 2 h, 5 °C/min, N2 | CC:KOH:urea = 1:0.3:0.3 | / | [22] |
| Corn cob | / | Chemical, KOH | 480 °C, 1 h, N2 | 85 wt% H3PO4: corn cob 40:60, 45 °C 24 h, KOH:char, (3:1 wt), 790 °C, 1 h, N2 | / | [64] |
4. Structural and Surface Properties of Corn Waste-Based Activated Carbon
4.1. Surface Morphology and Surface Chemistry
4.1.1. Presence of Functional Groups
4.1.2. Porosity and Textural Properties
4.1.3. Determination of Surface Charge
| Activated Carbon | SBET (m2/g) | Pore Diameter | pHpzc, pH Slurry, Zeta Potential (mV) | Reference |
|---|---|---|---|---|
| Activated carbon/corn cob (KOH/soap activation) | 2630 | Micropores (78.3%): 0.2–2 nm Mesopores (14.3%): 2–53 nm Macropores (7.4%): 53–100 nm | / | [71] |
| Activated corn cobs | 249 | 1.853 nm | / | [74] |
| AF-CHAC | 442.70 | / | 6.8 | [50] |
| CCA1 | 712.16 | 2.095 nm | / | [51] |
| CCA2 | 776.23 | 3.209 nm | ||
| CCA3 | 623.26 | 3.959 nm | ||
| CCA4 | 1262.50 | 4.881 nm | ||
| PAC1 | 5.82 | 5.320 nm | / | [51] |
| PAC2 | 4.21 | 5.034 nm | ||
| PAC3 | 6.46 | 5.969 nm | ||
| PAC4 | 13.04 | 4.111 nm | ||
| PAC5 | 75.60 | 1.924 nm | ||
| CCC | 11.16 | / | 2.03 | [47] |
| CCAC | 2797.75 | / | 3.49 | |
| CKAC | 1212.93 | / | pHpzc less than 2 | |
| Activated carbon from corn cob | 1054.2 | 2.41 nm | / | [17] |
| AC KOH (2% KOH) | 498.0 | 3.87 nm | / | [48] |
| AC KOH (3% KOH) | 566.1 | 4.84 nm | ||
| AC KOH (4% KOH) | 565.8 | 4.63 nm | ||
| AC KOH (5% KOH) | 565.3 | 4.14 nm | ||
| ACF | 1824 | 1–4 nm | 3.85 | [58] |
| Activated carbons from corn cob (ZnCl2 and NH4Cl) | 924.9 | 1.17 nm | / | [69] |
| Porous carbon material prepared from corn straw-KOH activation agents | 2131.181 | 0.5–1 nm (10 nm) | 4.76 | [15] |
| CCAC CSAC | 840.2542 816.4197 | <0.154 mm | / | [61] |
| MCCAC-Fe3O4 | 282 | / | 7.5 | [14] |
| CAC-Al | 146.64 | 2.747 nm | / | [18] |
| Cal-CCW | 88 | 0.58 nm | 16.8 mV | [7] |
| Base-CCW | 296 | 0.46 nm | −37.5 mV | |
| Oxide-CCW | 231 | 0.52 nm | 23.75 mV | |
| Cal-Oxide-CCW (HNO3) | 8 | 0.56 nm | ||
| CM-550 | 196 | 1.36 nm | 7.5 (pH Slurry) | [75] |
| KM-33 (33% wt of KOH) | 619 | 1.14 nm | 8.4 (pH Slurry) | |
| KM-50 (33% wt of KOH) | 1236 | 0.83 nm | 8.9 (pH Slurry) | |
| KM-66 (33% wt of KOH) | 1523 | 0.99 nm | 9.3 (pH Slurry) | |
| KM-75 (33% wt of KOH) | 1414 | 1.10 nm | 9.9 (pH Slurry) | |
| AC | 563.93 | / | / | [9] |
| AF | 217.87 | |||
| 3-20CCACz, (3:1), 20% ZnCl2 | 1270 | 2.10 nm | / | [76] |
| 1-20CCACz (1:1), 20% ZnCl2 | 616 | 1.87 nm | ||
| 3-40CCACz (3:1) 40% ZnCl2 | 828 | 1.74 nm | ||
| 2-20CCACp (H3PO4) | 553 | 2.28 nm | ||
| 3-20CCACs (H2SO4) | 556 | 1.09 nm | ||
| CS-AC | 7.335 | 98.53 (average pore width, Å, 9.85 nm) | / | [77] |
| CS-HC-AC | 331.233 | 24.36(average pore width, Å, 2.44 nm) | ||
| CCs-AC | 598.6 | 2.12 nm | 4.98 | [8] |
| ACP | 474 | 1.95 nm | 5.21 | [57] |
| ACP-Zn (50% H3PO4/ZnCl2) | 227 | 3.84 nm | 3.37 | |
| ACP-Zn-Fe (50% H3PO4/ZnCl2/FeCl3) | 186 | 5.86 nm | 4.21 | |
| Corn cob activated carbon (impregnation ratio 1:1 H3PO4) | SL(m2∕g) 810 (Langmuir isotherm) | / | 3.8 | [21] |
| Corn cob activated carbon | / | / | 3.0 | [59] |
| CCAK7 | 915 | 1.15 | / | [22] |
| CCAKN7 | 1395 | 0.95 |
5. Adsorption Performance of Activated Carbon for Heavy Metal Removal
5.1. Impact of pH
5.2. Impact of Contact Time and Kinetic Modeling
5.3. Impact of the Dose of Adsorbent
5.4. Impact of Initial Concentration
| Activated Carbon | Adsorbate | qmax | Adsorption Parameters (pH, Contact Time, Adsorption Dose, Initial Concentration-Ic) | Reference |
|---|---|---|---|---|
| Amine-functionalized corn husk activated carbon | Pb (II), Cu (II), Ni (II) | 2.814 mg/g, 0.724 mg/g, 0.337 mg/g | pH 2–11 optimal value (o.v.) 8 1–7 g/L o.v. 4 g/L 0–120 min o.v. 60 min Ic/ | [50] |
| CCA PAC1 | Pb (II) | / | pH 0–7 o.v. 7 0–0.1 g o.v./ 0–400 min o.v./ Ic/ | [51] |
| Activated carbon from corn cobs | Fe, Mn | / | pH 2–8 o.v. 4 0.1–2 g o.v. 1 g (Fe) 0.5 (Mn) 10–120 min o.v. 60 min (Fe) 30 min (Mn) Ic/ | [19] |
| CCC CCAC CKAC-KMnO4 | Hg | 175.88 mg/g 227.32 mg/g | pH 2–6 o.v. 4 (CCAC) 3 (CKAC) 0.20–1.00 g/L 0.40 g/L (CCAC) (CKAC) 15–1440 min. 120 min (CCAC) 60 min (CKAC) Ic 40–120 mg/L o.v. 100 mg/L (CCAC, CKAC) | [47] |
| CCAC | Hg | 2.39 mg/g | pH 3–11 o.v. 7 10–80 mg/L o.v. 20 mg/L 0–120 min o.v. 120 min Ic 20–100 µg/L o.v. 60 µg/L | [17] |
| AC-KOH (4) | Cr (VI) | 88.106 mg/g | pH 4.5 2.5 g/L 0–48 h o.v. 2 h Ic 50–300 mg/L | [48] |
| ACP-Zn (50% H3PO4/ZnCl2) ACP-Zn-Fe (50% H3PO4/ZnCl2/FeCl3) | Cr (VI) | 24.8 mg/g 30.3 mg/g | pH 2–8 o.v. 2–2.5 0.25 to 2.0 g/L o.v. 1 g/L 15–300 min o.v. 240 min Ic 5–30 mg/L o.v. 12 mg/L | [57] |
| ACF-H3PO4 | Pb2+, Cu2+ | 206.01 mg/g (Pb2+) 212.29 mg/g (Cu2+) | pH 2–5 o.v. 4–5 25 mg 0 to 360 min o.v. 100 min Ic 25 to 700 mg/L | [58] |
| Corn cob-based AC | Cr (VI) | 34.48 mg/g (298 K) | pH 2–9 o.v 1–2 0.2 to 2.0 g/L o.v 0.7 g/L 0–7 h o.v. 4 h Ic m 8–20 mg/L | [69] |
| Corn cob activated carbon (H3PO4) 1:1 | Pb2+, Cu2+ | 8 mg/g | pH 2–8 o.v. 5.3 (Pb2+) 5.7 (Cu2+) 0.07–0.2 g o.v. 0.1 g (Pb2+) 0.2 g (Cu2+) 5–120 min o.v. 60 min (Pb2+), 90 min (Cu2+) Ic 10–50 mg/L | [21] |
| Corn cob activated carbon (H3PO4) | Cu2+, Ni2+ | 0.39 mmol/g Cu2+ 0.28 mmol/g Ni2+ | pH 2–7 o.v 4 3–480 min 240 min Cu2+ 100 min Ni2+ Ic. 0.10 to 2.00 mmol/L | [59] |
| CCs-AC-H3BO3 | Cr (VI) | 123.7 mg/g | pH 2–8 o.v. 2 5 g/L 0–780 min o.v.4 h Ic 50–1000 mg/L | [60] |
| Carbon derived from corn straw | Cr (VI) | 175.44 mg/g | pH 1–9 o.v. 3 1 g/L 0–600 min o.v. 480 min Ic 100–350 mg/L | [15] |
| GACC (H3PO4, H3BO3, HCl) | Cd2+, Ni2+, Zn2+ | 0.21 mg/g, 0.19 mg/g, 0.28 mg/g | 0.1–2 g o.v. 1 g 0–12 h o.v. 6 h Ic. 2–10 mg/L | [12] |
| CCAC, (H3PO4, H3BO3) | Cr (VI) | 9.6246 mg/g | pH- 1–13 o.v. 7 0.5–2 g/L o.v. 1.0 g/L 5–60 min o.v. 60 min Ic 0.5–10 mg/L | [61] |
| MCAC | Ni (II) | / | pH/ 0.2–1 g. o.v. 0.8 g 15–150 min o.v. 120 min Ic o.v. 9.75 mg/L | [20] |
| Activated carbon produced from maize plant biomass | As (III) | / | pH 6 0.50–3.00 g/L o.v. 0.5 g/L 40.00–90.00 min o.v. 90 min Ic 10.00–30.00 mg/L o.v. 10 mg/L | [11] |
| MCCAC | Cr (VI) | 120 μm 57.372 mg/g 600 μm 52.246 mg/g 1200 μm 54.94 mg/g | pH 2–11 o.v. 2 0.01 g/10 mL-0.1 g/10 mL o.v. 0.1 g/10 mL Ic. 100–1000 mg/L | [14] |
| CAC Al-CAC (AlCl3) | Fe (III) | 500 mg/g 250 mg/g | pH 4–10 o.v. 8 0.01–2 g/L o.v. 0.1 g/L 0–30 min o.v. 5 min Ic 5–40 mg/L | [18] |
| MLAC | Pb (II) | 3.7136 mg/g | pH 2–8 o.v. 4 0.5 g 0–120 min o.v. 30 min Ic. 5–30 mg/L | [82] |
| CHAC CA-CHAC | Cu (II) | 612.52 mg/g 643.92 mg/g | pH 3–7 o.v.7 2–6 mg o.v. 5 mg 15–75 min o.v. 45 min(CHAC) and 30 min (CA-CHAC) Ic. 5–45 mg/L | [62] |
| AC-Ag-SiO2 | Cu2+, Pb2+, Cd2+ Zn2+ | 84.75 mg/g, 81.3 mg/g, 87.72 mg/g, 81.97 mg/g | pH 2–12 o.v. 5.5 0.1–0.4 g o.v. 0.1 g 0–280 min o.v. 200 min Ic. 10 mg/L to 100 mg/L | [16] |
| CCAK7 CCAKN7 | U(VI) | 46.32 mg/g 51.66 mg/g | pH 6 55 min Ic 20–100 ppm | [22] |
| CC-AC | Cr(VI) Ni(II) | 28.90 mg/g 6.27 mg/g | pH 2,7 o.v. 2 0–140 min o.v. 105 min, 0.1–0.3 g/100 mL o.v. 0.289 g, Ic 20–140 ppm o.v.37.2 ppm Cr (VI) pH 7,12 o.v. 12, 0–260 min o.v. 135 min, 0.2–1 g/100 mL o.v.0.94 g, Ic 20–100 ppm o.v.31 ppm Ni (II) | [64] |
5.5. Impact of Temperature and Thermodynamic Studies
6. Mechanism of Heavy Metal Removal Using Corn Waste–Based Carbon Material
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Younas, F.; Younas, S.; Bibi, I.; Farooqi, Z.U.R.; Hameed, M.A.; Mohy-Ud-Din, W.; Shehzad, M.T.; Hussain, M.M.; Shakil, Q.; Shahid, M.; et al. A critical review on the separation of heavy metal(loid)s from the contaminated water using various agricultural wastes. Int. J. Phytoremediat. 2024, 26, 349–368. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Hoang, A.T.; Kumar, S.; Lichtfouse, E.; Cheng, C.K.; Varma, R.S.; Senthilkumar, N.; Phong Nguyen, P.Q.; Nguyen, X.P. Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: An update of recent trends. Chemosphere 2022, 302, 134825. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Hou, X.-X.; Deng, Q.-F.; Ren, T.-Z.; Yuan, Z.-Y. Adsorption of Cu2+ and methyl orange from aqueous solutions by activated carbons of corncob-derived char wastes. Environ. Sci. Pollut. Res. 2013, 20, 8521–8534. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, A.; Bozbeyoglu, P.; Imamoglu, M.; Baltaci, C.; Duran, C.; Bulut, V.N. Characterization of the Adsorption Mechanism of Cadmium(II) and Methylene Blue upon Corncobs Activated Carbon. Anal. Lett. 2023, 56, 433–448. [Google Scholar] [CrossRef]
- Tan, G.; Mao, Y.; Wang, H.; Xu, N. A comparative study of arsenic(V), tetracycline and nitrate ions adsorption onto magnetic biochars and activated carbon. Chem. Eng. Res. Des. 2020, 159, 582–591. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 1984; ISBN 9789240045064. [Google Scholar]
- Bayuo, J.; Rwiza, M.J.; Mtei, K.M. Modeling and optimization of trivalent arsenic removal from wastewater using activated carbon produced from maize plant biomass: A multivariate experimental design approach. Biomass Convers. Biorefin. 2024, 14, 24809–24832. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.; Hefny, M.; Ahmed, H.M.; Abdel-Haleem, F. Removal of Cadmium, Nickel, and Zinc from aqueous solutions by activated carbon prepared from corncob—Waste agricultural materials. Egypt. J. Chem. 2021, 65, 677–687. [Google Scholar] [CrossRef]
- Kwikima, M.M.; Mateso, S.; Chebude, Y. Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review. Sci. Afr. 2021, 13, e00934. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Bioresour. Technol. 2013, 134, 94–100. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Nyirenda, J.; Kalaba, G.; Munyati, O. Synthesis and characterization of an activated carbon-supported silver-silica nanocomposite for adsorption of heavy metal ions from water. Results Eng. 2022, 15, 100553. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg(II) from aqueous solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef] [PubMed]
- El-Bendary, N.; El-Etriby, H.K.; Mahanna, H. High-performance removal of iron from aqueous solution using modified activated carbon prepared from corn cobs and luffa sponge. Desalination Water Treat. 2021, 213, 348–357. [Google Scholar] [CrossRef]
- Ermadani; Viviani, A.; Yasdi; Yanopa, S.; Suryanto; Arsyad, A.; Sarman. The use of activated charcoal from corn cobs as adsorbent of heavy metals from groundwater. Rev. Bras. Eng. Biossistemas 2023, 17, 1191. [Google Scholar] [CrossRef]
- Lala, M.A.; Olomowewe, Z.O.; Adeniyi, A.T.; Giwa, A. Maize cob-derived activated carbon as an adsorbent for the removal of nickel (II) cation from aqueous solution: Optimization and kinetic studies. Arab. J. Basic Appl. Sci. 2023, 30, 573–582. [Google Scholar] [CrossRef]
- Kouassi, N.L.B.; N’goran, K.P.D.A.; Blonde, L.D.; Diabate, D.; Albert, T. Simultaneous Removal of Copper and Lead from Industrial Effluents Using Corn Cob Activated Carbon. Chem. Afr. 2023, 6, 733–745. [Google Scholar] [CrossRef]
- Giraldo, L.; Serafin, J.; Dziejarski, B.; Moreno-Piraján, J.C. Activated carbon from biomass waste as potential materials for uranium removal. Chem. Eng. Sci. 2025, 306, 121222. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef]
- Islamoglu, S.; Yilmaz, L.; Ozbelge, H.O. Development of a Precipitation Based Separation Scheme for Selective Removal and Recovery of Heavy Metals from Cadmium Rich Electroplating Industry Effluents. Sep. Sci. Technol. 2006, 41, 3367–3385. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L. Heavy Metals Removal from Water and Wastewater. In Heavy Metals—Recent Advances; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health Part A 2004, 39, 455–464. [Google Scholar] [CrossRef]
- Twizerimana, P.; Wu, Y. Overview of integrated electrocoagulation-adsorption strategies for the removal of heavy metal pollutants from wastewater. Discov. Chem. Eng. 2024, 4, 14. [Google Scholar] [CrossRef]
- AlJaberi, F.Y. Operating cost analysis of a concentric aluminum tubes electrodes electrocoagulation reactor. Heliyon 2019, 5, e02307. [Google Scholar] [CrossRef] [PubMed]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.N. Biofouling formation and structure on original and modified PVDF membranes: Role of microbial species and membrane properties. RSC Adv. 2017, 7, 37990–38000. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; David Ng, K.M.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef]
- Deischter, J.; Wolter, N.; Palkovits, R. Tailoring Activated Carbons for Efficient Downstream Processing: Selective Liquid-Phase Adsorption of Lysine. ChemSusChem 2020, 13, 3614–3621. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, M.; Fan, J.; Zhou, Y.; Zhang, L. Regeneration Performance of Activated Carbon for Desulfurization. Appl. Sci. 2020, 10, 6107. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Li, X.; Zhou, Z.; Wei, B. Ultrasonic–Thermal Regeneration of Spent Powdered Activated Carbon. Sustainability 2023, 15, 9060. [Google Scholar] [CrossRef]
- Abdel daiem, M.M.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Ocampo-Pérez, R. Single, competitive, and dynamic adsorption on activated carbon of compounds used as plasticizers and herbicides. Sci. Total Environ. 2015, 537, 335–342. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. Activated Carbon and Adsorption. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 22–34. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Radenković, M.; Petrović, J.; Pap, S.; Kalijadis, A.; Momčilović, M.; Krstulović, N.; Živković, S. Waste biomass derived highly-porous carbon material for toxic metal removal: Optimisation, mechanisms and environmental implications. Chemosphere 2024, 347, 140684. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Christica, I.; Julia, R. Activated Carbon Utilization from Corn Cob (Zea mays) as a Heavy Metal Adsorbent in Industrial Waste. Asian J. Pharm. Res. Dev. 2018, 6, 1–4. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Danish, M.; Anastopoulos, I.; Iwuozor, K.O. Recent progress on corn (Zea mays L.)-based materials as raw, chemically modified, carbonaceous, and composite adsorbents for aquatic pollutants: A review. J. Anal. Appl. Pyrolysis 2023, 172, 106004. [Google Scholar] [CrossRef]
- Czajkowski, Ł.; Wojcieszak, D.; Olek, W.; Przybył, J. Thermal properties of fractions of corn stover. Constr. Build. Mater. 2019, 210, 709–712. [Google Scholar] [CrossRef]
- Zhong, X.; Yuan, R.; Zhang, B.; Wang, B.; Chu, Y.; Wang, Z. Full fractionation of cellulose, hemicellulose, and lignin in pith-leaf containing corn stover by one-step treatment using aqueous formic acid. Ind. Crops Prod. 2021, 172, 113962. [Google Scholar] [CrossRef]
- Sun, Y.; Webley, P.A. Preparation of activated carbons from corncob with large specific surface area by a variety of chemical activators and their application in gas storage. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar] [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2023, 17, 159. [Google Scholar] [CrossRef]
- Choi, J.Y.; Nam, J.; Yun, B.Y.; Kim, Y.U.; Kim, S. Utilization of corn cob, an essential agricultural residue difficult to disposal: Composite board manufactured improved thermal performance using microencapsulated PCM. Ind. Crops Prod. 2022, 183, 114931. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Qu, B.; Liu, X.; Yi, W.; Zhang, H. Study on Adsorption Properties of Modified Corn Cob Activated Carbon for Mercury Ion. Energies 2021, 14, 4483. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, L.; Ma, H.; Zhou, F.; Yang, K.; Wu, G. Corn stalk-based activated carbon synthesized by a novel activation method for high-performance adsorption of hexavalent chromium in aqueous solutions. J. Colloid. Interface Sci. 2020, 578, 650–659. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, L.; Shi, X.; Wang, Z. Preparation and analysis of activated carbon from sewage sludge and corn stalk. Adv. Powder Technol. 2016, 27, 684–691. [Google Scholar] [CrossRef]
- Ismail, M.S.; Yahya, M.D.; Auta, M.; Obayomi, K.S. Facile preparation of amine-functionalized corn husk derived activated carbon for effective removal of selected heavy metals from battery recycling wastewater. Heliyon 2022, 8, e09516. [Google Scholar] [CrossRef]
- Olorundare, O.F.; Krause, R.W.M.; Okonkwo, J.O.; Mamba, B.B. Potential application of activated carbon from maize tassel for the removal of heavy metals in water. Phys. Chem. Earth Parts A/B/C 2012, 50–52, 104–110. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Latiff, M.F.P.M.; Abustan, I.; Ahmad, M.A.; Yahaya, N.K.E.M.; Khalid, A.M. Effect of preparation conditions of activated carbon prepared from corncob by CO2 activation for removal of Cu (II) from aqueous solution. In Proceedings of the 1st International Conference on Advanced Science, Engineering and Technology, Penang, Malaysia, 21–22 December 2015; p. 030001. [Google Scholar] [CrossRef]
- Speranza, G. The Role of Functionalization in the Applications of Carbon Materials: An Overview. C 2019, 5, 84. [Google Scholar] [CrossRef]
- El-Shafey, E.I.; Ali, S.N.F.; Al-Busafi, S.; Al-Lawati, H.A.J. Preparation and characterization of surface functionalized activated carbons from date palm leaflets and application for methylene blue removal. J. Environ. Chem. Eng. 2016, 4, 2713–2724. [Google Scholar] [CrossRef]
- Rao, V.D.; Raghutu, R.; Rao, K.S.; Kumar, R.; Padma, D.V.; Sastry, S.V.A.R. Efficient biosorption of cadmium ions from wastewater using iron oxide–maize shell activated bio-carbon nanocomposites. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Ammar, N.S.; Fathy, N.A.; Ibrahim, H.S.; Mousa, S.M. Micro-mesoporous modified activated carbon from corn husks for removal of hexavalent chromium ions. Appl. Water Sci. 2021, 11, 154. [Google Scholar] [CrossRef]
- Silva, M.C.; Crespo, L.H.S.; Cazetta, A.L.; Silva, T.L.; Spessato, L.; Almeida, V.C. Activated carbon fibers of high surface area from corn husk: Mono and multicomponent adsorption studies of Pb2+ and Cu2+ ions from aqueous solution. J. Mol. Liq. 2024, 405, 124919. [Google Scholar] [CrossRef]
- Campos, N.F.; Guedes, G.A.J.C.; Oliveira, L.P.S.; Gama, B.M.V.; Sales, D.C.S.; Rodríguez-Díaz, J.M.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Competitive adsorption between Cu2+ and Ni2+ on corn cob activated carbon and the difference of thermal effects on mono and bicomponent systems. J. Environ. Chem. Eng. 2020, 8, 104232. [Google Scholar] [CrossRef]
- Bozbeyoglu, P.; Gündoğdu, A. Adsorption of hexavalent chromium from aqueous solution onto corn cobs—Activated carbon. Turk. J. Anal. Chem. 2023, 5, 107–117. [Google Scholar] [CrossRef]
- Li, H.; Gao, P.; Cui, J.; Zhang, F.; Wang, F.; Cheng, J. Preparation and Cr(VI) removal performance of corncob activated carbon. Environ. Sci. Pollut. Res. 2018, 25, 20743–20755. [Google Scholar] [CrossRef]
- Gamay, K.P.; Ongayo, G.D. From Waste to Resource: Assessing the Biosorption Efficiency of Citric Acid-Modified Activated Carbon from Corn (Zea mays) Husks in Aqueous Copper (II) Ion Solutions. Int. J. Res. Innov. Appl. Sci. 2024, IX, 468–479. [Google Scholar] [CrossRef]
- Adebayo, G.; Adegoke, H.; Jamiu, W.; Balogun, B.; Jimoh, A. Adsorption of Mn(II) and Co(II) ions from aqueous solution using Maize cob activated carbon: Kinetics and Thermodynamics Studies. J. Appl. Sci. Environ. Manag. 2016, 19, 737. [Google Scholar] [CrossRef]
- Kamel, M.; Bastaweesy, A.M.; Hefny, R.A. Optimized Removal of Cr (VI) and Ni (II) From Wastewater Using Corncob-Derived Activated Carbon. Water Air Soil Pollut. 2025, 236, 93. [Google Scholar] [CrossRef]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; Ulson de Souza, A.A.; de Souza, S.M.d.A.G.U.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Bläker, C.; Muthmann, J.; Pasel, C.; Bathen, D. Characterization of Activated Carbon Adsorbents—State of the Art and Novel Approaches. ChemBioEng Rev. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Tang, S.; Chen, Y.; Xie, R.; Jiang, W.; Jiang, Y. Preparation of activated carbon from corn cob and its adsorption behavior on Cr(VI) removal. Water Sci. Technol. 2016, 73, 2654–2661. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. A review of avocado waste-derived adsorbents: Characterizations, adsorption characteristics, and surface mechanism. Chemosphere 2022, 296, 134036. [Google Scholar] [CrossRef]
- Cao, Q.; Xie, K.; Lv, Y.; Bao, W. Process effects on activated carbon with large specific surface area from corn cob. Bioresour. Technol. 2006, 97, 110–115. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Julien, F.; Baudu, M.; Mazet, M. Relationship between chemical and physical surface properties of activated carbon. Water Res. 1998, 32, 3414–3424. [Google Scholar] [CrossRef]
- Aliyu, S.; Salahudeen, N.; Rasheed, A.A. Adsorptive Removal of Lead (II) Pollutants from Wastewater Using Corncob-Activated Carbon. J. Eng. Sci. 2024, 11, H1–H10. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. An insight into the KOH activation mechanism through the production of microporous activated carbon for the removal of Pb2+ cations. Appl. Surf. Sci. 2009, 255, 3723–3730. [Google Scholar] [CrossRef]
- Duan, X.-L.; Yuan, C.-G.; Jing, T.-T.; Yuan, X.-D. Removal of elemental mercury using large surface area micro-porous corn cob activated carbon by zinc chloride activation. Fuel 2019, 239, 830–840. [Google Scholar] [CrossRef]
- Ekinci, S.; Onat, E.; Atiç, S. Optimized Synthesis and Characterization of Activated Carbon from Corn Silk and Corn Silk Hydrochar. ChemistrySelect 2025, 10, e202405854. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.C.; Kyzas, G.Z. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Abou-Hadid, A.F.; El-Behairy, U.A.; Elmalih, M.M.; Amdeha, E.; El Naggar, A.M.A.; Taha, M.H.; Hussein, A.E.M. Conversion of corn shell as biomass solid waste into carbon species for efficient decontamination of wastewater via heavy metals adsorption. Biomass Convers. Biorefin. 2024, 14, 16435–16449. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.-H.; Tzeng, J.-H.; Anotai, J.; Jacobson, A.R.; Lin, Y.-T. Systematic optimization of biochars derived from corn wastes, pineapple leaf, and sugarcane bagasse for Cu(II) adsorption through response surface methodology. Bioresour. Technol. 2023, 382, 129131. [Google Scholar] [CrossRef] [PubMed]
- Abas, S.N.; Ismail, M.H.; Kamal, L.M.; Izhar, S. Adsorption Process of Heavy Metals by Low-Cost Adsorbent: A Review. World Appl. Sci. J. 2013, 28, 1518–1530. [Google Scholar]
- Nadeem, U. Adsorption of Pb(II) from aqueous solutions by activated carbon prepared from agricultural waste: Maize leaves. Eur. Chem. Bull. 2013, 2, 927–931. [Google Scholar]
- Anastopoulos, I.; Ighalo, J.O.; Adaobi Igwegbe, C.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Pashalidis, I.; Kalderis, D. Sunflower-biomass derived adsorbents for toxic/heavy metals removal from (waste) water. J. Mol. Liq. 2021, 342, 117540. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water—A review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs. commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- Tahir, D.; Heryanto, H. Novel corn stalks derived activated carbon for promising adsorption of lead (II) from aqueous solution: Characterization, IoT real-time monitoring, and performance investigation. Surf. Interfaces 2025, 61, 106138. [Google Scholar] [CrossRef]


| Metal | Permissible Limits in Drinking Water [10] | Sources | Related Health Problems | Reference |
|---|---|---|---|---|
| As | 10 g/L | naturally occurring in groundwater, mining | Skin, kidney, and bladder cancer, nausea, diarrhea, and muscle cramps | [9,11] |
| Cd | 0.003 mg/L | electroplating, alloys, photographic development, ceramic, naturally occurring | vomiting, diarrhea, (acute) asthma, and tubular dysfunction (chronic inhalation) | [12,13] |
| Cr | 0.05 mg/L (VI) | dyes and pigment manufacturing, wood preserving, electroplating | lung cancer, dermatitis, mutagenic | [14,15] |
| Cu | 2 mg/L | urban and industrial wastewater | stomach and intestine problems, neurotoxicity, jaundice, and liver toxicity | [7,16] |
| Hg | 1 μg/L | coalfired power plants, cement plants, and steel plants | adverse effects on the digestive tract, kidneys, capillaries, and nervous system | [17] |
| Fe | 0.3 mg/L * | coatings, car, aeronautics, and steel industries | / | [18,19] |
| Mn | 0.08 mg/L | naturally occurring, mining | neurological effects | [19] |
| Ni | bioavailable 4 μg/L (EU) | naturally occurring, electroplating, alloys, mining, waste incineration | skin irritation, dermatitis (dermal contact), nausea, vomiting, and diarrhea (ingestion), liver damage, and heart failure | [12,20] |
| Pb | 0.01 mg/L | tanneries, electronics, electroplating, and petrochemical industries | nervous system disorders, kidney failure, cancer, and cardiovascular disease | [16,21] |
| Zn | 3 mg/L | zinc water tanks, industrial wastewater | loss of appetite, decreased sense of taste and smell, slow wound healing, and skin sores | [12,16] |
| U | 30 µg/L | radioactive waste | Kidney, bone, lung damage | [10,22] |
| Process Methods for Removing Metal Ions from Water | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Chemical precipitation | -Treatment of water with high metal ion content. -Could be performed at ambient temperature -Simple operation, -Low costs | -Requires a high amount of precipitating agent -Does not remove complex metals -Generation of a large quantity of sludge, -The need to add chemical reagents to adjust the pH value -Corrosive effect on equipment when using strong alkaline precipitators | [23,24,25] |
| Ion exchange | -For the wastewater treatment with low metal ion content. -Reusing resin after regeneration -Easy to automate | -Expensive resin, -Required wash-out solvent for impregnated resins -Slow operation rate | [24,26] |
| Electrochemical treatment | -Selective removal of metal ions -No additional reagents are required -No generation of sludge -The coagulant is formed in situ | -High energy costs -Consumption, degradation, and passivation of electrodes | [25,27,28,29] |
| Membrane filtration | -The removal of trace concentrations of metal ions -High degree of metal ion removal -Does not require chemical reagents -Ensure good water quality | -The cost of the technology and the possibility of membrane fouling limit their large-scale use -Loss of efficiency during multiple processes | [25,30,31] |
| Adsorption | -Simple process -Wide range of applications -Large specific surface area and porosity -Susceptible to regeneration -Low costs | -Low selectivity -Decrease in adsorptive capacity following multiple steps -Adsorptive power decreases at a high concentration of pollutants | [25,32,33,34,35] |
| Adsorbent | Adsorbate | Temperature | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 | Reference |
|---|---|---|---|---|---|---|
| ACF | Pb2+, Cu2+ | 303 K | −26.12 Pb2+, −22.03 Cu2+ | 53.94 Pb2+ 23.39 Cu2+ | 264.87 J/mol K Pb2+ 149.96 J/mol K Cu2+ | [58] |
| 313 K | −29.28 Pb2+, −23.33 Cu2+ | |||||
| 323 K | −31.78 Pb2+ −25.66 Cu2+ | |||||
| 333 K | −34.08 Pb2+ −26.23 Cu2+ | |||||
| CCs-AC | Cr (VI) | 5 °C 15 °C 25 °C 35 °C 50 °C | 0.85 0.18 −0.26 −0.62 −1.85 | 12 kJ/mol | 40.69 J/mol K | [60] |
| Carbon derived from corn straw | Cr (VI) | 298 K 313 K 328 K | −2.42 −3.57 −4.86 | 21.70 kJ/mol | 0.08 kJ/mol K | [15] |
| CCAC | Cr (VI) | 288 K 293 K 298 K 303 K 308 K | −1.929 −1.663 −1.805 −1.843 −1.710 | 6.6875 kJ/mol | 6.5913 J/mol K | [61] |
| CAC AL-CAC | Fe (III) | 35 °C | −32.33, −26.47 (CAC, AL-CAC) | −3.03 (CAC) −6.56 (AL-CAC) | 81.51 J/mol K (CAC) 90.37 J/mol K (AL-CAC) | [18] |
| 40 °C | −33.12, −27.08 (CAC, AL-CAC) | |||||
| 45 °C | −34.05, −27.85 (CAC, AL-CAC) | |||||
| CS-C | Cd (II) | 25 °C 30 °C 40 °C 50 °C | −17.48 −17.97 −19.57 −21.12 | 26.83 | 148.7 J/mol K | [79] |
| AC-Ag-SiO2 | Cu2+, Pb2+, Cd2+, Zn2+ | 298 K | −6.85 Cu2+ −11.62 Pb2+ −5.45 Cd2+ −8.72 Zn2+ | 30.24 Cu2+ | 123.6 J/mol K Cu2+ | [16] |
| 308 K | −7.53 Cu2+ −11.36 Pb2+ −6.57 Cd2+ −11.69 Zn2+ | 25.89 Pb2+ | 126.4 J/mol K Pb2+ | |||
| 318 K | −8.87 Cu2+ −14.10 Pb2+ −7.59 Cd2+ −12.68 Zn2+ | 22.81 Cd2+ | 95.1 J/mol K Cd2+ | |||
| 328 K | −10.57 Cu2+ −15.57 Pb2+ −8.26 Cd2+ −13.72 Zn2+ | 39.12 Zn2+ | 162.4 J/mol K Zn2+ | |||
| CCAKN7 | U(VI) | 298 K 303 K 308 K 313 K | –32.45 −37.75 −39.84 −41.66 | −94.65 | −158.32 J/mol K | [22] |
| CCAK7 | 298 K 303 K 308 K 313 K | –32.38 −28.34 −26.32 –23.45 | −65.34 | −94.57 J/mol K | ||
| CC | 298 K 303 K 308 K 313 K | −30.21 −28.12 −24.37 −21.32 | −52.81 | −77.34 J/mol K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radenković, M.; Kovačević, M.; Radojičić, V.; Tošić, M.; Momčilović, M.; Živković, S. Corn Residue-Based Activated Carbon for Heavy Metal Removal: A Review of Adsorptive Performance and Properties. Processes 2025, 13, 3406. https://doi.org/10.3390/pr13113406
Radenković M, Kovačević M, Radojičić V, Tošić M, Momčilović M, Živković S. Corn Residue-Based Activated Carbon for Heavy Metal Removal: A Review of Adsorptive Performance and Properties. Processes. 2025; 13(11):3406. https://doi.org/10.3390/pr13113406
Chicago/Turabian StyleRadenković, Marina, Marija Kovačević, Vuk Radojičić, Miloš Tošić, Miloš Momčilović, and Sanja Živković. 2025. "Corn Residue-Based Activated Carbon for Heavy Metal Removal: A Review of Adsorptive Performance and Properties" Processes 13, no. 11: 3406. https://doi.org/10.3390/pr13113406
APA StyleRadenković, M., Kovačević, M., Radojičić, V., Tošić, M., Momčilović, M., & Živković, S. (2025). Corn Residue-Based Activated Carbon for Heavy Metal Removal: A Review of Adsorptive Performance and Properties. Processes, 13(11), 3406. https://doi.org/10.3390/pr13113406







