Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics
Abstract
1. Introduction
2. The Structure of Graphitic Carbon Nitride and the Mechanism of Photocatalytic Degradation of Antibiotics
2.1. The Basic Structure of Graphitic Carbon Nitride
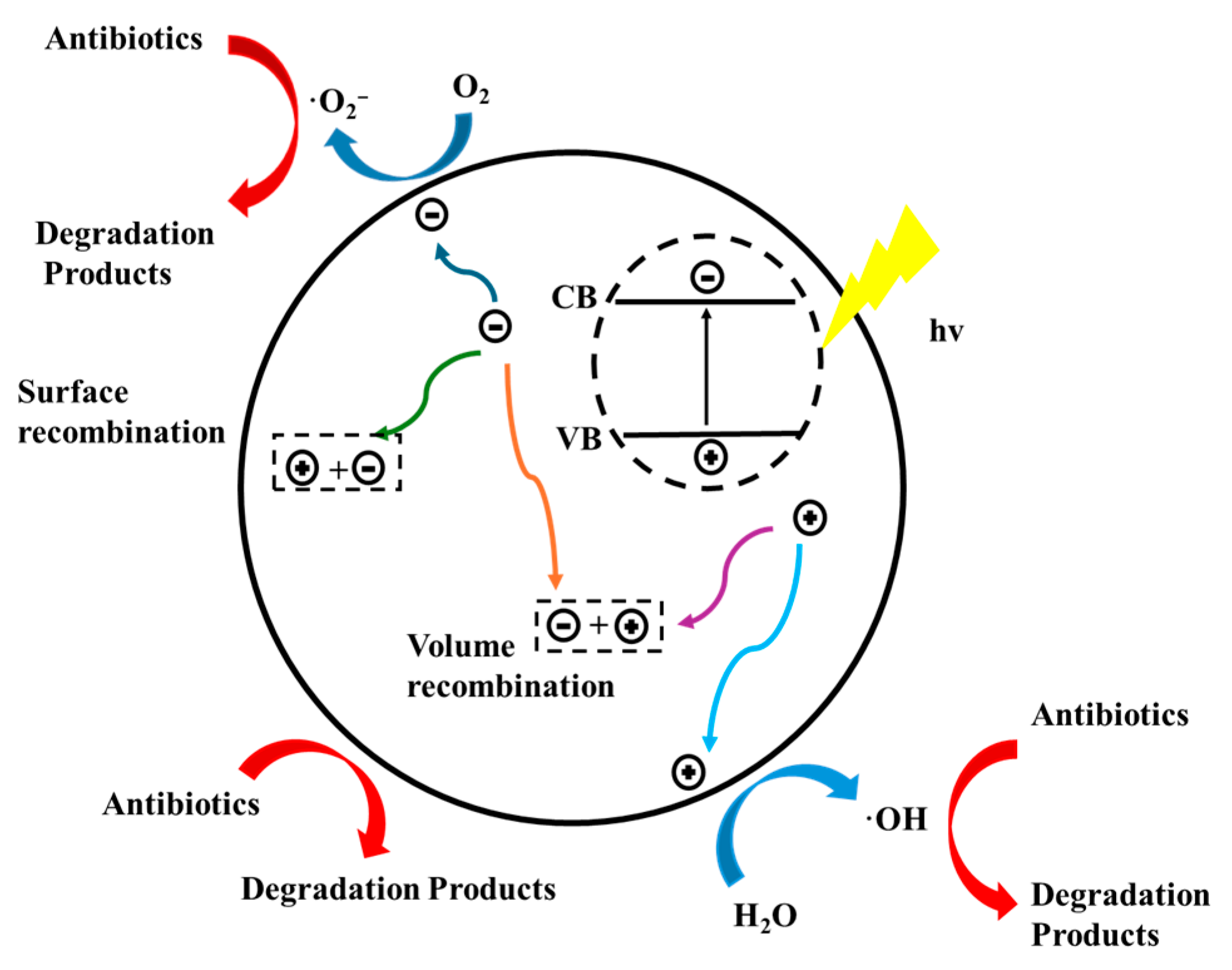
2.2. The Mechanism of Photocatalytic Degradation of Antibiotics by Graphitic Carbon Nitride
3. The Influence of Graphitic Carbon Nitride Precursors on Antibiotic Degradation
4. The Influence of Different Preparation Methods on the Degradation of Antibiotics by Graphitic Carbon Nitride
| Name | Principle of Preparation | Typical Product Characteristics | Scalability | Conclusion |
|---|---|---|---|---|
| Thermal Condensation Method | Carbon nitride is obtained by directly condensing nitrogen-rich precursors under high temperature in an air or inert gas atmosphere. | High crystallinity, but very low specific surface area (<10 m2/g), bulk morphology. | The process is simple, easily scalable to the kilogram level, and demonstrates excellent scalability. | Its low specific surface area severely limits the number of active sites, resulting in low intrinsic catalytic efficiency. Subsequent exfoliation or acid treatment is often required, which increases overall complexity and cost. |
| Solvothermal Method | The precursor is dissolved in a heat-conducting medium, and the mixed solution is placed in a high-pressure reaction kettle to prepare carbon nitride under specific temperature and pressure conditions. | Controllable morphology (nanosheets, microspheres), good dispersion, medium specific surface area. | Safety risks associated with high-pressure environments and the batch processing mode are major bottlenecks for scale-up production, resulting in poor scalability. | The high time cost and capital investment are traded for better morphology control. Its “mild” conditions come at the cost of sacrificing production efficiency and throughput; cost-effectiveness is a primary concern. |
| Template Method | The structure of the template material is used to guide the morphology and characteristics of carbon nitride. | Very high specific surface area (>100 m2/g), tunable pore structure. | The template removal step poses significant environmental issues and incurs additional purification costs, resulting in moderate scalability. | Performance enhancement comes at the cost of complex processes, high cost, and environmental unfriendliness. |
| Sol–Gel Method | A colloid (sol) is formed in the solution, which is then converted into solid material through drying and heat treatment. | High purity, homogeneous composition, medium to high specific surface area. | Shrinkage and cracking issues during the drying process are difficult to control at scale, adversely affecting product consistency and resulting in moderate scalability. | The advantage of “lower temperature” is often offset by expensive precursors and long process times. Low cost-effectiveness; more suitable for lab-scale preparation of materials requiring special purity. |
| High-Energy Microwave Method | Rapid heating of materials is achieved through microwave irradiation. | Rapid nucleation often leads to non-uniform crystallinity, easily forms ultra-thin nanosheets, nanorods, spheres, and other morphologies. | The process is characterized by poor reaction controllability and low yield, thus limited to small-scale laboratory preparation. | Despite outstanding time and energy efficiency, the fatal flaws of low yield and difficulty in scaling up currently prevent its application in actual production. |
5. The Influence of Modification Methods of Graphitic Carbon Nitride on Antibiotic Degradation
5.1. Crystal Structure Optimization
5.2. Element Doping
5.3. Surface Modification
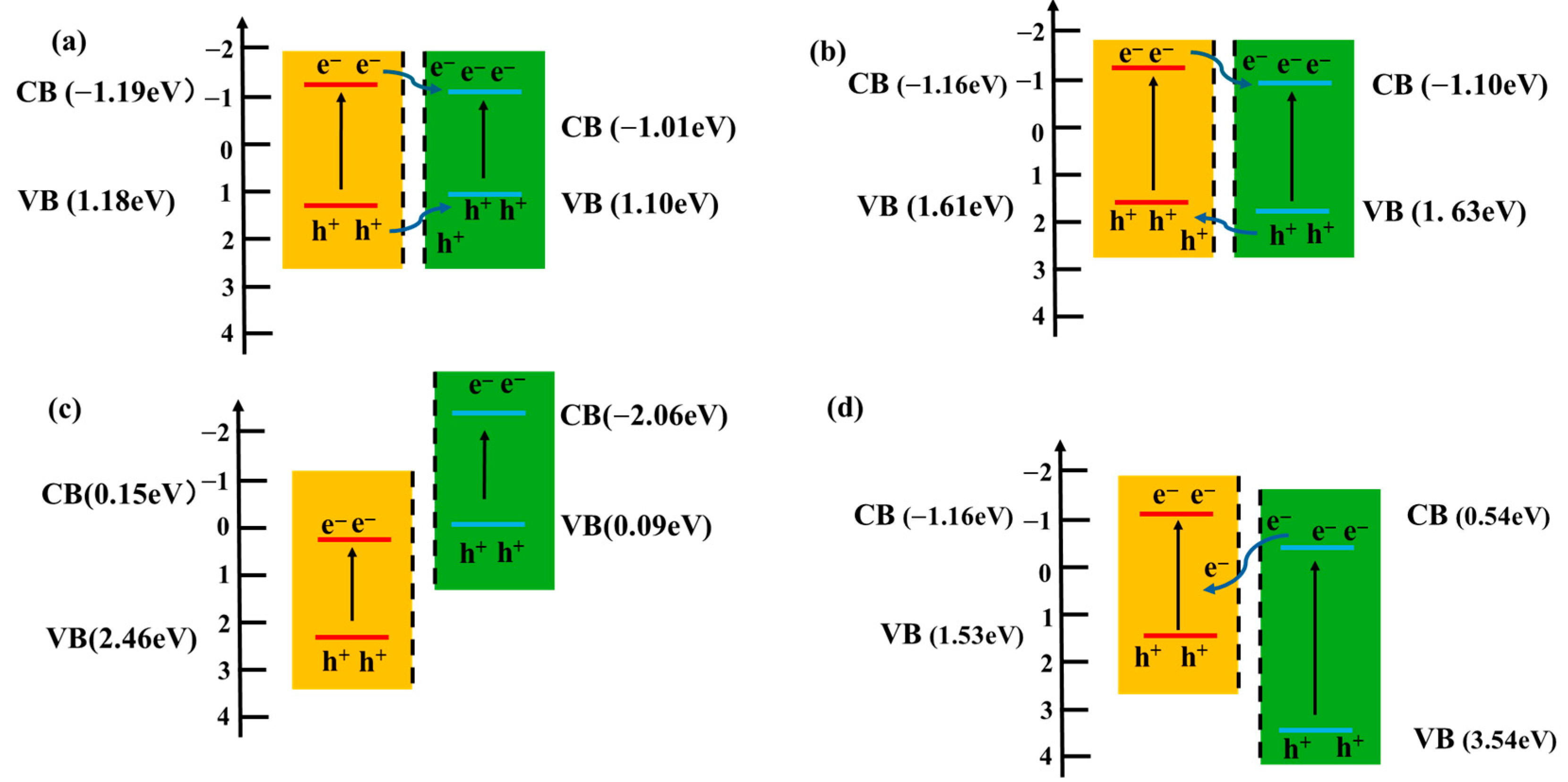
5.4. Constructing Heterojunctions
6. Future Development Trends of Graphitic Carbon Nitride
6.1. Application in Environmental Engineering
6.2. Machine Learning-Assisted Design of g-C3N4 Materials
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antos, J.; Piosik, M.; Ginter-Kramarczyk, D.; Zembrzuska, J.; Kruszelnicka, I. Tetracyclines Contamination in European Aquatic Environments: A Comprehensive Review of Occurrence, Fate, and Removal Techniques. Chemosphere 2024, 353, 141519. [Google Scholar] [CrossRef]
- Zhu, M.-C.; Lu, Y.-Z.; Chen, S.-W.; Hu, Z.-X.; Wang, J.-W.; Li, N.; Zeng, R.J.-X. Carbon Nano-Onions Acting as Artificial Pili Enhance Chloramphenicol Degradation in an Anaerobic Membrane Bioreactor. Chem. Eng. J. 2023, 475, 146110. [Google Scholar] [CrossRef]
- Işıtan, A. Sustainable Adsorption of Amoxicillin and Sulfamethoxazole onto Activated Carbon Derived from Food and Agricultural Waste: Isotherm Modeling and Characterization. Processes 2025, 13, 2528. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W. Degradation of Veterinary Antibiotics by the Ozonation Process: Product Identification and Ecotoxicity Assessment. J. Hazard. Mater. 2024, 469, 134026. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Zhao, G.; Su, P.; Wang, J.; Li, Y.; Zhou, W.; Mu, Y.; Zhang, J.; Liu, W. A Critical Review on Antibiotics Removal by Persulfate-Based Oxidation: Activation Methods, Catalysts, Oxidative Species, and Degradation Routes. Process. Saf. Environ. Prot. 2024, 187, 622–643. [Google Scholar] [CrossRef]
- Phuong, N.M.; Hai, P.V.; Viet, N.M.; Pham, T.-D.; Ha, T.T.V.; Hoang, D.H.; Minh, D.N.; Noi, N.V.; Rene, E.R.; Minh, T.D. Synthesis and Characterization of Z-Scheme Heterojunction CoWO4/RGO/g-C3N4 as a Visible Light-Driven Photocatalyst for Novel Removal of Organic Pollutant. J. Environ. Eng. 2023, 149, 04022098. [Google Scholar] [CrossRef]
- Liu, W.; Hui, J.; Cheng, X.; Zhang, L.; Li, Y.; Li, C.; Qi, C. Experimental Study on the Activation Energy of Coal Oxidation Under Different Oxygen Concentrations. Processes 2025, 13, 2889. [Google Scholar] [CrossRef]
- Zhao, B.; Zhong, W.; Chen, F.; Wang, P.; Bie, C.; Yu, H. High-Crystalline g-C3N4 Photocatalysts: Synthesis, Structure Modulation, and H2-Evolution Application. Chin. J. Catal. 2023, 52, 127–143. [Google Scholar] [CrossRef]
- Roy, R.; Chacko, A.R.; Abraham, T.; Korah, B.K.; John, B.K.; Punnoose, M.S.; Mohan, C.; Mathew, B. Recent Advances in Graphitic Carbon Nitrides (g-C3N4) as Photoluminescence Sensing Probe: A Review. ChemistrySelect 2022, 7, e202200876. [Google Scholar] [CrossRef]
- Dai, C.; Feng, Z.; Hu, Q.; Qiu, J.; You, J.; Guo, R.; Liu, X.; Zhang, H. Recent Progress in Modification and Composite Strategies of Graphitic Carbon Nitride as Catalysts for Heterogeneous Photo-Fenton Reaction. Mater. Sci. Semicond. Process. 2023, 167, 107807. [Google Scholar] [CrossRef]
- Moeinifard, B.; Najafi Chermahini, A. Fabrication of Au-Doped Mesoporous TiO2 Supported on g-C3N4 as an Efficient Light-Assisted Catalyst for Oxidative Desulfurization of Model Fuels with Different Sulfur Content. Int. J. Mech. Mater. Eng. 2025, 20, 14. [Google Scholar] [CrossRef]
- Ya, Z.; Zhang, S.; Xu, D.; Wang, H.; Li, M. Coupling Plastic Upgrading and Photocatalysis: Catalytic Mechanisms and Design Principles. ACS Catal. 2025, 15, 5339–5369. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, J.; Zhang, W.; Zhou, M.; Zhu, X.; Liu, Z.; Li, Y.; Guan, Z.; Lee, C.-S.; Wong, P.K.; et al. Modified-Pollen Confined Hybrid System: A Promising Union for Visible-Light-Driven Photocatalytic Antibiotic Degradation. Appl. Catal. B Environ. Energy 2023, 330, 122621. [Google Scholar] [CrossRef]
- Chand, H.; Kumar, A.; Goswami, S.; Krishnan, V. Comparison of Catalytic Activity of Graphitic Carbon Nitrides Derived from Different Precursors for Carbon Dioxide Conversion. Fuel 2023, 357, 129757. [Google Scholar] [CrossRef]
- Yu, Q.; Ren, X.; Pan, J.; Wang, Q.; Li, Y.; Shi, N. Chemical Bonds in Precursors Regulate G-C3N4 Structure and Its Photocatalytic Performance. J. Alloys Compd. 2022, 910, 164953. [Google Scholar] [CrossRef]
- Chamorro-Posada, P.; Dante, R.C.; Vázquez-Cabo, J.; Dante, D.G.; Martín-Ramos, P.; Rubiños-López, Ó.; Sánchez-Arévalo, F.M. From Urea to Melamine Cyanurate: Study of a Class of Thermal Condensation Routes for the Preparation of Graphitic Carbon Nitride. J. Solid State Chem. 2022, 310, 123071. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Sun, X.; Zheng, X.; Zhang, X.; Guan, X. Enhanced Photocatalytic Performance and Mechanism of N-Deficiently Porous g-C3N4 in Organic Pollutant Degradation. Mater. Res. Bull. 2023, 169, 112510. [Google Scholar] [CrossRef]
- Ba, G.; Hu, H.; Bi, F.; Yu, J.; Liu, E.; Ye, J.; Wang, D. Engineering Nitrogen Vacancies and Cyano Groups into C3N4 Nanosheets for Highly Efficient Photocatalytic H2O2 Production. Appl. Catal. B Environ. Energy 2024, 361, 124645. [Google Scholar] [CrossRef]
- Lei, S.; Wang, W.; Wang, C.; Li, W.; Xu, Z.; Li, G.; An, T. Photo-Transformation of Graphitic Carbon Nitride Synthesized from Different Precursors: Influence on Environmental Fate and Photocatalytic Water Disinfection. J. Water Process Eng. 2024, 61, 105279. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. The Effect of Precursor Selection on the Microwave-Assisted Synthesis of Graphitic Carbon Nitride. Catal. Today 2022, 424, 113868. [Google Scholar] [CrossRef]
- Zhai, Y.; Lv, L.; Fan, J. Synthesis of CuO/ZnWO4 Heterojunction Structure for H2S Gas Sensor with Ultra-High Response Value at Room Temperature. Processes 2025, 13, 2727. [Google Scholar] [CrossRef]
- Kailasam, K.; Epping, J.D.; Thomas, A.; Losse, S.; Junge, H. Mesoporous Carbon Nitride–Silica Composites by a Combined Sol–Gel/Thermal Condensation Approach and Their Application as Photocatalysts. Energy Environ. Sci. 2011, 4, 4668. [Google Scholar] [CrossRef]
- Muhmood, T.; Uddin, A. Fabrication of Spherical-Graphitic Carbon Nitride via Hydrothermal Method for Enhanced Photo-Degradation Ability towards Antibiotic. Chem. Phys. Lett. 2020, 753, 137604. [Google Scholar] [CrossRef]
- Wu, X.; Fan, H.; Wang, W.; Lei, L.; Chang, X. Ordered and Ultralong Graphitic Carbon Nitride Nanotubes Obtained via In-Air CVD for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2021, 4, 13263–13271. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Xue, J.; Wang, R.; Dong, J.; Yin, Z.; Guo, C.; Wang, H. Electrochemically Assisted Preparation of Graphitic Carbon Nitride Nanosheet Membranes for Efficient Water Purification. Chem. Eng. J. 2024, 490, 151638. [Google Scholar] [CrossRef]
- Kamble, G.; Ganai, A.M.; Lakshmi, D.V.; Rao, N.N.; Rajarikam, N.; Rao, P.V. Impact of Pyrolysis Temperature on Physicochemical Properties of Carbon Nitride Photocatalyst. Semicond. Sci. Technol. 2023, 36, 055020. [Google Scholar] [CrossRef]
- Shenoy, S.; Chuaicham, C.; Sekar, K.; Sasaki, K. Seamless Carbon Nitride Growth on Bimetallic Oxide for Antibiotic Residue Degradation. Environ. Chem. Lett. 2024, 23, 33–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, D.; Wang, Z.; Yi, L.; Sun, J.; Liu, D.; Yu, X.; Chen, Y. Bandgap Engineering of Carbon Nitride by Formic Acid Assisted Thermal Treatment for Photocatalytic Degradation of Tetracycline Hydrochloride. Chem. Eng. J. 2024, 485, 149830. [Google Scholar] [CrossRef]
- Xiao, Y.; Geng, A.; Zhu, J.; Xu, X.; Xu, X. Metal Doped Graphitic Carbon Nitride Prepared by a Bubbling Template Method for Photo-Degradation of Organic Pollutants. J. Phys. Appl. Phys. 2022, 55, 434002. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Liao, J.; Xiang, X.; Song, H.; Yang, A.; Ibupoto, Z.H.; Lv, K. Photocatalytic Degradation of Sulfonamides in Suspensions of Coral-like Graphene Carbon Nitride with Nitrogen Vacancies. Chemosphere 2024, 352, 141313. [Google Scholar] [CrossRef]
- Mohamed, H.H.; Aziz, M.; Youssef, T.E.; Alomair, N.A. Microwave Synthesized G-C3N4 Nanofibers with Modified Properties for Enhanced Solar Light Photocatalytic Performance. Inorg. Chem. Commun. 2024, 168, 112975. [Google Scholar] [CrossRef]
- Li, P. The Study of G-C3N4 with Modified Precursor Prepared by Microwave and Its Properties. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2024. [Google Scholar]
- Briševac, D.; Gabelica, I.; Radovanović-Perić, F.; Tolić Čop, K.; Matijašić, G.; Ljubas, D.; Ćurković, L. Influence of Different Precursors on Properties and Photocatalytic Activity of G-C3N4 Synthesized via Thermal Polymerization. Materials 2025, 18, 2522. [Google Scholar] [CrossRef]
- Latif, M.J.; Ali, S.; Jamil, S.; Bibi, S.; Jafar, T.; Rasheed, A.; Noreen, S.; Bashir, A.; Rauf Khan, S. Comparative Catalytic Reduction and Degradation with Biodegradable Sodium Alginate Based Nanocomposite: Zinc Oxide/N-Doped Carbon Nitride/Sodium Alginate. Int. J. Biol. Macromol. 2023, 254, 127954. [Google Scholar] [CrossRef] [PubMed]
- Medina-Llamas, M.; Bianchi, E.; Mozzati, M.C.; Tedesco, C.; Milanese, C.; Speltini, A.; Profumo, A.; Armenise, V.; Milella, A.; Listorti, A.; et al. Synthesis of Carbon Nitride Polymorphs by Sacrificial Template Method: Correlation between Physicochemical Properties and Photocatalytic Performance. ChemSusChem 2024, 18, e202400918. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhang, Z.; Pan, Y.; Leung, M.K.H.; Zhang, Y.; Chen, K. Carbon Nitride Gels: Synthesis, Modification, and Water Decontamination Applications. Gels 2025, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, J.; Huang, Q.; Wang, X.; Li, Z.; Li, M.; Zhou, W. Element Engineering in Graphitic Carbon Nitride Photocatalysts. Renew. Sustain. Energy Rev. 2024, 199, 114482. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; An, S.; Li, K.; Zhang, J.; Pei, M.; Song, C.; Guo, X. Ultrathin Crystalline Carbon Nitride Nanosheets for Highly Efficient Photocatalytic Pollutant Removal and Hydrogen Production. ACS Appl. Nano Mater. 2023, 6, 11601–11611. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Gong, Y.; Zhu, C.; Zhang, L.; Tang, H.; He, W.; Wang, B. Bi(Ⅲ) and Ce(Ⅳ) Functionalized Carbon Nitride Photocatalyst for Antibiotic Degradation: Synthesis, Toxicity, and Mechanism Investigations. Chemosphere 2023, 333, 138888. [Google Scholar] [CrossRef]
- Yuan, A.; Lei, H.; Xi, F.; Liu, J.; Qin, L.; Chen, Z.; Dong, X. Graphene Quantum Dots Decorated Graphitic Carbon Nitride Nanorods for Photocatalytic Removal of Antibiotics. J. Colloid Interface Sci. 2019, 548, 56–65. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Li, C.; Dai, L.; Hao, Z.; Yu, J.; He, H.; Si, C.; Shen, Z.; Qiu, Z.; et al. Metal-Free Graphitic Carbon Nitride/Black Phosphorus Quantum Dots Heterojunction Photocatalyst for the Removal of ARG Contamination. Adv. Compos. Hybrid Mater. 2023, 6, 145. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Hakki, A.; Macphee, D.E.; Liu, P.; Hu, S. The Influence of Zeolites Fly Ash Bead/TiO2 Composite Material Surface Morphologies on Their Adsorption and Photocatalytic Performance. Appl. Surf. Sci. 2017, 392, 687–696. [Google Scholar] [CrossRef]
- Wang, K.; Shu, Z.; Zhou, J.; Zhao, Z.; Wen, Y.; Sun, S. Enhancing Piezocatalytic H2O2 Production through Morphology Control of Graphitic Carbon Nitride. J. Colloid Interface Sci. 2023, 648, 242–250. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Thakur, C.; Pal, D. Synthesis of Exfoliated Graphitic Carbon Nitride (g-C3N4) for Tetracycline Hydrochloride (TCH) Degradation: Photocatalytic Efficiency and Mechanisms. Arab. J. Sci. Eng. 2025, 50, 10039–10050. [Google Scholar] [CrossRef]
- Zhan, X.; Zeng, Y.; Zhang, H.; Wang, X.; Jin, D.; Jin, H.; Luo, S.; Yang, L.; Hong, B. The Coral-like Carbon Nitride Array: Rational Design for Efficient Photodegradation of Tetracycline under Visible Light. J. Environ. Chem. Eng. 2022, 11, 109201. [Google Scholar] [CrossRef]
- Oh, W.-D.; Chang, V.W.C.; Hu, Z.-T.; Goei, R.; Lim, T.-T. Enhancing the Catalytic Activity of G-C3N4 through Me Doping (Me = Cu, Co and Fe) for Selective Sulfathiazole Degradation via Redox-Based Advanced Oxidation Process. Chem. Eng. J. 2017, 323, 260–269. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Bai, X.; Rao, X.; Zhang, Y. N, S-Doped Graphene Quantum Dots Grafted Graphitic Carbon Nitride to Boost Its Photocatalytic Hydrogen Evolution and Antibacterial Activity. Nano 2022, 17, 2250066. [Google Scholar] [CrossRef]
- Singh, S.; Singhal, R.; Yadav, R.K.; Gupta, N.K. In-Situ ‘X’ Doped ‘GCN’ Photocatalyst Enables Selective Aldehyde Synthesis via Photo-Oxidation of Benzyl Alcohol under Ambient Conditions. Diam. Relat. Mater. 2024, 149, 111609. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, L.; Luo, J.; Lu, X.; Jia, B.; Liang, L.; Zhang, J. Photocatalytic Degradation of Ciprofloxacin by Gd-Co/g-C3N4 under Low-Power Light Source: Degradation Pathways and Mechanistic Insights. J. Water Process Eng. 2024, 58, 104849. [Google Scholar] [CrossRef]
- Kong, Y.; Li, D.; Zhang, C.; Han, W.; Xue, Y.; Zhang, W.; Sun, H.; Wang, S.; Duan, X. Synergistic Silver Doping and N Vacancy Promoting Photocatalytic Performances of Carbon Nitride for Pollutant Oxidation and Hydrogen Production. Chem. Eng. J. 2023, 479, 147676. [Google Scholar] [CrossRef]
- Bhoyar, T.; Saraswat, N.; Jyothirmai, M.V.; Gupta, A.; Malla, S.K.; Park, J.; Vidyasagar, D.; Umare, S.S. Nitrogen-Doped Graphitic Carbon Dots Embedded in Carbon Nitride Scaffolds for Water Decontamination. ACS Appl. Nano Mater. 2023, 6, 3484–3496. [Google Scholar] [CrossRef]
- Gan, J.; Ma, X.; Qi, X.; Qin, Q.; Gong, Y.; Han, J.; Jin, T. Sunlight-Induced Multifunctional Photocatalyst of Gold-Deposited Graphitic Carbon Nitride with Enhanced Efficiency of Antibacterial, Antiviral, and Antibiotic Degradation. J. Environ. Chem. Eng. 2023, 12, 111810. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Sun, Z.; Liu, Q.; Ma, J. Carbon Quantum Dot Implanted Graphite Carbon Nitride Nanorod with Enhanced Photocatalytic Activity: Mechanisms, Degradation Pathway and DFT Calculation. Mater. Sci. Semicond. Process. 2023, 172, 108056. [Google Scholar] [CrossRef]
- Deng, A.; Sun, Y.; Gao, Z.; Yang, S.; Liu, Y.; He, H.; Zhang, J.; Liu, S.; Sun, H.; Wang, S. Internal Electric Field in Carbon Nitride-Based Heterojunctions for Photocatalysis. Nano Energy 2023, 108, 108228. [Google Scholar] [CrossRef]
- Lei, Y.; Ye, J.; García-Antón, J.; Liu, H. Recent Advances in the Built-in Electric-Field-Assisted Photocatalytic Dry Reforming of Methane. Chin. J. Catal. 2023, 53, 72–101. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, W.; Huang, K.; Guo, H.; Wang, Z.; Lian, C.; Zhang, J.; Wu, D.; Lei, Z.; Liu, Z.; et al. Engineering Built-In Electric Field Microenvironment of CQDs/g-C3N4 Heterojunction for Efficient Photocatalytic CO2 Reduction. Adv. Sci. 2024, 11, 2403607. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, J.; Yan, X.; Li, L.; Liu, G.; Ji, M.; Wang, B.; She, Y.; Li, H.; Xia, J. Construction of Carbonized Polymer Dots/Potassium Doped Carbon Nitride Nanosheets Van Der Waals Heterojunction by Ball Milling Method for Facilitating Photocatalytic CO2 Reduction Performance in Pure Water. Appl. Catal. B Environ. Energy 2024, 351, 123993. [Google Scholar]
- Tofaz, T.; Lu, X.-J.; Chen, S.; Li, J.-H.; Ding, H.-Y.; Ullah, I.; Habib, S.; Murtaza, G.; Xu, A.-W. 4,4′,4″-Nitrilotribenzoic Acid/Graphitic Carbon Nitride Type II Heterostructures for Highly Efficient Photocatalytic Hydrogen Evolution from Water Splitting. ACS Appl. Energy Mater. 2024, 7, 12016–12026. [Google Scholar] [CrossRef]
- Ramos Corona, A.; Rodríguez López, J.; Rangel Segura, R.; Martínez Garcia, M.M.; Flores, E.; Rodríguez Gattorno, G.; Alvarado Gil, J.J. Microwave-Assisted Synthesis of CdS-MOF MIL-101 (Fe) Composite: Characterization and Photocatalytic Performance. Inorg. Chem. 2024, 63, 19536–19552. [Google Scholar] [CrossRef]
- Malefane, M.E.; Mafa, P.J.; Managa, M.; Nkambule, T.T.I.; Kuvarega, A.T. Understanding the Principles and Applications of Dual Z-Scheme Heterojunctions: How Far Can We Go? J. Phys. Chem. Lett. 2023, 14, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, Y.; Binas, V.; Shen, S. Interface Engineering of Z-Scheme Heterojunction for Photocatalytic Water Splitting. Fundam. Res. 2024, 5, 2204–2208. [Google Scholar] [CrossRef]
- Dang, J.; Zhang, J.; Shen, Y.; Wang, L.; Guo, F.; Li, Y.; Guan, W. Fabrication of Magnetically Recyclable Fe3O4/BiOCl/BiOBr Nanocomposite with Z-Scheme Heterojunction for High-Efficiency Photocatalytic Degradation of Tetracycline. Mater. Sci. Semicond. Process. 2023, 158, 107371. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Liu, S.; Zhang, B.; Zhu, H.; Chen, H.; Wen, B.; Chen, L. Preparation of TiO2-Graphitized Carbon Composite Photocatalyst and Their Degradation Properties for Tetracycline Antibiotics. J. Mol. Struct. 2022, 1270, 133897. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, J.; Pang, L.; Wangjin, Y.; Li, M.; Zhao, Z.; Huang, L. Removal of Antibiotics and Antibiotic Resistance Genes from Urban Rivers Using a Photocatalytic-and-Bionic Artificial Ecosystem. J. Clean. Prod. 2022, 348, 131311. [Google Scholar] [CrossRef]
- Rapti, I.; Boti, V.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Psychiatric Pharmaceuticals in Hospital WWTP Secondary Effluents Using G-C3N4 and g-C3N4/MoS2 Catalysts in Laboratory-Scale Pilot. Catalysts 2023, 13, 252. [Google Scholar] [CrossRef]
- Wang, W.; Qu, Y.; Li, D.; Zhang, A.; Yan, H.; Feng, Z.; Yao, W. The Defect Chemistry and Machine Learning Study 5d Transition Metal Doped on Graphitic Carbon Nitride for Bifunctional Oxygen Electrocatalyst with Low Overpotential. Int. J. Hydrogen Energy 2024, 79, 702–714. [Google Scholar] [CrossRef]
| Precursor | Preparation Process and Structure Morphology | Performance Characteristics |
|---|---|---|
| Urea | Pyrolysis at 500–600 °C produces a dispersed sheet structure with high porosity. | Specific surface area (BET) typically ranging from 60 to 100 m2/g, a band gap of approximately 2.7 eV [17]. |
| Melamine | Pyrolysis above 500 °C yields a dense and bulky block-like morphology with an internally ordered layered structure. | Exhibits good crystallinity, demonstrates an ordered layered structure, and possesses a band gap of approximately 2.7 eV [18]. |
| Thiourea | Thiourea pyrolysis at 550–650 °C yields porous sheet-like architectures. | Sulfur doping effectively narrows the bandgap and enhances visible light absorption [19]. |
| Cyanamide | Copyrolysis at 500 °C results in amorphous bulk-aggregated morphology. | It exhibits an amorphous bulk-aggregated morphology with low specific surface area and a band gap of approximately 2.7 eV [20]. |
| Optimization Method | Description | Main Advantages | Exemplary Studies |
|---|---|---|---|
| Crystal Structure Optimization | Morphology control to increase specific surface area and reduce defects | Improved photocatalytic performance and enhanced electron-hole transport efficiency | The prepared crystalline polymeric carbon nitride (CCN) with an ultrathin two-dimensional nanosheet structure efficiently removes various high-concentration organic pollutants (50 mg·L−1) and achieves synergistic removal of organic contaminants and heavy metal ions. Within 40 min, the removal rate for all organic pollutants—including antibiotics and dyes—exceeds 95%. |
| Element Doping | Doping with metals and non-metals to adjust the band structure and create impurity levels | Expanded light absorption range and reduced recombination rate of photogenerated electron-hole pairs | A series of Bi/Ce/g-C3N4 photocatalysts with different doping ratios were prepared by direct calcination and applied for the photocatalytic degradation of Rhodamine B (RhB) and sulfamethoxazole (SMX). Experimental results demonstrated that the photocatalytic performance of Bi/Ce/g-C3N4 surpassed that of single-component samples. Under optimal conditions, the degradation rates of RhB (20 min) and SMX (120 min) by Bi/Ce/g-C3N4 reached 98.3% and 70.5%, respectively. |
| Surface Modification | Optimizing electronic structure through noble metal deposition and molecular/ionic modification | Increased light absorption capacity and facilitated rapid separation of electron-hole pairs | A metal-free composite photocatalyst comprising zero-dimensional (0D) graphene quantum dots (GQDs) decorated graphitic carbon nitride nanorods (g-CNNR) was successfully prepared via a hydrothermal method. Physicochemical characterization revealed that the GQDs/g-CNNR photocatalyst exhibits high crystallinity, enhanced visible-light absorption, and a staggered band alignment, which collectively facilitate the generation, migration, and separation of photoinduced electrons and holes. These advantages contribute to the significantly improved photocatalytic activity of GQDs/g-CNNR for efficient antibiotic degradation. Its photocatalytic reaction rate is 3.46 and 2.03 times higher than that of g-C3N4. |
| Heterojunction Construction | Combining different semiconductors to form heterostructures that reduce electron-hole recombination | Enhanced photoelectric conversion efficiency and extended spectral response range | A non-metallic heterojunction composite photocatalyst (H-g-C3N4/BPQDs) was synthesized using g-C3N4 and black phosphorus quantum dots (BPQDs) as raw materials through a process involving hydrothermal impregnation, high-temperature calcination, and ice-assisted ultrasonication. The obtained H-g-C3N4/BPQDs were applied for the removal of antibiotics and biofouling from water under visible-light irradiation. Owing to the porous structure and high specific surface area of H-g-C3N4, the resulting Type II heterojunction structure enhanced visible-light absorption, accelerated interfacial charge transfer, and suppressed the recombination of photogenerated electron-hole pairs. Under visible-light irradiation, the degradation efficiency of H-g-C3N4/BPQDs for tetracycline (TC) exceeded 91% within 30 min. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhu, M.; Chen, D.; Wu, J.; Gao, S.; Zhao, Y.; Yang, J.; Li, S.; Meng, J. Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics. Processes 2025, 13, 3365. https://doi.org/10.3390/pr13103365
Lu X, Zhu M, Chen D, Wu J, Gao S, Zhao Y, Yang J, Li S, Meng J. Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics. Processes. 2025; 13(10):3365. https://doi.org/10.3390/pr13103365
Chicago/Turabian StyleLu, Xiaoning, Mingchao Zhu, Dongdong Chen, Jiayang Wu, Shuangqian Gao, Yimin Zhao, Junling Yang, Shuping Li, and Jiang Meng. 2025. "Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics" Processes 13, no. 10: 3365. https://doi.org/10.3390/pr13103365
APA StyleLu, X., Zhu, M., Chen, D., Wu, J., Gao, S., Zhao, Y., Yang, J., Li, S., & Meng, J. (2025). Preparation, Modification, and Application of Graphitic Carbon Nitride in Photocatalytic Degradation of Antibiotics. Processes, 13(10), 3365. https://doi.org/10.3390/pr13103365





