Abstract
A stable atmosphere is very important for long-term life science experiments with small mammals in sealed habitats. In this study, we constructed a multi-stage, solid-state purification system to regulate and control the supply of oxygen and remove carbon dioxide, humidity, and trace contaminants in a sealed metal box. Lithium hydroxide was used as the material to absorb CO2; molecular sieves and humidity-indicating silica gel were used as dehumidification material; and activated carbon-based sorbent was used to remove ammonia and hydrogen sulfide. We evaluated the performance of the system by implementing a sealed culture test with mice for 7 days. The pressure, relative humidity, oxygen concentration, carbon dioxide concentration, and ammonia/hydrogen sulfide concentration in the sealed box were maintained at 101–103 kPa, 30–36%, 19–21%, <1000 ppm, and the main goal of this study was to test for single gas absorption. In the future, we will investigate the competitive effect of multi-gas mixtures.
1. Introduction
Space life science research has evolved considerably since the 20th century, with rodents used as model organisms in experiments [1]. The primary focus of early studies was to evaluate the basic survival skills of organisms in the harsh conditions of space [2,3]. Over time, research expanded to investigate more complex physiological aspects, including vital life processes like growth and development of organisms in microgravity and radiation environments [4]. Most rodent experiments have been conducted on returnable satellites or space stations, such as Russia’s biological satellites Cosmos and Bion [5,6,7]. In 1993, the International Space Station (ISS) was collaboratively established by the United States, European Union member states, Japan, and 15 other countries [8]. To this date, it is the largest space station ever constructed. Therefore, to support these research needs, space agencies such as NASA, ASI, and JAXA have designed rodent breeding facilities and environmental control and life support systems specifically for space animal experiments. They conduct experiments on rodents, including rats and mice, in space to study changes in the physiological states of animals [9,10,11,12].
There has been a long-standing focus on optimizing the environmental conditions of animal facilities. The environmental control systems of animal experiment facilities in various countries are shown in Figure 1. NASA’s first animal facility for piloted spaceflight is the Animal Enclosure Module (AEM) [13]. The AEM lacks a dedicated environmental control system; instead, it depends on the spacecraft’s environmental control system, as shown in Figure 1b. Air enters the animal enclosures through a HEPA/charcoal filter located at the back of the AEM [14]. It then passes through a glass fiber pad filter treated with phosphoric acid and a charcoal bed designed to collect waste and reduce odors [14]. Finally, the air is exhausted into the interior of the spacecraft.
NASA initially developed the RAHF (Research Animal Holding Facility). During the SL-3 flight, the RAHF identified two hardware issues: food and fecal contamination, as well as odor leakage [15]. The environmental control system of the RAHF device is shown in Figure 1a. The RAHF enhanced sealing by adding gaskets, coarse filters, and fans to improve ventilation throughout the housing cage. Before releasing gases into the space laboratory, all gases exiting the RAHF must pass through two HEPA filters, each with at least 99.97% removal efficiency for particles ≥ 0.3 μm [14,16]. The Bondinam™ is a HEPA coarse filter treated with a phosphoric acid solution that effectively suppresses odors mainly from ammonia in rodent urine [16]. In 2016, the Japan Aerospace Exploration Agency (JAXA) developed the Multiple Artificial-gravity Research System (MARS) as a platform for animal experimentation. The HCU (Habitat Cage Unit) is an onboard living cage within the MARS system [17], as shown in Figure 1c. The HCU floor stays clean because mice feces tumbles into the hole. Paper sheets on the bottom and walls of the HCU are coated with photocatalytic thermal spray to provide deodorization and antibacterial effects [18]. Mice produce droplet-shaped urine, which is quickly absorbed by the paper sheets through airflow. The cage’s air circulation is maintained by the HCU’s fan, which generates an airflow of less than 0.2 m/s [17,19]. The Habitat Supply Unit (BOS) for the Bion-M1 biological satellite, developed by the Russian Institute of Biomedical Problems (IBMP), is a cylindrical structure, as shown in Figure 1d. Waste is removed through a grid on the opposite side of the cylinder wall [11,20].
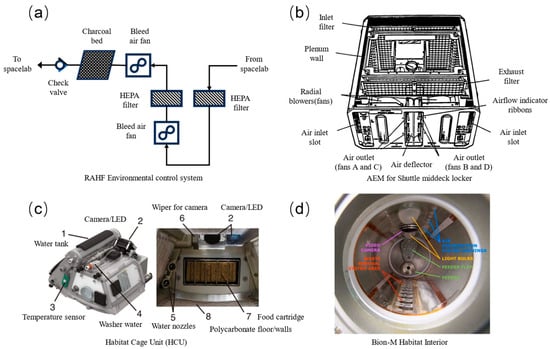
Figure 1.
Environmental control systems: (a) RAHF environmental control system [15], (b) AEM for shuttle middeck locker [13], (c) Habitat Cage Unit [17], and (d) Bion-M habitat interior [11,20].
The small size, rapid reproduction, and well-established research models of mice have made them the preferred subjects for space mammal experiments. Research teams worldwide have developed various feeding systems extensively used in space life science to ensure the long-term survival of mice in space. The U.S. National Aeronautics and Space Administration (NASA) recently created animal cultivation facilities, including the Research Animal Habitat Facility (RAHF) [15], the Rodent Habitat Hardware System (RHHS) [21], and the Animal Experiment Module (AEM) [13]. The Mice Drawer System (MDS) was jointly developed by Thales Alenia Space and the Italian Space Agency (ASI) [10]. The Block Obespecheniya Soderzhaniya (BOS; Russian for—literally—“Unit for the Provision of Housing”) installed on the Bion-M1 (launched in 2013) and the Advanced Animal Housing Facility in the Japanese Experiment Module (JEM-AAHF) [14], developed jointly by the Institute of Environmental Medicine at Nagoya University (led by Professor Satoru Watanabe) and Kawasaki Heavy Industries, installed in the Japanese Experiment Module of the International Space Station, all rely on filtered cabin air for gas exchange.
These systems were developed by the Russian Institute of Biomedical Problems (IMBP). However, our experiment showed that long-term feeding causes animal odors and the production of harmful metabolic gases, including ammonia, volatile organic compounds (VOCs), and hydrogen sulfide. If gases are not removed quickly, they can easily cause air pollution inside the space station cabin, which may compromise the experiment’s accuracy and even endanger the astronauts’ health. These toxic gases have become a major hidden danger that limits the continuation of small mammal experiments in space. Modules: the electronic control system and the gas distribution system are responsible for monitoring and optimizing gas composition in a closed environment. To further assess the adsorption kinetics and purification efficiency of various gas purification materials—including color-changing silica gel, 5A molecular sieves, 13X molecular sieves, lithium hydroxide, synthetic zeolite, activated carbon, and phosphoric acid-impregnated activated carbon—adsorption performance tests were conducted. The concentrations of the leading exhaust gases in the purified air were below the limit values after multiple rounds of ground simulation tests, meeting indoor air quality (IAQ) standards. Additionally, the purified gas was sent to the Beijing Institute of Physical and Chemical Research for quantitative analysis of volatile organic compounds (VOCs), and the results were consistent with the established technical specifications.
2. Materials and Methods
Gas monitoring and purification devices are essential parts of closed-loop mouse housing systems. These devices mainly include two modules: a gas concentration-monitoring and control module and a purification module for harmful gas. The gas concentration-monitoring and control module constantly measures the levels of various gases in the housing, adjusts oxygen levels, and controls environmental humidity to meet the physiological needs of the experimental animals. The gas purification module efficiently removes harmful gases such as ammonia, hydrogen sulfide, and excess carbon dioxide produced by mouse metabolism during housing, ensuring stable air quality over time.
2.1. Design of Gas Circuit Systems
This device connects to the space mouse closed breeding system via sealed pipes with quick disconnect couplings, creating a closed gas circulation loop to ensure continuous life support for the experimental animals. The design is entirely airtight to prevent negative effects on the gas environment inside the space station cabin during operation.
Figure 2 illustrates the three main components of the system: the oxygen supply unit, the mixture chamber, and the hazardous gas purification unit. The gas circuits and structural design are interconnected. For instance, to provide a consistent and adjustable oxygen supply, the oxygen supply unit includes an oxygen cylinder, cylinder-mounting clamps, a shut-off valve, a pressure reducer, a safety valve, a proportional valve, and connecting conduits, among other parts. The hazardous gas purification system includes HEPA filters to eliminate particles and specialized purification reagents designed to target common metabolic waste gases like carbon dioxide, ammonia, and hydrogen sulfide. The order of adsorbent in the purification unit was not randomly arranged, and we designed it to use the by-products of adsorption process in the previous stage. Firstly, the process gas flowed through the bed of synthetic zeolite and 5A molecular sieve. The main role of this bed was pre-dehumidification and to take out part of polar gases such as ammonia to obtain a relatively dry gas stream in the next stage. After that, the gas flowed through LiOH bed to remove CO2. However, we should note that LiOH would generate water vapor according to Equation (3) in the manuscript, and this would be included in the gas stream. Therefore, to avoid the influence caused by the moisture in the gas stream, we placed another stage of the cleaning system, which was composed of color-changing silica gel and another 5A molecular sieve, after the LiOH bed to remove the moisture generated in the reaction. At last, the gas passed through the activated carbon bed to adsorb VOCs or odors on the carbon, and obtained high quality air to return to the animal habitat. We arranged the CO2 scrubber between the upstream adsorbents and downstream adsorbents to pre-dry the incoming gas and post-dry the effluent gas. This system provides long-term control and safety for the experimental environment by effectively removing animal odors and dangerous metabolic gases. Gas mixing and environmental parameter monitoring are conducted using a gas pump and sensor module within the mixing chamber.
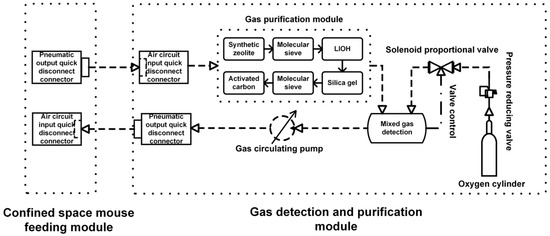
Figure 2.
Flow scheme of multi-stage gas purification system for sealed mouse habitat. Gas stream is passed sequentially through (1) synthetic zeolite (Na–Al–silicate); (2) 5A molecular sieve; (3) LiOH bed for CO2 chemisorption; (4) humidity-indicating silica gel for moisture removal; (5) second bed of 5A molecular sieve; and (6) activated carbon (and its modification with phosphoric acid) for final removal of NH3, H2S, and other trace contaminants.
Solid purification materials should be selected as the dedicated adsorption medium for harmful gas purification components to prevent leakage risks under extreme conditions, especially considering the microgravity characteristics of the space environment.
2.2. Design of Electronic Control Systems
The gas monitoring and purification control board serves as the core of this device. It includes multiple gas sensors to monitor the concentrations of critical gases such as oxygen, carbon dioxide, water vapor, ammonia, and hydrogen sulfide in real time. To achieve automatic balancing of oxygen levels within the enclosed breeding environment, the system can adjust the opening of the proportional valve in a closed-loop fashion based on the detected oxygen concentration. Figure 3 displays the system configuration of the gas sensor circuit board and the ground simulation experiment. The component connection diagram of the ground environment control module is shown in Figure 3a. The gas purification system was designed as a small rectangular sealed cartridge (external dimensions: 324 × 104 × 188 mm for length × width × height). In the sealed culture experiments lasting 7 days, the airflow through the cartridge was provided by a miniature circulation fan, and the flow rate was controlled at about 0.8–1.2 L/min to ensure sufficient contact between the air stream and the adsorbent layers. The activity space for experimental mice is represented by the “mouse cage box.” The gas purification room contains various adsorbent materials designed to remove harmful gases. The testing room is fitted with built-in gas sensor components that monitor changes in the composition of purified gases in real time. Additionally, the system is connected to a high-pressure oxygen cylinder, which supplies oxygen intermittently through a proportional valve. A closed gas circulation loop is created by connecting all functional units sequentially with gas pipelines.
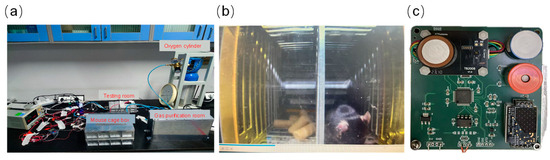
Figure 3.
The test diagram for ground simulation: (a) Connections between components of the experimental environment, (b) mouse status during the experiment, and (c) circuit board for gas detection sensor.
Steady-state oxygen partial pressure output occurs when oxygen consumption by the rodents falls below the threshold. The system uses a polling mechanism to collect data from multiple sensors and standardizes the data format to reduce communication load, enhance human–machine interaction, and improve data retrieval consistency, despite the system’s limited hardware resources.
Gas concentration was measured with commercial sensors, and the main specifications are listed in Table 1. CO2 concentration was measured with a non-dispersive infrared sensor (SenseAir S8, SenseAir AB, Delsbo, Sweden), O2 concentration was measured with a fluorescence-based optical sensor (LOX-02, Scott Specialist Technology (SST), Glasgow, UK), and NH3 and H2S concentrations were measured with electrochemical sensors (EC-SENSE, EC-SENSE GmbH, Magdeburg, Germany). As seen in Table 1, the range of the detection of these sensors were CO2: 0.04–2%, O2: 0–25%, NH3: 0–100 ppm, and H2S: 0–100 ppm; these values are the saturation limits of the sensors. In addition, the precision and temperature/humidity requirements of these sensors are listed in Table 1, and all of the measurements obtained in this study were performed within the working range of these sensors.

Table 1.
Sensor information and descriptions.
2.3. Gas Adsorption and BET Surface Area Measurements
Adsorption isotherms were recorded at 25 °C (room temperature) by static volumetric method from BSD-660MC A3MC gas adsorption analyzer (Beishide Instrument Technology Co., Ltd., Beijing, China). Before the measurement, all samples were degassed under vacuum at 120 °C for 12 h to remove the adsorbed moisture and impurities. The isotherms were recorded in the range of 0–1 bar. The specific surface areas of zeolite 13X and LiOH were determined by nitrogen adsorption at 77 K and calculated by the BET method. The pore size distribution was obtained from the adsorption branch of the N2 isotherm by the NLDFT model.
The BET surface area value of LiOH was measured as around 892 m2/g. It is our pleasure to state that the LiOH sample used in this study has been received as lithium hydroxide powder, and no carrier or support was used in the experiment. The surface area value is included the fine powder information of lithium hydroxide powder. The information on commercial adsorption materials used in the gas purification simulation adsorption test is presented in Table 2:

Table 2.
Material information for the purification simulation test.
3. Testing of Materials and Experimental Outcomes
CO2 and various harmful trace gases like NH3 and H2S are continuously produced by mice metabolism [22]. These gases can affect the rods’ vital signs and the environmental odor. Excessive NH3 can irritate the mice’s respiratory tract mucosa, and long-term exposure can weaken their immune systems [23]. Additionally, high levels of CO2 may alter blood pH, potentially impacting metabolic research results [24]. Environments with high humidity can cause food spoilage and promote microbial and bacterial growth in the mice’s living space. The odor from ammonia volatilization from mouse urine can significantly affect daily life for astronauts inside the spacecraft. Furthermore, gas production can compromise experimental accuracy and threaten the survival of rodents in space. Therefore, it is crucial to effectively remove metabolic gases from rodents. Methods such as adsorption and catalysis are most effective for gas removal, with physical approaches typically using solutions as absorbents being most common [25]. All purification systems in this setup employ solid materials as adsorbent media to neutralize harmful gases, especially in the unique microgravity environment of space.
Textural properties of the adsorbents were determined by N2 physisorption at 77 K with a Specific Surface Area and Pore Size Analyzer (BSD-660S, Beishide Instrument Technology Co., Ltd., Beijing, China). The model of the equipment is BSD-660MC A3MC and the manufacturer is BSD Instruments. All samples were outgassed under vacuum at 120 °C for 12 h before measurements. Specific surface area was calculated by the BET method. Pore size distribution was calculated from the desorption branch of the isotherm using the Barrett–Joyner–Halenda (BJH) model.
The BJH method is reliable only for the middle pore range (2–50 nm) and does not provide an accurate representation of the micropore (<2 nm) structure of materials such as zeolites. The apparent lack of distinct peaks for micropores in the PSDs of zeolitic materials is a well-known artifact of this limitation; the microporosity of zeolites is better reflected by the Type I isotherm shape and high uptake at low relative pressures. In addition, the “tails” increase with pore size in some of the PSD plots due to the network percolation effect, or may represent interparticle voids in powdered samples.
3.1. The Treatment of CO2 Gas and Testing Results
The most common method for purifying CO2 is to use hydroxides of alkali metals or alkaline earth metals for absorption. Although Ca(OH)2 is often used to absorb CO2, calcium’s atomic mass is an important factor in space experiments because of its relatively high value. Lithium has a smaller atomic mass and LiOH offers a high stoichiometric CO2 capacity (≈0.92 kg CO2 per kg LiOH, ≈0.47 m3 kg−1 at STP) together with fast, diffusion-limited kinetics under 15–60 °C and 25–100 kPa; accordingly, we use LiOH as the primary CO2 scavenger in this system. Thus, they are consistent with our 7-day sealed-chamber data where CO2 < 1000 ppm [26,27]. As a result, LiOH is the main adsorbent for carbon dioxide in this system. The principle of CO2 absorption is as follows:
Additionally, 13X molecular sieves exhibit exceptional adsorption capabilities for CO2 gas. Van der Waals forces and electrostatic interactions are the main factors driving the physical adsorption of CO2 by 13X molecular sieves [28]. The adsorption–desorption isotherms and pore size distribution of lithium hydroxide and 13X molecular sieves, as shown by static capacity testing for CO2 gas, are depicted in Figure 4. Figure 4a shows that the isotherm of 13X molecular sieves is a typical Type I isotherm. The isotherm can be well described by the Langmuir model and indicates a relatively homogeneous distribution of adsorption sites, or sites with very similar adsorption energies, in the well-defined crystalline framework of a zeolite. The adsorption capacity nears saturation as pressure increases, with plenty of adsorption sites available at moderate pressures. The microporous structure of 13X molecular sieves is further confirmed by the BET specific surface area of 516.1 m2/g at reasonably low relative pressures. LiOH demonstrates chemical adsorption toward CO2 [29]. For LiOH, the uptake occurs via heterogeneous conversion to Li2CO3, rather than physisorption; hence, surface area parameters and physisorption isotherms are not employed to size the LiOH bed.
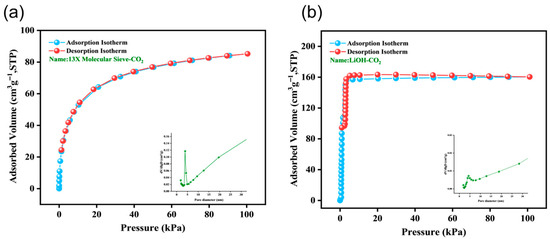
Figure 4.
The adsorption isotherms and pore size distribution of CO2 gas adsorption and desorption by adsorbents (Testing temperature: 25 °C): (a) 13X molecular sieve and (b) LiOH.
3.2. The Treatment of NH3 Gas and Testing Results
Currently, the main methods for treating NH3 include adsorption, catalytic conversion, biological treatment, and plasma techniques [30,31]. For sealed microgravity habitats, we focus on using solid-state adsorption beds instead of liquids (to preclude leakage risks) and to facilitate cartridge-style replacement; this is in keeping with the overall NH3 separation technology and related concepts summarized in the recent reviews [30]. In adsorption, materials with porous structures and surface acidic groups are used to remove ammonia gas by hydrogen bonding with ammonia molecules, which offers significant advantages for the effective treatment of low-concentration ammonia gas [31]. This approach offers significant advantages for the effective treatment of low-concentration ammonia gas. Traditional unmodified activated carbon primarily removes ammonia through physical adsorption, using van der Waals forces to trap ammonia molecules inside micropores (<2 nm) and mesopores (2–50 nm). Pretreating with phosphoric acid (H3PO4) introduces many oxygen-containing acidic functional groups (such as carboxyl groups and phenolic hydroxyl groups) onto the carbon surface, increasing the number of active sites and thereby enhancing ammonia adsorption efficiency. As a result, ammonia adsorption on phosphoric acid-modified activated carbon involves both physical and chemical mechanisms [32]. The synthesis of synthetic zeolite mimics the natural mineralization process and is characterized by a distinct tetrahedral framework structure. The commercial synthetic zeolite (spherical pellets, ∼5 mm diameter; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, Catalog CAS:1318-02-1) used in this study is a sodium aluminosilicate with the typical formula1Na2O·Al2O3·xSiO2·yH2O as reported by the manufacturer. This composition is consistent with a low-silica (Si/Al ratio typically ∼1.0), sodium-form (Na+) zeolite. Given its typical commercial classification and formula, it is very likely to have the LTA (Linde Type A) framework structure in addition to that of the 5A (LTA, Ca2+) and 13X (FAU, Na+) zeolites studied in this work. Mechanistically, ammonia removal by aluminosilicate zeolites involves (i) ion exchange of NH4+ with framework charge-balancing cations and (ii) molecular NH3 adsorption under alkaline conditions. Under the sealed-chamber conditions in this work (RH 30–36%), a thin adsorbed water layer should develop on the sorbent so that gas-phase NH3 partially protonates to NH4+ and ion exchange should dominate near neutral pH. For clinoptilolite/13X-type zeolites, the selectivity of exchange is Na+ > Ca2+ > K+ > Mg2+; and when at high ammonium loading, Ca2+ can exceed Na+ only after Na+ depletion. By contrast, at elevated alkalinity, the free-ammonia fraction increases and molecular NH3 adsorption can become significant and possibly suppress ion exchange. These trends, established here for zeolite–ammonium systems, explain our capacity ranking where 13X > synthetic zeolite > activated carbons, while the phosphoric acid-modified carbon represents an additional carbon-oxygenated phosphorus adsorbent that exhibits acid–base chemisorption atop pore-filling physisorption. We therefore attribute the higher NH3 uptake of 13X in our tests to its high Na content and strong electrostatic interactions with polar NH3/NH4+, and this is in agreement with our mechanism framework [33].
The adsorption–desorption isotherms and pore size distributions of traditional activated carbon, synthetic zeolite, phosphate-modified activated carbon, and 13X molecular sieve data are illustrated in Figure 5 under static capacity test conditions for NH3 gas. The adsorption isotherms of both types of activated carbon show Type IV characteristics. Specifically, traditional activated carbon exhibits a gradual upward trend, while phosphoric acid-modified activated carbon demonstrates a higher initial adsorption capacity in the low-pressure region. This indicates the presence of microporous structures or strong adsorption sites, reflecting a multilayer adsorption process. The adsorption and desorption curves of activated carbon do not fully overlap (incomplete desorption), as shown in Figure 5a,c. The remaining adsorption capacity exceeds the capacity at the same pressure. Compared to the adsorption branch, the desorption branch forms a distinct delayed ring. The mesoporous structure of activated carbon is further confirmed by the lag ring, which is related to the pore architecture. This curve suggests that activated carbon mainly exhibits mesoporous characteristics, potentially combined with contributions from micropores. The adsorption–desorption curves of synthetic zeolite and 13X molecular sieves for NH3, as shown in Figure 5b,d, also display typical Type I isotherms. This Type I shape indicates predominant micropore filling, and the attainment of saturation capacity suggests a uniform pore system, with capacity approaching saturation as pressure increases. According to test results, the maximum adsorption capacity of activated carbon for NH3 per unit mass is 63.5 cm3/g, the value for synthetic zeolite is 98.1 cm3/g, and that of phosphate-modified activated carbon is 125.5 cm3/g. The 13X molecular sieve has a maximum capacity of 162.9 cm3/g. It is clear that ammonia adsorption capacity is significantly higher in phosphoric acid-modified activated carbon and the 13X molecular sieve than in traditional activated carbon and synthetic zeolite. Therefore, prioritizing 13X molecular sieve and phosphoric acid-modified activated carbon in ammonia purification treatment schemes is recommended.
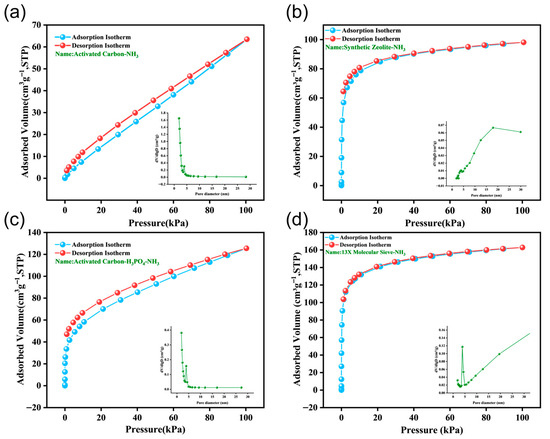
Figure 5.
The adsorption isotherms and pore size distribution of adsorbents for NH3 gas adsorption and desorption (Testing temperature: 25 °C): (a) Traditionally used activated carbon, (b) synthetic zeolite, (c) activated carbon modified with phosphoric acid, and (d) 13X molecular sieve.
3.3. The Treatment of H2O Humidity and Testing Results
Absorption drying and condensation (freeze drying) are common methods for drying gases. In adsorption drying, the process uses the physical absorption properties of porous materials to remove moisture. Silicone materials are high-molecular-weight polymers with a basic structure of siloxane chains, with side chains attached to other organic groups through silicon atoms [34]. Fine-pore silica gel (SiO2) acts as a carrier for color-changing silica gel, while cobalt chloride (CoCl2) serves as an indicator. This process relies on physical adsorption and capillary condensation. The color of cobalt chloride shifts from blue (dry) to pink (moist) as it reacts with water to form a hydrate (CoCl2→CoCl2·6H2O), providing a visual cue for humidity levels. A silico–aluminate of the calcium–sodium type, 5A molecular sieve, has a silicon-to-aluminum ratio of approximately 2 (SiO2/Al2O3). It selectively adsorbs small molecules like H2O, CO2, and NH3 through electrostatic interactions and pore size separation effects. Strong polar locations are provided by the calcium ions, which maintain a high adsorption capacity even at low partial pressures and have a high affinity for polar molecules (such as water).
The adsorption–desorption isotherms and pore size distribution of 5A molecular sieve and color-changing silica gel are depicted in Figure 6 under the dynamic weight method and DVS testing conditions at atmospheric pressure for H2O gas. Figure 6a illustrates that the 5A molecular sieve demonstrates a Type I isotherm, which is indicative of adsorption within micropores (micropore filling). Microporous materials are characterized by a high initial adsorption capacity and a rapid saturation of adsorption in low relative pressure regions (P/P0 < 0.1). The adsorption–desorption curve of color-changing silica gel (Figure 6b) exhibits a Type II isotherm, with a significant increase in adsorption capacity at medium to high relative pressures (P/P0 > 0.4), which is consistent with the characteristics of a Type II isotherm. This is characterized by a slow increase in adsorption at low relative pressures (P/P0), a rapid rise at medium to high relative pressures, a turning point (where multilayer adsorption begins after monolayer saturation), and continued growth near P/P0 = 1, indicating that the silica gel is a type of mesoporous or macroporous material. The effective diffusion coefficients (D/r2) of water vapor adsorption were calculated based on the kinetics data by fitting to the diffusion model for spherical particles.
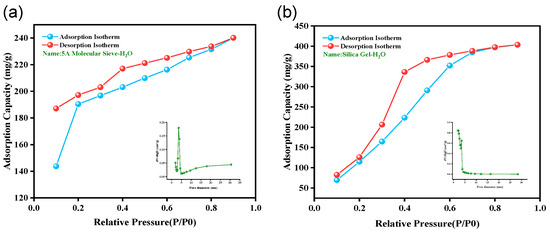
Figure 6.
Adsorption isotherms and pore size distribution of adsorbents for H2O gas adsorption and desorption (Testing temperature: 25 °C): (a) 5A molecular sieve and (b) color-changing silica gel.
The solid sphere model diffusion coefficient equation is as follows:
For the 5A molecular sieve, the (D/r2) value is 4.78793 × 10−5 s−1, while the color-changing silica gel has a value of 2.85569 × 10−5 s−1. The 5A molecular sieve has a higher diffusion coefficient, which leads to more rapid gas drying and quicker adsorption/desorption rates. While color-changing silica gel has an adsorption capacity of 403.528 (mg/g), 5A molecular sieves have an adsorption capacity of 240.148 (mg/g). Color-changing silica gel has a greater total capacity and adsorbs more water at high humidity (P/P0 = 0.9). Nevertheless, 5A molecular sieves exhibit an adsorption capacity of 143.7 mg/g at low-humidity conditions (e.g., P/P0 = 0.1), as indicated by the geometry of the isotherm curves, whereas silica gel only reaches 69.4 mg/g. 5A molecular sieves are more susceptible to low-humidity conditions, resulting in a more rapid and comprehensive dehydrating process. At high humidity, color-changing silica gel demonstrates more substantial adsorption increases; however, it performs inferiorly in comparison to 5A molecular sieves in low-humidity conditions. Consequently, in order to optimize drying performance for gas drying applications, it is advisable to combine 5A molecular sieves with color-changing silica gel.
4. Results of the Experiment
The gas environment control module was validated through the ground simulation test shown in Figure 3 to meet the gas regulation requirements for small mammal devices in a sealed environment. Moisture removal within the device was achieved using a dual dehydrating component that included a 5A molecular sieve and color-changing silica gel. The system configuration is as follows: for effective adsorption of ammonia and other volatile gases, a combination of phosphoric acid-activated carbon and 13X molecular sieve was used. Additionally, lithium hydroxide absorbent was employed to efficiently eliminate carbon dioxide. The experiment involved operating the system continuously for seven days while placing four 12 g C57BL female mice in a housing box. The gas sensor control module dynamically monitored key gas parameters, such as relative humidity, ammonia concentration, hydrogen sulfide concentration, carbon dioxide concentration, environmental pressure, and oxygen levels during the test.
Figure 7 shows the environmental parameters that changed during the system’s continuous operation over seven days. Figure 7b displays the trend of relative humidity, which remains within 30–36%, meeting the requirements for small rodent experiments. From Figure 7c, it can be seen that the oxygen concentration was well controlled by the closed-loop control system, and the oxygen concentration was maintained in the range of 19–21%, which meets the oxygen requirements of small mammals. Figure 7d,e presents the monitoring results for ammonia and hydrogen sulfide levels. Both gases are effectively adsorbed, and their concentrations stay consistent over time. Considering the gas sensors’ detection range (about 1 ppm detection limit), the concentrations of both gases stayed below the detection limit throughout the experiment, with no significant increases. Figure 7f depicts the trend of carbon dioxide levels in the environment. The activity pattern of rodents—being inactive during the day and active at night—is observable. Additionally, there is no significant accumulation of carbon dioxide, as the concentration stays below 1000 ppm.
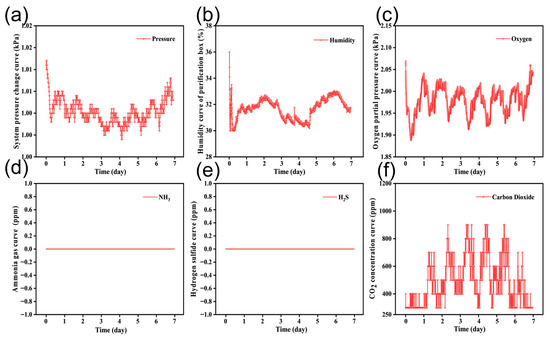
Figure 7.
Environmental conditions of the 7-day sealed culture system. (a) The pressure of system in real time, which the system maintained in the range 101–103 kPa. (b) The change in relative humidity, which was kept within 30–36%. (c) The oxygen concentration, which the system kept within 19–21%. (d) The carbon dioxide concentration, which the system kept below 1000 ppm. (e) The ammonia concentration, not detected. (f) The hydrogen sulfide concentration, below detection limit.
Under the combined action of a variety of adsorption and purification materials (including lithium hydroxide for CO2 removal), the system is capable of maintaining a stable and suitable closed gas environment within a 7-day closed cycle. This provides reliable support conditions for life science experiments, and the overall test results illustrate this.
5. Conclusions
The primary focus of this study was to address the challenges of gas odor and harmful gas treatment during the long-term housing of small mammals (mice) in a confined space. To resolve these issues, a multi-stage solid-state adsorption purification system has been developed and implemented. The system uses various high-efficiency adsorbent materials, such as synthetic zeolite, 5A molecular sieves, 13X molecular sieves, phosphoric acid-modified activated carbon, and color-changing silica gel. It achieves precise control and stable regulation of key environmental parameters, including oxygen concentration, humidity, and toxic metabolic gases (e.g., NH3, H2S, CO2), while ensuring the system structure remains airtight. The system successfully meets the survival and experimental needs of rodents by maintaining proper environmental parameters during continuous closed-loop operation, as shown by the results of a 7-day ground simulation test. The overall design’s reliability and purification efficiency are confirmed by the fact that the system’s air pressure, humidity, and oxygen partial pressure stay within the set ranges, and harmful gas levels remain below detection limits without significant buildup. Sensor monitoring data backs these findings.
One of the strengths of this work is that we successfully integrated and validated the multi-stage purification cartridge into a relevant functional closed-loop system and proved its applicability to live animals. However, a limitation of the present work is that it focused on single component adsorption isotherms to describe the performance of the base-line sorbent bed. It should be noted that real closed habitats will have to deal with multi-gas mixtures and competitive adsorption. In particular, water vapor competes intensely with ammonia on hydrophilic zeolites. One of the advantages of our design is that we prevented this “competing species problem” by the multi-stage design in which desiccants (5A zeolite and silica gel) remove moisture upstream of the 13X zeolite bed used for CO2/NH3 sorption and phosphoric acid-modified activated carbon used for the remaining NH3 and trace contaminants. This design reduces the cross-competition between these species.
In the future, the work will extend towards the regeneration of the adsorbents to sustain the operation for prolonged missions. For example, molecular sieves and activated carbon can be regenerated, respectively, by thermal desorption and pressure swing adsorption (PSA), while LiOH is irreversibly consumed. The potential of the regenerative materials (e.g., zeolites for NH3) and replaceable cartridges (e.g., LiOH for CO2) seems to be very promising for sustainability. The research not only establishes a solid foundation for gas environment assurance and technical capabilities for future in-orbit and ground-based biological simulation experiments but also provides technical support for environmental control in sealed biological experiment chambers. To enhance the overall system’s sustainable operation, future research could explore the system’s regeneration capacity and material replacement mechanisms during long-duration missions, as well as incorporate the performance characteristics of materials under microgravity conditions to optimize the layout and structural parameters of purification materials.
Author Contributions
Conceptualization, R.Y. and F.L.; methodology, F.L.; software, R.Y.; validation, R.Y. and F.L.; formal analysis, R.Y. and H.S.; investigation, H.S. and L.Z.; resources, F.L.; data curation, R.Y.; writing—original draft preparation, R.Y.; writing—review and editing, F.L. and S.Y.; visualization, Q.Z. and L.Z.; supervision, T.Z.; project administration, S.Y.; and funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (2021YFA0719303) and the China Manned Space Engineering Program (KJZ-YY-NSM0601).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Laboratory Animal Welfare Ethics Review Committee, Institute of Zoology, Chinese Academy of Sciences (protocol code: IOZ-IACUC-2024-084, approval date: 2025.03.18).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
During the preparation of this manuscript/study, the author(s) used Deep L and Quillbot for the purposes of translation and polishing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ronca, A.E.; Lowe, M.G. Rodents as a Model for Research in Space. In Handbook of Space Pharmaceuticals; Pathak, Y.V., dos Santos, M.A., Zea, L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 679–700. [Google Scholar]
- Morey-Holton, E.; Hill, E.; Souza, K. Animals and spaceflight: From survival to understanding. J. Musculoskelet. Neuronal Interact. 2006, 7, 17–25. [Google Scholar]
- Duan, E.-k.; Cao, Y.; Lei, X.; Ma, C. The research progress and prospect of mammalian reproduction and embryonic development under space conditions. Sci. Sin. Vitae 2021, 51, 116–125. [Google Scholar] [CrossRef]
- Burgess, C.; Dubbs, C. (Eds.) High-altitude research. In Animals in Space: From Research Rockets to the Space Shuttle; Springer New York: New York, NY, USA, 2007; pp. 85–119. [Google Scholar]
- West, J.B. Historical aspects of the early Soviet/Russian manned space program. J. Appl. Physiol. 2001, 91, 1501–1511. [Google Scholar] [CrossRef]
- Savina, E.A.; Podymov, V.K.; Alekseev, E.I. Hypothalamo-Pituitary Neurosecretory System of Rats After a 22-Day Space Flight. In Neurosecretion and Neuroendocrine Activity; Bargmann, W., Oksche, A., Polenov, A.L., Scharrer, B., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; 279p. [Google Scholar]
- Andreev-Andrievskiy, A.; Shenkman, B.; Popova, A.; Dolguikh, O.; Anokhin, K.; Soldatov, P.; Ilyin, E.; Sychev, V. Experimental studies with mice on the program of the biosatellite BION-M1 mission. Aviakosm. I Ekol. Meditsina Aerosp. Environ. Med. 2014, 48, 14–27. [Google Scholar]
- Voels, S.A.; Eppler, D.B. The International Space Station as a platform for space science. Adv. Space Res. 2004, 34, 594–599. [Google Scholar] [CrossRef]
- Sun, G.-S.; Tou, J.C.; Yu, D.; Girten, B.E.; Cohen, J. The past, present, and future of National Aeronautics and Space Administration spaceflight diet in support of microgravity rodent experiments. Nutrition 2014, 30, 125–130. [Google Scholar] [CrossRef]
- Cancedda, R.; Liu, Y.; Ruggiu, A.; Tavella, S.; Biticchi, R.; Santucci, D.; Schwartz, S.; Ciparelli, P.; Falcetti, G.; Tenconi, C.; et al. The Mice Drawer System (MDS) Experiment and the Space Endurance Record-Breaking Mice. PLoS ONE 2012, 7, e32243. [Google Scholar] [CrossRef]
- Andreev-Andrievskiy, A.; Popova, A.; Boyle, R.; Alberts, J.; Vinogradova, O.; Dolgov, O.; Anokhin, K.; Tsvirkun, D.; Soldatov, P.; Nemirovskaya, T.; et al. Mice in Bion-M 1 Space Mission: Training and Selection. PLoS ONE 2014, 9, e104830. [Google Scholar] [CrossRef]
- Shimbo, M.; Kudo, T.; Hamada, M.; Jeon, H.; Imamura, Y.; Asano, K.; Okada, R.; Tsunakawa, Y.; Mizuno, S.; Yagami, K.-I.; et al. Ground-based assessment of JAXA mouse habitat cage unit by mouse phenotypic studies. Exp. Anim. 2016, 65, 175–187. [Google Scholar] [CrossRef]
- Niederwieser, T.; Gerren, R.A.; Koenig, P.; Tozer, S.; Stodieck, L.S.; Rieger, S.; Hoehn, A. AEM-E—A small life support system for the transport of rodents to the ISS. In Proceedings of the 44th International Conference on Environmental Systems, Tuscon, AZ, USA, 13–17 July 2014. [Google Scholar]
- Bonting, S.L.; Kishiyama, J.S.; Arno, R.D. Facilities for Animal Research in Space. Adv. Space Biol. Med. 1991, 1, 279–325. [Google Scholar] [CrossRef]
- Hogan, R.P.; Dalton, B.P. Performance of the Research Animal Holding Facility (RAHF) and General Purpose Work Station (GPWS) and Other Hardware in the Microgravity Environment. SAE Trans. 1991, 100, 1817–1829. [Google Scholar]
- Savage, P.D.; Jahns, G.C.; Dalton, B.P.; Hogan, R.P.; Wray, A.E. The Rodent Research Animal Holding Facility as a Barrier to Environmental Contamination. SAE Trans. 1989, 98, 879–886. [Google Scholar]
- Shiba, D.; Mizuno, H.; Yumoto, A.; Shimomura, M.; Kobayashi, H.; Morita, H.; Shimbo, M.; Hamada, M.; Kudo, T.; Shinohara, M.; et al. Development of new experimental platform ‘MARS’—Multiple Artificial-gravity Research System—To elucidate the impacts of micro/partial gravity on mice. Sci. Rep. 2017, 7, 10837. [Google Scholar] [CrossRef]
- Mao, X.W.; Byrum, S.; Nishiyama, N.C.; Pecaut, M.J.; Sridharan, V.; Boerma, M.; Tackett, A.J.; Shiba, D.; Shirakawa, M.; Takahashi, S.; et al. Impact of Spaceflight and Artificial Gravity on the Mouse Retina: Biochemical and Proteomic Analysis. Int. J. Mol. Sci. 2018, 19, 2546. [Google Scholar] [CrossRef]
- Obata, K.; Abe, C.; Shiba, D.; Shirakawa, M.; Kudo, T.; Takahashi, S. Feasibility of a Short-Arm Centrifuge for Mouse Hypergravity Experiments. PLoS ONE 2015, 10, e0133981. [Google Scholar] [CrossRef]
- Berg-Johansen, B.; Liebenberg, E.C.; Li, A.; Macias, B.R.; Hargens, A.R.; Lotz, J.C. Spaceflight-induced bone loss alters failure mode and reduces bending strength in murine spinal segments. J. Orthop. Res. 2016, 34, 48–57. [Google Scholar] [CrossRef]
- Orr, S.; Rhonda, W.; Adams, T.; Raychev, R.; Griko, Y. Environmental Enrichment in the ISS Rodent Habitat Hardware System. Int. J. Biosci. Med. 2017, 1, 6. [Google Scholar] [CrossRef]
- Burnett, C.M.L.; Grobe, J.L. Dietary effects on resting metabolic rate in C57BL/6 mice are differentially detected by indirect (O2/CO2 respirometry) and direct calorimetry. Mol. Metab. 2014, 3, 460–464. [Google Scholar] [CrossRef]
- Yanagawa, T.; Kikuchi, Y. Determination of the No-Observed-Adverse-Effect Level by the AIC. Sankhy Indian J. Stat. Ser. B 1995, 57, 285–297. [Google Scholar]
- Iversen, N.K.; Malte, H.; Baatrup, E.; Wang, T. The normal acid–base status of mice. Respir. Physiol. Neurobiol. 2012, 180, 252–257. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, Y.; Zhang, J.; Chen, L.; Meng, X.; Xiao, F.-S. Adsorptive and catalytic properties in the removal of volatile organic compounds over zeolite-based materials. Chin. J. Catal. 2016, 37, 800–809. [Google Scholar] [CrossRef]
- Zhuo, Z.; Fu, P.; Zhao, B. Studies on Reaction Kinetics of LiOH for Absorbing CO2. In Proceedings of the 2009 Asia-Pacific Power and Energy Engineering Conference, Wuhan, China, 27–31 March 2009; pp. 1–4. [Google Scholar]
- Zilberman, P. The CO2 Absorber Based on LiOH. Acta Medica Marisiensis 2015, 61, 4–6. [Google Scholar] [CrossRef]
- Punpee, S.; Tongpadungrod, P.; Suttikul, T.; Phalakornkule, C. Characteristics of CO2 adsorption and desorption on activated carbon in comparison with zeolite 13X and carbon molecular sieve and applications in biogas upgrading using vacuum pressure swing adsorption. J. Chem. Technol. Biotechnol. 2023, 98, 2677–2690. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ghaemi, A.; Qasemnazhand, M. Lithium hydroxide as a high capacity adsorbent for CO2 capture: Experimental, modeling and DFT simulation. Sci. Rep. 2023, 13, 7150. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, L.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. A Mini-Review on NH3 Separation Technologies: Recent Advances and Future Directions. Energy Fuels 2022, 36, 14516–14533. [Google Scholar] [CrossRef]
- He, Y.; Guan, B.; Zhuang, Z.; Chen, J.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Shu, K.; et al. Advances in ammonia (NH3) adsorption and storage: Materials, mechanisms, and applications. Adsorption 2025, 31, 48. [Google Scholar] [CrossRef]
- Premjet, S.; Dana, S.; Obeng, A.; Premjet, D. Enzymatic response to structural and chemical transformations in Hibiscus sabdariffa var. altissima bark and core during phosphoric acid pretreatment. Bioresources 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.-J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Rochow, E.G.; Rochow, T.G. The Properties and Molecular Weights of Some Silicone Polymers. J. Phys. Chem. 1951, 55, 9–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).