Next-Generation Wastewater Treatment: Omics and AI-Driven Microbial Strategies for Xenobiotic Bioremediation and Circular Resource Recovery
Abstract
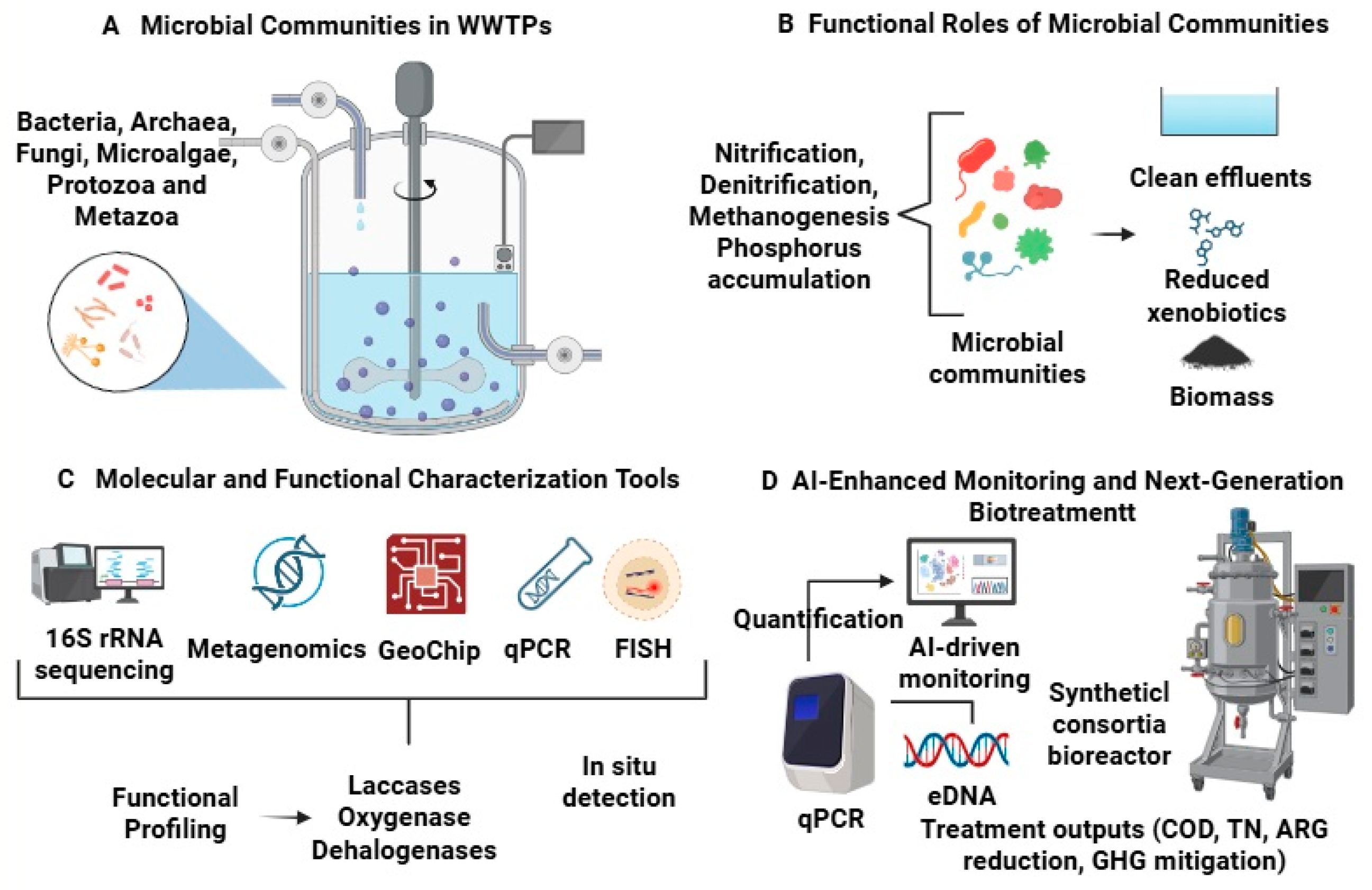
1. Introduction
2. Microbial Diversity and Ecology in WWTPs
3. Xenobiotic Bioremediation in WWTPs
3.1. Organic Pollutants Degradation
3.2. Textile Dyes and Industrial Effluents
3.3. Pesticide Biodegradation
3.4. Heavy Metal Bioremediation
3.5. Urban Wastewater and Emerging Pathogens
4. Molecular Tools for Characterization of Microbial Communities
4.1. eDNA and Metagenomics
4.2. 16S rRNA Gene Sequencing
4.3. eDNA-Based Performance Monitoring
4.4. Functional Gene Analysis
4.5. Molecular and Enzymatic Surveillance Tools
5. Case Studies of Microbial Communities in WWTPs
5.1. Municipal WWTPs
5.2. Industrial WWTPs
5.3. Onsite WWTPS
5.3.1. Anaerobic Baffled Reactors (ABRs)
5.3.2. Hybrid and Constructed Wetland Systems
5.3.3. Plant–Microbe Interactions and Microbial Indicators
6. Challenges and Future Perspectives
6.1. Microbial Ecology of WWTPs
6.2. Need for Integrative Omics and AI Approaches
6.3. Engineering of Synthetic Microbial Consortia
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renganathan, P.; Gaysina, L.A.; Holguín-Peña, R.J.; Sainz-Hernández, J.C.; Ortega-García, J.; Rueda-Puente, E.O. Phycoremediated microalgae and cyanobacteria biomass as biofertilizer for sustainable agriculture: A holistic biorefinery approach to promote circular bioeconomy. Biomass 2024, 4, 1047–1077. [Google Scholar] [CrossRef]
- Renganathan, P.; Gaysina, L.A.; García Gutiérrez, C.; Rueda Puente, E.O.; Sainz-Hernández, J.C. Harnessing engineered microbial consortia for xenobiotic bioremediation: Integrating multi-omics and AI for next-generation wastewater treatment. J. Xenobiotics 2025, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.B.; Greene, K.; Vasquez-Arroyo, E.; Awais, M.; Gomez-Sanabria, A.; Kyle, P.; Palatnik, R.R.; Schaeffer, R.; Zhou, P.; Aissaoui, B.; et al. Toward enhancing wastewater treatment with resource recovery in integrated assessment and computable general equilibrium models. Environ. Sci. Technol. Lett. 2024, 11, 654–663. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Khan, M.I.; Moussa, S.B.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive review of industrial wastewater treatment techniques. Environ. Sci. Pollut. Res. 2024, 31, 51064–51097. [Google Scholar] [CrossRef]
- Emergen Research. Biological Wastewater Treatment Market Share Report. Available online: https://www.emergenresearch.com/industry-report/biological-wastewater-treatment/market-share (accessed on 8 August 2025).
- Kalli, M.; Noutsopoulos, C.; Mamais, D. The fate and occurrence of antibiotic-resistant bacteria and antibiotic resistance genes during advanced wastewater treatment and disinfection: A review. Water 2022, 15, 2084. [Google Scholar]
- Lan, L.; Wang, Y.; Chen, Y.; Wang, T.; Zhang, J.; Tan, B. A review on the prevalence and treatment of antibiotic resistance genes in hospital wastewater. Toxics 2025, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Tang, M.; Zhou, J.; Zhang, T. The microbial dark matter and “wanted list” in worldwide wastewater treatment plants. Microbiome 2024, 11, 59. [Google Scholar] [CrossRef]
- Liang, Y.Y. Role of spacers in osmotic membrane desalination: Advances, challenges, practical and artificial intelligence-driven solutions. Process Saf. Environ. Prot. 2025, 201, 107587. [Google Scholar] [CrossRef]
- De Simone Souza, H.H.; De Morais Lima, P.; Medeiros, D.L.; Vieira, J.; Magalhães Filho, F.J.; Paulo, P.L.; Fullana-i-Palmer, P.; Boncz, M.Á. Environmental assessment of on-site source-separated wastewater treatment and reuse systems for resource recovery in a sustainable sanitation view. Sci. Total Environ. 2023, 895, 165122. [Google Scholar]
- Mutuku, C.; Gazdag, Z.; Melegh, S. Occurrence of antibiotics and bacterial resistance genes in wastewater: Resistance mechanisms and antimicrobial resistance control approaches. World J. Microbiol. Biotechnol. 2022, 38, 152. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, J.L.; Tang, G.X.; Chen, Y.S.; Huang, Y.H.; Hu, R.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Li, Y.W.; et al. Microbial consortium degrading of organic pollutants: Source, degradation efficiency, pathway, mechanism and application. J. Clean. Prod. 2024, 141913. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, G.; Xie, H.; Zhai, Y.; Li, X. Advances and solutions in biological treatment for antibiotic wastewater with resistance genes: A review. J. Environ. Manag. 2024, 368, 122115. [Google Scholar] [CrossRef]
- Dueholm, M.K.; Nierychlo, M.; Andersen, K.S.; Rudkjøbing, V.; Knutsson, S.; Albertsen, M.; Nielsen, P.H. MiDAS 4: A global catalogue of full-length 16S rRNA gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 2022, 13, 1908. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, B.; Ning, D.; Zhang, Y.; Dai, T.; Wu, L.; Li, T.; Liu, W.; Zhou, J.; Wen, X. Seasonal dynamics of the microbial community in two full-scale wastewater treatment plants: Diversity, composition, phylogenetic group based assembly and co-occurrence pattern. Water Res. 2021, 200, 117295. [Google Scholar]
- Vestergaard, S.Z.; Dottorini, G.; Peces, M.; Murguz, A.; Dueholm, M.K.; Nierychlo, M.; Nielsen, P.H. Microbial core communities in activated sludge plants are strongly affected by immigration and geography. Environ. Microbiome 2024, 19, 63. [Google Scholar] [CrossRef]
- Chen, K.; Wang, H.; Valverde-Pérez, B.; Zhai, S.; Vezzaro, L.; Wang, A. Optimal control towards sustainable wastewater treatment plants based on multi-agent reinforcement learning. Chemosphere 2021, 279, 130498. [Google Scholar] [CrossRef] [PubMed]
- Frontistis, Z.; Lykogiannis, G.; Sarmpanis, A. Machine learning implementation in membrane bioreactor systems: Progress, challenges, and future perspectives: A review. Environments 2023, 10, 127. [Google Scholar] [CrossRef]
- Zamfir, F.; Carbureanu, M.; Mihalache, S.F. Application of machine learning models in optimizing wastewater treatment processes: A review. Appl. Sci. 2024, 15, 8360. [Google Scholar] [CrossRef]
- Gao, P.; Xu, W.; Sontag, P.; Li, X.; Xue, G.; Liu, T.; Sun, W. Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl. Microbiol. Biotechnol. 2016, 100, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkova, N.M.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.A.; Kharitonov, S.L.; Klimina, K.M.; Melnikova, N.V.; Kudryavtseva, A.V. Microbial community structure of activated sludge in treatment plants with different wastewater compositions. Front. Microbiol. 2016, 7, 90. [Google Scholar] [CrossRef]
- Wu, L.; Ning, D.; Zhang, B.; Li, Y.; Zhang, P.; Shan, X.; Zhang, Q.; Brown, M.R.; Li, Z.; Van Nostrand, J.D.; et al. Global diversity and biogeography of bacterial communities in wastewater treatment plants. Nat. Microbiol. 2019, 4, 1183–1195. [Google Scholar] [CrossRef]
- Wang, X.; Ya, T.; Zhang, M.; Liu, L.; Hou, P.; Lu, S. Cadmium (II) alters the microbial community structure and molecular ecological network in activated sludge system. Environ. Pollut. 2019, 255, 113225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, X.; Zhu, L. Structure and function of the microbial consortia of activated sludge in typical municipal wastewater treatment plants in winter. Sci. Rep. 2017, 7, 17930. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, S.N.; Lee, P.K. Microbial communities in full-scale wastewater treatment systems exhibit deterministic assembly processes and functional dependencies over time. Environ. Sci. Technol. 2021, 55, 5312–5323. [Google Scholar] [CrossRef]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Mühldorfer, K.; Sauer, S.; Grossart, H.P.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-length 16S rRNA sequencing. Sci. Rep. 2019, 9, 9673. [Google Scholar] [CrossRef] [PubMed]
- LaMartina, E.L.; Mohaimani, A.A.; Newton, R.J. Urban wastewater bacterial communities assemble into seasonal steady states. Microbiome 2021, 9, 116. [Google Scholar] [CrossRef]
- Begmatov, S.; Dorofeev, A.G.; Kadnikov, V.V.; Beletsky, A.V.; Pimenov, N.V.; Ravin, N.V.; Mardanov, A.V. The structure of microbial communities of activated sludge of large-scale wastewater treatment plants in the city of Moscow. Sci. Rep. 2022, 12, 3458. [Google Scholar] [CrossRef]
- Suazo, K.S.; Dobbeleers, T.; Dries, J. Bacterial community and filamentous population of industrial wastewater treatment plants in Belgium. Appl. Microbiol. Biotechnol. 2024, 108, 43. [Google Scholar] [CrossRef]
- Han, T.; Zheng, J.; Han, Y.; Xu, X.; Li, M.; Schwarz, C.; Zhu, L. Comprehensive insights into key microbial assemblages in activated sludge exposed to textile-dyeing wastewater stress. Sci. Total Environ. 2021, 791, 148145. [Google Scholar] [CrossRef]
- Meerbergen, K.; Van Geel, M.; Waud, M.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Assessing the composition of microbial communities in textile wastewater treatment plants in comparison with municipal wastewater treatment plants. MicrobiolOpen 2017, 6, e00413. [Google Scholar] [CrossRef] [PubMed]
- Singleton, C.M.; Petriglieri, F.; Kristensen, J.M.; Kirkegaard, R.H.; Michaelsen, T.Y.; Andersen, M.H.; Kondrotaite, Z.; Karst, S.M.; Dueholm, M.S.; Nielsen, P.H.; et al. Connecting structure to function with the recovery of over 1000 high-quality metagenome-assembled genomes from activated sludge using long-read sequencing. Nat. Commun. 2021, 12, 2009. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Saleem, F.; Li, E.; Chan, A.W.; Naphtali, J.; Naphtali, P.; Paschos, A.; Schellhorn, H.E. Comparative analysis of metagenomic (amplicon and shotgun) DNA sequencing to characterize microbial communities in household on-site wastewater treatment systems. Water 2023, 15, 271. [Google Scholar] [CrossRef]
- Osunmakinde, C.O.; Selvarajan, R.; Mamba, B.B.; Msagati, T.A. Profiling bacterial diversity and potential pathogens in wastewater treatment plants using high-throughput sequencing analysis. Microorganisms 2019, 7, 506. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Suresh, K.; Jiang, G. Bacterial bioremediation of heavy metals in wastewater: A review of processes and applications. J. Water Process Eng. 2022, 48, 102884. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Rangasamy, G.; Hariharan, R.; Hemavathy, R.V.; Deepika, P.D.; Anand, K.; Karthika, S. Strategies for enhancing the efficacy of anaerobic digestion of food industry wastewater: An insight into bioreactor types, challenges, and future scope. Chemosphere 2023, 310, 136856. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, C.; Ma, G.; Zhang, X.; Guo, H.; Zhang, T.; Zhang, Y.; Hu, Y.; Li, D.; Li, Y.Y.; et al. Anaerobic treatment of nitrogenous industrial organic wastewater by carbon–neutral processes integrated with anaerobic digestion and partial nitritation/anammox: Critical review of current advances and future directions. Bioresour. Technol. 2024, 131648. [Google Scholar] [CrossRef]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Liu, Y.; Zhou, L.; Jiang, L.H.; Tan, X.F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent advances in bacterial degradation of hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Gnanasekaran, L.; Kumar, S.; Durvasulu, R.; Sundaram, T.; Rajendran, S.; Nangan, S.; Kanagaraj, K. Utilization of fungal and bacterial bioremediation techniques for the treatment of toxic waste and biowaste. Front. Mater. 2024, 11, 1416445. [Google Scholar] [CrossRef]
- Nejad, Z.G.; Borghei, S.M.; Yaghmaei, S. Kinetic studies of Bisphenol A in aqueous solutions by enzymatic treatment. Int. J. Environ. Sci. Technol. 2019, 16, 821–832. [Google Scholar] [CrossRef]
- Yu, X.; Bai, M.; Li, X.; Yang, P.; Wang, Q.; Wang, Z.; Weng, L.; Ye, H. Tetracycline removal by immobilized indigenous bacterial consortium using biochar and biomass: Removal performance and mechanisms. Bioresour. Technol. 2024, 413, 131463. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.I.; Talwar, M.P.; Bagewadi, Z.K.; Hoskeri, R.S.; Ninnekar, H.Z. Enhanced degradation of 2-nitrotoluene by immobilized cells of Micrococcus sp. strain SMN-1. Chemosphere 2013, 90, 1920–1924. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Aslam, A.; Kanwal, F.; Javied, S.; Nisar, N.; Torriero, A.A. Microbial biosorption: A sustainable approach for metal removal and environmental remediation. Int. J. Environ. Sci. Technol. 2025, 22, 1–32. [Google Scholar] [CrossRef]
- Moyo, S.; Makhanya, B.P.; Zwane, P.E. Use of bacterial isolates in the treatment of textile dye wastewater: A review. Heliyon 2022, 8, e09792. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Lewis, T.; Abbassi, R.; Khan, S.J. Electrode dependent anaerobic ammonium oxidation in microbial fuel cell integrated hybrid constructed wetlands: A new process. Sci. Total Environ. 2020, 698, 134248. [Google Scholar] [CrossRef]
- Grafias, P.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Diamadopoulos, E. Pilot treatment of olive pomace leachate by vertical-flow constructed wetland and electrochemical oxidation: An efficient hybrid process. Water Res. 2010, 44, 2773–2780. [Google Scholar] [CrossRef]
- Tizazu, S.; Tesfaye, G.; Andualem, B.; Wang, A.; Guadie, A. Evaluating the potential of thermo-alkaliphilic microbial consortia for azo dye biodegradation under anaerobic–aerobic conditions: Optimization and microbial diversity analysis. J. Environ. Manage. 2022, 323, 116235. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Suarez, C.; Paul, C.J.; Simachew, A. Evaluation of integrated anaerobic/aerobic conditions for treating dye-rich synthetic and real textile wastewater using a soda lake derived alkaliphilic microbial consortia. Water 2024, 16, 2937. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Nor, M.H.; Sabaruddin, M.F.; Bay, H.N.; Lim, C.K.; Aris, A.; Ibrahim, Z. Development and performance of BAC-ZS bacterial consortium as biofilm onto macrocomposites for raw textile wastewater treatment. Malays. J. Fundam. Appl. Sci. 2018, 14, 257–262. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; Lopez-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Gangola, S.; Sharma, A.; Joshi, S.; Bhandari, G.; Prakash, O.; Govarthanan, M.; Kim, W.; Bhatt, P. Novel mechanism and degradation kinetics of pesticides mixture using Bacillus sp. strain 3C in contaminated sites. Pestic. Biochem. Physiol. 2022, 181, 104996. [Google Scholar] [CrossRef]
- Aswathi, A.; Pandey, A.; Sukumaran, R.K. Rapid degradation of the organophosphate pesticide chlorpyrifos by a novel strain of Pseudomonas nitroreducens AR-3. Bioresour. Technol. 2019, 292, 122025. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, C.V.; Kumar, M.; Khanna, S. Biotransformation of chlorpyrifos and bioremediation of contaminated soil. Int. Biodeterior. Biodegrad. 2008, 62, 204–209. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Salem, S.H.; Abd, H.I.; Akl, B.A.; Fayez, M.; Maher, M.; Aioub, A.A.; Sitohy, M. Insights into the role of hexa-bacterial consortium for bioremediation of soil contaminated with chlorantraniliprole. Environ. Sci. Eur. 2024, 36, 1–16. [Google Scholar] [CrossRef]
- Góngora-Echeverría, V.R.; García-Escalante, R.; Rojas-Herrera, R.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide bioremediation in liquid media using a microbial consortium and bacteria-pure strains isolated from a biomixture used in agricultural areas. Ecotoxicol. Environ. Saf. 2020, 200, 110734. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, A.; Pandey, A.; Madhavan, A.; Sukumaran, R.K. Chlorpyrifos induced proteome remodelling of Pseudomonas nitroreducens AR-3 potentially aid efficient degradation of the pesticide. Environ. Technol. Innov. 2021, 21, 101307. [Google Scholar] [CrossRef]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of heavy metal (Cd and Cr)-polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, I.; Cárdenas-González, J.F.; Rodríguez Pérez, A.S.; Oviedo, J.T.; Martínez-Juárez, V.M. Bioremoval of different heavy metals by the resistant fungal strain Aspergillus niger. Bioinorg. Chem. Appl. 2018, 2018, 3457196. [Google Scholar] [CrossRef]
- Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Rueda Puente, E.O. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass 2025, 25, 25. [Google Scholar] [CrossRef]
- Ortiz-Bernad, I.; Anderson, R.T.; Vrionis, H.A.; Lovley, D.R. Vanadium respiration by Geobacter metallireducens: Novel strategy for in situ removal of vanadium from groundwater. Appl. Environ. Microbiol. 2004, 70, 3091–3095. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowski, H.A.; Ward, P.M.; Barkay, T. Novel reduction of mercury(II) by mercury-sensitive dissimilatory metal reducing bacteria. Environ. Sci. Technol. 2006, 40, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiang, G.; Xiao, W.; Yang, Z.; Zhao, B. Microbial mediated remediation of heavy metals toxicity: Mechanisms and future prospects. Front. Plant Sci. 2024, 15, 1420408. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Bonetta, S.; Pignata, C.; Gasparro, E.; Richiardi, L.; Bonetta, S.; Carraro, E. Impact of wastewater treatment plants on microbiological contamination for evaluating the risks of wastewater reuse. Environ. Sci. Eur. 2022, 34, 20. [Google Scholar] [CrossRef]
- Yu, J.; Tang, S.N.; Lee, P.K. Universal dynamics of microbial communities in full-scale textile wastewater treatment plants and system prediction by machine learning. Environ. Sci. Technol. 2023, 57, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Numberger, D.; Zoccarato, L.; Woodhouse, J.; Ganzert, L.; Sauer, S.; Márquez, J.R.; Domisch, S.; Grossart, H.P.; Greenwood, A.D. Urbanization promotes specific bacteria in freshwater microbiomes including potential pathogens. Sci. Total Environ. 2022, 845, 157321. [Google Scholar] [CrossRef]
- Rathore, S.; Varshney, A.; Mohan, S.; Dahiya, P. An innovative approach of bioremediation in enzymatic degradation of xenobiotics. Biotechnol. Genet. Eng. Rev. 2022, 38, 1–32. [Google Scholar] [CrossRef]
- Ferraro, G.B.; Bonomo, C.; Brandtner, D.; Mancini, P.; Veneri, C.; Briancesco, R.; Coccia, A.M.; Lucentini, L.; Suffredini, E.; Bongiorno, D.; et al. Characterisation of microbial communities and quantification of antibiotic resistance genes in Italian wastewater treatment plants using 16S rRNA sequencing and digital PCR. Sci. Total Environ. 2024, 933, 173217. [Google Scholar] [CrossRef]
- Manz, W.; Wagner, M.; Amann, R.; Schleifer, K.H. In situ characterization of the microbial consortia active in two wastewater treatment plants. Water Res. 1994, 28, 1715–1723. [Google Scholar] [CrossRef]
- Nair, N.; Patwardhan, S.; Wagh, N.S.; Lakkakula, J.; Mketo, N. Elucidation of microbial diversity in wastewater treatment system through molecular tools: Molecular tools, techniques, and applications in wastewater treatment. In Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2022; pp. 71–84. [Google Scholar]
- Yasir, M. Analysis of microbial communities and pathogen detection in domestic sewage using metagenomic sequencing. Diversity 2020, 13, 6. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring antibiotic resistance genes in wastewater treatment: Current strategies and future challenges. Sci. Total Environ. 2021, 783, 146964. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Johnson, T.A.; Lyra, C.; Stedtfeld, R.D.; Tamminen, M.; Tiedje, J.M.; Virta, M. High-throughput quantification of antibiotic resistance genes from an urban wastewater treatment plant. FEMS Microbiol. Ecol. 2016, 92, fiw014. [Google Scholar] [CrossRef]
- Urrea-Valencia, S.; Melo, A.L.; Gonçalves, D.R.; Galvão, C.W.; Etto, R.M. Molecular techniques to study microbial wastewater communities. Braz. Arch. Biol. Technol. 2021, 64, e21200193. [Google Scholar] [CrossRef]
- Sasi, R.; Suchithra, T.V. Wastewater microbial diversity versus molecular analysis at a glance: A mini-review. Braz. J. Microbiol. 2023, 54, 3033–3039. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, S.S.; Xiao, Z.C.; Min, Y.; Tian, T.; Zheng, Y.M.; Zhao, Q.B.; Yuan, Z.H.; Yu, H.Q. Re-evaluation and modification of dehydrogenase activity tests in assessing microbial activity for wastewater treatment plant operation. Water Res. 2023, 246, 120737. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Zhao, G.; Xu, X.; Zhang, Q.; Shen, Q.; Fang, Z.; Huang, L.; Ji, F. Microbial community structures and functions of wastewater treatment systems in plateau and cold regions. Bioresour. Technol. 2018, 249, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Correa-Galeote, D.; Roibás-Rozas, A.; Mosquera-Corral, A.; Juárez-Jiménez, B.; González-López, J.; Rodelas, B. Revealing the dissimilar structure of microbial communities in different WWTPs that treat fish-canning wastewater with different NaCl content. J. Water Process Eng. 2021, 44, 102328. [Google Scholar] [CrossRef]
- Yoo, K.; Yoo, H.; Lee, J.; Choi, E.J.; Park, J. Exploring the antibiotic resistome in activated sludge and anaerobic digestion sludge in an urban wastewater treatment plant via metagenomic analysis. J. Microbiol. 2020, 58, 123–130. [Google Scholar] [CrossRef]
- Liu, Z.; Klümper, U.; Liu, Y.; Yang, Y.; Wei, Q.; Lin, J.G.; Gu, J.D.; Li, M. Metagenomic and metatranscriptomic analyses reveal activity and hosts of antibiotic resistance genes in activated sludge. Environ. Int. 2019, 129, 208–220. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.; Gao, Y.; Wang, W.; Guo, G.; Duan, Y.; Zhou, S.; Tang, Z. Metagenomic insight into the enrichment of antibiotic resistance genes in activated sludge upon exposure to nanoplastics. Environ. Pollut. 2024, 363, 125260. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Su, Z.; Zeng, Y.; Bao, Y.; Zheng, Y.; Guo, H.; Yang, Y.; Wen, D. Wastewater treatment plant effluent discharge decreases bacterial community diversity and network complexity in urbanized coastal sediment. Environ. Pollut. 2023, 322, 121122. [Google Scholar] [CrossRef]
- Tumendelger, A.; Alshboul, Z.; Lorke, A. Methane and nitrous oxide emission from different treatment units of municipal wastewater treatment plants in Southwest Germany. PLoS ONE 2019, 14, e0209763. [Google Scholar] [CrossRef]
- De Haas, D.; Andrews, J. Nitrous oxide emissions from wastewater treatment—Revisiting the IPCC 2019 refinement guidelines. Environ. Chall. 2022, 8, 100557. [Google Scholar] [CrossRef]
- Wagner, J.; Coupland, P.; Browne, H.P.; Lawley, T.D.; Francis, S.C.; Parkhill, J. Evaluation of PacBio sequencing for full-length bacterial 16S rRNA gene classification. BMC Microbiol. 2016, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- LaMartina, E.L.; Schmoldt, A.L.; Newton, R.J. Full-length 16S rRNA gene sequences from raw sewage samples spanning geographic and seasonal gradients in conveyance systems across the United States. Microbiol. Resour. Announc. 2022, 11, e00319-22. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, E.; Dimian, M.; Covașă, M. Nanopore sequencing assessment of bacterial pathogens and associated antibiotic resistance genes in environmental samples. Microorganisms 2023, 11, 2834. [Google Scholar] [CrossRef]
- Galagoda, R.; Chanto, M.; Takemura, Y.; Tomioka, N.; Syutsubo, K.; Honda, R.; Yamamoto-Ikemoto, R.; Matsuura, N. Quantitative 16S rRNA gene amplicon sequencing for comprehensive pathogenic bacterial tracking in a municipal wastewater treatment plant. ACS ES&T Water 2023, 3, 923–933. [Google Scholar] [CrossRef]
- Su, Z.; Liu, T.; Guo, J.; Zheng, M. Nitrite oxidation in wastewater treatment: Microbial adaptation and suppression challenges. Environ. Sci. Technol. 2023, 57, 12557–12570. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Gajendiran, V.; Mani, K. Meta-analysis to identify the core microbiome in diverse wastewater. Int. J. Environ. Sci. Technol. 2022, 19, 5079–5096. [Google Scholar] [CrossRef]
- Stoeck, T.; Pan, H.; Dully, V.; Forster, D.; Jung, T. Towards an eDNA metabarcode-based performance indicator for full-scale municipal wastewater treatment plants. Water Res. 2018, 144, 322–331. [Google Scholar] [CrossRef]
- Lukyanov, V.; Gaysina, L.; Bukin, Y.; Renganathan, P.; Tupikin, A. DNA-metabarcoding of cyanobacteria and microalgae in chernozem soils of temperate continental climate of the forest-steppe zone of Eurasia under different degrees of agrotechnology intensification. World J. Microbiol. Biotechnol. 2024, 40, 351. [Google Scholar] [CrossRef]
- Tu, Q.; Yu, H.; He, Z.; Deng, Y.E.; Wu, L.; Van Nostrand, J.D.; Zhou, A.; Voordeckers, J.; Lee, Y.J.; Qin, Y.; et al. GeoChip 4: A functional gene-array-based high-throughput environmental technology for microbial community analysis. Mol. Ecol. Resour. 2014, 14, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yin, H.; Van Nostrand, J.D.; Voordeckers, J.W.; Tu, Q.; Deng, Y.; Yuan, M.; Zhou, A.; Zhang, P.; Xiao, N.; et al. Functional gene array-based ultrasensitive and quantitative detection of microbial populations in complex communities. mSystems 2019, 4, e00296-19. [Google Scholar] [CrossRef]
- Joshi, D.R.; Zhang, Y.; Zhang, H.; Gao, Y.; Yang, M. Characteristics of microbial community functional structure of a biological coking wastewater treatment system. J. Environ. Sci. 2018, 63, 105–115. [Google Scholar] [CrossRef]
- Jie, S.; Li, M.; Gan, M.; Zhu, J.; Yin, H.; Liu, X. Microbial functional genes enriched in the Xiangjiang River sediments with heavy metal contamination. BMC Microbiol. 2016, 16, 179. [Google Scholar] [CrossRef]
- Mirabile, A.; Sangiorgio, G.; Bonacci, P.G.; Bivona, D.; Nicitra, E.; Bonomo, C.; Bongiorno, D.; Stefani, S.; Musso, N. Advancing pathogen identification: The role of digital PCR in enhancing diagnostic power in different settings. Diagnostics 2024, 14, 1598. [Google Scholar] [CrossRef]
- Silvester, R.; Woodhall, N.; Nurmi, W.; Muziasari, W.; Farkas, K.; Cross, G.; Malham, S.K.; Jones, D.L. High-throughput qPCR profiling of antimicrobial resistance genes and bacterial loads in wastewater and receiving environments. Environ. Pollut. 2025, 373, 126096. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Noman, E.A.; Alsahari, M.; Al-Maqtari, Q.; Vo, D.V. Potential of complex microbial community in aerobic granular sludge as a bio-startup approach for next-generation wastewater treatment. Appl. Water Sci. 2024, 14, 215. [Google Scholar] [CrossRef]
- Leiviskä, T.; Risteelä, S. Analysis of pharmaceuticals, hormones and bacterial communities in a municipal wastewater treatment plant—Comparison of parallel full-scale membrane bioreactor and activated sludge systems. Environ. Pollut. 2022, 292, 118433. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, A.; Pierpaoli, M.; Jankowska, K.; Fudala-Ksiazek, S.; Remiszewska-Skwarek, A.; Uczkiewicz, A. Insights into the microbial community of treated wastewater, its year-round variability and impact on the receiver, using cultivation, microscopy and amplicon-based methods. Sci. Total Environ. 2022, 829, 154630. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Chang, H.; Yang, H.; Zhang, W.; Zhang, Y.; Sun, H. Deciphering the microbial community structures and functions of wastewater treatment at high-altitude area. Front. Bioeng. Biotechnol. 2023, 11, 1107633. [Google Scholar] [CrossRef]
- Talat, A.; Bashir, Y.; Khalil, N.; Brown, C.L.; Gupta, D.; Khan, A.U. Antimicrobial resistance transmission in the environmental settings through traditional and UV-enabled advanced wastewater treatment plants: A metagenomic insight. Environ. Microbiome 2025, 20, 27. [Google Scholar] [CrossRef]
- Xu, S.; Yao, J.; Ainiwaer, M.; Hong, Y.; Zhang, Y. Analysis of bacterial community structure of activated sludge from wastewater treatment plants in winter. Biomed. Res. Int. 2018, 2018, 8278970. [Google Scholar] [CrossRef]
- Freeman, C.N.; Russell, J.N.; Yost, C.K. Temporal metagenomic characterization of microbial community structure and nitrogen modification genes within an activated sludge bioreactor system. Microbiol. Spectr. 2024, 12, e02832-e23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sehgal, R.; Gupta, R. Microbes and wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–255. [Google Scholar]
- Jałowiecki, Ł.; Chojniak, J.M.; Dorgeloh, E.; Hegedusova, B.; Ejhed, H.; Magnér, J.; Płaza, G.A. Microbial community profiles in wastewaters from onsite wastewater treatment systems technology. PLoS ONE 2016, 11, e0147725. [Google Scholar] [CrossRef]
- Wigginton, S.K.; Brannon, E.Q.; Kearns, P.J.; Lancellotti, B.V.; Cox, A.; Moseman-Valtierra, S.; Loomis, G.W.; Amador, J.A. Nitrifying and denitrifying microbial communities in centralized and decentralized biological nitrogen removing wastewater treatment systems. Water 2020, 12, 1688. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Wu, Y. Performance of an anaerobic baffled filter reactor in the treatment of algae-laden water and the contribution of granular sludge. Water 2013, 6, 122–138. [Google Scholar] [CrossRef]
- Nasr, F.A.; Doma, H.S.; Nassar, H.F. Treatment of domestic wastewater using an anaerobic baffled reactor followed by a duckweed pond for agricultural purposes. Environment 2009, 29, 270–279. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Zhu, G. Performance, process kinetics and functional microbial community of biocatalyzed electrolysis-assisted anaerobic baffled reactor treating carbohydrate-containing wastewater. RSC Adv. 2018, 8, 41150–41162. [Google Scholar] [CrossRef]
- Jamshidi, S.; Akbarzadeh, A.; Woo, K.S.; Valipour, A. Wastewater treatment using integrated anaerobic baffled reactor and Bio-rack wetland planted with Phragmites sp. and Typha sp. J. Environ. Health Sci. Eng. 2014, 12, 131. [Google Scholar] [CrossRef]
- Song, S.; Wang, B.; Yang, T.; Gu, Y.; Sheng, S.; Zhao, D.; An, S.; Li, A. Performance and bacteria communities of a full-scale constructed wetland treating the secondary effluent after multi-years’ operation. Processes 2023, 11, 1469. [Google Scholar] [CrossRef]
- Khajah, M.; Bydalek, F.; Babatunde, A.O.; Wenk, J.; Webster, G. Nitrogen removal performance and bacterial community analysis of a multistage step-feeding tidal flow constructed wetland. Front. Water 2023, 5, 1128901. [Google Scholar] [CrossRef]
- Zhang, Y.; Carvalho, P.N.; Lv, T.; Arias, C.; Brix, H.; Chen, Z. Microbial density and diversity in constructed wetland systems and the relation to pollutant removal efficiency. Water Sci. Technol. 2016, 73, 679–686. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Louca, S.; Jacques, S.M.; Pires, A.P.; Leal, J.S.; Srivastava, D.S.; Parfrey, L.W.; Farjalla, V.F.; Doebeli, M. High taxonomic variability despite stable functional structure across microbial communities. Nat. Ecol. Evol. 2016, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.; Borenstein, E. Taxa-function robustness in microbial communities. Microbiome 2018, 6, 1. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular ‘omics’ for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef]
- Arıkan, M.; Muth, T. Integrated multi-omics analyses of microbial communities: A review of the current state and future directions. Mol. Omics 2023, 19, 607–623. [Google Scholar] [CrossRef]
- Jiang, D.; Armour, C.R.; Hu, C.; Mei, M.; Tian, C.; Sharpton, T.J.; Jiang, Y. Microbiome multi-omics network analysis: Statistical considerations, limitations, and opportunities. Front. Genet. 2019, 10, 995. [Google Scholar] [CrossRef]
- Zampieri, G.; Campanaro, S.; Angione, C.; Treu, L. Metatranscriptomics-guided genome-scale metabolic modeling of microbial communities. Cell Rep. Methods 2023, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Schiml, V.C.; Delogu, F.; Kumar, P.; Kunath, B.; Batut, B.; Mehta, S.; Johnson, J.E.; Grüning, B.; Pope, P.B.; Jagtap, P.D.; et al. Integrative meta-omics in Galaxy and beyond. Environ. Microbiome 2023, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Jafar, R.; Awad, A.; Jafar, K.; Shahrour, I. Predicting effluent quality in full-scale wastewater treatment plants using shallow and deep artificial neural networks. Sustainability 2022, 14, 15598. [Google Scholar] [CrossRef]
- Voipan, D.; Voipan, A.E.; Barbu, M. Evaluating machine learning-based soft sensors for effluent quality prediction in wastewater treatment under variable weather conditions. Sensors 2025, 25, 1692. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Y.; Wei, Q.; Yin, H. A hybrid deep learning approach to improve real-time effluent quality prediction in wastewater treatment plant. Water Res. 2024, 250, 121092. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.R.; Kou, R.; Li, Y.; Fu, J.; Zhao, L.; Liu, H. Prediction of effluent quality in a wastewater treatment plant by dynamic neural network modeling. Process Saf. Environ. Prot. 2022, 158, 515–524. [Google Scholar] [CrossRef]
- Safder, U.; Kim, J.; Pak, G.; Rhee, G.; You, K. Investigating machine learning applications for effective real-time water quality parameter monitoring in full-scale wastewater treatment plants. Water 2022, 14, 3147. [Google Scholar] [CrossRef]
- Wang, D.; Thunéll, S.; Lindberg, U.; Jiang, L.; Trygg, J.; Tysklind, M.; Souihi, N. A machine learning framework to improve effluent quality control in wastewater treatment plants. Sci. Total Environ. 2021, 784, 147138. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef] [PubMed]
- McCarty, N.S.; Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, A.; Zhuang, G. Construction of environmental synthetic microbial consortia: Based on engineering and ecological principles. Front. Microbiol. 2022, 13, 829717. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Fan, H.; Dong, Y.; Wang, Y.; Bai, Z.; Zhuang, X. Engineering artificial microbial consortia based on division of labor promoted simultaneous removal of Cr(VI)-atrazine combined pollution. J. Hazard. Mater. 2023, 443, 130221. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.S. Microalgae-based wastewater treatment—microalgae-bacteria consortia, multi-omics approaches and algal stress response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef]
- Li, S.N.; Zhang, C.; Li, F.; Ren, N.Q.; Ho, S.H. Recent advances of algae-bacteria consortia in aquatic remediation. Crit. Rev. Environ. Sci. Technol. 2023, 53, 315–339. [Google Scholar] [CrossRef]
| Pollutants | Representative Taxa | Enzymes/Mechanisms | Reported Efficiency/Conditions | Limitations/Challenges | Reference |
|---|---|---|---|---|---|
| Hydrocarbons/Polycyclic Aromatic Hydrocarbons (PAHs) | Pseudomonas aeruginosa, P. putida, Bacillus subtilis, Rhodococcus spp. | Oxygenases, dehydrogenases, ring-cleavage enzymes | 90–95% total petroleum hydrocarbon removal within 7–10 d (aerobic); consortia 20–40% faster mineralization; immobilized cells show 30% higher kinetics | Efficiency decreases under fluctuating influent load; immobilized consortia stability remains uncertain | [39,40,41,42,43,44] |
| Textile dyes | Bacillus firmus, Klebsiella oxytoca, Aspergillus spp., Thermoalkaliphilic consortia | Azoreductases, peroxidases | 90% decolorization in 24–72 h (anaerobic–aerobic); thermo-alkaliphiles: 98% under salinity/pH stress; biofilm consortia: 78.6% in raw textile effluent | Real effluents with dye–metal–salt mixtures inhibit microbial activity; scalability issues persist | [45,46,47,48,49,50,51,52] |
| Agrochemicals | Pseudomonas nitroreducens AR-3, Bacillus spp., Serratia marcescens, Alcaligenes aquatilis | Hydrolases, dehalogenases, oxidoreductases | AR-3: 97% chlorpyrifos in 8 h (100 mg L−1); mixed consortia: 75–87% chlorpyrifos in 20 d; Bacillus-based consortia: 99.3% chlorantraniliprole in 20 d; biobed consortia: >90% atrazine/carbofuran/glyphosate removal | Persistence varies among pesticide classes; mixed pesticide loads reduce efficiency | [53,54,55,56,57,58,59] |
| Heavy metals | Aspergillus niger, Penicillium simplicissimum, Saccharomyces cerevisiae, Candida albicans, Geobacter metallireducens, Shewanella oneidensis | Biosorption proteins, reductases, metal transporters | A. niger: 98% Cd, 43% Cr uptake; dead biomass: 100% Cr(VI)/Zn(II); S. cerevisiae: 40–80 mg g−1 Pb uptake; G. metallireducens: V reduced from 50 µM to 6 µM in aquifer trial; CRISPR strains: improved Cd, Cu, Hg, Ni, Fe uptake | Mixed-metal stress and sulfide toxicity constrain scalability; GMOs raise biosafety concerns | [60,61,62,63,64,65,66] |
| Plastics/Microplastics | Pseudomonas, Rhodococcus, Aspergillus, Ideonella sakaiensis | Esterases, hydrolases, laccases | Degrades polyethylene terephthalate, polyethylene, polystyrene, polyurethane; efficiencies under WWTP conditions remain poorly established | Efficiencies poorly established under WWTP conditions; slow kinetics | [67,68] |
| Emerging pathogens | Mixed sludge microbiomes, beneficial consortia | Competitive exclusion, predation, ARG suppression | Opportunistic pathogens (Legionella, Leptospira) persist at 102–107 CFU L−1; AI models achieve >90% accuracy in outbreak prediction | Opportunistic pathogens persist; ARG suppression remains incomplete | [23,28,69,70,71,72] |
| Technique | Target Biomarker | Strengths | Limitations | Applications | Performance | References |
|---|---|---|---|---|---|---|
| 16S rRNA gene sequencing | 16S rRNA gene (bacteria/archaea) | High taxonomic resolution; detects uncultured taxa | Limited functional insight; primer bias; rare taxa may be overlooked | Community profiling; diversity analysis; microbiome detection | Detects ≥1% relative abundance taxa; ~104 cells mL−1 detection limit | [15,27,73,74,75] |
| Full-length 16S rRNA sequencing | Complete 16S rRNA gene | Species-level classification; enhanced taxonomic accuracy | Higher cost; requires long-read platforms and advanced analysis | Pathogen detection, species-level taxonomic profiling | Error rates as low as 0.007%; species resolution >60% vs. <5% for short reads | [15,27] |
| Metagenomics (Shotgun) | Total DNA (coding and non-coding genes) | Functional and taxonomic insights; ARG/metabolic pathway detection | Computationally intensive; higher sequencing cost | Functional profiling; ARG monitoring; novel gene discovery | ~107 reads/sample; detects 100s of ARG subtypes; sequencing depth >20 Gb | [76,77] |
| Digital PCR (dPCR) | Specific genes (e.g., ARGs, 16S, pathogens) | Ultra-sensitive absolute quantification; high precision | Target-specific; requires prior sequence knowledge | ARG quantification; rare gene detection | Detects down to 0.1% rare alleles; CV <5%; LoD ~10–100 copies µL−1 | [75,77,78] |
| Quantitative PCR (qPCR) | Targeted genes (ARGs, pathogens, PAOs) | Rapid, sensitive, quantitative | Limited multiplexing; primer design critical | Monitoring of target pathogens or ARGs; process optimization | LoD ~102–103 copies mL−1; efficiency 90–105% | [73,77,78] |
| Fluorescence in situ hybridization (FISH) | rRNA sequences, functional genes | Visualizes morphology and spatial organization; detects active cells | Probe design required; limited multiplexing; labor-intensive | In situ localization; biofilm/community structure analysis | Resolution ~0.5–1 µm; requires ≥103–104 cells for detection | [74,75,79] |
| Functional gene microarrays (GeoChip) | Known functional gene (N, P, S cycling) | High-throughput; sensitive to metabolic shifts | Limited to predefined genes; less sensitive than metagenomics | Functional diversity; nutrient cycling assessment | >365,000 genes; detects genes down to 10 pg DNA | [79,80] |
| High-throughput qPCR arrays | Multiple ARGs, mobile elements, integrons | Parallel quantification of many genes, seasonal/spatial analysis | Limited to known targets, primer intensive | ARG hotspot mapping; risk assessment | Detects up to 300–400 ARGs in parallel; sensitivity 102–103 copies/mL; quantification range 6–8 log units | [77,78] |
| Dehydrogenase activity (DHA) assay | Enzymatic activity (dehydrogenases) | Simple, cost-effective, proxy for total microbial activity | No taxonomic specificity; affected by stressors | Process monitoring; toxicity assessment | LoD ~0.05 µg formazan/g sludge; activity correlates with microbial biomass | [81] |
| WWTP Type | Dominant Phyla/Genera (Range %) | Key Environmental Factors | Functional/Ecological Outcomes | Performance | References |
|---|---|---|---|---|---|
| Municipal | Bacteroidetes (30–44%), Proteobacteria (15–84%), Firmicutes (20–37%); core genera: Comamonas, Pseudomonas, Acidovorax, Acinetobacter | Seasonal shifts (e.g., winter increased Proteobacteria, and decreased Bacteroidetes); influent source variability | Conserved “core microbiome” ensures nutrient removal redundancy; sludge bulking connected to filamentous Saprospiraceae, Flavobacterium, Tetrasphaera | COD: 80–95%; TN: 65–85%; TP: 60–80%; Pathogen log-reduction: 2–3 | [104,105,106,107,108,109,110] |
| Industrial | Proteobacteria (24–95%), Bacteroidetes (0.5–45%), Firmicutes (4–67%); enriched: Planctomycetes, Chloroflexi, Thaumarchaeota | High dye load, surfactants, heavy metals, extreme pH, salinity | Reduced richness; limited nitrifiers/denitrifiers; inhibited metabolic pathways for lower treatment efficiency | COD: 50–70%; TN: 30–50%; TP: <40%; Dye decolorization: 60–80% (lab scale) | [31,32,70,111] |
| Onsite (ABR, CW, hybrid systems) | Proteobacteria, Firmicutes, Actinobacteria; variable with plant species and depth | Variable inflows, shallow groundwater, plant–microbe interactions | COD removal 80–95%; nutrient removal enhanced in CWs with diverse vegetation; microbial biomass and Shannon diversity correlated with BOD/N removal | COD: 75–95%; BOD: 80–95%; TN: 50–70%; TP: 45–65% | [112,113,114,115,116,117,118,119,120] |
| Model Type | Target Parameters | Reported Accuracy | Reference |
|---|---|---|---|
| Random Forest, Artificial Neural Networks (ANN), Convolutional Neural Networks (CNN), Recurrent Neural Networks (RNN) | COD, BOD5, nitrate, phosphate | Correlation coefficients up to 0.96; ensemble models outperform single learners | [130] |
| Long Short-Term Memory (LSTM), Gated Recurrent Units (GRU), Transformer | Effluent Quality Index (EQI) | Transformer achieved highest accuracy under dry conditions; GRU performed better under rainfall/storm conditions | [131] |
| Hybrid Deep Learning Model (Temporal Convolutional Network + LSTM) | Total nitrogen (TN) | Up to 33% higher accuracy vs. stand-alone DL; ~63% improvement vs. traditional ML | [132] |
| Dynamic Neural Networks (Nonlinear Autoregressive with Exogenous Inputs—NARX; Principal Component Analysis integrated NARX—PCA-NARX) | COD, TN | High accuracy: RMSE = 2.9 mg L−1 (COD), 0.8 mg L−1 (TN) | [133] |
| Multi-Attention RNN | Effluent total nitrogen (TNeff) | Forecast accuracy: 98.1% (1 h ahead), 96.3% (3 h ahead) | [134] |
| Random Forest, Deep Neural Network (DNN) | Total suspended solids (TSS), phosphate | Identified key operational variables; enabled integration with real-time control | [135] |
| Challenges | Opportunities | References |
|---|---|---|
| Microbial ecology uncertainty; “microbial dark matter”; functional redundancy | Integrative omics approaches for taxonomy-to-function linkage | [121,122,123,124,125,126] |
| Antibiotic resistance genes (ARGs) and pathogens persist despite treatment | High-throughput monitoring: qPCR, dPCR, HT-qPCR arrays, eDNA diagnostics | [73,75,77,78,103] |
| Greenhouse gas emissions (CH4, N2O) from microbial guilds | AI and digital twins for predictive GHG and energy optimization | [128,129,130,131,132,133] |
| Influent variability; bulking and foaming reduce stability | Synthetic biology and CRISPR-based engineered consortia | [104,107,134,135,136,137] |
| Scalability and cost barriers in enzymatic or advanced strategies | Hybrid strategies: immobilized enzymes, nanomaterials, AI-guided selection | [138,139,140,141] |
| Regulatory and biosafety issues (GMOs, nanomaterials) | Circular resource recovery: energy, nutrient, and material valorization | [10,11,14,15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renganathan, P.; Gaysina, L.A. Next-Generation Wastewater Treatment: Omics and AI-Driven Microbial Strategies for Xenobiotic Bioremediation and Circular Resource Recovery. Processes 2025, 13, 3218. https://doi.org/10.3390/pr13103218
Renganathan P, Gaysina LA. Next-Generation Wastewater Treatment: Omics and AI-Driven Microbial Strategies for Xenobiotic Bioremediation and Circular Resource Recovery. Processes. 2025; 13(10):3218. https://doi.org/10.3390/pr13103218
Chicago/Turabian StyleRenganathan, Prabhaharan, and Lira A. Gaysina. 2025. "Next-Generation Wastewater Treatment: Omics and AI-Driven Microbial Strategies for Xenobiotic Bioremediation and Circular Resource Recovery" Processes 13, no. 10: 3218. https://doi.org/10.3390/pr13103218
APA StyleRenganathan, P., & Gaysina, L. A. (2025). Next-Generation Wastewater Treatment: Omics and AI-Driven Microbial Strategies for Xenobiotic Bioremediation and Circular Resource Recovery. Processes, 13(10), 3218. https://doi.org/10.3390/pr13103218







