From Waste to Wonder: Valorization of Colombian Plant By-Products for Peroxidase Production and Biotechnological Innovation
Abstract
1. Introduction
2. Agricultural By-Products as a Source of Peroxidases
2.1. Guinea Grass (Panicum maximum)
2.2. Royal Palm (Roystonea regia)
2.3. African Palm (Elaeis guineensis)
2.4. Lemongrass (Cymbopogon citratus)
2.5. Sleepy Plant (Mimosa pudica)
2.6. Sweet Potato (Ipomea batata)
3. Peroxidases: Definition, Purification and Biochemical Properties
3.1. Peroxidases
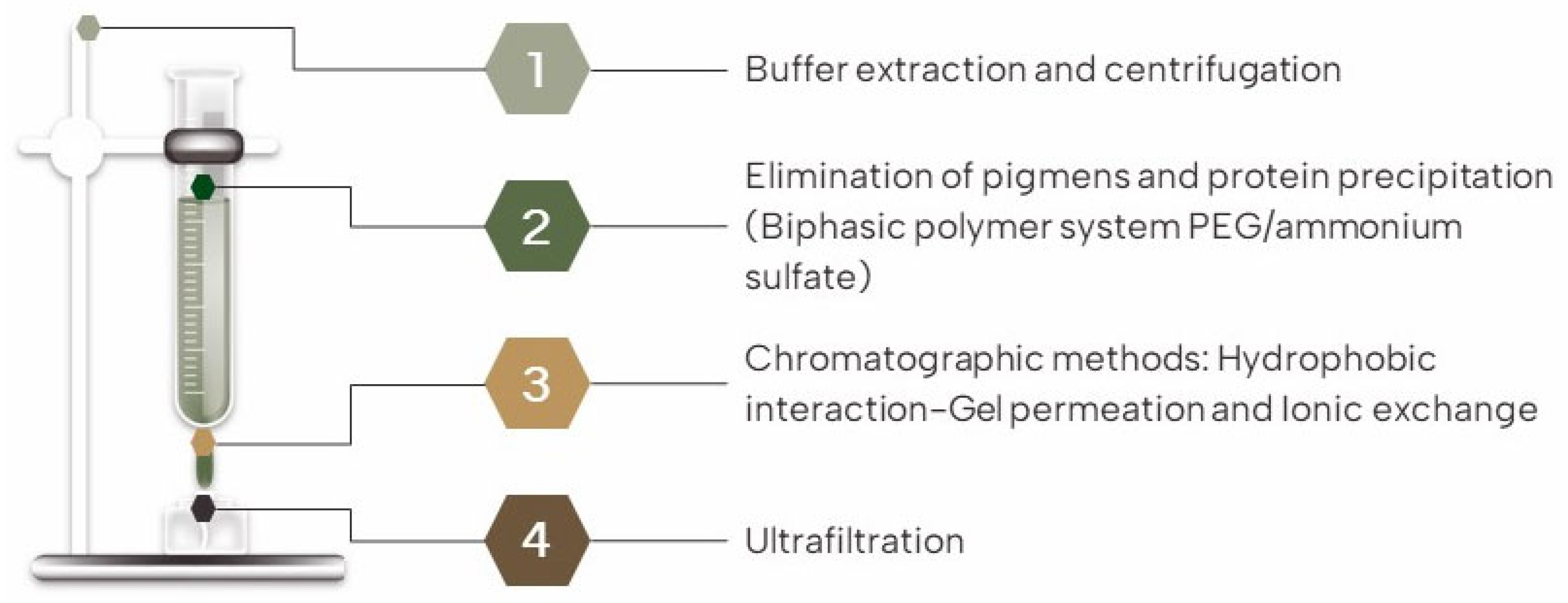
3.2. Extraction and Purification Techniques
3.3. Biochemical Properties
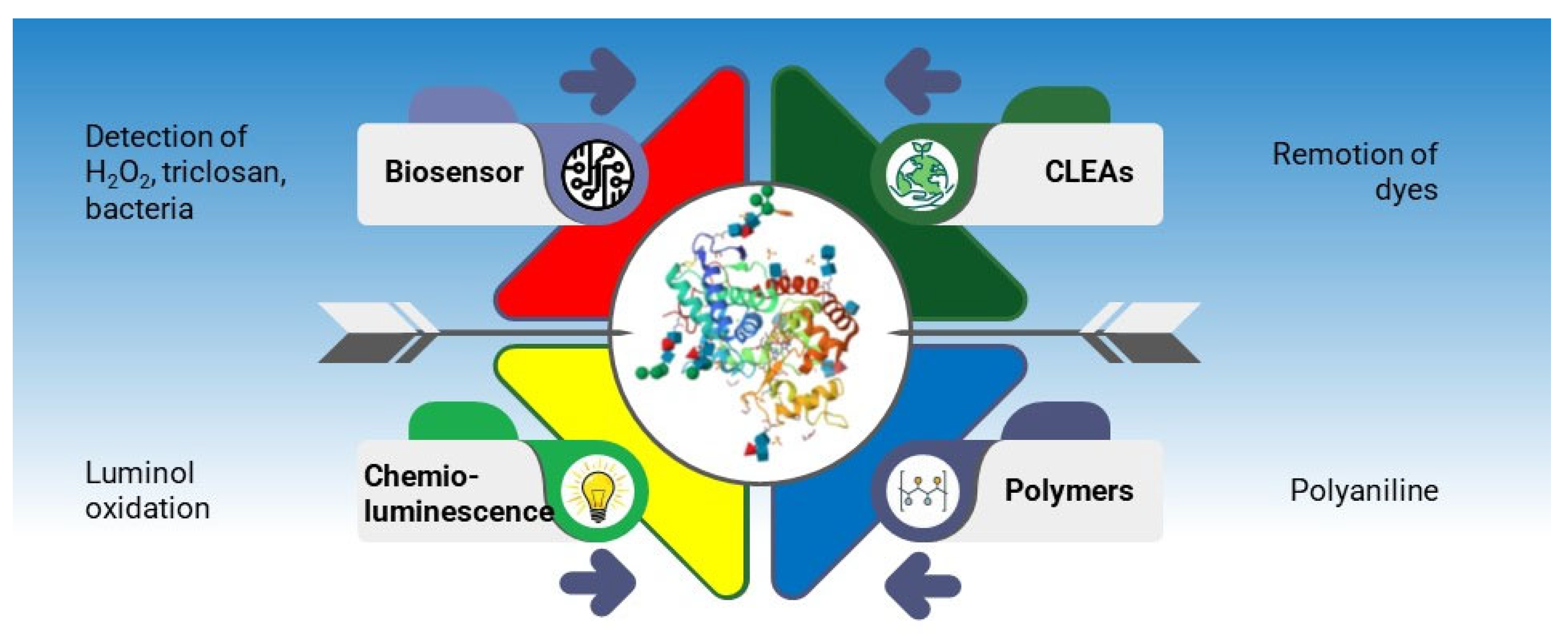
4. Biotechnological Applications of Peroxidases
4.1. Electrochemical Biosensing
4.2. Synthesis of Polyaniline
4.3. Chemiluminescence Assays
4.4. Cross-Linked Enzymatic Aggregates
5. Sustainability and the Circular Bioeconomy in the Context of Colombian Plants Peroxidases
6. Conclusions
- (i)
- Scaling up POD production: Future studies should focus on optimizing extraction and purification methods for large-scale, cost-effective production. This includes developing continuous processing technologies, green extraction approaches, and robust immobilization strategies to meet industrial demands.
- (ii)
- Structural characterization: Detailed studies using X-ray crystallography, cryo-EM, and computational modeling are needed to understand the structural basis of the exceptional thermal stability and substrate specificity of Colombian PODs. These insights will enable rational protein engineering and design of tailored biocatalysts.
- (iii)
- Integration into circular bioeconomy policies: Collaboration with policymakers, industries, and local communities is essential to incorporate POD-based technologies into Colombia’s circular bioeconomy framework, promoting sustainable waste valorization and contributing to national and global climate action goals.
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, Z.; Li, Y.; Zhang, H.; Qin, P.; Hu, X.; Wang, J.; Wei, G.; Chen, C. Lighting Up Agricultural Sustainability in the New Era through Nanozymology: An Overview of Classifications and Their Agricultural Applications. J. Agric. Food Chem. 2022, 70, 13445–13463. [Google Scholar] [CrossRef]
- Nesterov, D.; Barrera-Martínez, I.; Martínez-Sánchez, C.; Sandoval-González, A.; Bustos, E. Approaching the circular economy: Biological, physicochemical, and electrochemical methods to valorize agro-industrial residues, wastewater, and industrial wastes. J. Environ. Chem. Eng. 2024, 12, 113335. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of Agricultural Lignocellulosic Plant Byproducts through Enzymatic and Enzyme-Assisted Extraction of High-Value-Added Compounds: A Review. Am. Chem. Soc. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Sánchez, A. A Perspective of Solid-State Fermentation As Emergent Technology for Organic Waste Management in the Framework of Circular Bioeconomy. ACS Sustain. Resour. Manag. 2024, 1, 1630–1638. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, W.; Paneru, R.; Yang, Q.; Gong, W.; Tjeng, S.T.; Goroncy, A.; Dai, Q.; Zhang, J.; Kammen, D.M.; et al. Transformative and sustainable approach to waste plastic mixture valorization. Chem. Eng. J. 2024, 499, 156558. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Jiang, W.; Zhang, Q.; Sun, P. Life-Cycle Greenhouse Gas Emissions and Primary Energy Demand of Agricultural Greenhouse Plastics: Proposed Optimization toward Agricultural Plastic Sustainability. ACS Sustain. Chem. Eng. 2024, 12, 8415–8424. [Google Scholar] [CrossRef]
- Mubayi, V.; Ahern, C.B.; Calusinska, M.; O’Malley, M.A. Toward a Circular Bioeconomy: Designing Microbes and Polymers for Biodegradation. Am. Chem. Soc. 2024, 13, 1978–1993. [Google Scholar] [CrossRef]
- Jin, C.; Hu, J.; Wu, J.; Liang, H.; Li, J. Innovative and Economically Beneficial Use of Corn and Corn Products in Electrochemical Energy Storage Applications. Am. Chem. Soc. 2021, 9, 10678–10703. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Basso, A.; Brady, D. New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chem. Soc. Rev. 2021, 50, 5850–5862. [Google Scholar] [CrossRef]
- Svetozarević, M.; Šekuljica, N.; Knežević-Jugović, Z.; Mijin, D. Agricultural waste as a source of peroxidase for wastewater treatment: Insight in kinetics and process parameters optimization for anthraquinone dye removal. Environ. Technol. Innov. 2021, 21, 101289. [Google Scholar] [CrossRef]
- Lui, M.Y.; Wong, C.Y.Y.; Choi, A.W.T.; Mui, Y.F.; Qi, L.; Horváth, I.T. Valorization of Carbohydrates of Agricultural Residues and Food Wastes: A Key Strategy for Carbon Conservation. ACS Sustain. Chem. Eng. 2019, 7, 17799–17807. [Google Scholar] [CrossRef]
- Gonzalez, L.V.P.; Gómez, S.P.M.; Giraldo Abad, P.A. Exploitation of Agroindustrial Waste in Colombia. Rev. Investig. Agrar. Ambient. 2017, 8, 141–150. [Google Scholar] [CrossRef]
- Mathé, C.; Barre, A.; Jourda, C.; Dunand, C. Evolution and expression of class III peroxidases. Arch. Biochem. Biophys. 2010, 500, 58–65. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Alhaqbani, N.; Mohamed, M.B.; Kamal, A.M.; Albassam, A.A.; Lakshminarayana, G. Structural optical, dielectric and electrical characteristics of flexible blended polymers based on PMMA/PVAc/TBAI and milled PANI for energy storage applications and optoelectronic devices. J. Mol. Liq. 2024, 414, 126131. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cleas combi-cleas and ‘smart’ magnetic cleas: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef]
- Asrafi, R.; Kumar, Y.; Bist, Y.; Saxena, D.C.; Sharanagat, V.S. Esterified porous starch from guinea grass seed for enhanced facile microencapsulation of bioactive materials. Carbohydr. Polym. Technol. Appl. 2024, 7, 100490. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, H.; Huang, Q.; Liu, X.; Zhou, Y.; Diao, X.; Li, Q.X. Comparison of three palm tree peroxidases expressed by Escherichia coli: Uniqueness of African oil palm peroxidase. Protein Expr. Purif. 2021, 179, 105806. [Google Scholar] [CrossRef]
- Skaharov, I.; Vesga-Blanco, K.; Sakharova, I. Substrate Specificity of African Oil Palm Tree Peroxidase. Biochemistry 2002, 67, 1043–1047. [Google Scholar] [CrossRef]
- Leon, J.C.; Alpeeva, I.S.; Chubar, T.A.; Galaev, I.Y.; Csoregi, E.; Sakharov, I.Y. Purification and substrate specificity of peroxidase from sweet potato tubers. Plant Sci. 2002, 163, 1011–1019. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of Action, Sources, and Application of Peroxidases; Elsevier Ltd.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Ferreira, L.M.C.; Lima, D.; Marcolino-Junior, L.H.; Bergamini, M.F.; Kuss, S.; Campanhã Vicentini, F. Cutting-Edge Biorecognition Strategies to Boost the Detection Performance of COVID-19 Electrochemical Biosensors: A Review; Elsevier B.V.: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Centeno, D.A.; Solano, X.H.; Castillo, J.J. A new peroxidase from leaves of guinea grass (Panicum maximum): A potential biocatalyst to build amperometric biosensors. Bioelectrochemistry 2017, 116, 33–38. [Google Scholar] [CrossRef]
- Kor, L.; Diazgranados, M. Identifying important plant areas for useful plant species in Colombia. Biol. Conserv. 2023, 284, 110187. [Google Scholar] [CrossRef]
- Sánchez, M.; Castañeda, R.; Castañeda, S.M. Usos y potencialidad de la Higuerilla (Ricinus communis) en sistemas agroforestales en Colombia. Pubvet 2016, 10, 507–512. [Google Scholar] [CrossRef]
- Jaramillo, M.A.; Reyes-Palencia, J.; Jiménez, P. Floral biology and flower visitors of cocoa (Theobroma cacao L.) in the upper Magdalena Valley, Colombia. Flora 2024, 313, 152480. [Google Scholar] [CrossRef]
- Garcia-Vallejo, M.C.; Alzate, C.A.C. Life cycle assessment of the cassava simplified value chain in Colombia and the use of cassava residues as energy carriers. Ind. Crop. Prod. 2024, 210, 118135. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Ardila, G.B.; Sakharova, I.V. Peroxidasa de plantas tropicales. Rev. Colomb. Quim. 1999, 28, 97–106. [Google Scholar]
- Sakharov, I.Y.; Vesga, M.K.; Galaev, I.Y.; Sakharova, I.V.; Pletjushkina, O.Y. Peroxidase from Leaves of Royal Palm Tree Roystonea Regia: Purification and Some Properties. 2001. Available online: www.elsevier.com/locate/plantsci (accessed on 6 October 2025).
- Rodríguez, A.; Pina, D.G.; Yélamos, B.; León, J.J.C.; Zhadan, G.G.; Villar, E.; Gavilanes, F.; Roig, M.G.; Sakharov, I.Y.; Shnyrov, V.L. Thermal stability of peroxidase from the African oil palm tree Elaeis guineensis. Eur. J. Biochem. 2002, 269, 2584–2590. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, K.; Gundabala, V. Development of a Lemongrass/Silver Nanocomposite for Controlling a Foodborne Pathogen—Escherichia coli. ACS Food Sci. Technol. 2022, 2, 1850–1861. [Google Scholar] [CrossRef]
- Don, S.M.; Rambli, M.; Nore, B.F. Antioxidant content following fermentation of lemongrass for herbal beverage development. Food Sci. Technol. 2024, 61, 1–14. [Google Scholar] [CrossRef]
- Tazi, A.; Zinedine, A.; Rocha, J.M.; Errachidi, F. Review on the pharmacological properties of lemongrass (Cymbopogon citratus) as a promising source of bioactive compounds. Pharmacol. Res.—Nat. Prod. 2024, 3, 100046. [Google Scholar] [CrossRef]
- Dhanush, C.; Aravindh, S.; Jesreena, J.S.; Nagadharshini, R.; Jano, N.; Almeer, R.; Velu, S.K.P. Biomimetic Synthesis of Carbon Dots from Mimosa pudica Leaves for Enhanced Bioimaging. Waste Biomass Valorization 2024, 16, 713–721. [Google Scholar] [CrossRef]
- Hagihara, T.; Mano, H.; Miura, T.; Hasebe, M.; Toyota, M. Calcium-mediated rapid movements defend against herbivorous insects in Mimosa pudica. Nat. Commun. 2022, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adurosakin, O.E.; Iweala, E.J.; Otike, J.O.; Dike, E.D.; Uche, M.E.; Owanta, J.I.; Ugbogu, O.C.; Chinedu, S.N.; Ugbogu, E.A. Ethnomedicinal Uses, Phytochemistry, Pharmacological Activities and Toxicological Effects of Mimosa Pudica—A Review; Elsevier B.V.: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Collado, L.S.; Mabesa, R.C.; Corke, H. Genetic variation in the physical properties of sweet potato starch. J. Agric. Food Chem. 1999, 47, 4195–4201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, P.; Zhao, T.; Huang, X.; Zong, J.; Luo, Q.; Peng, C.; Wu, X.; Qiu, F.; Zhao, D.; et al. Biosynthesis of Scopoletin in Sweet Potato Confers Resistance against Fusarium oxysporum. J. Agric. Food Chem. 2024, 72, 7749–7764. [Google Scholar] [CrossRef] [PubMed]
- Arrazola-Paternina, G.; Alvis-Bermúdez, A.; García-Mogollon, C. Efecto del tratamiento de escaldado sobre la actividad enzimática de la polifenoloxidasa en dos variedades de batata (Ipomoea batatas Lam.). Rev. Colomb. Cienc. Hortícolas 2016, 10, 80–88. [Google Scholar] [CrossRef]
- Jiménez-Villalba, K.; Arrieta-Banquet, L.; Salcedo-Mendoza, J.; Contreras-Lozano, K. Characterization of batatas flours and starches (Ipomoea batatas Lam.) from the colombian caribbean coast. Rev. U.D.C.A Actual. Divulg. Cient. 2019, 22, e1185. [Google Scholar] [CrossRef]
- Ayala, M.; Roman, R.; Vazquez-Duhalt, R. A catalytic approach to estimate the redox potential of heme-peroxidases. Biochem. Biophys. Res. Commun. 2007, 357, 804–808. [Google Scholar] [CrossRef]
- Škulj, S.; Kožić, M.; Barišić, A.; Vega, A.; Biarnés, X.; Piantanida, I.; Barisic, I.; Bertoša, B. Comparison of two peroxidases with high potential for biotechnology applications—HRP vs. APEX2. Comput. Struct. Biotechnol. J. 2024, 23, 742–751. [Google Scholar] [CrossRef]
- Minibayeva, F.; Beckett, R.P.; Kranner, I. Roles of Apoplastic Peroxidases in Plant Response to Wounding; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Watanabe, L.; Nascimento, A.S.; Zamorano, L.S.; Shnyrov, V.L.; Polikarpov, I. Purification, crystallization and preliminary X-ray diffraction analysis of royal palm tree (Roystonea regia) peroxidase. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Dequaire, M.; Limoges, B.; Moiroux, J.; Savéant, J.M. Mediated electrochemistry of horseradish peroxidase. Catalysis and inhibition. J. Am. Chem. Soc. 2002, 124, 240–253. [Google Scholar] [CrossRef]
- Guarin-Guio, P.A.; Cano-Calle, H.D.; Castillo-León, J.J. Detección electroquímica de peróxido de hidrógeno usando peroxidasa de pasto Guinea (Panicum maximum) inmovilizada sobre electrodos serigrafiados de puntos cuánticos. Rev. Ion 2019, 32, 67–76. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Sumana, G.; Malhotra, B.D. Horse radish peroxidase immobilized polyaniline for hydrogen peroxide sensor. Polym. Adv. Technol. 2011, 22, 903–908. [Google Scholar] [CrossRef]
- Stepanov, E.V.; Shcherbakov, I.A. Physicochemical Methods of Studying Hydrogen Peroxide for Biomedical Applications. Phys. Wave Phenom. 2023, 31, 92–97. [Google Scholar] [CrossRef]
- Barbosa, L.M.M.; Carneiro, T.d.S.; Favoreto, M.W.; Borges, C.P.F.; Reis, A.; Meireles, S.S.; Loguercio, A.D. Whitening toothpastes with hydrogen peroxide concentrations vs. at-home bleaching. Clin. Oral. Investig. 2024, 28, 436. [Google Scholar] [CrossRef]
- Malevich, D.; Mypati, S.; Ray, S.G.; Dinh, C.T.; Barz, D.P.J. Surfactant-tolerant cathodes for electrochemical generation of hydrogen peroxide for wastewater treatment. J. Appl. Electrochem. 2024, 55, 231–241. [Google Scholar] [CrossRef]
- Alpeeva, I.S.; Niculescu-Nistor, M.; Leon, J.C.; Csöregi, E.; Sakharov, I.Y. Palm tree peroxidase-based biosensor with unique characteristics for hydrogen peroxide monitoring. Biosens. Bioelectron. 2005, 21, 742–748. [Google Scholar] [CrossRef]
- Castillo, J.; Gáspár, S.; Sakharov, I.; Csöregi, E. Bienzyme biosensors for glucose, ethanol and putrescine built on oxidase and sweet potato peroxidase. Biosens. Bioelectron. 2003, 18, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Forzato, C.; Vida, V.; Berti, F. Biosensors and Sensing Systems for Rapid Analysis of Phenolic Compounds from Plants: A Comprehensive Review. Biosensors 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Orduz, A.E.; Rozo, C.E. Characterization of the non-covalent conjugate graphene-folic acid using Raman spectroscopy and computational methods. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2018, 42, 96–103. [Google Scholar] [CrossRef]
- Vallés, C.; Zhang, X.; Cao, J.; Lin, F.; Young, R.J.; Lombardo, A.; Ferrari, A.C.; Burk, L.; Mülhaupt, R.; Kinloch, I.A. Graphene/Polyelectrolyte Layer-by-Layer Coatings for Electromagnetic Interference Shielding. ACS Appl. Nano Mater. 2019, 2, 5272–5281. [Google Scholar] [CrossRef]
- Villamizar, E.N.; Ríos, C.A.; Castillo, J.J. A hydrogen peroxide biosensor based on the immobilization of the highly stable royal palm tree peroxidase (Roystonea regia) with chitosan and glutaraldehyde on screen-printed graphene electrodes. J. Mex. Chem. Soc. 2016, 60, 135–140. [Google Scholar] [CrossRef]
- Orduz, A.E.; Gutiérrez, J.A.; Blanco, S.I.; Castillo, J.J. Amperometric detection of triclosan with screen-printed carbon nanotube electrodes modified with Guinea Grass (Panicum maximum) peroxidase. Univ. Sci. 2019, 24, 363–379. [Google Scholar] [CrossRef]
- Guarín, P.; Cristancho, J. Rapid electrochemical detection of Staphylococcus aureus based on screen-printed gold electrodes modified with cysteine and Guinea grass (Panicum maximum) peroxidase. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 835–844. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Vorobiev, A.C.; Leon, J.J.C. Synthesis of polyelectrolyte complexes of polyaniline and sulfonated polystyrene by palm tree peroxidase. Enzym. Microb. Technol. 2003, 33, 661–667. [Google Scholar] [CrossRef]
- Mazhugo, Y.M.; Caramyshev, A.V.; Shleev, S.V.; Sakharov, I.Y.; Yaropolov, A.I. Enzymatic synthesis of a conducting complex of polyaniline and poly(2-acrylamido-2-methyl-1-propanesulfonic acid) using palm tree peroxidase and its properties. Appl. Biochem. Microbiol. 2005, 41, 247–250. [Google Scholar] [CrossRef]
- Caramyshev, A.V.; Evtushenko, E.G.; Ivanov, V.F.; Barceló, A.R.; Roig, M.G.; Shnyrov, V.L.; van Huystee, R.B.; Kurochkin, I.N.; Vorobiev, A.K.; Sakharov, I.Y. Synthesis of conducting polyelectrolyte complexes of polyaniline and poly(2-acrylamido-3-methyl-1-propanesulfonic acid) catalyzed by pH-stable palm tree peroxidase. Biomacromolecules 2005, 6, 1360–1366. [Google Scholar] [CrossRef]
- Blau, R.; Shelef, O.; Shabat, D.; Satchi-Fainaro, R. Chemiluminescent probes in cancer biology. Nat. Rev. Bioeng. 2023, 1, 648–664. [Google Scholar] [CrossRef]
- Zvereva, M.V.; Zhmurova, A.V. The Use of a Chemiluminescence in the Assessment of the Nanomaterials Antioxidant Activity; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Yan, X.L.; Xue, X.-X.; Deng, X.-M.; Jian, Y.-T.; Luo, J.; Jiang, M.-M.; Zheng, X.-J. Chemiluminescence strategy induced by HRP-sandwich structure based on strand displacement for sensitive detection of DNA methyltransferase. Microchem. J. 2020, 158, 105183. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Zhang, P.; Zhang, X.; Fu, A. An ultra-sensitive chemiluminescence immunosensor of carcinoembryonic antigen using HRP-functionalized mesoporous silica nanoparticles as labels. Sens. Actuators B Chem. 2011, 155, 557–561. [Google Scholar] [CrossRef]
- Sakharov, I. Long-term chemiluminescent signal is produced in the course of luminol peroxidation catalyzed by peroxidase isolated from leaves of african oil palm tree. Biochemistry 2001, 66, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Alpeeva, I.S.; Sakharov, I.Y. Luminol oxidation catalyzed by royal palm leaf peroxidase. Appl. Biochem. Microbiol. 2007, 43, 25–28. [Google Scholar] [CrossRef]
- Parveen, S.; Asgher, M.; Bilal, M. Lignin peroxidase-based cross-linked enzyme aggregates (LiP-CLEAs) as robust biocatalytic materials for mitigation of textile dyes-contaminated aqueous solution. Environ. Technol. Innov. 2021, 21, 101226. [Google Scholar] [CrossRef]
- Morales, A.; Barbosa, O.; Rueda, N.; Fonseca, Z.; Torres, R.; Rodrigues, R.C.; Ortiz, C.; Fernandez-Lafuente, R. Optimization and characterization of CLEAs of the very thermostable dimeric peroxidase from Roystonea regia. RSC Adv. 2015, 5, 53047–53053. [Google Scholar] [CrossRef]
- Terres, J.; Battisti, R.; Andreaus, J.; De Jesus, P.C. Decolorization and degradation of Indigo Carmine dye from aqueous solution catalyzed by horseradish peroxidase. Biocatal. Biotransform. 2014, 32, 64–73. [Google Scholar] [CrossRef]
- Perez, A.V.; Gaitan-Oyola, J.A.; Vargas-Delgadillo, D.P.; Castillo, J.J.; Barbosa, O.; Fernandez-Lafuente, R. Synthesis and Characterization of Cross-Linked Aggregates of Peroxidase from Megathyrsus maximus (Guinea Grass) and Their Application for Indigo Carmine Decolorization. Molecules 2024, 29, 2696. [Google Scholar] [CrossRef]
- Andhalkar, V.V.; Ahorsu, R.; De María, P.D.; Winterburn, J.; Medina, F.; Constantí, M. Valorization of Lignocellulose by Producing Polyhydroxyalkanoates under Circular Bioeconomy Premises: Facts and Challenges. Am. Chem. Soc. 2022, 10, 16459–16475. [Google Scholar] [CrossRef]
- Türker, Y.Ö. The impact of the right of access to information on sustainable development goals under the Aarhus Convention. J. Environ. Manag. 2024, 370, 122918. [Google Scholar] [CrossRef]

| Plant Part | Source of POD | POD Activity (U/g) |
|---|---|---|
| Fruits | Almond (Terminalia catappa) | <1.0 |
| Cocoa (Theobroma cacao) | 11.6 | |
| Coffee (Coffea arabica) | 22.9 | |
| Cocoa palm (Cocos nucifera) | 1.2 | |
| Tree tomato (Cyphomandra betacca) | 16.2 | |
| Totumo (Crescentia cujete) | 5.8 | |
| Roots | Celery (Apium graveolens) | 58.0 |
| Arracacha (Arracacia xanthorrhiza) | <1.0 | |
| Sweet potato (Ipomea batatas) | 1800.0 | |
| Coriander (Coriandrum sativum) | 35.0 | |
| Bore (Colocasa esculenta) | 370.0 | |
| Ginger (Zingeber officinale) | 11.6 | |
| Red radish (Rapharus sativas) | 121.3 | |
| Horseradish (Armoracia rusticana) | 2600.0 | |
| Cassava (Manihot esculenta) | 1.7 | |
| Leaves | Oleander (Nerium oleander) | 98.3 |
| Pear cactus (Monstera delisiosa) | 179.0 | |
| Banana (Musa sapientum) | 49.7 | |
| Bamboo (Bambusa guadua) | <1.0 | |
| Spanish moss (Tillandsia recurvata) | 5.2 | |
| Boojum tree (Cereus hexagonus) | 19.0 | |
| Marigold (Calendula oficionales) | 231.2 | |
| Bottlebrush (Callistemon lanceolatu) | <1.0 | |
| Sugar cane (Sacharum officinarum) | 104.0 | |
| Sleepy plant (Mimosa pudica) | 460.0 | |
| Fique (Agave fourcroides) | 19.6 | |
| Fern (Adiamtum obliguum) | <1.0 | |
| Castor bean plant (Ricinum communis L.) | 440.0 | |
| Lemongrass (Cymbopogon citratus) | 390.0 | |
| Fan palm (copernica pectori) | 220.0 | |
| African oil palm (eleais guineensis) | 566.0 | |
| Date palm (Phoenix dactilera) | 580.0 | |
| Royal palm (Roystonea regia) | 694.0 | |
| Coconut palm (Cocos nucifera) | 48.6 | |
| Corozo palm (Acrocomia aculeata) | 570.0 | |
| Wine palm (Scheelea butyracea) | 173.4 | |
| Thatch palm (Astrocarium sp.) | 220.0 | |
| Macaw palm (Bactris sp.) | 196.0 | |
| Palma mararai (Aiphanes cariotifolia) | 1145.0 | |
| Guinea Grass (Panicum maximum) | 980.0 | |
| Parsley (Petroselinum sativum) | 35.0 |
| Plant Source | Extraction Technique | Yield/Specific Activity | Advantages | Drawbacks | Applications |
|---|---|---|---|---|---|
| Royal palm (Roystonea regia) | Homogenization → Ammonium sulfate precipitation → Hydrophobic interaction + ion-exchange chromatography | 6170 U/mg [32] | High purity, excellent thermal stability | Time-consuming, high cost, not easily scalable | Electrochemical biosensors, high-temperature industrial catalysis |
| Guinea grass (Panicum maximum) | Biphasic polymer system (PEG/ammonium sulfate) → Size-exclusion chromatography | 2000–3000 U/mg [26] | Good stability preservation, moderate cost | Polymer disposal issues, requires optimization | Biosensors for H2O2, environmental monitoring |
| Sweet potato (Ipomea batatas) | Homogenization → Pigment removal → Hydrophobic interaction + ion-exchange chromatography | 1800 U/mg [20] | High substrate specificity, compatible with food industry | Moderate yield, pigment interference can complicate extraction | Food biosensors, wastewater treatment |
| African oil palm (Elaeis guineensis) | Homogenization → Ammonium sulfate precipitation → Chromatographic purification | 2500 U/mg [33] | Good thermal stability, wide pH tolerance | Limited studies on scalability | Environmental remediation, CLEA synthesis |
| Lemongrass (Cymbopogon citratus) | Aqueous extraction → Ammonium sulfate precipitation → Chromatography | 1200 U/mg [34] | Easy implementation, accessible raw material | Enzyme unstable at pH > 7 | Biosensors for H2O2 and phenolic compounds |
| Sleepy plant (Mimosa pudica) | Aqueous extraction → Ammonium sulfate precipitation → DEAE-Toyopearl chromatography | 460 U/mg [38] | Very low detection limit with gold electrodes | Narrow pH stability range | Biosensors for sensitive biomedical detection |
| PODs Source | pH Optimum | Temperature Optimum (°C) | Inactivation Constant (min−1) | Substrate Specificity | Reference |
|---|---|---|---|---|---|
| Royal palm | 7.0–9.0 | 90 | 1.5 × 10−2 | Ferulic acid ABTS | [32] |
| African oil palm | 4.0–9.0 | 72 | 2.0 × 10−3 | Ferulic acid ABTS | [19] |
| Guinea grass | 7.0–9.0 | 66 | 8.0 × 10−3 | Guaiacol ABTS o-dianisidine | [26] |
| Lemongrass | 4.0–6.0 | 66 | 1.0 × 10−2 | Guaiacol o-dianisidine | [34] |
| Sweet potato | 8.0 | 60 | 7.0 × 10−3 | Ferulic acid ABTS o-Phenylene diamine | [20] |
| Sleepy plant | 4.0 | 55 | 7.0 × 10−3 | - | [38] |
| Horseradish | 6.0–6.5 | 25–30 | 1.0 × 10−3 | o-dianisidine ABTS | [50] |
| Plant Source | Electrode Material | Detection Limit (μM) | Linear Range (mM) | Reference |
|---|---|---|---|---|
| Royal palm | Graphene/chitosan | 87 | 0.1–5 | [59] |
| Guinea grass | Graphene | 150 | 0.1–3.5 | [26] |
| Lemongrass | Graphene | 50 | 0.5–4 | [49] |
| Sweet potato | Graphene oxide | 460 | 0.25–5 | [20] |
| Sleepy plant | Gold nanoparticles | 0.4 | 0.5–5 | [37] |
| Horseradish | Carbon paste | 50 | 0.05–10 | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, J.J. From Waste to Wonder: Valorization of Colombian Plant By-Products for Peroxidase Production and Biotechnological Innovation. Processes 2025, 13, 3198. https://doi.org/10.3390/pr13103198
Castillo JJ. From Waste to Wonder: Valorization of Colombian Plant By-Products for Peroxidase Production and Biotechnological Innovation. Processes. 2025; 13(10):3198. https://doi.org/10.3390/pr13103198
Chicago/Turabian StyleCastillo, John J. 2025. "From Waste to Wonder: Valorization of Colombian Plant By-Products for Peroxidase Production and Biotechnological Innovation" Processes 13, no. 10: 3198. https://doi.org/10.3390/pr13103198
APA StyleCastillo, J. J. (2025). From Waste to Wonder: Valorization of Colombian Plant By-Products for Peroxidase Production and Biotechnological Innovation. Processes, 13(10), 3198. https://doi.org/10.3390/pr13103198







