Numerical Investigation of Wet Coke Particles Drying in a Silo Dryer Using CFD-DEM Simulation
Abstract
1. Introduction
2. Methodology and Simulation Conditions
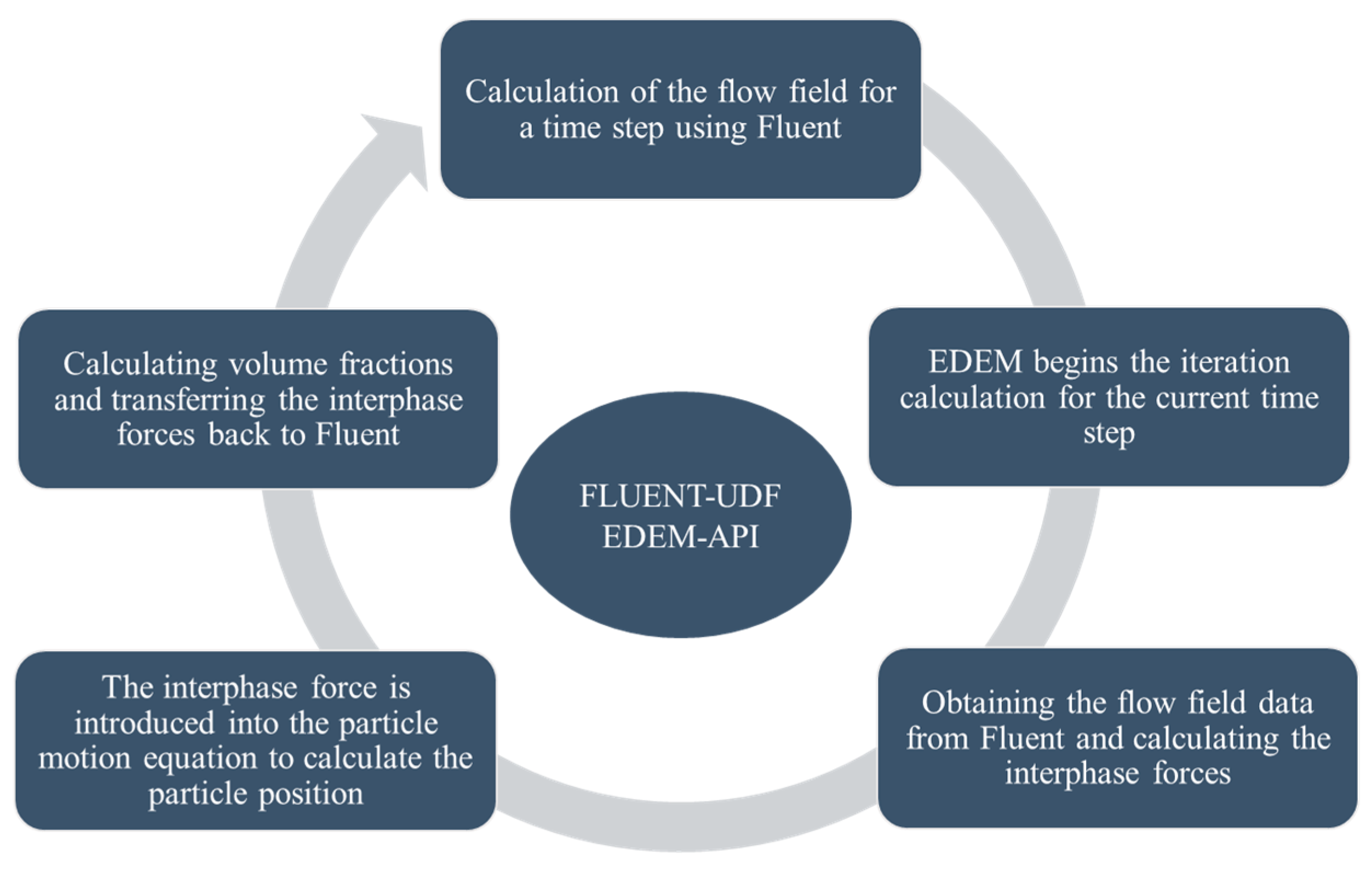
2.1. Mathematical Model
2.1.1. Governing Equations for the Gas Phase
2.1.2. Governing Equations for the Solid Phase
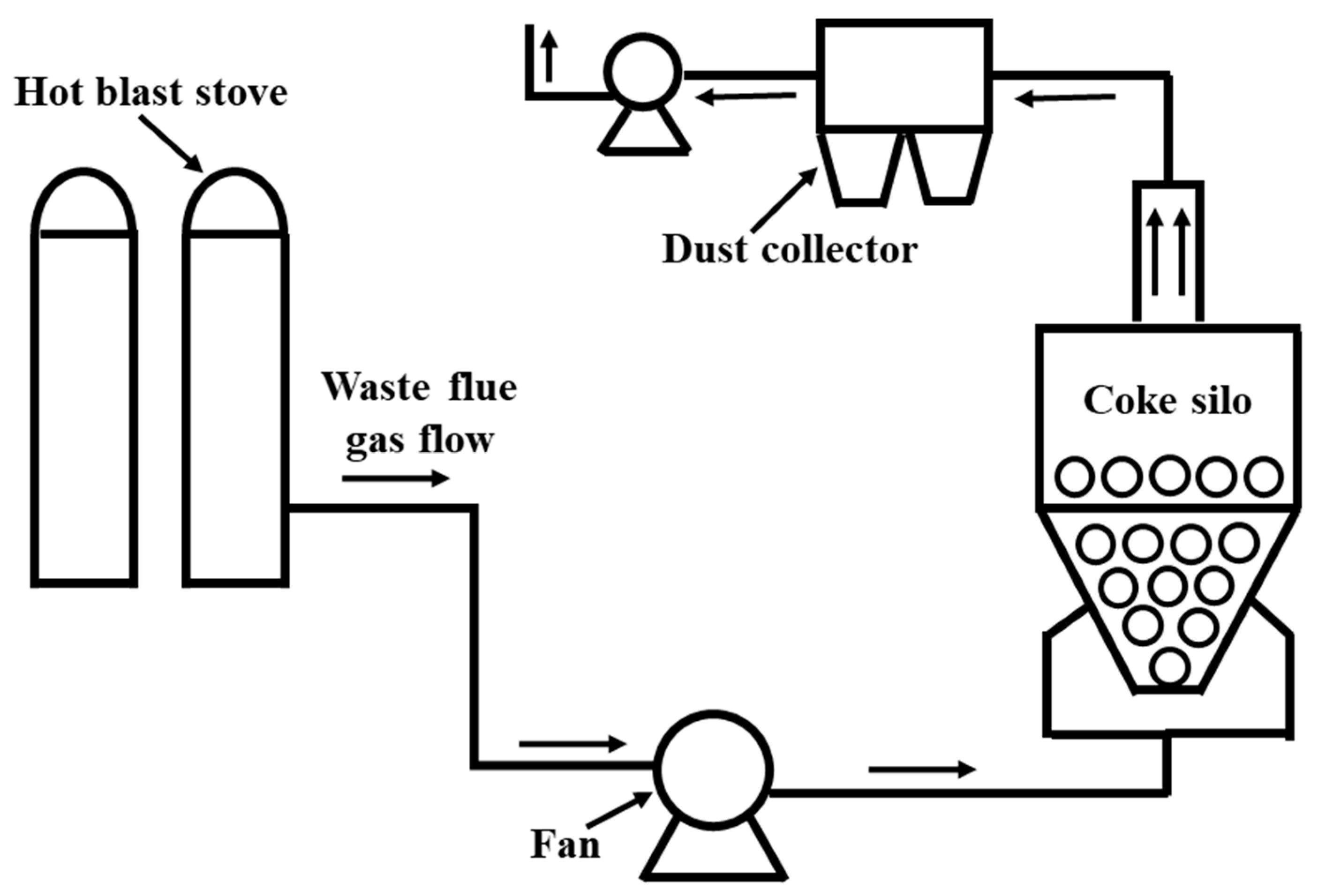
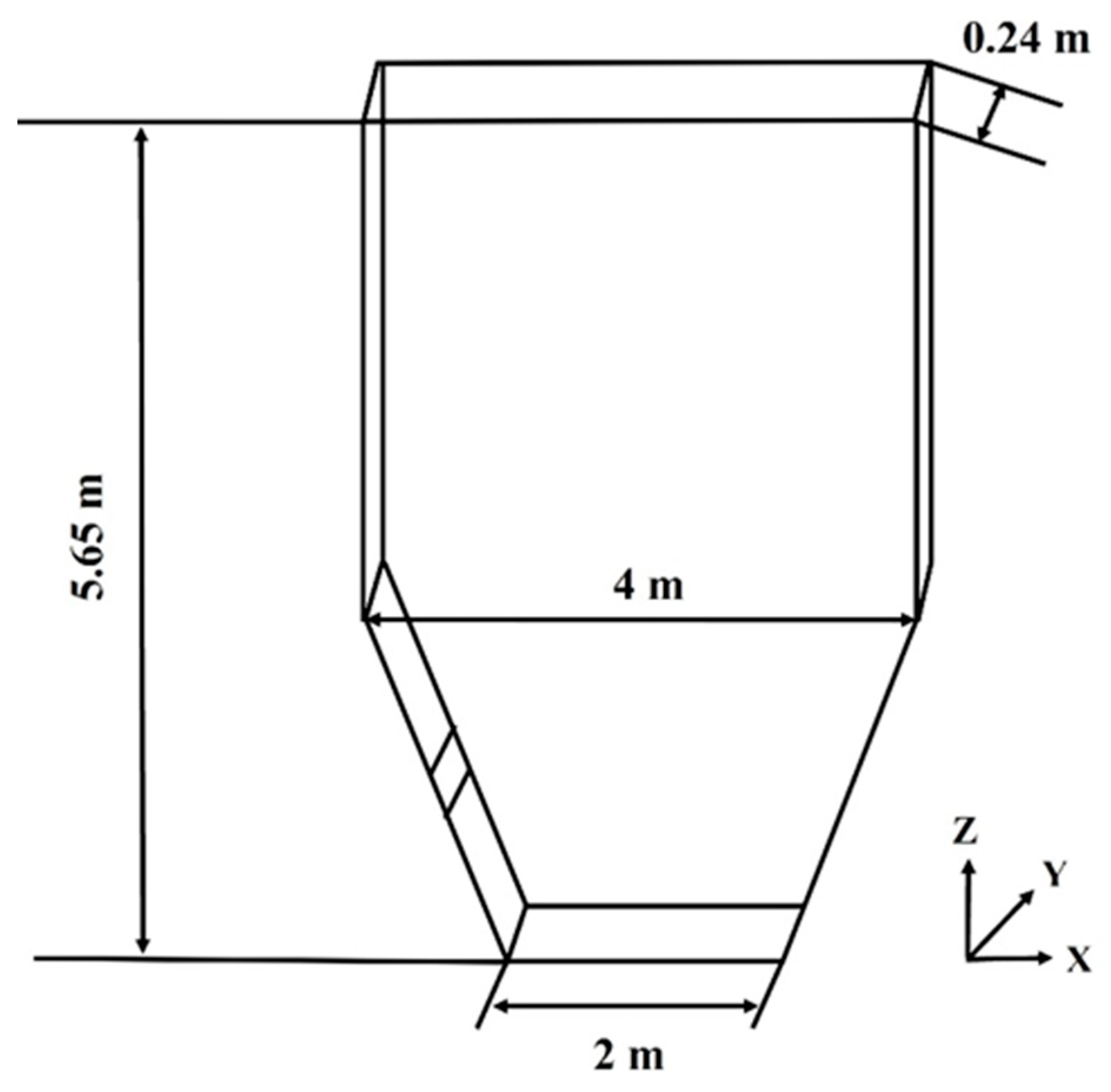
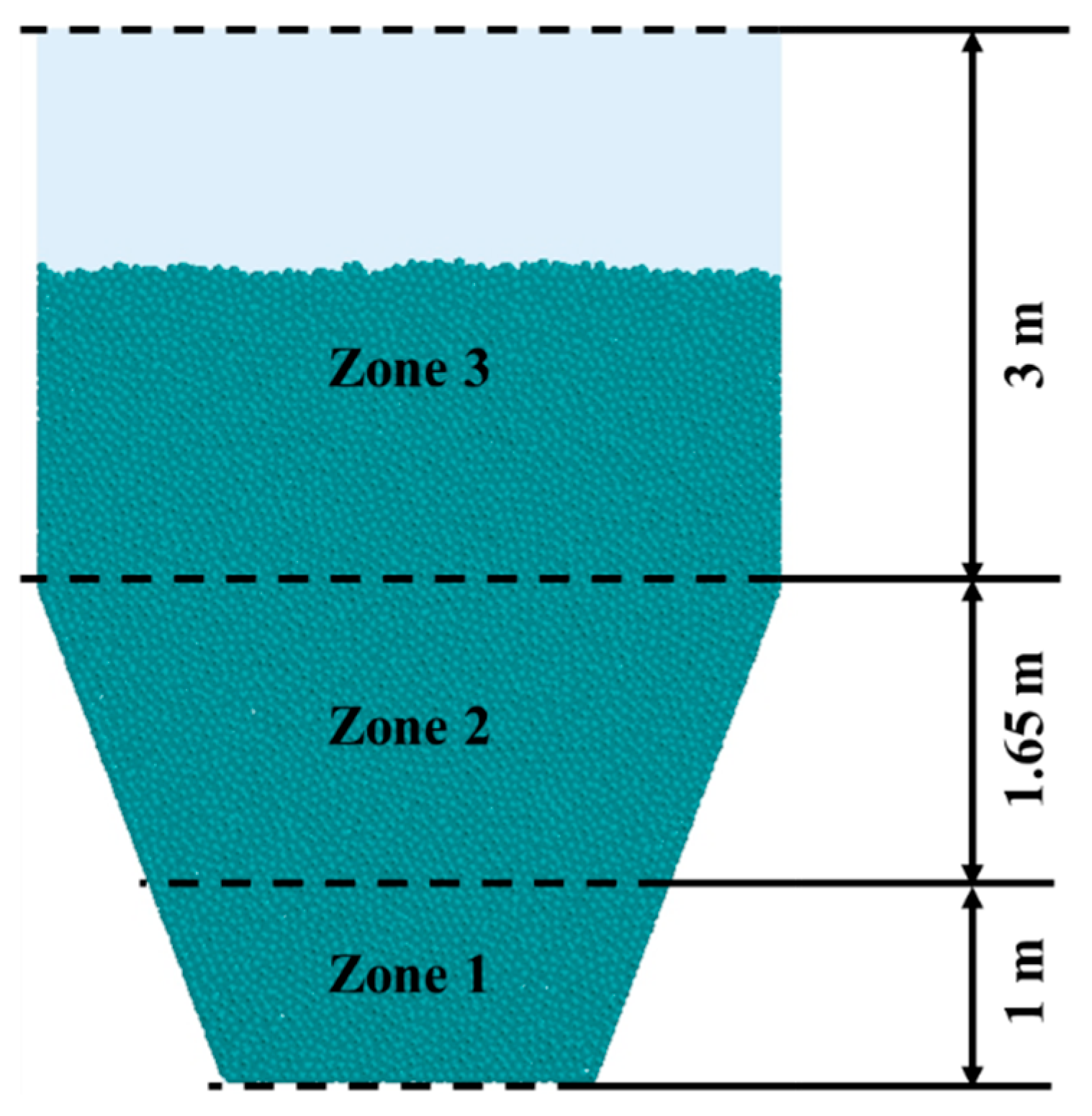
2.2. Simulation Setup
2.3. Boundary Conditions
3. Results and Discussion
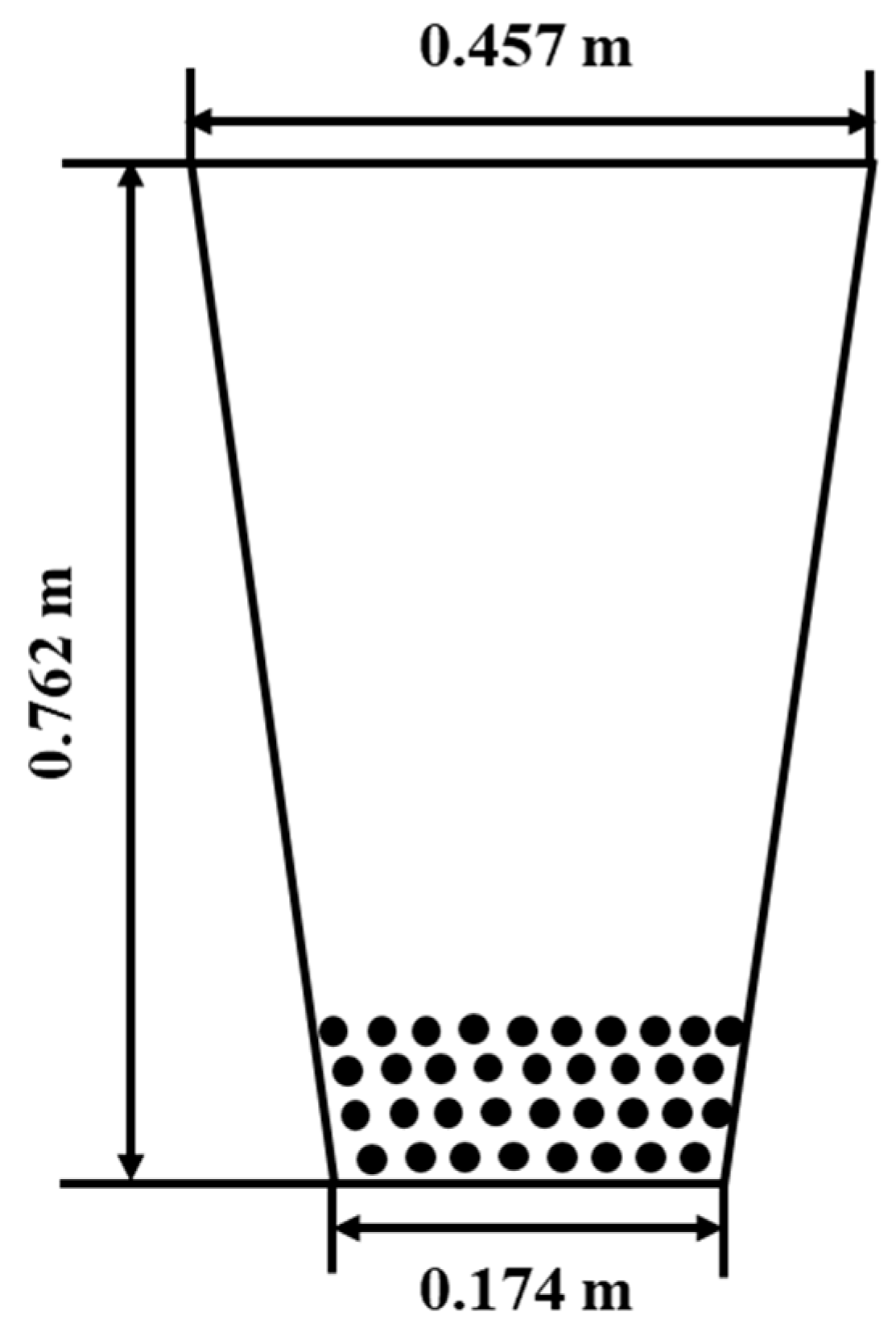
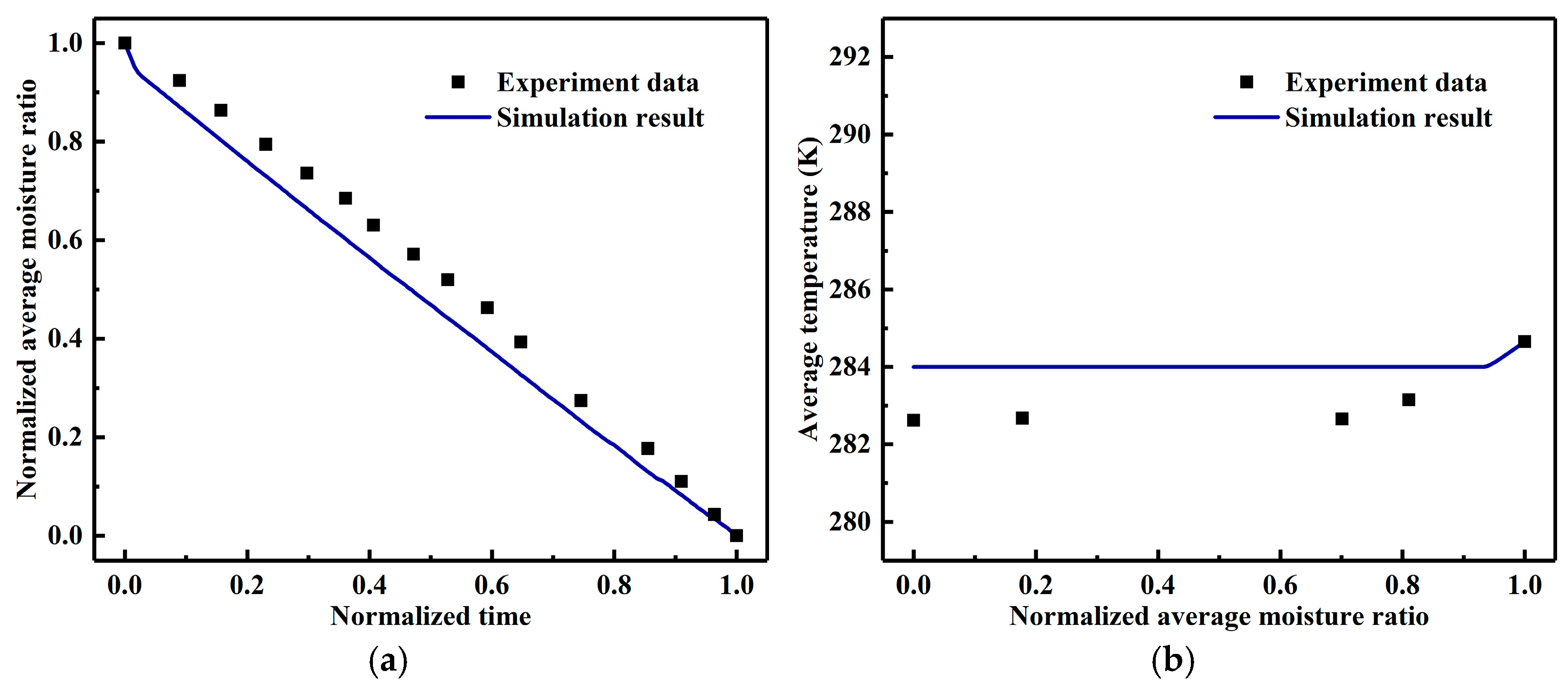
3.1. Model Validation
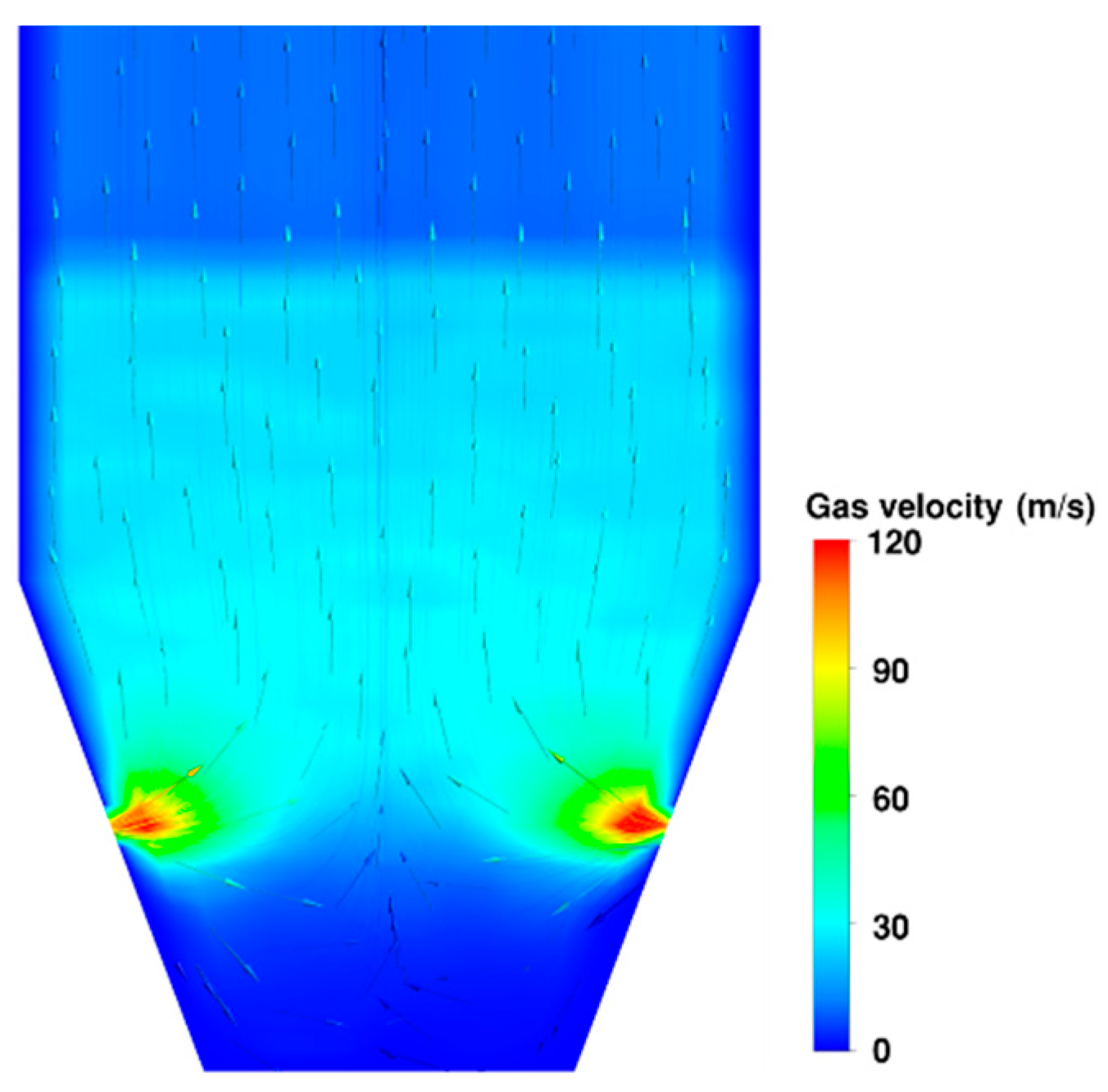
3.2. Flow Behaviour
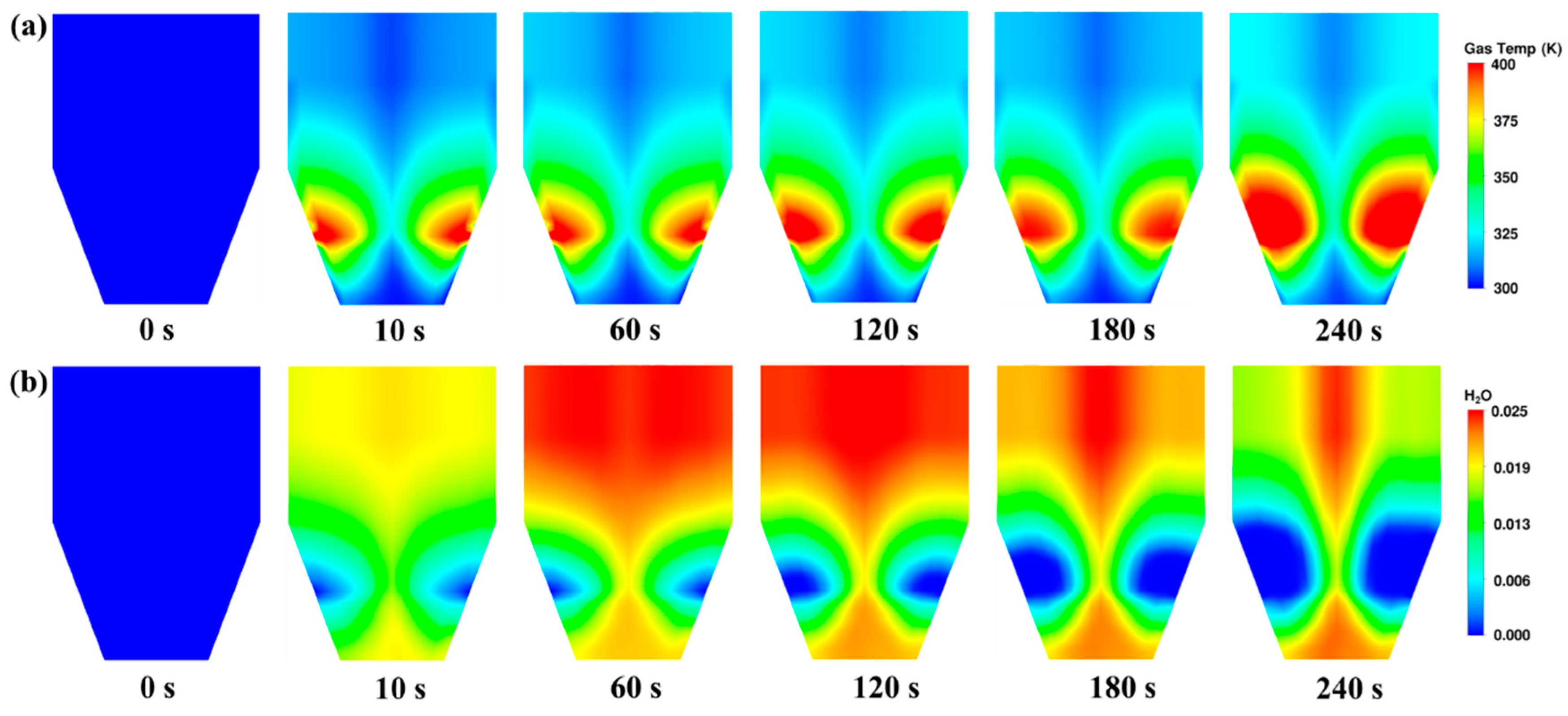
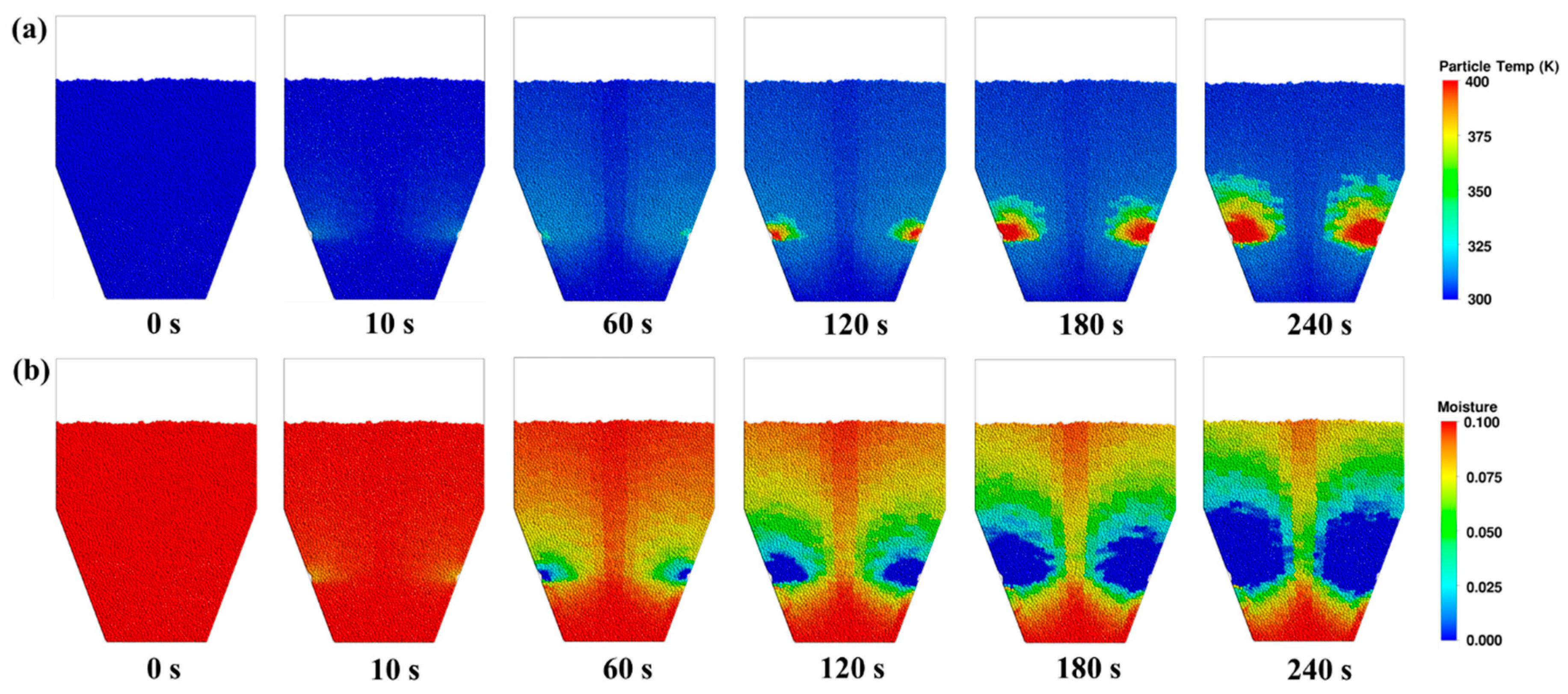
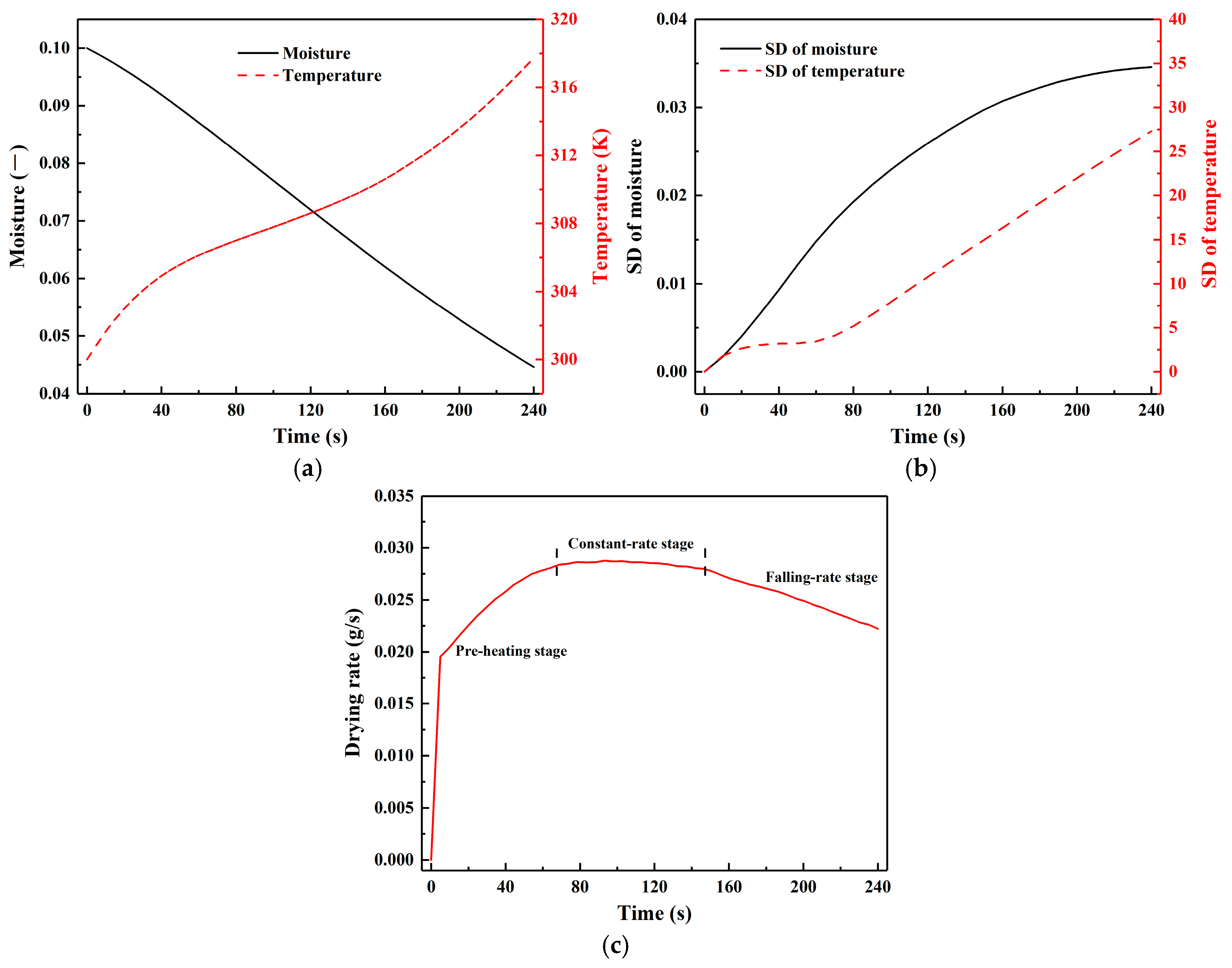
3.3. Heat and Mass Transfer Behaviours
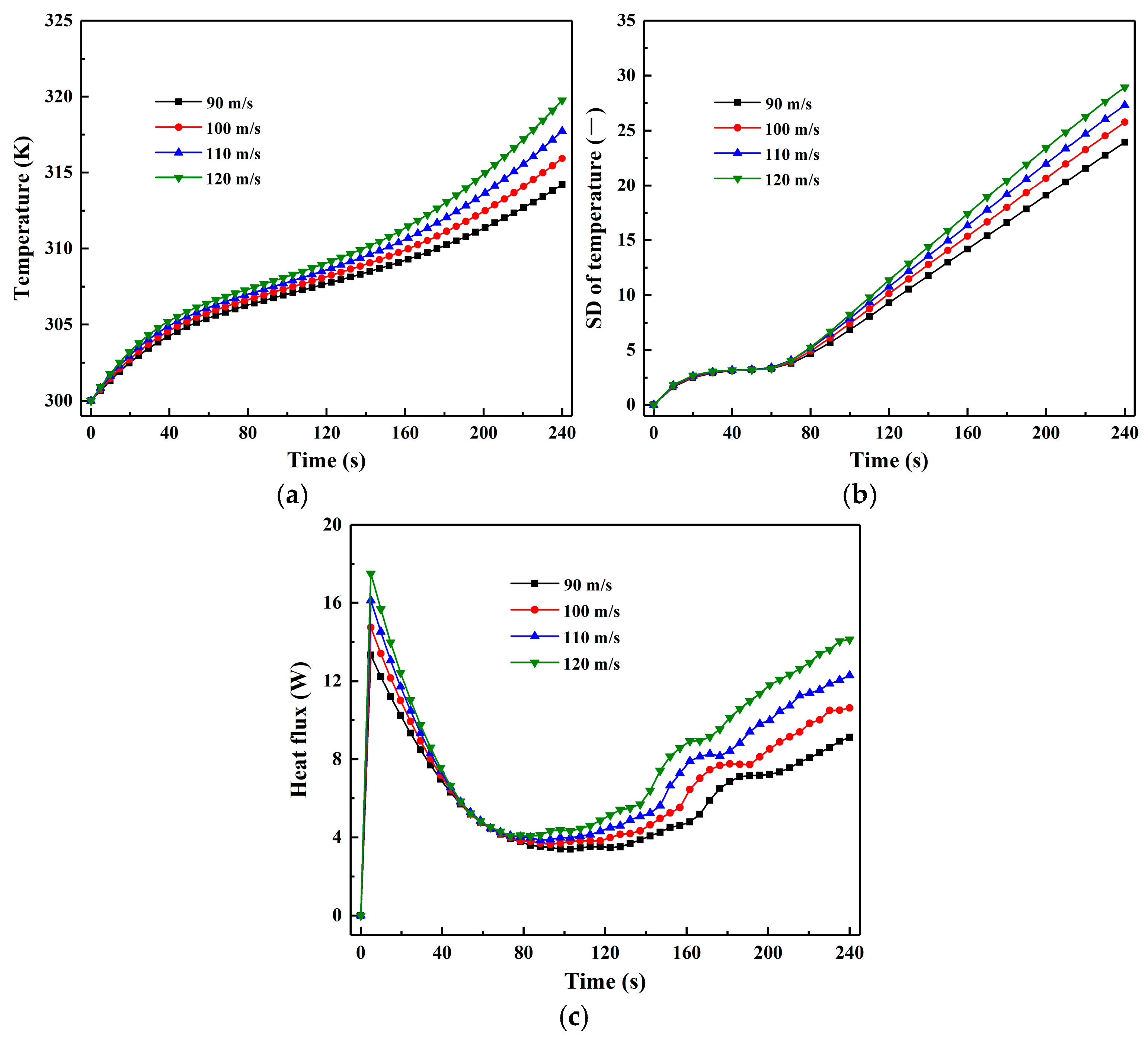
3.4. Effect of Inlet Gas Velocity
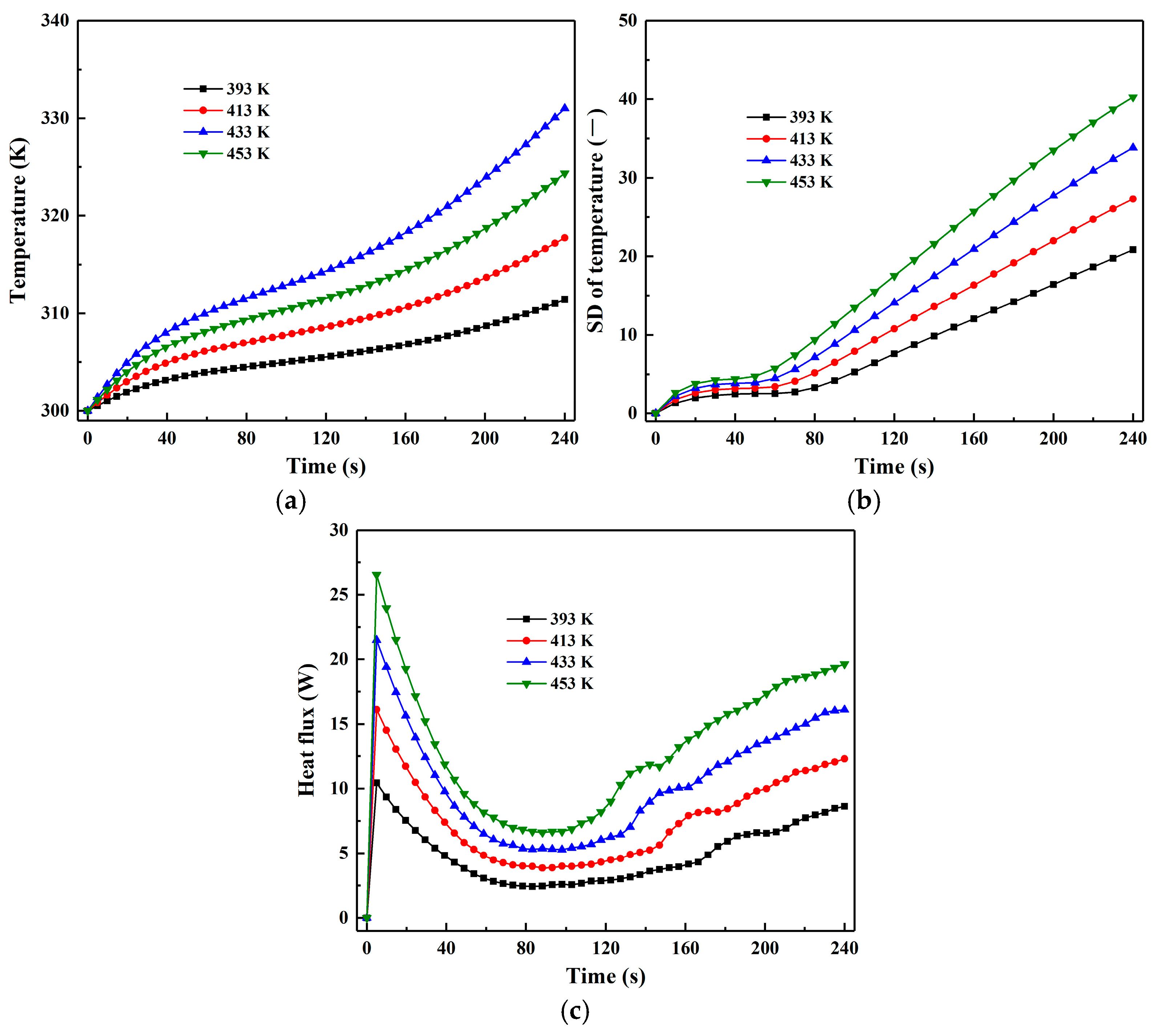
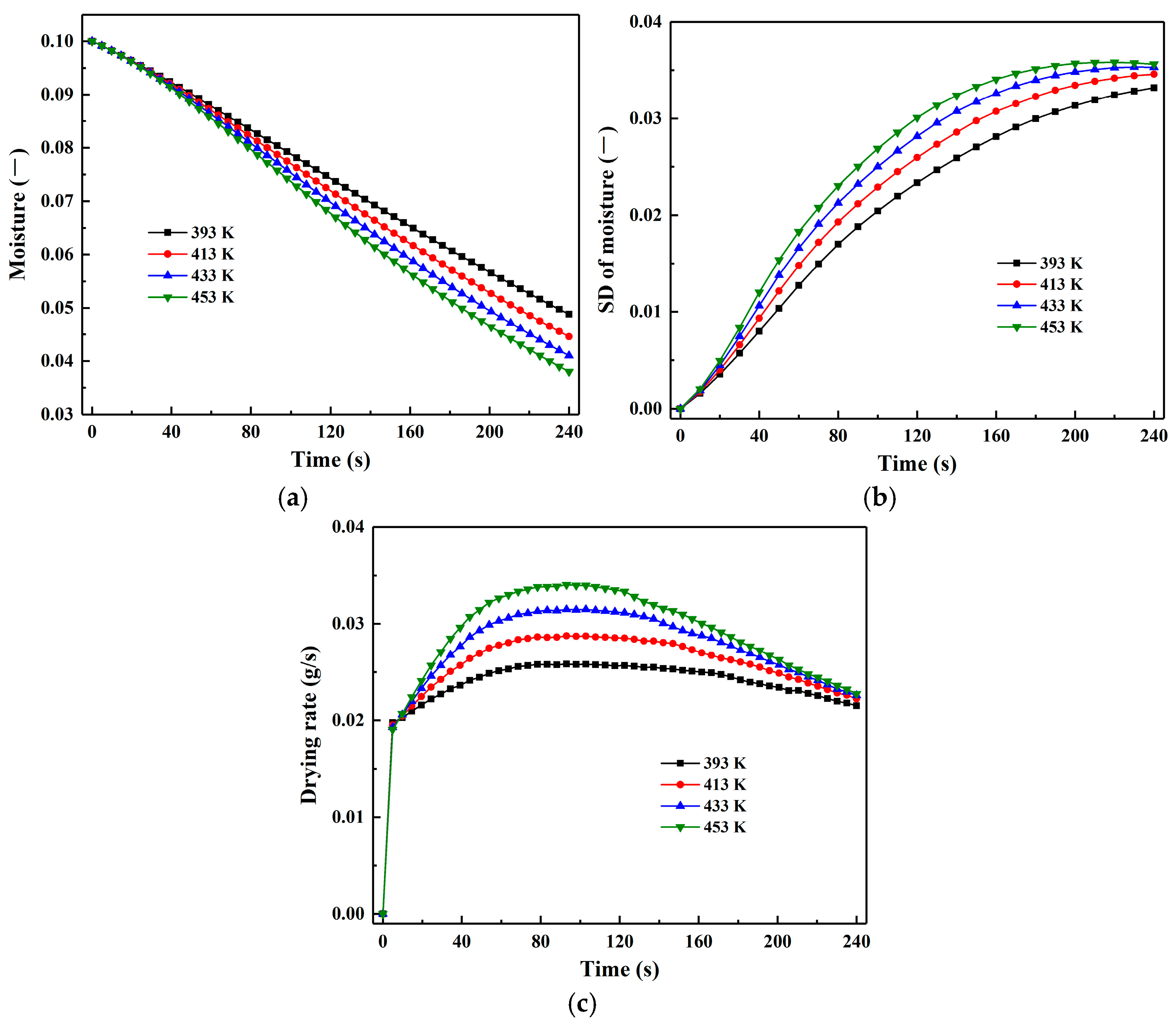
3.5. Effect of Inlet Gas Temperature
4. Conclusions
- (1)
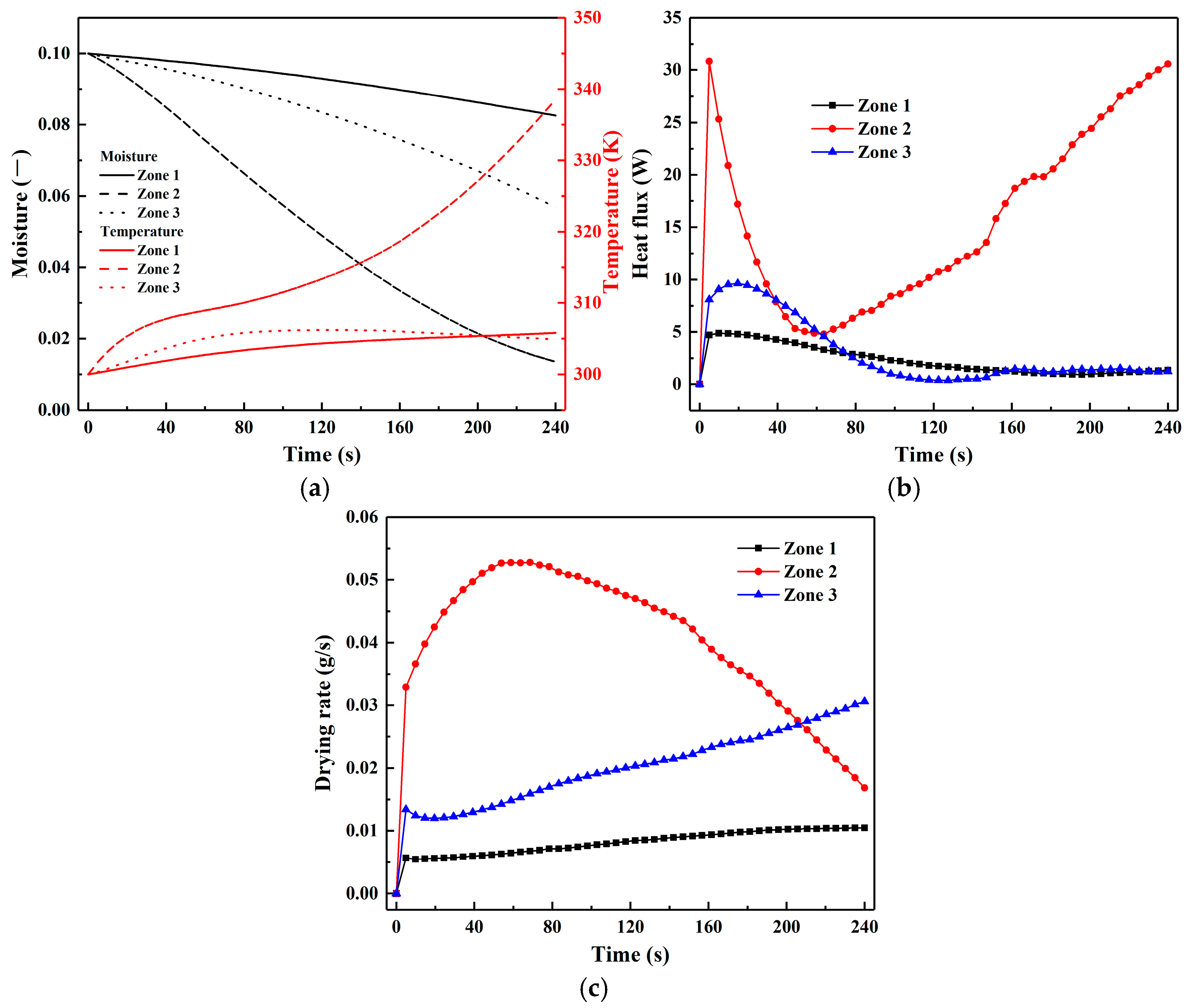
- The uneven distribution of gas flow within the CS dryer results in insufficient drying of particles in the centre and bottom regions, and forms a distinct dead zone at the bottom. As the drying process progresses, the spatial non-uniformity in particle moisture content and temperature distribution gradually intensifies. In comparison to zones 1 and 3, particles in zone 2 exhibit relatively high drying rates and heat fluxes, zones 1 and 3 show similar trends in temperature rise and heat flux, with zone 1 demonstrating the lowest drying rate and the smallest reduction in moisture content.
- (2)
- Increasing the inlet gas velocity enhances the particle drying rate and heat flux, thereby accelerating moisture reduction and particle temperature rise. However, higher inlet gas velocities decrease the uniformity of particle moisture distribution, slightly reducing drying product quality. Additionally, increased inlet gas velocity shortens the constant-rate period and prolongs the falling-rate period. The effect of inlet gas velocity on particle heat flux becomes significant mainly after the constant-rate period.
- (3)
- In comparison, inlet gas temperature has a more significant impact on the drying process. Increasing inlet gas temperature substantially enhances particle heat flux throughout the drying duration, leading to a more rapid rise in both particle temperature and its SD. The particle drying rate also increases with higher inlet gas temperature, accompanied by a shortening of the constant-rate period and an extension of the pre-heating and falling-rate periods.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cil, B.; Topuz, A. Fluidized bed drying of corn, bean and chickpea. J. Food Process Eng. 2010, 33, 1079–1096. [Google Scholar] [CrossRef]
- Lang, W.; Sokhansanj, S. Bulk volume shrinkage during drying of wheat and canola. J. Food Process Eng. 1993, 16, 305–314. [Google Scholar] [CrossRef]
- Jang, J.; Arastoopour, H. CFD simulation of a pharmaceutical bubbling bed drying process at three different scales. Powder Technol. 2014, 263, 14–25. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, C.H.; Park, Y.C.; Shun, D.; Bae, D.H.; Park, J. Drying efficiency of indonesian lignite in a batch-circulating fluidized bed dryer. Dry. Technol. 2014, 32, 268–278. [Google Scholar] [CrossRef]
- Aziz, H.; Sansare, S.; Duran, T.; Gao, Y.J.; Chaudhuri, B. On the applicability of the coarse grained coupled CFD-DEM model to predict the heat transfer during the fluidized bed drying of pharmaceutical granules. Pharm. Res. 2022, 39, 1991–2003. [Google Scholar] [CrossRef]
- Gavel, D.J. A review on nut coke utilisation in the ironmaking blast furnaces. Mater. Sci. Technol. 2016, 33, 381–387. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Tian, Y.S.; Du, P.; Zhou, J.L.; Wang, G.H. A study on the characteristics of coke in the hearth of a superlarge blast furnace. PLoS ONE 2021, 16, e0247051. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Yu, X.B.; Shen, Y.S. Transient CFD study of wet burden charging on dynamic in-furnace phenomena in an ironmaking blast furnace: Impacts and remedies. Powder Technol. 2022, 408, 117708. [Google Scholar] [CrossRef]
- Lan, C.C.; Hao, Y.J.; Shao, J.N.; Zhang, S.H.; Liu, R.; Lyu, Q. Effect of H2 on Blast Furnace Ironmaking: A Review. Metals 2022, 12, 1864. [Google Scholar] [CrossRef]
- Yilmaz, C.; Wendelstorf, J.; Turek, T. Modeling and simulation of hydrogen injection into a blast furnace to reduce carbon dioxide emissions. J. Clean. Prod. 2017, 154, 488–501. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, B.; Shen, X.; Xie, Z.H.; Sun, F.R. Thermodynamic optimization opportunities for the recovery and utilization of residual energy and heat in China’s iron and steel industry: A case study. Appl. Therm. Eng. 2015, 86, 151–160. [Google Scholar] [CrossRef]
- Cywicka-Jakiel, T. Fast-neutron and gamma-ray transmission method for coke-humidity determination. Appl Energy. 2003, 74, 305–311. [Google Scholar] [CrossRef]
- Ravichandar, D.; Naha, T.K.; Pillai, M.S.; Nagashanmugam, K.B. The influence of porosity on the moisture adsorption capacity of coke and installation of drying system to minimize coke moisture fluctuation. Trans. Indian Inst. Met. 2015, 69, 61–65. [Google Scholar] [CrossRef]
- Wang, S.; Luo, K.; Hu, C.S.; Lin, J.J.; Fan, J.R. CFD-DEM simulation of heat transfer in fluidized beds: Model verification, validation, and application. Chem. Eng. Sci. 2019, 197, 280–295. [Google Scholar] [CrossRef]
- Zhuang, Y.Q.; Chen, X.M.; Luo, Z.H.; Xiao, J. CFD-DEM modeling of gas-solid flow and catalytic MTO reaction in a fluidized bed reactor. Comput. Chem. Eng. 2014, 60, 1–16. [Google Scholar] [CrossRef]
- Uglietti, R.; Bracconi, M.; Maestri, M. Coupling CFD-DEM and microkinetic modeling of surface chemistry for the simulation of catalytic fluidized systems. React. Chem. Eng. 2018, 3, 527–539. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.Q.; Shao, Y.J.; Li, K.X. Coupling of CFD-DEM and reaction model for 3D fluidized beds. Powder Technol. 2019, 353, 72–83. [Google Scholar] [CrossRef]
- Cai, W.J.; Zhang, Q.H.; Wang, S.Y.; Lu, H.L. Numerical investigation of the two-fluid model and computational fluid dynamics-discrete element method in supercritical methanol fluidized beds. Ind. Eng. Chem. Res. 2022, 61, 14983–14999. [Google Scholar] [CrossRef]
- Wei, G.C.; Zhang, H.; An, X.Z.; E, D.Y. Numerical investigation on the mutual interaction between heat transfer and non-spherical particle dynamics in the blast furnace raceway. Int. J. Heat Mass Tran. 2020, 153, 119577. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.S. CFD-DEM modelling of raceway dynamics and coke combustion in an ironmaking blast furnace. Fuel 2021, 302, 121167. [Google Scholar] [CrossRef]
- Wei, G.C.; Zhang, H.; An, X.Z.; Jiang, S.Q. Influence of particle shape on microstructure and heat transfer characteristics in blast furnace raceway with CFD-DEM approach. Powder Technol. 2020, 361, 283–296. [Google Scholar] [CrossRef]
- Alobaid, F.; Epple, B. Improvement, validation and application of CFD/DEM model to dense gas-solid flow in a fluidized bed. Particuology 2013, 11, 514–526. [Google Scholar] [CrossRef]
- Deb, S.; Tafti, D. Investigation of flat bottomed spouted bed with multiple jets using DEM-CFD framework. Powder Technol. 2014, 254, 387–402. [Google Scholar] [CrossRef]
- Azmir, J.; Hou, Q.F.; Yu, A.B. Discrete particle simulation of food grain drying in a fluidised bed. Powder Technol. 2018, 323, 238–249. [Google Scholar] [CrossRef]
- Azmir, J.; Hou, Q.F.; Yu, A.B. CFD-DEM simulation of drying of food grains with particle shrinkage. Powder Technol. 2019, 343, 792–802. [Google Scholar] [CrossRef]
- Azmir, J.; Hou, Q.F.; Yu, A.B. CFD-DEM study of the effects of food grain properties on drying and shrinkage in a fluidised bed. Powder Technol. 2020, 360, 33–42. [Google Scholar] [CrossRef]
- Aziz, H.; Ahsan, S.N.; De Simone, G.; Gao, Y.J.; Chaudhuri, B. Computational modeling of drying of pharmaceutical wet granules in a fluidized bed dryer using coupled CFD-DEM approach. AAPS PharmSciTech 2022, 23, 59. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Ku, X.K.; Lin, J.Z.; Yu, Z.S. Influence of reactor configuration and operating conditions on heat transfer and drying behavior of wet particles in a spouted bed. Powder Technol. 2025, 461, 121117. [Google Scholar] [CrossRef]
- Liu, H.R.; He, Y.R.; Tang, T.Q.; Zhai, M. Prediction of the non-uniform drying of wet grain in a fluidised-bed based on a modified CFD-DEM drying model. Biosyst. Eng. 2023, 236, 103–119. [Google Scholar] [CrossRef]
- Jin, H.; Wu, Q.; Wang, S.; He, Y. Heat and mass transfer performance of non-spherical wet particles in a fluidized bed dryer. Appl. Therm. Eng. 2024, 236, 121780. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comp. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- Wei, G.C.; Zhang, H.; An, X.Z.; Xiong, B.; Jiang, S.Q. CFD-DEM study on heat transfer characteristics and microstructure of the blast furnace raceway with ellipsoidal particles. Powder Technol. 2019, 346, 350–362. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Wu, G.; An, X.; Zhang, H. DEM-CFD investigation on the blast furnace raceway characteristics: Heat/mass transfer, dynamics and mechanisms. Powder Technol. 2024, 435, 119411. [Google Scholar] [CrossRef]
- Mahmoodi, B.; Hosseini, S.H.; Ahmadi, G. CFD-DEM simulation of a pseudo-two-dimensional spouted bed comprising coarse particles. Particuology 2019, 43, 171–180. [Google Scholar] [CrossRef]
- Gidaspow, D.; Rukmini, B.; Ding, J. Hydrodynamics of Circulating Fluidized Beds: Kinetic Theory Approach; Illinois Inst. of Tech., Dept. of Chemical Engineering: Chicago, IL, USA, 1990. [Google Scholar]
- Wu, Z.; Shi, Y.; Zhou, F.; Li, B.; Zhang, X. Numerical Simulation of Pellet Drying Process in Updraught Drying of Chain Grate Based on CFD-DEM. Trans. Indian Inst. Met. 2021, 74, 1751–1761. [Google Scholar] [CrossRef]
- Cundall, P.A.; Strack, O.D.L. A discrete numerical model for granular assemblies. Geotechnique 1979, 29, 47–65. [Google Scholar] [CrossRef]
- E, D.Y.; Zhou, P.; Guo, S.Y.; Zeng, J.; Xu, Q.; Guo, L.J.; Hou, Q.F.; Yu, A.B. Particle-scale study of coke combustion in the raceway of an ironmaking blast furnace. Fuel 2021, 311, 122490. [Google Scholar] [CrossRef]
- E, D.Y.; Zhou, P.; Ji, L.Y.; Cui, J.X.; Xu, Q.; Guo, L.J.; Yu, A.B. Particle-scale modelling of injected hydrogen and coke co-combustion in the raceway of an ironmaking blast furnace. Fuel 2023, 336, 126778. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Muzzio, F.J.; Tomassone, M.S. Modeling of heat transfer in granular flow in rotating vessels. Chem. Eng. Sci. 2006, 61, 6348–6360. [Google Scholar] [CrossRef]
- Sousani, M.; Hobbs, A.M.; Anderson, A.; Wood, R.J.P.T. Accelerated heat transfer simulations using coupled DEM and CFD. Powder Technol. 2019, 357, 367–376. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Yu, A.B.; Zulli, P. Particle scale study of heat transfer in packed and bubbling fluidized beds. AIChE J. 2009, 55, 868–884. [Google Scholar] [CrossRef]
- Jin, H.Y.; Wang, S.; He, Y.R. Investigation of drying process of non-spherical particle in a pulsed fluidized bed. Particuology 2025, 98, 134–141. [Google Scholar] [CrossRef]
- Ranz, W.E. Evaporation form drops. Chem. Eng. Prog. 1952, 48, 141–146. [Google Scholar]
- Pan, K.S.; Jen, W.H.; Wang, T.F.; Lo, K.Y.; Hsu, W.Y.; Huang, A.N.; Kuo, H.P. CFD-DEM studies of continuous particle drying in a bubbling fluidized bed with large hole perforated plates. Can. J. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Choicharoen, K.; Devahastin, S.; Soponronnarit, S. Numerical simulation of multiphase transport phenomena during impinging stream drying of a particulate material. Dry. Technol. 2012, 30, 1227–1237. [Google Scholar] [CrossRef]
- Khomwachirakul, P.; Devahastin, S.; Swasdisevi, T.; Soponronnarit, S. Simulation of flow and drying characteristics of high-moisture particles in an impinging stream dryer via CFD-DEM. Dry. Technol. 2015, 34, 403–419. [Google Scholar] [CrossRef]
- Hou, Q.F.; E, D.Y.; Yu, A.B. Discrete particle modeling of lateral jets into a packed bed and micromechanical analysis of the stability of raceways. AIChE J 2016, 62, 4240–4250. [Google Scholar] [CrossRef]
- Lu, Y.X.; Liu, S.H.; Zhang, X.R.; Jiang, Z.Y.; E, D.Y. A probabilistic statistical method for the determination of void morphology with CFD-DEM approach. Energies 2020, 13, 4041. [Google Scholar] [CrossRef]
- Briens, L.; Bojarra, M. Monitoring fluidized bed drying of pharmaceutical granules. AAPS PharmSciTech 2010, 11, 1612–1618. [Google Scholar] [CrossRef]
- Nadda, M.; Banaeizadeh, A.; Avilala, P.; Roy, S.; Yadav, A. Coupled computational fluid dynamics-discrete element method (CFD-DEM) modeling of moisture removal in a fluidized bed dryer for heterogeneously coated solids. Chem. Eng. Sci. 2025, 316, 121811. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Bishop, C.; Glasser, B.J. Fluidized bed drying of a pharmaceutical powder: A parametric investigation of drying of dibasic calcium phosphate. Dry. Technol. 2017, 35, 1602–1618. [Google Scholar] [CrossRef]
- Chen, H.; Rustagi, S.; Diep, E.; Langrish, T.A.G.; Glasser, B.J. Scale-up of fluidized bed drying: Impact of process and design parameters. Powder Technol. 2018, 339, 8–16. [Google Scholar] [CrossRef]




| Parameters | Values |
|---|---|
| Number of particles | 18,000 |
| Diameter of particles (dp) | 0.06 m |
| Density of particles (ρp) | 1000 kg/m3 |
| Initial temperature of particles (Tp) | 300 K |
| Initial moisture content of particles | 10 wt% |
| Thermal conductivity of particles (kp) | 1.7 W/(m·K) |
| Specific heat capacity of particles (cp) | 850 J/(kg·K) |
| Restitution coefficient | 0.8 |
| Particle–particle/wall sliding friction (μs) | 0.4 |
| Particle–particle/wall rolling friction (μr) | 0.05 |
| Particle Young’s modulus (E) | 1.0 × 107 Pa |
| Particle Poisson ratio (ν) | 0.25 |
| Inlet gas velocity (Ug) | 90–120 m/s |
| Inlet gas temperature (Tg) | 393–453 K |
| Gas density (ρg) | 1.2 kg/m3 |
| Gas dynamic viscosity (μg) | 1.8 × 10−5 kg/(m·s) |
| Gas thermal conductivity (kg) | 0.0262 W/(m·K) |
| Gas specific heat capacity (cg) | 1006 J/(kg·K) |
| Fluid time step (∆tf) | 8.0 × 10−3 s |
| Solid time step (∆ts) | 2.0 × 10−4 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Wu, Y.; Cui, J.; E, D. Numerical Investigation of Wet Coke Particles Drying in a Silo Dryer Using CFD-DEM Simulation. Processes 2025, 13, 3164. https://doi.org/10.3390/pr13103164
Zhou P, Wu Y, Cui J, E D. Numerical Investigation of Wet Coke Particles Drying in a Silo Dryer Using CFD-DEM Simulation. Processes. 2025; 13(10):3164. https://doi.org/10.3390/pr13103164
Chicago/Turabian StyleZhou, Peng, Yiliu Wu, Jiaxin Cui, and Dianyu E. 2025. "Numerical Investigation of Wet Coke Particles Drying in a Silo Dryer Using CFD-DEM Simulation" Processes 13, no. 10: 3164. https://doi.org/10.3390/pr13103164
APA StyleZhou, P., Wu, Y., Cui, J., & E, D. (2025). Numerical Investigation of Wet Coke Particles Drying in a Silo Dryer Using CFD-DEM Simulation. Processes, 13(10), 3164. https://doi.org/10.3390/pr13103164






