Abstract
Polymeric membranes are a highly viable technology for wastewater treatment, water purification, and other filtration operations. Accordingly, flat membranes were developed from extruded nanocomposites of polyamide 6 (PA6) and carbon nanotubes (MWCNT), varying the filler content to 1, 3, and 5 parts per hundred resin (phr). The membranes were produced using the phase inversion process through the immersion–precipitation technique. In total, eight membrane compositions were developed with solvent/polymer ratios of 80/20 (weight %). Calcium chloride (CaCl2) was used as a pore-forming agent at a content of 10 phr. Thus, the characterizations performed were: solution viscosity, FTIR, contact angle measurement, SEM, AFM, water permeability test, and water vapor permeation test. The results showed that the high viscosity of membranes, excessive gelation time, and higher MWCNT contents contributed to a decrease and/or absence of flow. Through SEM images and water flow measurements, the significant influence of CaCl2 was observed in modifying the membrane morphology (more interconnected porous structures), ensuring the presence of flow. The AFM images also confirm this phenomenon through the increase in roughness. Water vapor transmission increased with higher MWCNT content. These results demonstrate that PA6 and MWCNT membranes were effective for water filtration, only in those where CaCl2 was used, and for water vapor initially.
1. Introduction
In light of global challenges, the United Nations Sustainable Development Goals (SDGs) provide a framework for advancing environmentally responsible practices. Within this context, membrane separation processes (MSP) play a central role, as they align with SDGs related to the sustainable management of water and sanitation, while also contributing to natural resource conservation and sustainable development [1,2]. Membranes, which are semi-permeable barriers of polymeric or inorganic origin, regulate the transport of solutes and are widely used in industrial sectors such as water treatment due to their energy efficiency, selectivity, and operational simplicity. Although they present high initial costs, continuous technological advances aim to improve their durability and performance, thereby enhancing cost-effectiveness [3].
Most polymeric membranes for microfiltration and ultrafiltration are produced through the phase inversion method, especially the immersion–precipitation technique. In this process, a polymer solution is cast into a thin film or extruded into hollow fibers and immersed in a non-solvent bath, where phase separation generates a microporous structure [4,5]. Among the polymers commonly used for this purpose are polyamide, polysulfone, polyetherimide, polyacrylonitrile, polycarbonate, polyethylene, and others. Polyamide, in particular, stands out for its excellent mechanical, thermal, and barrier properties, flame retardancy, dimensional stability, and intrinsic hydrophilicity, making it highly suitable for microfiltration and reverse osmosis membranes [6,7,8,9,10,11,12].
Despite these advantages, membranes based on polyamide 6 (PA6) face limitations, as their longer precipitation time during phase inversion increases polymer density, reduces pore size, and consequently lowers flux rates. As an alternative, porogenic agents have been introduced to accelerate precipitation, modifying membrane morphology and improving permeability [13]. Additionally, in the field of membranes, studies [14,15,16] have explored nanocomposites incorporating various nanofillers, such as carbon nanotubes and metal oxide nanoparticles. These nanofillers impart properties like conductivity, mechanical strength, and hydrophilicity to the membranes, while also achieving altered morphology that influences flow behavior [17,18].
Nanocomposites are now recognized as a new class of advanced materials in which at least one phase has nanoscale dimensions, leading to significant improvements in functional and mechanical properties [19,20,21]. Studies [22,23,24,25,26,27] have explored the use of carbon nanotubes in polymeric membranes for applications such as microfiltration, ultrafiltration, nanofiltration, and desalination. Their unique structure allows for rapid water transport while maintaining ion rejection, in addition to imparting antifouling behavior—stemming from their diverse surface chemistry and inherent antibacterial effects, electrical conductivity, and mechanical reinforcement [12,22,23,27,28,29,30,31,32,33,34].
Although PA6 membranes with carbon nanotubes (MWCNT) have been studied [33], and the effect of pore-forming agents such as CaCl2 on polymer solutions is also known, few studies have evaluated the combination of these two additives. The simultaneous presence of MWCNT and CaCl2 may produce synergistic effects, altering membrane porosity, surface morphology, and permeability. Therefore, this study aims to investigate how the combined addition of MWCNT and CaCl2 influences the structural and functional properties of PA6 membranes, addressing a gap in the understanding of the combined behavior of these components.
2. Methodology
2.1. Materials
PA6 (polyamide 6) granules, commercially identified as B300®, with a density of 1.13 g/cm3 and a viscosity range of 140–160 g/mL, were supplied by Polyform (São José dos Campos, São Paulo, Brazil). As the filler material, MWCNT (multi-walled carbon nanotubes) were used, synthesized via chemical vapor deposition (CVD). These were provided as a black powder with an internal diameter ranging from 3 to 5 nm, an external diameter of 8–15 nm, and a length of 3–12 µm. The MWCNTs have a specific surface area greater than 233 m2/g, a density of 0.15 g/cm3, and an electrical conductivity of 100 S/cm, and were supplied by Advanced 2D Materials (Singapore). Formic acid, with 85% purity and a density of 1.22 g/mL, was used as the solvent for membrane preparation and was supplied by Contemporary Chemical Dynamics Ltd.a (Indaiatuba, São Paulo, Brazil). Dihydrated calcium chloride (CaCl2·2H2O), provided by Vetec Chemistry Ltd.a. (Duque de Caxias, Rio de Janeiro, Brazil), served as the porogenic agent.
2.2. Preparation of Nanocomposites
Prior to extrusion processing, the PA6 matrix was dried at 80 °C for 24 h to remove moisture and prevent degradation caused by hydrolysis due to temperature and shear stress [35]. PA6 and the nanocomposites were extruded using a modular, co-rotating twin-screw extruder, model ZSK (D = 18 mm, L/D = 40), from Coperion Werner & Pfleiderer (Stuttgart, Germany). The temperature profile applied was 210 °C, 220 °C, 220 °C, 230 °C, 230 °C, 240 °C, 240 °C, with a screw rotation speed of 250 rpm and a controlled feeding rate of 2 kg/h. The processing parameters were based on the literature [36]. The screw profile was specifically designed for nanocomposite processing, featuring distributive and dispersive mixing elements to ensure better homogenization of the MWCNT. The MWCNT contents added to the polymer matrix were 1, 3, and 5 phr (parts per hundred resin).
2.3. Obtaining Membranes
As reported in the literature [13], PA6 and the nanocomposites were dissolved in formic acid at a ratio of 80/20 (solvent/polymer by weight). CaCl2 was used at a concentration of 10 pcr, due to its superior performance in terms of flow in a previous study [37]. After complete dissolution, the solutions were left to rest for 24 h. Prior to solution preparation, PA6 and the nanocomposites were pre-dried in an oven at 80 °C under vacuum for 24 h to ensure complete moisture removal. The membranes were labeled as shown in Table 1. Before membrane formation, the viscosity of each solution was measured using a Q860M21 rotary viscometer from Quimis (Diadema, São Paulo, Brazil) at room temperature.

Table 1.
Nomenclature of the flat membranes produced.
The membrane preparation process is illustrated in Figure 1. The obtained polymer solution was poured and manually spread onto a glass plate using a glass rod. Immediately afterward, the plate containing the polymer film was exposed to air for 5 min, as recommended by the study of Medeiros, K. M. et al. (2017) [13]. The plate was then immersed in a distilled water bath at room temperature, ensuring the plate was fully submerged. After approximately 3 min of precipitation, the membranes were removed, rinsed with distilled water, and dried at room temperature.
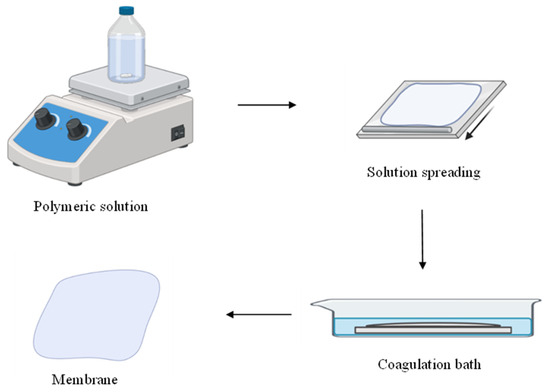
Figure 1.
Process of obtaining a membrane using the immersion–precipitation technique.
It is important to note that ambient humidity was not controlled; therefore, some of the observed effects may be related to the presence of moisture.
2.4. Membrane Characterization
The developed materials were chemically analyzed using Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR), employing a Bruker Alpha II spectrometer (Bruker, Atibaia, São Paulo, Brazil). The analysis covered a scanning range from 4000 to 400 cm−1, with a resolution of 4 cm−1 and 32 scans per sample.
The contact angle analysis was conducted using the sessile drop method in static mode, with five images captured at each point, spaced 1 s apart. This was performed using a portable contact angle meter, model Phoenix-i, from Surface Electro Optics (Gyeonggi-do, Republic of Korea).
The morphology of the membranes was analyzed using a scanning electron microscope (SEM) VEGAN MIRA LMU (TESCAN, Brno, Czech Republic), operated at 15 keV under vacuum. The top surface of the PA6/CaCl2 sample and the cross-section of the samples exhibiting flow were evaluated. In the latter case, the samples were fractured in liquid nitrogen to preserve their morphology and prevent plastic deformation. Additionally, the samples were gold-coated for the analysis. From the cross-sectional images, pore size distribution graphs were generated based on measurements of at least 40 randomly selected pores from each membrane.
Atomic Force Microscopy (AFM) analysis was conducted using an SPM 9700-Shimadzu system (Shimadzu Corporation, Kyoto, Japan), operating in dynamic mode with a scanning frequency of 1 Hz.
The membranes, each measuring approximately 13.0 cm2, were subjected to permeability tests using distilled water in a filtration system, as shown in Figure 2. The tests were conducted under pressures of 0.5 bar, 1 bar, 1.5 bar, and 2 bar, with permeate samples collected every minute for a duration of 60 min. The mass flux of the permeate (J), used to assess membrane performance, was determined using Equation (1):
m = mass of permeate (kg); A = membrane area (m2); t = time (h)
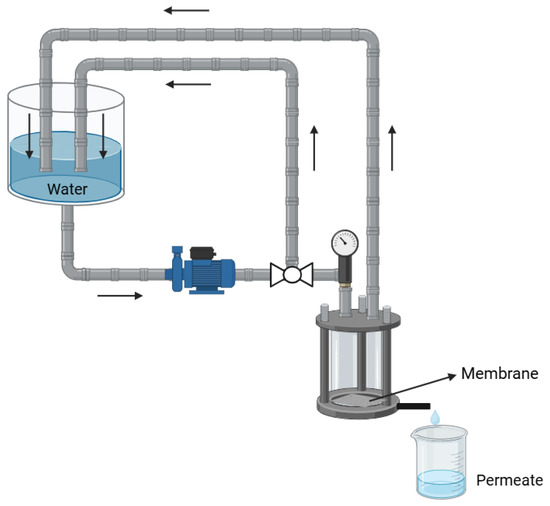
Figure 2.
Filtration system for water flow.
The water vapor permeation test was conducted in accordance with ASTM E96 standards. The membranes were cut and adhered to a glass container, which was filled with distilled water to half of its total volume. Initially, the system was weighed and placed in a sealed environment with a desiccant agent (silica gel). The test was performed in triplicate for each composition. The containers were weighed at 24 h intervals over a period of 7 days. Thus, the water vapor transmission (WVT) was calculated using Equation (2).
G = weight change (g); t = time at which G occurred (h); A = test area (“mouth” of the cup) (m2); G/t = slope of the straight line (weight vs. time graph) (g/h).
It is important to note that, in preliminary experiments, it was observed that all membranes prepared without CaCl2 did not exhibit water flux, suggesting that they are likely dense membranes. As a result, SEM characterization and contact angle measurements were performed only on the samples that allowed water permeation. To assess the effect of the absence of CaCl2 while maintaining the presence of MWCNT, the PA6/1 MWCNT membrane was selected for evaluation of its morphology and hydrophilicity.
3. Results and Discussion
3.1. Viscosity
Table 2 presents the viscosity values measured for each prepared solution. In formulations containing CaCl2, an increase in viscosity was observed when compared with neat PA6. For instance, the PA6/5 MWCNT/CaCl2 solution exhibited a viscosity approximately 12 times higher than that of pure PA6. In contrast, in the absence of CaCl2, the PA6/5 MWCNT solution reached a viscosity nearly 24 times greater than pure PA6.

Table 2.
Viscosity of polymer solutions.
It is important to note that viscosity plays an essential role in the phase inversion process, during which the polymer solution, upon immersion in the coagulation bath, separates into a polymer-rich phase and a polymer-lean phase. This process leads to the development of various membrane morphologies: dense, porous, or mixed. An increase in viscosity slows down the solvent–non-solvent exchange kinetics due to reduced molecular mobility within the system. This slower exchange allows for better control over phase separation, often resulting in membranes with a denser and more uniform structure, but generally at the expense of membrane permeability [38,39].
When comparing membranes with and without CaCl2, a reduction in viscosity is observed in MWCNT-containing solutions upon the addition of the salt, aligning with findings reported in the literature [6,40]. For PA6 solutions, the presence of Ca2+ and Cl− ions increases viscosity due to electrostatic or coordination interactions with the amide groups of the polymer chains, restricting their mobility and making the solution more resistant to flow [41,42]. On the other hand, when MWCNTs are incorporated, viscosity normally increases substantially because of nanotube aggregation caused by strong Van der Waals interactions. In this case, the addition of CaCl2 decreases viscosity, since the ions act as dispersing agents, weakening the attractive forces between nanotubes, limiting agglomerate formation, and consequently reducing flow resistance [43,44].
This reduction in viscosity facilitates faster diffusion between the solvent and non-solvent in the coagulation bath, promoting the formation of more interconnected pores. As a result, membranes prepared from PA6/MWCNT/CaCl2 solutions tend to exhibit higher permeability compared with those produced without CaCl2 [45], as will be discussed in the following sections.
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
Figure 3 shows the FTIR spectra of the membranes fabricated using nanocomposites, both with and without the addition of CaCl2. All spectra exhibit a similar pattern, primarily corresponding to the characteristic bonds of polyamide 6. The characteristic absorption bands of polyamide were identified at the following wavenumbers: 3294 cm−1 (N–H stretching), 2934 cm−1 (axial deformation of CH2–NH), 1635 cm−1 (axial deformation of the amide carbonyl group), 1538 cm−1 (C–N stretching and N–H bending), 1201 cm−1 (α-form of polyamide), 1170 cm−1 (skeletal motion of the CO–NH bond), 1074 cm−1 (C–C stretching), 1029 cm−1 (vibrations associated with C–C, C–OH, and C–H bonds), 959 cm−1 and 928 cm−1 (α-phase of polyamides, CONH α-bond), 834 cm−1 (CH2 rocking), and 685 cm−1 (C–N stretching and N–H deformation) [7,46,47,48].
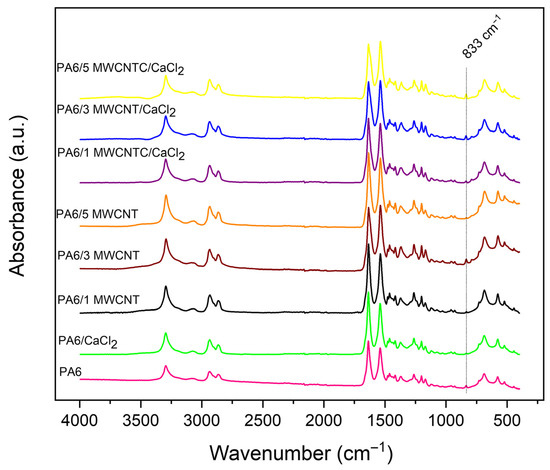
Figure 3.
FTIR spectra for the produced membranes.
Additional absorption bands related to formic acid were also identified at 2865 cm−1 (C–H vibration), 1415 cm−1 (in-plane bending of the C–OH group), and 1263 cm−1 (C–O stretching). The first two peaks attributed to polyamide (3294 cm−1 and 2934 cm−1) may also include contributions from formic acid. In such cases, overlapping absorption bands from both materials can occur, complicating spectral interpretation [7,40,49]. Furthermore, in the spectral region around 833 cm−1, an increase in peak intensity was observed in membranes with higher MWCNT content. This is likely due to the greater concentration of carbon nanotubes, which leads to stronger absorption of vibrations associated with C–C bonds in the MWCNT structure [48].
Based on the presented spectra, no distinct peaks associated with CaCl2 were detected in the membranes containing this salt. This absence can be explained because the CaCl2 is soluble in water and is completely removed during the immersion–precipitation step, preventing its retention within the final membrane structure.
During immersion in water, reactions occur that accelerate the phase inversion process, promoting pore formation in PA6 membranes. This behavior can be attributed to the presence of chloride ions (Cl−) from CaCl2, which act as pore-forming agents by destabilizing the polymer solution and accelerating pore formation during the coagulation bath, as reported in the literature [13].
3.3. Contact Angle
The contact angle results for the fabricated membranes are shown in Figure 4. Surfaces with angles between 0° and 90° are considered hydrophilic, while those with angles between 90° and 180° are classified as hydrophobic [50]. Based on this definition, the developed membranes showed no significant variation in contact angle, considering the experimental error. However, membranes containing CaCl2 and those with 1 and 5 phr of MWCNT exhibited slightly lower contact angles, suggesting a potentially higher degree of wettability.
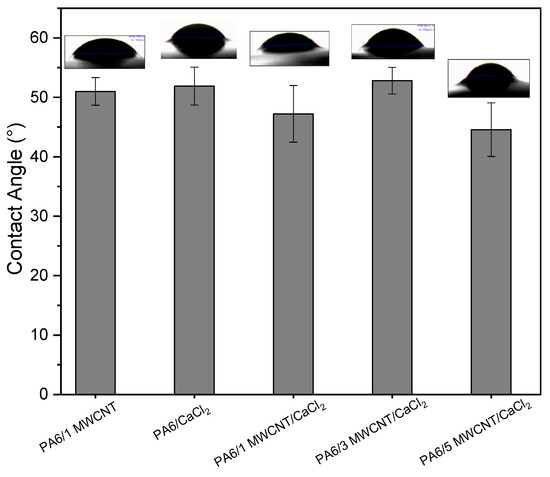
Figure 4.
Contact angle for distilled water of PA6/1 MWCNT, PA6 membranes, and nanocomposites with CaCl2.
This behavior can be attributed to a combination of factors: the presence of CaCl2 during phase inversion promotes the exposure of PA6 polar groups on the membrane surface, increasing the surface energy, and the salt also contributes to the dispersion of MWCNT, reducing the formation of hydrophobic agglomerates. The presence of CaCl2 further facilitates MWCNT dispersion by reducing solution viscosity and modifying polymer–polymer interactions. This creates a more favorable environment for uniform distribution of the nanotubes, enhancing both surface uniformity and the formation of a well-defined porous structure. This enhanced wettability could contribute to improved water flux due to better absorption characteristics.
3.4. Scanning Electron Microscopy (SEM)
The SEM images (Figure 5, Figure 6 and Figure 7) reveal that the membranes exhibit an asymmetric microporous structure characterized by spongy pores. Each membrane features a thin top layer, known as the selective skin layer, supported by a porous substrate. Notably, the PA6/1 MWCNT (Figure 6) membrane displays a dense skin layer and a sublayer with closed pores. This morphology results from delayed phase inversion, which occurs due to the low affinity between the solvent and non-solvent, leading to a slow precipitation process. Consequently, the skin layer forms a barrier during solvent exchange, promoting the development of closed-cell structures. This structure of the PA6/1 MWCNT membrane results from the slow precipitation process, which promotes more closed cells and smaller or fewer pores on the upper surface [51,52].
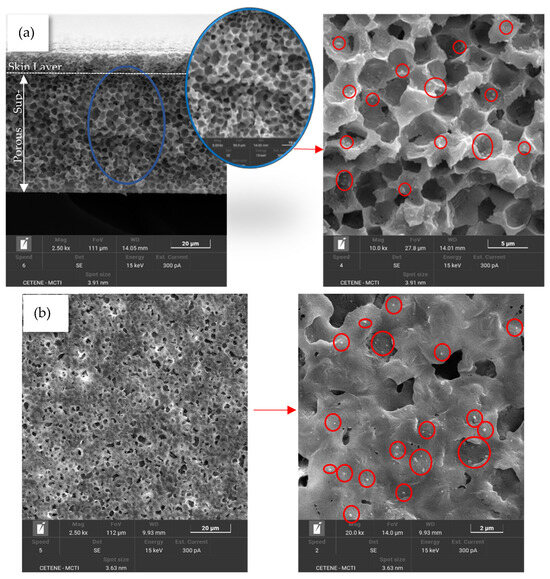
Figure 5.
SEM images of the cross-section of the PA6/CaCl2 membrane (a) and of the top surface (b).
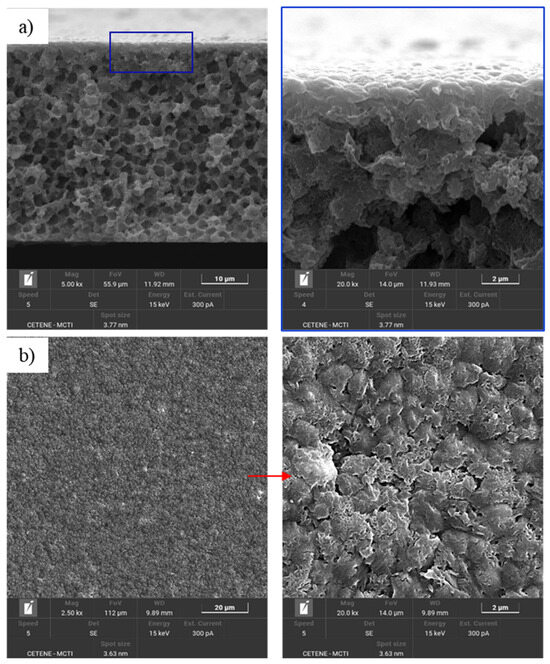
Figure 6.
SEM images of the cross-section with dense skin layer (a) and of the top surface (b) of the PA6/1 MWCNT.
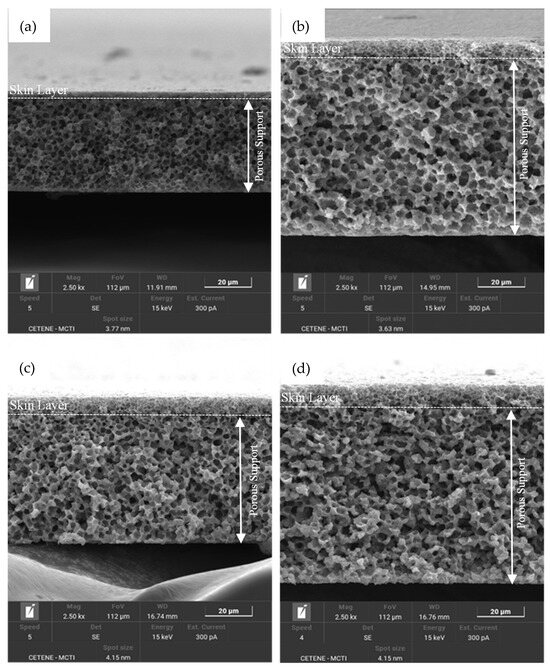
Figure 7.
SEM images of the cross-section of the membranes: (a) PA6/1 MWCNT; (b) PA6/1 MWCNT/CaCl2; (c) PA6/3 MWCNT/CaCl2; (d) PA6/5 MWCNT/CaCl2.
In contrast, membranes containing CaCl2 (Figure 5 and Figure 7) show a thicker skin layer with pores (as further supported by the data in Table 3) and greater pore connectivity throughout the cross-section. This structural difference likely plays a significant role in observed permeation behavior, as discussed in the subsequent sections of this study. Furthermore, particles were observed in the polymer matrix of the PA6/CaCl2 membrane, both on the surface and in the cross-section, as highlighted by the red circles in Figure 5. As previously noted, this particular membrane exhibited an increased viscosity compared to pure PA6, which slows down the polymer’s solubility in the solvent. This effect can lead to incomplete dissolution or localized polymer precipitation [6,53].

Table 3.
Total and filter skin thickness of membranes characterized by SEM.
As observed in Table 2, no direct correlation was found between solution viscosity and the total thickness of the membranes. This indicates that factors other than viscosity have a significant influence on the final thickness, such as gelation time, partial solvent evaporation before immersion, solvent/non-solvent exchange kinetics, and polymer matrix contraction during precipitation. At 1 phr, the low amount of MWCNT likely did not provide sufficient structural effect to prevent membrane contraction during coagulation. At 5 phr, the high concentration of nanotubes tends to promote agglomeration, which hinders polymer chain reorganization, leading to thicker membranes. In the case of 3 phr, a relatively more efficient dispersion of the carbon nanotubes is observed, facilitated by the presence of CaCl2, resulting in a more homogeneous and compact structure, and consequently, reduced thickness. Additionally, the viscosity reduction promoted by CaCl2 in this formulation with 3 phr of MWCNT likely accelerated solvent/non-solvent exchange, leading to faster precipitation and therefore thinner membranes. Thus, the 3 phr MWCNT content appears to be a critical concentration at which a balance between the amount of nanotubes and the action of the salt on dispersion occurs, optimizing the interaction with the PA6 matrix.
As mentioned, the PA6/1 MWCNT membrane exhibited a dense skin layer without visible pores, making it impossible to determine the pore size distribution for this layer (Figure 8). In contrast, the other membranes feature a thicker selective skin and a porous sublayer with pore size variations throughout the thickness. These morphological variations across the cross-section play a crucial role in membrane selectivity by regulating the transport of water and solutes [37,52]. Additionally, the membrane morphology showed consistent characteristics despite the increased concentration of MWCNT.
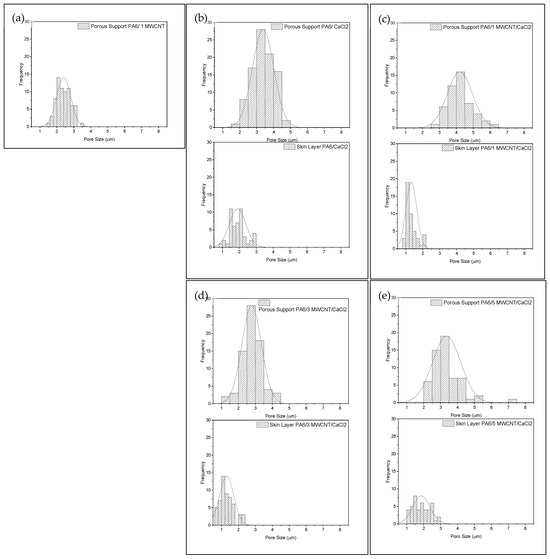
Figure 8.
Pore size distribution of skin layer and porous support of the membranes PA6/1 MWCNT (a), PA6/CaCl2 (b), PA6/1 MWCNT/CaCl2 (c), PA6/3 MWCNT/CaCl2 (d), and PA6/5 MWCNT/CaCl2 (e).
An important factor to consider is the exposure time of the membranes to air before immersion in the coagulation bath, known as the gelation time. This period directly affects the membrane morphology. Figure 8 illustrates that the selective skin layer contains smaller pores compared to the porous substrate layer. During the 5 min gelation time, increased evaporation of formic acid from the casting solution occurs, contributing to the development of this anisotropic structure. Similar behavior was reported by Ebrahimi et al. [54], who investigated the effect of gelation time on PA6 membranes, where the solution also contained CaCl2.
3.5. Atomic Force Microscopy (AFM)
Figure 9 presents AFM images of the produced membranes, where lighter and darker regions correspond to higher and lower surface areas, respectively. According to the literature [55,56], greater variations between peaks and valleys indicate increased surface roughness, which is often associated with a larger superficial pore diameter in membranes. Furthermore, based on Wenzel’s model (applicable for materials with contact angles between 0° and 90°), surface roughness inversely influences the contact angle: as roughness increases, water droplet absorption and wettability improve, leading to a decrease in the contact angle [57].
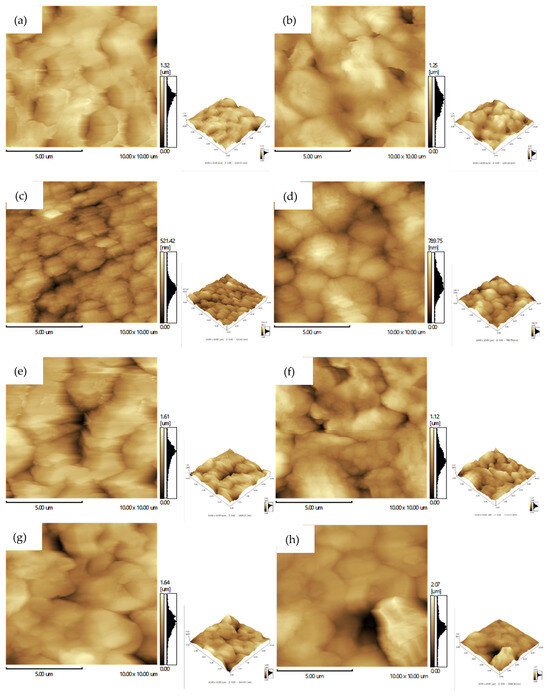
Figure 9.
AFM images of the membrane surfaces: (a) PA6, (b) PA6/CaCl2, (c) PA6/1 MWCNT, (d) PA6/3 MWCNT, (e) PA6/5 MWCNT, (f) PA6/1 MWCNT/CaCl2, (g) PA6/3 MWCNT/CaCl2, (h) PA6/5 MWCNT/CaCl2.
It was observed that the amount of MWCNT has a direct impact on the membrane surface roughness: higher MWCNT content corresponds to increased surface roughness, as shown in Table 4. This trend suggests that MWCNTs play a significant role in altering the surface morphology. Additionally, the presence of CaCl2 further contributed to roughness enhancement, particularly when combined with MWCNT, leading to even more textured surfaces. This effect can be attributed to the synergy between MWCNT and CaCl2, as observed in the PA6/MWCNT/CaCl2-based membranes. The incorporation of MWCNT disrupts the uniformity of the polymer chains on the surface, while CaCl2 accelerates the phase inversion kinetics, promoting the formation of surface pores. As a result, surface roughness increases, enhancing water penetration and spreading within the pores, intensifying capillary action, and consequently leading to higher water flux through the membrane [53,55,57,58]. However, the behavior of the PA6/1 MWCNT/CaCl2 membrane was distinct, presenting lower roughness than pure PA6. Apparently, the content of 1 phr of MWCNT was a reduced amount in the membrane formulation, not sufficient to disrupt the regularity of the polymer matrix. Nevertheless, it was enough to slow down the precipitation kinetics, leading to the formation of a dense surface with smaller topographical variations. This initial effect was later counterbalanced at higher MWCNT concentrations in the PA6/MWCNT/CaCl2 membranes, when the increased amount of nanotubes and the presence of CaCl2 promoted greater surface heterogeneity, leading to greater surface roughness, as seen in Table 4.

Table 4.
Average roughness (Ra) of the membranes.
3.6. Water Flow Measurements
Membranes without chloride showed no measurable flux, likely due to the absence of pores or the presence of closed pores within their structure, which would block permeate transport. Another explanation could be the formation of a dense superficial layer with closed pores, as observed in the morphology of the PA6/1 MWCNT membrane (Figure 6a). This behavior may be linked to an excessively long gelation time during membrane fabrication. Ebrahimi et al. [54] also reported that prolonged gelation time in PA6 membranes leads to reduced water uptake due to smaller pore sizes, thereby limiting water absorption and potentially acting as a barrier to hydrodynamic water flow. Another key factor influencing membrane flux is the viscosity of the solutions, which can affect the morphological development of the membranes. According to several studies reported in the literature [29,59,60,61], higher viscosity reduces water diffusion and prolongs the solvent de-solvation time. This leads to membranes with a denser, thicker selective layer and lower porosity, as well as an increased formation of isolated micropores within the porous substrate, as illustrated in Figure 6a. These isolated pores do not contribute to permeate transport, thus diminishing membrane flux. Additionally, higher concentrations of MWCNT tend to agglomerate, potentially blocking membrane pores and further impeding water permeation [62].
However, upon the addition of CaCl2 to the solution, permeate flux was observed in both the PA6 membranes and their nanocomposites. Figure 10 presents the flux curves measured with distilled water at pressures of 0.5, 1, 1.5, and 2 bar for these membranes. It is evident that membranes containing carbon nanotubes exhibited higher permeate flux compared to pure PA6, regardless of the applied pressure, following this trend: PA6/1 MWCNT/CaCl2 > PA6/5 MWCNT/CaCl2 > PA6/3 MWCNT/CaCl2 > PA6/CaCl2. Notably, the first two membranes showed the lowest contact angles (indicating greater hydrophilicity), which likely contributed to their improved water flux performance.
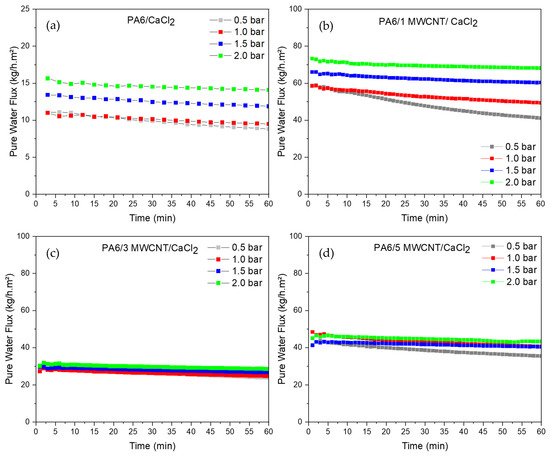
Figure 10.
Water flux measurement curves for the membranes (a) PA6/CaCl2, (b) PA6/1 MWCNT/CaCl2, (c) PA6/3 MWCNT/CaCl2, (d) PA6/5 MWCNT/CaCl2 at pressures of 0.5 bar, 1 bar, 1.5 bar, and 2.0 bar.
The literature [29,59] reports that membranes containing carbon nanotubes exhibit high flux rates because water molecules can pass through the nanotubes at ultrafast speeds. Despite the relatively hydrophobic nature of the carbon nanotubes, due to their nonpolar characteristics, they offer minimal resistance (friction) to permeation. However, it is important to emphasize that the distribution and orientation of the nanotubes within the membrane structure can significantly affect flux, potentially hindering rather than facilitating water permeation.
Overall, a trend of decreasing permeate flux is observed in the curves corresponding to pressures of 0.5 and 1 bar. In contrast, the flux curves at 1.5 and 2 bar show greater stability after 40 min, with 2 bar yielding the highest flux values. This behavior, marked by a reduction in flux rate over time, can be attributed to mechanical compaction of the membrane under applied pressure or to swelling of the polymer matrix, which gradually reduces pore size and eventually leads to a steady-state flux [63,64,65]. Additionally, since PA6 is a hydrophilic material, it tends to absorb water, which further decreases pore size and, as a result, lowers permeate flux. It is also worth noting that the curves confirm a common trend: higher pressure results in higher flux, a pattern consistent with previous studies on PA6 membranes [13,66].
The PA6/5 MWCNT membrane exhibited a distinct behavior compared to the others. While most membranes showed a gradual increase in flux with rising pressure, resulting in progressively higher curves, Figure 10d reveals a decline in performance at 1.5 and 2.0 bar. Specifically, the flux at 1.0 bar was nearly identical to that at 1.5 bar and only slightly lower than at 2.0 bar. This behavior may be attributed to the membrane’s internal structure. The simultaneous addition of carbon nanotubes and the pore-forming agent may have increased the pore density (or introduced more defects) in the porous substrate. Such structural changes could compromise the membrane’s mechanical strength under higher pressures. However, it is important to note that this membrane did not show the highest flux values at lower pressures (0.5 and 1.0 bar) when compared, for instance, to the PA6/1 MWCNT/CaCl2 membrane. This suggests that while the additives may have increased the number of pores, these pores were likely less interconnected than those in the membrane shown in Figure 10b.
It is also noteworthy that the membranes containing 3 and 5 phr of MWCNT did not show significant changes in flux in response to increasing pressure. This suggests that the incorporation of the filler enhances resistance to applied pressure, helping maintain the structural integrity of the pores. These membranes exhibited flux rates ranging between 20–35 kg/h·m2 and 35–50 kg/h·m2, respectively. In comparison, the PA6/1 MWCNT/CaCl2 membrane demonstrated higher performance, with flux values ranging from 40 to 75 kg/h·m2. A near twofold increase in flux was observed for this membrane as the pressure increased from 0.5 to 2 bar.
3.7. Water Vapor Permeability
The water vapor transmission rates (WVTR) of the fabricated membranes are shown in Figure 11. The highest values were observed for the membranes containing CaCl2 and for the PA6/5 MWCNT membrane, both reaching 32.7 g/h·m2. These WVTR results align with the previously reported water flux data and are consistent with the porous structures observed in the SEM images.
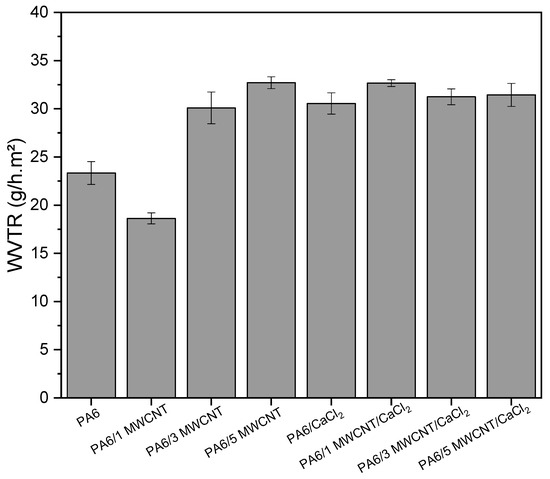
Figure 11.
Water vapor transmission rate of the developed membranes.
Additionally, the PA6/5 MWCNT membrane exhibited a WVTR comparable to that of the PA6/1 MWCNT/CaCl2 membrane, despite showing no water flux. This suggests that the membrane likely contains extremely small pores, allowing water vapor, due to its smaller kinetic radius, to permeate more easily. It is also evident that increasing the MWCNT content leads to higher vapor transmission rates, clearly indicating that the nanotubes enhance water vapor permeability. A higher concentration of MWCNT introduces more defects and polymer/nanotube interfaces, which expand the diffusion pathways for vapor. Moreover, MWCNTs may act as additional nanochannels for transporting small molecules like water vapor. Their inner walls provide a low-friction environment, allowing for more efficient transport and contributing to improved permeability [67,68].
4. Conclusions
Based on the preliminary results, it can be concluded that the interplay between solution viscosity, phase inversion dynamics of PA6, and the addition of CaCl2 strongly influenced the structural development of the membranes, as well as their ability to sustain water flux and vapor transmission. Membranes prepared without CaCl2 were ineffective in facilitating water transport, confirming its essential role as a pore-promoting agent and precipitation accelerator. When incorporated together with MWCNTs, CaCl2 not only reduced solution viscosity but also enabled the formation of a more favorable porous network, leading to an apparent synergistic effect that improved flux compared to membranes containing CaCl2 alone. AFM analysis revealed that both MWCNT and CaCl2 significantly altered the surface topography of the membranes, which may influence mechanical properties, wettability, and performance across various applications. It was also observed that the addition of MWCNT improved pressure resistance, particularly in membranes containing CaCl2. However, in the absence of the salt, the nanotubes were not effective in enabling water flux, although they did enhance water vapor transmission, suggesting potential for future applications in gas separation. Therefore, further research is necessary to better understand and optimize PA6/MWCNT membranes. This includes adjusting solvent ratios, exploring alternative nanotube incorporation techniques, reducing gelation time, and fine-tuning MWCNT content to fully harness the potential of this nanocomposite for use in functional membrane technologies.
Author Contributions
Conceptualization, C.M.M.S., V.d.N.M., P.T.V.d.S., R.A.D. and C.B.B.L.; methodology, C.M.M.S., R.A.d.P., V.d.N.M., P.T.V.d.S. and R.A.D.; validation, R.A.d.P., V.d.N.M., R.A.D., P.T.V.d.S. and C.B.B.L.; formal analysis, C.M.M.S., R.A.d.P., R.A.D., V.d.N.M., P.T.V.d.S. and C.B.B.L.; investigation, C.M.M.S., R.A.d.P., R.A.D., V.d.N.M. and P.T.V.d.S.; resources, E.M.A.; data curation, E.M.A., C.B.B.L. and R.M.R.W.; writing—original draft preparation, C.M.M.S.; writing—review and editing, C.M.M.S. and C.B.B.L.; visualization, E.M.A., R.A.d.P., R.M.R.W., R.A.D., V.d.N.M. and C.B.B.L.; supervision, E.M.A.; project administration, E.M.A.; funding acquisition, E.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work received financial support from CNPq, particularly from the Nanotechnology and New Materials Program, under process number 408779/2022-5. The authors acknowledge the CNPq for the research grants awarded to Carlos Bruno (Process No. 350025/2023-1), Renate Wellen (Process No. 303426/2021-7), and Edcleide Araújo (Process No. 312014/2020).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel), CNPq (National Council for Scientific and Technological Development), and FAPESQ (Paraíba State Research Support Foundation) for the financial support. We also express our gratitude to the Federal University of Campina Grande (UFCG) for providing the infrastructure and resources necessary to carry out this research.
Conflicts of Interest
The authors confirm that there are no conflicts of interest related to the execution or publication of this study.
References
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and solvents used in membrane fabrication: A review focusing on sustainable membrane development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Iroegbu, A.O.C.; Bordado, J.C. Polymer-Based Membranes and Composites for Safe, Potable, and Usable Water: A Survey of Recent Advances. Chem. Afr. 2020, 3, 593–608. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Wang, H. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef]
- Bezerra, M.G.; Landeira, M.A.C.; Leite, A.M.D.; Viana, K.M.d.S. Membranas de Poliamida 6 por inversão de fases: Formação de membranas pelos métodos de imersão em banho coagulante e por evaporação de solvente. Braz. J. Dev. 2020, 6, 76611–76626. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- dos Santos Filho, E.A.; de Medeiros, K.M.; Araújo, E.M.; Ferreira, R.D.S.B.; Oliveira, S.S.L.; Medeiros, V.D.N. Membranes of polyamide 6/clay/salt for water/oil separation. Mater. Res. Express 2019, 6, 105313. [Google Scholar] [CrossRef]
- de Medeiros, K.M.; Araújo, E.M.; Lira, H.d.L.; Lima, D.d.F.; de Lima, C.A.P. Membranas microporosas híbridas assimétricas: Influência da argila na morfologia das membranas. Rev. Mater. 2017, 22, e11812. [Google Scholar] [CrossRef]
- Bezerra, E.B.; Leite, A.M.D.; Araújo, E.M.; De Mélo, T.J.A. Obtenção e caracterização de membranas obtidas a partir de blendas poliméricas de poliamida 6. Polimeros 2014, 24, 381–387. [Google Scholar] [CrossRef]
- Leite, A.M.D.; Araújo, E.M.; Lira Hde, L.; Barbosa, R.; Ito, E.N. Obtenção de Membranas Microporosas a partir de Nanocompósitos de Poliamida 6/Argila Nacional. Parte 1: Influência da Presença da Argila na Morfologia das Membranas. Polímeros Ciênc. Tecnol. 2009, 19, 271–277. [Google Scholar] [CrossRef]
- Marques, A.V.S.; Barbosa, A.S.; Mais, L.F.; Rodrigues, M.G.F.; Barbosa, T.L.A.; Luna, C.B.B. Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment. Membranes 2025, 15, 298. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.S.; Lai, J.Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Ferreira, R.D.S.B.; Pereira, C.H.D.Ó.; Da Paz, R.A.; Damião Leite, A.M.; Araújo, E.M.; Lira, H.D.L. Influence of processing type in the morphology of membranes obtained from PA6/MMT nanocomposites. Adv. Mater. Sci. Eng. 2014, 2014, 659148. [Google Scholar] [CrossRef]
- De Medeiros, K.M.; Araújo, E.M.; De Lucena Lira, H.; De Farias Lima, D.; De Lima, C.A.P. Hybrid membranes of polyamide applied in treatment of waste water. Mater. Res. 2017, 20, 308–316. [Google Scholar] [CrossRef]
- Ahmad, A.; Sabir, A.; Iqbal, S.S.; Felemban, B.F.; Riaz, T.; Bahadar, A.; Hossain, N.; Khan, R.U.; Inam, F. Novel antibacterial polyurethane and cellulose acetate mixed matrix membrane modified with functionalized TiO2 nanoparticles for water treatment applications. Chemosphere 2022, 301, 134711. [Google Scholar] [CrossRef]
- Valamohammadi, E.; Behdarvand, F.; Mohammadi, T.; Tofighy, M.A.; Moghiseh, Z. Effects of carbon nanotubes on structure, performance and properties of polymer nanocomposite membranes for water/wastewater treatment applications: A comprehensive review. Polym. Bull. 2023, 80, 11589–11632. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric Membranes Incorporated With ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Ciência e Engenharia de Materiais—Uma Introdução, 9th ed.; LTC-Livros Técnicos e Científicos Editora Ltd.a.: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Tewari, P.K. Nanocomposite Membrane Technology: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional nanocomposites and their potential applications: A review. J. Polym. Res. 2021, 28, 36. [Google Scholar] [CrossRef]
- Nitodas, S.F.; Das, M.; Shah, R. Applications of Polymeric Membranes with Carbon Nanotubes: A Review. Membranes 2022, 12, 454. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Madenli, E.C.; Ciftci, Z.I. Effects of the carbon nanotube and polymer amounts on ultrafiltration membranes. Environ. Eng. Res. 2022, 27, 210626. [Google Scholar] [CrossRef]
- Vargas-Figueroa, C.; Pino-Soto, L.; Beratto-Ramos, A.; Tapiero, Y.; Rivas, B.L.; Berrio, M.E.; Melendrez, M.F.; Bórquez, R.M. In-Situ Modification of Nanofiltration Membranes Using Carbon Nanotubes for Water Treatment. Membranes 2023, 13, 616. [Google Scholar] [CrossRef]
- Paul, S.; Roy, S.; Mitra, S. Carbon nanotube enhanced selective micro filtration of butanol. Sep. Purif. Technol. 2024, 330, 125462. [Google Scholar] [CrossRef]
- Batool, M.; Abbas, M.A.; Khan, I.A.; Khan, M.Z.; Saleem, M.; Khan, A.U.; Deen, K.M.; Batool, M.; Khan, A.L.; Zhu, S.; et al. Response Surface Methodology Modeling Correlation of Polymer Composite Carbon Nanotubes/Chitosan Nanofiltration Membranes for Water Desalination. ACS ES T Water 2023, 3, 1406–1421. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Johnson, D.J. Membrane modification with carbon nanomaterials for fouling mitigation: A review. Adv. Colloid Interface Sci. 2024, 327, 103140. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Novel ultrafiltration membranes prepared from a multi-walled carbon nanotubes/polymer composite. J. Membr. Sci. 2010, 362, 374–383. [Google Scholar] [CrossRef]
- Bak, H.; Cho, S.Y.; Yun, Y.S.; Jin, H.J. Electrically conductive transparent films based on nylon 6 membranes and single-walled carbon nanotubes. Curr. Appl. Phys. 2010, 10, S468–S472. [Google Scholar] [CrossRef]
- He, X.; Kumakiri, I. Carbon Membrane Technology: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Zainab, G.; Iqbal, N.; Babar, A.A.; Huang, C.; Wang, X.; Yu, J.; Ding, B. Free-standing, spider-web-like polyamide/carbon nanotube composite nanofibrous membrane impregnated with polyethyleneimine for CO2 capture. Compos. Commun. 2017, 6, 41–47. [Google Scholar] [CrossRef]
- Asadi, A.; Gholami, F.; Nazari, S.; Dolatshah, M. Preparation of antifouling and antibacterial polyvinylidene fluoride membrane by incorporating functionalized multiwalled carbon nanotubes. J. Water Process Eng. 2022, 49, 103042. [Google Scholar] [CrossRef]
- Luna, C.B.B.; da Silva Barbosa Ferreira, E.; Siqueira, D.D.; Araújo, E.M.; do Nascimento, E.P.; Medeiros, E.S.; de Mélo, T.J.A. Electrical nanocomposites of PA6/ABS/ABS-MA reinforced with carbon nanotubes (MWCNTf) for antistatic packaging. Polym. Compos. 2022, 43, 3639–3658. [Google Scholar] [CrossRef]
- da Silva, F.S.; Luna, C.B.B.; da Silva Barbosa Ferreira, E.; de Matos Costa, A.R.; Wellen, R.M.R.; Araújo, E.M. Polyamide 6 (PA6)/carbon nanotubes (MWCNT) nanocomposites for antistatic application: Tailoring mechanical and electrical properties for electronic product protection. J. Polym. Res. 2024, 31, 15. [Google Scholar] [CrossRef]
- De Medeiros, K.M. Membranas Microporosas Híbridas de Poliamida Aplicadas no Tratamento de Emulsões Oleosas da Indústria Petrolífera. Ph.D. Thesis, Universidade Federal de Campina Grande (UFCG), Campina Grande, Brazil, 2014. [Google Scholar]
- Helali, N.; Rastgar, M.; Farhad Ismail, M.; Sadrzadeh, M. Development of underwater superoleophobic polyamide-imide (PAI) microfiltration membranes for oil/water emulsion separation. Sep. Purif. Technol. 2020, 238, 116451. [Google Scholar] [CrossRef]
- Dias, R.A.; da Silva Barbosa Ferreira, R.; da Nóbrega Medeiros, V.; Araujo, B.A.; da Silva, P.T.V.; da Paz, R.A.; Araújo, E.M.; de Lucena Lira, H. Influence of Solvent Content in the Coagulation Bath and of Polyvinylpyrrolidone on the Properties of PSF/PES Blend Membranes For Water/Oil Separation. Polym. Adv. Technol. 2025, 36, e70210. [Google Scholar] [CrossRef]
- Santos Filho, E.A.; Florindo Salviano, A.; Araújo, B.A.; de Medeiros, K.M.; Nóbrega Medeiros, V.; Araújo, E.M.; Lira, H.L. Influence of Additives on Hybrids Membranes Morphology for Water Treatment. Diffus. Found. 2017, 14, 86–106. [Google Scholar] [CrossRef]
- Rambabu, K.; Velu, S. Improved performance of CaCl2 incorporated polyethersulfone ultrafiltration membranes. Period. Polytech. Chem. Eng. 2016, 60, 181–191. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Li, B.; Gong, W.; Jing, X.; Zheng, B. Effect of NaCl concentration on the dispersion, stability and rheological properties of MWNTs by CMC. J. Dispers. Sci. Technol. 2021, 42, 2043–2052. [Google Scholar] [CrossRef]
- Dai, K.; Shi, L.; Fang, J.; Zhang, D.; Yu, B. NaCl adsorption in multi-walled carbon nanotubes. Mater. Lett. 2005, 59, 1989–1992. [Google Scholar] [CrossRef]
- Poolachira, S.; Velmurugan, S. Effect of solvents in the formation of PES-based asymmetric flat sheet membranes in phase inversion method: Phase separation and rheological studies. Iran. Polym. J. (Engl. Ed.) 2023, 32, 365–376. [Google Scholar] [CrossRef]
- Aparna, S.; Purnima, D.; Adusumalli, R.B. Effect of short carbon fiber content and water absorption on tensile and impact properties of PA6/PP blend based composites. Polym. Compos. 2020, 41, 5167–5181. [Google Scholar] [CrossRef]
- Essabir, H.; El Mechtali, F.Z.; Nekhlaoui, S.; Raji, M.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Compatibilization of PA6/ABS blend by SEBS-g-MA: Morphological, mechanical, thermal, and rheological properties. Int. J. Adv. Manuf. Technol. 2020, 110, 1095–1111. [Google Scholar] [CrossRef]
- Aitha, S.; Vasanthan, N. Effect of cellulose nanocrystals on crystallization, morphology and phase transition of polyamide 6. Compos. Interfaces 2020, 27, 371–384. [Google Scholar] [CrossRef]
- Fauzi, A.; Hapidin, D.A.; Munir, M.M.; Iskandar, F.; Khairurrijal, K. A superhydrophilic bilayer structure of a nylon 6 nanofiber/cellulose membrane and its characterization as potential water filtration media. RSC Adv. 2020, 10, 17205–17216. [Google Scholar] [CrossRef]
- Rosa, M.J.; De Pinho, M.N. Membrane surface characterisation by contact angle measurements using the immersed method. J. Membr. Sci. 1997, 131, 167–180. [Google Scholar] [CrossRef]
- Huang, N.Y.; Wang, C.C.; Chen, C.Y. Investigation of the gas permeation properties of a polyether sulfone asymmetric membrane via the phase inversion method. J. Appl. Polym. Sci. 2022, 139, e52762. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Lin, Y.; Liu, X.; Yao, H.; Yu, L.; Wang, H.; Wang, X. Fabrication of polysulfone membrane with sponge-like structure by using different non-woven fabrics. Sep. Purif. Technol. 2022, 297, 121553. [Google Scholar] [CrossRef]
- Gunjal, H.V.; Singh, G. Innovative PEEK membrane structure fabrication using non-solvent induced phase separation. J. Polym. Res. 2025, 32, 150. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K. Fabrication of Polyamide-6 Membranes—The Effect of Gelation Time towards Their Morphological, Physical, and Transport Properties. Membranes 2022, 12, 315. [Google Scholar] [CrossRef]
- Barbosa Ferreira, R.d.S.; Salviano, A.F.; Lima Oliveira, S.S.; Araújo, E.M.; Medeiros, V.d.N.; Lira, H.d.L. Treatment of effluents from the textile industry through polyethersulfone membranes. Water 2019, 11, 2540. [Google Scholar] [CrossRef]
- Dias, R.A.; Ferreira, R.S.B.; Medeiros, V.d.N.; Araujo, B.A.; Araújo, E.M.; Lira, H.d.L. Flat membranes of polyethersulfone/polysulfone blends in water/oil separation. Polym. Bull. 2023, 80, 4289–4305. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Ghalamchi, L.; Aber, S.; Vatanpour, V.; Kian, M. A novel antibacterial mixed matrixed PES membrane fabricated from embedding aminated Ag3PO4/g-C3N4 nanocomposite for use in the membrane bioreactor. J. Ind. Eng. Chem. 2019, 70, 412–426. [Google Scholar] [CrossRef]
- Mattia, D.; Lee, K.P.; Calabrò, F. Water permeation in carbon nanotube membranes. Curr. Opin. Chem. Eng. 2014, 4, 32–37. [Google Scholar] [CrossRef]
- Rizzuto, C.; Pugliese, G.; Bahattab, M.A.; Aljlil, S.A.; Drioli, E.; Tocci, E. Multiwalled carbon nanotube membranes for water purification. Sep. Purif. Technol. 2018, 193, 378–385. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Chen, W.; Shen, Z.; Ding, M.; Lin, T.; Tao, H.; Kong, Q.; Yang, G.; Xie, Z. A polyamide membrane with tubular crumples incorporating carboxylated single-walled carbon nanotubes for high water flux. Desalination 2020, 479, 114330. [Google Scholar] [CrossRef]
- Zeni, M.; Bellincanta, T.; Poletto, P.; Thürmer, M.B.; Duarte, J.; Toscan, A. Preparação e Caracterização de Membranas Poliméricas a partir da Blenda Polisulfona/Poliuretano. Polímeros 2011, 21, 229–232. [Google Scholar] [CrossRef]
- de Cavalho, T.C.; Medeiros, V.d.N.; Leite, A.M.D.; de Araújo, E.M.; Lira, H.L. Membranas de poliétersulfona/argila e sua permeabilidade à água. Rev. Mater. 2017, 22, e11825. [Google Scholar] [CrossRef]
- Cunha, C.T.C. Desenvolvimento de Membranas a Partir de Blendas de PA6/PEAD/Compatibilizantes. Master’s Thesis, UFCG, Campina Grande, Brazil, 2011. [Google Scholar]
- Inukai, S.; Cruz-Silva, R.; Ortiz-Medina, J.; Morelos-Gomez, A.; Takeuchi, K.; Hayashi, T.; Tanioka, A.; Araki, T.; Tejima, S.; Noguchi, T.; et al. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube·polyamide nanocomposite. Sci. Rep. 2015, 5, 13562. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lyu, Q.; Si, Y.; Tong, T.; Lin, L.C.; Yang, F.; Tang, C.Y.; Dong, Y. Superhydrophobic Carbon Nanotube Network Membranes for Membrane Distillation: High-Throughput Performance and Transport Mechanism. Environ. Sci. Technol. 2022, 56, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Visvini, G.A.; Mathioudakis, G.N.; Soto Beobide, A.; Piperigkou, Z.; Giannakas, A.E.; Messaritakis, S.; Sotiriou, G.; Voyiatzis, G.A. Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents. Polymers 2023, 15, 4432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).