Hydrocracking of Algae Oil and Model Alkane into Jet Fuel Using a Catalyst Containing Pt and Solid Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Catalyst Syntheses
2.3. Characterization Method
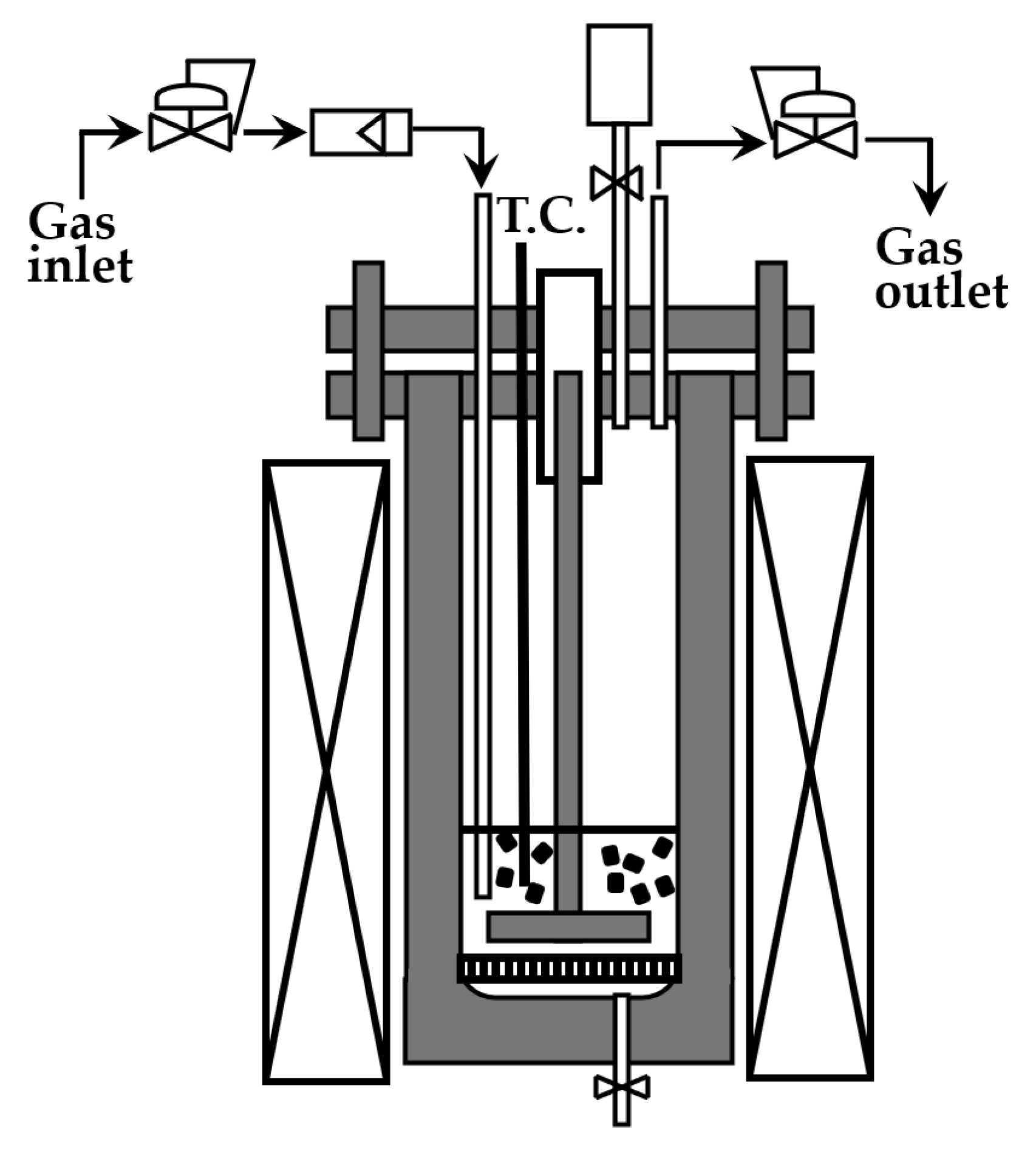
2.4. Reaction Procedure
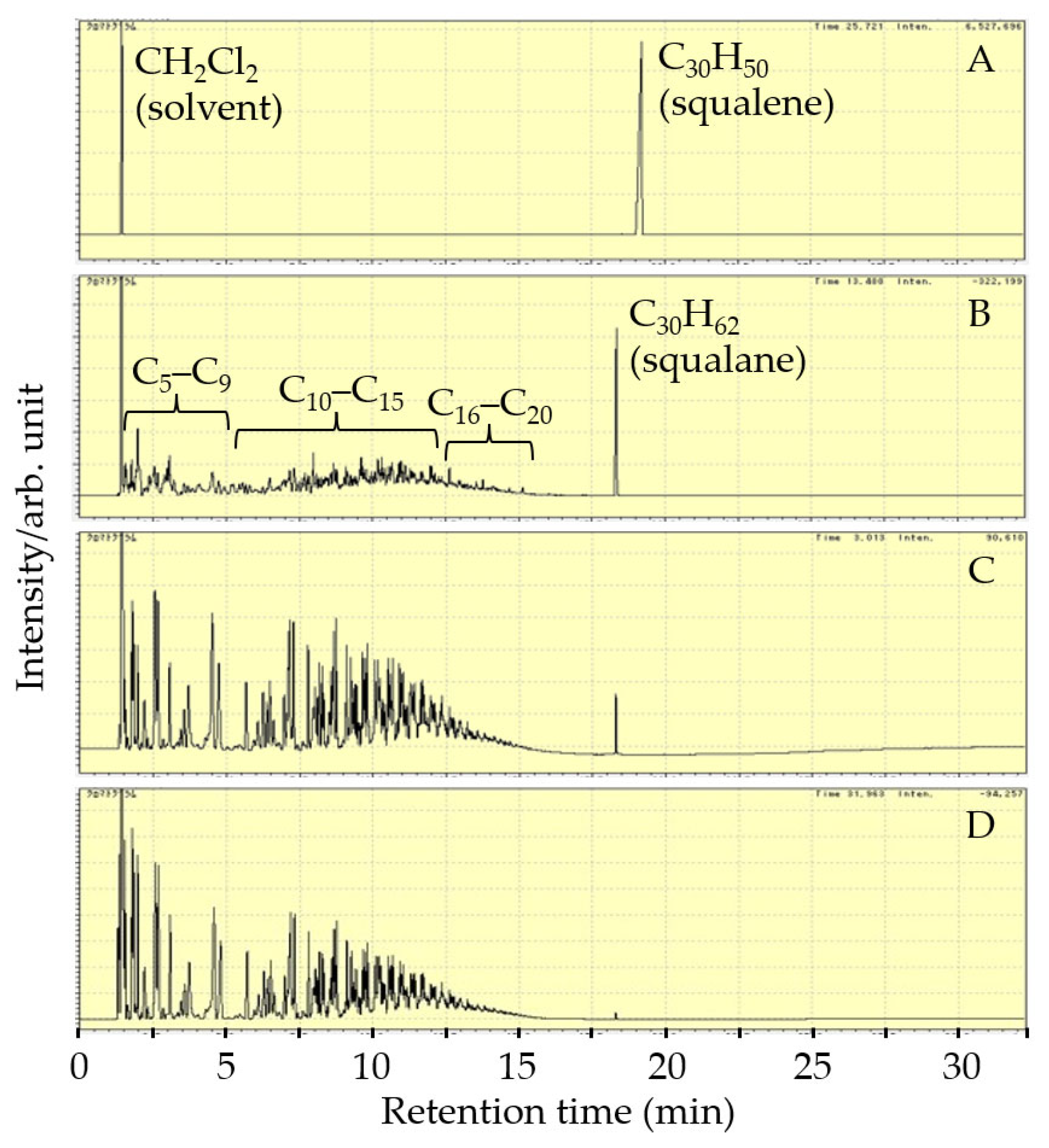
2.5. Product Analysis
3. Results and Discussion
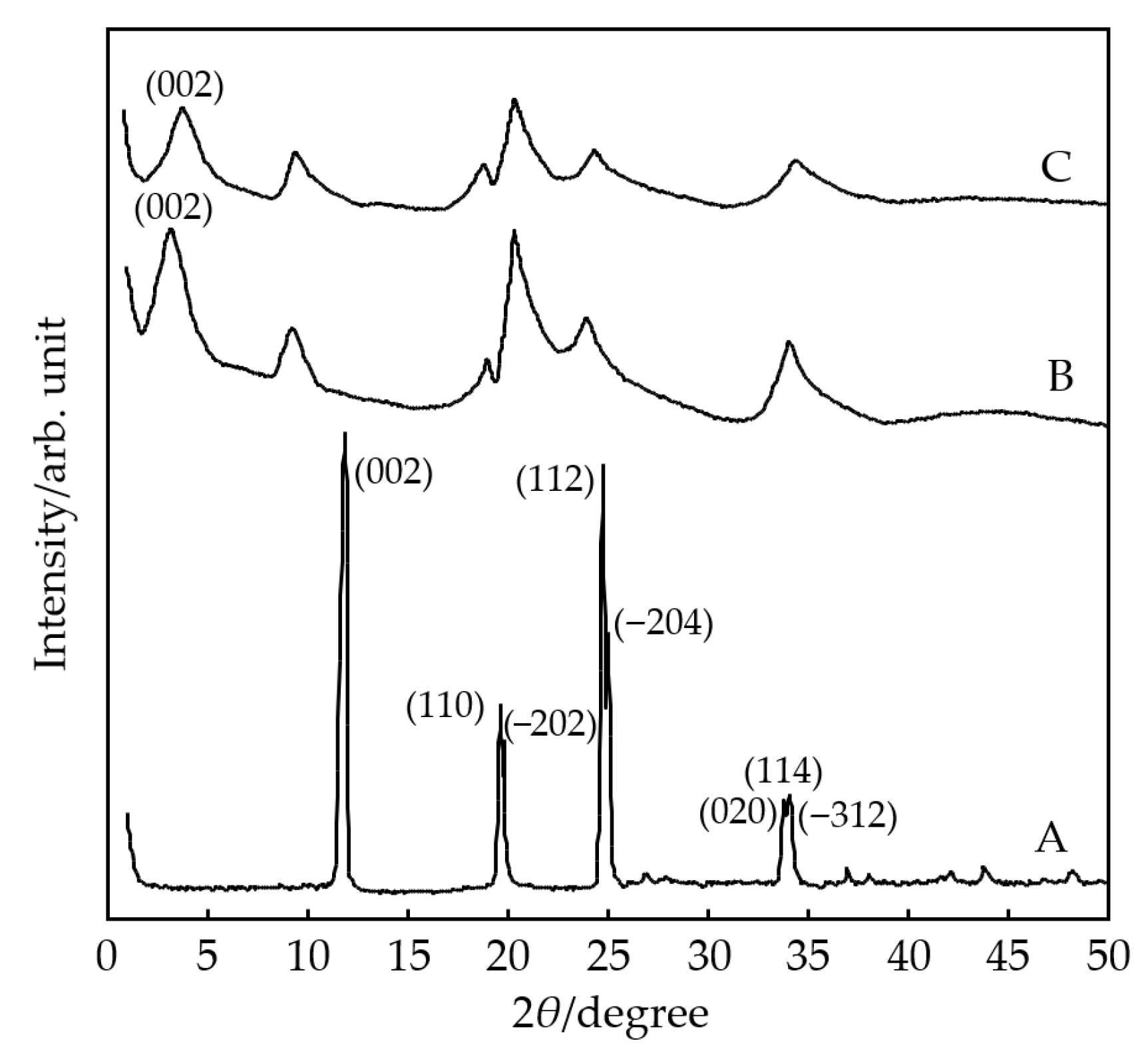
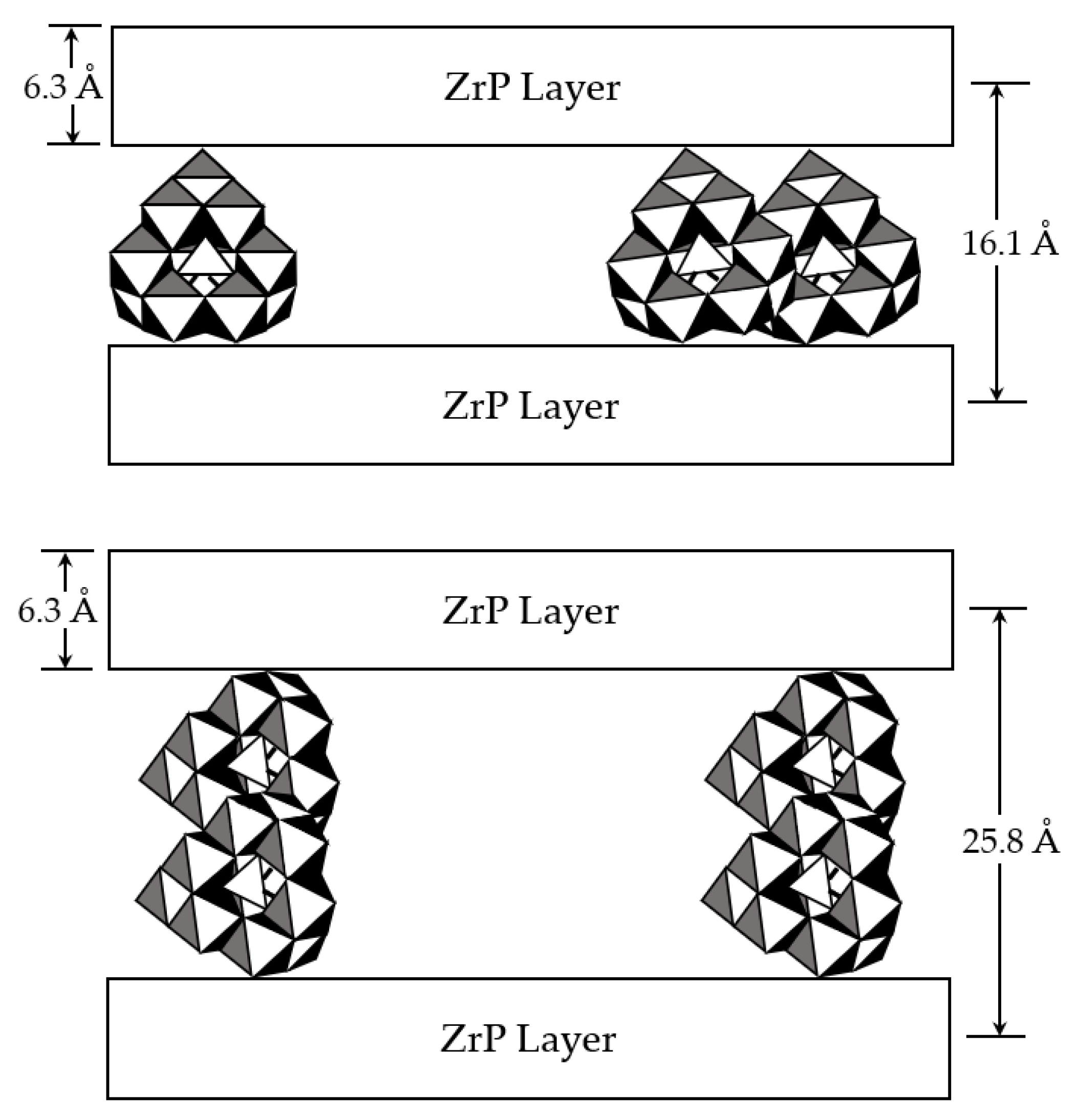
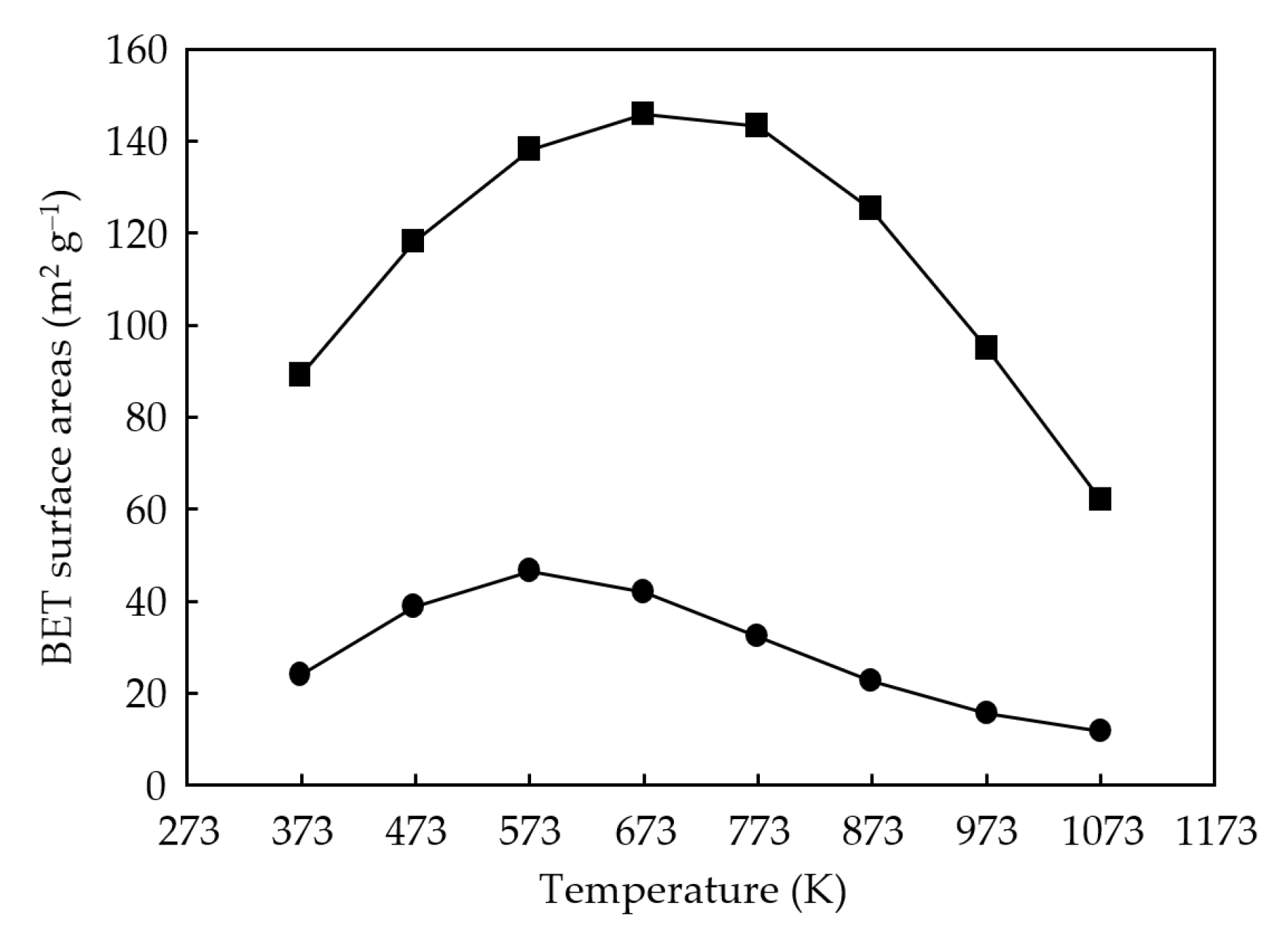
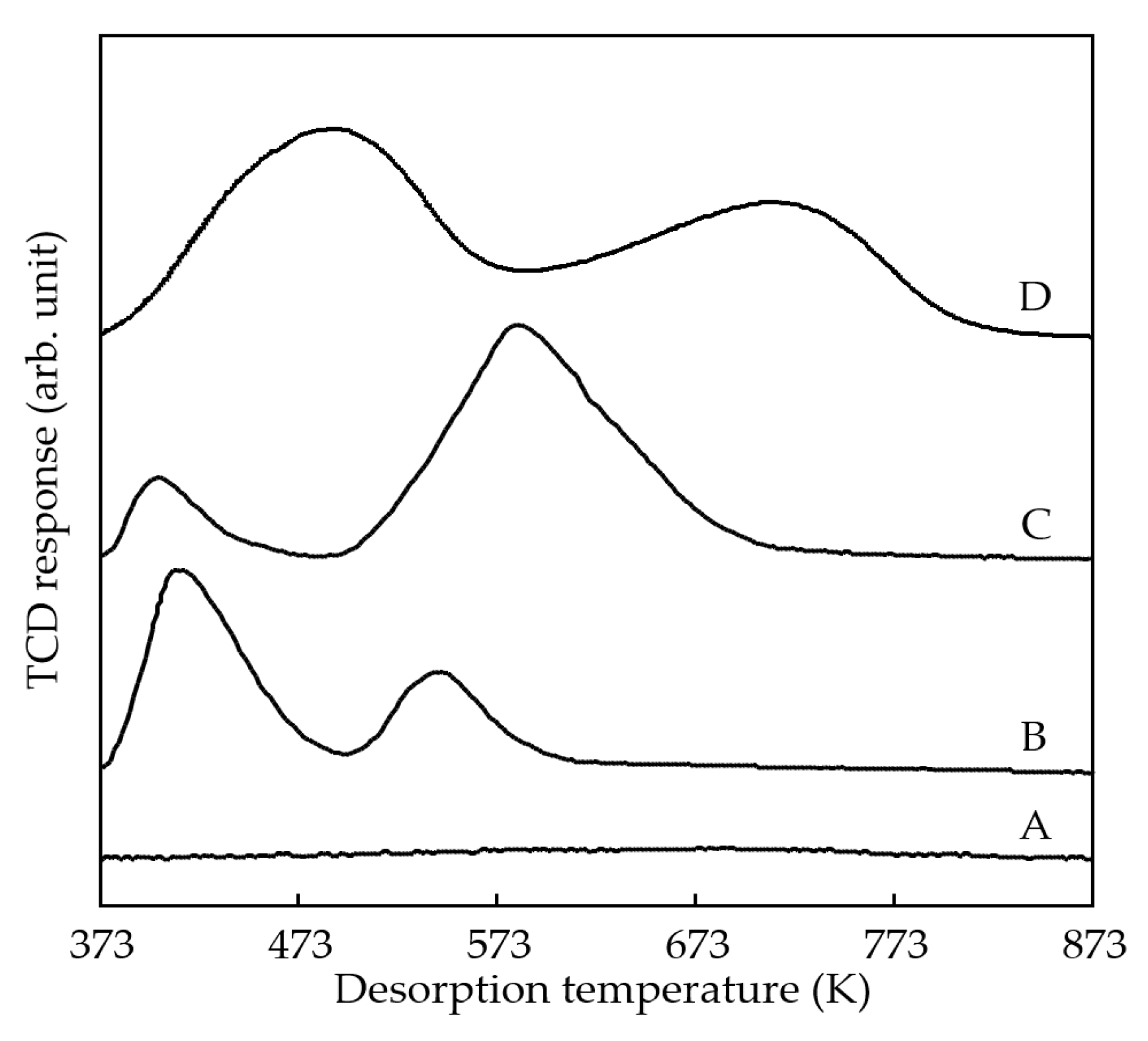
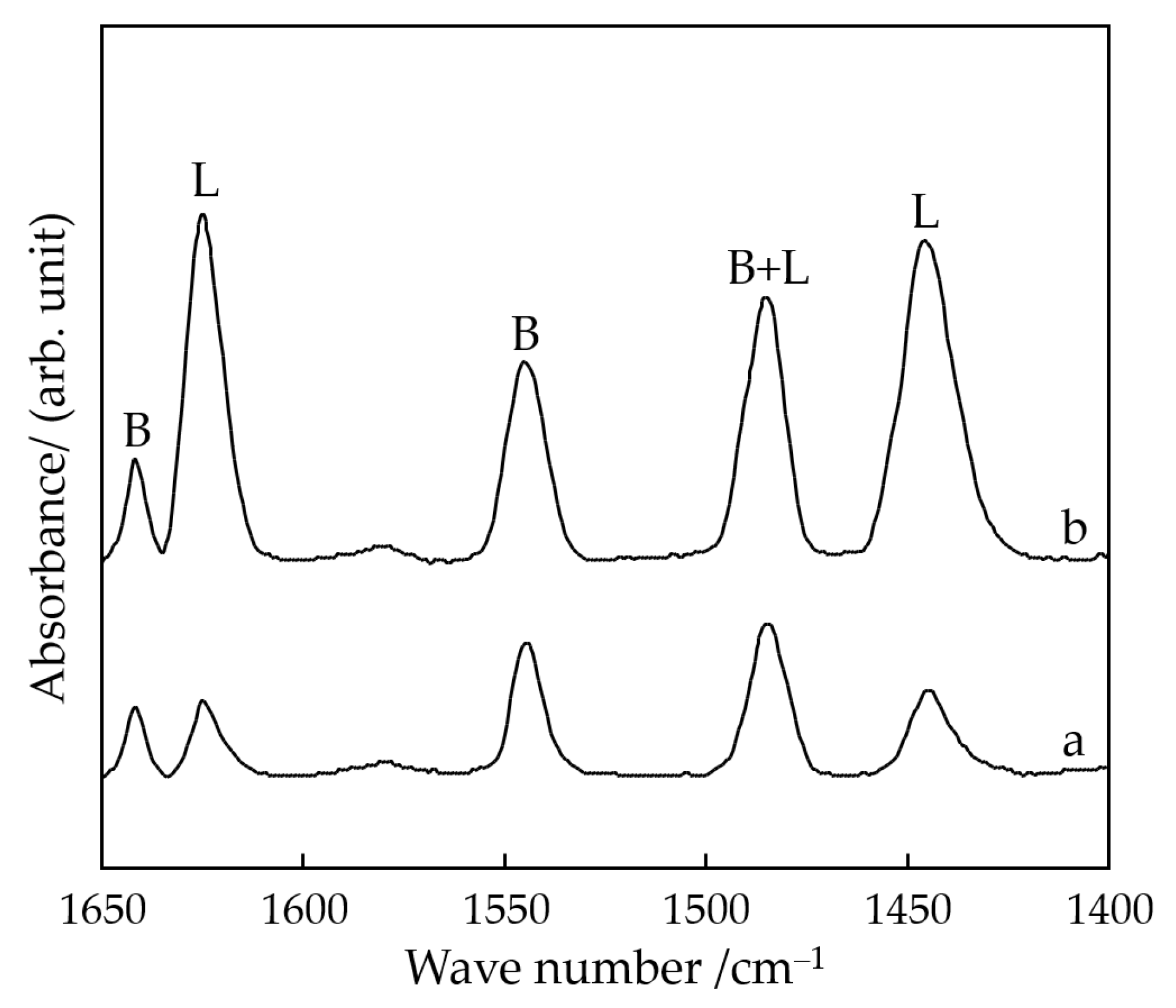
3.1. Catalyst Synthesis and Characterization
3.2. Hydrocracking of Squalene over Various Catalysts
3.3. Designing Reaction Process for Squalene Hydrocracking on Pt/Al2O3-α-ZrP
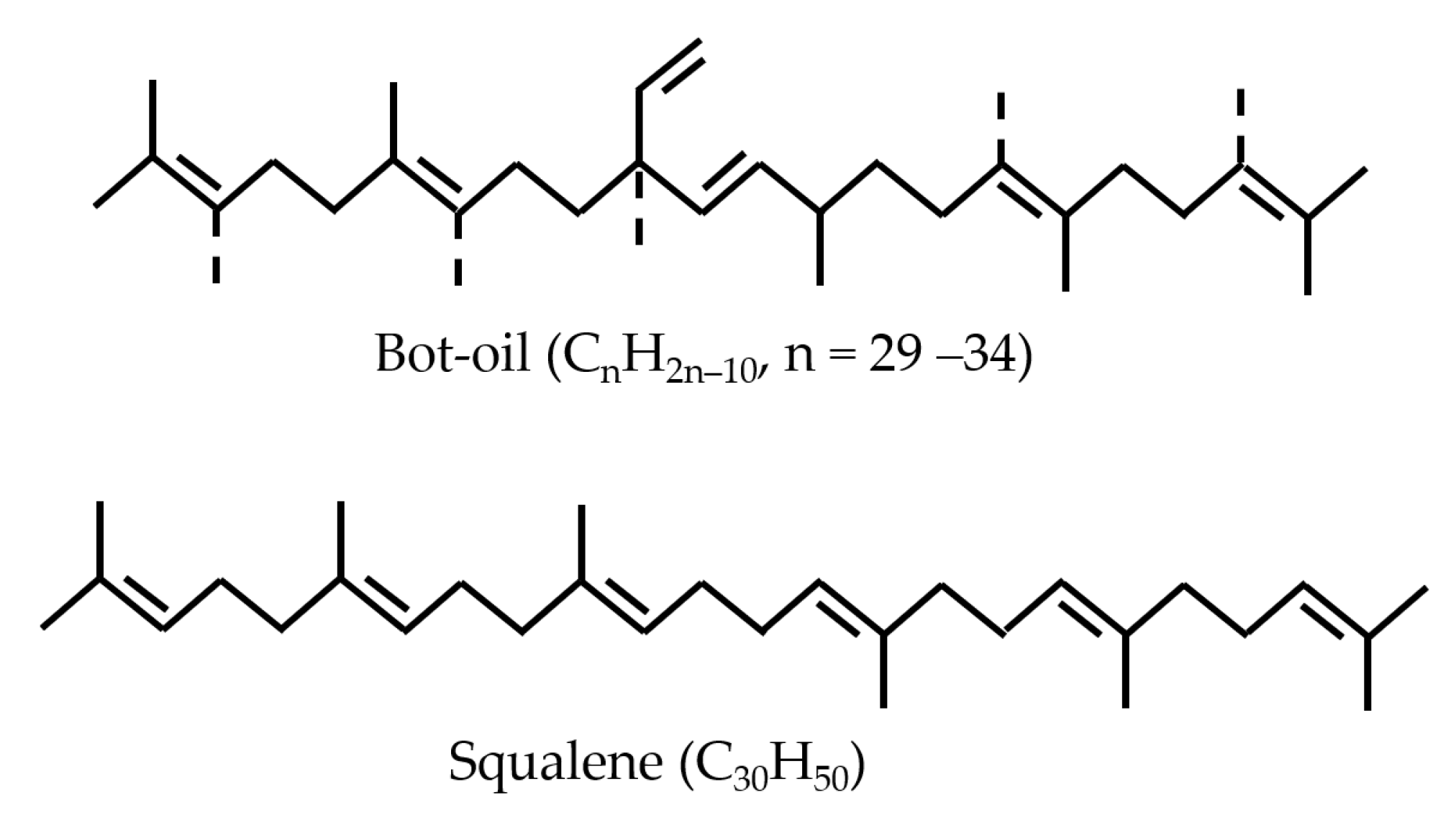
3.4. Hydrocracking of Bot-Oil over Pt/Al2O3-α-ZrP in a Two-Step Process
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.S.; Pitari, G.; Grewe, V.; Gierens, K.; Penner, J.E.; Petzold, A.; Prather, M.J.; Schumann, U.; Bais, A.; Berntsen, T.; et al. Transport Impacts on Atmosphere and Climate: Aviation. Atmos. Environ. 2010, 44, 4678–4734. [Google Scholar] [CrossRef]
- Yang, X.; Guo, F.; Xue, S.; Wang, X. Carbon Distribution of Algae-based Alternative Aviation Fuel Obtained by Different Pathways. Renew. Sustain. Energy Rev. 2016, 54, 1129–1147. [Google Scholar] [CrossRef]
- Gutiérrez-Antonioa, C.; Gómez-Castrob, F.I.; Lira-Floresa, J.A.; Hernández, S. A review on the Production Processes of Renewable Jet Fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary Advancements in Carbon Dioxide Valorization via Metal-organic Framework-based Strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Kieckhäfer, K.; Quante, G.; Müller, C.; Spengler, T.S.; Lossau, M.; Jonas, W. Simulation-Based Analysis of the Potential of Alternative Fuels towards Reducing CO2 Emissions from Aviation. Energies 2018, 11, 186. [Google Scholar] [CrossRef]
- Crawford, J.T.; Chan, C.W.; Budsberg, E.; Morgan, H.; Bura, R.; Gustafson, R. Hydrocarbon Bio-jet Fuel from Bioconversion of Poplar Biomass: Techno-economic Assessment. Biotechnol. Biofuels 2016, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable Bio-jet Fuel Production for Aviation: A Review. Fuel 2019, 254, 115599. [Google Scholar] [CrossRef]
- Wang, M.; Dewil, R.; Maniatis, K.; Wheeldon, J.; Tan, T.; Baeyens, J.; Fang, Y. Biomass-derived Aviation Fuels: Challenges and Perspective. Prog. Energy Combust. Sci. 2019, 74, 31–49. [Google Scholar] [CrossRef]
- Swaina, P.K.; Das, L.M.; Naik, S.N. Biomass to Liquid: A Prospective Challenge to Research and Development in 21st Century. Renew. Sustain. Energy Rev. 2011, 15, 4917–4933. [Google Scholar] [CrossRef]
- Liu, Y.; Sotelo-Boyás, R.; Murata, K.; Minowa, T.; Sakanishi, K. Hydrotreatment of Vegetable Oils to Produce Bio-hydrogenated Diesel and Liquefied Petroleum Gas Fuel over Catalysts Containing Sulfided Ni-Mo and Solid Acids. Energy Fuel 2011, 25, 4675–4685. [Google Scholar] [CrossRef]
- Eagan, N.M.; Kumbhalkar, M.D.; Buchanan, J.S.; Dumesic, J.A.; Huber, G.W. Chemistries and Processes for the Conversion of Ethanol into Middle-distillate Fuels. Nat. Rev. Chem. 2019, 3, 223–249. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, W.; Xu, K.; Liu, Y.; Wu, J.; Zhang, F. Understanding the Structure−Activity Relationships in Catalytic Conversion of Polyolefin Plastics by Zeolite-Based Catalysts: A Critical Review. ACS Catal. 2022, 12, 14882–14901. [Google Scholar] [CrossRef]
- Georgianna, D.R.; Mayfield, S.P. Exploiting Diversity and Synthetic Biology for the Production of Algal Biofuels. Nature 2012, 488, 329–335. [Google Scholar] [CrossRef]
- Yoshida, M.; Tanabe, Y.; Yonezawa, N.; Watanabe, M.M. Energy Innovation Potential of Oleaginous Microalgae. Biofuels 2012, 3, 761–781. [Google Scholar] [CrossRef]
- Xu, W.; Ding, K.; Hu, L. A Mini Review on Pyrolysis of Natural Algae for Bio-Fuel and Chemicals. Processes 2021, 9, 2042. [Google Scholar] [CrossRef]
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V.M.; Fernández-Siurob, I.; Garcia-Casillas, L.A.; Velázquez-Juárez, G. Dunaliella salina as a Potential Biofactory for Antigens and Vehicle for Mucosal Application. Processes 2022, 10, 1776. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Comparison of Growth Rate and Nutrient Content of Five Microalgae Species Cultivated in Greenhouses. Plants 2019, 8, 279. [Google Scholar] [CrossRef]
- Hillen, L.W.; Pollard, G.; Wake, L.V.; White, N. Hydrocracking of the Oils of Botryococcus braunii to Transport Fuels. Biotechnol. Bioeng. 1982, 24, 193–205. [Google Scholar] [CrossRef]
- Nakaji, Y.; Oya, S.; Watanabe, H.; Watanabe, M.M.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Production of Gasoline Fuel from Alga-Derived Botryococcene by Hydrogenolysis over Ceria-Supported Ruthenium Catalyst. ChemCatChem 2017, 9, 2701–2708. [Google Scholar] [CrossRef]
- Yamamoto, S.; Mandokoro, Y.; Nagano, S.; Nagakubo, M.; Atsumi, K.; Watanabe, M.M. Catalytic Conversion of Botryococcus braunii Oil to Diesel Fuel under Mild Reaction Conditions. J. Appl. Phycol. 2014, 26, 55–64. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Tan, T. The Production of Bio-jet Fuel from Botryococcus braunii Liquid over a Ru/CeO2 Catalyst. RSC Adv. 2016, 6, 99842–99850. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Watanabe, M.M.; Inaba, M.; Takahara, I. Hydrocracking of Algae Oil into Aviation Fuel-Range Hydrocarbons Using a Pt–Re Catalyst. Energy Fuels 2014, 28, 6999–7006. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Hydrocracking of Algae Oil to Aviation Fuel-Range Hydrocarbons over NiMo-supported Catalysts. Catal. Today 2019, 332, 115–121. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A.W.; Abbas, H.F. Heterogeneous Catalysts for Advanced Bio-Fuel Production through Catalytic Biomass Pyrolysis Vapor Upgrading: A Review. RSC Adv. 2015, 5, 22234–22255. [Google Scholar] [CrossRef]
- Tiwari, A.; Rajesh, V.M.; Yadav, S. Biodiesel Production in Micro-reactors: A review. Energy Sustain. Dev. 2018, 43, 143–161. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Sales-Cruz, M.; Lopez-Arenas, T.; Viveros-García, T.; Pérez-Cisneros, E.S. An Intensified Reactive Separation Process for Bio-Jet Diesel Production. Processes 2019, 7, 655. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Costantino, U.; Vivani, R. Layered and Pillared Metal (IV) Phosphates and Phosphonates. Adv. Mater. 1996, 8, 291–303. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sue, H.J.; Clearfield, A. Preparation of α-Zirconium Phosphate Nanoplatelets with Wide Variations in Aspect Ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M. Synthesis of Mixed Alcohols from Synthesis Gas over Alkali and Fischer–Tropsch Metals Modified MoS2/Al2O3-montmorillonite Catalysts. React. Kinet. Mech. Catal. 2014, 113, 187–200. [Google Scholar] [CrossRef]
- Majhi, D.; Das, K.; Bariki, R.; Sahu, P.; Bhoi, Y.P.; Mishra, B.G. Preparation and Catalytic Application of Sulfonated Polyvinyl Alcohol-Al-pillared α-Zirconium Phosphate (SPV-AAZP) Hybrid Material towards Synthesis of 4,6-Diarypyrimidin-2(1H)-ones. J. Porous Mater. 2020, 27, 355–368. [Google Scholar] [CrossRef]
- Jones, D.J.; Leloup, J.M.; Ding, Y.; Roziere, J. Enhancement of the Protonic Conductivity of α-M(IV)(HPO4)2·H2O, M (IV) = Zr, Sn, by Intercalation of the Aluminum Keggin Ion, [Al13O4(OH)24·12H2O ]7+. Solid State Ion. 1993, 61, 117–123. [Google Scholar] [CrossRef]
- Liu, Y.; Hanaoka, T.; Miyazawa, T.; Murata, K.; Okabe, K.; Sakanishi, K. Fischer–Tropsch Synthesis in Slurry-phase Reactors over Mn- and Zr-modified Co/SiO2 Catalysts. Fuel Process. Technol. 2009, 90, 901–908. [Google Scholar] [CrossRef]
- Pica, M. Zirconium Phosphate Catalysts in the XXI Century: State of the Art from 2010 to Date. Catalysts 2017, 7, 190. [Google Scholar] [CrossRef]
- Segawa, K. Structure and Surface Chemistry of Crystalline Zirconium Phosphate Catalysts. Mater. Chem. Phys. 1987, 17, 181–200. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Z. Alumina-pillared α-Zirconium Phosphate Prepared by in-situ Polymerization Method. Microporous Mesoporous Mater. 1998, 24, 213–222. [Google Scholar] [CrossRef]
- Fu, G.; Nazar, L.F.; Bain, A.D. Aging Processes of Alumina Sol-Gels: Characterization of New Aluminium Polyoxycations by 27Al NMR Spectroscopy. Chem. Mater. 1991, 3, 602–610. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Hanaoka, T.; Inaba, M.; Sakanishi, K. Syntheses of New Peroxo-polyoxometalates Intercalated Layered Double Hydroxides for Propene Epoxidation by Molecular Oxygen in Methanol. J. Catal. 2007, 248, 277–287. [Google Scholar] [CrossRef]
- Hoppe, R.; Alberti, G.; Costantino, U.; Dionigi, C.; Schulz-Ekloff, G.; Vivani, R. Intercalation of Dyes in Layered Zirconium Phosphates. 1. Preparation and Spectroscopic Characterization of α-Zirconium Phosphate Crystal Violet Compounds. Langmuir 1997, 13, 7252–7257. [Google Scholar] [CrossRef]
- Liu, Y.; Murata, K.; Inaba, M.; Mimura, N. Selective Oxidation of Propylene to Acetone by Molecular Oxygen over Mx/2H5–x[PMo10V2O40]/HMS (M = Cu2+, Co2+, Ni2+). Catal. Commun. 2003, 4, 281–285. [Google Scholar] [CrossRef]
- Weitkamp, J. Catalytic Hydrocracking—Mechanisms and Versatility of the Process. ChemCatChem 2012, 4, 292–306. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Layer α-Zirconium Phosphate: An Efficient Catalyst for the Synthesis of Solketal from Glycerol. Appl. Clay Sci. 2019, 174, 120–126. [Google Scholar] [CrossRef]
- Shimomura, O.; Sasaki, S.; Kume, K.; Kawano, S.; Shizuma, M.; Ohtaka, A.; Nomura, R. Release Behavior of Benzimidazole-Intercalated α-Zirconium Phosphate as a Latent Thermal Initiator in the Reaction of Epoxy Resin. Catalysts 2019, 9, 69. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, J.; Algarra, M.; Guimarães, V.; Bobos, I.; Rodríguez-Castellón, E. The Application of Functionalized Pillared Porous Phosphate Heterostructures for the Removal of Textile Dyes from Wastewater. Materials 2017, 10, 1111. [Google Scholar] [CrossRef]
- Babu, B.H.; Lee, M.; Hwang, D.W.; Kim, Y.; Chae, H.J. An Integrated Process for Production of Jet-fuel Range Olefins from Ethylene using Ni-AlSBA-15 and Amberlyst-35 Catalysts. Appl. Catal. A-Gen. 2017, 530, 48–55. [Google Scholar] [CrossRef]
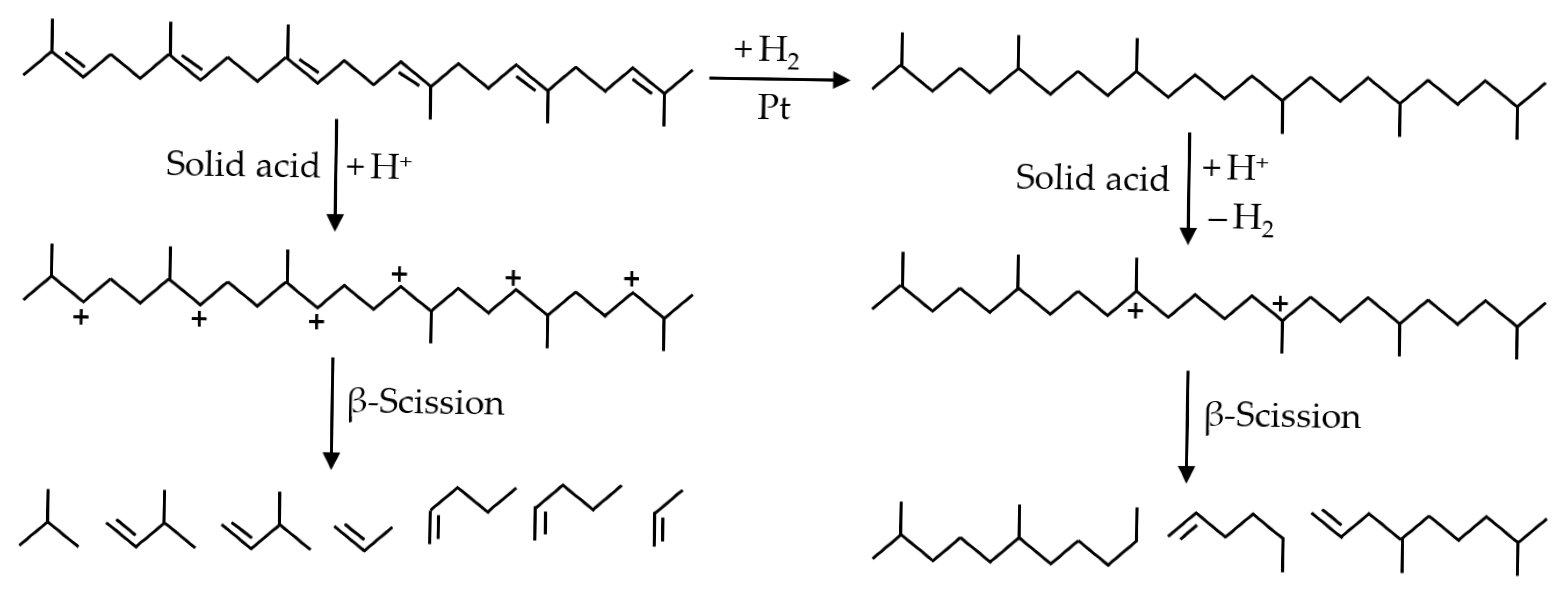
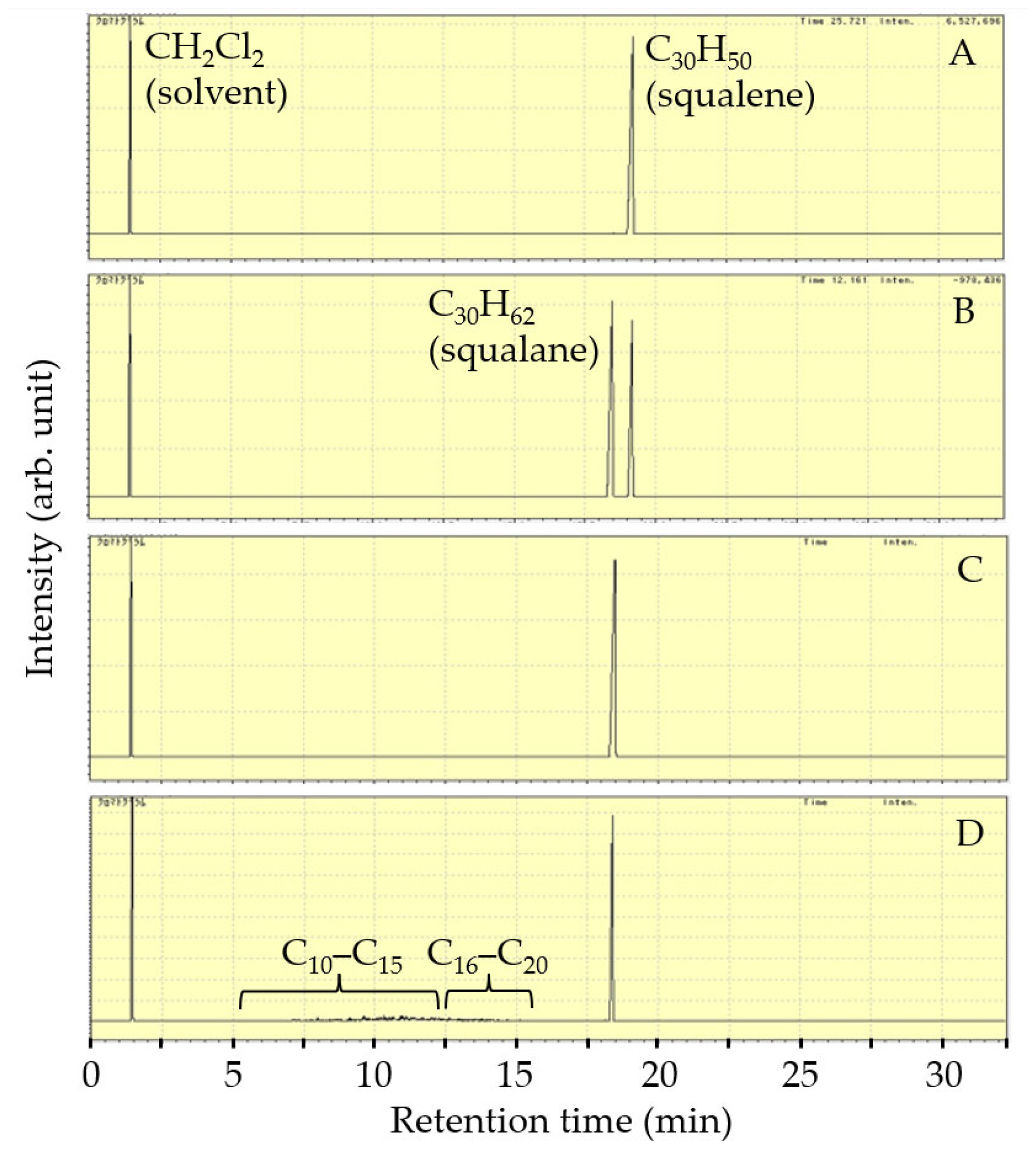
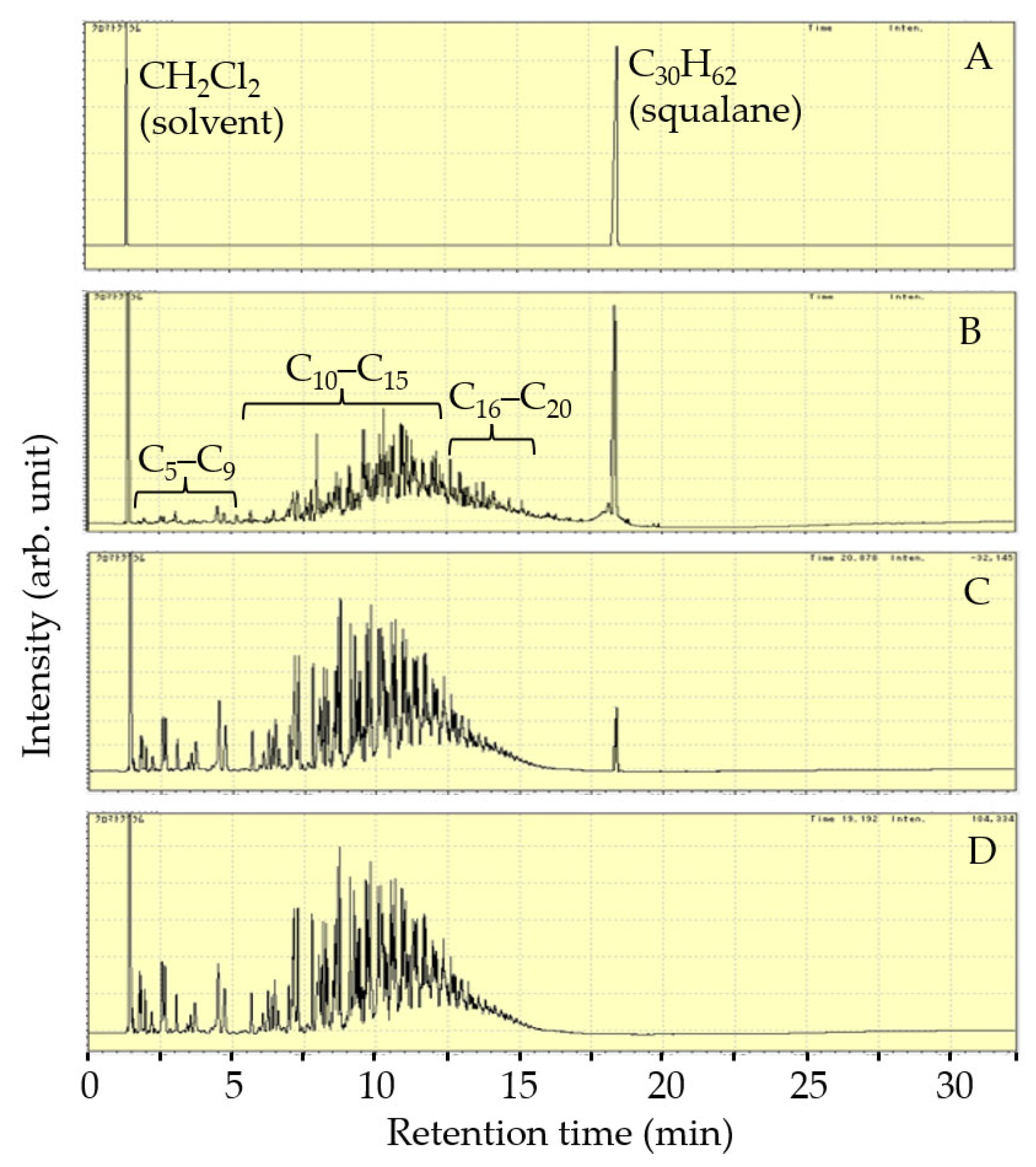
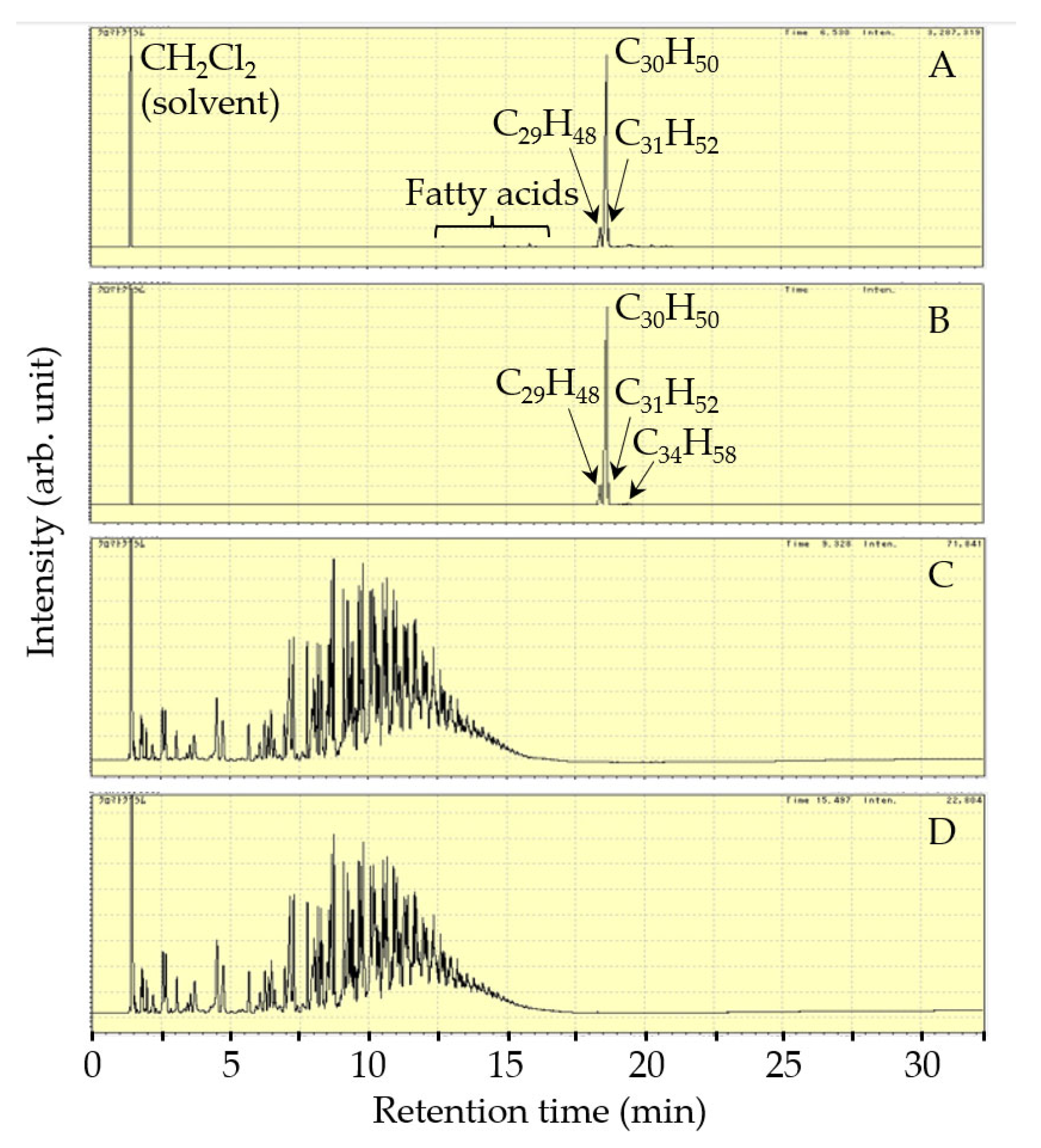
| Catalyst | Reactor | Reaction Temperature (K) | H2 Pressure (MPa) | Product Yield (%) | Reference | ||
|---|---|---|---|---|---|---|---|
| Gasoline | Jet Fuel | Diesel | |||||
| CoMo | batch | 673 | 20 | 67 | 15 | 15 | [18] |
| Ru/CeO2 | batch | 513 | 6 | 70 | 15 | 10 | [19] |
| HY | Flow | 533 | 0.1 | 8 | 6 | 85 | [20] |
| Ru/CeO2 | batch | 513 | 3.5 | N.A. | 41 | 35 | [21] |
| PtRe/SiO2–Al2O3 | batch | 603 | 5.5 | 17 | 50 | 17 | [22] |
| NiMo/Clay | batch | 573 | 4 | 32 | 52 | 5 | [23] |
| Pt/Al2O3-α-ZrP | batch | 623 | 2 | 10.6 | 66.2 | 20.1 | This study |
| Sample | Temperature (K) | d (002) (Å) | BET Surface Area (m2/g) | Average Pore Size (Å) | Total Vp (cm3/g) | Micro Vp (cm3/g) |
|---|---|---|---|---|---|---|
| α-ZrP | 373 | 7.6 | 22 | 7.6 | 0.065 | 0.012 |
| 573 | 7.4 | 51 | 7.3 | 0.078 | 0.014 | |
| 673 | 7.1 | 43 | 7.2 | 0.059 | 0.011 | |
| 773 | – | 31 | – | 0.028 | – | |
| Alpoly-α-ZrP | 373 | 25.8 | 87 | 36.2 | 0.211 | 0.136 |
| 573 | 25.4 | 138 | 34.8 | 0.234 | 0.156 | |
| 673 | 25.3 | 143 | 33.6 | 0.251 | 0.169 | |
| 773 | 25.1 | 140 | 30.4 | 0.207 | 0.132 |
| Catalyst | C30H50 Conv. (%) | Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| C30H62 | C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | ||
| Pt/α-ZrP | 100 | 9.5 | 7.2 | 48.7 | 30.6 | 1.7 | 0.2 |
| Pt/Al2O3-α-ZrP | 100 | 0.1 | 5.5 | 35.6 | 52.8 | 5.3 | 0.2 |
| Al2O3-α-ZrP | 100 | 0 | 44.8 | 51.3 | 3.4 | 0 | 0 |
| Pt/SiO2 | 100 | 92.4 | 3.2 | 2.1 | 1.2 | 0.4 | 0.1 |
| Pt/H-ZSM-5 | 100 | 0 | 23.8 | 49.6 | 21.3 | 0.2 | 0 |
| Reaction Time (h) | C30H50 Conv. (%) | Yield (%) | C10–C15 /C1–C29 | |||||
|---|---|---|---|---|---|---|---|---|
| C30H62 | C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | |||
| 1 | 100 | 48.6 | 2.3 | 16.6 | 29.3 | 2.9 | 0.4 | 0.57 |
| 2 | 100 | 4.7 | 5.1 | 34.0 | 51.1 | 4.6 | 0.3 | 0.54 |
| 3 | 100 | 0.1 | 5.5 | 35.6 | 52.8 | 5.3 | 0.2 | 0.53 |
| Reaction Time (h) | C30H50 Conv. (%) | Yield (%) | C10–C15 /C1–C29 | |||||
|---|---|---|---|---|---|---|---|---|
| C30H62 | C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | |||
| 0.5 | 51.1 | 51.1 | 0 | 0 | 0 | 0 | 0 | – |
| 1 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | – |
| 5 | 100 | 97.4 | 0.2 | 0 | 1.7 | 0.6 | 0 | 0.68 |
| Reaction Temperature (K) | C30H50 Conv. (%) | Yield (%) | C10–C15 /C1–C29 | |||||
|---|---|---|---|---|---|---|---|---|
| C30H62 | C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | |||
| 523 | 100 | 97.4 | 0.2 | 0 | 1.7 | 0.6 | 0 | 0.68 |
| 573 | 100 | 5.1 | 4.6 | 36.1 | 50.4 | 3.5 | 0.2 | 0.53 |
| 623 | 100 | 0 | 6.4 | 38.9 | 50.6 | 3.3 | 0.1 | 0.51 |
| 723 | 100 | 0 | 10.6 | 52.2 | 32.5 | 1.2 | 0 | 0.34 |
| Reaction Time in 2nd Step (h) | C30H50 Conv. (%) | Yield (%) | C10–C15 /C1–C29 | |||||
|---|---|---|---|---|---|---|---|---|
| C30H62 | C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | |||
| 1 | 100 | 32.5 | 1.2 | 3.0 | 42.8 | 19.3 | 0.9 | 0.64 |
| 2 | 100 | 4.1 | 2.8 | 10.1 | 62.3 | 20.2 | 0.3 | 0.65 |
| 3 | 100 | 0 | 3.1 | 12.4 | 65.0 | 19.1 | 0.2 | 0.65 |
| Type of Bot-Oil | Conv. (%) | Fraction in Total Hydrocarbon Products (%) | C10–C15 /C1–C29 | |||||
|---|---|---|---|---|---|---|---|---|
| C1–C4 | C5–C9 | C10–C15 | C16–C20 | C21–C29 | C30+ | |||
| Bot-oil-C | 100 | 2.8 | 10.6 | 66.2 | 20.1 | 0.3 | 0 | 0.66 |
| Bot-oil-P | 100 | 2.9 | 10.5 | 66.1 | 20.2 | 0.2 | 0 | 0.66 |
| Catalyst | Cycle Number | Conversion (%) | C10–C15 Fraction in Hydrocarbon Products (%) | Carbon Balance |
|---|---|---|---|---|
| Fresh | 1 | 100 | 66.2 | 83.6 |
| Used | 2 | 100 | 66.3 | 92.2 |
| 3 | 100 | 66.1 | 93.5 | |
| 4 | 100 | 66.3 | 94.1 | |
| 5 | 100 | 66.2 | 93.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y. Hydrocracking of Algae Oil and Model Alkane into Jet Fuel Using a Catalyst Containing Pt and Solid Acid. Processes 2025, 13, 3129. https://doi.org/10.3390/pr13103129
Liu Y. Hydrocracking of Algae Oil and Model Alkane into Jet Fuel Using a Catalyst Containing Pt and Solid Acid. Processes. 2025; 13(10):3129. https://doi.org/10.3390/pr13103129
Chicago/Turabian StyleLiu, Yanyong. 2025. "Hydrocracking of Algae Oil and Model Alkane into Jet Fuel Using a Catalyst Containing Pt and Solid Acid" Processes 13, no. 10: 3129. https://doi.org/10.3390/pr13103129
APA StyleLiu, Y. (2025). Hydrocracking of Algae Oil and Model Alkane into Jet Fuel Using a Catalyst Containing Pt and Solid Acid. Processes, 13(10), 3129. https://doi.org/10.3390/pr13103129







