Adsorption of Ammonium by Coal and Coal Fly Ash Derived from Hawthorn Tree from Aquatic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Materials
2.3. Physical-Chemical Properties
2.4. Scanning Electron Microscopy-Energy Dispersive X-Ray Analysis
2.5. Surface Charge
2.6. Fourier-Transform Infrared Spectroscopy
2.7. Batch Adsorption–Desorption Studies
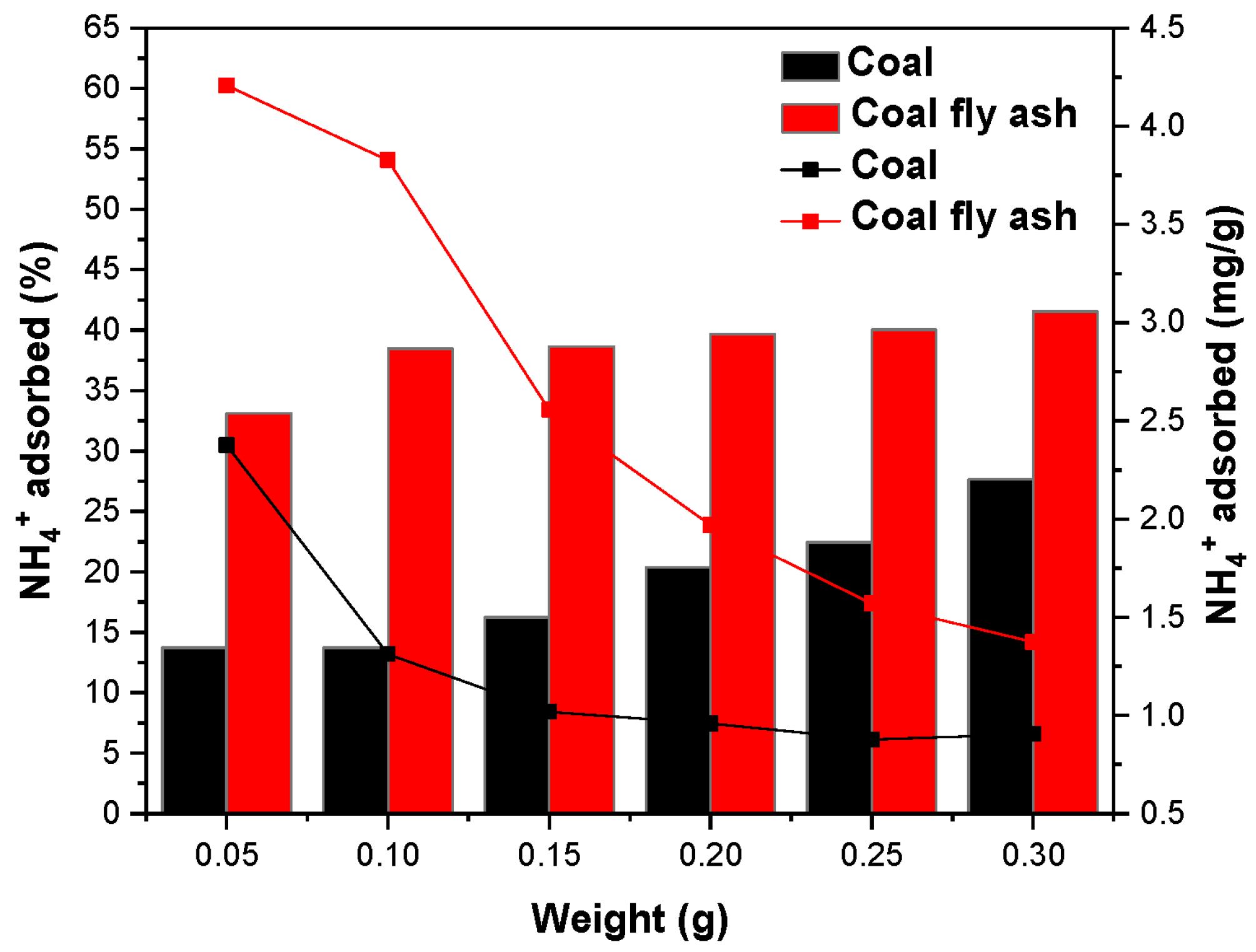
2.7.1. Effect of Adsorbent Dose
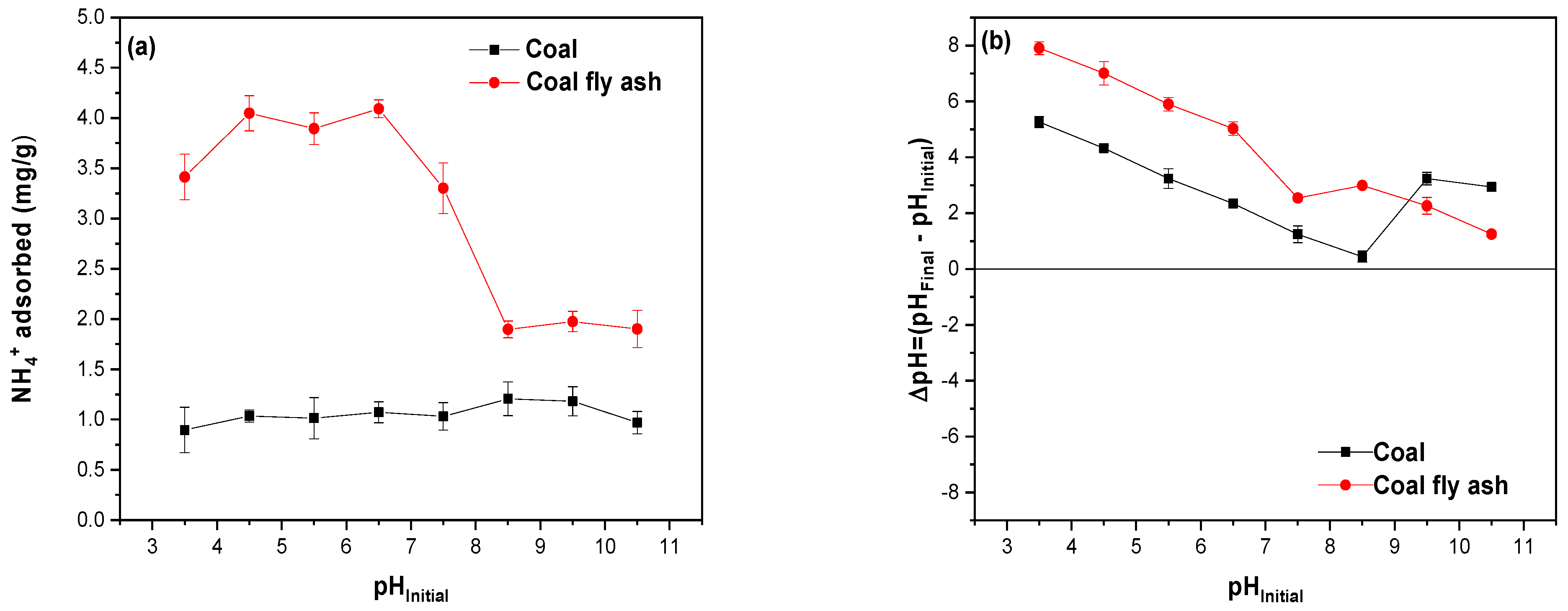
2.7.2. Effect of pH
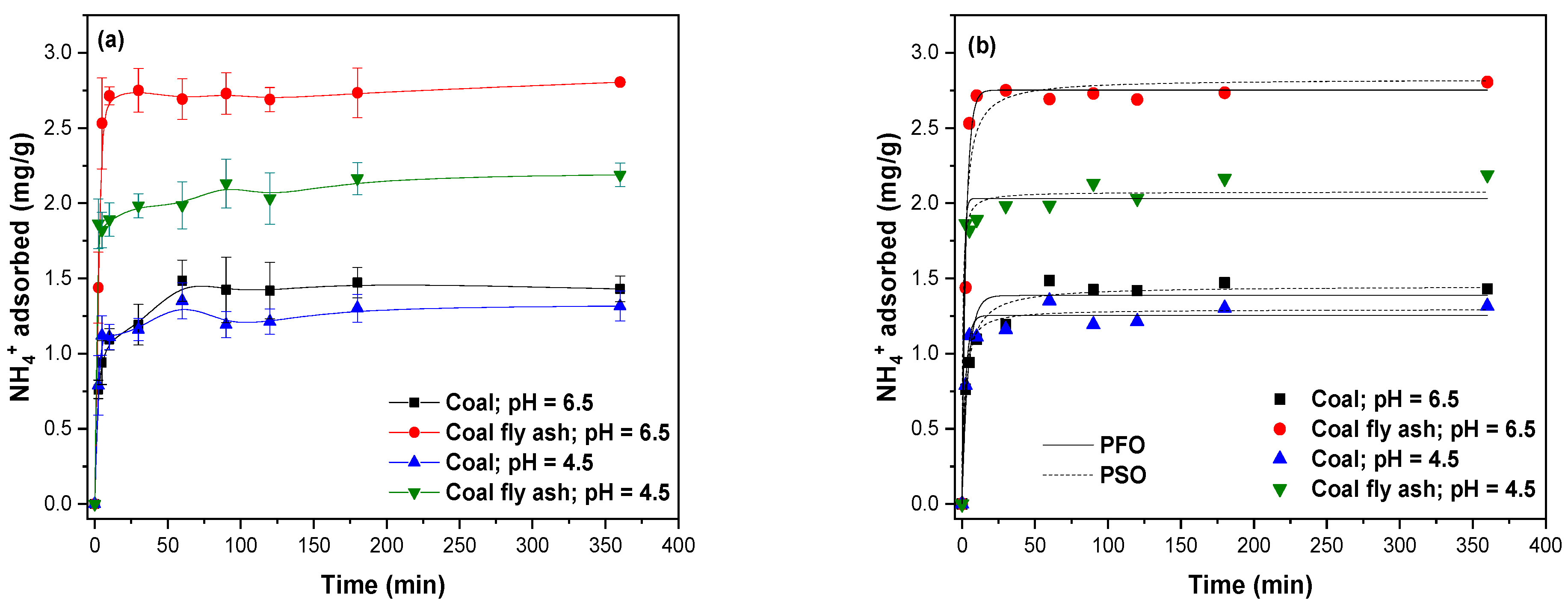
2.7.3. Adsorption Kinetics
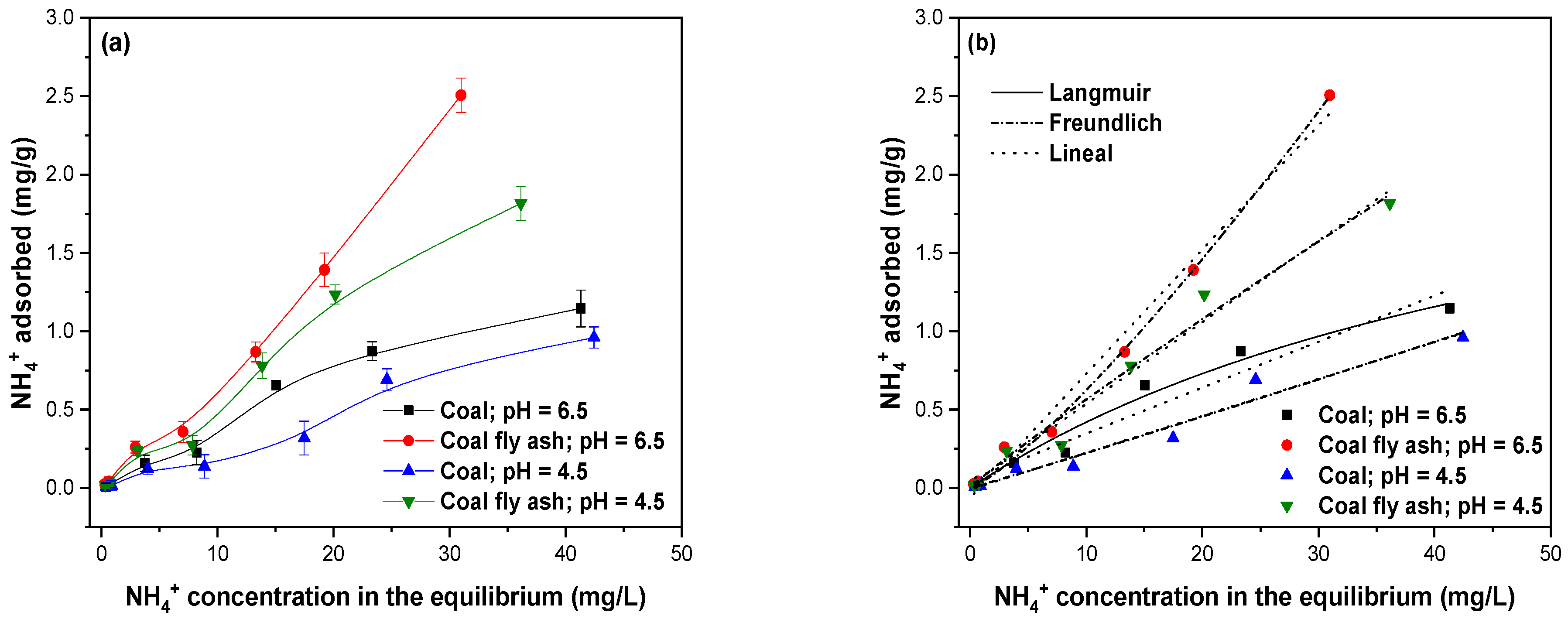
2.7.4. Adsorption Isotherms
2.7.5. Desorption Studies
2.7.6. Adsorption Kinetics and Isotherm Models
2.8. Data Analysis
3. Results and Discussion
3.1. Characterization of the Materials
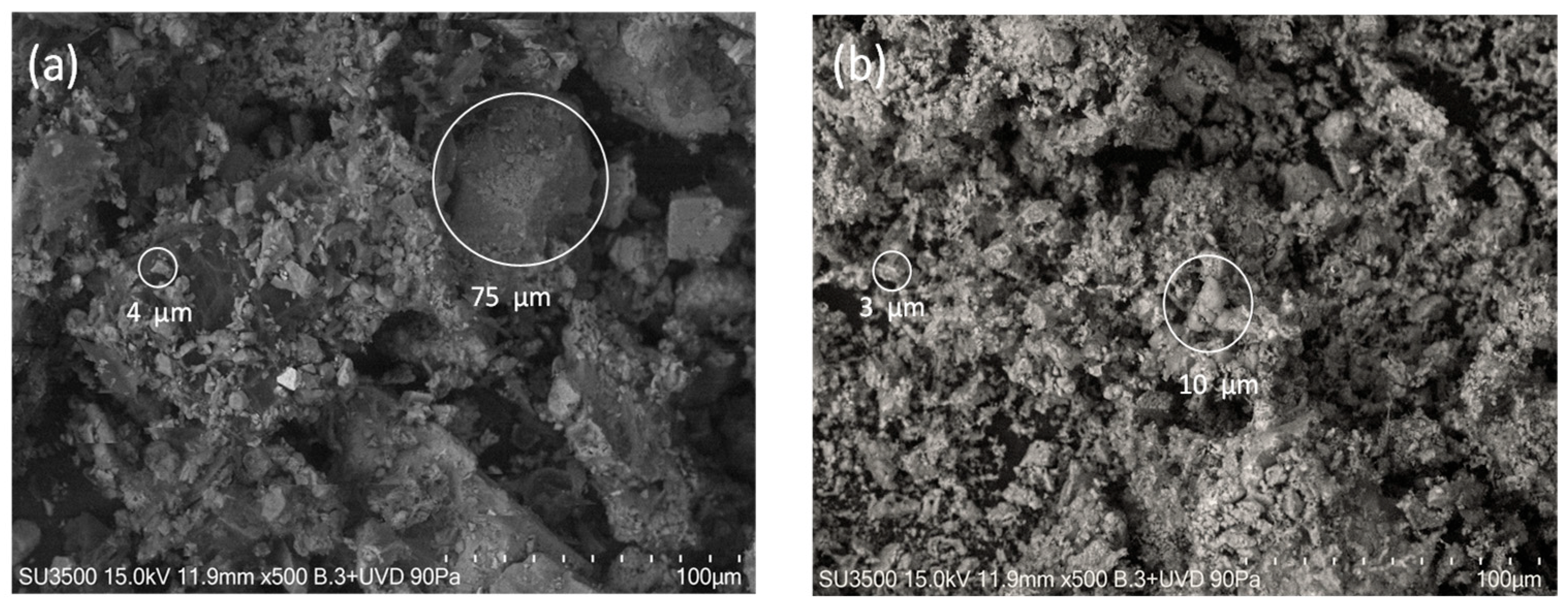
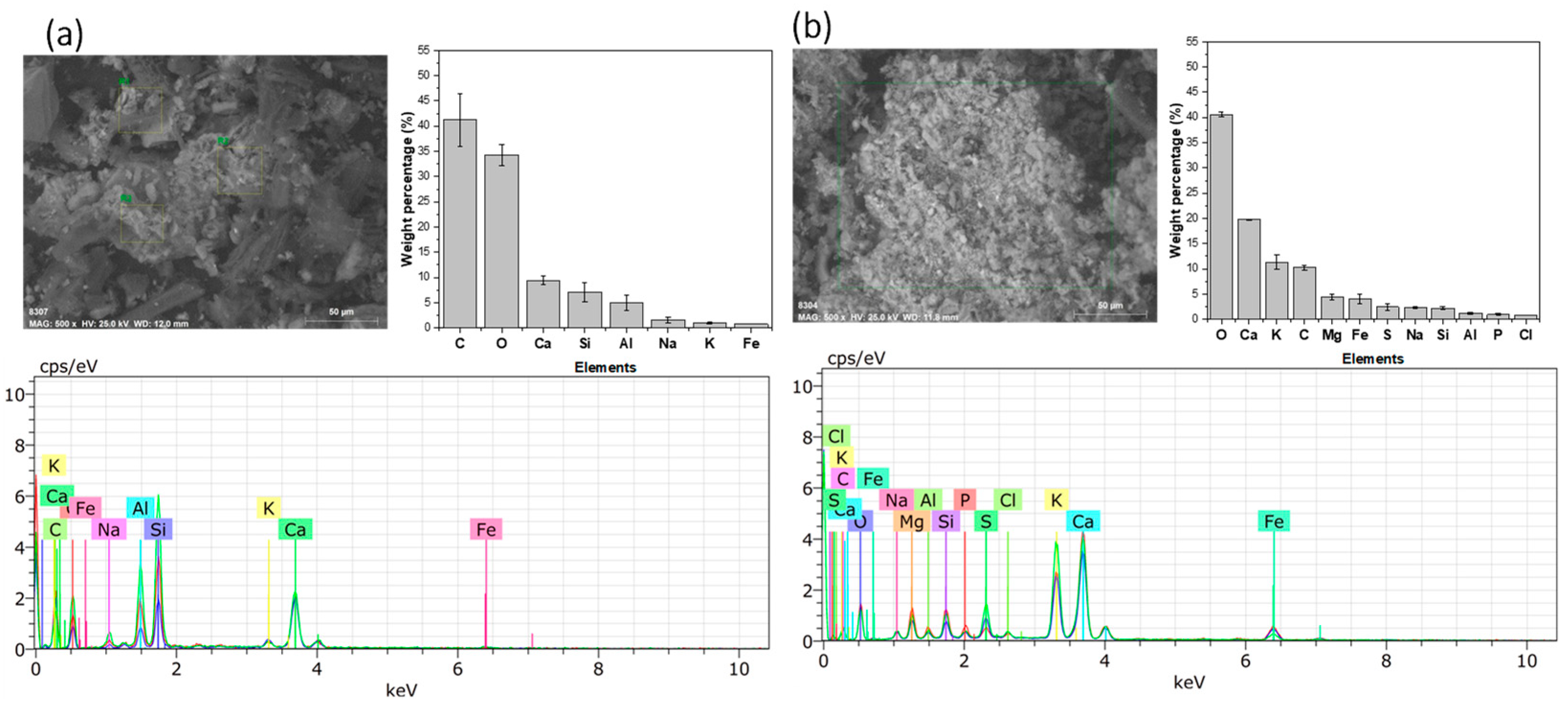
3.2. SEM-EDX Analysis
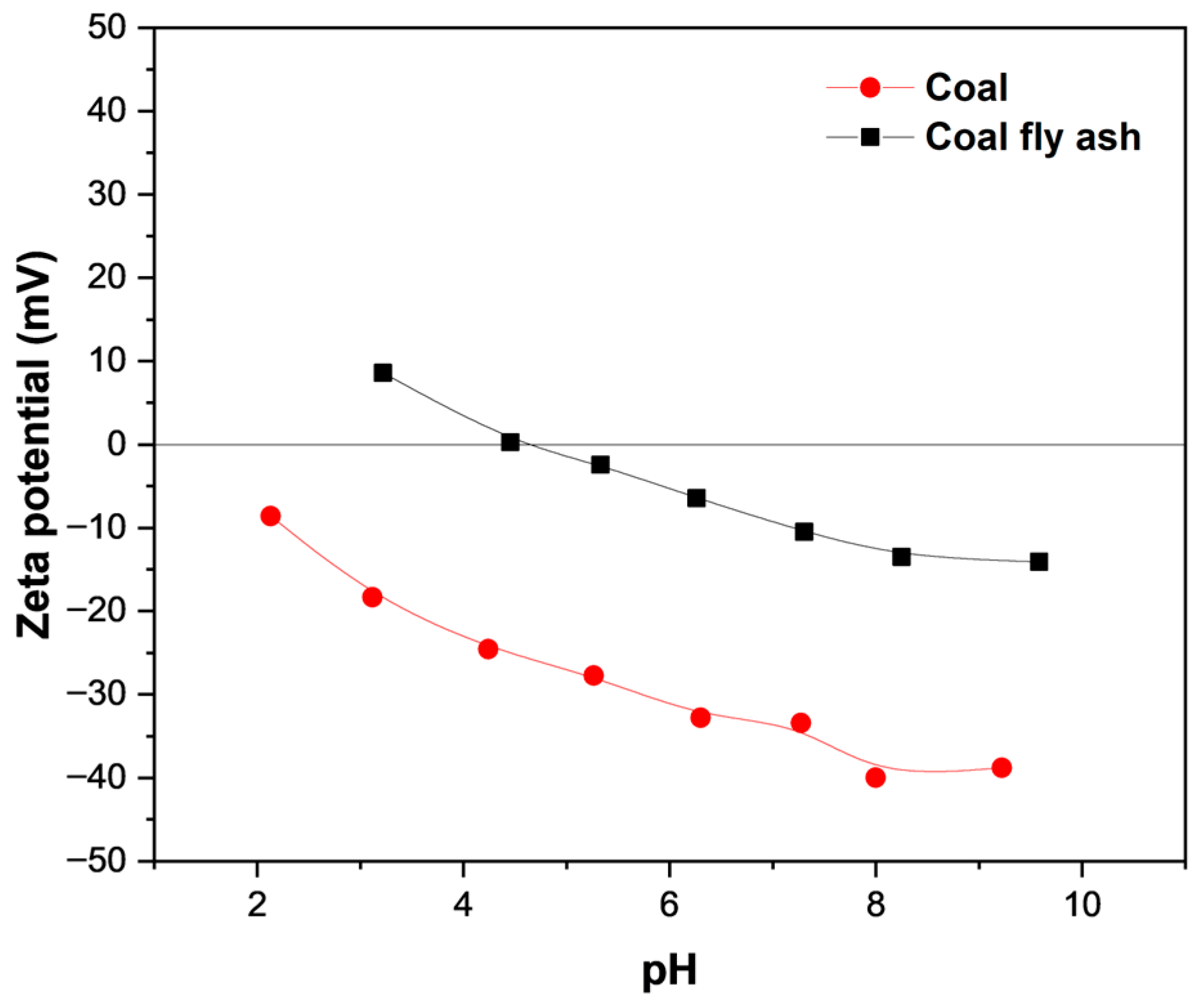
3.3. Zeta Potential
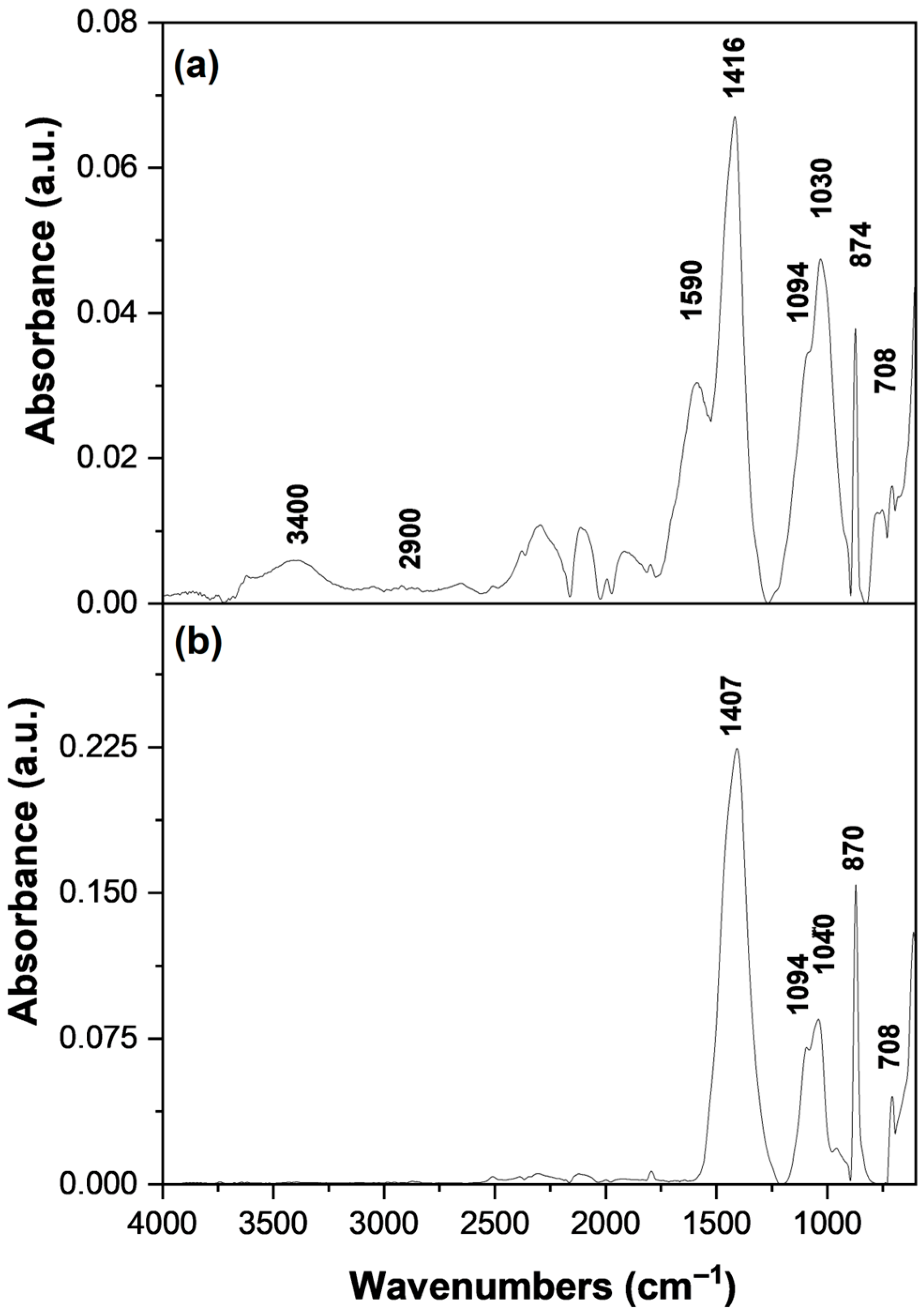
3.4. FT-IR Analysis
3.5. Effect of Adsorbent Dosage on NH4+ Removal
3.6. Effect of Solution pH on NH4+ Removal
3.7. Adsorption Kinetics of NH4+
3.8. Adsorption Isotherms of NH4+
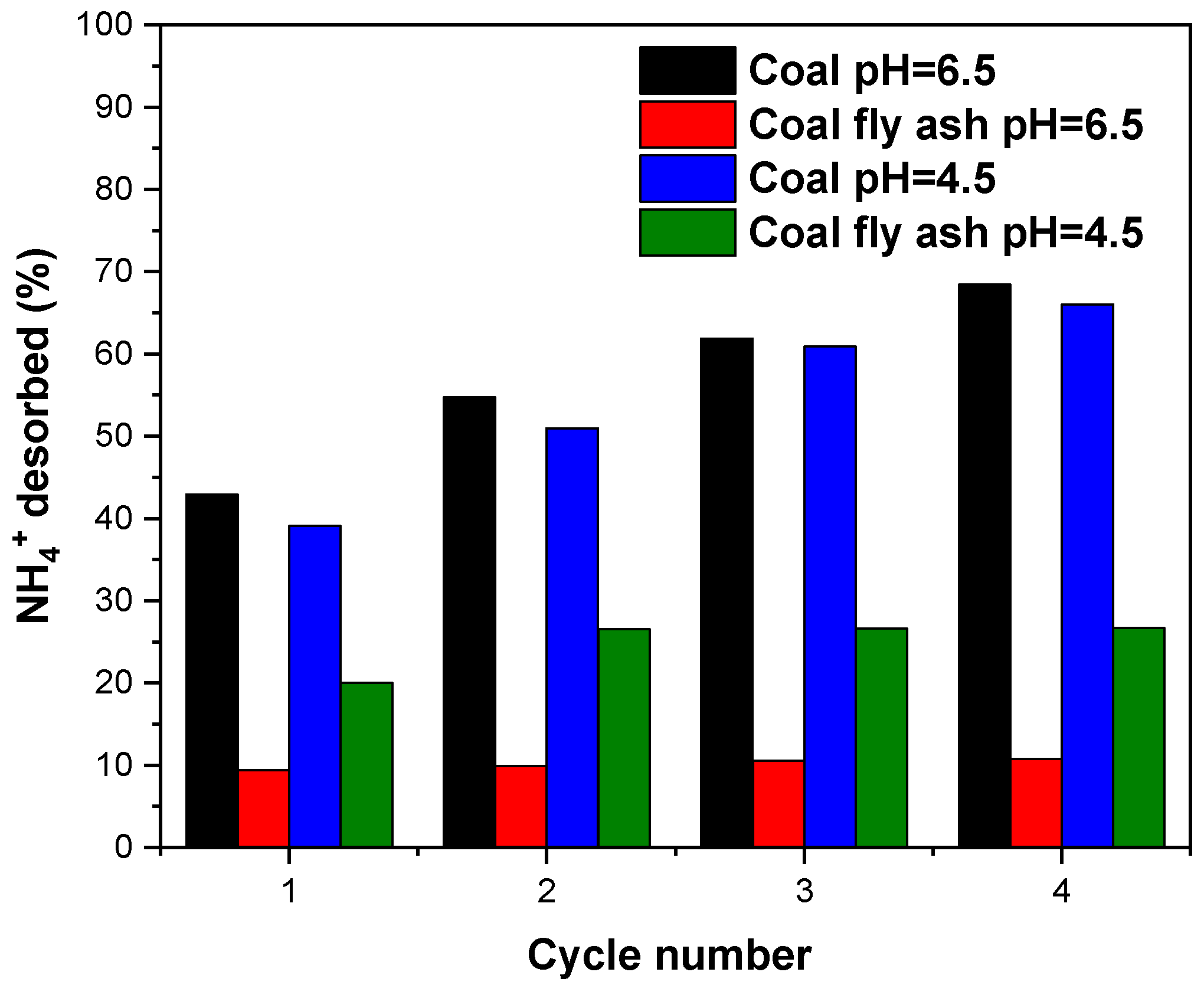
3.9. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V. Textbook of Environment and Ecology; Springer: Singapore, 2024; ISBN 9789819988457. [Google Scholar]
- Marañón, E.; Ulmanu, M.; Fernández, Y.; Anger, I.; Castrillón, L. Removal of Ammonium from Aqueous Solutions with Volcanic Tuff. J. Hazard. Mater. 2006, 137, 1402–1409. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia Toxicity in Fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Li, H.; Mei, X.; Nangia, V.; Guo, R.; Liu, Y.; Hao, W.; Wang, J. Effects of Different Nitrogen Fertilizers on the Yield, Water- and Nitrogen-Use Efficiencies of Drip-Fertigated Wheat and Maize in the North China Plain. Agric. Water Manag. 2021, 243, 106474. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current Trends and Future Prospects of Ammonia Removal in Wastewater: A Comprehensive Review on Adsorptive Membrane Development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Joseph, J.; Sajeesh, A.K.; Nagashri, K.; Gladis, E.H.E.; Sharmila, T.M.; Dhanaraj, C.J. Determination of Ammonia Content in Various Drinking Water Sources in Malappuram District, Kerala and Its Removal by Adsorption Using Agricultural Waste Materials. Mater. Today Proc. 2021, 45, 811–819. [Google Scholar] [CrossRef]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing Ammonium from Water Using Modified Corncob-Biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, J.; Zhang, Y.; Zhang, H.; Luo, Y.; Su, Y. Identification of Ammonium Source for Groundwater in the Piedmont Zone with Strong Runoff of the Hohhot Basin Based on Nitrogen Isotope. Sci. Total Environ. 2023, 882, 163650. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.A.; Rohman, A.; Ahsan, S.A.; Firmansyah, F.; Perdananugraha, G.M.; Rusydi, A.F. Evaluation of Ammonium Issues in Indonesian Groundwater: Potential Sources and Removal Methods. IOP Conf. Ser. Earth Environ. Sci. 2023, 1201, 012108. [Google Scholar] [CrossRef]
- Chen, S.K.; Lee, Y.Y.; Liao, T.L. Assessment of Ammonium–N and Nitrate–N Contamination of Shallow Groundwater in a Complex Agricultural Region, Central Western Taiwan. Water 2022, 14, 2130. [Google Scholar] [CrossRef]
- Buazar, H.; Larki, A.; Pourreza, N. Digital Colorimetric Detection of Ammonium in Water Samples after Microextraction Procedure Using Deep Eutectic Solvent, Based on DLLME Method. J. Mol. Liq. 2024, 404, 124938. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Pham, A. Bronchiectasis, a Long-Term Sequela of Ammonia Inhalation: A Case Report and Review of the Literature. Burns 2009, 35, 451–453. [Google Scholar] [CrossRef]
- Haralambous, A.; Maliou, E.; Malamis, M. The Use of Zeolite for Ammonium Uptake. Water Sci. Technol. 1992, 25, 139–145. [Google Scholar] [CrossRef]
- Atsbha, M.W.; Liu, R.; Nir, O. Circular Hybrid Membrane Process Treating High-Salinity Ammonium-Rich Pharmaceutical Wastewater. Chem. Eng. J. 2024, 497, 154690. [Google Scholar] [CrossRef]
- Jo, J.Y.; Kim, J.G.; Tsang, Y.F.; Baek, K. Removal of Ammonium, Phosphate, and Sulfonamide Antibiotics Using Alum Sludge and Low-Grade Charcoal Pellets. Chemosphere 2021, 281, 130960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, X.; Song, X.; Cao, X.; Xu, Z.; Wang, Y.; Li, J.; Wu, N.; Bai, J. Manganese Oxide–Loaded Activated Carbon for Ammonium Removal from Wastewater: The Roles of Adsorption and Oxidation. Environ. Sci. Pollut. Res. 2023, 30, 110161–110174. [Google Scholar] [CrossRef]
- Amanollahi, H.; Moussavi, G.; Ostovar, S.; Giannakis, S. From Waste to Wealth: Using MgO Nanoparticles to Transform Ammonium into a Valuable Resource. J. Water Process Eng. 2023, 56, 104331. [Google Scholar] [CrossRef]
- Zare, K.; Sadegh, H.; Shahryari-Ghoshekandi, R.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Equilibrium and Kinetic Study of Ammonium Ion Adsorption by Fe3O4 Nanoparticles from Aqueous Solutions. J. Mol. Liq. 2016, 213, 345–350. [Google Scholar] [CrossRef]
- Vu, T.M.; Nguyen, T.M.P.; Van, H.T.; Hoang, V.H.; Nga, L.T.Q.; Hoang, L.P.; Ravindran, B. High Removal Efficiency of Ammonium from Aqueous Solution by Colloidal Silver Nanoparticles: Batch Adsorption. Urban Water J. 2024, 21, 323–336. [Google Scholar] [CrossRef]
- Bellahsen, N.; Varga, G.; Halyag, N.; Kertész, S.; Tombácz, E.; Hodúr, C. Pomegranate Peel as a New Low-Cost Adsorbent for Ammonium Removal. Int. J. Environ. Sci. Technol. 2021, 18, 711–722. [Google Scholar] [CrossRef]
- Affandi, K.A.; Bagastyo, A.Y.; Fitriana, A.R. View of Removal of Ammonium and Phosphate in the Simulated Wastewater by Using Coal Fly Ash Adsorbent. Sustinere J. Environ. Sustain. 2021, 5, 24–35. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Zhao, R.; Wang, X.; Pan, C.; Jiang, Y.; Zhang, X.; Ge, B. Adsorption Behavior and Performance of Ammonium onto Sorghum Straw Biochar from Water. Sci. Rep. 2022, 12, 5358. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Paramisparam, P.; Musah, A.A.; Jalloh, M.B. Charcoal and Sago Bark Ash Regulates Ammonium Adsorption and Desorption in an Acid Soil. Sustainability 2023, 15, 1368. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Cai, Z. Dual Role of Coal Fly Ash in Copper Ion Adsorption Followed by Thermal Stabilization in a Spinel Solid Solution. RSC Adv. 2018, 8, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, H.; Guo, Y.; Wang, L.; Cheng, F. Converting Waste Coal Fly Ash into Effective Adsorbent for the Removal of Ammonia Nitrogen in Water. J. Mater. Sci. 2018, 53, 12731–12740. [Google Scholar] [CrossRef]
- Serra, M.T. Especies Arbóreas y Arbustivas Para Las Zonas Aridas y Semiáridas de América Latina. In Red Latinoamericana de Cooperación Técnica en Sistemas Agroforestales; FAO: Santiago, Chile, 1997. [Google Scholar]
- Cruz, J.P.; Schulze, D.C.C.; Honeyman, P. Chilean Mediterranean Forest, Their Values and Destiny Facing Global Change. In Forest Management of Mediterranean Forest Under the New Context of Climate Change; Universidad Mayor: Santiago, Chile, 2005; pp. 78–88. ISBN 9781624178689. [Google Scholar]
- Sadzawka, R.A.; Carrasco, R.A.M.; Grez, Z.R.; De La Luz Mora, G.M. Métodos de Análisis de Compost; Comisión de Normalización y Acreditación (CNA); Sociedad Chilena de La Ciencia Del Suelo: Santiago, Chile, 2005. [Google Scholar]
- Liu, Y.; Miao, J.; Han, H.; Xu, P. Differences in Influence of Particle Size on the Adsorption Capacity between Deformed and Undeformed Coal. ACS Omega 2021, 6, 5886–5897. [Google Scholar] [CrossRef]
- Batra, V.S.; Urbonaite, S.; Svensson, G. Characterization of Unburned Carbon in Bagasse Fly Ash. Fuel 2008, 87, 2972–2976. [Google Scholar] [CrossRef]
- Koch, F.C.; McMeekin, T.L. A New Direct Nesslerization Micro-Kjeldahl Method and a Modification of the Nessler-Folin Reagent for Ammonia. J. Am. Chem. Soc. 1924, 46, 2066–2069. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Klumpp, E.; Arancibia-Miranda, N.; Poblete-Grant, P.; Jara, A.; Bol, R.; de La Luz Mora, M. Describing Phosphorus Sorption Processes on Volcanic Soil in the Presence of Copper or Silver Engineered Nanoparticles. Minerals 2021, 11, 373. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Acharath Mohanakrishnan, A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, Chemical, and Geotechnical Properties of Coal Fly Ash: A Global Review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Gaffney, J.S.; Marley, N.A. The Impacts of Combustion Emissions on Air Quality and Climate—From Coal to Biofuels and Beyond. Atmos. Environ. 2009, 43, 23–36. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zevenhoven, R.; Bhattacharya, P.; Sajwan, K.S.; Kikuchi, R. Mercury Flow via Coal and Coal Utilization By-Products: A Global Perspective. Resour. Conserv. Recycl. 2008, 52, 571–591. [Google Scholar] [CrossRef]
- Silva-Yumi, J.; Escudey, M.; Gacitua, M.; Pizarro, C. Kinetics, Adsorption and Desorption of Cd(II) and Cu(II) on Natural Allophane: Effect of Iron Oxide Coating. Geoderma 2018, 319, 70–79. [Google Scholar] [CrossRef]
- Can, Ö.; Baklacioglu, T.; Özturk, E.; Turan, O. Artificial Neural Networks Modeling of Combustion Parameters for a Diesel Engine Fueled with Biodiesel Fuel. Energy 2022, 247, 123473. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, S.; Longhurst, P.; Yang, W.; Zheng, S. Molecular Structure Characterization of Bituminous Coal in Northern China via XRD, Raman and FTIR Spectroscopy. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 255, 119724. [Google Scholar] [CrossRef] [PubMed]
- da Silva Veiga, P.A.; Cerqueira, M.H.; Gonçalves, M.G.; da Silva Matos, T.T.; Pantano, G.; Schultz, J.; de Andrade, J.B.; Mangrich, A.S. Upgrading from Batch to Continuous Flow Process for the Pyrolysis of Sugarcane Bagasse: Structural Characterization of the Biochars Produced. J. Environ. Manag. 2021, 285, 12145. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of Sewage Sludge Properties on the Biochar Characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; MacQuarrie, S.; Hawboldt, K. Study of Surface Heterogeneity and Nitrogen Functionalizing of Biochars: Molecular Modeling Approach. Carbon N. Y. 2021, 171, 161–170. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar Bound Urea Boosts Plant Growth and Reduces Nitrogen Leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and Characterization of Coal Fly Ash and Palm Oil Fuel Ash Modified Artisanal and Small-Scale Gold Mine (ASGM) Tailings Based Geopolymer Using Sugar Mill Lime Sludge as Ca-Based Activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; He, Z.; Wei, J.; Ren, S.; Wu, W. Structural Evolution Characteristics of Lignite during Pyrolysis Based on Alkaline-Oxygen Oxidation, NMR and FTIR. J. Anal. Appl. Pyrolysis 2023, 172, 105980. [Google Scholar] [CrossRef]
- Nair, R.R.; Mondal, M.M.; Weichgrebe, D. Biochar from Co-Pyrolysis of Urban Organic Wastes—Investigation of Carbon Sink Potential Using ATR-FTIR and TGA. Biomass Convers. Biorefinery 2022, 12, 4729–4743. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of Chemical Functional Groups in Macerals across Different Coal Ranks via Micro-FTIR Spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Meng, D.; Yue, C.; Wang, T.; Chen, X. Evolution of Carbon Structure and Functional Group during Shenmu Lump Coal Pyrolysis. Fuel 2021, 287, 119538. [Google Scholar] [CrossRef]
- Zhao, Y.; Luan, H.; Yang, B.; Li, Z.; Song, M.; Li, B.; Tang, X. Adsorption of Low-Concentration Ammonia Nitrogen from Water on Alkali-Modified Coal Fly Ash: Characterization and Mechanism. Water 2023, 15, 956. [Google Scholar] [CrossRef]
- Xia, L.; Cao, J.; Stüeken, E.E.; Zhi, D.; Wang, T.; Li, W. Unsynchronized Evolution of Salinity and PH of a Permian Alkaline Lake Influenced by Hydrothermal Fluids: A Multi-Proxy Geochemical Study. Chem. Geol. 2020, 541, 119581. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Yang, F.; Long, D.; Jian, Y.; Pu, S. The Preparation of {001}TiO2/TiOF2 via a One-Step Hydrothermal Method and Its Degradation Mechanism of Ammonia Nitrogen. Materials 2022, 15, 6465. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Musyoka, N.; Dladla, B.S.; Nxumalo, E.N.; Mhlanga, S.D. Microwave-Assisted Synthesis of Coal Fly Ash-Based Zeolites for Removal of Ammonium from Urine. RSC Adv. 2020, 10, 2416–2427. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; Carroll, D.M.O. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nano Zerovalent Iron Particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Shaheen, U.; Ye, Z.L.; Abass, O.K.; Zamel, D.; Rehman, A.; Zhao, P.; Huang, F. Evaluation of Potential Adsorbents for Simultaneous Adsorption of Phosphate and Ammonium at Low Concentrations. Microporous Mesoporous Mater. 2024, 379, 113301. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.L.; Xiong, J.W.; Hong, C.; Hu, H.Q.; Violante, A. Adsorption of Phosphate onto Ferrihydrite and Ferrihydrite-Humic Acid Complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of Ammonium by Different Natural Clay Minerals: Characterization, Kinetics and Adsorption Isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Aghoghovwia, M.P.; Hardie, A.G.; Rozanov, A.B. Characterisation, Adsorption and Desorption of Ammonium and Nitrate of Biochar Derived from Different Feedstocks. Environ. Technol. 2022, 43, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Suazo-Hernández, J.; Sepúlveda, P.; Manquián-Cerda, K.; Ramírez-Tagle, R.; Rubio, M.A.; Bolan, N.; Sarkar, B.; Arancibia-Miranda, N. Synthesis and Characterization of Zeolite-Based Composites Functionalized with Nanoscale Zero-Valent Iron for Removing Arsenic in the Presence of Selenium from Water. J. Hazard. Mater. 2019, 373, 810–819. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Urdiales, C.; Poblete-Grant, P.; Pesenti, H.; Cáceres-Jensen, L.; Sarkar, B.; Bolan, N.; de la Luz Mora, M. Effect of Particle Size of Nanoscale Zero–Valent Copper on Inorganic Phosphorus Adsorption–Desorption in a Volcanic Ash Soil. Chemosphere 2023, 340, 139836. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Mlih, R.; Bustamante, M.; Castro-Castillo, C.; Mora, M.d.l.L.; Sepúlveda-Parada, M.d.l.Á.; Mella, C.; Cornejo, P.; Ruiz, A. Immobilization of Inorganic Phosphorus on Soils by Zinc Oxide Engineered Nanoparticles. Toxics 2025, 13, 363. [Google Scholar] [CrossRef]
- Do, T.T.; Van, H.T.; Pham, Q.H.; Nguyen, T.H. Adsorption of Ammonium from Aqueous Solution Using Coffee Husk-Derived Activated Charcoal Composite with Fe3O4. Results Surf. Interfaces 2025, 18, 100471. [Google Scholar] [CrossRef]
- Jiang, Q.; He, J.; Wang, Y.; Chen, B.; Tian, K.; Yang, K.; Wei, H.; Xu, X. Efficient Removal of Ammonia–Nitrogen in Wastewater by Zeolite Molecular Sieves Prepared from Coal Fly Ash. Sci. Rep. 2024, 14, 21064. [Google Scholar] [CrossRef]
- Godifredo, J.; Ferrer, J.; Seco, A.; Barat, R. Zeolites for Nitrogen Recovery from the Anaerobic Membrane Bioreactor Permeate: Zeolite Characterization. Water 2023, 15, 1007. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Li, W.; Xu, X.; Zhong, Q. Adsorption Removal of Ammonium and Phosphate from Water by Fertilizer Controlled Release Agent Prepared from Wheat Straw. Chem. Eng. J. 2011, 171, 1209–1217. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium Biosorption onto Sawdust: FTIR Analysis, Kinetics and Adsorption Isotherms Modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Adsorption of Ammonium Ion by Coconut Shell-Activated Carbon from Aqueous Solution: Kinetic, Isotherm, and Thermodynamic Studies. Environ. Sci. Pollut. Res. 2013, 20, 533–542. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, T.; Shi, X.; Wen, G.; Sun, Y. Removal of Ammonium Ion from Water by Na-Rich Birnessite: Performance and Mechanisms. J. Environ. Sci. 2017, 57, 402–410. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of Ammonia Nitrogen in Low Temperature Domestic Wastewater by Modification Bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]
| Coal | Coal Fly Ash | |
|---|---|---|
| pHH2O (1:5) | 8.4 | 11.9 |
| Electrical conductivity (1:5) dS/m | 1.2 | 37.1 |
| Humidity (%) | 4.70 | 0.60 |
| Organic matter (%) | 90.50 | 1.68 |
| Total nitrogen (%) | 1.10 | 0.06 |
| NH4+ (mg/kg) | 9.20 | 1.10 |
| P2O5 (%) | 0.30 | 2.37 |
| K2O (%) | 1.45 | 8.80 |
| CaO (%) | 3.89 | 27.00 |
| MgO (%) | 0.18 | 4.81 |
| BET specific surface area (m2/g) | 4.409 | 5.411 |
| Pore radius (Å) | 17.66 | 18.72 |
| Pore volume (g/cm3) | 0.0138 | 0.0126 |
| Coal | Coal Fly Ash | Coal | Coal Fly Ash | |
|---|---|---|---|---|
| pH | 4.5 | 6.5 | ||
| qe (%) | 20.35 | 33.48 | 23.76 | 44.67 |
| qexp (mg/g) | 1.39 ± 0.10 | 2.19 ± 0.05 | 1.54 ± 0.03 | 2.79 ± 0.18 |
| Pseudo-first-order | ||||
| qe (mg/g) | 1.24 ± 0.03 | 2.03 ± 0.04 | 1.39 ± 0.05 | 2.75 ± 0.04 |
| k1 (1/min) | 0.409 ± 0.059 | 0.913 ± 0.243 | 0.238 ± 0.04 | 0.361 ± 0.03 |
| r2 | 0.965 | 0.970 | 0.956 | 0.986 |
| χ2 | 0.008 | 0.014 | 0.013 | 0.013 |
| Pseudo-second-order | ||||
| qe (mg/g) | 1.30 ± 0.03 | 2.08 ± 0.04 | 1.45 ± 0.03 | 2.83 ± 0.09 |
| k2 (g/mg/min) | 0.565 ± 0.140 | 1.128 ± 0.402 | 0.259 ± 0.04 | 0.247 ± 0.07 |
| h (mg/g/min) | 0.988 ± 0.000 | 4.88 ± 0.00 | 0.544 ± 0.00 | 1.978 ± 0.000 |
| r2 | 0.970 | 0.983 | 0.981 | 0.951 |
| χ2 | 0.006 | 0.008 | 0.005 | 0.041 |
| Coal | Coal Fly Ash | Coal | Coal Fly Ash | |
|---|---|---|---|---|
| pH | 4.5 | 6.5 | ||
| Langmuir | ||||
| qmax (mg/g) | N/A | N/A | 2.79 ± 0.85 | N/A |
| kL | N/A | N/A | 0.018 ± 0.008 | N/A |
| r2 | N/A | N/A | 0.978 | N/A |
| χ2 | N/A | N/A | 0.005 | N/A |
| Freundlich | ||||
| kF (mg/g) | 0.021 ± 0.011 | 0.066 ± 0.022 | 0.070 ± 0.025 | 0.037 ± 0.007 |
| n | 0.97 ± 0.140 | 1.07 ± 0.12 | 1.30 ± 0.18 | 0.82 ± 0.04 |
| r2 | 0.965 | 0.979 | 0.966 | 0.996 |
| χ2 | 0.006 | 0.012 | 0.008 | 0.004 |
| Linear | ||||
| K (L/g) | 0.024 ± 0.002 | 0.052 ± 0.004 | 0.029 ± 0.03 | 0.079 ± 0.004 |
| r2 | 0.966 | 0.977 | 0.943 | 0.986 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suazo-Hernández, J.; Burgos, N.; Sepúlveda-Parada, M.d.L.Á.; Castro-Rojas, J.; Poblete-Grant, P.; Castro-Castillo, C.; Mlih, R.; Urdiales, C.; Schoffer, T.; Joseph, C.G.; et al. Adsorption of Ammonium by Coal and Coal Fly Ash Derived from Hawthorn Tree from Aquatic Systems. Processes 2025, 13, 3118. https://doi.org/10.3390/pr13103118
Suazo-Hernández J, Burgos N, Sepúlveda-Parada MdLÁ, Castro-Rojas J, Poblete-Grant P, Castro-Castillo C, Mlih R, Urdiales C, Schoffer T, Joseph CG, et al. Adsorption of Ammonium by Coal and Coal Fly Ash Derived from Hawthorn Tree from Aquatic Systems. Processes. 2025; 13(10):3118. https://doi.org/10.3390/pr13103118
Chicago/Turabian StyleSuazo-Hernández, Jonathan, Nicol Burgos, María de Los Ángeles Sepúlveda-Parada, Jorge Castro-Rojas, Patricia Poblete-Grant, Carmen Castro-Castillo, Rawan Mlih, Cristian Urdiales, Tomás Schoffer, Collin G. Joseph, and et al. 2025. "Adsorption of Ammonium by Coal and Coal Fly Ash Derived from Hawthorn Tree from Aquatic Systems" Processes 13, no. 10: 3118. https://doi.org/10.3390/pr13103118
APA StyleSuazo-Hernández, J., Burgos, N., Sepúlveda-Parada, M. d. L. Á., Castro-Rojas, J., Poblete-Grant, P., Castro-Castillo, C., Mlih, R., Urdiales, C., Schoffer, T., Joseph, C. G., & Ruiz, A. (2025). Adsorption of Ammonium by Coal and Coal Fly Ash Derived from Hawthorn Tree from Aquatic Systems. Processes, 13(10), 3118. https://doi.org/10.3390/pr13103118











