Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Standards
2.3. Extraction of Sugars and Acids
2.4. Determination of Organic Acids
2.5. Determination of Sugar Content
2.6. Statistical Analysis
3. Results and Discussion
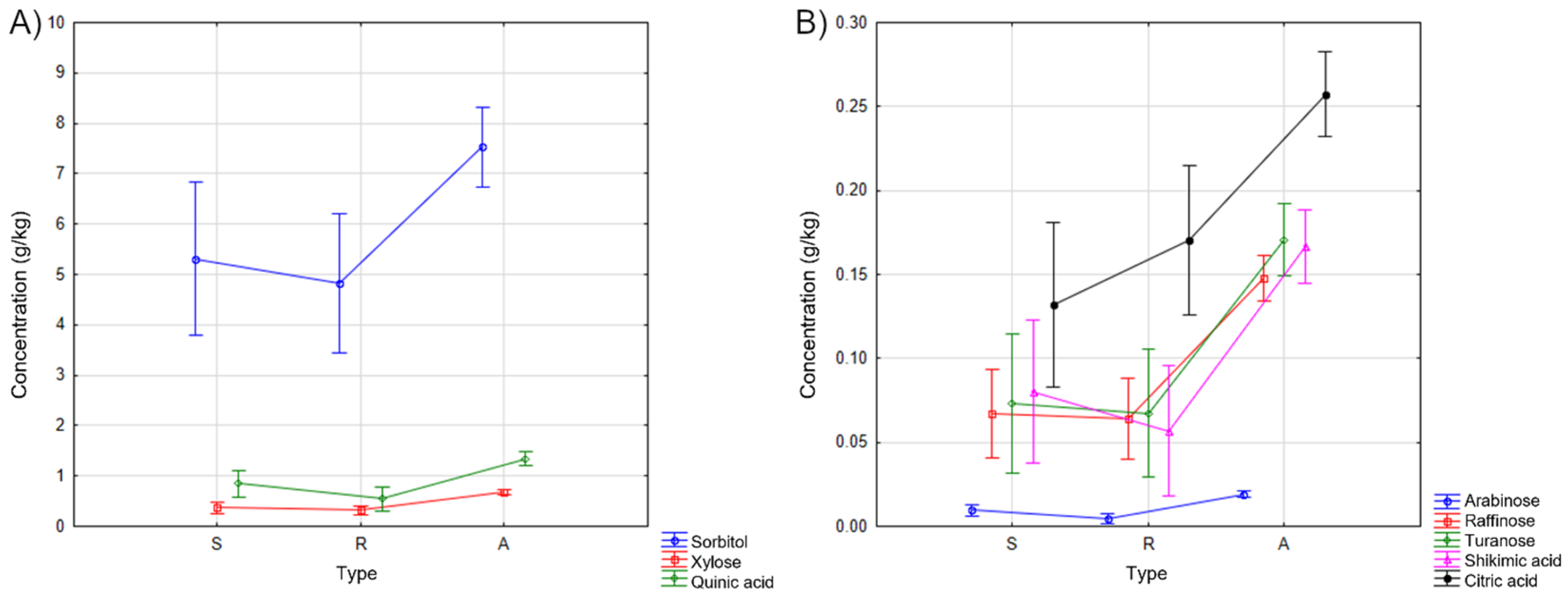
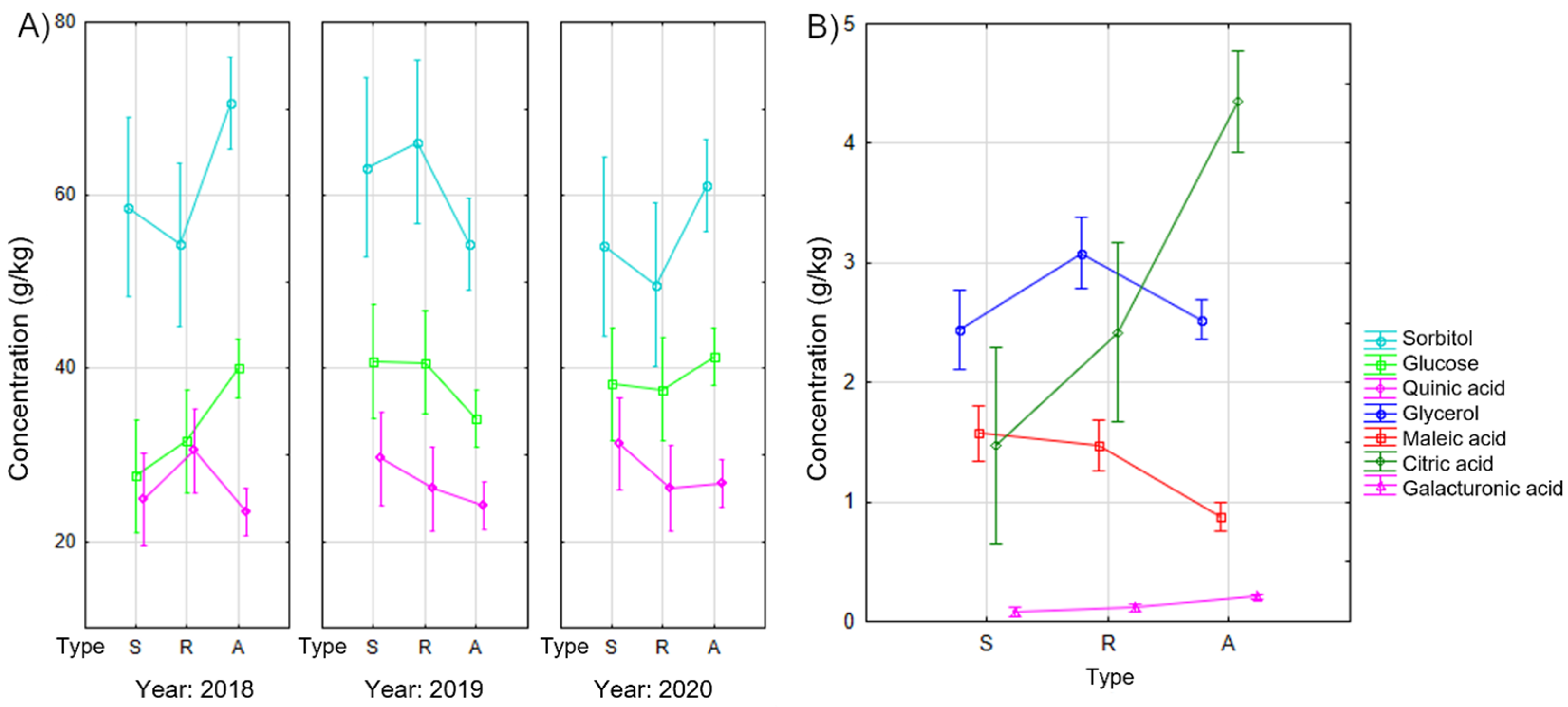
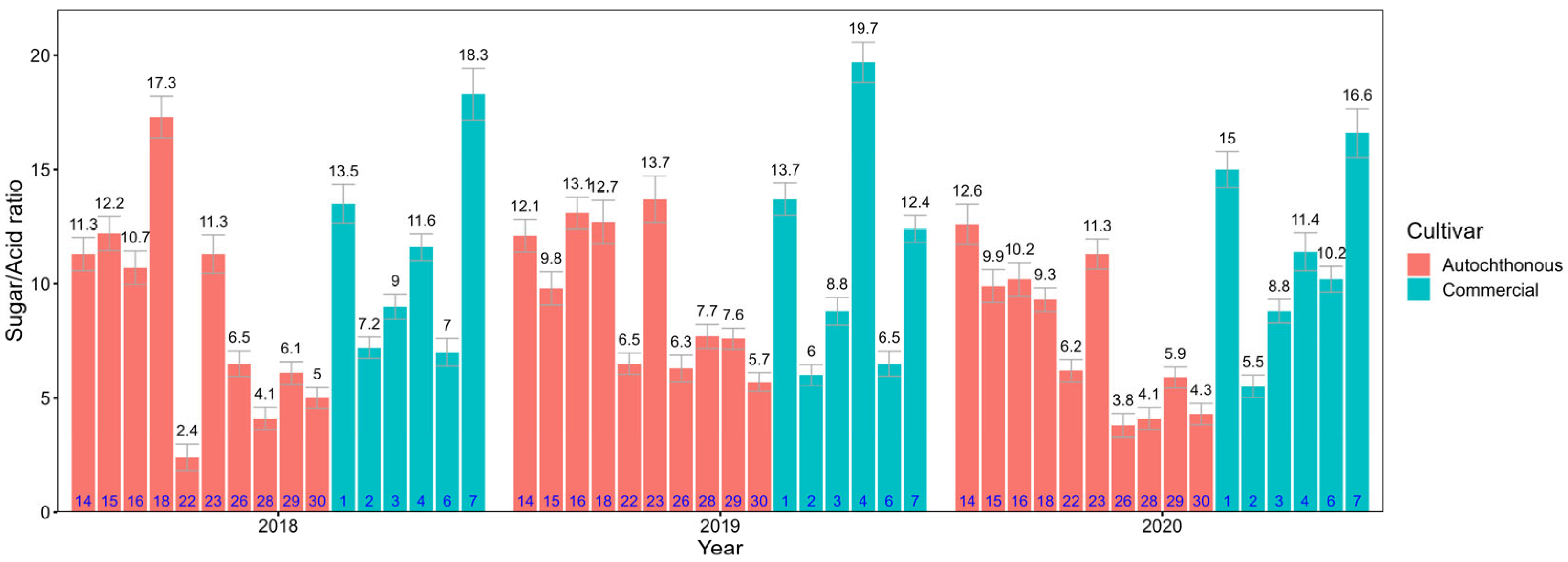
3.1. Sugar and Organic Acid Content in Apple Fruit
3.2. Sugar and Organic Acid Content in Apple Leaves
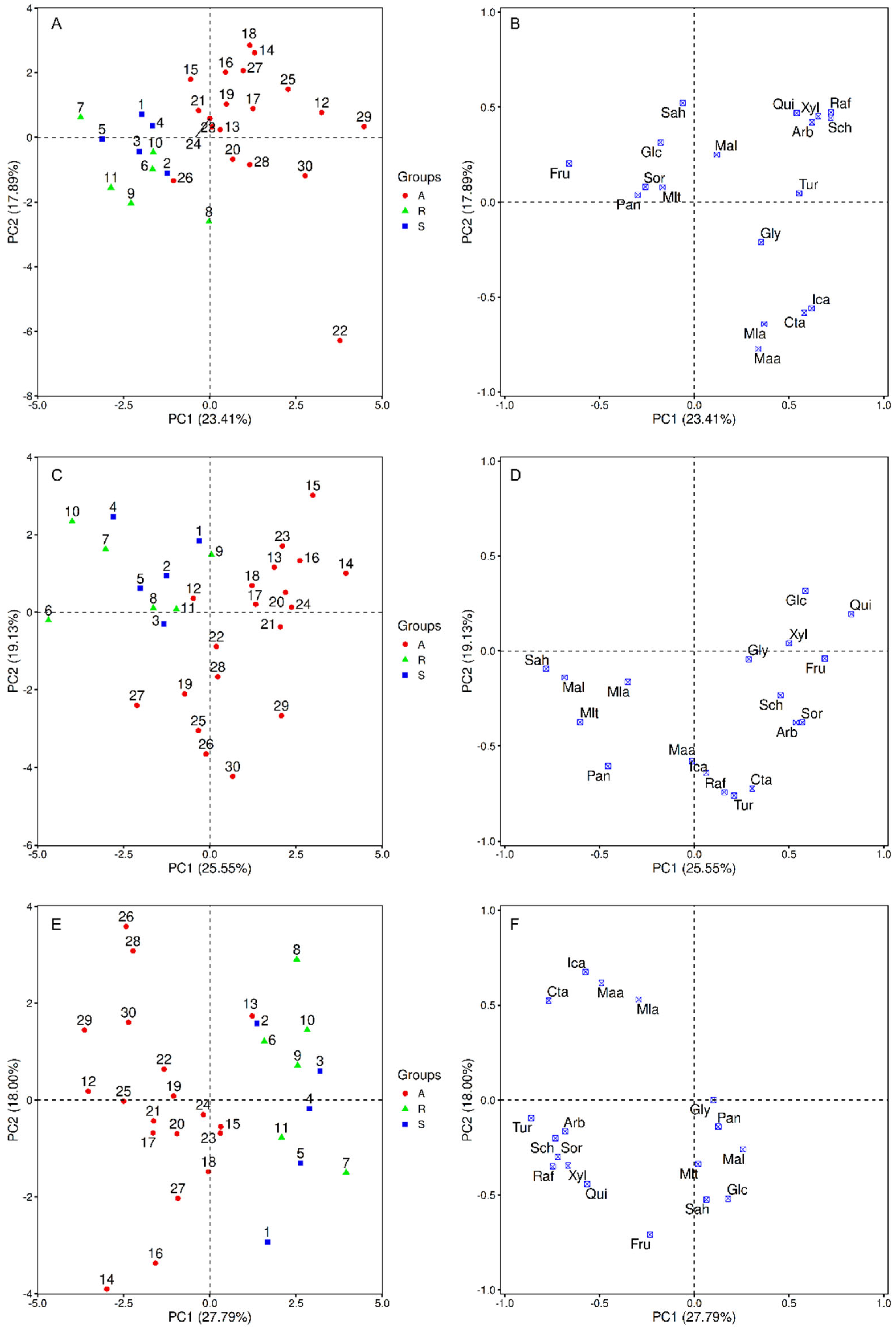
3.3. Multivariate Analysis of Variance (MANOVA)
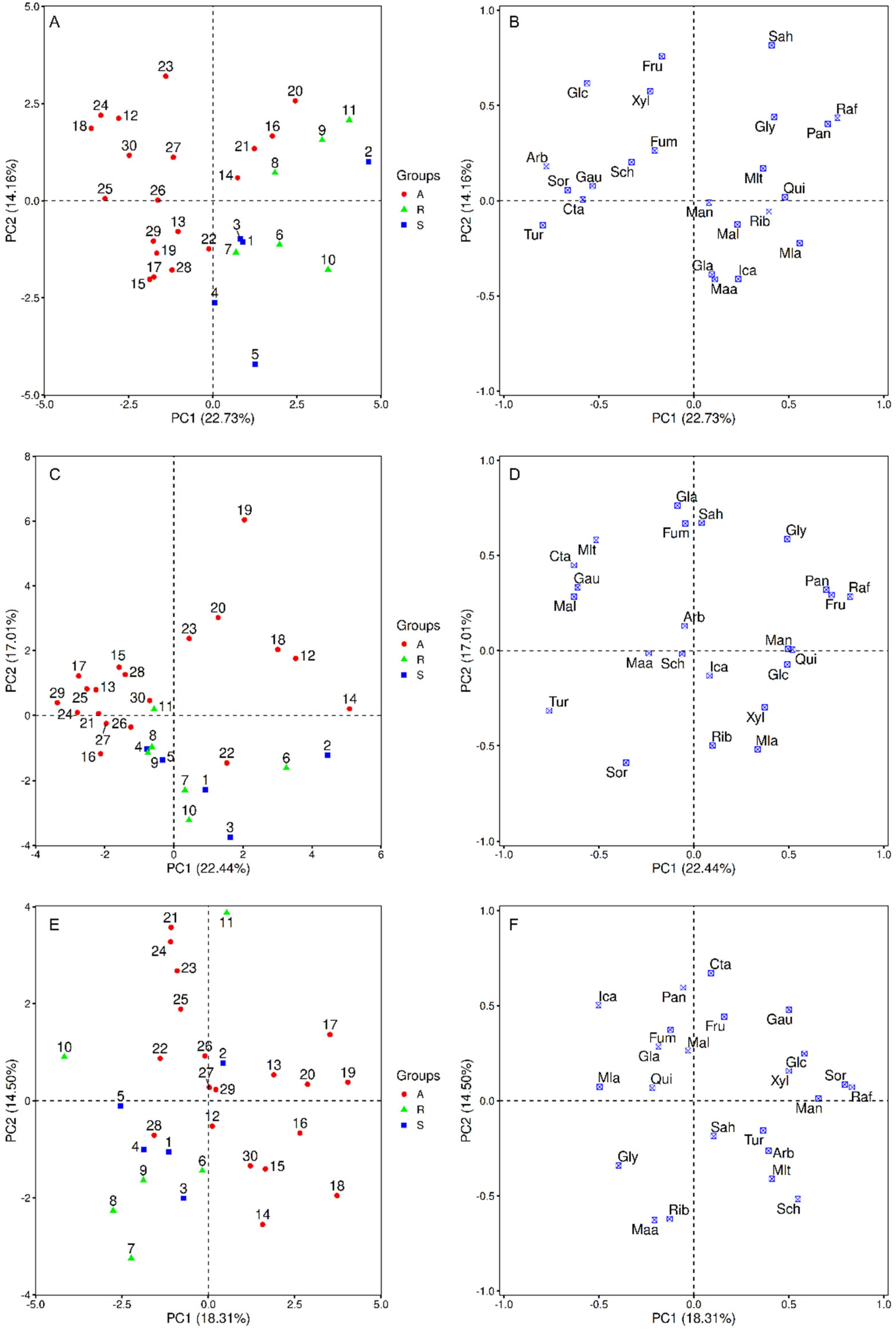
3.4. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Musacchi, S.; Serra, S. Apple Fruit Quality: Overview on Pre-Harvest Factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess. Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding Apple (Malus x Domestica Borkh). In Breeding Plantation Tree Crops: Temperate Species; Springer: New York, NY, USA, 2009; pp. 33–81. ISBN 978-0-387-71202-4. [Google Scholar]
- Tschida, A.; Stadlbauer, V.; Schwarzinger, B.; Maier, M.; Pitsch, J.; Stübl, F.; Müller, U.; Lanzerstorfer, P.; Himmelsbach, M.; Wruss, J.; et al. Nutrients, Bioactive Compounds, and Minerals in the Juices of 16 Varieties of Apple (Malus domestica) Harvested in Austria: A Four-Year Study Investigating Putative Correlations with Weather Conditions during Ripening. Food Chem. 2021, 338, 128065. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, N.; Varming, C.; Agerlin Petersen, M.; Viereck, N.; Schütz, B.; Toldam-Andersen, T.B.; Randazzo, A.; Balling Engelsen, S. Ancient Danish Apple Cultivars—A Comprehensive Metabolite and Sensory Profiling of Apple Juices. Metabolites 2019, 9, 139. [Google Scholar] [CrossRef]
- Meland, M.; Aksic, M.F.; Frøynes, O.; Konjic, A.; Lasic, L.; Pojskic, N.; Gasi, F. Genetic Identity and Diversity of Apple Accessions within a Candidate Collection for the Norwegian National Clonal Germplasm Repository. Horticulturae 2022, 8, 630. [Google Scholar] [CrossRef]
- Mratinić, E.; Fotirić Akšić, M. Evaluation of Phenotipyc Diversity of Apple (Malus Sp.) GermplasmThrough the Principle Component Analysis. Genetika 2011, 43, 331–340. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The Influence of Organic/Integrated Production on the Content of Phenolic Compounds in Apple Leaves and Fruits in Four Different Varieties over a 2-Year Period: Organic/Integrated Production and Content of Phenolic Compounds in Apple. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- Bazoche, P.; Combris, P.; Giraud-Heraud, E.; Seabra Pinto, A.; Bunte, F.; Tsakiridou, E. Willingness to Pay for Pesticide Reduction in the EU: Nothing but Organic? Eur. Rev. Agric. Econ. 2014, 41, 87–109. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Natić, M.; Dabić Zagorac, D.; Jakanovski, M.; Smailagić, A.; Čolić, S.; Meland, M.; Fotirić Akšić, M. Fruit Quality Attributes of Organically Grown Norwegian Apples Are Affected by Cultivar and Location. Plants 2024, 13, 147. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Mendes, G.C.; Seabra, R.M.; Ferreira, M.A. Study of the Organic Acids Composition of Quince (Cydonia oblonga Miller) Fruit and Jam. J. Agric. Food Chem. 2002, 50, 2313–2317. [Google Scholar] [CrossRef]
- Cebulj, A.; Cunja, V.; Mikulic-Petkovsek, M.; Veberic, R. Importance of Metabolite Distribution in Apple Fruit. Sci. Hortic. 2017, 214, 214–220. [Google Scholar] [CrossRef]
- Baccichet, I.; Chiozzotto, R.; Bassi, D.; Gardana, C.; Cirilli, M.; Spinardi, A. Characterization of Fruit Quality Traits for Organic Acids Content and Profile in a Large Peach Germplasm Collection. Sci. Hortic. 2021, 278, 109865. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Cheng, L. Developmental Changes of Carbohydrates, Organic Acids, Amino Acids, and Phenolic Compounds in ‘Honeycrisp’ Apple Flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of Physiological Characteristics, Soluble Sugars, Organic Acids and Volatile Compounds in ‘Orin’ Apples (Malus domestica) at Different Ripening Stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef]
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N.; Postles, J.; Mottram, D.S. Sugars in Crop Plants. Ann. Appl. Biol. 2011, 158, 1–25. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, İ.; Yayli, N.; Şat, İ.G.; Mehmet, Ö.Z.; Hatipoglu Serdar, G. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti. Agrobo. 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Role of Organic Acids in the Integration of Cellular Redox Metabolism and Mediation of Redox Signalling in Photosynthetic Tissues of Higher Plants. Free Radic. Biol. Med. 2018, 122, 74–85. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate Valves: Old Shuttles with New Perspectives. Plant Biol. J. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Berüter, J. Carbohydrate Metabolism in Two Apple Genotypes That Differ in Malate Accumulation. J. Plant Physiol. 2004, 161, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What Controls Fleshy Fruit Acidity? A Review of Malate and Citrate Accumulation in Fruit Cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant Sugars Are Crucial Players in the Oxidative Challenge during Abiotic Stress: Extending the Traditional Concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Derridj, S.; Garrec, J.-P.; Lombarkia, N. Sugars on Leaf Surfaces Used as Signals by the Insect and the Plant: Implications in Orchard Protection against Cydia pomonella L. (Lepidoptera, Tortricidae). In Moths: Types, Ecological Significance, and Control Methods; Cauterruccio, L., Ed.; Insects and Other Terrestrial Arthropods: Biology, Chemistry and Behavior; Nova Science Publishers: New York, NY, USA, 2012; pp. 1–38. ISBN 978-1-61470-626-7. [Google Scholar]
- Lombarkia, N.; Derridj, S. Resistance of Apple Trees to Cydia pomonella Egg-laying Due to Leaf Surface Metabolites. Entomol. Exp. Appl. 2008, 128, 57–65. [Google Scholar] [CrossRef]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-Talk Interactions of Sucrose and Fusarium Oxysporum in the Phenylpropanoid Pathway and the Accumulation and Localization of Flavonoids in Embryo Axes of Yellow Lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef]
- Surekha Devi, V.; Sharma, H.C.; Arjuna Rao, P. Influence of Oxalic and Malic Acids in Chickpea Leaf Exudates on the Biological Activity of CryIAc towards Helicoverpa Armigera. J. Insect Physiol. 2013, 59, 394–399. [Google Scholar] [CrossRef]
- Jakobek, L.; Barron, A.R. Ancient Apple Varieties from Croatia as a Source of Bioactive Polyphenolic Compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Šavikin, K.; Živković, J.; Zdunić, G.; Gođevac, D.; Ðorđević, B.; Dojčinović, B.; Đorđević, N. Phenolic and Mineral Profiles of Four Balkan Indigenous Apple Cultivars Monitored at Two Different Maturity Stages. J. Food Compos. Anal. 2014, 35, 101–111. [Google Scholar] [CrossRef]
- Dramićanin, A.M.; Andrić, F.L.; Poštić, D.Ž.; Momirović, N.M.; Milojković-Opsenica, D.M. Sugar Profiles as a Promising Tool in Tracing Differences between Potato Cultivation Systems, Botanical Origin and Climate Conditions. J. Food Compos. Anal. 2018, 72, 57–65. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Parameters of Inner Quality of the Apple Scab Resistant and Susceptible Apple Cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, Acid- and Phenol Contents in Apple Cultivars from Organic and Integrated Fruit Cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet Taste in Apple: The Role of Sorbitol, Individual Sugars, Organic Acids and Volatile Compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Cai, Y.; Yang, Q.; Ogutu, C.O.; Liao, L.; Han, Y. Analysis of Sorbitol Content Variation in Wild and Cultivated Apples. J. Sci. Food Agric. 2020, 100, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Schrader, L.E.; Zhang, J.; Sun, J.; Xu, J.; Elfving, D.C.; Kahn, C. Postharvest Changes in Internal Fruit Quality in Apples with Sunburn Browning. J. Amer. Soc. Hort. Sci. 2009, 134, 148–155. [Google Scholar] [CrossRef]
- Hajizadeh, H.S.; Kazemi, M. Investigation of Approaches to Preserve Postharvest Quality and Safety in Fresh-Cut Fruits and Vegetables. Res. J. Environ. Sci. 2012, 6, 93–106. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical Compositional Characterization of Some Apple Cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Sadar, N.; Urbanek Krajnc, A.; Tojnko, S.; Tijskens, L.M.M.; Schouten, R.E.; Unuk, T. Development and Distribution of Quality Related Compounds in Apples during Growth. Sci. Hortic. 2016, 213, 222–231. [Google Scholar] [CrossRef]
- Berüter, J.; Feusi, M.E.S.; Rüedi, P. Sorbitol and Sucrose Partitioning in the Growing Apple Fruit. J. Plant Physiol. 1997, 151, 269–276. [Google Scholar] [CrossRef]
- Šircelj, H.; Tausz, M.; Grill, D.; Batič, F. Biochemical Responses in Leaves of Two Apple Tree Cultivars Subjected to Progressing Drought. J. Plant Physiol. 2005, 162, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Nemeskéri, E.; Kovács-Nagy, E.; Nyéki, J.; Sárdi, É. Responses of Apple Tree Cultivars to Drought: Carbohydrate Composition in the Leaves. Turk. J. Agric. 2015, 39, 949–957. [Google Scholar] [CrossRef]
- Sivaci, A. Seasonal Changes of Total Carbohydrate Contents in Three Varieties of Apple (Malus sylvestris Miller) Stem Cuttings. Sci. Hortic. 2006, 109, 234–237. [Google Scholar] [CrossRef]
- Wang, Z.; Stutte, G.W. The Role of Carbohydrates in Active Osmotic Adjustment in Apple under Water Stress. J. Am. Soc. Hortic. Sci. 1992, 117, 816–823. [Google Scholar] [CrossRef]
- Li, T.H.; Li, S.H. Leaf Responses of Micropropagated Apple Plants to Water Stress: Nonstructural Carbohydrate Composition and Regulatory Role of Metabolic Enzymes. Tree Physiol. 2005, 25, 495–504. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Comparison of Bioactive Compounds and Health Promoting Properties of Fruits and Leaves of Apple, Pear and Quince. Sci. Rep. 2021, 11, 20253. [Google Scholar] [CrossRef]
- Yamada, K.; Osakabe, Y. Sugar Compartmentation as an Environmental Stress Adaptation Strategy in Plants. Semin. Cell Dev. Biol. 2018, 83, 106–114. [Google Scholar] [CrossRef]
- Zupan, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Sugar and Phenol Content in Apple with or without Watercore: Effect of Watercore on Apple Phenols and Sugars. J. Sci. Food Agric. 2016, 96, 2845–2850. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, Y.; Zhang, W.; Mu, W. Sucrose Isomers as Alternative Sweeteners: Properties, Production, and Applications. Appl. Microbiol. Biotechnol. 2019, 103, 8677–8687. [Google Scholar] [CrossRef]
- Carrington, Y.; Guo, J.; Le, C.H.; Fillo, A.; Kwon, J.; Tran, L.T.; Ehlting, J. Evolution of a Secondary Metabolic Pathway from Primary Metabolism: Shikimate and Quinate Biosynthesis in Plants. Plant J. 2018, 95, 823–833. [Google Scholar] [CrossRef]
- Mratinić, E.; Fotirić-Akšić, M. Phenotypic Diversity of Apple (Malus Sp.) Germplasm in South Serbia. Braz. Arch. Biol. Technol. 2012, 55, 349–358. [Google Scholar] [CrossRef]
- Horvacki, N.; Andrić, F.; Gašić, U.; Đurović, D.; Tešić, Ž.; Fotirić Akšić, M.; Milojković-Opsenica, D. Phenolic Compounds as Phytochemical Tracers of Varietal Origin of Some Autochthonous Apple Cultivars Grown in Serbia. Molecules 2022, 27, 7651. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, C.; Schwaninger, H.; Chao, C.T.; Ma, Y.; Duan, N.; Khan, A.; Ban, S.; Xu, K.; Cheng, L.; et al. Phased Diploid Genome Assemblies and Pan-Genomes Provide Insights into the Genetic History of Apple Domestication. Nat. Genet. 2020, 52, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Peace, C.; Rudell, D.; Verma, S.; Evans, K. QTLs Detected for Individual Sugars and Soluble Solids Content in Apple. Mol. Breed. 2015, 35, 135. [Google Scholar] [CrossRef]
- Zheng, L.-J.; Nie, J.-Y.; Yan, Z.; Xu, G.-F.; Wang, K.; Gao, Y.; Ye, M.-L. Studies on the Characteristics of the Composition and Content of Soluble Sugars in Apple Fruit. Acta Hortic. Sin. 2015, 42, 950–960. [Google Scholar] [CrossRef]
- Hammer, K.; Gladis, T.; Diederichsen, A. In Situ and On-Farm Management of Plant Genetic Resources. Eur. J. Agron. 2003, 19, 509–517. [Google Scholar] [CrossRef]
| Number | Cultivar | Cultivar Type | Month of Collection |
|---|---|---|---|
| 1. | Red Delicious | Standard | September (second half) |
| 2. | Granny Smith | Standard | September (second half) |
| 3. | Idared | Standard | September (second half) |
| 4. | Golden Delicious | Standard | September (second half) |
| 5. | Jonagold | Standard | September (second half) |
| 6. | Prima | Resistant | August (second half) |
| 7. | Gala Galax | Resistant | August (second half) |
| 8. | Williams Pride | Resistant | July |
| 9. | Rewena | Resistant | September (first half) |
| 10. | Topaz | Resistant | September (second half) |
| 11. | Remura | Resistant | September (first half) |
| 12. | Zaječarska duguljasta | Autochthonous | September (second half) |
| 13. | Mionička tikvara | Autochthonous | September (first half) |
| 14. | Zaječarski delišes | Autochthonous | September (second half) |
| 15. | Gružanjska letnja kolačara | Autochthonous | July |
| 16. | Šećeruša | Autochthonous | September |
| 17. | Pamuklija | Autochthonous | September (first half) |
| 18. | Demirka | Autochthonous | September (first half) |
| 19. | Jesenji jablan | Autochthonous | September (second half) |
| 20. | Kadumana | Autochthonous | September (first half) |
| 21. | Buzlija | Autochthonous | September (second half) |
| 22. | Krtajka | Autochthonous | August (second half) |
| 23. | Hajdučica | Autochthonous | September (second half) |
| 24. | Vrtiglavska slatkača | Autochthonous | September (first half) |
| 25. | Kopaoničanka | Autochthonous | September (second half) |
| 26. | Bela kalaćuša | Autochthonous | September (second half) |
| 27. | Loznička tikvara | Autochthonous | September (second half) |
| 28. | Šipura | Autochthonous | August (second half) |
| 29. | Šipina | Autochthonous | September |
| 30. | Kožara | Autochthonous | September (first half) |
| Content (mg/g) Fresh Weight | ||||||||||
| Year | Cv. Type | Glycerol | Sorbitol | Arabinose | Glucose | Xylose | Fructose | Sucrose | Raffinose | Turanose |
| 2018 | A | 0.09 ± 0.04 | 5.9 ± 3.4 | 0.028 ± 0.012 | 16.5 ± 8.0 | 0.77 ± 0.24 | 50.1 ± 9.9 | 25.7 ± 7.7 | 0.18 ± 0.08 | 0.16 ± 0.12 |
| S | 0.11 ± 0.10 | 6.4 ± 1.6 | 0.015 ± 0.009 | 19.6 ± 9.0 | 0.32 ± 0.16 | 65.8 ± 7.8 | 27.1 ± 6.0 | 0.07 ± 0.05 | 0.08 ± 0.07 | |
| R | 0.07 ± 0.09 | 5.5 ± 1.9 | 0.005 ± 0.004 | 18.4 ± 7.2 | 0.37 ± 0.20 | 62.7 ± 15 | 25.0 ± 7.2 | 0.06 ± 0.03 | 0.06 ± 0.06 | |
| 2019 | A | 0.38 ± 0.27 | 9.5 ± 3.9 | 0.014 ± 0.003 | 21.3 ± 7.1 | 0.41 ± 0.24 | 73.6 ± 12 | 25.6 ± 7.8 | 0.12 ± 0.06 | 0.16 ± 0.08 |
| S | 0.15 ± 0.06 | 6.5 ± 2.8 | 0.009 ± 0.005 | 20.3 ± 5.1 | 0.16 ± 0.15 | 64.7 ± 12 | 31.2 ± 6.6 | 0.06 ± 0.02 | 0.07 ± 0.02 | |
| R | 0.26 ± 0.14 | 4.6 ± 2.1 | 0.003 ± 0.002 | 16.4 ± 8.4 | 0.18 ± 0.08 | 57.1 ± 7.2 | 34.0 ± 11 | 0.07 ± 0.02 | 0.07 ± 0.06 | |
| 2020 | A | 0.14 ± 0.08 | 7.2 ± 2.5 | 0.015 ± 0.005 | 24.2 ± 8.5 | 0.86 ± 0.24 | 53.2 ± 7.4 | 25.0 ± 7.1 | 0.14 ± 0.05 | 0.19 ± 0.08 |
| S | 0.10 ± 0.10 | 3.3 ± 0.9 | 0.005 ± 0.002 | 33.7 ± 3.3 | 0.63 ± 0.19 | 47.9 ± 6.6 | 30.6 ± 6.1 | 0.07 ± 0.03 | 0.07 ± 0.02 | |
| R | 0.11 ± 0.06 | 4.4 ± 2.7 | 0.005 ± 0.002 | 24.8 ± 14 | 0.40 ± 0.11 | 48.2 ± 5.4 | 25.3 ± 6.9 | 0.07 ± 0.03 | 0.07 ± 0.06 | |
| Content (mg/g) Fresh Weight | ||||||||||
| Year | Cv. Type | Maltose | Maltorioseosose | Panose | Quinic | Shikimic | Maleic | Malic | Citric | Isocitric |
| 2018 | A | 0.12 ± 0.11 | 0.26 ± 0.14 | 0.006 ± 0.005 | 1.41 ± 0.58 | 0.14 ± 0.05 | 0.04 ± 0.03 | 10.4 ± 5.4 | 0.25 ± 0.13 | 0.04 ± 0.03 |
| S | 0.12 ± 0.12 | 0.28 ± 0.11 | 0.009 ± 0.005 | 0.92 ± 0.65 | 0.07 ± 0.05 | 0.03 ± 0.02 | 10.3 ± 4.0 | 0.13 ± 0.07 | 0.02 ± 0.02 | |
| R | 0.07 ± 0.08 | 0.26 ± 0.08 | 0.007 ± 0.006 | 0.56 ± 0.14 | 0.07 ± 0.05 | 0.05 ± 0.03 | 11.4 ± 2.6 | 0.19 ± 0.11 | 0.02 ± 0.02 | |
| 2019 | A | 0.12 ± 0.06 | 0.26 ± 0.15 | 0.008 ± 0.003 | 1.39 ± 0.65 | 0.20 ± 0.16 | 0.06 ± 0.04 | 10.9 ± 4.2 | 0.25 ± 0.09 | 0.03 ± 0.03 |
| S | 0.17 ± 0.09 | 0.34 ± 0.08 | 0.008 ± 0.002 | 0.82 ± 0.54 | 0.07 ± 0.06 | 0.05 ± 0.04 | 10.4 ± 3.6 | 0.14 ± 0.08 | 0.03 ± 0.03 | |
| R | 0.15 ± 0.11 | 0.32 ± 0.14 | 0.008 ± 0.003 | 0.55 ± 0.30 | 0.04 ± 0.02 | 0.07 ± 0.04 | 12.0 ± 4.5 | 0.18 ± 0.07 | 0.02 ± 0.02 | |
| 2020 | A | 0.12 ± 0.06 | 0.27 ± 0.11 | 0.006 ± 0.005 | 1.23 ± 0.50 | 0.16 ± 0.05 | 0.05 ± 0.02 | 13.0 ± 5.3 | 0.27 ± 0.12 | 0.05 ± 0.04 |
| S | 0.16 ± 0.06 | 0.36 ± 0.12 | 0.009 ± 0.005 | 0.80 ± 0.43 | 0.10 ± 0.05 | 0.04 ± 0.02 | 10.4 ± 4.0 | 0.14 ± 0.04 | 0.03 ± 0.02 | |
| R | 0.13 ± 0.10 | 0.27 ± 0.11 | 0.007 ± 0.006 | 0.54 ± 0.09 | 0.07 ± 0.02 | 0.04 ± 0.02 | 9.9 ± 3.4 | 0.14 ± 0.05 | 0.03 ± 0.02 | |
| Content (mg/g) Dry Weight | ||||||||||||
| Year | Cv. Type | Glycerol | Sorbitol | Arabinose | Glucose | Xylose | Fructose | Sucrose | Raffinose | Turanose | Maltose | |
| 2018 | A | 2.52 ± 0.65 | 70.7 ± 16.3 | 0.03 ± 0.01 | 40.0 ± 8.0 | 0.021 ± 0.016 | 5.5 ± 1.5 | 0.42 ± 0.20 | 0.08 ± 0.12 | 0.26 ± 0.14 | 0.11 ± 0.07 | |
| S | 2.26 ± 0.23 | 58.5 ± 13.4 | 0.02 ± 0.01 | 28.1 ± 3.0 | 0.008 ± 0.007 | 3.5 ± 1.8 | 0.36 ± 0.27 | 0.13 ± 0.11 | 0.10 ± 0.17 | 0.17 ± 0.05 | ||
| R | 3.11 ± 0.72 | 64.1 ± 10.2 | 0.03 ± 0.01 | 36.0 ± 2.1 | 0.022 ± 0.015 | 4.8 ± 1.1 | 0.56 ± 0.16 | 0.19 ± 0.10 | 0.10 ± 0.14 | 0.11 ± 0.07 | ||
| 2019 | A | 2.42 ± 0.70 | 54.8 ± 10.9 | 0.02 ± 0.01 | 33.6 ± 10.0 | 0.018 ± 0.011 | 3.7 ± 1.2 | 0.46 ± 0.36 | 0.11 ± 0.16 | 0.17 ± 0.13 | 0.15 ± 0.06 | |
| S | 2.05 ± 0.16 | 65.1 ± 9.7 | 0.03 ± 0.01 | 43.0 ± 7.5 | 0.029 ± 0.036 | 3.6 ± 1.0 | 0.22 ± 0.05 | 0.14 ± 0.15 | 0.24 ± 0.18 | 0.11 ± 0.06 | ||
| R | 2.42 ± 0.20 | 52.9 ± 9.6 | 0.02 ± 0.01 | 37.1 ± 3.9 | 0.012 ± 0.030 | 4.0 ± 0.6 | 0.43 ± 0.03 | 0.15 ± 0.25 | 0.20 ± 0.10 | 0.13 ± 0.07 | ||
| 2020 | A | 2.73 ± 0.68 | 60.6 ± 10.2 | 0.04 ± 0.08 | 39.5 ± 6.3 | 0.039 ± 0.026 | 5.4 ± 0.7 | 0.44 ± 0.18 | 0.22 ± 0.09 | 0.09 ± 0.09 | 0.10 ± 0.05 | |
| S | 3.90 ± 0.81 | 49.9 ± 1.8 | 0.02 ± 0.01 | 40.4 ± 2.4 | 0.025 ± 0.016 | 5.6 ± 1.0 | 0.42 ± 0.18 | 0.18 ± 0.06 | 0.04 ± 0.02 | 0.10 ± 0.08 | ||
| R | 2.70 ± 0.79 | 71.5 ± 5.2 | 0.10 ± 0.01 | 43.4 ± 8.4 | 0.050 ± 0.016 | 5.6 ± 0.8 | 0.42 ± 0.17 | 0.36 ± 0.04 | 0.19 ± 0.26 | 0.10 ± 0.08 | ||
| Content (mg/g) Dry Weight | ||||||||||||
| Year | Cv. Type | Panose | Maltotriose | Quinic | Shikimic | Malic | Maleic | Citric | Isocitric | Quinic | Galactouronic | Glucuronic |
| 2018 | A | 0.18 ± 0.14 | 0.21 ± 0.07 | 23.3 ± 6.4 | 2.6 ± 1.6 | 18.1 ± 4.6 | 0.73 ± 0.29 | 4.2 ± 1.7 | 0.17 ± 0.09 | 0.15 ± 0.05 | 0.02 ± 0.01 | 23.3 ± 6.4 |
| S | 0.26 ± 0.27 | 0.24 ± 0.05 | 24.1 ± 7.7 | 2.2 ± 0.8 | 17.4 ± 3.8 | 1.23 ± 0.36 | 1.9 ± 0.5 | 0.19 ± 0.06 | 0.07 ± 0.02 | 0.04 ± 0.07 | 24.1 ± 7.7 | |
| R | 0.29 ± 0.15 | 0.24 ± 0.06 | 28.4 ± 4.3 | 1.9 ± 0.7 | 18.0 ± 3.9 | 0.86 ± 0.36 | 3.4 ± 1.0 | 0.23 ± 0.15 | 0.08 ± 0.04 | 0.02 ± 0.01 | 28.4 ± 4.3 | |
| 2019 | A | 0.22 ± 0.18 | 0.24 ± 0.06 | 24.7 ± 4.9 | 3.0 ± 1.5 | 24.4 ± 4.5 | 1.00 ± 0.39 | 4.3 ± 1.8 | 0.19 ± 0.11 | 0.24 ± 0.11 | 0.03 ± 0.02 | 24.7 ± 4.9 |
| S | 0.12 ± 0.20 | 0.20 ± 0.06 | 26.8 ± 8.3 | 3.0 ± 0.7 | 27.0 ± 6.8 | 1.52 ± 0.34 | 2.6 ± 0.4 | 0.25 ± 0.14 | 0.15 ± 0.05 | 0.02 ± 0.01 | 26.8 ± 8.3 | |
| R | 0.23 ± 0.02 | 0.23 ± 0.06 | 26.0 ± 6.2 | 3.2 ± 1.1 | 25.7 ± 8.1 | 1.09 ± 0.42 | 3.9 ± 1.6 | 0.28 ± 0.16 | 0.21 ± 0.10 | 0.03 ± 0.01 | 26.0 ± 6.2 | |
| 2020 | A | 0.41 ± 0.38 | 0.21 ± 0.12 | 26.3 ± 6.0 | 2.1 ± 1.2 | 20.6 ± 3.1 | 1.16 ± 0.57 | 4.2 ± 2.0 | 0.27 ± 0.12 | 0.20 ± 0.07 | 0.02 ± 0.01 | 26.3 ± 6.0 |
| S | 0.45 ± 0.10 | 0.15 ± 0.08 | 27.6 ± 7.6 | 1.8 ± 1.2 | 22.1 ± 4.5 | 1.22 ± 0.68 | 3.0 ± 0.2 | 0.24 ± 0.11 | 0.17 ± 0.03 | 0.02 ± 0.01 | 27.6 ± 7.6 | |
| R | 0.30 ± 0.38 | 0.33 ± 0.18 | 26.6 ± 5.1 | 2.8 ± 0.7 | 22.8 ± 7.1 | 0.94 ± 0.61 | 3.2 ± 1.1 | 0.13 ± 0.15 | 0.22 ± 0.11 | 0.02 ± 0.02 | 26.6 ± 5.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvacki, N.M.; Jakanovski, M.V.; Krstić, Đ.D.; Nedić, J.M.; Dramićanin, A.M.; Fotirić-Akšić, M.M.; Milojković-Opsenica, D.M. Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia. Processes 2025, 13, 3093. https://doi.org/10.3390/pr13103093
Horvacki NM, Jakanovski MV, Krstić ĐD, Nedić JM, Dramićanin AM, Fotirić-Akšić MM, Milojković-Opsenica DM. Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia. Processes. 2025; 13(10):3093. https://doi.org/10.3390/pr13103093
Chicago/Turabian StyleHorvacki, Nikola M., Mihajlo V. Jakanovski, Đurđa D. Krstić, Jelena M. Nedić, Aleksandra M. Dramićanin, Milica M. Fotirić-Akšić, and Dušanka M. Milojković-Opsenica. 2025. "Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia" Processes 13, no. 10: 3093. https://doi.org/10.3390/pr13103093
APA StyleHorvacki, N. M., Jakanovski, M. V., Krstić, Đ. D., Nedić, J. M., Dramićanin, A. M., Fotirić-Akšić, M. M., & Milojković-Opsenica, D. M. (2025). Evaluation of Sugar and Organic Acid Composition of Apple Cultivars (Malus domestica Borkh.) Grown in Serbia. Processes, 13(10), 3093. https://doi.org/10.3390/pr13103093






