Analysis of Trace Rare Earth Elements in Uranium-Bearing Nuclear Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
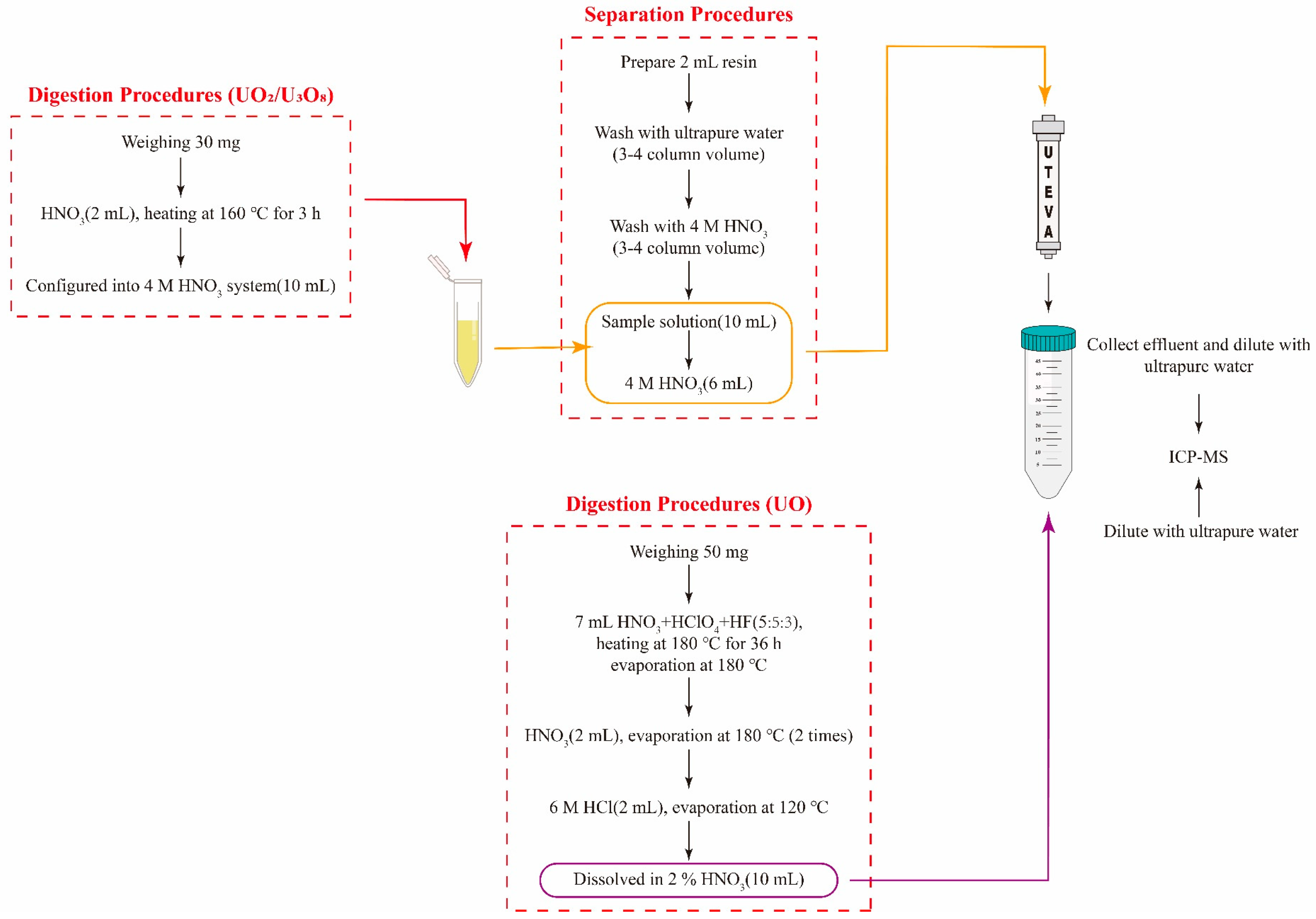
2.2. Analysis Procedures for Uranium Oxides and Uranium Ore
2.3. Instrumentation
3. Results and Discussion
3.1. Optimization of Digestion Conditions
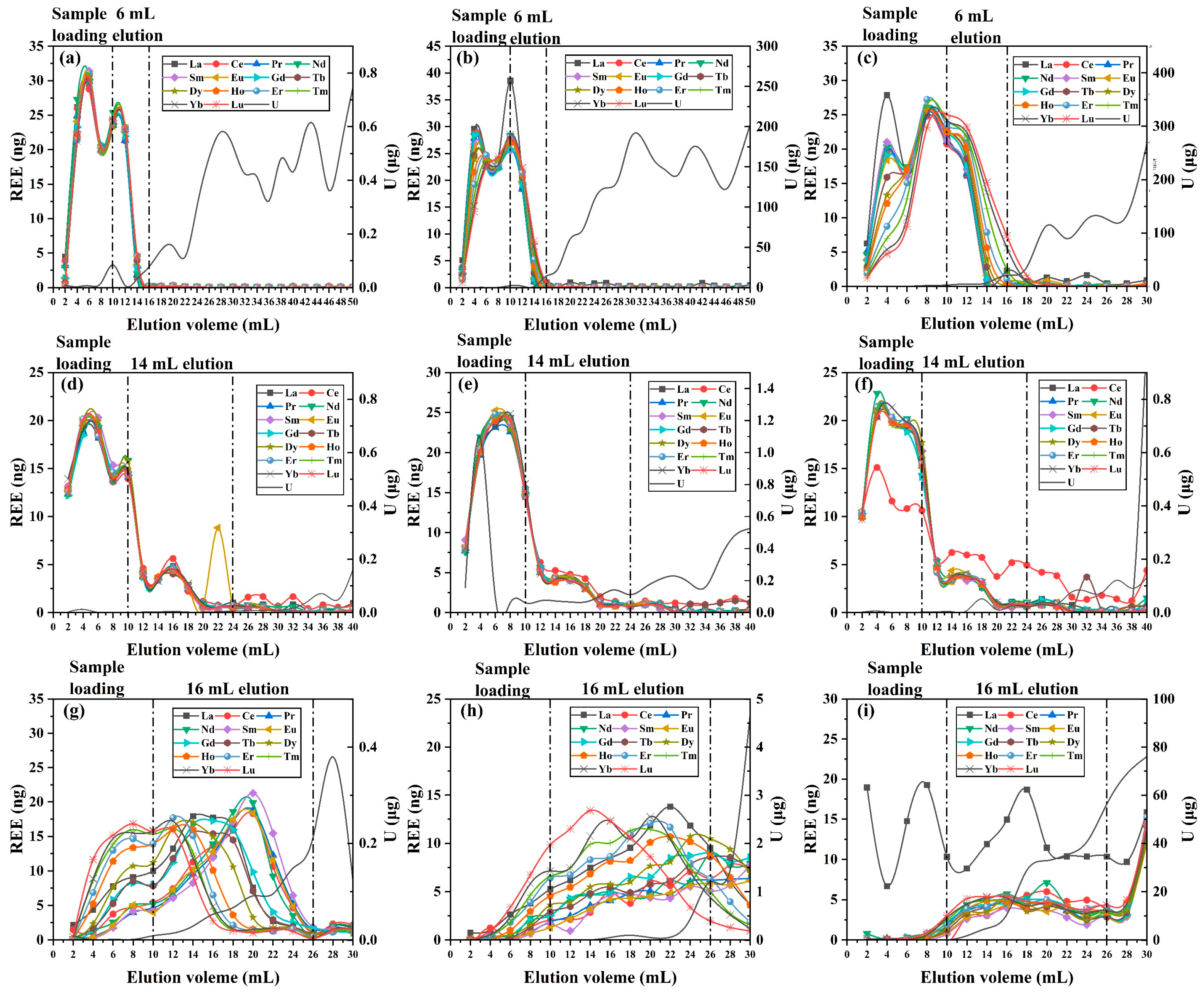
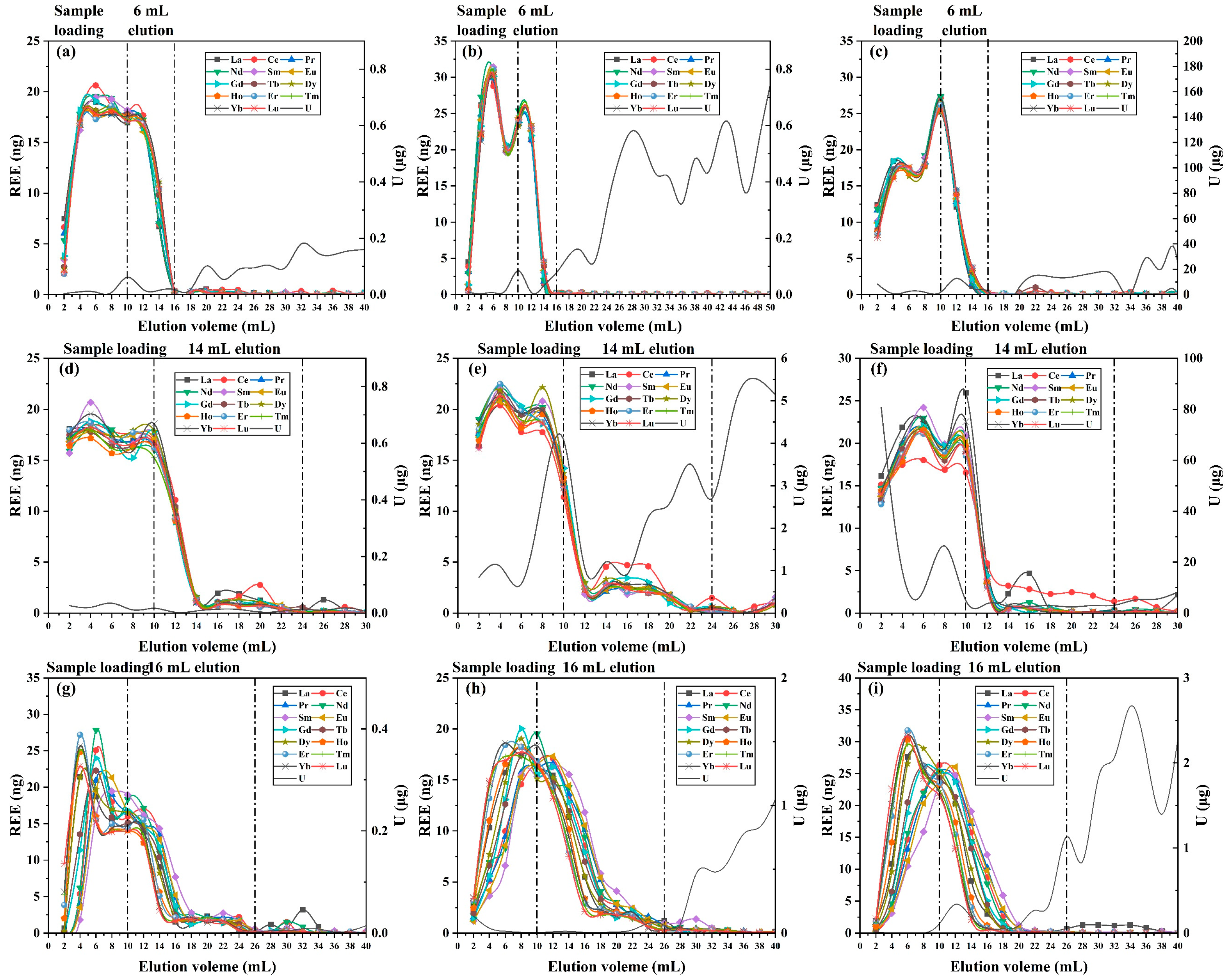
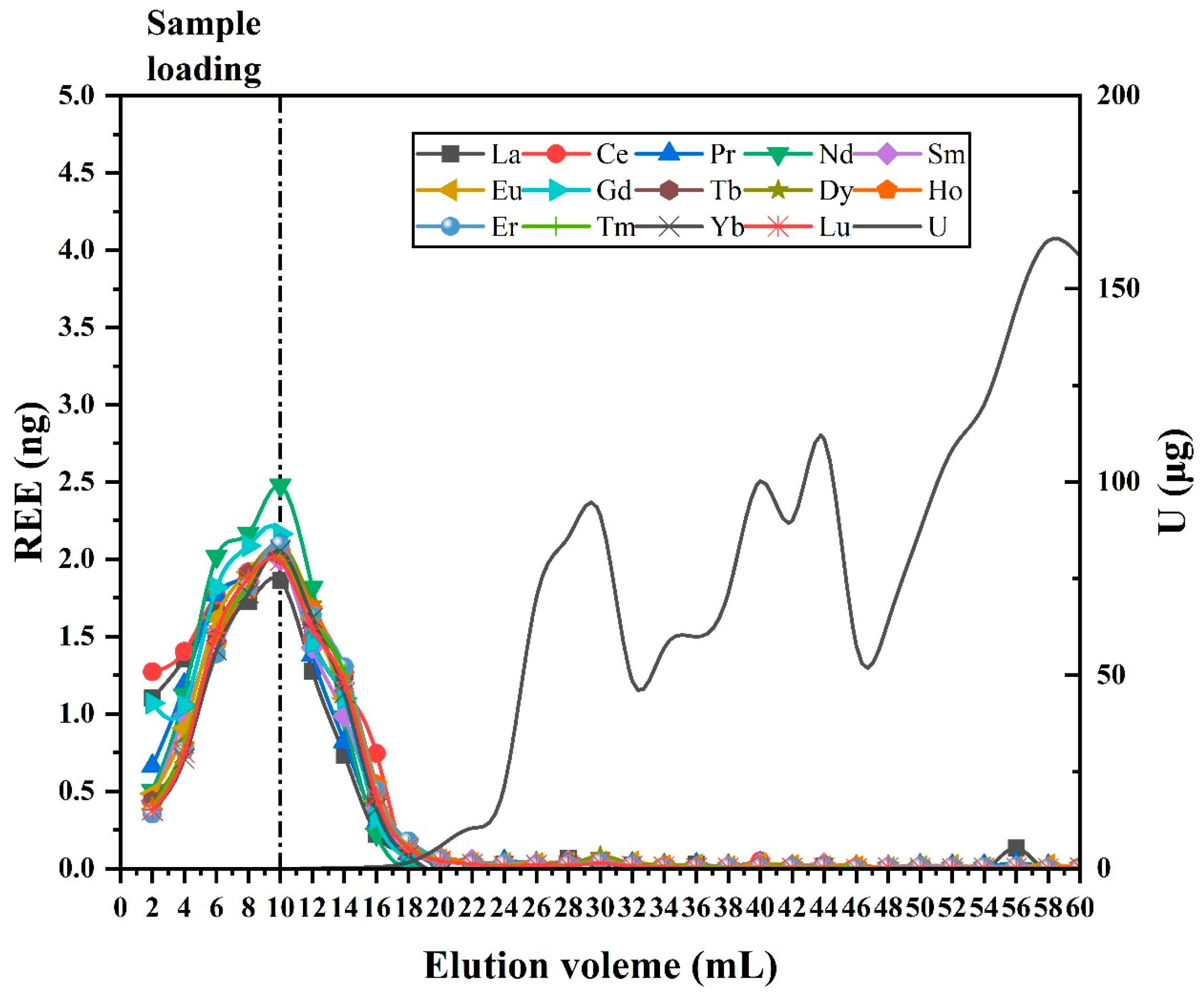
3.2. Optimization of Uranium Matrix Removal
3.3. Optimization of Inner Diameter Conditions
3.4. Analysis of REEs in High-Uranium Matrix
3.5. Validation of the Developed Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asai, S.; Limbeck, A. LA-ICP-MS of rare earth elements concentrated in cation-exchange resin particles for origin attribution of uranium ore concentrate. Talanta 2015, 135, 41–49. [Google Scholar] [CrossRef]
- Spano, T.L.; Simonetti, A.; Balboni, E.; Dorais, C.; Burns, P.C. Trace element and U isotope analysis of uraninite and ore concentrate: Applications for nuclear forensic investigations. Appl. Geochem. 2017, 84, 277–285. [Google Scholar] [CrossRef]
- Corcoran, L.; Simonetti, A.; Spano, T.; Lewis, S.; Dorais, C.; Simonetti, S.; Burns, P. Multivariate Analysis Based on Geochemical, Isotopic, and Mineralogical Compositions of Uranium-Rich Samples. Minerals 2019, 9, 537. [Google Scholar] [CrossRef]
- Mercadier, J.; Cuney, M.; Lach, P.; Boiron, M.; Bonhoure, J.; Richard, A.; Leisen, M.; Kister, P. Origin of uranium deposits revealed by their rare earth element signature. Terra Nova 2011, 23, 264–269. [Google Scholar] [CrossRef]
- Keegan, E.; Richter, S.; Kelly, I.; Wong, H.; Gadd, P.; Kuehn, H.; Alonso-Munoz, A. The provenance of Australian uranium ore concentrates by elemental and isotopic analysis. Appl. Geochem. 2008, 23, 765–777. [Google Scholar] [CrossRef]
- Varga, Z.; Katona, R.; Stefánka, Z.; Wallenius, M.; Mayer, K.; Nicholl, A. Determination of rare-earth elements in uranium-bearing materials by inductively coupled plasma mass spectrometry. Talanta 2010, 80, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Britt, H.C.; Dairiki, J.M.; Lougheed, R.W.; McNabb, D.P.; Prussin, S.G. Review of the Status of Cumulative Fission Yields from 239Pu(n,f) of Interest to Nuclear Forensics; Lawrence Livermore National Laboratory (LLNL): Livermore, CA, USA, 2010. [Google Scholar]
- Moody, K.J.; Grant, P.M.; Hutcheon, I.D. Nuclear Forensic Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mayer, K.; Wallenius, M.; Varga, Z. Nuclear Forensic Science: Correlating Measurable Material Parameters to the History of Nuclear Material. Chem. Rev. 2013, 113, 884–900. [Google Scholar] [CrossRef]
- He, H.; Zhao, X.; Zhang, Y.; Zhao, L.; Hu, R.; Li, L. Determination of rare earth elements in uranium ores by ICP-MS after total dissolution with NH4F and matrix separation with TRU resin. J. Radioanal. Nucl. Chem. 2023, 332, 1909–1916. [Google Scholar] [CrossRef]
- Natarajan, V.; Dhawale, B.A.; Rajeswari, B.; Hon, N.S.; Thulasidas, S.K.; Porwal, N.K.; Godbole, S.V.; Manchanda, V.K. Determination of metallic impurities in U3O8 using energy dispersive X-ray fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 817–819. [Google Scholar] [CrossRef]
- Santoliquido, P.M. The determination of trace elements in uranium oxide (U3O8) by inductively coupled plasma emission spectrometry and graphite furnace atomic absorption spectrometry. J. Res. Natl. Bur. Stand. 1988, 93, 452–454. [Google Scholar] [CrossRef]
- Kin, F.D.; Prudêncio, M.I.; Gouveia, M.Â.; Magnusson, E. Determination of rare earth elements in geological reference materials: A comparative study by INAA and ICP-MS. Geostand. Newsl. 1999, 23, 47–58. [Google Scholar] [CrossRef]
- Navarro, M.S.; Ulbrich, H.H.G.J.; Andrade, S.; Janasi, V.A. Adaptation of ICP–OES routine determination techniques for the analysis of rare earth elements by chromatographic separation in geologic materials: Tests with reference materials and granitic rocks. J. Alloys Compd. 2002, 344, 40–45. [Google Scholar] [CrossRef]
- Gao, J.; Manard, B.T.; Castro, A.; Montoya, D.P.; Xu, N.; Chamberlin, R.M. Solid-phase extraction microfluidic devices for matrix removal in trace element assay of actinide materials. Talanta 2017, 167, 8–13. [Google Scholar] [CrossRef]
- Quarles, C.D.; Manard, B.T.; Wylie, E.M.; Xu, N. Trace elemental analysis of bulk uranium materials using an inline automated sample preparation technique for ICP-OES. Talanta 2018, 190, 460–465. [Google Scholar] [CrossRef]
- Trommetter, G.; Dumoulin, D.; Billon, G. Direct determination of rare earth elements in natural water and digested sediment samples by inductively coupled plasma quadrupole mass spectrometry using collision cell. Spectrochim. Acta Part B At. Spectrosc. 2020, 171, 105922. [Google Scholar] [CrossRef]
- Quemet, A.; Brennetot, R.; Chevalier, E.; Prian, E.; Laridon, A.-L.; Mariet, C.; Fichet, P.; Laszak, I.; Goutelard, F. Analysis of twenty five impurities in uranium matrix by ICP-MS with iron measurement optimized by using reaction collision cell, cold plasma or medium resolution. Talanta 2012, 99, 207–212. [Google Scholar] [CrossRef]
- Wu, Y.; Pena, L.D.; Goldstein, S.L.; Basak, C.; Bolge, L.L.; Jones, K.M.; McDaniel, D.K.; Hemming, S.R. A user-friendly workbook to facilitate rapid and accurate rare earth element analyses by ICP-MS for multispiked samples. Geochem. Geophys. Geosyst. 2020, 21, e2020GC009042. [Google Scholar] [CrossRef]
- Campbell, K.; Judge, E. Digestion and trace metal analysis of uranium nitride. J. Radioanal. Nucl. Chem. 2022, 331, 209–214. [Google Scholar] [CrossRef]
- Ding, X.; Bu, W.; Ni, Y.; Shao, X.; Xiong, K.; Yang, C.; Hu, S. Determination of trace rare earth elements in uranium ore samples by triple quadrupole inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2021, 36, 2144–2152. [Google Scholar] [CrossRef]
- Ni, Y.; Bu, W.; Xiong, K.; Ding, X.; Wang, H.; Liu, X.; Long, K.; Hu, S. Trace impurity analysis in uranium materials by rapid separation and ICP-MS/MS measurement with matrix matched external calibration. Microchem. J. 2021, 169, 106615. [Google Scholar] [CrossRef]
- Baghaliannejad, R.; Aghahoseini, M.; Amini, M.K. Determination of rare earth elements in uranium materials by ICP-MS and ICP-OES after matrix separation by solvent extraction with TEHP. Talanta 2021, 222, 121509. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, K.; Durani, S. Separation and inductively coupled plasma optical emission spectrometric (ICP-OES) determination of trace impurities in nuclear grade uranium oxide. J. Radioanal. Nucl. Chem. 2010, 285, 659–665. [Google Scholar] [CrossRef]
- Aziz, A.; Jan, S.; Waqar, F.; Mohammad, B.; Hakim, M.; Yawar, W. Selective ion exchange separation of uranium from concomitant impurities in uranium materials and subsequent determination of the impurities by ICP-OES. J. Radioanal. Nucl. Chem. 2010, 284, 117–121. [Google Scholar] [CrossRef]
- Lee, C.H.; Suh, M.Y.; Choi, K.S.; Kim, J.S.; Song, B.C.; Jee, K.Y.; Kim, W.H. Separation of fission products from spent pressurized water reactor fuels by anion exchange and extraction chromatography for inductively coupled plasma atomic emission spectrometric analysis. Anal. Chim. Acta 2001, 428, 133–142. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Dietz, M.L.; Chiarizia, R.; Diamond, H.; Maxwell, S.L.; Nelson, M.R. Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: Application to the characterization of high-level nuclear waste solutions. Anal. Chim. Acta 1995, 310, 63–78. [Google Scholar] [CrossRef]
- Rotzinger, F.P. Mechanism for the substitution of an aqua ligand of UO2(OH2)52+ by chloride. J. Chem. Theory Comput. 2008, 4, 1654–1658. [Google Scholar] [CrossRef]
- Pinto, F.G.; Tronto, J.; Lepri, F.G.; Tormen, L.; Saint’Pierre, T.D.; Costa, L.M.; Beinner, M.A.; da Silva, J.B.B. Study of ultrasonic and pneumatic nebulizers for ICP-MS determination of rare earth elements in geological samples. Atom. Spectrosc. 2015, 36, 116–127. [Google Scholar] [CrossRef]
- Nakamura, K.; Chang, Q. Precise determination of ultra-low (sub-ng g−1) level rare earth elements in ultramafic rocks by quadrupole ICP-MS. Geostand. Geoanal. Res. 2007, 31, 185–197. [Google Scholar] [CrossRef]
- Yokoyama, T.; Makishima, A.; Nakamura, E. Separation of thorium and uranium from silicate rock samples using two commercial extraction chromatographic resins. Anal. Chem. 1999, 71, 135–141. [Google Scholar] [CrossRef]
- Cong, H.; Liu, C.; Li, R.; Liu, Y.; Dou, Q.; Fu, H.; Zhang, L.; Zhou, W.; Li, Q.; Li, W. Trace impurities analysis in UF4 via standard addition and 103Rh internal standardization techniques combined with ICP-MS. J. Radioanal. Nucl. Chem. 2019, 322, 2025–2032. [Google Scholar] [CrossRef]
- Krajkó, J.; Varga, Z.; Wallenius, M.; Mayer, K. Pre-concentration of trace levels of rare-earth elements in high purity uranium samples for nuclear forensic purposes. Radiochim. Acta 2016, 104, 471–479. [Google Scholar] [CrossRef]
- Hubley, N.T.; Brockman, J.D.; Robertson, J.D. Evaluation of ammonium bifluoride fusion for rapid dissolution ineutron energy spectrumneutron energy spectrum post-detonation nuclear forensic analysis. Radiochim. Acta 2017, 105, 629–635. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Z.; Liu, Y.; Chen, H.; Gao, S.; Gaschnig, R.M. Total Rock Dissolution Using Ammonium Bifluoride (NH4HF2) in Screw-Top Teflon Vials: A New Development in Open-Vessel Digestion. Anal. Chem. 2012, 84, 10686–10693. [Google Scholar] [CrossRef]
- Bürger, S.; Riciputi, L.R.; Bostick, D.A. Determination of impurities in uranium matrices by time-of-flight ICP-MS using matrix-matched method. J. Radioanal. Nucl. Chem. 2007, 274, 491–505. [Google Scholar] [CrossRef]
- Lehto, J.; Hou, X. Chemistry and Analysis of Radionuclides: Laboratory Techniques and Methodology; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Marinov, G.; Marinova, A.; Milanova, M.; Happel, S.; Lebedev, N.A.; Drokhlyansky, A.; Mirzayev, N.; Karaivanov, D.V.; Filosofov, D.V. Sorption of Rare-Earth Elements and Ac on UTEVA Resin in Different Acid Solutions. Solvent Extr. Ion Exch. 2017, 35, 280–291. [Google Scholar] [CrossRef]
- Ostapenko, V.; Vasiliev, A.; Lapshina, E.; Ermolaev, S.; Aliev, R.; Totskiy, Y.; Zhuikov, B.; Kalmykov, S. Extraction chromatographic behavior of actinium and REE on DGA, Ln and TRU resins in nitric acid solutions. J. Radioanal. Nucl. Chem. 2015, 306, 707–711. [Google Scholar] [CrossRef]
- Balboni, E.; Simonetti, A.; Spano, T.; Cook, N.D.; Burns, P.C. Rare-earth element fractionation in uranium ore and its U(VI) alteration minerals. Appl. Geochem. 2017, 87, 84–92. [Google Scholar] [CrossRef]
- Treadway, J.W.; Wyndham, K.D.; Jorgenson, J.W. Highly efficient capillary columns packed with superficially porous particles via sequential column packing. J. Chromatogr. A 2015, 1422, 345–349. [Google Scholar] [CrossRef]
- Khoo, H.T.; Leow, C.H. Advancements in the preparation and application of monolithic silica columns for efficient separation in liquid chromatography. Talanta 2021, 224, 121777. [Google Scholar] [CrossRef]
- Gritti, F.; Guiochon, G. Kinetic investigation of the relationship between the efficiency of columns and their diameter. J. Chromatogr. A 2011, 1218, 1592–1602. [Google Scholar] [CrossRef]
- Wu, N.; Bradley, A.C. Effect of column dimension on observed column efficiency in very high pressure liquid chromatography. J. Chromatogr. A 2012, 1261, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Deb, S.B.; Saxena, M.K. Determination of trace impurities in advanced metallic nuclear fuels by inductively coupled plasma time-of-flight mass spectrometry (ICP-TOF-MS). J. Anal. At. Spectrom. 2016, 31, 1480–1489. [Google Scholar] [CrossRef]
- Durani, S.; Krishnakumar, M.; Satyanarayana, K. Solid phase separation and ICP-OES/ICP-MS determination of rare earth impurities in nuclear grade uranium oxide. J. Radioanal. Nucl. Chem. 2012, 294, 215–220. [Google Scholar] [CrossRef]

| Parameter | Operating Conditions | Unit |
|---|---|---|
| RF power | 1300 | W |
| Sampling depth | 120 | mm |
| Plasma gas flow rate | 13 | L/min |
| Auxiliary gas flow rate | 0.8 | L/min |
| Nebulizer gas flow rate | 0.8 | L/min |
| Cool gas flow rate | 18 | L/min |
| Element | UO2-50 ng | UO2-100 ng | UO2-200 ng | U3O8-50 ng | U3O8-100 ng | U3O8-200 ng |
|---|---|---|---|---|---|---|
| La | 89.3 ± 4.3% | 84.9 ± 2.3% | 88.2 ± 3.6% | 91.0 ± 2.5% | 91.5 ± 4.6% | 86.9 ± 4.5% |
| Ce | 91.1 ± 6.6% | 84.5 ± 1.7% | 87.4 ± 4.1% | 92.7 ± 1.7% | 91.2 ± 4.6% | 86.0 ± 4.0% |
| Pr | 88.7 ± 3.6% | 85.3 ± 2.0% | 86.1 ± 3.7% | 91.3 ± 3.3% | 91.2 ± 4.7% | 86.3 ± 4.2% |
| Nd | 93.5 ± 3.6% | 85.8 ± 2.5% | 87.4 ± 4.3% | 87.3 ± 5.9% | 91.1 ± 3.9% | 87.3 ± 4.5% |
| Sm | 90.2 ± 5.0% | 86.3 ± 1.5% | 85.9 ± 4.1% | 88.7 ± 2.6% | 92.3 ± 4.7% | 84.0 ± 4.2% |
| Eu | 87.0 ± 3.5% | 84.4 ± 2.2% | 87.0 ± 3.2% | 91.4 ± 3.0% | 91.4 ± 4.1% | 84.4 ± 4.0% |
| Gd | 87.6 ± 3.8% | 83.4 ± 2.1% | 86.6 ± 2.9% | 86.4 ± 4.7% | 90.8 ± 4.2% | 82.2 ± 4.5% |
| Tb | 87.9 ± 3.6% | 83.8 ± 1.8% | 86.2 ± 3.2% | 90.0 ± 3.3% | 90.5 ± 4.3% | 84.2 ± 4.4% |
| Dy | 87.3 ± 5.3% | 84.6 ± 2.6% | 86.4 ± 4.0% | 89.2 ± 3.8% | 92.6 ± 5.1% | 84.7 ± 4.7% |
| Ho | 87.5 ± 4.0% | 84.9 ± 1.5% | 86.1 ± 3.2% | 89.7 ± 2.8% | 91.2 ± 4.6% | 83.0 ± 4.4% |
| Er | 89.5 ± 3.9% | 84.6 ± 2.1% | 86.9 ± 2.9% | 91.6 ± 3.3% | 91.4 ± 4.1% | 84.9 ± 4.4% |
| Tm | 89.0 ± 3.5% | 83.7 ± 1.9% | 86.4 ± 3.3% | 91.2 ± 2.5% | 90.3 ± 4.7% | 83.3 ± 4.3% |
| Yb | 91.9 ± 4.0% | 84.7 ± 2.5% | 87.8 ± 3.0% | 91.5 ± 3.2% | 91.5 ± 3.8% | 85.0 ± 4.8% |
| Lu | 88.4 ± 3.6% | 84.7 ± 1.9% | 86.6 ± 3.1% | 90.3 ± 2.3% | 90.7 ± 5.1% | 84.8 ± 4.7% |
| Average | 89.2 ± 4.2% | 84.7 ± 2.0% | 86.8 ± 3.5% | 90.2 ± 3.2% | 91.3 ± 4.5% | 84.8 ± 4.4% |
| Element | Uranium Oxides (ppt) | Uranium Ores (ppt) |
|---|---|---|
| La | 9.05 | 5.47 |
| Ce | 16.2 | 14.0 |
| Pr | 4.01 | 1.28 |
| Nd | 8.56 | 5.82 |
| Sm | 6.54 | 1.80 |
| Eu | 5.53 | 1.69 |
| Gd | 7.25 | 3.17 |
| Tb | 2.90 | 0.540 |
| Dy | 5.06 | 2.03 |
| Ho | 3.49 | 0.487 |
| Er | 4.61 | 2.23 |
| Tm | 2.89 | 0.363 |
| Yb | 3.09 | 1.26 |
| Lu | 2.30 | 0.273 |
| U | 8.15 | 4.34 |
| LOD | Uranium Decontamination Factor | REE Recovery | Reference |
|---|---|---|---|
| ≤16.2 ppt | 1.56 × 105 | 84~91% (all REEs) | The developed method |
| ≤0.2 ng/g | ~2 × 106 | >94% (Sm, Eu, Gd, Dy) | [6] |
| ≤2 ng/g | 3 × 104 | >92% (Sm, Eu, Gd, Dy) | [18] |
| ≤0.98 ppt | ~1 × 103 | >98% (all REEs) | [21] |
| \ | 1.2 × 103 | \ | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shao, Y.; Xin, F.; Li, C.; Zhang, J.; Li, X.; Luo, M.; Xu, D.; Ma, L. Analysis of Trace Rare Earth Elements in Uranium-Bearing Nuclear Materials. Processes 2025, 13, 3089. https://doi.org/10.3390/pr13103089
Li Z, Shao Y, Xin F, Li C, Zhang J, Li X, Luo M, Xu D, Ma L. Analysis of Trace Rare Earth Elements in Uranium-Bearing Nuclear Materials. Processes. 2025; 13(10):3089. https://doi.org/10.3390/pr13103089
Chicago/Turabian StyleLi, Ziao, Yang Shao, Futao Xin, Chun Li, Jilong Zhang, Xi Li, Min Luo, Diandou Xu, and Lingling Ma. 2025. "Analysis of Trace Rare Earth Elements in Uranium-Bearing Nuclear Materials" Processes 13, no. 10: 3089. https://doi.org/10.3390/pr13103089
APA StyleLi, Z., Shao, Y., Xin, F., Li, C., Zhang, J., Li, X., Luo, M., Xu, D., & Ma, L. (2025). Analysis of Trace Rare Earth Elements in Uranium-Bearing Nuclear Materials. Processes, 13(10), 3089. https://doi.org/10.3390/pr13103089










