Waste to Value: L-Asparaginase Production from Agro-Industrial Residues
Abstract
1. Introduction
2. Agro-Industrial Residues as Substrate
3. Microbial Sources for L-Asparaginase Production
| Class | Microorganism | Reactor | Optimal Conditions | Activity/ Specific Activity | Ref. |
|---|---|---|---|---|---|
| Archaea | Thermococcus sibiricus * | Shaker | 90 °C and pH 9.0 | 83.361 U/mL | [32] |
| Pyrococcus * furiosus | Shaker | 50 °C and pH 8.0 | 12,321.4 U/mL | [33] | |
| Pyrococcus abyssi * | Shaker | 80 °C and pH 8.0 | 1175 U/mg | [34] | |
| Actinomycetes | Streptomyces koyangensis (SK4) | Shaker | - | 136 U/mL | [35] |
| Streptomyces gulbargensis | Shaker | 40 °C and pH 9.0 | 3.23 U/mL | [30] | |
| Bacteria | Bacillus paralicheniformis (AUMC B-516) | Shaker | 35 °C and pH 8.0 | 116.4 U/mL | [36] |
| Bacillus licheniformis (ASN51) | Shaker | 37 °C and pH 8.0 | 499 U/mg | [37] | |
| Escherichia coli (MF-107) | Shaker | 35 °C and pH 7.5–8.0 | 9.16 U/mg | [38] | |
| Pectobacterium carotovorum | Shaker | 37 °C and pH 8.7 | 20 U/mL | [39] | |
| Pseudomonas aeruginosa | SSF | 37 °C and pH 7.4 | 1900 IU/mg | [40] | |
| Sphingomonas leidyi (VN01) | Shaker | 37 °C | 156 IU/mg | [41] | |
| Zymomonas mobilis (CP4) | Shaker | 30 °C | 16.55 IU/L | [42] | |
| Yeast | Trichoderma viride | Shaker | 37 °C and pH 7.5 | 71.3 U/mL | [43] |
| Meyerozyma guilliermondii | Shaker | 37 °C and pH 7.0 | 26.01 U/mL | [44] | |
| Candida utilis (ATCC 9950) | Fermenter (Batch) | 245.6 U/mL | [45] | ||
| Saccharomyces cerevisiae * | Shaker | 40 °C and pH 8.6 | 196.2 U/mg | [46] | |
| Leucosporidium scottii (L115) | Shaker | - | 178.1 U/gdcw−1 | [47] | |
| Lachancea thermotolerans | Shaker | 37 °C and pH 8.6 | 313.8 U/mg | [48] | |
| Cyberlindnera subsufficiens (GULAMMS8) | Shaker | - | 57.54 U/mL | [49] | |
| Meyerozyma guilliermondii | Shaker | 37 °C and pH 7.0 | 26.01 U/mL | [44] | |
| Fungi | Aspergillus oryzae (IOC 3999) | Shaker | 60 °C and pH 5.0 | 1443.57 U/mL | [50] |
| Aspergillus niger (INCQS 40018) | Shaker | 40 °C and pH 5.0 | 0.6712 U/mL | [23] | |
| Penicillium brevicompactum (NRC 829) | Shaker | 37 °C and pH 8.0 | 132.4 U/mg | [51] | |
| Cladosporium sp. | Shaker | - | 255-428 U/mL | [52] | |
| Aspergillus caespitosus; Aspergillus oryzae | Shaker | - | 0.0249 and 0.0139 U/mL | [18] | |
| Fusarium equiseti (AHMF4) | Shaker | - | 40.78 U/mL | [53] | |
| Penicillium sizovae (2DSST1) and Fusarium proliferatum (DCFS10) | Shaker | - | 3.68 and 1.86 U/mL | [54] | |
| Aspergillus sydowii and Fusarium oxysporum | Shaker | - | 146 and 143 U/mL | [55] | |
| Aspergillus niger | SSF | 50 °C and pH 9.0 | 187.19 U/mg | [56] | |
| Fereydounia khargensis (IBRC-M 30116) | Shaker | - | 61.3 U/mL | [57] | |
| Aspergillus oryzae | SSF | - | 16.122 U/g | [58] | |
| Aspergillus caespitosus (CCDCA 11593) | SSF | - | 2.75 U/mL | [59] |
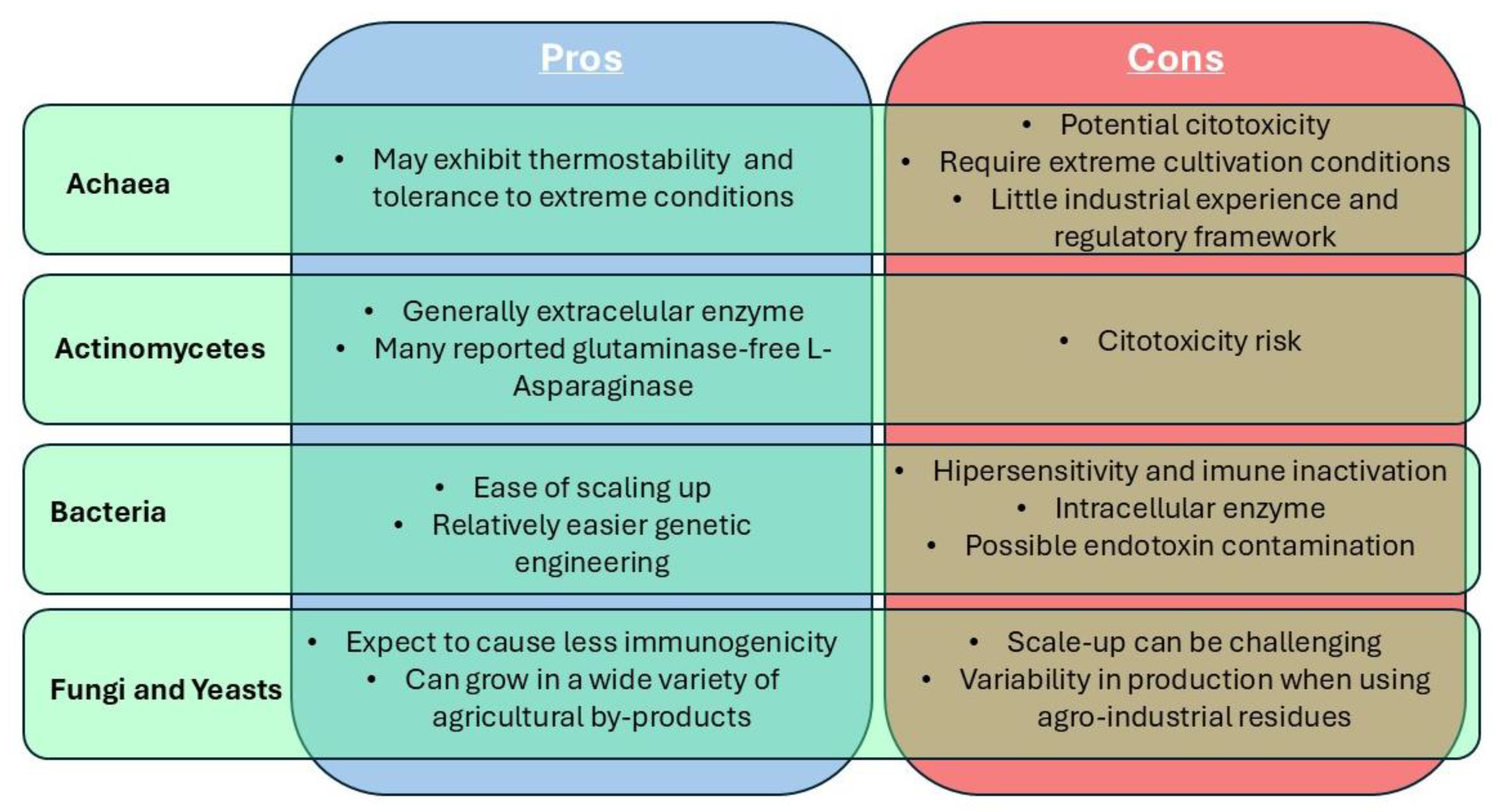
3.1. Archaea
3.2. Actinomycetes
3.3. Bacteria
3.4. Fungi and Yeasts
3.5. Advances in Protein Engineering and Bioprospecting
4. Perspectives on L-Asparaginase Production Using Agro-Industrial Residues
4.1. Technological and Sustainability Trends
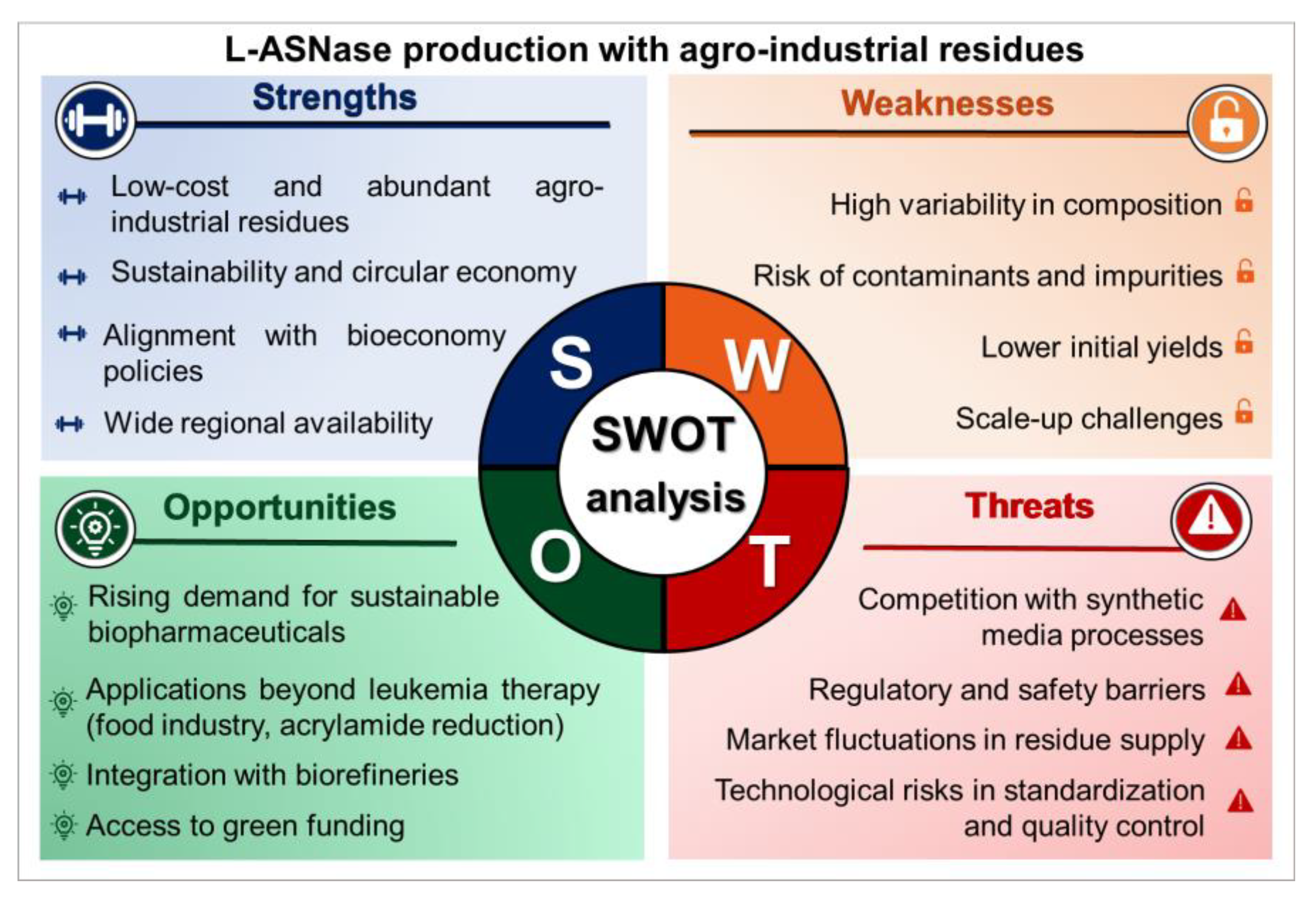
4.2. Regulatory Challenges and SWOT Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| gds | Grams of dry substrate |
| gdcw | Grams of dry cell weight |
| SSF | Solid State Fermentation |
| SmF | Submerged Fermentation |
References
- Maese, L.; Rau, R.E. Current Use of Asparaginase in Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma. Front. Pediatr. 2022, 10, 902117. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, F.; Jahanafrooz, Z.; Mokhtarzadeh, A. Microbial L-asparaginase as a promising enzyme for treatment of various cancers. App. Microb. Biotech. 2022, 106, 5335–5347. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.T.; Belén, L.H.; Leteller, P.; Calle, Y.; Pessoa, A.; Farías, J.G. l-Asparaginase as the gold standard in the treatment of acute lymphoblastic leukemia: A comprehensive review. Med. Oncol. 2023, 40, 150. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Biswas, S.; Ghosh, R.; Modak, R. Recent advances in L-asparaginase enzyme production and formulation development for acrylamide reduction during food processing. Food Chem. X 2024, 25, 102055. [Google Scholar] [CrossRef]
- Shishparenok, A.N.; Gladilina, Y.A.; Zhdanov, D.D. Engineering and Expression Strategies for Optimization of L-asparaginase Development and Production. Int. J. Mol. Sci. 2023, 24, 15220. [Google Scholar] [CrossRef]
- Suresh, S.A.; Ethiraj, S.; Rajnish, K.N. A systematic review of recent trends in research on therapeutically significant l-asparaginase and acute lymphoblastic leukemia. Mol. Biol. Rep. 2022, 49, 11281–11287. [Google Scholar] [CrossRef]
- Hosseini, K.; Zivari-Ghader, T.; Akbarzadehlaleh, P.; Ebrahimi, V.; Sharafabad, B.E.; Dilmaghanit, A. A Comprehensive Review of L-asparaginase: Production, Applications and Therapeutic Potential in Cancer Treatment. Appl. Biochem. Microbiol. 2024, 60, 599–613. [Google Scholar] [CrossRef]
- Castro, D.; Marques, A.S.C.; Almeida, M.R.; Paiva, G.B.; Bento, H.B.S.; Pedrolli, D.B.; Freire, M.G.; Tavares, A.P.M.; Santos-Ebinuma, V.C. L-asparaginase production review: Bioprocess design and biochemical characteristics. Appl. Microbiol. Biotechnol. 2021, 105, 4515–4534. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; Filho, J.G.O.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of agro-industrial waste for sustainable green production: A review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Vućurović, D.; Bajić, B.; Trivunović, Z.; Dodić, J.; Zeljko, M.; Jevtić-Mućibabić, R.; Dodić, S. Biotechnological Utilization of Agro-Industrial Residues and By-Products—Sustainable Production of Biosurfactants. Foods 2024, 13, 711. [Google Scholar] [CrossRef]
- Silva, S.O.; Mafra, A.K.C.; Pelissari, F.M.; de Lemos, L.R.; Molina, G. Biotechnology in Agro-Industry: Valorization of Agricultural Wastes, By-Products and Sustainable Practices. Microorganisms 2025, 13, 1789. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.-A.; Odocheanu, R.; Dudu, A.I.; Dulf, F.V.; Toșa, M.I.; Vodnar, D.C. Towards circular economy: Agro-industrial by-products enabling in situ enzyme production for food and pharmaceutical applications. Sustain. Chem. Pharm. 2025, 44, 101974. [Google Scholar] [CrossRef]
- Liu, T.; He, M.; Shi, R.; Yin, H.; Luo, W. Biofuel–Pharmaceutical Co-Production in Integrated Biorefineries: Strategies, Challenges, and Sustainability. Fermentation 2025, 11, 312. [Google Scholar] [CrossRef]
- Nadar, C.G.; Fletcher, A.; de Almeida Moreira, B.R.; Hine, D.; Yadav, S. Waste to protein: A systematic review of a century of advancement in microbial fermentation of agro-industrial byproducts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13375. [Google Scholar] [CrossRef]
- Mudaliar, S.; Kumar, V.; Verma, P. Bioprospecting of microbial L-asparaginase: Sustainable production from waste and its application for acrylamide reduction in food industry. Process. Saf. Environ. Prot. 2025, 195, 106737. [Google Scholar] [CrossRef]
- Fernandes, M.L.P.; Veríssimo, L.A.A.; de Souza, A.C.; Schwan, R.F.; Dias, D.R. Low-Cost Agro-Industrial Sources as a Substrate for the Production of L-asparaginase Using Filamentous Fungi. Biocatal. Agric. Biotechnol. 2021, 34, 102037. [Google Scholar] [CrossRef]
- Raina, D.; Kumar, V.; Saran, S. A critical review on exploitation of agro-industrial biomass as substrates for the therapeutic microbial enzymes production and implemented protein purification techniques. Chemosphere 2022, 294, 133712. [Google Scholar] [CrossRef]
- Sharma, D.; Mishra, A. Synergistic effects of ternary mixture formulation and process parameters optimization in a sequential approach for enhanced L-asparaginase production using agro-industrial wastes. Environ. Sci. Pollut. Res. 2023, 31, 17858–17873. [Google Scholar] [CrossRef]
- Vimal, A.; Kumar, A. Optimized Production of Medically Significant Enzyme L-Asparaginase Under Submerged and Solid-State Fermentation From Agricultural Wastes. Curr. Microbiol. 2022, 79, 394. [Google Scholar] [CrossRef]
- Acharya, R.; Kanungo, T.; Gupta, N. Screening and Purification of L-asparaginase Production by Aspergillus quadrilineatus Using Agro Wastes and Vegetable Peels. Curr. Trends Biotechnol. Pharm. 2025, 19, 2322–2329. [Google Scholar] [CrossRef]
- Honfoga, J.N.B.; Nascimento, P.A.; Ferreira, A.N.; da Silva, E.C.; Bauer, L.C.; Teixeira, J.M.; Santana, N.B.; Bonomo, R.C.F. Production and characterization of L-asparaginase by Aspergillus niger: A sustainable use of pitaya (Hylocereus spp.) by-products. Sustain. Chem. Environ. 2025, 10, 100255. [Google Scholar] [CrossRef]
- Correa, H.T.; Vieira, W.F.; Pessoa, A., Jr.; Cardoso, V.L.; Coutinho Filho, U. Production of Biosurfactant and l-Asparaginase Using Dry Tenebrio molitor Larvae as Innovative Biomass for Fermentation and Liquid Extractive Process. Waste Biomass Valorization 2023, 14, 4061–4069. [Google Scholar] [CrossRef]
- De Moraes, L.B.S.; Mota, G.C.P.; Molina-Miras, A.; Sánchez-Mirón, A.; del Carmen Cerón-García, M.; Gálvez, A.O.; de Souza Bezerra, R.; García-Camacho, F. Enhancing high-value metabolite production and asparaginase activity in Haematococcus pluvialis: Impact of different organic carbon sources and feeding strategies. Biomass Convers. Biorefin. 2025. [Google Scholar] [CrossRef]
- Da Silva, L.F.; de Pádua, A.P.S.L.; de Oliveira Ferro, L.; Agamez-Montalvo, G.S.; Bezerra, J.D.P.; Moreira, K.A.; de Souza-Motta, C.M. Cacti as low-cost substrates to produce L-asparaginase by endophytic fungi. World J. Microbiol. Biotechnol. 2022, 38, 247. [Google Scholar] [CrossRef]
- Tong, W.H.; Rizzari, C. Back to the future: The amazing journey of the therapeutic anti-leukemia enzyme asparaginase Erwinia chrysanthemi. Haematologica 2023, 108, 2606–2615. [Google Scholar] [CrossRef]
- Alam, S.; Pranaw, K.; Tiwari, R.; Khare, S.K. Green Bio-Processes Enzymes in Industrial Food Processing; Springer: Singapore, 2019; pp. 55–81. [Google Scholar] [CrossRef]
- Da Cunha, M.C.; dos Santos Aguilar, J.G.; de Melo, R.R.; Nagamatsu, S.T.; Ali, F.; de Castro, R.J.S.; Sato, H.H. Fungal L-asparaginase: Strategies for Production and Food Applications. Food Res. Int. 2019, 126, 108658. [Google Scholar] [CrossRef]
- Amena, S.; Vishalakshi, N.; Prabhakar, M.; Dayanand, A.; Lingappa, K. Production, purification and characterization of L-asparaginase from Streptomyces gulbargensis. Braz. J. Microbiol. 2010, 41, 173–178. [Google Scholar] [CrossRef]
- Kabeer, S.S.; Francis, B.; Vishnupriya, S.; Kattatheyil, H.; Joseph, K.J.; Krishnan, K.P.; Mohamed Hatha, A.A. Characterization of L-asparaginase from Streptomyces koyangensis SK4 with acrylamide-minimizing potential in potato chips. Braz. J. Microbiol. 2023, 54, 1645–1654. [Google Scholar] [CrossRef]
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’darov, M. A Novel L-asparaginase from Hyperthermophilic Archaeon Thermococcus sibiricus: Heterologous Expression and Characterization for Biotechnology Application. Int. J. Mol. Sci. 2021, 22, 9894. [Google Scholar] [CrossRef]
- Saeed, H.; Hemida, A.; El-Nikhely, N.; Abdel-Fattah, M.; Shalaby, M.; Hussein, A.; Eldoksh, A.; Ataya, F.; Aly, N.; Labrou, N.; et al. Highly efficient Pyrococcus furiosus recombinant L-asparaginase with no glutaminase activity: Expression, purification, functional characterization, and cytotoxicity on THP-1, A549 and Caco-2 cell lines. Int. J. Biol. Macromol. 2020, 156, 812–828. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Khan, J.A.; Al-Ghamdi, M.A.; Khan, M.I.; Zeyadi, M.A. Studies on the recombinant production and anticancer activity of thermostable L- asparaginase I from Pyrococcus abyssi. Braz. J. Biol. 2021, 82, e244735. [Google Scholar] [CrossRef]
- Saleena, S.K.; Johnson, J.I.; Joseph, J.K.; Padinchati, K.K.; Abdulla, M.H.A. Production and optimization of L-asparaginase by Streptomyces koyangensis SK4 isolated from Arctic sediment. J. Basic Microbiol. 2023, 63, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; El-Aref, H.M.; Ezzeldin, A.M.; Ewida, R.M.; Al-Bedak, O.A.M. Enhanced production and purification of L-asparaginase from Bacillus paralicheniformis AUMC B-516 with potent cytotoxicity against MCF-7 cell lines. AMB Express 2025, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, I.; Shahzad, S.; Yasmin, A.; Almusallam, S.Y.; Al-Megrin, W.A.I. Efficient Production, Purification and Characterization of Therapeutically Significant L-asparaginase from Bacillus licheniformis ASN51. Poli. J. Environ. Stud. 2023, 32, 4267–4280. [Google Scholar] [CrossRef]
- Shahnazari, M.; Bigdeli, R.; Dashbolaghi, A.; Ahangari Cohan, R.; Shoari, A.; Hosseini, H.; Nouri Inanlou, D.; Asgary, V. Biochemical and Biological Evaluation of an L-asparaginase from Isolated Escherichia coli MF-107 as an Anti-Tumor Enzyme on MCF7 Cell Line. Iran. Biomed. J. 2022, 26, 279–290. [Google Scholar] [CrossRef]
- Jetti, J.; Jetti, A.; Perla, R. Production of L-Aparaginase by Using Pectobacterium carotovorum. J. Prob. Health 2017, 5, 1000168. [Google Scholar] [CrossRef]
- El-Bessoumy, A.A.; Sarhan, M.; Mansour, J. Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. BMB Rep. 2004, 37, 387–393. [Google Scholar] [CrossRef]
- Deepak, V.M.M.; Vaishnavi, L.; Palaniswamy, R. Isolation and screening of L-asparaginase producing Sphingomonas sp. from soil sample. Asian J. Pharm. Clin. Res. 2025, 18, 58–62. [Google Scholar] [CrossRef]
- Neto, D.C.; Buzato, J.B.; Borsato, D. L-asparaginase production by Zymomonas mobilis during molasses fermentation: Optimization of culture conditions using factorial design. Acta Sci. Technol. 2006, 28, 151–153. [Google Scholar] [CrossRef]
- Elshafei, A.; El-Ghonemy, D.H. Extracellular glutaminase-free L-asparaginase from Trichoderma viride f2: Purification, biochemical characterization and evaluation of its potential in mitigating acrylamide formation in starchy fried food. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e4336. Available online: https://office2.jmbfs.org/index.php/JMBFS/article/view/4336/346 (accessed on 27 July 2025).
- Ratuchne, A.; Izidoro, S.C.; Beitel, S.M.; Lacerda, L.T.; Knob, A. A new extracellular glutaminase and urease-free L-asparaginase from Meyerozyma guilliermondii. Braz. J. Microbiol. 2023, 54, 715–723. [Google Scholar] [CrossRef]
- Momeni, V.; Alemzadeh, I.; Vosoughi, M. Enhancement of L-asparaginase Production by Candida utilis in a 13 L Fermenter and its Purification. Int. J. Eng. 2015, 28, 1134–1139. Available online: https://www.ije.ir/article_72558.html (accessed on 27 July 2025). [CrossRef]
- Costa, I.M.; Schultz, L.; de Araujo Bianchi Pedra, B.; Leite, M.S.M.; Farsky, S.H.P.; de Oliveira, M.A.; Pessoa, A.; Monteiro, G. Recombinant L-asparaginase 1 from Saccharomyces cerevisiae: An allosteric enzyme with antineoplastic activity. Sci. Rep. 2016, 6, 36239. [Google Scholar] [CrossRef]
- Moguel, I.S.; Yamakawa, C.K.; Brumano, L.P.; Pessoa, A., Jr.; Mussatto, S.I. Selection and Optimization of Medium Components for the Efficient Production of L-asparaginase by Leucosporidium scottii L115—A Psychrotolerant Yeast. Fermentation 2022, 8, 398. [Google Scholar] [CrossRef]
- Aktar, B.Y.; Aysan, A.; Turunen, O.; Yağci, T.; Solğun, H.A.; Binay, B. L-asparaginase from Lachancea Thermotolerans: Effect of Lys99Ala on Enzyme Performance and in vitro Antileukemic Efficacy. Biotechnol. J. 2024, 19, e202400507. [Google Scholar] [CrossRef] [PubMed]
- Marathe, A.; Charya, L. Goan mangrove yeast: A source of therapeutic enzyme L-asparaginase. Folia Microbiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.C.; Aguilar, J.G.D.S.; Orrillo Lindo, S.M.D.R.; de Castro, R.J.S.; Sato, H.H. L-asparaginase from Aspergillus oryzae spp.: Effects of production process and biochemical parameters. Prep. Biochem. Biotechnol. 2022, 52, 253–263. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Hassan, M.M.; Abouzeid, M.A.-E.; Mahmoud, D.A.; Elghonemy, D.H. Purification, characterization and antitumor activity of L-asparaginase from Penicillium brevicompactum NRC 829. Micro. Res. J. Inter. 2012, 2, 158–174. [Google Scholar] [CrossRef]
- Johar, D.; Hefny, H.M.; Mansy, M.S.; Mekawey, A.A.I.; Abdulrahman, M.S.; Zaky, S. Cytotoxicity of L-asparaginase from eucaryotic Cladosporium species against breast and colon cancer in vitro. J. Egypt. Natl. Cancer Inst. 2025, 37, 33. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.A.; Awad, M.F.; El-Shenawy, F.S.; El-Bondkly, A.M.A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci. 2021, 28, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Souza, P.; Cardoso, S.; Cruvinel, K.; Abrunhosa, L.S.; Ferreira Filho, E.X.; Inácio, J.; Pinho, D.B.; Pessoa, A.; OMagalhães, P. Filamentous Fungi Producing L-asparaginase with Low Glutaminase Activity Isolated from Brazilian Savanna Soil. Pharmaceutics 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.I.; Ouf, S.A.; Eweis, M.; Soliman, D.M. Optimization of L-asparaginase Production from Some Filamentous Fungi with Potential Pharmaceutical Properties. Egypt. J. Bot. 2018, 58, 355–369. [Google Scholar] [CrossRef]
- Da Silva Duarte, S.M.; de Carvalho Silva, A.K.; Assunção Borges, K.R.; Borges Cordeiro, C.; Lindoso Lima, F.J.; da Silva, M.A.C.N.; de Souza Andrade, M.; do Desterro Soares Brandão Nascimento, M. Production, Characterization Purification, and Antitumor Activity of L-asparaginase from Aspergillus niger. Fermentation 2024, 10, 226. [Google Scholar] [CrossRef]
- Fazeli, N.; Alimadadi, N.; Nasr, S. Screening and Optimization of Process Parameters for the Production of L-asparaginase by Indigenous Fungal-Type Strains. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 409–416. [Google Scholar] [CrossRef]
- Ali, S.; Maqsood, S.; Ahmad, M.U.; Shabbir, I.; Raish, M.; Batool, F.; Rehman, A.U.; Liaqat, I.; Abbasi, B.B.K.; Irfan, A.; et al. Enhanced production of extracellular L-asparaginase in batch culture via nitrous acid-induced mutagenesis of Aspergillus oryzae. Microb. Cell Factories 2025, 24, 172. [Google Scholar] [CrossRef]
- Rabelo, N.G.; Simões, L.A.; de Andrade Teixeira Fernandes, N.; Souza, A.C.; Fernandes, M.L.P.; Veríssimo, L.A.A.; Schwan, R.F.; Dias, D.R. Optimization of L-asparaginase Production from Aspergillus caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification. Appl. Microbiol. 2025, 5, 19. [Google Scholar] [CrossRef]
- Guo, J.; Coker, A.R.; Wood, S.P.; Cooper, J.B.; Chohan, S.M.; Rashid, N.; Akhtar, M. Structure and function of the thermostable L-asparaginase from Thermococcus kodakarensis. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73, 889–895. [Google Scholar] [CrossRef]
- Sharma, A.; Kaushik, V.; Goel, M. Insights into the Distribution and Functional Properties of L-asparaginase in the Archaeal Domain and Characterization of Picrophilus torridus Asparaginase Belonging to the Novel Family Asp2like1. ACS Omega 2022, 7, 40750–40765. [Google Scholar] [CrossRef]
- Zuo, S.; Xue, D.; Zhang, T.; Jiang, B.; Mu, W. Biochemical characterization of an extremely thermostable L-asparaginase from Thermococcus gammatolerans EJ3. J. Mol. Catal. B Enzym. 2014, 109, 122–129. [Google Scholar] [CrossRef]
- Narayana, K.J.; Kumar, K.G.; Vijayalakshmi, M. L-asparaginase production by Streptomyces albidoflavus. Indian J. Microbiol. 2008, 48, 331–336. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; El-Shweihy, N.M. Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines. Sci. Rep. 2020, 10, 7942. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Nivedita, S.; Behera, S.S.; Behera, H.T.; Gouda, S.K.; Raina, V.; Achary, K.G.; Behera, S.K.; Ray, L. L-asparaginase producing novel Streptomyces sp. HB2AG: Optimization of process parameters and whole genome sequence analysis. 3 Biotech 2023, 13, 201. [Google Scholar] [CrossRef]
- Vimal, A.; Kumar, A. Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol. Genet. Eng. Rev. 2017, 33, 40–61. [Google Scholar] [CrossRef]
- Chand, S.; Mahajan, R.V.; Prasad, J.P.; Sahoo, D.K.; Mihooliya, K.N.; Dhar, M.S.; Sharma, G. A Comprehensive Review on Microbial L-asparaginase: Bioprocessing, Characterization, and Industrial Applications. Biotechnol. Appl. Biochem. 2020, 67, 619–647. [Google Scholar] [CrossRef]
- Eyvazi, S.; Hakemi-Vala, M.; Hashemi, A.; Bejestani, F.B.; Elahi, N. Emergence of NDM-1-Producing Escherichia coli in Iran. Arch. Clin. Infect. Dis. 2017, 13, e62029. [Google Scholar] [CrossRef]
- Ashok, A.; Doriya, K.; Rao, J.V.; Qureshi, A.; Tiwari, A.K.; Kumar, D.S. Microbes Producing L-asparaginase free of Glutaminase and Urease isolated from Extreme Locations of Antarctic Soil and Moss. Sci. Rep. 2019, 9, 1423. [Google Scholar] [CrossRef]
- Costa-Silva, T.A.; Camacho-Córdova, D.I.; Agamez-Montalvo, G.S.; Parizotto, L.A.; Sánchez-Moguel, I.; Pessoa, A., Jr. Optimization of culture conditions and bench-scale production of anticancer enzyme L-asparaginase by submerged fermentation from Aspergillus terreus CCT 7693. Prep. Biochem. Biotechnol. 2018, 49, 95–104. [Google Scholar] [CrossRef]
- Muneer, F.; Siddique, M.H.; Azeem, F.; Rasul, I.; Muzammil, S.; Zubair, M.; Afzal, M.; Nadeem, H. Microbial L-asparaginase: Purification, characterization and applications. Arch. Microbiol. 2020, 202, 967–981. [Google Scholar] [CrossRef]
- Lefin, N.; Miranda, J.; Munhoz Costa, I.; Pedroso Reynaldo, A.; Monteiro, G.; Zamorano, M.; Pessoa, A., Jr.; Farias, J.G. Optimized Amino Acid-Enhanced Medium for Efficient L-asparaginase II Production in E. coli: From Shake Flask to Bioreactor. Fermentation 2025, 11, 239. [Google Scholar] [CrossRef]
- Yang, X.; Rao, Y.; Zhang, M.; Wang, J.; Liu, W.; Cai, D.; Chen, S. Efficient production of L-asparaginase in Bacillus licheniformis by optimizing expression elements and host. Chin. J. Biotechnol. 2023, 39, 1096–1106. [Google Scholar] [CrossRef]
- Suresh, J.V.; Raju, K.J. Studies on the Production of L-asparaginase by Aspergillus Terreus MTCC 1782 Using Agro-Residues under Mixed Substrate Solid State Fermentation. J. Chem. Biol. Phys. Sci. JCBPS 2012, 3, 314. [Google Scholar]
- Soni, S.K.; Soni, R. Policy and Regulatory Frameworks for Green Biorefinery: Driving Sustainable Waste Valorization. In Green Biorefinery Solutions: Transforming Biodegradable Waste into Resources; Springer: Berlin/Heidelberg, Germany, 2025; pp. 315–349. [Google Scholar] [CrossRef]
- Lefin, N.; Miranda, J.; Beltrán, J.F.; Belén, L.H.; Effer, B.; Pessoa, A., Jr.; Farias, J.G.; Zamorano, M. Current State of Molecular and Metabolic Strategies for the Improvement of L-asparaginase Expression in Heterologous Systems. Front. Pharmacol. 2023, 14, 1208277. [Google Scholar] [CrossRef]
- Brumano, L.P.; da Silva, F.V.S.; Costa-Silva, T.A.; Apolinário, A.C.; Santos, J.H.P.M.; Kleingesinds, E.K.; Monteiro, G.; de Oliveira Rangel-Yagui, C.; Benyahia, B.; Junior, A.P. Development of L-asparaginase Biobetters: Current Research Status and Review of the Desirable Quality Profiles. Front. Bioeng. Biotechnol. 2019, 6, 212. [Google Scholar] [CrossRef]
- Chakraborty, M.; Shivakumar, S. Bioprospecting of the Agaricomycete Ganoderma Australe GPC191 as Novel Source for L-asparaginase Production. Sci. Rep. 2021, 11, 6192. [Google Scholar] [CrossRef]
- Correa, H.T.; Vieira, W.F.; Pinheiro, T.M.A.; Cardoso, V.L.; Silveira, E.; Sette, L.D.; Pessoa, A.; Filho, U.C. L-asparaginase and Biosurfactants Produced by Extremophile Yeasts from Antarctic Environments. Ind. Biotechnol. 2020, 16, 107–116. [Google Scholar] [CrossRef]
- Gurunathan, B.; Sahadevan, R. Design of Experiments and Artificial Neural Network Linked Genetic Algorithm for Modeling and Optimization of L-asparaginase Production by Aspergillus Terreus MTCC 1782. Biotechnol. Bioprocess Eng. 2011, 16, 50–58. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hamouda, R.A.; Elshafey, N. Artificial Intelligence-Based Optimization for Extracellular L-Glutaminase Free L-asparaginase Production by Streptomyces Violaceoruber under Solid State Fermentation Conditions. Sci. Rep. 2024, 14, 29625. [Google Scholar] [CrossRef]
- Moguel, I.S.; Yamakawa, C.K.; Pessoa, A., Jr.; Mussatto, S.I. L-asparaginase Production by Leucosporidium Scottii in a Bench-Scale Bioreactor with Co-Production of Lipids. Front. Bioeng. Biotechnol. 2020, 8, 576511. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Nunes, J.C.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P. Recent Strategies and Applications for L-asparaginase Confinement. Molecules 2020, 25, 5827. [Google Scholar] [CrossRef]
- Veríssimo, N.V.; Vicente, F.A.; de Oliveira, R.C.; Likozar, B.; de Souza Oliveira, R.P.; Pereira, J.F.B. Ionic Liquids as Protein Stabilizers for Biological and Biomedical Applications: A Review. Biotechnol. Adv. 2022, 61, 108055. [Google Scholar] [CrossRef]
- Veríssimo, N.V.P.; Mussagy, C.U.; Bento, H.B.S.; Pereira, J.F.B.; de Carvalho Santos-Ebinuma, V. Ionic Liquids and Deep Eutectic Solvents for the Stabilization of Biopharmaceuticals: A Review. Biotechnol. Adv. 2024, 71, 108316. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wan, X.; Geng, X.; Xue, D.; Xie, Z.; Chen, C. Microbial L-asparaginase for Application in Acrylamide Mitigation from Food: Current Research Status and Future Perspectives. Microorganisms 2021, 9, 1659. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.M.; El-Fakharany, E.M.; Abdelkhalek, E.S.; Sidkey, N.M. L-asparaginase and L-Glutaminase: Sources, Production, and Applications in Medicine and Industry. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 179. [Google Scholar] [CrossRef]
- Sahu, S.; Agrawal, S.; Sahu, A. Regulatory Policies on Use of Food Enzymes. In Enzymes Beyond Traditional Applications in Dairy Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 519–535. [Google Scholar] [CrossRef]
- Veríssimo, N.V.; Mussagy, C.U.; Oshiro, A.A.; Mendonça, C.M.N.; de Carvalho Santos-Ebinuma, V.; Pessoa, A.; de Souza Oliveira, R.P.; Pereira, J.F.B. From Green to Blue Economy: Marine Biorefineries for a Sustainable Ocean-Based Economy. Green Chem. 2021, 23, 9377–9400. [Google Scholar] [CrossRef]
- Rojas, L.F.; Zapata, P.; Ruiz-Tirado, L. Agro-Industrial Waste Enzymes: Perspectives in Circular Economy. Curr. Opin. Green Sustain. Chem. 2022, 34, 100585. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent Advances in Sugarcane Industry Solid By-Products Valorization. Waste Biomass Valorization 2017, 8, 241–266. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Suriya, J.; Krishnan, M.; Manivasagan, P.; Kim, S.-K. Production of Enzymes from Agricultural Wastes and Their Potential Industrial Applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 125–148. ISBN 1043-4526. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Bioprocesses for Enzyme Production Using Agro-Industrial Wastes: Technical Challenges and Commercialization Potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 61–93. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent Advances in Production of Lignocellulolytic Enzymes by Solid-State Fermentation of Agro-Industrial Wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407. [Google Scholar] [CrossRef]
- Lalor, F.; Fitzpatrick, J.; Sage, C.; Byrne, E. Sustainability in the Biopharmaceutical Industry: Seeking a Holistic Perspective. Biotechnol. Adv. 2019, 37, 698–707. [Google Scholar] [CrossRef]
- Sarkis, M.; Fyfe, A.T.; Kontoravdi, C.; Papathanasiou, M.M. Towards a Net Zero, Socially Sustainable and Eco-Efficient Biopharma Industry: How Far Are We? Curr. Opin. Chem. Eng. 2024, 44, 101027. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R.; Renganathan, S. Applications of Asparaginase in Food Processing. In Green Bioprocesses: Enzymes in Industrial Food Processing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–98. [Google Scholar] [CrossRef]
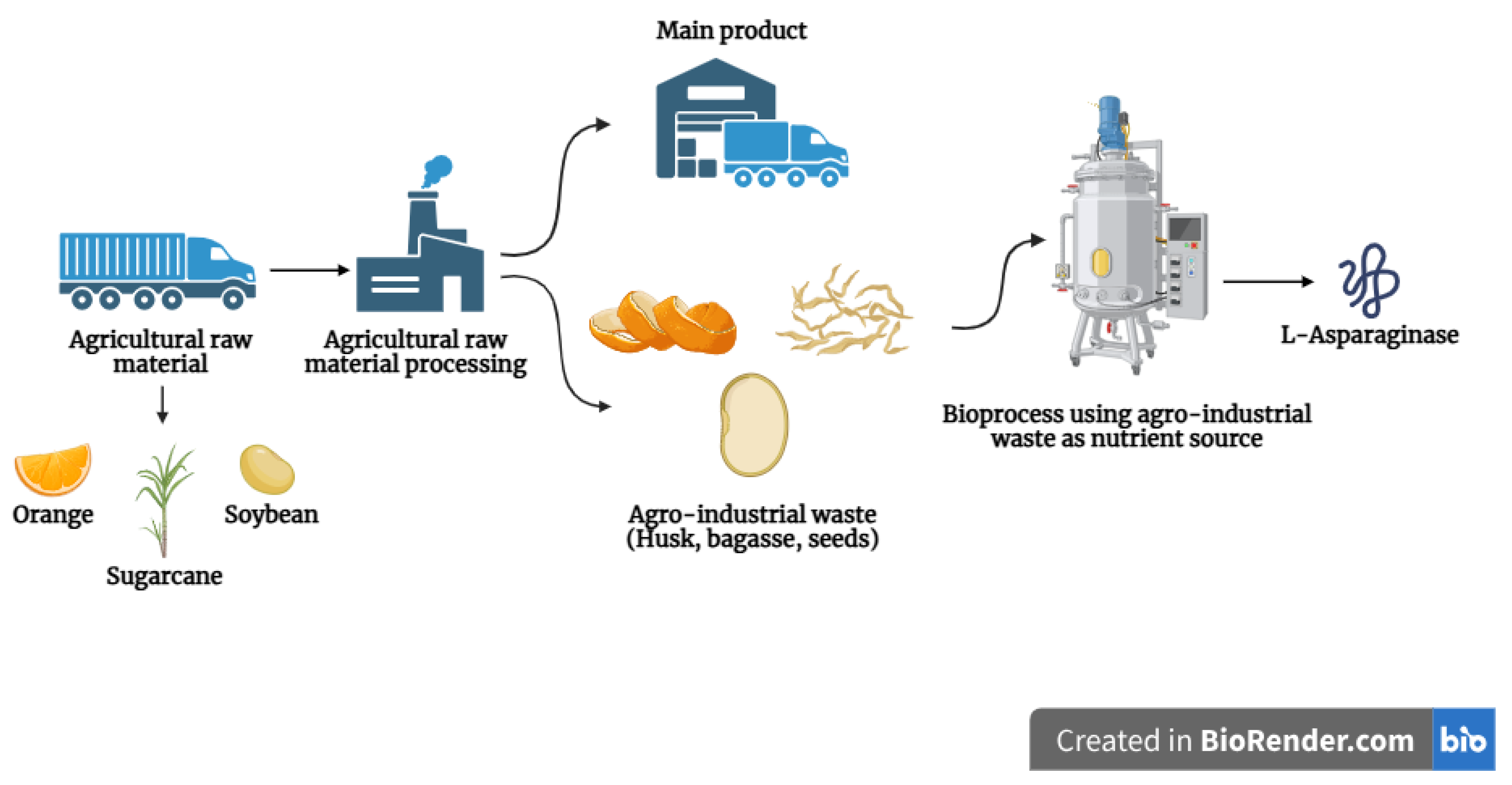
| Residue | Microorganism | SSF/SmF | Pretreatment/Supplementation | Best Results | Ref. |
|---|---|---|---|---|---|
| Wheat, arhar, kulthi brans | Purpureocillium lilacinum | SSF | RSM optimization | 248.234 U/gds (optimized); 190.439 U/gds (main effects) | [21] |
| Soybean meal, corn gluten, groundnut de-oiled cake | Aspergillus niger | SSF | Ternary mixture; ANN optimization | 141.45 ± 5.24 IU/gds | [20] |
| Pitaya peel waste | Aspergillus niger | SmF (induction) | Moisture optimization ~60% | ≤0.6712 IU/mL | [23] |
| Papaya peel | Aspergillus quadrilineatus | SmF (screening) | Initial screening | ≈2.99 U/mL | [22] |
| Carrot peel | Aspergillus quadrilineatus | SmF | Selected after screening for nutritional profile | Best in mass culture (not specified) | [22] |
| Defatted Tenebrio molitor biomass (insect) | Penicillium sp. LAMAI-505 | SSF | Direct use of defatted biomass | ≈2.75 U/g | [24] |
| Cane molasses/Glycerol | Haematococcus pluvialis | SmF/photo-heterotrophic culture | Carbon pulses (molasses or glycerol) | Intracellular qualitative changes | [25] |
| Cactus cladode flours (Opuntia/Nopalea) | Aspergillus oryzae | SSF | Low flour concentration (0.2% w/v), acidic pH | Not quantified | [26] |
| Category | Trends and Perspectives | Potential Impacts | Ref. |
|---|---|---|---|
| Sustainability and green bioprocesses | Development of processes with low energy consumption, sustainable solvents, and reuse of inputs. | Cost reduction, lower environmental impact, and contribution to the circular economy. Increased industrial feasibility and compliance with environmental regulations. | [17,18,23,74] |
| Funding and public policies | Incentives for bioeconomy projects and financing of clean technologies at national and international levels. | Greater investment attractiveness and acceleration of industrial implementation. | [75] |
| Emerging technologies | Integration with advanced bioreactors (e.g., continuous), automation, and omics-based tools. | Better process control, improved scalability, and real-time optimization. | [67,76] |
| Metabolic engineering and bioprospection | Use of novel bioprospected or genetically modified microorganisms and robust recombinant systems to enhance enzyme yield from residues. | Higher productivity and improved efficiency in the utilization of heterogeneous substrates. | [8,66,77,78,79] |
| AI and digitalization | Application of AI, machine learning, big data, and digital twins to optimize media formulation, predict yields, and control processes. | Reduced R&D time and costs, more robust processes, and faster scale-up. | [77,80,81] |
| Integration into biorefineries | Combined production of L-asparaginase, bioenergy, enzymes, and biopolymers within multiproduct platforms. | Product diversification and greater economic valorization of residues. | [79,82,83] |
| Stability and formulation | Use of biocompatible additives, encapsulation, and stabilization technologies for residue-derived L-asparaginase. | Improved stability, longer shelf life, and expanded possibilities for industrial use. | [4,77,84,85,86] |
| Expanded applications | Extension of applications beyond pharmaceuticals, including the food industry (acrylamide reduction) and biotechnology. | Market expansion and broader commercial potential for the enzyme. | [29,87,88] |
| Regulation and safety | Increasing demand for traceability and validation in the use of residues as substrates. | Significant regulatory barriers, but opportunities for green and sustainable certification. | [75,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corvello, E.; Gambarato, B.C.; Veríssimo, N.V.P.; Rodrigues, T.Q.J.; Pesconi, A.D.R.; Carvalho, A.K.F.; Bento, H.B.S. Waste to Value: L-Asparaginase Production from Agro-Industrial Residues. Processes 2025, 13, 3088. https://doi.org/10.3390/pr13103088
Corvello E, Gambarato BC, Veríssimo NVP, Rodrigues TQJ, Pesconi ADR, Carvalho AKF, Bento HBS. Waste to Value: L-Asparaginase Production from Agro-Industrial Residues. Processes. 2025; 13(10):3088. https://doi.org/10.3390/pr13103088
Chicago/Turabian StyleCorvello, Enzo, Bruno C. Gambarato, Nathalia V. P. Veríssimo, Thiago Q. J. Rodrigues, Alice D. R. Pesconi, Ana K. F. Carvalho, and Heitor B. S. Bento. 2025. "Waste to Value: L-Asparaginase Production from Agro-Industrial Residues" Processes 13, no. 10: 3088. https://doi.org/10.3390/pr13103088
APA StyleCorvello, E., Gambarato, B. C., Veríssimo, N. V. P., Rodrigues, T. Q. J., Pesconi, A. D. R., Carvalho, A. K. F., & Bento, H. B. S. (2025). Waste to Value: L-Asparaginase Production from Agro-Industrial Residues. Processes, 13(10), 3088. https://doi.org/10.3390/pr13103088









