Eco-Friendly Oxidative–Adsorptive Desulfurization for Real Diesel Fuel Using Green MnO2 Biowaste-Extracted Calcite in Digital Basket Reactor
Abstract
1. Introduction
2. Material Used and Experimental Design
2.1. Chemicals
2.1.1. Feedstock
2.1.2. Oxidizing Agent
2.1.3. Catalyst Component
2.1.4. Adsorbent
2.2. Preparation of MnO2/Calcite Nanocatalyst
2.3. Characterization of MnO2/Calcite Nanocatalyst and AC
2.4. Experimental Procedure
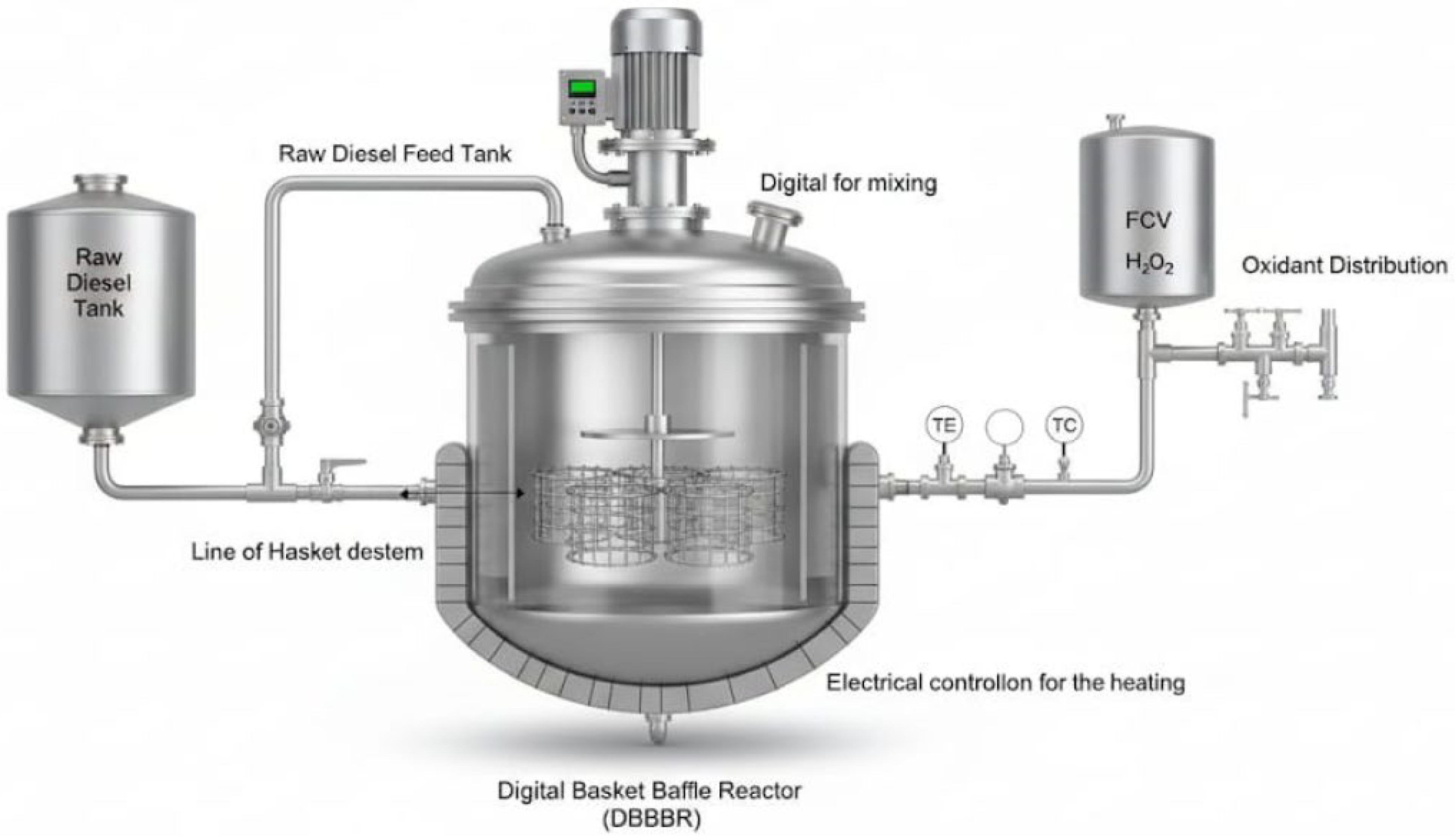
2.4.1. Design of Basket Reactor
2.4.2. Oxidative–Adsorptive Desulfurization (ODS) Process in DBR
3. Results and Discussion
3.1. Characterization of the Designed Nano-Catalyst (MnO2/Calcite)
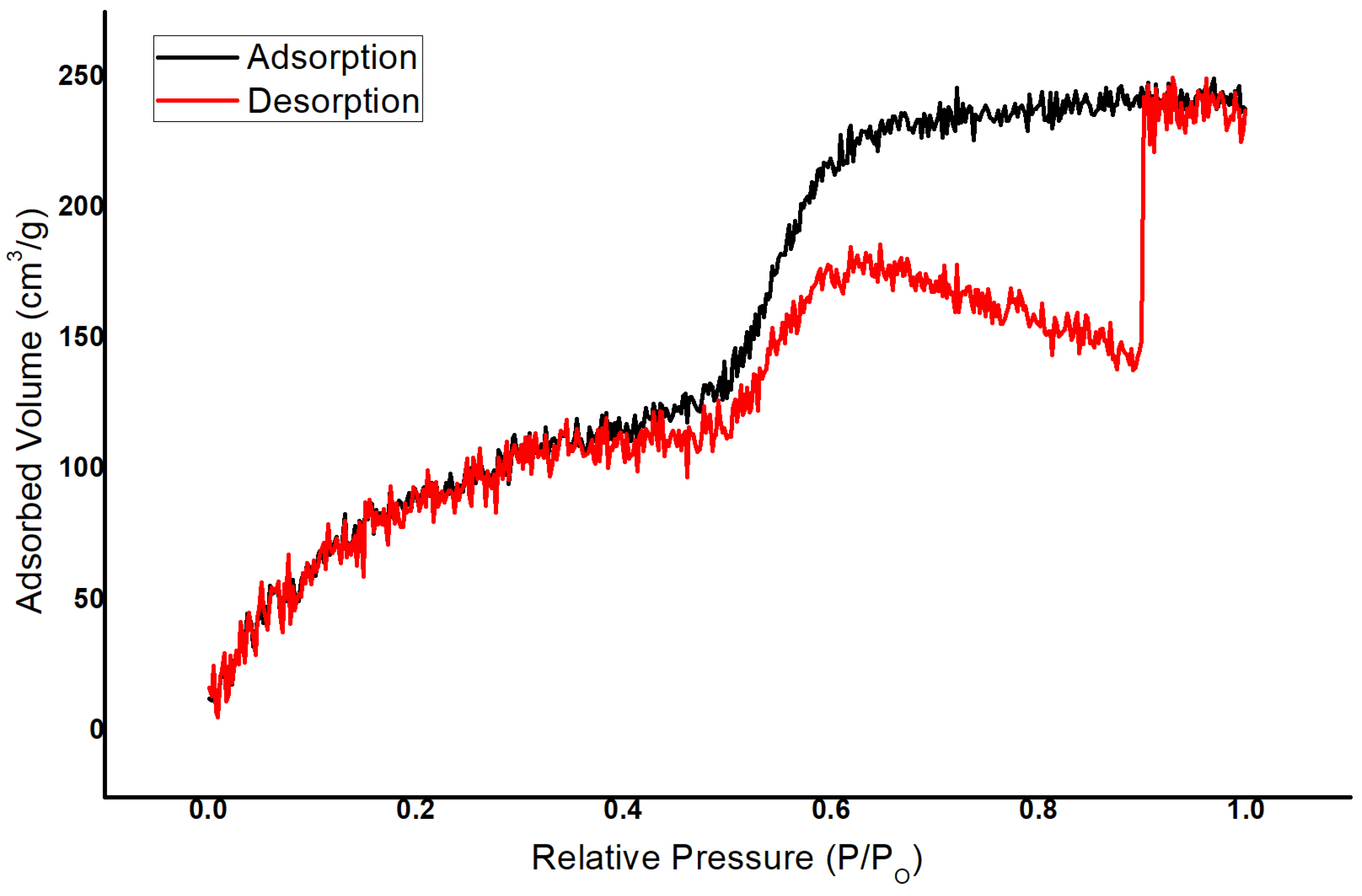
3.1.1. Surface Area Analysis
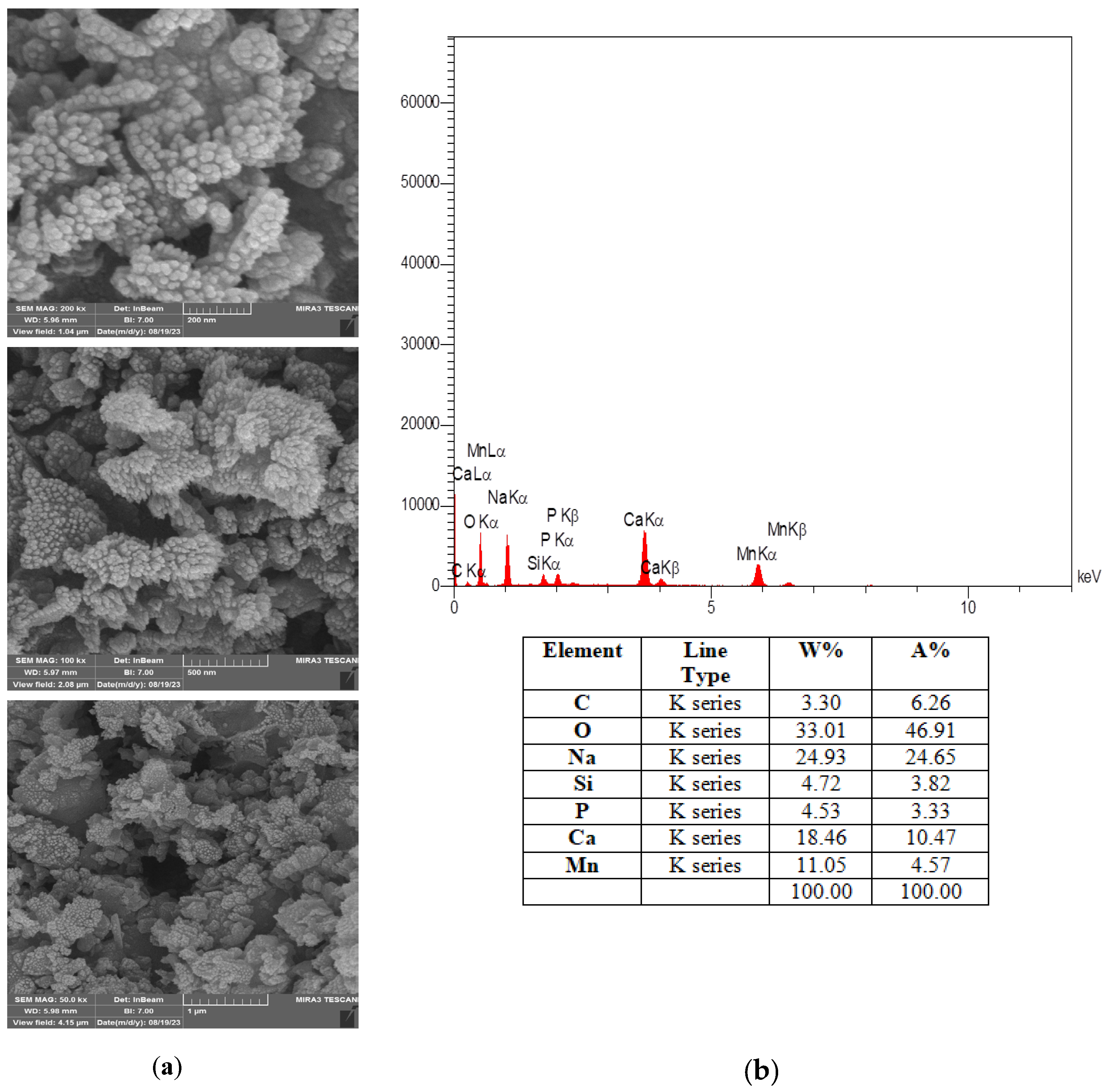
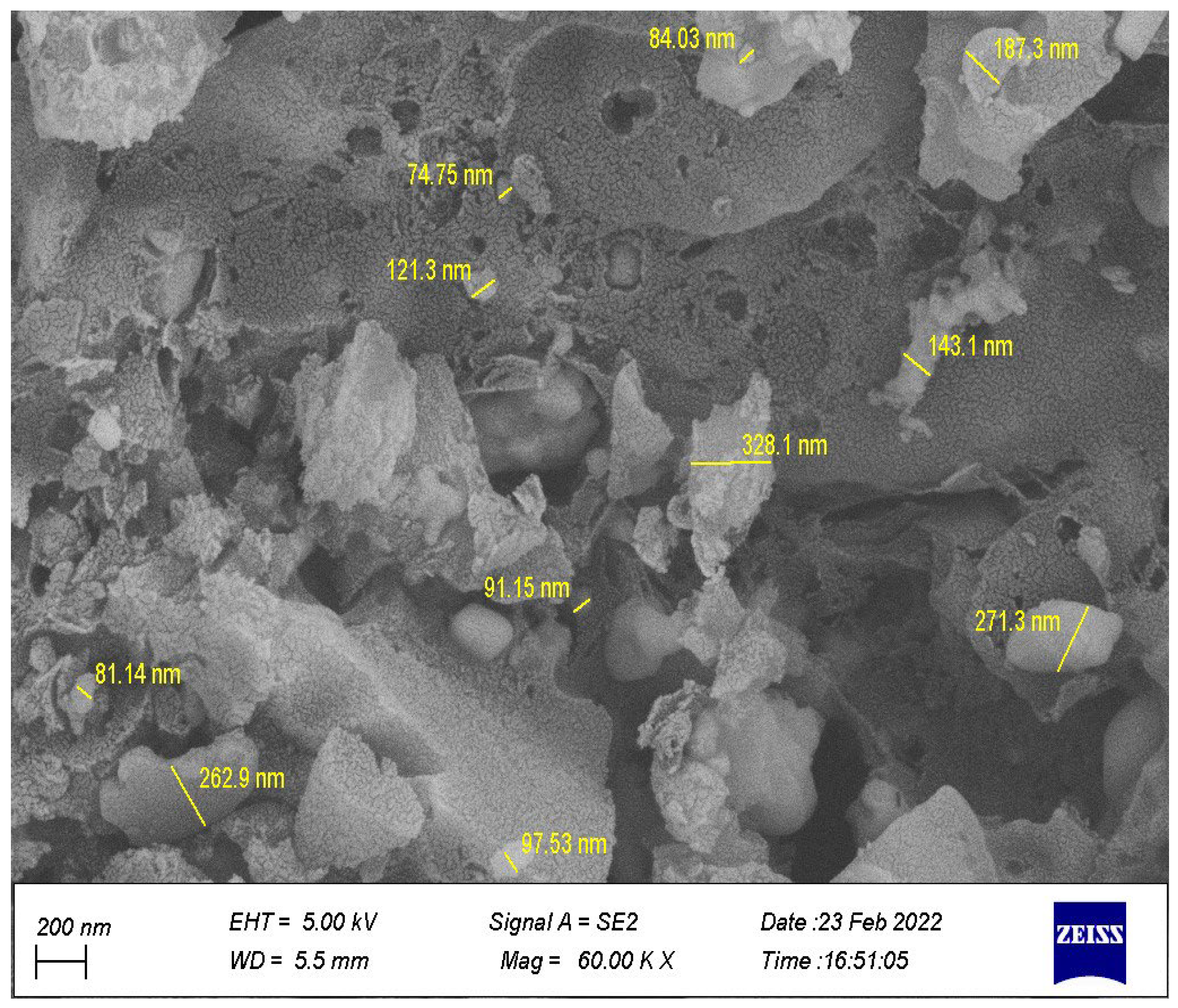
3.1.2. Morphological and Elemental Analysis of the Synthesized Catalyst
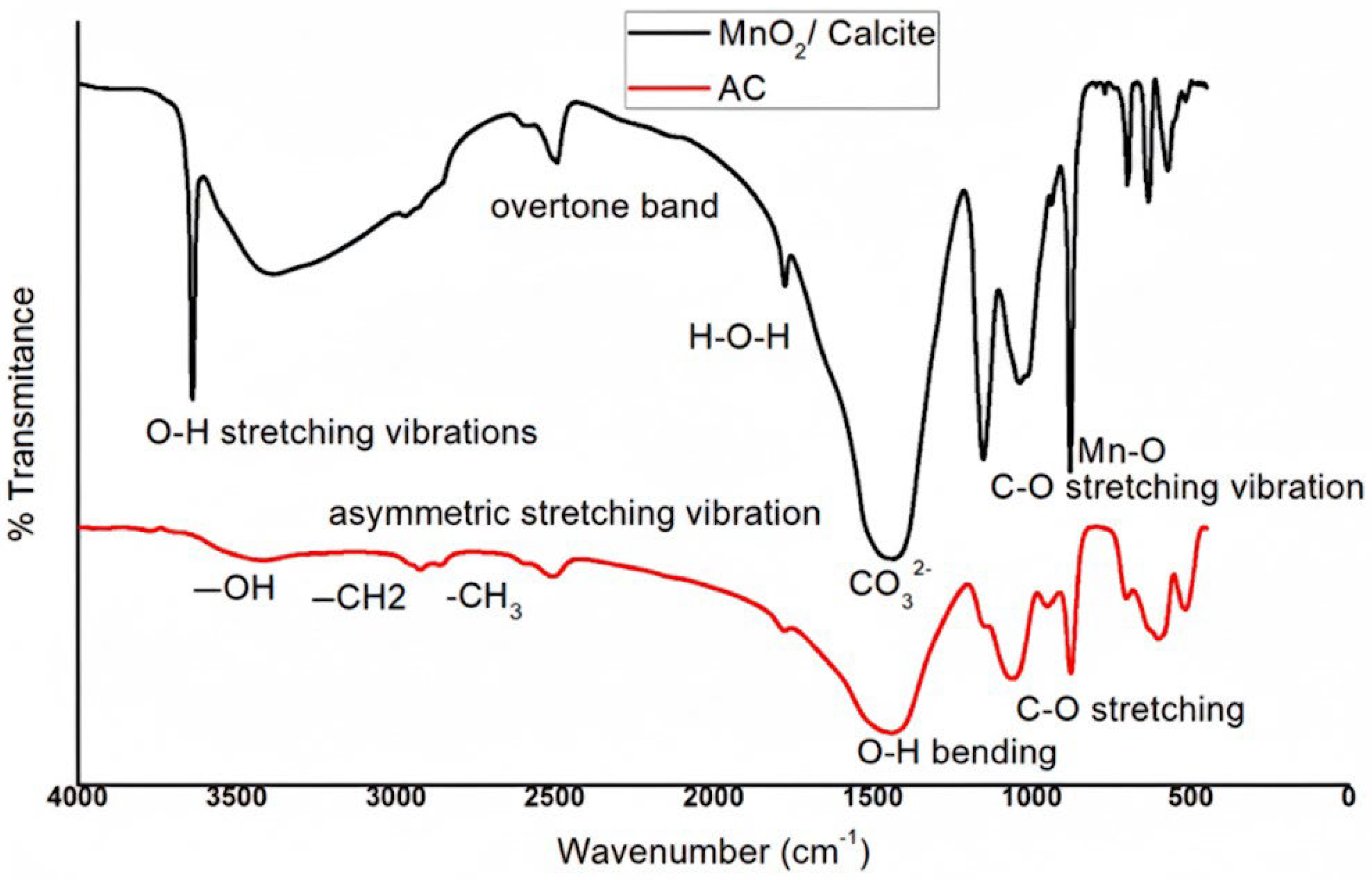
3.1.3. FTIR Spectral Analysis and Functional Group Identification
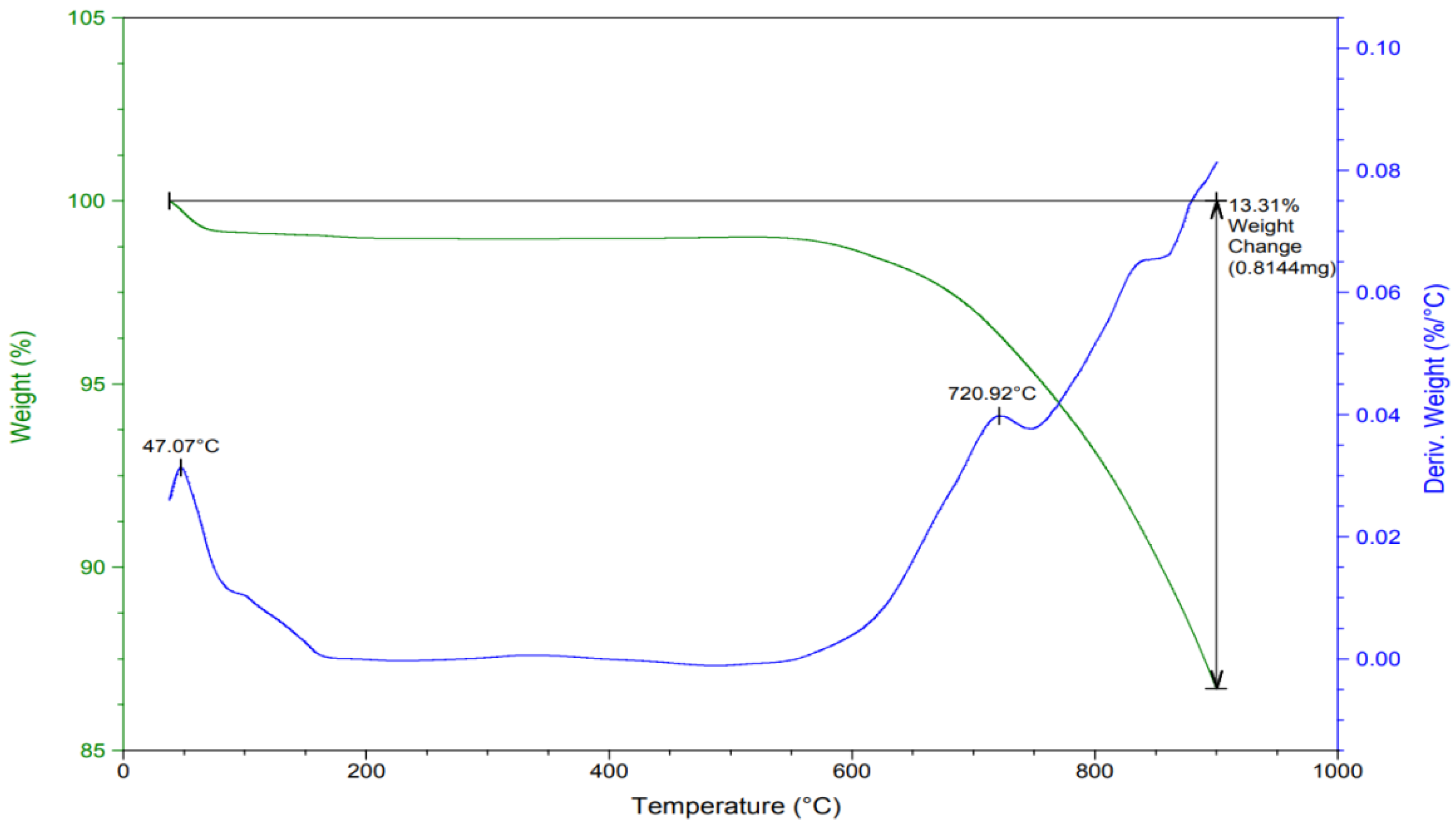
3.1.4. Thermal Stability and Decomposition Behavior: TGA and DTA Analysis
3.2. Characterization of the Adsorbent (Activated Carbon)
3.2.1. X-Ray Diffraction (XRD) Analysis of Activated Carbon
3.2.2. Surface Morphology of Activated Carbon: SEM Analysis
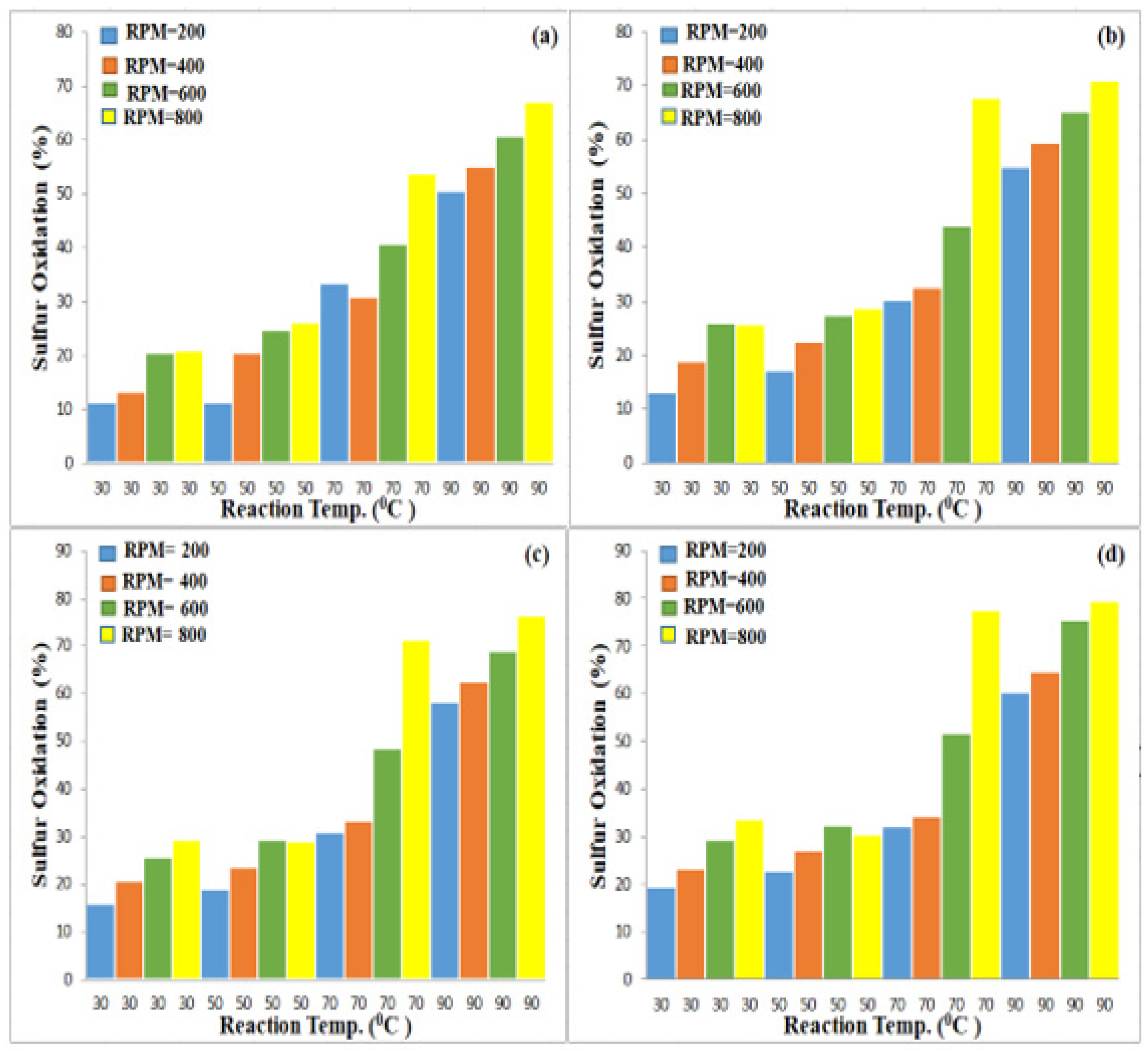
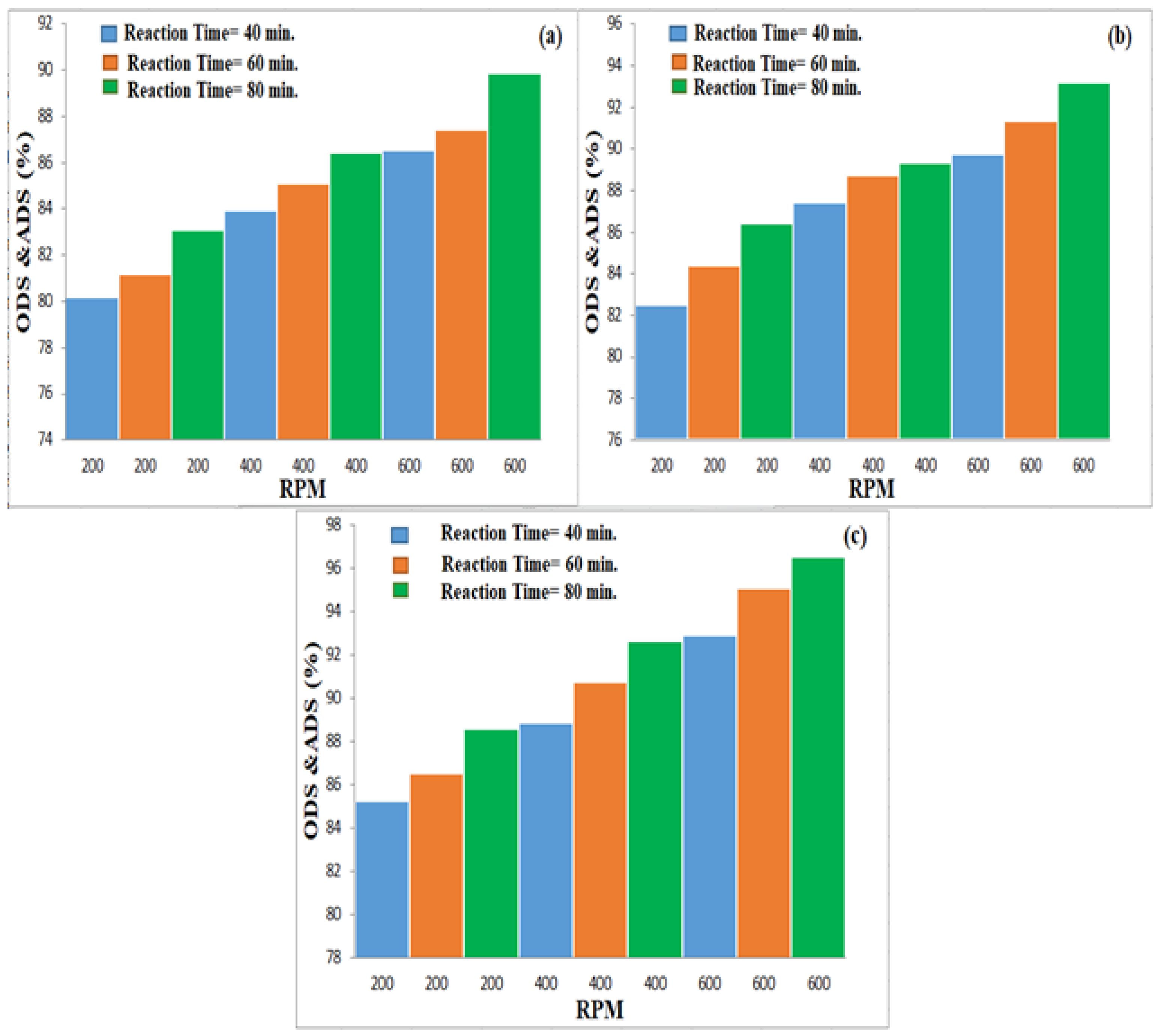
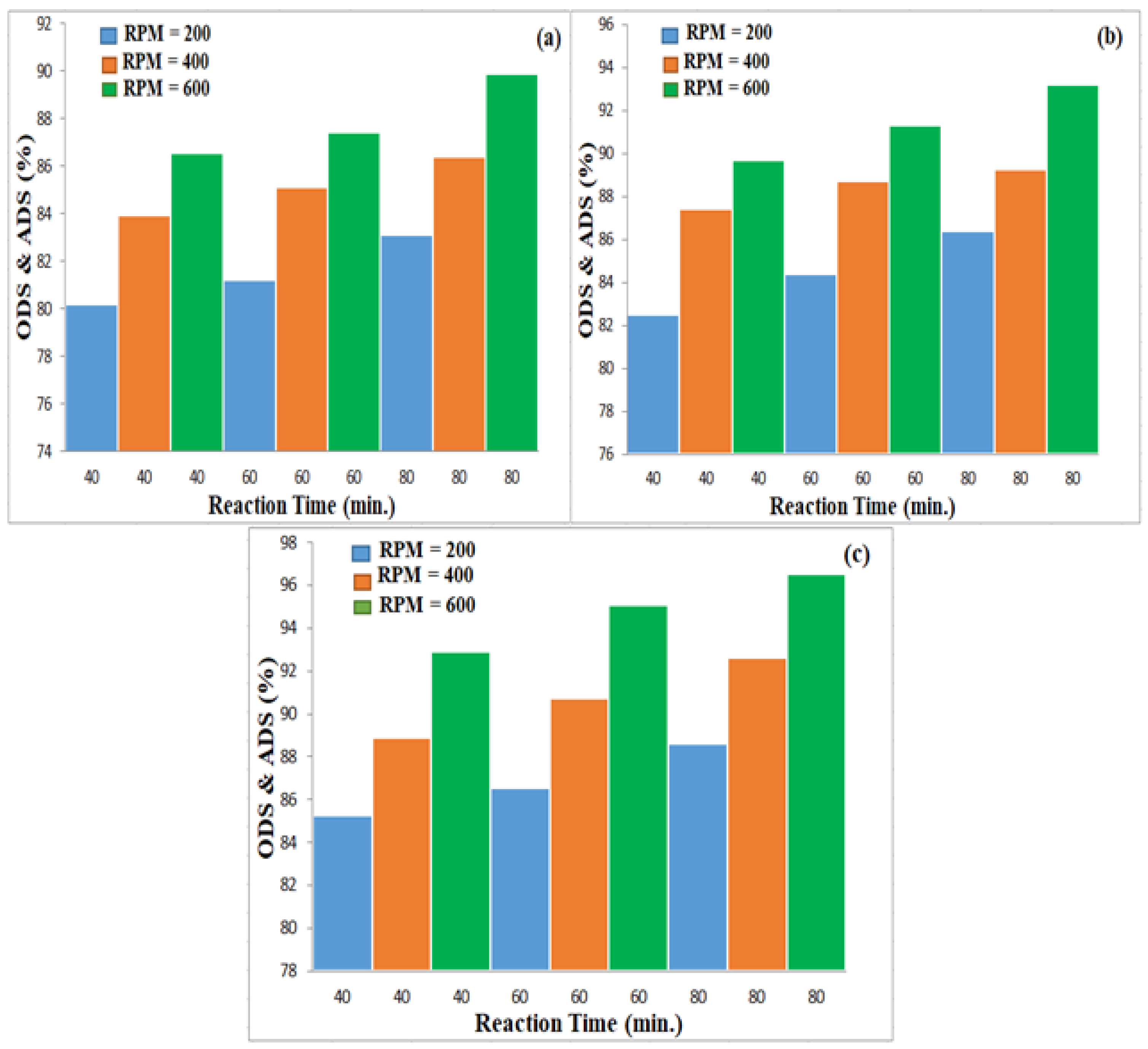
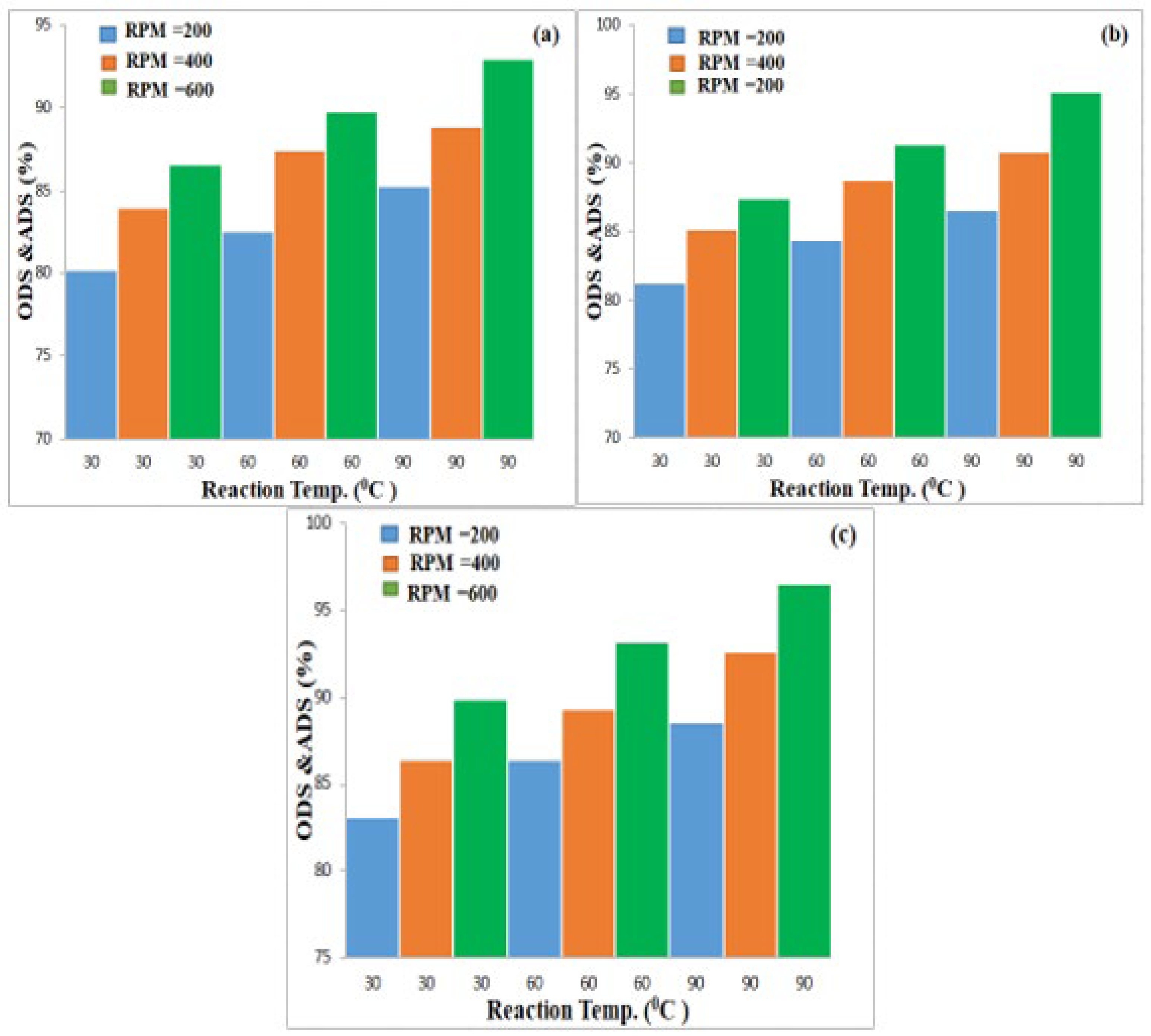
3.3. Effect of Experimental Parameters on ODS
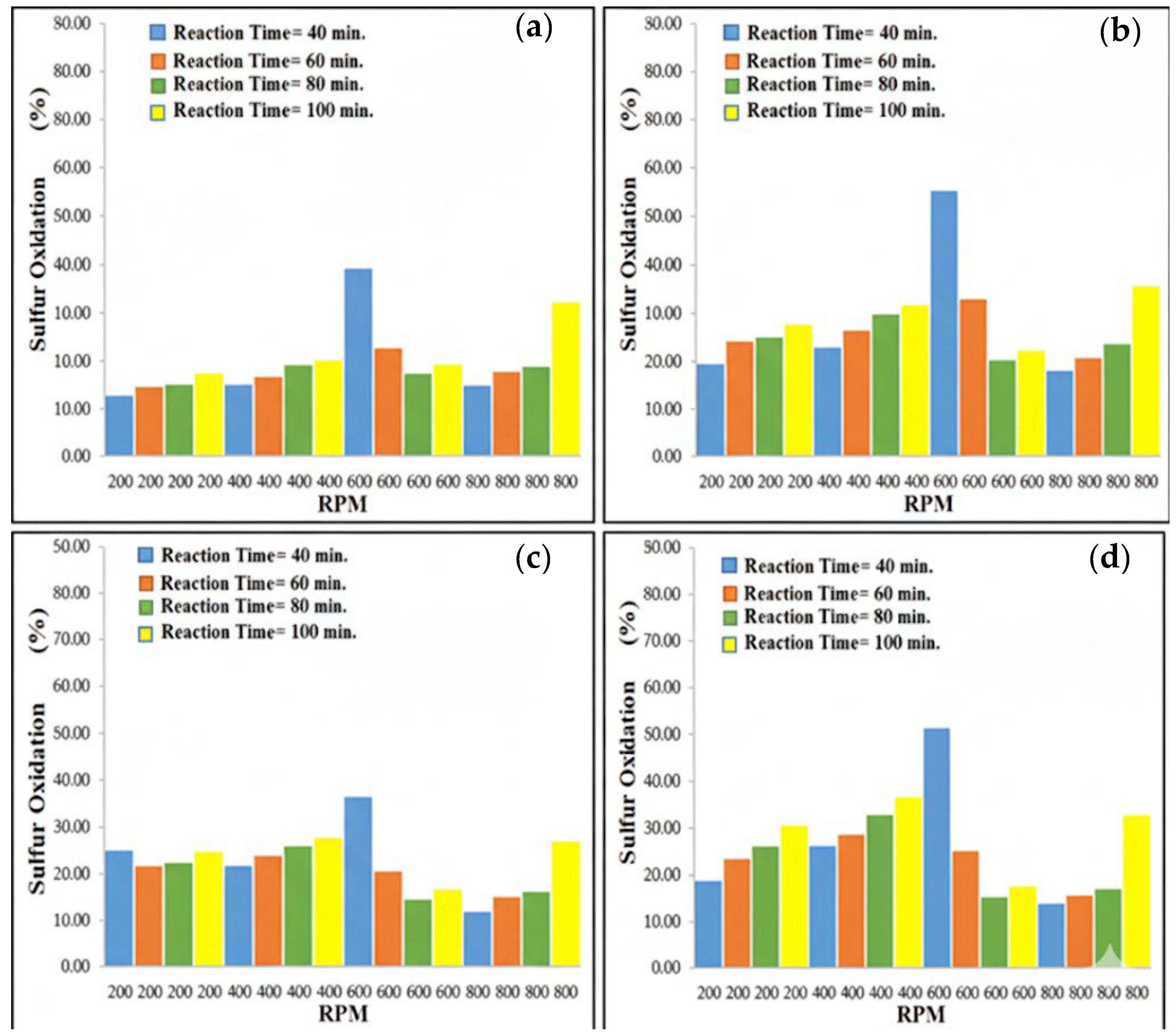
3.3.1. Effect of Magnetic Stirrer Speed
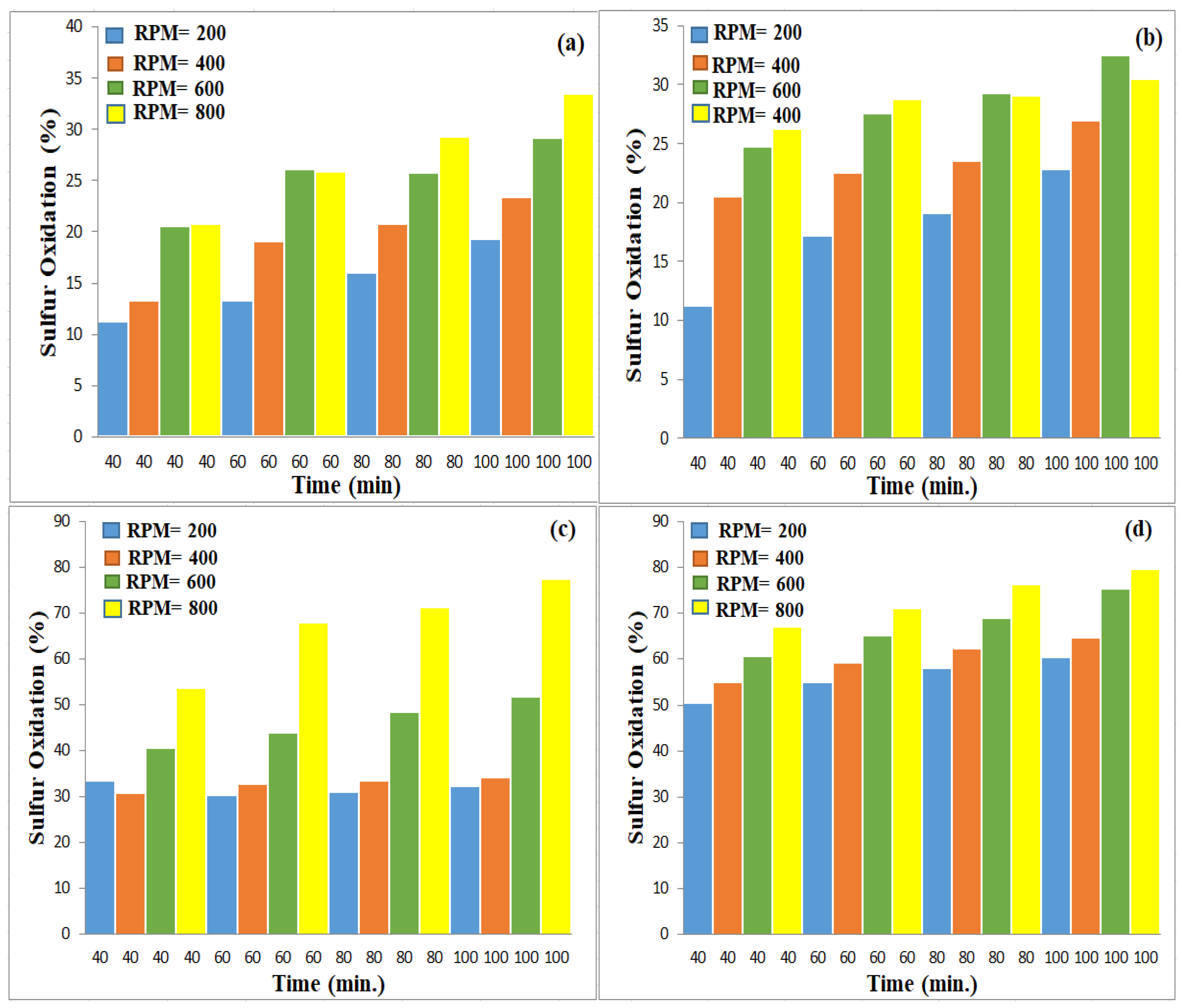
3.3.2. Effect of Oxidation Time
3.3.3. Effect of Oxidation Temperature
3.4. Effect of Studied Variables on ADS by AC Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U.S. Environmental Protection Agency (EPA). Ultra-Low Sulfur Diesel (ULSD) Regulations. 2020. Available online: https://www.epa.gov/diesel-fuel-standards/diesel-fuel-standards-and-rulemakings (accessed on 15 September 2025).
- European Commission. Directive 2016/2284 on the Reduction of National Emissions of Certain Atmospheric Pollutants. 2021. Available online: https://eur-lex.europa.eu/TodayOJ/ (accessed on 15 September 2025).
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel, and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Hussein, A.K.; Al-Tabbakh, B.A.; Hameed, S.A.; Mujtaba, I.M.; Saeed, L.I.; Humadi, J.I. Production of green fuel using a new synthetic magnetite mesoporous nano-silica composite catalyst for oxidative desulfurization: Experiments and process modeling. Catalysts 2024, 14, 529. [Google Scholar] [CrossRef]
- Kitashov, Y.N.; Nazarov, A.V.; Zorya, E.I.; Muradov, A.V. Alternative methods for the removal of sulfur compounds from petroleum fractions. Chem. Technol. Fuels Oils 2019, 55, 584–589. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Capel-Sanchez, M.C.; Fierro, J.L.G. Oxidative desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef]
- Humadi, J.I.; Mohammed, W.T. Fast, ultradeep, and continuous desulfurization of heavy gasoil in novel oscillatory basket central baffled reactor using MnO2-incorporated Fe2O3-supported activated carbon catalyst. Fuel 2025, 400, 135716. [Google Scholar] [CrossRef]
- Boshagh, F.; Rahmani, M.; Zhu, W. Recent advances and challenges in developing technological methods assisting oxidative desulfurization of liquid fuels: A review. Energy Fuels 2022, 36, 12961–12985. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, Y.; Zhi, Y.; Yan, L.; Lu, S. Combined extraction–oxidation system for oxidative desulfurization (ODS) of a model fuel. Energy Fuels 2015, 29, 618–625. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Afonso, C.A. Deep desulfurization of diesel fuel using ionic liquids: Current status and future challenges. Green Chem. 2010, 12, 1139–1149. [Google Scholar] [CrossRef]
- Hamad, K.I.; Humadi, J.I.; Abdulkareem, H.A.; Al-Salihi, S.; Farhan, O.I. Efficient immobilization of acids into activated carbon for high durability and continuous desulfurization of diesel fuel. Energy Sci. Eng. 2023, 11, 3662–3679. [Google Scholar] [CrossRef]
- Alheety, M.A.; Al-Jibori, S.A.; Karadağ, A.; Akbaş, H.; Ahmed, M.H. A novel synthesis of MnO2 nanoflowers as an efficient heterogeneous catalyst for oxidative desulfurization of thiophenes. Nano-Struct. Nano-Objects 2019, 20, 100392. [Google Scholar] [CrossRef]
- Patil, R.B.; Yadav, A.D.; Kanamadi, C.M.; Patil, S.P. Electrochemical advancements: MnO2-based electrode materials for supercapacitors. Ionics 2025, 31, 1203–1231. [Google Scholar] [CrossRef]
- Mostafa, M.M.; Morshedy, A.S. Novel calcium carbonate-titania nanocomposites for enhanced sunlight photocatalytic desulfurization process. J. Environ. Manag. 2019, 250, 109462. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Alalwan, H.A. Catalytic oxidative and adsorptive desulfurization of heavy naphtha fraction. Korean Chem. Eng. Res. 2019, 57, 283–288. [Google Scholar] [CrossRef]
- Fang, H.B.; Zhao, J.T.; Fang, Y.T.; Huang, J.J.; Wang, Y. Selective oxidation of hydrogen sulfide to sulfur over activated carbon-supported metal oxides. Fuel 2013, 108, 143–148. [Google Scholar] [CrossRef]
- Hameed, S.A.; Ben Amar, R.; Hamad, K.I.; Jarullah, A.T.; Mujtaba, I.M. Design of Highly Efficient Nickel-Cobalt-Manganese-Molybdenum (NCMM) Nano-Catalysts Supported on Activated Carbon for Desulfurization Process. Catalysts 2023, 13, 1196. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Futalan, C.M.; Dayrit, R.A.; Choi, A.E.S.; Wan, M.W. Evaluation of continuously mixed reactor configurations in the oxidative-adsorptive desulfurization of diesel fuel: Optimization and parametric studies. J. Clean. Prod. 2018, 203, 664–673. [Google Scholar] [CrossRef]
- Patel, H. Comparison of batch and fixed bed column adsorption: A critical review. Int. J. Environ. Sci. Technol. 2022, 19, 10409–10426. [Google Scholar] [CrossRef]
- Humadi, J.I.; Nawaf, A.T.; Jarullah, A.T.; Ahmed, M.A.; Hameed, S.A.; Mujtaba, I.M. Design of new nano-catalysts and digital basket reactor for oxidative desulfurization of fuel: Experiments and modelling. Chem. Eng. Res. Des. 2023, 190, 634–650. [Google Scholar] [CrossRef]
- Ahmad, W.; Ur Rahman, A.; Ahmad, I.; Yaseen, M.; Mohamed Jan, B.; Stylianakis, M.M.; Kenanakis, G.; Ikram, R. Oxidative desulfurization of petroleum distillate fractions using manganese dioxide supported on magnetic reduced graphene oxide as catalyst. Nanomaterials 2021, 11, 203. [Google Scholar] [CrossRef]
- Gado, K.; Hamad, K.I.; Kawi, S. Microporous activated carbon catalyst for an efficient and deactivation resistive supercritical water upgrading process of sour crude oil. Fuel 2023, 353, 129202. [Google Scholar]
- Afzalinia, A.; Mirzaie, A.; Nikseresht, A.; Musabeygi, T. Ultrasound-assisted oxidative desulfurization process of liquid fuel by phosphotungstic acid encapsulated in an interpenetrating amine-functionalized Zn(II)-based MOF as catalyst. Ultrason. Sonochem. 2017, 34, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Green Chem. 2014, 16, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeong, K.E.; Jeong, S.Y.; Park, Y.K.; Kim, D.H.; Jeon, J.K. Utilization of a by-product produced from oxidative desulfurization process over Cs-mesoporous silica catalysts. J. Hazard. Mater. 2011, 192, 1260–1267. [Google Scholar] [CrossRef]
- Yao, Y.; Li, S.; Chen, C.; Zheng, D.; Wu, Z.; Yu, C.; Pu, S.; Liu, F.Q. Catalytic oxidation and desulfurization of calcium-hydroxide gypsum wet flue gas using modified MIL-53(Fe). Energies 2022, 15, 5851. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shaterian, S.; Ghafari, H. Novel synthesis of MnO2-supported on magnetic reduced graphene oxide as catalyst for oxidative desulfurization of petroleum distillate fractions. Materials 2021, 14, 4515. [Google Scholar] [CrossRef]
- Do Prado, N.T.; Heitmann, A.P.; Mansur, H.S.; Mansur, A.A.; Oliveira, L.C.A.; de Castro, C.S. PET-modified red mud as catalysts for oxidative desulfurization reactions. J. Hazard. Mater. 2017, 324, 194–204. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Gao, J.; Xu, W.; Song, C. Spectroscopic identification of physisorbed and chemisorbed water species on metal oxides. Appl. Surf. Sci. 2021, 563, 150035. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Liu, C. Characterization of aliphatic hydrocarbon contamination using FTIR spectroscopy. Fuel Process. Technol. 2020, 209, 106571. [Google Scholar]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier transform infrared (FTIR) spectroscopy in characterization of green synthesized nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, T.; Sonthalia, A.; Varuvel, E.G. Effect of calcite/activated carbon-based post-combustion CO2 capture system in a biodiesel-fueled CI engine—An experimental study. Energy Sources A 2019, 41, 1972–1982. [Google Scholar] [CrossRef]
- Staerz, A.; Seo, H.G.; Defferriere, T.; Tuller, H.L. Silica: Ubiquitous poison of metal oxide interfaces. J. Mater. Chem. A 2022, 10, 2618–2636. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Sun, Y.; Fang, S.; Wu, Z.; Gao, E.; Li, J. Unveiling the role of strong metal–support interactions in gold-catalyzed CO oxidation on MnO2 polymorphs. Langmuir 2024, 40, 23739–23753. [Google Scholar] [CrossRef]
- Humadi, J.I.; Shihab, M.A.; Hasan, A.A.; Mohammed, A.M. Experimental and ANN modeling of kerosene fuel desulfurization using a manganese oxide–tin oxide catalyst. Chem. Eng. Res. Des. 2024, 211, 160–167. [Google Scholar] [CrossRef]
- Al-Abadleh, H.A.; Al-Hosney, H.A.; Grassian, V.H. Oxide and carbonate surfaces as environmental interfaces: The importance of water in surface composition and surface reactivity. J. Mol. Catal. A Chem. 2005, 228, 47–54. [Google Scholar] [CrossRef]
- Gao, X.; Zhong, Z.; Huang, L.; Mao, Y.; Wang, H.; Liu, J.; Zhu, M. The role of transition metal doping in enhancing hydrogen storage capacity in porous carbon materials. Nano Energy 2023, 118, 109038. [Google Scholar] [CrossRef]
- Park, E.J.; Seo, H.O.; Kim, Y.D. Influence of humidity on the removal of volatile organic compounds using solid surfaces. Catal. Today 2017, 295, 3–13. [Google Scholar] [CrossRef]
- Habibi, B.; Pashazadeh, S.; Saghatforoush, L.A.; Pashazadeh, A. A thioridazine hydrochloride electrochemical sensor based on zeolitic imidazolate framework-67-functionalized bio-mobile crystalline material-41 carbon quantum dots. New J. Chem. 2021, 45, 14739–14750. [Google Scholar] [CrossRef]
- Opoku, B.K.; Isaac, A.; Micheal, A.A.; Bentum, J.K.; Muyoma, W.P. Characterization of chemically activated carbons produced from coconut and palm kernel shells using SEM and FTIR analyses. Am. J. Appl. Chem. 2021, 9, 90–96. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, Y.; Park, D. Decomposition pathways of metal carbonate catalysts: A combined TGA and FTIR study. Appl. Catal. B Environ. 2020, 268, 118454. [Google Scholar] [CrossRef]
- Darroudi, T.; Searcy, A.W. Effect of carbon dioxide pressure on the rate of decomposition of calcite (CaCO3). J. Phys. Chem. 1981, 85, 3971–3974. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Guo, X.; Tang, J.; Chen, Z.; Ha, M.N.; Ke, Q. Manganese oxide-based catalysts for the sustainable synthesis of value-added chemicals through oxidation processes: A critical review and perspectives for the future. Green Chem. 2024, 26, 2365–2383. [Google Scholar] [CrossRef]
- Dose, W.M.; Donne, S.W. Manganese dioxide structural effects on its thermal decomposition. Mater. Sci. Eng. B 2011, 176, 1169–1177. [Google Scholar] [CrossRef]
- Tanimu, A.; Tanimu, G.; Ganiyu, S.A.; Gambo, Y.; Alasiri, H.; Alhooshani, K. Metal-free catalytic oxidative desulfurization of fuels—A review. Energy Fuels 2022, 36, 3394–3419. [Google Scholar] [CrossRef]
- Park, J.; Lu, W.; Sastry, A.M. Numerical simulation of stress evolution in lithium manganese dioxide particles due to coupled phase transition and intercalation. J. Electrochem. Soc. 2010, 158, A201. [Google Scholar] [CrossRef]
- Shanmugasundaram, E.; Ravi, A.; Ganesan, V.; Narayanan, V.; Vellaisamy, K.; Pandikannan, S.; Thambusamy, S. Peanut shell-derived activated carbon incorporated with nitrogen anode and cobalt cathode materials (“two-in-one” strategy) for asymmetric supercapacitor (N-PAC//PVA-KOH//Co-PAC) applications. RSC Sustain. 2025, 3, 413–426. [Google Scholar] [CrossRef]
- Wang, L.; Liu, B.; Li, H.; Yang, W.; Ding, Y.; Sinogeikin, S.V.; Mao, W.L. Long-range ordered carbon clusters: A crystalline material with amorphous building blocks. Science 2012, 337, 825–828. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Ren, X.; Hussain, M.I.; Chang, Y.; Ge, C. State-of-the-art review on amorphous carbon nanotubes: Synthesis, structure, and application. Int. J. Mol. Sci. 2023, 24, 17239. [Google Scholar] [CrossRef] [PubMed]
- Sapnik, A.F.; Sun, C.; Laulainen, J.E.; Johnstone, D.N.; Brydson, R.; Johnson, T.; Collins, S.M. Mapping nanocrystalline disorder within an amorphous metal–organic framework. Commun. Chem. 2023, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ait Hamoudi, S.; Hamdi, B.; Brendlé, J. Tetracycline removal from water by adsorption on geomaterial, activated carbon and clay adsorbents. Ecol. Chem. Eng. S 2021, 28, 303–328. [Google Scholar] [CrossRef]
- Nawaf, A.T.; Humadi, J.I.; Hassan, A.A.; Habila, M.A.; Haldhar, R. Improving fuel quality and environment using new synthetic (Mn3O4/AC-nanoparticles) for oxidative desulfurization in digital baffle batch reactor. S. Afr. J. Chem. Eng. 2025, 52, 8–19. [Google Scholar] [CrossRef]
- Bashkova, S.; Baker, F.S.; Wu, X.; Armstrong, T.R.; Schwartz, V. Activated carbon catalyst for selective oxidation of hydrogen sulphide: On the influence of pore structure, surface characteristics, and catalytically-active nitrogen. Carbon 2007, 45, 1354–1363. [Google Scholar] [CrossRef]
- Herold, F.; Prosch, S.; Oefner, N.; Brunnengräber, K.; Leubner, O.; Hermans, Y.; Etzold, B.J. Nanoscale hybrid amorphous/graphitic carbon as key towards next-generation carbon-based oxidative dehydrogenation catalysts. Angew. Chem. Int. Ed. 2021, 60, 5898–5906. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Dittmann, D.; Saal, L.; Zietzschmann, F.; Mai, M.; Altmann, K.; Al-Sabbagh, D.; Braun, U. Characterization of activated carbons for water treatment using TGA-FTIR for analysis of oxygen-containing functional groups. Appl. Water Sci. 2022, 12, 203. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Zhou, K.; Li, H. Porous carbon materials based on biomass for acetone adsorption: Effect of surface chemistry and porous structure. Appl. Surf. Sci. 2018, 459, 657–664. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, Q.; Hand, D.W.; Perram, D.; Taylor, R. Adsorption and regeneration on activated carbon fiber cloth for volatile organic compounds at indoor concentration levels. J. Air Waste Manag. Assoc. 2009, 59, 31–36. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, L.; Zhang, J.; Zhu, J.; Du, L.; Wang, Y. Kinetics and Mechanism of Catalytic Oxidation Desulfurization of Gasoline Liquefied Petroleum Gas in MeroxTM Process with Microfluidics. Chem. Eng. Technol. 2022, 45, 2186–2194. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.; Sun, Z.; Yu, G.; Xu, Y.; Shi, S. An efficient photocatalytic oxidative desulfurization system with hierarchical MIL-101(Fe) as photocatalyst for diesel. Fuel 2023, 332, 126130. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Wei, S.; He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Qiu, L. Performances, kinetics and mechanisms of catalytic oxidative desulfurization from oils. RSC Adv. 2016, 6, 103253–103269. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.J.; Xu, J.H.; Luo, G.S. Oxidative desulfurization of DBT with H2O2 catalysed by TiO2/porous glass. Green Chem. 2016, 18, 771–781. [Google Scholar] [CrossRef]
- Nawaf, A.T.; Humadi, J.I.; Jarullah, A.T.; Ahmed, M.A.; Hameed, S.A.; Mujtaba, I.M. Design of nano-catalyst for removal of phenolic compounds from wastewater by oxidation using modified digital basket baffle batch reactor: Experiments and modeling. Processes 2023, 11, 1990. [Google Scholar] [CrossRef]
- Hori, H.; Ogi, K.; Fujita, Y.; Yasuda, Y.; Nagashima, E.; Matsuki, Y.; Nomiya, K. Oxidative removal of dibenzothiophene and related sulfur compounds from fuel oils under pressurized oxygen at room temperature with hydrogen peroxide and a phosphorus-free catalyst: Sodium decatungstate. Fuel Process. Technol. 2018, 179, 175–183. [Google Scholar] [CrossRef]
- Lou, J.; Wang, Q.; Wu, P.; Wang, H.; Zhou, Y.G.; Yu, Z. Transition-metal mediated carbon–sulfur bond activation and transformations: An update. Chem. Soc. Rev. 2020, 49, 4307–4359. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Qazi, A.B.; Zhang, L.; Liu, X. Metal-based ionic liquids in oxidative desulfurization: A critical review. Environ. Sci. Technol. 2021, 55, 1419–1435. [Google Scholar] [CrossRef]
- Huang, D.; Zhai, Z.; Lu, Y.C.; Yang, L.M.; Luo, G.S. Optimization of composition of a directly combined catalyst in dibenzothiophene oxidation for deep desulfurization. Ind. Eng. Chem. Res. 2007, 46, 1447–1451. [Google Scholar] [CrossRef]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef]
- Sahraei, S. Assessment of reaction parameters in the oxidative desulfurization reaction. Energy Fuels 2023, 37, 15373–15393. [Google Scholar] [CrossRef]
- Cui, H.; Turn, S.Q.; Reese, M.A. Removal of sulfur compounds from utility pipelined synthetic natural gas using modified activated carbons. Catal. Today 2009, 139, 274–279. [Google Scholar] [CrossRef]
- Salih, Y.; Othman, C.S.; Hamasalih, L.O. The optimization of the adsorption desulfurization process for dibenzothiophene in a model oil using different ratios of hybrid MOF: AC micro adsorbers. J. Sulfur Chem. 2023, 44, 694–711. [Google Scholar] [CrossRef]
- Othman, C.S.; Salih, Y.; Hamasalih, L.O. Adsorption desulfurization of dibenzothiophene in a model and diesel fuel by hybrid activated charcoal/mixed metal oxide. Pet. Sci. Technol. 2023, 41, 2121–2140. [Google Scholar] [CrossRef]
- Castro, P.S.; Zuniga, G.M.; Holmes, W.; Buchireddy, P.R.; Gang, D.D.; Revellame, E.; Zappi, M.; Hernandez, R. Review of the adsorbents/catalysts for the removal of sulfur compounds from natural gas. Gas Sci. Eng. 2023, 115, 205004. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T. Desulfurization of transportation fuels by adsorption. Catal. Rev. 2004, 46, 111–150. [Google Scholar] [CrossRef]
- Song, J.; Zhu, L.; Yu, S.; Li, G.; Wang, D. The synergistic effect of adsorption and Fenton oxidation for organic pollutants in water remediation: An overview. RSC Adv. 2024, 14, 33489–33511. [Google Scholar] [CrossRef]
- Humadi, J.I.; Mohammed, W.T. Experimental and artificial intelligence-machine learning modeling of ultra-deep diesel desulfurization in oscillatory central bed baffled reactor using protected and unprotected dual active carbonaceous catalyst. Sustain. Chem. Clim. Action 2025, 7, 100111. [Google Scholar] [CrossRef]
- Falah, H.; Hamad, K.I.; Jarullah, A. Oxidation of phenol by CWPO method using nickel manganese oxidecatalyst prepared in glycerol solvent using microwave in batch reactor. Al-Rafidain J. Eng. Sci. 2025, 3, 396–409. [Google Scholar] [CrossRef]


| Physical Property | Values |
|---|---|
| Specific gravity at 15.5 °C | 0.8333 |
| API at 60 F | 38.31 |
| Total sulfur, (ppm) | 9 |
| Kinematic viscosity, (mm2/s) | 3.15 |
| Cetane index | 53.90 |
| Flash point, (°C) | 61 |
| Pour point, (°C) | <−20 |
| Distillation, (°C) | |
| Initial boiling point, IBP (°C) | 165 |
| 10% | 202 |
| 50% | 276 |
| 90% | 338 |
| Final boiling point, FBP (°C) | 357 |
| Description | Specification |
|---|---|
| Material of the reactor | Stainless steel |
| Dimension of reactor | D = 8 cm, H = 10 cm |
| Length of the rod | 35 cm |
| Dimension of basket | H = 1 cm, L = 1 cm, W = 1 cm |
| Impeller type | Four-basket impeller |
| Impeller diameter | 90.0 mm |
| Reactor working volume | 250 mL |
| Baffle | 4, distributed along the wall of the reactor (height 8 cm) |
| Preheater | Electrical heater |
| Insulator material | Glass wool |
| Property | Adsorbent (AC) | Catalyst (MnO2/Calcite) |
|---|---|---|
| BET | 912.974 m2/g | 5.140 m2/g |
| Total pore volume (p/p0 = 0.9900) | 0.499 cm3/g | 0.010 cm3/g |
| Mean pore diameter | 2.18 nm | 7.89 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humadi, J.I.; Hamad, K.I.; Abdulkareem, H.A.; Ismael, M.N.; Jarullah, A.T.; Ahmed, M.A.; Hameed, S.A.; Nawaf, A.T.; Mujtaba, I.M. Eco-Friendly Oxidative–Adsorptive Desulfurization for Real Diesel Fuel Using Green MnO2 Biowaste-Extracted Calcite in Digital Basket Reactor. Processes 2025, 13, 3084. https://doi.org/10.3390/pr13103084
Humadi JI, Hamad KI, Abdulkareem HA, Ismael MN, Jarullah AT, Ahmed MA, Hameed SA, Nawaf AT, Mujtaba IM. Eco-Friendly Oxidative–Adsorptive Desulfurization for Real Diesel Fuel Using Green MnO2 Biowaste-Extracted Calcite in Digital Basket Reactor. Processes. 2025; 13(10):3084. https://doi.org/10.3390/pr13103084
Chicago/Turabian StyleHumadi, Jasim I., Khaleel I. Hamad, Hiba A. Abdulkareem, Maha Nazar Ismael, Aysar T. Jarullah, Mustafa A. Ahmed, Shymaa A. Hameed, Amer T. Nawaf, and Iqbal M. Mujtaba. 2025. "Eco-Friendly Oxidative–Adsorptive Desulfurization for Real Diesel Fuel Using Green MnO2 Biowaste-Extracted Calcite in Digital Basket Reactor" Processes 13, no. 10: 3084. https://doi.org/10.3390/pr13103084
APA StyleHumadi, J. I., Hamad, K. I., Abdulkareem, H. A., Ismael, M. N., Jarullah, A. T., Ahmed, M. A., Hameed, S. A., Nawaf, A. T., & Mujtaba, I. M. (2025). Eco-Friendly Oxidative–Adsorptive Desulfurization for Real Diesel Fuel Using Green MnO2 Biowaste-Extracted Calcite in Digital Basket Reactor. Processes, 13(10), 3084. https://doi.org/10.3390/pr13103084







