Disruption of Early Streptococcus mutans Biofilm Development on Orthodontic Aligner Materials
Abstract
1. Introduction
2. Materials and Methods
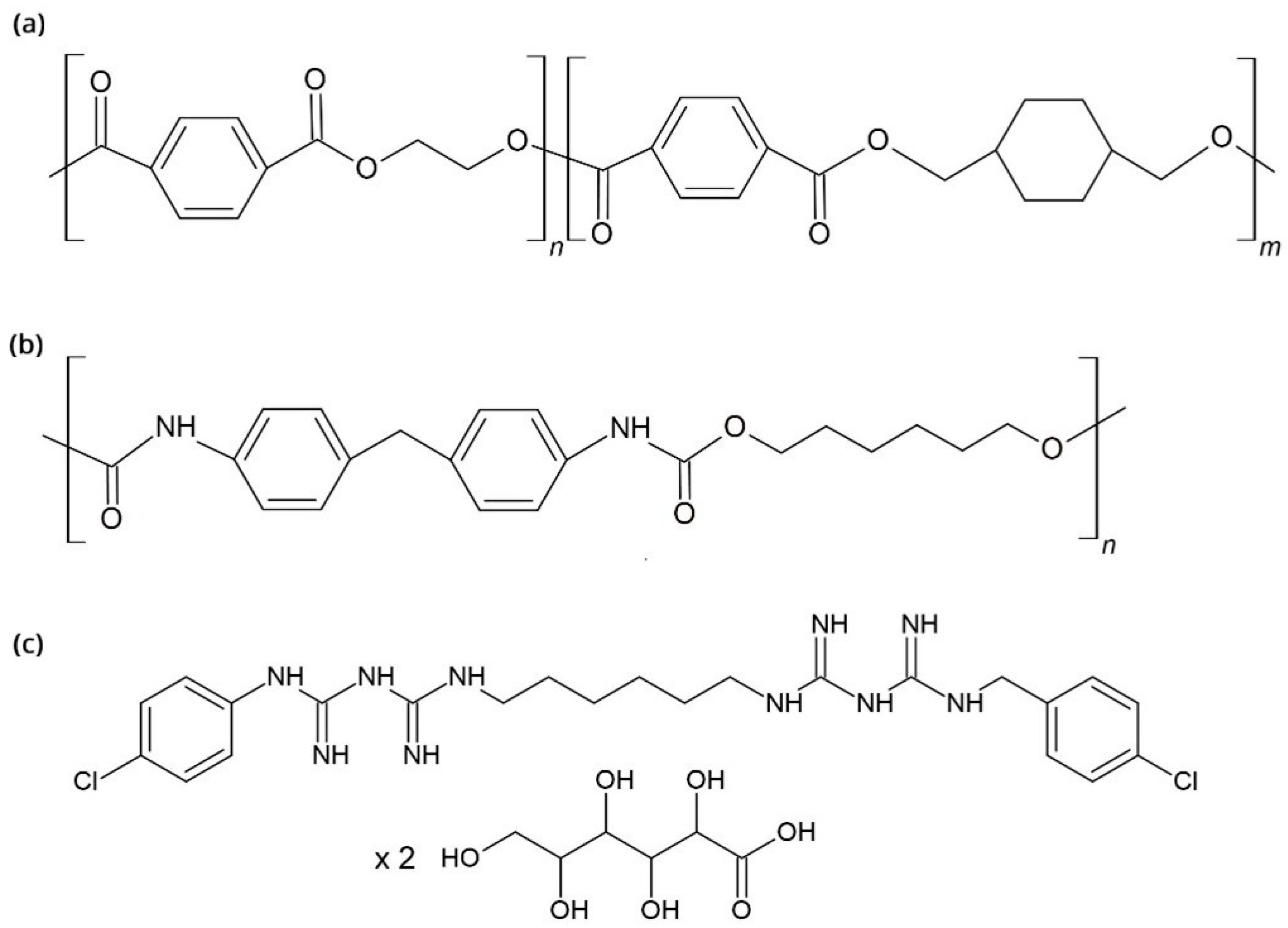
2.1. Aligner Materials
- PU plates (10 mm × 10 mm; Zendura, Bay Materials, Fremont, CA, USA)
- PETG plates (10 mm × 10 mm; Erkodur, Erkodent Erich Kopp GmbH, Pfalzgrafenweiler, Germany)
2.2. Antiseptic Solutions
- Curasept ADS 212 (Curasept, Saronno, Italy) with 0.12% CHX
- Perioplus (Curasept, Flawil, Switzerland) with 0.12% CHX
- Solution of pure 0.12% CHX prepared in distilled water. The antiseptics used in this study have been described in a previous publication [33].
2.3. Biofilm Treatment
2.4. Atomic Force Microscopy (AFM)
2.5. SFE
2.6. Scanning Electron Microscopy (SEM)
3. Results
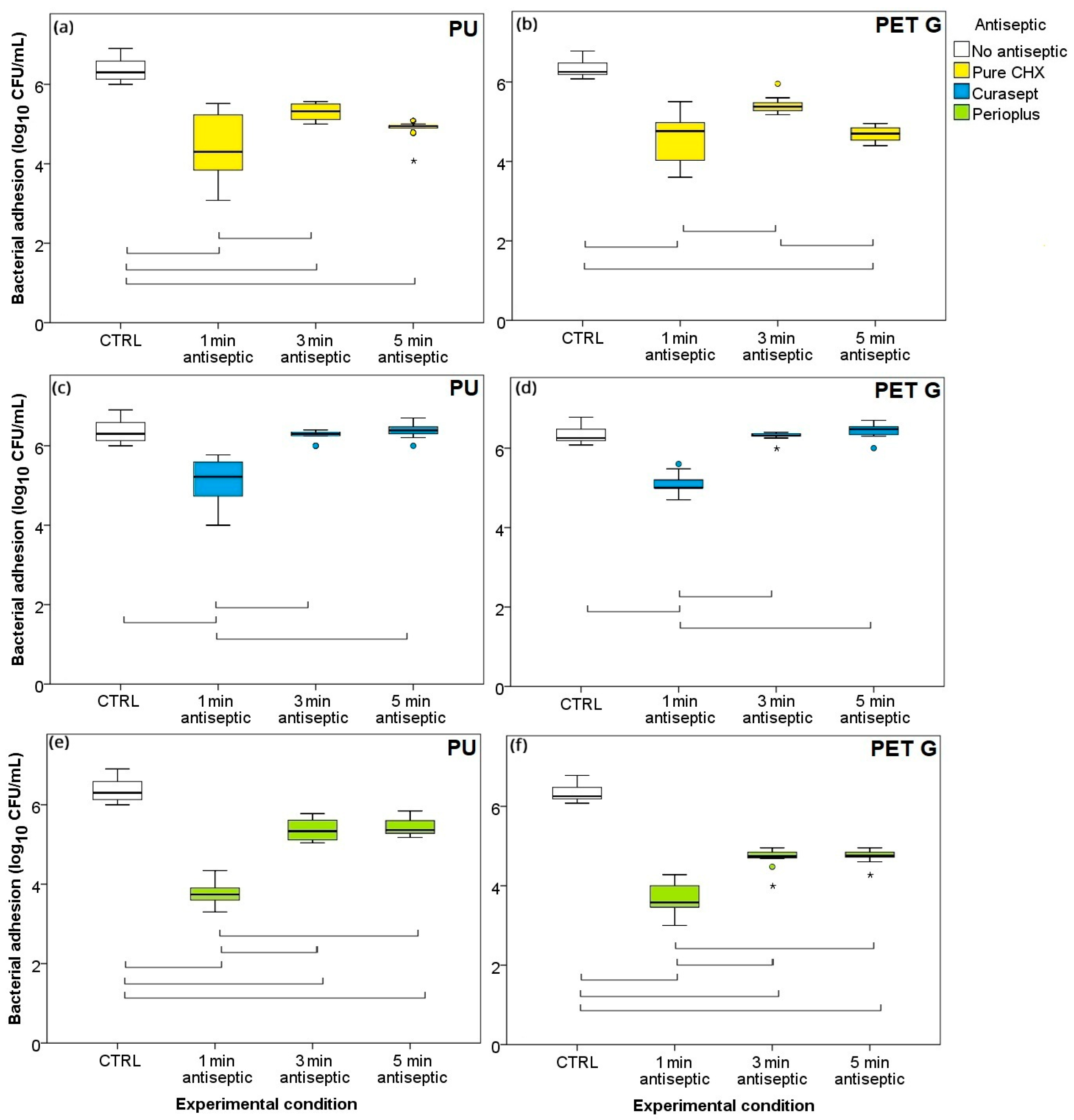
3.1. Time–Kill Curves
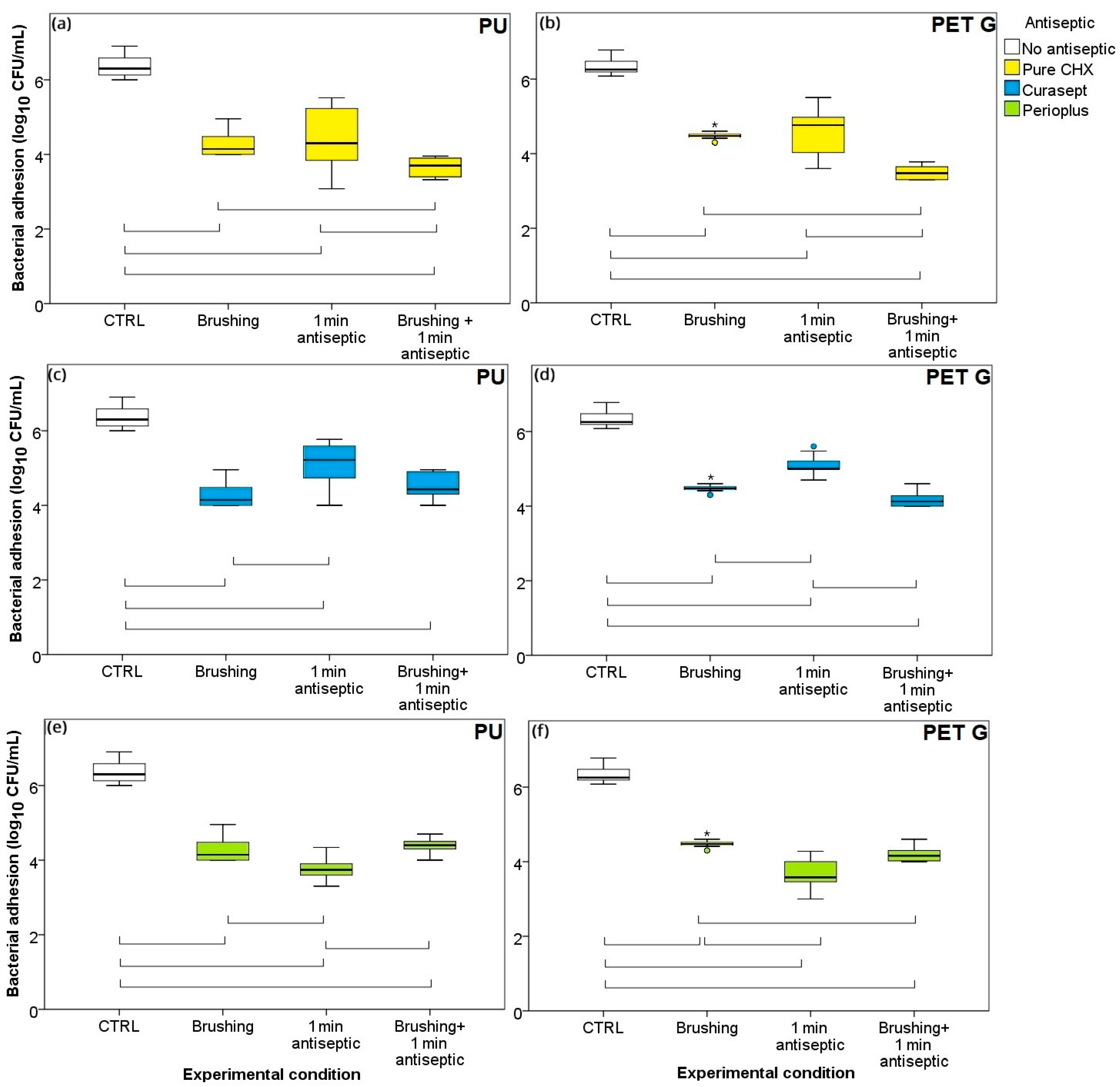
3.2. One Minute Exposure and Brushing: Individual Treatments vs. Combination
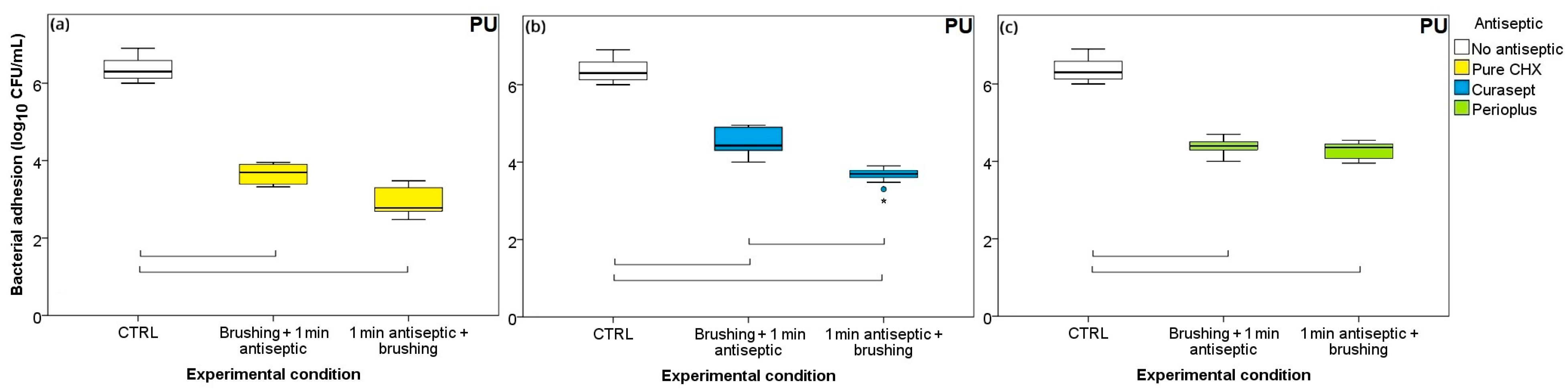
3.3. Ordering of Treatments: Brushing-First or Antiseptic-First?
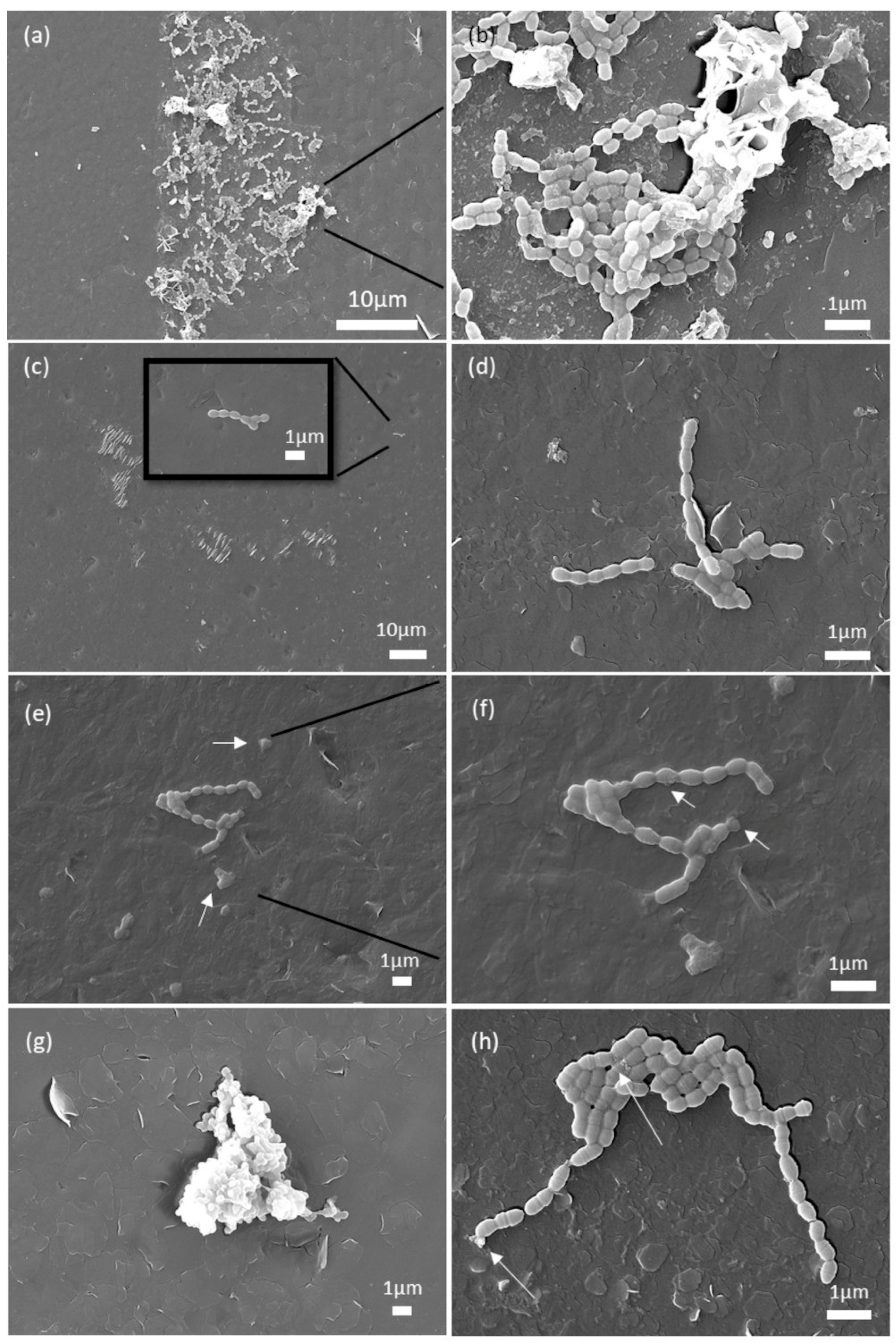
3.4. Analysis of the SEM Micrographs
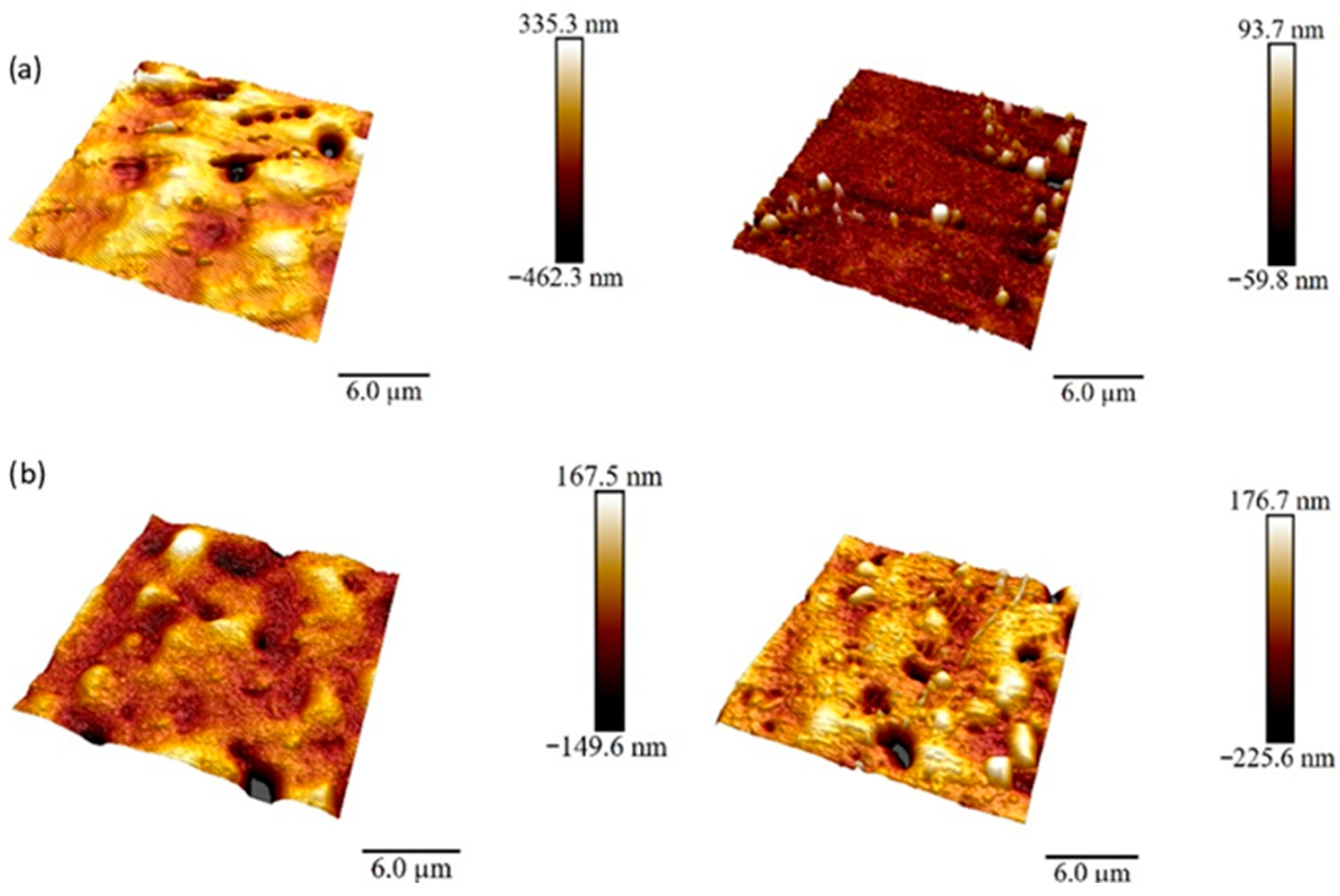
3.5. Surface Roughness Analysis
4. Discussion
4.1. Aligner Materials
4.2. Decreased Effectiveness of CHX After Longer Exposures
4.3. Optimum Treatment of the Biofilm
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PETG | poly(ethylene terephthalate glycol) |
| PU | polyurethane |
| CHX | chlorhexidine digluconate |
| SFE | surface free energy |
| CHDM | cyclohexanedimethanol |
| CFU/mL | colony-forming units/milliliter |
| BHI | Brain Heart Infusion |
| PBS | phosphate-buffered saline |
| AFM | Atomic force microscopy |
| SEM | Scanning electron microscopy |
| WCA | Static water contact angles |
| Rq | Root mean square roughness |
| Ra | arithmetical mean height |
| Rmax | maximum roughness depth |
| XRD | LDX-ray diffraction |
| DSC | differential scanning calorimetry |
References
- Zheng, M.; Liu, R.; Ni, Z.; Yu, Z. Efficiency, effectiveness and treatment stability of clear aligners: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2017, 20, 127–133. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in orthodontic clear aligner materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef] [PubMed]
- Cenzato, N.; Di Iasio, G.; Martìn Carreras-Presas, C.; Caprioglio, A.; Del Fabbro, M. Materials for Clear Aligners—A Comprehensive Exploration of Characteristics and Innovations: A Scoping Review. Appl. Sci. 2024, 14, 6533. [Google Scholar] [CrossRef]
- Charavet, C.; Gourdain, Z.; Graveline, L.; Lupi, L. Cleaning and Disinfection Protocols for Clear Orthodontic Aligners: A Systematic Review. Healthcare 2022, 10, 340. [Google Scholar] [CrossRef]
- Hamdoon, S.M.; AlSamak, S.; Ahmed, M.K.; Gasgoos, S. Evaluation of biofilm formation on different clear orthodontic retainer materials. J. Orthod. Sci. 2022, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Eliades, G.; Brantley, W.A. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 351–360. [Google Scholar] [CrossRef]
- Salehi, B.; Kregiel, D.; Mahady, G.; Sharifi-Rad, J.; Martins, N.; Rodrigues, C.F. Management of Streptococcus mutans-Candida spp. Oral Biofilms’ Infections: Paving the Way for Effective Clinical Interventions. J. Clin. Med. 2020, 9, 517. [Google Scholar] [CrossRef]
- Lindh, L.; Aroonsang, W.; Sotres, J.; Arnebrant, T. Salivary Pellicles. Monogr. Oral Sci. 2014, 24, 30–39. [Google Scholar] [CrossRef]
- Christersson, C.E.; Dunford, R.G.; Glantz, P.J.; Baier, R.E. Effect of critical surface tension on retention of oral microorganisms. Eur. J. Oral Sci. 1989, 97, 247–256. [Google Scholar] [CrossRef]
- Absolom, D.R.; Hawthorn, L.A.; Chang, G. Endothelialization of polymer surfaces. J. Biomed. Mater. Res. 1988, 22, 271–285. [Google Scholar] [CrossRef]
- Hasson, J.E.; Wiebe, D.H.; Sharefkin, J.B.; Abbott, W.M. Migration of adult human vascular endothelial cells: Effect of extracellular matrix proteins. Surgery 1986, 100, 384–391. [Google Scholar]
- Quirynen, M.; Bollen, C.M.L. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man: A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Zhang, N.; Bai, Y.; Ding, X.; Zhang, Y. Preparation and characterization of thermoplastic materials for invisible orthodontics. Dent. Mater. J. 2011, 30, 954–959. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Jiao, Y.; Zeng, X.; Wang, X.; Lu, Y.; Cheng, A.; Dai, P.; Zhao, X. Fine dispersion morphology of polystyrene/poly(ethylene terephthalate glycol) blending generation for controlled foaming behavior. RSC Adv. 2017, 7, 39138–39146. [Google Scholar] [CrossRef]
- Yan, C.; Kleiner, C.; Tabigue, A.; Shah, V.; Sacks, G.; Shah, D.; DeStefano, V. PETG: Applications in Modern Medicine. Eng. Regen. 2024, 5, 45–55. [Google Scholar] [CrossRef]
- Petrov, P.; Agzamova, D.; Pustovalov, V.; Zhikhareva, E.; Saprykin, B.; Chmutin, I.; Shmakova, N. Research into the effect of the 3D-printing mode on changing the properties of PETG transparent plastic. In Proceedings of the ESAFORM 2021—24th International Conference on Material Forming, Liège, Belgium, 14–16 April 2021. [Google Scholar] [CrossRef]
- Rynio, P.; Galant, K.; Wójcik, Ł.; Grygorcewicz, B.; Kazimierczak, A.; Falkowski, A.; Gutowski, P.; Dołęgowska, B.; Kawa, M. Effects of Sterilization Methods on Different 3D Printable Materials for Templates of Physician-Modified Aortic Stent Grafts Used in Vascular Surgery—A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 3539. [Google Scholar] [CrossRef] [PubMed]
- López-Jornet, P.; Plana-Ramon, E.; Leston, J.S.; Pons-Fuster, A. Short-term side effects of 0.2% alcohol-free chlorhexidine mouthrinse in geriatric patients: A randomized, double-blind, placebo-controlled study. Gerodontology 2012, 29, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hancock, E.B. Prevention. Ann. Periodontol. 1996, 1, 223–249. [Google Scholar] [CrossRef]
- Rölla, G.; Melsen, B. On the Mechanism of the Plaque Inhibition by Chlorhexidine. J. Dent. Res. 1975, 54, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.G.; Aalam, A.; Slots, J. Periodontal antimicrobials—Finding the right solutions. Int. Dent. J. 2005, 55, 3–12. [Google Scholar] [CrossRef]
- Davies, A. The mode of action of chlorhexidine. J. Periodontal Res. 1973, 8, 68–75. [Google Scholar] [CrossRef]
- Kampf, G. Chlorhexidine Digluconate. Antiseptic Steward; Springer International Publishing: Cham, Switzerland, 2024; pp. 653–806. [Google Scholar] [CrossRef]
- Chatzigiannidou, I.; Teughels, W.; Van De Wiele, T.; Boon, N. Oral biofilms exposure to chlorhexidine results in altered microbial composition and metabolic profile. Npj Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef]
- Daniele, V.; Macera, L.; Taglieri, G.; Spera, L.; Marzo, G.; Quinzi, V. Color Stability, Chemico-Physical and Optical Features of the Most Common PETG and PU Based Orthodontic Aligners for Clear Aligner Therapy. Polymers 2021, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Yang, S.B.; Moon, J.-H.; Ahn, H.-W.; Hong, J. A Polysaccharide-Based Antibacterial Coating with Improved Durability for Clear Overlay Appliances. ACS Appl. Mater. Interfaces 2018, 10, 17714–17721. [Google Scholar] [CrossRef]
- Moradinezhad, M.; Abbasi Montazeri, E.; Hashemi Ashtiani, A.; Pourlotfi, R.; Rakhshan, V. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida Albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: An in vitro study. BMC Oral Health 2024, 24, 1107. [Google Scholar] [CrossRef] [PubMed]
- Suter, F.; Zinelis, S.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. Roughness and wettability of aligner materials. J. Orthod. 2020, 47, 223–231. [Google Scholar] [CrossRef]
- Iijima, M.; Kohda, N.; Kawaguchi, K.; Muguruma, T.; Ohta, M.; Naganishi, A.; Murakami, T.; Mizoguchi, I. Effects of temperature changes and stress loading on the mechanical and shape memory properties of thermoplastic materials with different glass transition behaviours and crystal structures. Eur. J. Orthod. 2015, 37, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Qin, D.; Wei, F.; Xiaobing, L. Application of antibacterial nanoparticles in orthodontic materials. Nanotechnol. Rev. 2022, 11, 2433–2450. [Google Scholar] [CrossRef]
- Begić, G.; Petković Didović, M.; Lučić Blagojević, S.; Jelovica Badovinac, I.; Žigon, J.; Perčić, M.; Cvijanović Peloza, O.; Gobin, I. Adhesion of Oral Bacteria to Commercial d-PTFE Membranes: Polymer Microstructure Makes a Difference. Int. J. Mol. Sci. 2022, 23, 2983. [Google Scholar] [CrossRef]
- Worreth, S.; Bieger, V.; Rohr, N.; Astasov-Frauenhoffer, M.; Töpper, T.; Osmani, B.; Braissant, O. Cinnamaldehyde as antimicrobial in cellulose-based dental appliances. J. Appl. Microbiol. 2022, 132, 1018–1024. [Google Scholar] [CrossRef]
- Gergeta, D.; Badnjevic, M.; Karleusa, L.; Maglica, Z.; Spalj, S.; Gobin, I. Effect of Chlorhexidine Digluconate on Oral Bacteria Adhesion to Surfaces of Orthodontic Appliance Alloys. Appl. Sci. 2024, 14, 2145. [Google Scholar] [CrossRef]
- Pratten, J.; Smith, A.W.; Wilson, M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J. Antimicrob. Chemother. 1998, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.P.; Falkenberg, S.M.; Stasko, J.A.; Shircliff, A.L.; Lippolis, J.D.; Briggs, R.E. Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PLoS ONE 2020, 15, e0233973. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804. [Google Scholar] [CrossRef]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic Surfaces. Effects of Topography Length Scales on Wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and Ultralyophobic Surfaces: Some Comments and Examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Law, K.-Y. Water–surface interactions and definitions for hydrophilicity, hydrophobicity and superhydrophobicity. Pure Appl. Chem. 2015, 87, 759–765. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Drago, L.; Del Fabbro, M.; Bortolin, M.; Vassena, C.; De Vecchi, E.; Taschieri, S. Biofilm Removal and Antimicrobial Activity of Two Different Air-Polishing Powders: An In Vitro Study. J. Periodontol. 2014, 85, e363–e369. [Google Scholar] [CrossRef] [PubMed]
- McBain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Gilbert, P. Effects of a Chlorhexidine Gluconate-Containing Mouthwash on the Vitality and Antimicrobial Susceptibility of In Vitro Oral Bacterial Ecosystems. Appl. Environ. Microbiol. 2003, 69, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Day, M.J. Antibacterial activity of chlorhexidine. J. Hosp. Infect. 1993, 25, 229–238. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Davies, A.; Russell, A.D. Uptake of 14 C-chlorhexidine diacetate to Escherichia coli and Pseudomonas aeruginosa and its release by azolectin. FEMS Microbiol. Lett. 1989, 60, 327–332. [Google Scholar] [CrossRef]
- 47 Stickler, D.J.; Clayton, C.L.; Chawla, J.C. The resistance of urinary tract pathogens to chlorhexidine bladder washouts. J. Hosp. Infect. 1987, 10, 28–39. [Google Scholar] [CrossRef]
- Stickler, D.J.; Clayton, C.L.; Chawla, J.C. Assessment of Antiseptic Bladder Washout Procedures using a Physical Model of the Catheterised Bladder. Br. J. Urol. 1987, 60, 413–418. [Google Scholar] [CrossRef]
- Forbes, S.; Dobson, C.B.; Humphreys, G.J.; McBain, A.J. Transient and sustained bacterial adaptation following repeated sublethal exposure to microbicides and a novel human antimicrobial peptide. Antimicrob. Agents Chemother. 2014, 58, 5809–5817. [Google Scholar] [CrossRef]
- Jian, H.J.; Yu, J.; Li, Y.J.; Unnikrishnan, B.; Huang, Y.F.; Luo, L.J.; Ma, D.H.K.; Harroun, S.G.; Chang, H.T.; Lin, H.J.; et al. Highly adhesive carbon quantum dots from biogenic amines for prevention of biofilm formation. Chem. Eng. J. 2020, 386, 123913. [Google Scholar] [CrossRef]
- Fang, C.-T.; Chen, H.-C.; Chuang, Y.-P.; Chang, S.-C.; Wang, J.-T. Cloning of a Cation Efflux Pump Gene Associated with Chlorhexidine Resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2002, 46, 2024–2028. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria—Is there cause for concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Korbecka-Paczkowska, M.; Stasiewicz, M.; Mrozikiewicz, A.E.; Włodkowic, D.; Cielecka-Piontek, J. Activity of antiseptics against Pseudomonas aeruginosa and its adaptation potential. Antibiotics 2025, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.R.; Godwin, M.J.; Velsko, I.M.; Richards, V.P.; Burne, R.A. Spontaneously arising Streptococcus mutans variants with reduced susceptibility to chlorhexidine display genetic defects and diminished fitness. Antimicrob. Agents Chemother. 2019, 63, 17. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tamura, Y.; Yokota, T. Antiseptic and antibiotic resistance plasmid in Staphylococcus aureus that possesses ability to confer chlorhexidine and acrinol resistance. Antimicrob. Agents Chemother. 1988, 32, 932–935. [Google Scholar] [CrossRef]
- Senadheera, D.; Cvitkovitch, D.G. Quorum sensing and biofilm formation by Streptococcus mutans. Adv. Exp. Med. Biol. 2008, 631, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.R.; Vasudevan, S.; Shanmugam, K.; Lévesque, C.M.; Solomon, A.P.; Neelakantan, P. Combinatorial effects of trans-cinnamaldehyde with fluoride and chlorhexidine on Streptococcus mutans. J. Appl. Microbiol. 2021, 130, 382–393. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, C.; Ren, B.; Li, X.; Weir, M.D.; Masri, R.M.; Oates, T.W.; Cheng, L.; Xu, H.K.H. Formation of persisters in Streptococcus mutans biofilms induced by antibacterial dental monomer. J. Mater. Sci. Mater. Med. 2017, 28, 178. [Google Scholar] [CrossRef]
- Ogunro, O.B.; Richard, G.; Izah, S.C.; Ovuru, K.F.; Babatunde, O.T.; Das, M. Citrus aurantium: Phytochemistry, Therapeutic Potential, Safety Considerations, and Research Needs. In Herbal Medicine Phytochemistry: Applications and Trends; Izah, S.C., Ogwu, M.C., Akram, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–40. [Google Scholar] [CrossRef]
- García-Juárez, A.; Garzón-García, A.M.; Ramos-Enríquez, J.R.; Tapia-Hernández, J.A.; Ruiz-Cruz, S.; Canizales-Rodríguez, D.F.; Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Ocaño-Higuera, V.M.; Ornelas-Paz, J.D.J. Evaluation of Antioxidant and Antibacterial Activity of Gelatin Nanoparticles with Bitter Orange Peel Extract for Food Applications. Foods 2024, 13, 3838. [Google Scholar] [CrossRef]

| Sample | Contact Angle/° | ||
|---|---|---|---|
| Water | Formamide | Diiodomethane | |
| PETG | 77.3 ± 1.0 | 57.5 ± 1.1 | 39.9 ± 2.9 |
| PU | 80.4 ± 0.5 | 56.6 ± 0.4 | 42.6 ± 1.9 |
| Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Owens-Wendt Model | Wu’s Model | OCG Model | ||||||||
| PETG | 36.9 | 4.94 | 41.4 | 36.6 | 8.20 | 44.8 | 39.7 | 0.02 | 7.90 | 40.6 |
| PU | 36.9 | 3.65 | 40.6 | 36.4 | 7.40 | 43.8 | 38.3 | 0.23 | 4.90 | 40.4 |
| PETG | PU | |||||
|---|---|---|---|---|---|---|
| Image 1 | Image 2 | Average | Image 1 | Image 2 | Average | |
| Rq/nm | 98.0 | 16.8 | 57.4 | 41.2 | 49.6 | 45.4 |
| Ra/nm | 71.0 | 9.45 | 40.2 | 29.8 | 35.2 | 32.6 |
| Rmax/nm | 1180 | 335 | 758 | 561 | 758 | 660 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badnjević, M.; Petković Didović, M.; Jelovica Badovinac, I.; Lučić Blagojević, S.; Perčić, M.; Špalj, S.; Gobin, I. Disruption of Early Streptococcus mutans Biofilm Development on Orthodontic Aligner Materials. Processes 2025, 13, 3069. https://doi.org/10.3390/pr13103069
Badnjević M, Petković Didović M, Jelovica Badovinac I, Lučić Blagojević S, Perčić M, Špalj S, Gobin I. Disruption of Early Streptococcus mutans Biofilm Development on Orthodontic Aligner Materials. Processes. 2025; 13(10):3069. https://doi.org/10.3390/pr13103069
Chicago/Turabian StyleBadnjević, Matea, Mirna Petković Didović, Ivana Jelovica Badovinac, Sanja Lučić Blagojević, Marko Perčić, Stjepan Špalj, and Ivana Gobin. 2025. "Disruption of Early Streptococcus mutans Biofilm Development on Orthodontic Aligner Materials" Processes 13, no. 10: 3069. https://doi.org/10.3390/pr13103069
APA StyleBadnjević, M., Petković Didović, M., Jelovica Badovinac, I., Lučić Blagojević, S., Perčić, M., Špalj, S., & Gobin, I. (2025). Disruption of Early Streptococcus mutans Biofilm Development on Orthodontic Aligner Materials. Processes, 13(10), 3069. https://doi.org/10.3390/pr13103069










