Abstract
This paper focuses on the possibility of using phosphogypsum, which is a residue from the production of orthophosphoric acid as an additional source of calcium and the use of spent caustic as an alkaline activator for production of ceramic materials in construction industry. The use of the above-mentioned waste will allow to increase fraction of calcium, sodium and silicate needed for the geopolymerization process and improve properties of material. This review presents a description of the geopolymerization process and the influence of alkaline activator on the reactions occurring in ceramic materials. Collected information, which confirm the possibility of using post-production waste from chemical industry as components for the production of building materials.
1. Introduction
The construction industry is one of the most environmentally burdensome industries, which not only generates greenhouse gas emissions, but is also extremely energy-intensive. It is estimated that the annual global production of bricks will increase to 2.5 billion pieces by 2030 [1], while approximately 2.07 millions tons of carbon dioxide are generated, and the energy needed for production ranges from 0.54 MJ to 3.14 MJ per kilogram of brick. These values depend on the type of brick, kiln and fuel used the weight of the brick may vary from 2 kg to 4 kg) [2,3].
The production of bricks is based on the creation of amorphous structures of aluminosilicates, which are formed in the dehydroxylation reaction of mineral compounds, creating matrix connections between SiO2,– Al2O3 and CaO [4]. The pozzolanic reaction takes place in an alkaline environment at temperatures up to 500–700 °C [5,6]. The addition of active materials that can create pozzolanic bonds has a positive effect on the compressive strength, chemical corrosion, temperature shrinkage, but also on the setting time and heat of hydration of bricks and lime mortars [7,8,9,10].
For many years, in order to increase the amount of active ingredients in building materials, ashes from the incineration of e.g., sewage sludge or municipal waste have been used. It was noted that the addition of an appropriate amount of ashes has a positive effect on the mechanical and thermal properties of the obtained building materials [11,12,13,14]. In addition, their use allows to reduce the firing temperature. Taking into consideration the number of landfills and the amount of disposal wastes presented in Table 1, the use of waste as components in the production of building materials in the form of bricks or binders can become a new way to manage them in accordance with the assumptions of the promoted circular economy [14,15,16,17].

Table 1.
Number and capacity of recovery and disposal facilities for European Union (27 countries) [18].
Studies were carried out to check the effect of alkaline substances used as activators of the mineral binding process in ceramic materials. The increase in durability is one of many examples of the positive impact of alkali, along with an increase in fire resistance or a reduction in firing temperature, which directly reduce the carbon dioxide emissions [19,20,21].
The last two years have been full of papers focused on the use of phosphogypsum as additives in the production of building materials. Due to European Commission Decision of 3 May 2000 replacing Decision 94/3/EC establishing a list of wastes pursuant to Article 1(4) Council Directive 75/442/EEC on waste and Council Decision 94/904/EC establishing a list of hazardous waste pursuant to Article 1(4) of Council Directive 91/689/EEC on hazardous waste, phosphogypsum is classify with code 06 09 01 [22]. According to the data available in the literature, only about 15% of phosphogypsum from chemical production is reused, mainly in construction and road construction [23,24,25,26,27,28] Additionally, given that in 2023 the total amount of this waste in landfills worldwide exceeded 6 billion tons, and an average of 300 million tons per year increases, this is a growing problem [29,30,31,32]. It is estimated that by 2050, the total amount of waste phosphogypsum in landfills will exceed 11 billion tons, taking into consideration that for every ton of phosphoric acid produced, there are 4 tons of waste phosphogypsum [29]. According to statistics, nearly 60% of waste phosphogypsum is stored in landfills, and about 25% is dumped in the seas and oceans [25,33]. Closed landfills are usually covered with a layer of soil, but the fact that the problem is not visible does not mean that it disappears [34,35]. This proves the need to find new ways to manage phosphogypsum on a larger scale and implement new solutions in line with the idea of circular economy.
Due to the limited number of papers summarizing the current state of knowledge about the use of phosphogypsum as composites in the production of building materials, it was decided to collect the available information and articles in the form of this literature review. To the best of the authors’ knowledge, no such summary work has appeared in recent years, therefore this work may constitute a stimulation in the field of the use of phosphogypsum as building materials.
2. Pozzolanic Materials
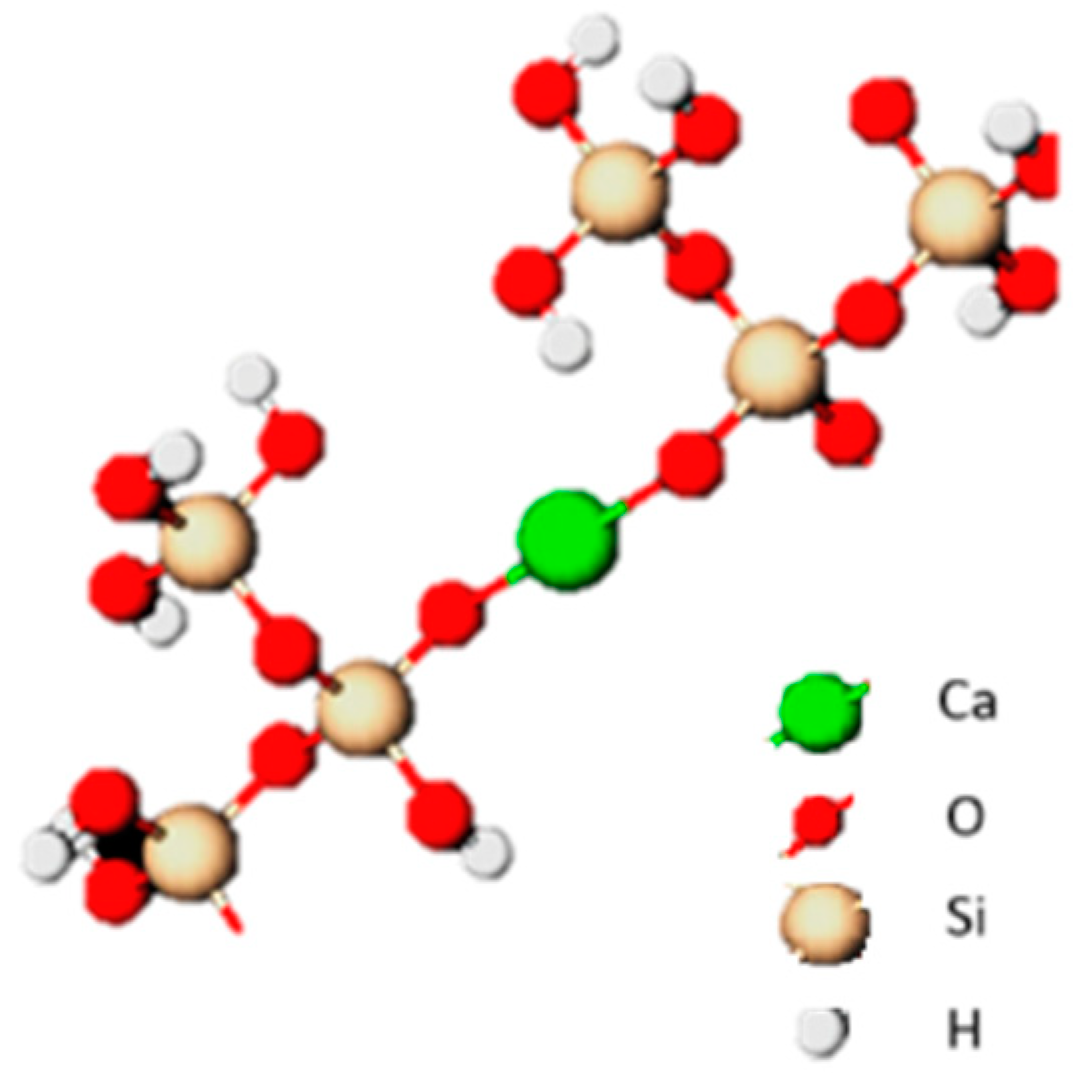
Pozzolans consist mainly of silica or aluminosilicate structures that combine with calcium in the presence of water. The finer grain size, the better their binding properties. The content of amorphous silica also has an undoubted influence. In the reaction of calcium with silicate ions and the hydration of the resulting calcium silicates, the CSH phase, which is sparingly soluble in water, is formed in an amorphous form and tobermorite in a crystalline form [36,37]. Additionally, the formation of hydrated calcium aluminates (C4AH13, C2AH8), hydrogehlenites (C2ASH8) and hydrogarnets (C3AS3-C3AH6) can be observed. The example of formation is presented in Figure 1. It is important that the reactions leading to the hardening of the mixture are initiated already at room temperature, however, they are relatively slow, so the material must “mature” to fully demonstrate its increased strength properties [8,37,38,39].
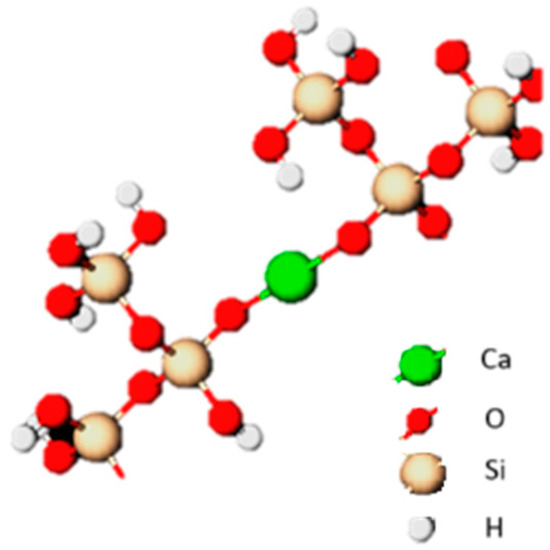
Figure 1.
Schematic representation of the C-S-H gel.
Pozzolanic materials in the concrete and cement industry are used as an additional source of minerals, mainly silica. For many years, a popular trend has been the cooperation of enterprises with producers of ceramic building materials in the disposal of fly ash or micro silica remaining after combustion processes, e.g., municipal waste or biomass [40,41]. This is the result of researches on alternative routes of cement production to reduce pollution from the production of standard cements. It was found that wastes from the energy and chemical industry exhibit the properties of pozzolanic materials involved in the growth of the C-A-S-H phase (C–CaO, A–Al2O3, S–SiO2, H–H2O) due to the high content of calcium, silicon and aluminum [42,43]. The calcium content in pozzolanic material influents directly into the overall strength, the length of the material setting time and the temperature of the hydration process. Taking into consideration that all these conditions are conducive to the stability of the final product and its functional properties, in Table 2 the information how the addition of the considered waste as phosphogypsum and spent caustic improve the properties of the material, are shown [10,44,45,46,47,48].

Table 2.
Summary of the dependence of selected parameters on the addition of waste.
Even in antiquity, it was noticed that some substances can act as an activator of the geopolymerization reaction for pozzolanic materials. One of them is seawater, which, thanks to the content of chloride and sulfate ions, increases the temperature of the system, which promotes the hydration process [55,56,57,58]. For the same purpose, alkaline activators are used, which additionally promote resistance to cracking of the material or propagation of cracks.
Taking into consideration the possibility of simultaneous formation of C-A-S-H and N-A-S-H phases (n–Na2O, A–Al2O3, S–SiO2, H–H2O), due to the use of a material containing calcium and an alkaline activator (most often NaOH), research is carried out to determine their compatibility. Depending on the amount of calcium and sodium, the phases form in different proportions and influence each other, what influences into thermodynamics, chemistry and efficiency of the entire process [59,60].
Due to the beneficial effect of fly ashes on the physicochemical properties of ceramic materials, many studies can be found in the literature focusing on the use of ashes not only from the energy industry, but also from the incineration of municipal waste and sewage, the management of phosphogypsum and other industrial and municipal waste, which contain large amounts of minerals in their composition, that can contribute to improving geopolymerization conditions [40,41].
3. Phosphogypsum as a Way to Increase the Mineral Fraction
One of the most problematic hazardous waste in landfills is phosphogypsum derived from the extraction method of orthophosphoric acid, otherwise known as the wet method. Orthophosphoric acid is an important raw material that is mainly used in the production of fertilizers, but also in medicine and the metallurgical industry. The annual global production of orthophosphoric acid has been at a level exceeding 60 million tons per year for many years [61,62]. The wet production method involves the extraction of phosphorus from phosphorus-containing minerals (mainly phosphates) using sulfuric acid. Thus, it uses the mechanism of the substitution reaction by replacing the acid residue of a weak acid with the acid residue of a strong acid. The main reactions in the process are shown below [63,64,65]:
The main component of phosphogypsum is calcium sulfate, but also calcium fluoride, calcium phosphate and silica. It is neutral to the environment in itself, however, taking into account the mineral composition of the raw materials used in the production of phosphoric acid, it may be contaminated with heavy metals (Cr, Zn, Cd, Pb) and radioactive elements (Ra-226, U-238) or residual amounts of orthophosphoric acid and hydrogen fluoride [65,66,67,68,69,70,71]. In addition to impurities in the form of oxides and other inorganic substances, phosphogypsum contains organic matter—including dioxins [64]. Impurities and significant acidity of phosphogypsum are the main problem that prevents it from being used as a substitute for natural gypsum [71,72,73,74].
Phosphogypsum also contains fractions that are desirable for their reuse. It is possible to determine the presence of calcium, iron and aluminum fractions, which undergo the following transformations in the extraction process:
Fe2O3 + 2 H3PO4 → 2 Fe3+ + 2 PO43− +3 H2O
Al2O3 + 2 H3PO4 → 2 Al3+ + 2 PO43− +3 H2O
Depending on the technological regime, concentrations and temperature of the extraction process, it is possible to obtain three main varieties of phosphogypsum. The dependence of the resulting forms on the process conditions is shown in the Table 3 [75,76,77,78,79,80]:

Table 3.
Dependence of the resulting forms of phosphogypsum on the parameters of the orthophosphoric acid production process.
Thanks to the above parameters, it is possible to control the transformation of the resulting sulfates and the size of the formed phosphogypsum. From the perspective of production efficiency and the economics of the phosphoric acid production process, it is necessary to strive to obtain coarse crystalline phosphogypsum and anhydrite, which are easy to filter and do not require large amounts of water, what conduct to obtain high concentration of acid. For this reason, relatively high temperatures of around 110 °C are used in the process [77,81,82,83].
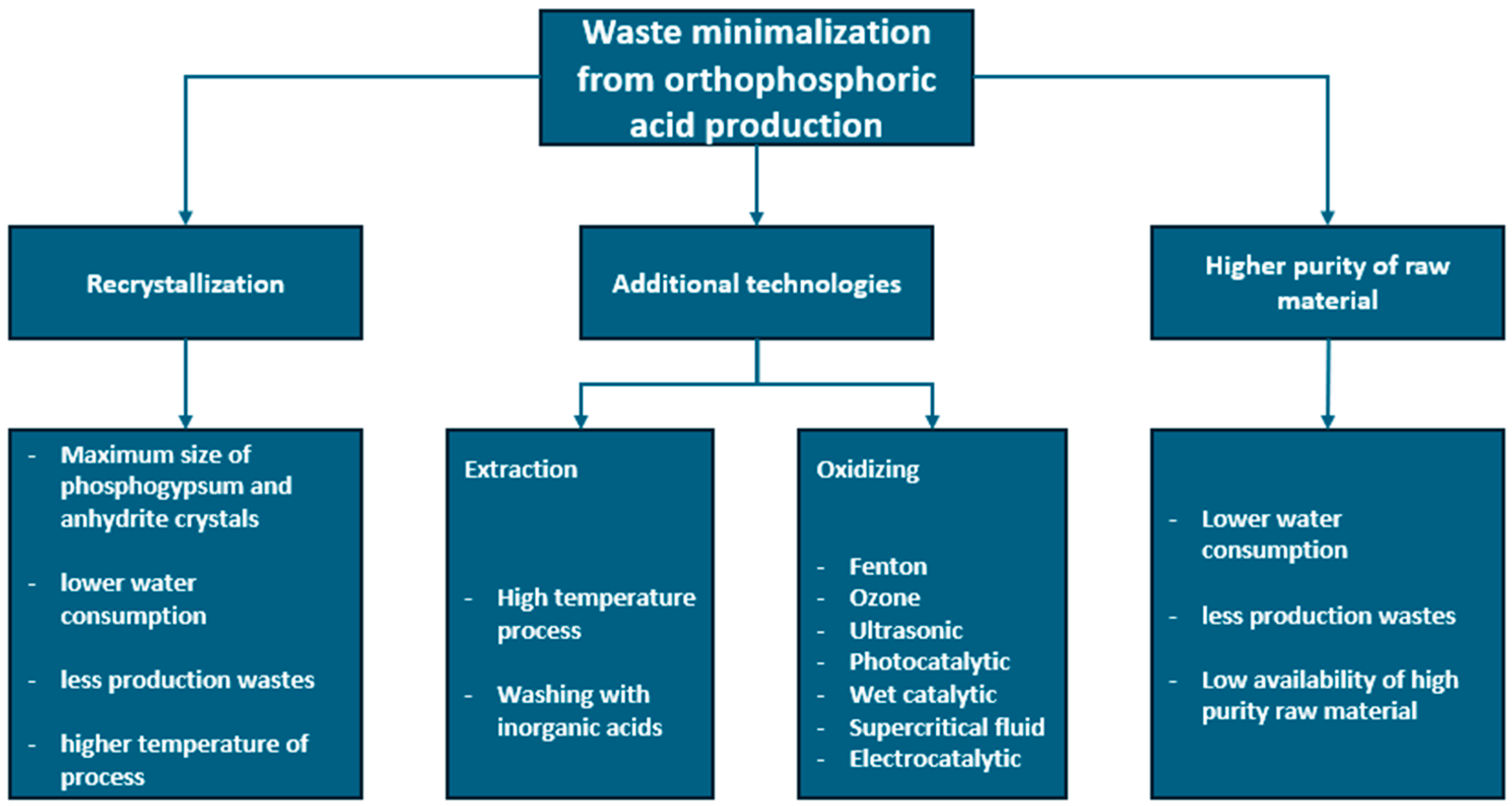
Bearing in mind that the annual global production of phosphogypsum reaches up to 280 Mt, the cleaning of phosphogypsum waste generates additional costs and does not eliminate the waste problem. In order to increase the efficiency of acid production and minimize the amount of waste, the technological principle of better use of the raw material is considered as the recrystallization of the resulting phosphogypsum and the use of raw materials of higher purity. The second idea is problematic due to the global crisis related to the depletion of phosphorus deposits and the fact that most of them are of medium or low purity. In order to maximize their use, research is carried out on the technology of extraction with other inorganic acids, or oxidation of phosphorus-containing minerals with various oxidizing agents, such as ozone, ultrasound, or microwaves [61,65,84,85]. The summary of the above information is presented in Figure 2.
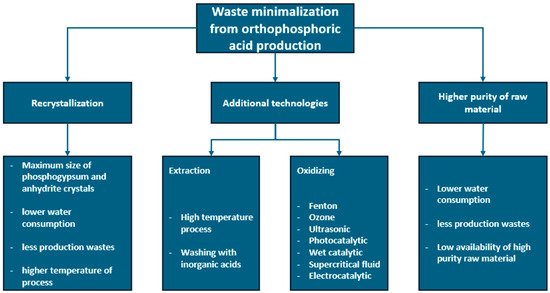
Figure 2.
Methods of minimizing waste and intensifying of orthophosphoric acid production.
4. Alkaline Activation
Spent caustic is a waste stream generated mainly in refineries and petrochemical plants in the process of purifying olefins from sulfides, hydrogen sulfide and other sulfur compounds (e.g., mercaptans), carbon monoxide and organic substances [53,54]. It has been classified as hazardous waste, mainly due to its high alkalinity (pH > 12) and the content of radioactive and toxic elements. It consists mainly of NaOH and Na2CO3—their total content can be even more than 20%, so it has great potential to be a precursor of the bond formation reaction between aluminum-silicate anions and calcium or sodium cations [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89].
Neutralization of the spent substance is usually done by wet air oxidation (WAO), which involves washing sulfur compounds and oxidizing them with high-pressure steam according to the following reactions [81,90,91]:
CaO · Al2O3 · 6 H2O + 3 (CaSO4 · 2 H2O) + 20 H2O -> 3 CaO · Al2O3 · 3 CaSO4 · 32 H2O
According to the available literature, the above properties allow the use of the spent caustic as a substitute for expensive pure NaOH [59,60,92,93,94].
The activation itself takes place by creating hydrates in the form of N-A-S-H or C-A-S-H, which take the form of a gel with properties that combine amorphous and zeolite structures [53,95,96,97]. Increasing the pH causes the separation of silicon and aluminum atoms from the solid form to the forming aluminosilicate matrix [97]. Due to the presence of sulfates in the composition of the substance, one of the crystalline forms is ettringite with the formula Ca6Al2(SO4)3(OH)12·26H2O, which allows to control the rate of hydration (binding) of the resulting mixture. For the same purpose, gypsum is added to the production of concrete [53,98].
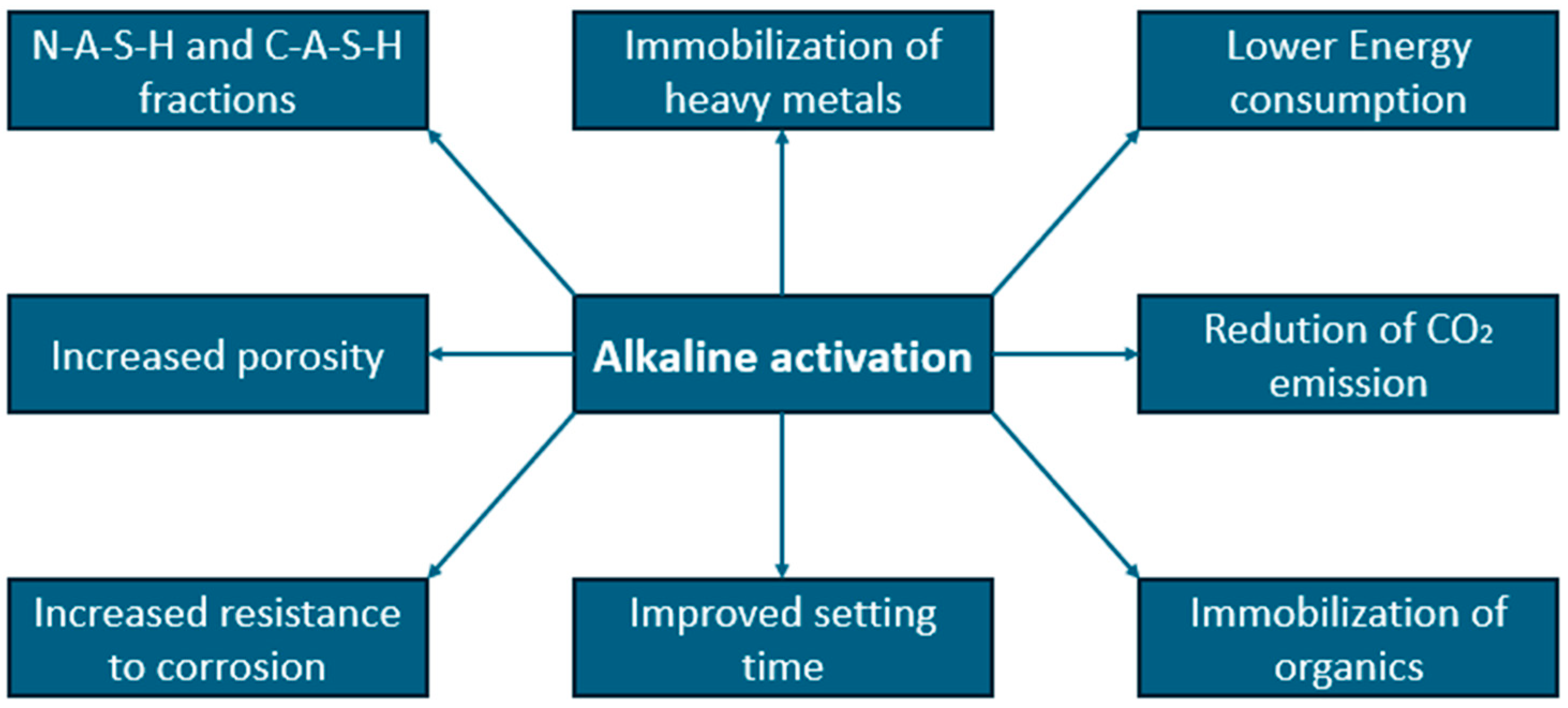
Alkaline activators have become a popular direction for the development of building materials due to their relatively large impact on improving the mechanical strength, durability and thermal resistance of the resulting materials compared to the cost of their production [99]. Technologies involving the use of alkaline activators have been developed since around the 1970s. From the very beginning, the use of waste materials containing large amounts of silica or alumina, such as sludge or fly ash, was taken into consideration [53,99]. The biggest problem is the control of the setting process, which also depends on the ratio of water and caustic solution [99,100]. It was observed that the higher pH of the alkaline activator, the more drastic acceleration the material hydration, even in the first minutes after activator addition. The kinetic of the reaction is connected with the sudden heat release. Taking into consideration available literature, main product (alkali activated) could be formed even in 12 h, while the standard setting process could take up to several days [100,101]. At the moment, it is a common method of producing binders all over the world, by smaller and larger manufacturers, and the scale of use of these additives depends on their availability. The main advantages of using alcali activators are presented in Figure 3.
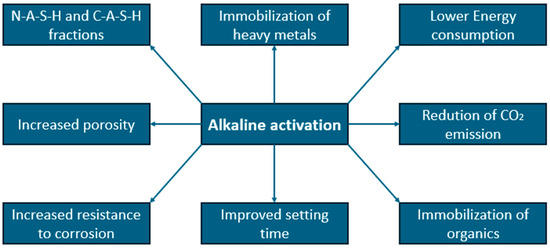
Figure 3.
Presentation of selected parameters, which are positively influenced by alkaline activator.
5. Waste or Component
Taking into consideration the properties of both substances described above—phosphogypsum and spent caustic, there is the idea of using them as components in the production of building material.
According to studies published so far, both the presence of phosphogypsum and spent caustic have a positive effect on compressive strength. The fresh and aged samples showed better compressive strength properties than the samples without the additives in the form of these wastes. In addition, it was confirmed that a higher content of Na2CO3, which is one of the main components of the spent caustic, has a positive effect on the above properties, m.in. by extending the setting time [53,54,96].
Considering that the bonds form gel systems at room temperature, the energy consumption in the material manufacturing process can be significantly reduced. According to literature data, even samples that do not require firing have better strength properties than those that do not contain spent caustic or phosphogypsum. This is due to the activation of phosphogypsum with an alkaline solution, which reduces the grain size of phosphogypsum and increases the gelation activity and thus also the density [19,53,102,103,104]. In addition, tetrahedral aluminosilicate structures enable the immobilization of heavy metals through the so-called encapsulation of heavy metal ions, which consists in encapsulating them in microporous matrices [102,105]. Similarly, in the case of organic substances, the resulting zeolite structures increase the absorption surface area of the material and immobilize them [54].
Taking into consideration the above, the simultaneous use of both mentioned wastes opens up new paths of research on the possibility of managing post-production waste into building materials. They have the potential to create a new alternative with the same or better performance parameters than the building materials used so far, while meeting all environmental standards [104,105,106].
As it shows in Table 4 with summary of articles used in this paper due to the year of publication, it seems to be clear that the topic of using phosphogypsum as a component to building materials is more and more popular in recent years but it is still not the main path to develop. There are also works focusing on other directions of use, mainly phosphogypsum, such as fillers or environmental functional materials, but at the moment their use in the production of building materials seems to be the most promising [107,108,109,110,111].

Table 4.
Summary of citations in scope of phosphogypsum used as a component to the building material production.
Referring to the table it could be stated phosphogypsum is never used as the only additive but mixed with other wastes used as additives enables the improvement of mechanical and chemical properties of the material and probably has influence of curing temperature. As there is no industrial-scale technology for the production of building materials with added phosphogypsum, the above information summarised in the Table 4 gives a broader spectrum of the advantages of using this waste.
Importantly, the table includes papers that did not use alkaline activator or used it in different concentrations. Any work that included the use of an activator represented the use of NaOH, or solutions with it.
As the work presented above focused on the use of several additives simultaneously with and without the use of an alkaline activator, it is difficult to know which component had a direct effect on specific improvements. An important point to note is that no work was found that described the use of only phosphogypsum and an alkaline activator.
6. Conclusions
Taking into consideration the increasing demand for construction materials and the increased involvement of the industry in minimizing the harmful impact on the environment, new ways and solutions are being sought to manage waste, reduce emissions and energy consumption. Construction is one of the industries that has the largest share in greenhouse gas emissions and energy absorption, which is why the entire range of research focuses on the production of eco-building materials.
- There are emerging trends in construction, that use waste, which properties favor the desired characteristics of building materials.
- Researches are carried out on the use of alkaline substances as activators for the formation of N-A-S-H and C-A-S-H aluminosilicate forms combines gel and zeolite forms.
- The addition of phosphogypsum and spent caustic affects the formation of gel forms, which results in an increased effect on the setting.
- Because of the formation of tetrahedral forms of aluminosilicates, it is possible to immobilize heavy metals and develop an absorbent surface by creating a zeolitic fraction for the immobilization of organic substances.
- The material binding stage begins at room temperature, so it is possible to reduce the energy consumption needed, e.g., for firing the material, due to the lack of such necessity.
- Further researches are needed to determine the leaches of organic and inorganic substances from the material, its radioactivity and the selection of the optimal composition in order to minimize the negative characteristics of the material.
Taking into consideration given amounts of phosphogypsum in landfills (between 3 and 8 billion tons, depending on the data) and that annual production is more than 300 million tons, the use of this material should be considered not only because of its potential to improve the properties of building materials, but also because of its availability and the negative margin of the waste itself [25,111].
Author Contributions
Conceptualization, A.L. and J.G.; writing—original draft preparation, A.L.; writing—review and editing, J.G.; supervision, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This research received no external funding.
References
- Wire, B. Global Concrete Block and Brick Manufacturing: Global Strategic Business Report; Global Industry Analysts, Inc.: San Jose, CA, USA, 2024. [Google Scholar]
- Available online: https://www.awbud.pl/ile-wazy-cegla-czy-ceglowka-wazy-kilogram/#:~:text=Standardowa%20ceg%C5%82a%20ceramiczna%20mo%C5%BCe%20wa%C5%BCy%C4%87%20od%202%20do,%C5%BCe%20ceg%C5%82a%20wa%C5%BCy%20zazwyczaj%20wi%C4%99cej%20ni%C5%BC%20jeden%20kilogram (accessed on 7 March 2024).
- Murmu, A.L.; Patel, A. Towards sustainable bricks production: An overview. Constr. Build. Mater. 2018, 165, 112–125. [Google Scholar] [CrossRef]
- Knapik, K. Wybrane właściwości fluidalnego popiołu lotnego przeznaczonego do wzmacniania podłoża gruntowego. Inżynieria Morska I Geotech. 2015, 3, 413–416. [Google Scholar]
- Pytel, Z. Influence of the thermal treatment parameters of clay raw materials on it pozzolanic activity. Probl. Nauk. -Badaw. Budownictwa 2001, 1, 387–396. [Google Scholar]
- Dodson, V.H. Pozzolans and the Pozzolanic Reaction, Concrete Admixtures; Springer: Boston, MA, USA, 1990; pp. 159–201. [Google Scholar]
- Tkaczewska, E. Metody badań aktywności pucolanowej dodatków mineralnych. Mater. Ceram. 2011, 63, 536–541. [Google Scholar]
- Dron, R. Bulletin de Liaison Laboratoires des Ponts et Chaussees; CFGI: Boston, MA, USA, 1978; p. 66. [Google Scholar]
- Kołakowski, J.; Szymański, E.; Wilczyńska, H. Prace Katedry Chemii i Technologii Materiałów Budowlanych; Politechnika Warszawska: Warsaw, Poland, 1996; pp. 1960–1970. [Google Scholar]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly ash for sustainable construction: A review of fly ash concrete and its beneficial use case studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, D.F.; Chiang, P.C. Utilization of sludge as brick material. Adv. Environ. Res. 2003, 7, 679–685. [Google Scholar] [CrossRef]
- Bubalo, A.; Vouk, D.; Curkovic, L.; Rogosic, M.; Nakić, D.; Cheeseman, C. Influence of combustion temperature on the performance of sewage sludge ash as a supplementary material in manufacturing bricks. Constr. Build. Mater. 2023, 404, 133126. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Bertelsen, I.; Jensen, P.E.; Kirkelund, G.M. Sewage sludge ash as resource for phosphorous and material for clay brick manufacturing. Constr. Build. Mater. 2020, 249, 118684. [Google Scholar] [CrossRef]
- Siemieniuk, J.; Szatyłowicz, E. Zmniejszenie emisji CO2 w procesie produkcji cementu. Civ. Environ. Eng. 2018, 9, 81–87. [Google Scholar]
- Okuno, N.; Takahashi, S. Full scale application of manufacturing bricks from sewage. Water Sci. Technol. 1997, 36, 243–250. [Google Scholar] [CrossRef]
- Zhang, Z.; Wong, Y.C.; Arulrajah, A.; Horpibulsuk, S. A review of studies on bricks using alternative materials and approaches. Constr. Build. Mater. 2018, 188, 1101–1118. [Google Scholar] [CrossRef]
- Seco, A.; Omer, J.; Marcelino, S.; Espuelas, S.; Prieto, E. Sustainable unfired bricks manufacturing from construction and demolition wastes. Constr. Build. Mater. 2018, 167, 154–165. [Google Scholar] [CrossRef]
- Eurostat Database. Number and Capacity of Recovery and Disposal Facilities by NUTS 2 Regions. Available online: https://data.europa.eu/data/datasets/pc4xatxpijivikn3tgf1rw?locale=en (accessed on 20 May 2024).
- Zhang, W.; Jin, Y.; Na, S.; Zhao, L.; Su, F.; Zhu, J. Improving mechanical properties of hemihydrate phosphogypsum via alkali-activated mineral admixtures. J. Clean. Prod. 2024, 454, 142234. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Zuaiter, H.A.; Najm, O. Shrinkage characteristics of sustainable mortar and concrete with alkali-activated slag and fly ash Binders: A focused review. Mater. Today Proc. 2024, in press. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Robert, P.M.; Petrou, M.F.; Nicolaides, D. Mechanical behavior of construction and demolition waste-based alkali activated materials exposed to fire conditions. Constr. Build. Mater. 2024, 415, 134994. [Google Scholar] [CrossRef]
- Decision-2000/532-EN-EUR-Lex (europa.eu). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32000D0532 (accessed on 20 May 2024).
- Tayibi, H.; Mohamed, C. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Abril, J.; Garcia-Tenorio, R.; Enamorado, S.; Hurtado, M.D.; Andreu, L.; Delgado, A. The cumulative effect of three decades of phosphogypsum amendments in reclaimed marsh soils from SW Spain: 226 Ra, 238 U and Cd contents in soils and tomato fruit. Sci. Total Environ. 2008, 403, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- El Aloui, E. Phosphogypsum (PG) is a waste or by product, ICCME22, November 2022. Available online: https://www.researchgate.net/publication/365704647_Phosphogypsum_PG_is_a_waste_or_by_product#:~:text=Phosphogypsum%20%28PG%29%20is%20a%20by-product%20resulting%20from%20the,production%20rate%20exceeds%2010%20million%20tons%20per%20year (accessed on 20 May 2024).
- Silva, L.F.O.; Oliviera, M.L.S.; Crissien, T.J.; Santosh, M.; Bolivar, J.; Shao, L.; Dotto, G.L.; Gasparotto, J.; Schindler, M. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 2022, 286, 131513. [Google Scholar] [CrossRef] [PubMed]
- Pliaka, M.; Gaidjis, G. Potential uses of phosphogypsum: A review. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. 2022, 57, 746–763. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.L. Exploring the potential reuse of phosphogypsum: A waste or a resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Wang, Z.-Y.; Huang, D.-M.; Huang, Q.-C.; Chen, Y.; Zhang, H.; Shui, Z.-H. Recovery and recycling core of phosphogypsum: Characteristic hazardous elements risk assessment and analysis. Process Saf. Environ. Prot. 2023, 170, 738–756. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yi, Y.; Tian, J.; Xuan, C.; Wang, Y.; Wen, J.; Cao, J. Effects of phosphogypsum on enzyme activity and microbial community in acid soil. Sci. Rep. 2023, 13, 6189. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, Y.; Yakhnenko, E.; Chubur, V.; Roubik, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Awad, S.; Essam, M.; Boukhriss, A.; Kamar, M.; Midani, M. Properties, Purification and Applications of Phosphogypsum: A Comprehensive Review Towards Circular Economy. Mater. Circ. Econ. 2024, 6, 9. [Google Scholar] [CrossRef]
- Parliamentary Question|Plan to Cover over Phosphogypsum Ponds in Huelva|E-001616/2023| uropean Parliament (europa.eu). Available online: https://www.europarl.europa.eu/doceo/document/E-9-2023-001616_EN.html (accessed on 20 May 2024).
- Staub, M.; Gourc, J.P.; Simonin, R. Influence of landfill cap cover characteristics on the mitigation of GHG emissions. In Proceedings of the 9th International Conference on Geosynthetics, Guarujá, Brazil, 1 February 2010. [Google Scholar]
- Błaszczyński, T. Wpływ uziarnienia popiołów lotnych na wytrzymałość spoiw geopolimerowych. Mater. Bud. 2017, 1, 96–99. [Google Scholar] [CrossRef]
- Błaszczyński, T. Właściwości spoiw geopolimerowych na bazie lotnych popiołów wapniowych. Builder 2021, 288, 100–103. [Google Scholar] [CrossRef]
- Available online: www.budownictwo.org/artykuly/szczegoly/45058_co-to-sa-pucolany (accessed on 7 March 2024).
- Radwan, M.M.; Nagi, S.M. Hydration behavior and formation of strätlingite compound (C2ASH8) in a bio-cement based on tri-calcium silicate and mono-calcium aluminate for dental applications: Influence of curing medium. Bull. Natl. Res. Cent. 2022, 46, 180. [Google Scholar] [CrossRef]
- Lin, C.; Wu, C.; Ho, H.M. Recovery of municipal waste incineration bottom ash and water treatment sludge to water permeable pavement. Materials 2006, 26, 970–9783. [Google Scholar] [CrossRef]
- Munoz, P.S.P.; Characa, M.S.; Davila, G.C.M.; Díaz, R.I. Use of fly ash in the production of geopolymers: A literature review. Innov. Infrastruct. Solut. 2022, 7, 236. [Google Scholar]
- Shaji, N.; Holmes, N.; Tyrer, M. Early Age Assessment of a New Course of Irish Fly Ash as a Cement Replacement. Appl. Sci. 2024, 14, 4128. [Google Scholar] [CrossRef]
- EN 450-1:2012; Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- Bubalo, A.; Vouk, D.; Stirmer, N.; Nad, K. Use of Sewage Sludge Ash in the Production of Innovative Bricks—An Example of a Circular Economy. Sustainability 2021, 13, 9330. [Google Scholar] [CrossRef]
- Thomas, M. Optimizing the Use of Fly Ash in Concrete; Portland Cement Association: Tokyo, Japan, 2007. [Google Scholar]
- Bouaissi, A.; Li, L.; Mohd, M.A.B.A.; Romisuhani, A. Fly Ash as a Cementitious Material for Concrete. Zero-Energy Buildings—New Approaches and Technologies; Intech Open: London, UK, 2020. [Google Scholar]
- Paya, J.; Monzo, J.; Borrachero, M.V.; Soriano, L. Sewage Sludge Ash, New Trends in Eco-efficient and Recycled Concrete. Civil and Structural Engineering; Woodhead Publishing: Cambridge, UK, 2018; pp. 121–152. [Google Scholar]
- Sah, P.K.; Sah, B.K.; Kumar, S.S. Sustainable utilization of sewage sludge ash in stabilizing subgrade soil: An appraisal. Environment. Dev. Sustain. 2024, 26. [Google Scholar] [CrossRef]
- Murali, G.; Azab, M. Recent research in utilization of phosphogypsum as building materials: Review. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- Turkel, S.; Aksin, E. A comparative study on the use of fly ash and phosphogypsum in the brick production. Sadhana 2012, 37, 595–607. [Google Scholar] [CrossRef]
- Bouchhima, L.; Rouis, M.J.; Choura, M. Engineering properties of wade sand-lime- cement-phosphogypsum building brick grade MW. Int. J. Eng. Adv. Technol. 2013, 2, 43–49. [Google Scholar]
- Vaičiukynienė-Palubinskaitė, D.; Nizeviciene, D.; Kantautas, A.; Bocullo, V.; Kielė, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; Leon-Patino, C.A.; Song, S. Co-disposal of MSWI fly ash and spent caustic through alkaline-activation consolidation. Cem. Concr. Compos. 2021, 116, 103824. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Q.; Zhang, Y.; Zhang, X. Synthesis and characterization of fly ash-based geopolymers activated with spent caustic. Gels 2022, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, W.; Borno, I.B.; Khan, R.I.; Siddique, S.; Haque, M.I.; Tahsin, A. Mimicking the cementation mechanism of ancient Roman seawater concrete using calcined clays. Appl. Clay Sci. 2022, 230, 106696. [Google Scholar] [CrossRef]
- MacFarlane, J.; Vanorio, T.; Monteiro, P. Multi-scale imaging, strength and permeability measurements: Understanding the durability of Roman marine concrete. Constr. Build. Mater. 2021, 272, 121812. [Google Scholar] [CrossRef]
- Pinter, F.; Vidovszky, I.; Weber, J.; Bayer, K. Mineralogical and microstructural characteristics of historic Roman cement renders from Budapest, Hungary. J. Cult. Herit. 2014, 15, 219–226. [Google Scholar] [CrossRef]
- Allard, C. Self-healing Roman concrete. Nat. Rev. Mater. 2023, 8, 80. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernandez-Jimenez, A.; Macphee, D.E. Compability studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Fang, K.; Xu, L.; Yang, M.; Chen, Q. One-step wet-process phosphoric acid by-product CaSO4 and its purification. Sep. Purif. Technol. 2023, 309, 123048. [Google Scholar] [CrossRef]
- Available online: https://www.statista.com/statistics/1289304/global-phosphoric-acid-production-capacity/ (accessed on 20 April 2024).
- Dorozhkin, S.V. Fundamentals of the Wet-Process Phosphoric Acid Production. 1. Kinetics and Mechanism of the Phosphate Rock Dissolution. Ind. Eng. Chem. Res. 1996, 35, 4328–4335. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, G.; Long, Y.; Liu, Z.; Tao, C.; Liu, R. Advanced oxidation processes for wet-process phosphoric acid: Enhanced phosphorus recovery and removal of organic matters. Hydrometallurgy 2022, 210, 105842. [Google Scholar] [CrossRef]
- Podraza, Z.; Krupa-Żuczek, K.; Wzorek, Z. Technologie Otrzymywania Kwasu Fosforowego (V), Chemia Czasopismo Techniczne; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2011. [Google Scholar]
- Biegaska, J.; Pitkowska, E.; Pala, A. Zagospodarowanie fosfogipsu na cele nieprzemysłowe. In Innowacyjne i Przyjazne dla Środowiska Techniki i Technologie Przeróbki Surowców Mineralnych; Bezpieczeństwo–Jakość–Efektywność; Instytut Techniki Górniczej KOMAG: Gliwice, Poland, 2014; pp. 107–117. [Google Scholar]
- Garrido, F.; Illera, V.; Garcia-Gonzales, M.T. Effect of the addition of gypsum- and lime- rich industrial by-products on Cd, Cu and Pb availability and leachability in metal-spiked acid soils. Appl. Geochem. 2005, 20, 397–408. [Google Scholar] [CrossRef]
- Sahu, S.K.; Ajmal, P.Y.; Bhangare, R.C.; Tiwari, M.; Pandit, G.G. Natural radioactivity assessment of a phosphate fertilizer plant area. J. Radiat. Res. Appl. Sci. 2014, 7, 123–128. [Google Scholar] [CrossRef]
- Hassoune, H.; Lachehab, A.; Hajjaji, K.; Mertah, O.; Kherbeche, A. Dynamic of Heavy Metals and Environmental Impact of Waste Phosphogypsum, Fate and Transport of Subsurface Pollutants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 57–77. [Google Scholar]
- Sadowski, R.F.; Kosieradzka-Federczyk, A. Paradoksy ekologiczne. In Odpady Miarą Sukcesu i Porażki Cywilizowanej Ludzkości; Krajowa Szkoła Administracji Publicznej: Warszawa, Poland, 2020. [Google Scholar]
- Afifi, E.M.; Hilal, M.A.; El-Reefy, S.A. Characterization of phosphogypsum wastes associated with phosphoric acid and fertilizers production. J. Environ. Radioact. 2009, 100, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2021, 242, 106778. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 168196. [Google Scholar] [CrossRef]
- Afifi, E.M.; Hilal, M.A.; Aly, H.F. Evaluation of U, Th, K and emanated radon in some NORM and TENORM samples. Radiat. Meas. 2006, 41, 627–633. [Google Scholar] [CrossRef]
- Podraza, Z.; Krupa-Żuczek, K.; Wzorek, Z. Ekstrakcyjna Metoda Otrzymywania Kwasu Fosforowego(V) z Odpadów Kostnych z Przemysłu Mięsnego, Chemia Czasopismo Techniczne; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2011. [Google Scholar]
- Donigiewicz, F.; Wadowski, M.; Skiba, T.; Hoffmann, K.; Hoffmann, J. Badania nad natężeniem ekstrakcyjnego kwasu fosforowego na potrzeby otrzymywania fosforanów paszowych. Proc. ECOpole 2010, 4, 121–126. [Google Scholar]
- Bojanowicz-Bablok, A. Challenges in phosphoric acid production. Possibilities Limit. Waste Manag. Przemysł Chem. 2013, 92, 450–457. [Google Scholar]
- Gobbitt, J.M. Yara Hemihydrate (HH) and Hemidihydrate (HDH) Processes for Phosphoric Acid Production. Procedia Eng. 2012, 46, 143–153. [Google Scholar] [CrossRef]
- Bouchkira, I.; Benjelloun, S.; Khamar, L.; Latifi, A. Thermodynamic modeling and parameter estimability analysis of a wet phosphoric acid process with impurities. Fluid Phase Equilibria 2022, 564, 113594. [Google Scholar] [CrossRef]
- Soboleva, I.V.; Lyashenko, S.E. Studying the Hemihydrate Stage of the Process of Preparation of Extractive Phosphoric Acid by the Dihydrate–Hemihydrate Method from Low-Grade Phosphorites. Theor. Found. Chem. Eng. 2023, 57, 704–708. [Google Scholar] [CrossRef]
- Kępiński, J. Technologia Chemiczna Nieorganiczna; PWN: Warszawa, Pland, 1964. [Google Scholar]
- Praca, Z. Technologia Chemiczna Nieorganiczna; Wydawnictwa Naukowo-Techniczne: Warszawa, Pland, 1965. [Google Scholar]
- Production of Phosphoric Acid, Best Available Techniques for Pollution Prevention and Control in the European Fertilizer Industry; European Fertilizer Manufacturers Association: Brussels, Belgium, 2000. Booklet No. 4 of 8. Available online: https://fertilizerseurope.com/wp-content/uploads/2019/08/Booklet_4_final.pdf (accessed on 20 May 2024).
- Calderon-Morales, B.; Garcia-Martinez, A.; Pineda, P.; Garcia-Tenorio, R. Valorization of phosphogypsum in cement-based materials: Limits and potential in eco-efficient construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Qu, G.; Jin, C.; Liu, Y.; Kuang, L.; Li, H.; Chen, X.; Wang, Z.; Cheng, Y. Application prospect of advanced oxidation technology in wet process phosphoric acid production. J. Environ. Chem. Eng. 2022, 10, 113594. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Available online: https://www.watertechonline.com/wastewater/article/15550510/treating-refinery-spent-caustic-with-electro-oxidation (accessed on 20 April 2024).
- Niknejad, A.S.; Bazgir, S.; Ardjmand, M.; Shirazi, M.M.A. Spent caustic wastewater treatment using direct contact membrane distillation with electro blown styrene-acrylonitrile membrane. Int. J. Environ. Sci. Technol. 2020, 18, 2283–2294. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.B.; Heris, S.Z.; Mohammadpourfard, M. Highly efficient treatment of petrochemical spent caustic effluent via electro-Fenton process for COD and TOC removal: Optimization and experimental. Biomass Convers. Biorefinery 2023, 14, 17481–17497. [Google Scholar] [CrossRef]
- Ahmadi, M.; Seyedin, S.H.; Sayedin, S.V. Modern Spent-caustic Wastewater Treatment Simulation by Aspen Plus in Electrolytic Medium. J. Multidiscip. Eng. Sci. Technol. 2019, 6, 10814–10823. [Google Scholar]
- Mohammadizadeh, Z.; Farshi, A.; Royaee, S.J.; Jodaree, N. An Extensive Review on Spent Caustic Treatment Method in Oil and Gas Industry. J. Iran. Chem. Eng. 2018, 17, 39. [Google Scholar]
- Palanisamy, M.K.; Murugesan, A.; Ismail, A.A.M.; Srinivasan, D. Alkali activation of fly ash in self-consolidating concrete at low molar concentration. Mag. Concr. Res. 2023, 76, 201–216. [Google Scholar] [CrossRef]
- Horvat, B.; Music, B. Green Transition in Slovenian Building and Civil Engineering Industry: 10 Years of Research on Alkali-Activated Materials and Alkali-Activated Foams. Socrat. Lect. 2024, 10, 136–146. [Google Scholar]
- Kryvenko, P.; Rudenko, I.; Sikora, P.; Sanytsky, M.; Konstantynovskyi, O.; Kropyvnytska, T. Alkali-activated cements as sustainable materials for repairing building construction: A review. J. Build. Eng. 2024, 90, 109399. [Google Scholar] [CrossRef]
- Kalifa, A.Z.; Cizer, O.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Khorshidi, H.; Zhang, C.; Ghasemi, M. Alkali-activated binder based on red-mud with class F fly ash and ground granulated blast-furnace slag under ambient temperature. Rev. Adv. Mater. Sci. 2023, 62, 20230114. [Google Scholar] [CrossRef]
- Nijland, T.G.; Larbi, J.A. Microscopic examination of deteriorated concrete. Non-Destr. Eval. Reinf. Concr. Struct. 2010, 1, 137–179. [Google Scholar]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Wang, S.; Pu, X.; Scrivener, K.L.; Pratt, P.L. Alkali-activated slag cement and concrete: A review of properties and problems. Adv. Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Thomas, R.J.; Ye, H.; Radlińska, A.; Peethamparan, S. Alkali-Activated Slag Cement Concrete A closer look at a sustainable alternative to protland cement. ACI Comm. 2016, 236, 33–38. [Google Scholar]
- Wang, H.; You, X.; Tian, J. Study on Pretreatment of Phosphogypsum and Preparation of Non Burning Building Materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 668, 012076. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. Advances in understanding and analyzing the anti-diffusion behavior in complete carbonation zone of MSWI bottom ash-based alkali-activated concrete. Constr. Build. Mater. 2018, 186, 1072–1081. [Google Scholar] [CrossRef]
- Mashifana, T. Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Rożek, P.; Florek, P.; Król, M.; Mozgawa, W. Immobilization of Heavy Metals in Boroaluminosilicate Geopolymers. Materials 2021, 14, 214. [Google Scholar] [CrossRef]
- Dong, E.; Fu, S.; Wu, C.; Lv, W.; Zhang, L.; Feng, Y.; Yu, R. Value-added utilization of phosphogypsum industrial by-products in producing green Ultra-High performance Concrete: Detailed reaction kinetics and microstructure evolution mechanism. Constr. Build. Mater. 2023, 389, 131726. [Google Scholar] [CrossRef]
- Wu, F.; Ren, Y.; Qu, G.; Liu, S.; Chen, B.; Liu, X.; Li, J. Utilization path of bulk industrial solid waste: A review on the multi-directional resource utilization path of phosphogypsum. J. Environ. Manag. 2022, 313, 114957. [Google Scholar] [CrossRef] [PubMed]
- Oubaha, S.; Machi, A.; Mabroum, S.; Taha, Y.; Benzaazoua, M.; Hakkou, R. Recycling of phosphogypsum and clay by-products from phosphate mines for sustainable alkali-activated construction materials. Constr. Build. Mater. 2024, 411, 134262. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, H.; Chen, B.; Cui, Q. Development and optimization of phosphogypsum-based geopolymer cement. Constr. Build. Mater. 2023, 369, 130577. [Google Scholar] [CrossRef]
- Avsar, C.; Gezerman, A.O. Industrial Waste Management: Economical Benefits of the Resource Utilization of Phosphogypsum. Int. J. Ind. Mark. 2022, 7, 1–9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).