Microcalorimetry as an Effective Tool for the Determination of Thermodynamic Characteristics of Fulvic–Drug Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Isothermal Titration Calorimetry
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carvalho, T.O.; Matias, A.E.B.; Braga, L.R.; Evangelista, S.M.; Prado, A.G.S. Calorimetric studies of removal of nonsteroidal anti-inflammatory drugs diclofenac and dipyrone from water. J. Therm. Anal. Calorim. 2011, 106, 475–481. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.E.; Fimmen, R.L.; Chin, Y.-P.; Mash, H.E.; Weavers, L.K. Fulvic acid mediated photolysis of ibuprofen in water. Water Res. 2011, 45, 4449–4458. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic, wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Tanishima, T.; Shinohara, H.; Kiri, K.; Takada, H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006, 40, 3297–3303. [Google Scholar] [CrossRef]
- Winker, M.; Faika, D.; Gulyas, H.; Otterpohl, R. A comparison of pharmaceutical concentrations in raw municipal wastewater and yellow water. Sci. Total Environ. 2008, 399, 96–104. [Google Scholar] [CrossRef]
- Vulava, V.M.; Cory, W.C.; Murphey, V.L.; Ulmer, C.Z. Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. Sci. Total Environ. 2016, 565, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Bialk, H.M.; Pedersen, J.A. NMR investigation of enzymatic coupling of sulfonamide antimicrobials with humic substances. Environ. Sci. Technol. 2008, 42, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Qiao, X.; Zhang, H.; Zhang, Y.; Zhou, C. Insights into photolytic mechanism of sulfapyridine induced by triplet-excited dissolved organic matter. Chemosphere 2016, 147, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Liu, L.C.; Chen, W.R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231, 1163–1171. [Google Scholar] [CrossRef]
- Sim, W.-J.; Lee, J.-W.; Oh, J.-E. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Boxall, A.B.A. Factors affecting the degradation of pharmaceuticals in agricultural soils. Environ. Toxicol. Chem. 2009, 28, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Le Guet, T.; Hsini, I.; Labanowski j Mondamert, L. Sorption of selected pharmaceuticals by a river sediment: Role and mechanisms of sediment or Aldrich humic substances. Environ. Sci. Pollut. Res. 2018, 25, 14532–14543. [Google Scholar] [CrossRef] [PubMed]
- Anielak, A.M.; Styszko, K.; Kwásny, J. The importance of humic substances in transporting “chemicals of emerging concern” in water and sewage environments. Molecules 2023, 28, 6483. [Google Scholar] [CrossRef] [PubMed]
- Anielak, A.M.; Styszko, K.; Kłeczek, A.; Łomínska-Płatek, D. Humic substances—Common carriers of micropollutants in municipal engineering. Energies 2022, 15, 8496. [Google Scholar] [CrossRef]
- Ruiz, S.H.; Wickramasekara, S.; Abrell, L.; Gao, X.; Chefetz, B.; Chorover, J. Complexation of trace organic contaminants with fractionated dissolved organic matter: Implications for mass spectrometric quantification. Chemosphere 2013, 91, 344–350. [Google Scholar] [CrossRef]
- Margon, A.; Pastrello, A.; Mosetti, D.; Cantone, P.; Leita, L. Interaction between diclofenac and soil humic cids, soil and sediment. Contamination 2009, 18, 489–496. [Google Scholar]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Park, H.S. Sorptive removal of ibuprofen from water using selected soil minerals and activated carbon. Int. J. Environ. Sci. Technol. 2012, 9, 85–94. [Google Scholar]
- Földényi, R.; Joó, S.; Tóth, J. Adsorption of diclofenac on activated carbon and its hypochlorination in the presence of dissolved organic matter. Int. J. Environ. Sci. Technol. 2017, 14, 1071–1080. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Influence of ionic strength, anions, cations, and natural organic matter on the adsorption of pharmaceuticals to silica. Chemosphere 2010, 80, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M.; Závodská, P. Diffusion of pharmaceuticals in agarose hydrogels enriched by humic acids. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131825. [Google Scholar] [CrossRef]
- Xu, J.; Koopal, L.K.; Fang, L.; Xiong, J.; Tan, W. Proton and copper binding to humic acids analyzed by XAFS spectroscopy and isothermal titration calorimetry. Environ. Sci. Technol. 2018, 52, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xu, Z.; Hu, M.; Zhang, H.; Peacock, C.L.; Liu, X.; Nie, N.; Xue, Q.; Lei, M.; Tie, B. Natural organic matter decreases uptake of W(VI), and reduces W(VI) to W (V), during adsorption to ferrihydrite. Chem. Geol. 2020, 540, 119567. [Google Scholar] [CrossRef]
- Klučáková, M.; Krouská, J.; Kalina, M. Physico-chemical aspects of metal-fulvic complexation. Processes 2024, 12, 989. [Google Scholar] [CrossRef]
- Kimuro, S.; Kirishima, A.; Kitatsuji, Y.; Miyakawa, K.; Akiyama, D.; Sato, N. Thermodynamic study of the complexation of humic acid by calorimetry. J. Chem. Thermodyn. 2019, 132, 352–362. [Google Scholar] [CrossRef]
- Čokeša, Đ.; Radmanović, S.; Potkonjak, N.; Marković, M.; Šerbula, S. Soil humic acid and arsenite binding by isothermal titration calorimetry and dynamic light scattering: Thermodynamics and aggregation. Chemosphere 2023, 315, 137687. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Vitorazi, L.; Berret, J.-F.; Stoll, S. Isothermal titration calorimetry as a powerful tool to quantify and better understand agglomeration mechanisms during interaction processes between TiO2 nanoparticles and humic acids. Environ. Sci. Nano 2015, 2, 541–550. [Google Scholar] [CrossRef]
- Tan, W.F.; Koopal, L.K.; Norde, W. Interaction between humic acid and lysozyme, studied by dynamic light scattering and isothermal titration calorimetry. Environ. Sci. Technol. 2009, 43, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Khil’ko, S.L.; Semenova, R.G. Interaction of humic acid salts with drug preparations. Solid Fuel Chem. 2016, 50, 390–394. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Y.-Y.; Li, X.-Y.; Chen, J.-J.; Sheng, G.-P. Rapidly probing the interaction between sulfamethazine antibiotics and fulvic acids. Environ. Pollut. 2018, 243, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Choudhary, S.; Kishore, N. Insights into the binding of the drugs diclofenac sodium and cefotaxime sodium to serum albumin: Calorimetry and spectroscopy. Eur. J. Pharm. Sci. 2012, 46, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ràfols, C.; Zarza, S.; Bosch, E. Molecular interactions between some non-steroidal anti-inflammatory drugs (NSAID’s) and bovine (BSA) or human (HSA) serum albumin estimated by means of isothermal titration calorimetry (ITC) and frontal analysis capillary electrophoresis (FA/CE). Talanta 2014, 130, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Talele, P.; Choudhary, S.; Kishore, N. Understanding thermodynamics of drug partitioning in micelles and delivery to proteins: Studies with naproxen, diclofenac sodium, tetradecyltrimethylammonium bromide, and bovine serum albumin. J. Chem. Thermodyn. 2016, 92, 182–190. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Sprague, S.E.; Smith, B.M.; Giffune, T.R. Binding thermodynamics of Diclofenac and Naproxen with human and bovine serum albumins: A calorimetric and spectroscopic study. J. Chem. Thermodyn. 2016, 103, 299–309. [Google Scholar] [CrossRef]
- Csapó, E.; Juhász, Á.; Varga, N.; Sebők, D.; Hornok, V.; Janovák, L.; Dékány, I. Thermodynamic and kinetic characterization of pH-dependent interactions between bovine serum albumin and ibuprofen in 2D and 3D systems. Colloid. Surface. A 2016, 504, 471–478. [Google Scholar] [CrossRef]
- Seal, P.; Sikdar, J.; Roy, A.; Haldar, R. Binding of ibuprofen to human hemoglobin: Elucidation of their molecular recognition by spectroscopy, calorimetry, and molecular modeling techniques. J. Biomol. Struct. Dyn. 2017, 36, 3137–3154. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Vaulot, C.; Reinert, L.; Guittonneau, S.; Gadiou, R.; Duclaux, L. Thermodynamic study of seven micropollutants adsorption onto an activated carbon cloth: Van’t Hoff method, calorimetry, and COSMO-RS simulations. Environ. Sci. Pollut. Res. 2017, 24, 10005–10017. [Google Scholar] [CrossRef]
- IHSS|International Humic Substances Society. Available online: https://humic-substances.org/acidic-functional-groups-of-ihss-samples/ (accessed on 15 November 2024).
- Liu, S.; Benedetti, M.F.; Han, W.; Korshin, G.V. Comparison of the properties of standard soil and aquatic fulvic and humic acids based on the data of differential absorbance and fluorescence spectroscopy. Chemosphere 2020, 261, 128189. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 2018, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M. Conductometric study of the dissociation behavior of humic and fulvic acids. React. Funct. Polym. 2018, 128, 24–28. [Google Scholar] [CrossRef]
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
- Thorn, A.K.; Cox, L.G. N-15 NMR spectra of naturally abundant nitrogen in soil and aquatic natural organic matter samples of the International Humic Substances Society. Org. Geochem. 2009, 40, 484–499. [Google Scholar] [CrossRef]
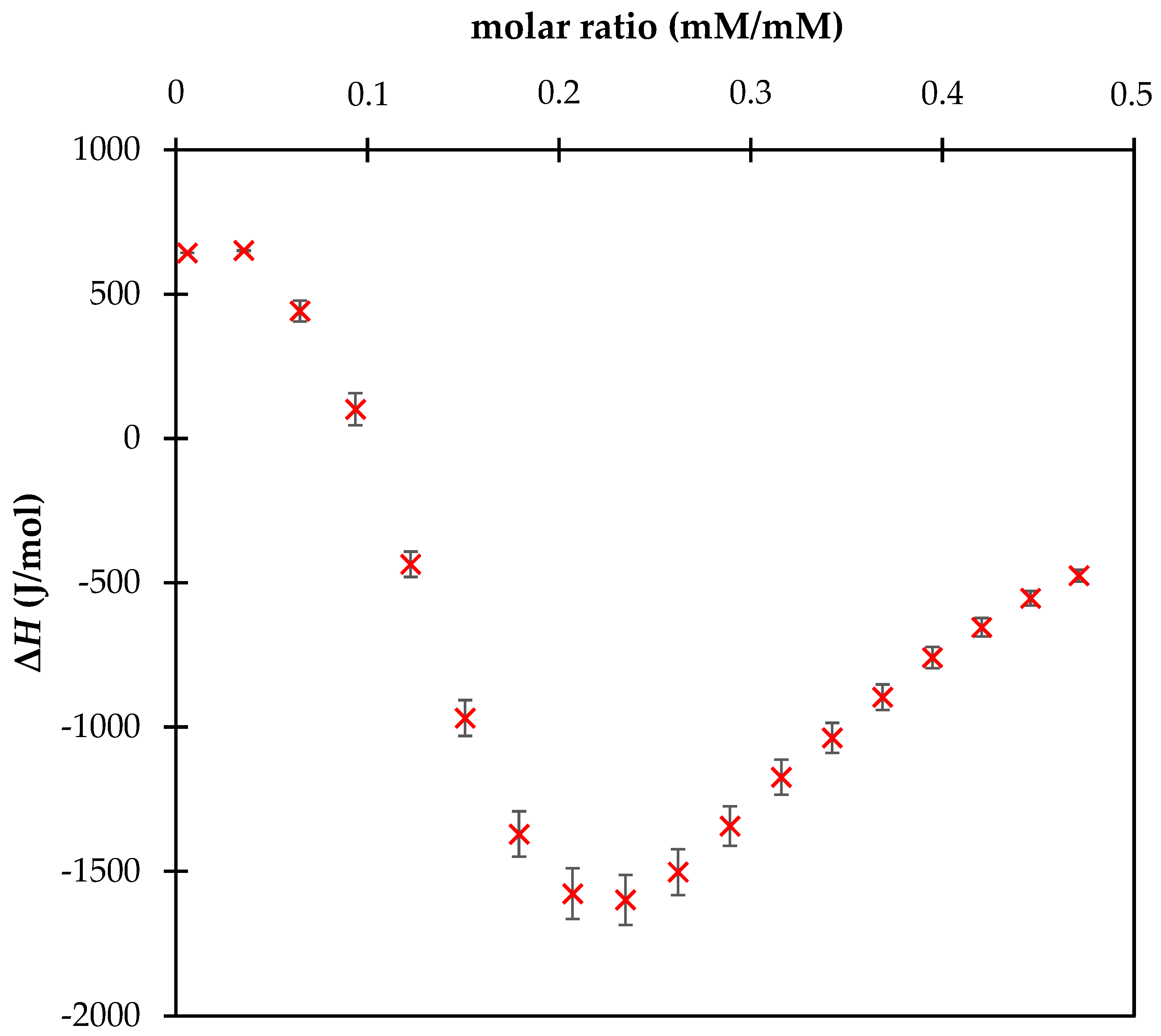

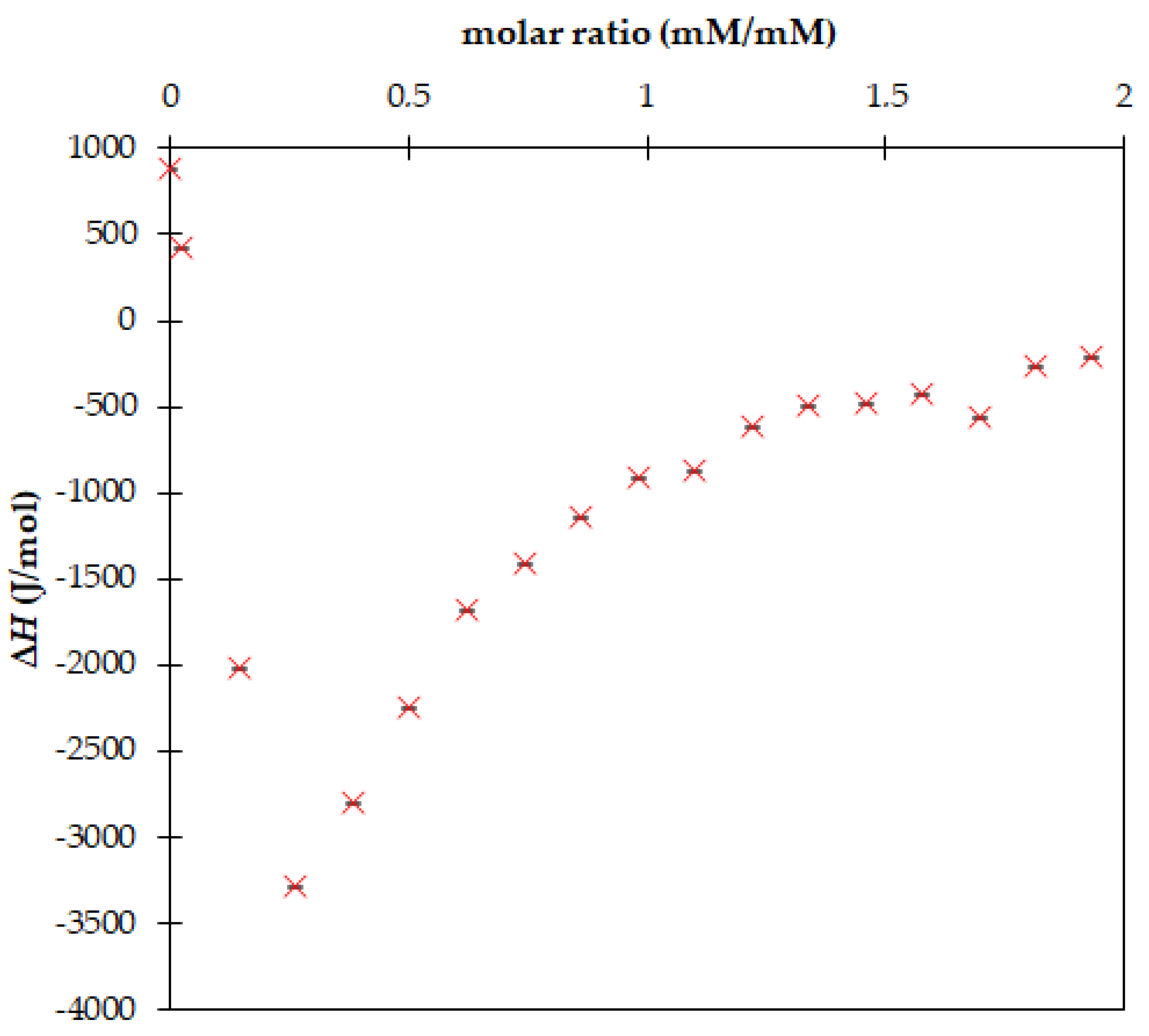
| Fulvic Acids | COOH (mmol/g) | OH (mmol/g) | Total Acidity (mmol/g) |
|---|---|---|---|
| Suwanee River | 5.77 | 1.47 | 7.24 |
| Pahokee Peat | 6.25 | 1.09 | 7.34 |
| Nordic Lake | 5.67 | 1.61 | 7.28 |
| Fulvic Acids | ΔH (J/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| Suwanee River | −5032 ± 277 | −27.7 ± 1.5 | 76.2 ± 4.2 |
| Pahokee Peat | −496 ± 43 | −20.6 ± 0.1 | 67.7 ± 0.4 |
| Nordic Lake | −1693 ± 149 | −23.7 ± 0.2 | 74.0 ± 0.2 |
| Fulvic Acids | ΔH (J/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| Suwanee River | −5356 ± 295 | −24.6 ± 1.3 | 64.7 ± 3.6 |
| Pahokee Peat | −3302 ± 117 | −20.4 ± 0.1 | 57.4 ± 0.2 |
| Nordic Lake | −8975 ± 95 | −23.5 ± 0.1 | 48.9 ± 0.5 |
| Fulvic Acids | ΔH (J/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| Suwanee River | −3709 ± 204 | −23.9 ± 1.3 | 67.8 ± 3.7 |
| Pahokee Peat | −9938 ± 645 | −23.7 ± 0.2 | 46.2 ± 1.5 |
| Nordic Lake | −7770 ± 249 | −23.8 ± 4.3 | 53.7 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klučáková, M.; Krouská, J. Microcalorimetry as an Effective Tool for the Determination of Thermodynamic Characteristics of Fulvic–Drug Interactions. Processes 2025, 13, 49. https://doi.org/10.3390/pr13010049
Klučáková M, Krouská J. Microcalorimetry as an Effective Tool for the Determination of Thermodynamic Characteristics of Fulvic–Drug Interactions. Processes. 2025; 13(1):49. https://doi.org/10.3390/pr13010049
Chicago/Turabian StyleKlučáková, Martina, and Jitka Krouská. 2025. "Microcalorimetry as an Effective Tool for the Determination of Thermodynamic Characteristics of Fulvic–Drug Interactions" Processes 13, no. 1: 49. https://doi.org/10.3390/pr13010049
APA StyleKlučáková, M., & Krouská, J. (2025). Microcalorimetry as an Effective Tool for the Determination of Thermodynamic Characteristics of Fulvic–Drug Interactions. Processes, 13(1), 49. https://doi.org/10.3390/pr13010049






