Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Cellulose Nanocrystal (CNCs) from Microcrystalline Cellulose
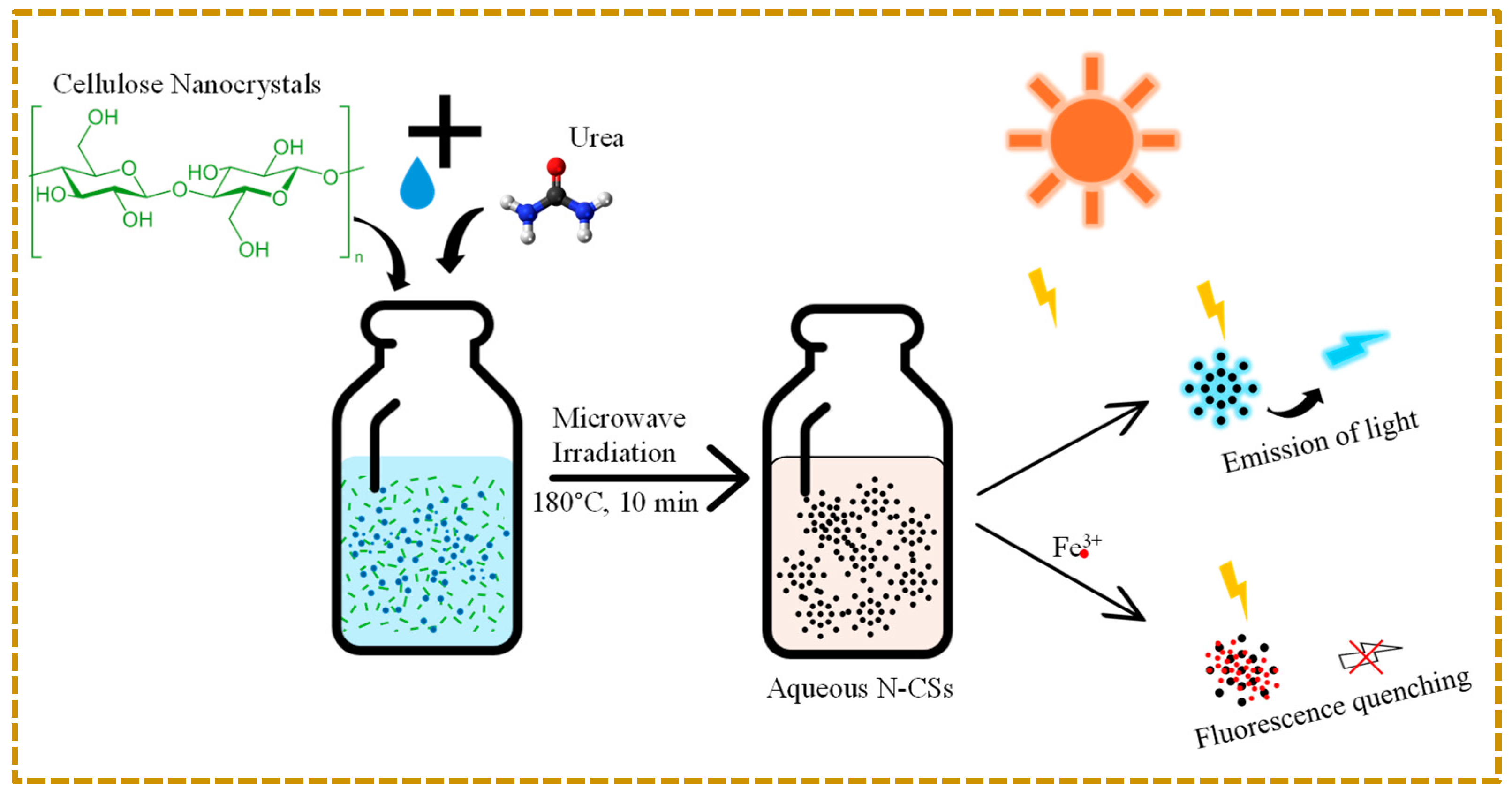
2.3. Synthesis of Nitrogen-Doped Carbon Quantum Dots (N-CQDs) from Cellulose Nanocrystals (CNCs)
2.4. Detection of Fe3+ Using N-CQDs
2.5. Characterization
3. Results
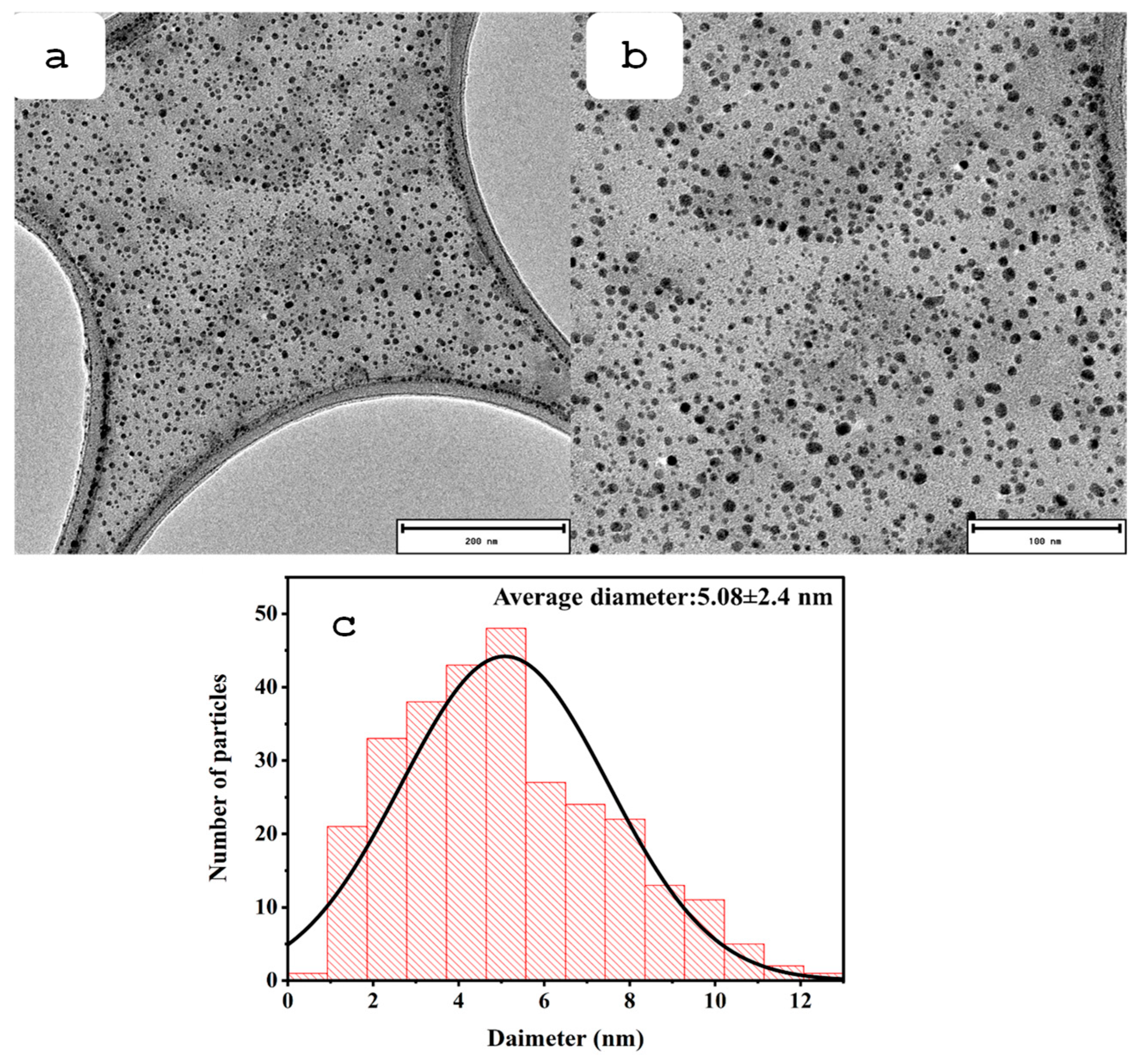
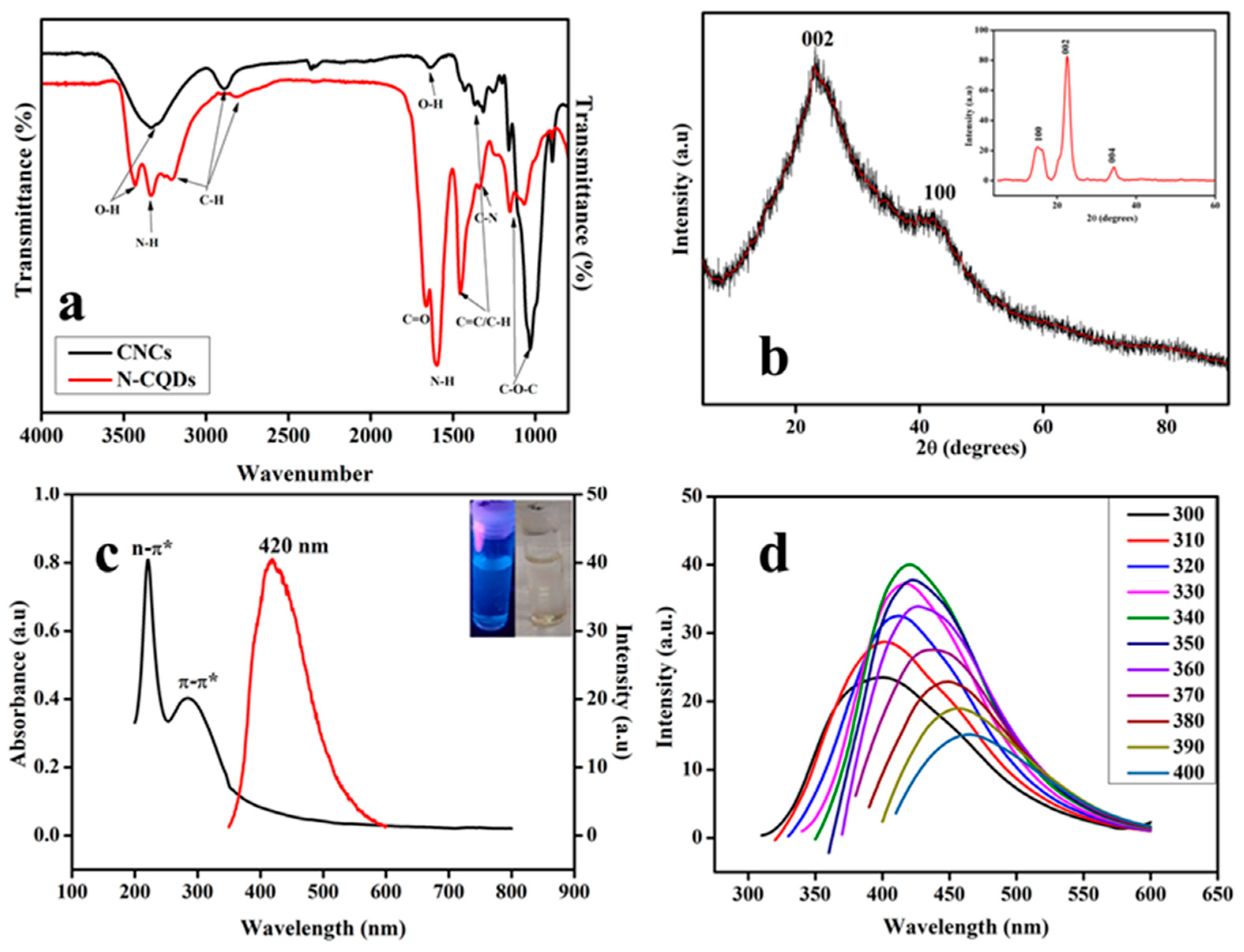
3.1. Properties of the Prepared N-CQDs
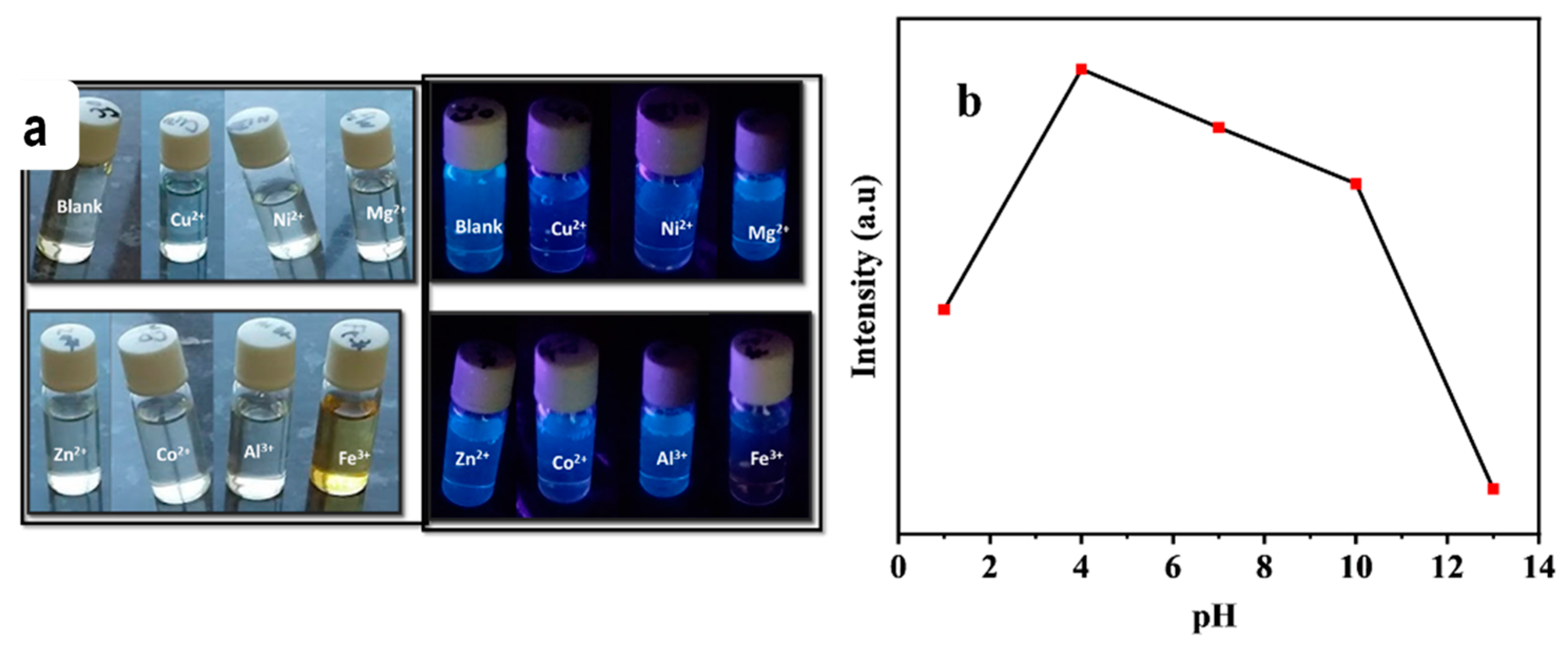
3.2. N-CQDs Sensitivity and Selectivity Towards Fe3+
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, M.A.; Ye, J.; Wang, G.; Shi, L.; Liu, Z.; Lu, H.; Zhang, S.; Ning, G. Bifunctional chemosensor based on a dye-encapsulated metal-organic framework for highly selective and sensitive detection of Cr2O72− and Fe3+ ions. Polyhedron 2020, 185, 114604. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Wu, N.; Xu, M.; Wang, M.; Zhang, L.; Yang, W. In-situ synthesis of carbon dots-embedded europium metal-organic frameworks for ratiometric fluorescence detection of Hg2+ in aqueous environment. Anal. Chem. Acta 2021, 1141, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Meng, F.; Wang, B.; Cheng, Y.; Zhu, C. N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+ detection. New J. Chem. 2015, 39, 3357–3360. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumari, M.; Chauhan, P.; Chaudhary, G.R. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021, 120, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, S.; Luo, Y.; Yuan, X.; Wei, Y.; Zhang, Q.; Qin, K.; Tu, Y. Green synthesis of weissella-derived fluorescence carbon dots for microbial staining, cell imaging and dual sensing of vitamin B12 and hexavalent chromium. Dye. Pigment. 2021, 184, 108818. [Google Scholar] [CrossRef]
- Wang, Y.; Lao, S.; Ding, W.; Zhang, Z.; Liu, S. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sensors Actuators B Chem. 2019, 284, 186–192. [Google Scholar] [CrossRef]
- Dutta, A.; Rooj, B.; Mondal, T.; Mukherjee, D.; Mandal, U. Detection of Co2+ via fluorescence resonance energy transfer between synthesized nitrogen-doped carbon quantum dots and Rhodamine 6G. J. Iran. Chem. Soc. 2020, 17, 1695–1704. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Q.; Jin, C.; Zhang, J.; Wang, B. A fluorescence turn-off chemosensor based on N-doped carbon quantum dots for detection of Fe3+ in aqueous solution. Mater. Lett. 2015, 141, 366–368. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Yang, J.; Wu, F.-G. On-off-on fluorescent nanosensor for Fe3+ detection and cancer/normal cell differentiation via silicon-doped carbon quantum dots. Carbon 2018, 134, 232–243. [Google Scholar] [CrossRef]
- Zulfajri, M.; Gedda, G.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Cranberry Beans Derived Carbon Dots as a Potential Fluorescence Sensor for Selective Detection of Fe3+ Ions in Aqueous Solution. ACS Omega 2019, 4, 15382–15392. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-T.; Yang, J.-X.; Song, J.-M.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Synthesis of high fluorescence graphene quantum dots and their selective detection for Fe3+ in aqueous solution. Sensors Actuators B Chem. 2017, 243, 863–872. [Google Scholar] [CrossRef]
- Deng, X.; Feng, Y.; Li, H.; Du, Z.; Teng, Q.; Wang, H. N-doped carbon quantum dots as fluorescent probes for highly selective and sensitive detection of Fe3+ ions. Particuology 2018, 41, 94–100. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Chai, X.; Zhao, P.; He, H.; Liu, Z. Green production of fluorescent carbon quantum dots based on pine wood and its application in the detection of Fe3+. J. Clean. Prod. 2020, 263, 121561. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Li, H.; Zhang, M.; Liao, Q.; Wang, L.; Zhang, Y.; Niu, X. A fluorescent probe for selective detection of Fe3+ by using a self-assembled nitrogen-doped carbon quantum dots-3,4,9,10-perylenetetracarboxylic acid composite. Ionics 2021, 27, 4907–4916. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, S.; Li, Y.; Li, X.; Fan, L.; Voelcker, N.H. Rhodamine-functionalized graphene quantum dots for detection of Fe3+ in cancer stem cells. ACS Appl. Mater. Interfaces 2015, 7, 23958–23966. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-J.; Pei, W.-W.; Wang, Q.-B.; Liu, G.-F.; Yan, B.; Yao, S.-Q.; Zhai, Q.-G. Decoration of bare carboxyl group on the pore surface of metal-organic frameworks for high selective fluorescence Fe3+ detection. J. Solid State Chem. 2019, 274, 18–25. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, C.; Hao, X.-R.; Li, X.-M.; Yu, X.-H.; Zheng, Y.-P.; Su, Z.-M. Two water-stable Cd(II)-MOFs as multiresponsive chemosensors with high sensitivity and selectivity for Fe3+, Cr2O72−, and MnO4− ions. J. Solid State Chem. 2021, 303, 122538. [Google Scholar] [CrossRef]
- Lu, M.; Duan, Y.; Song, Y.; Tan, J.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liq. 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-Sensitive carbon dots for detection of Fe3+ in aqueous solution and intracellular imaging of Fe3+ inside fungal cells. Front. Chem. 2020, 7, 911. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Zhuo, Y.; Miao, H.; Zhong, D.; Zhu, S.; Yang, X. One-step synthesis of high quantum-yield and excitation-independent emission carbon dots for cell imaging. Mater. Lett. 2015, 139, 197–200. [Google Scholar] [CrossRef]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon dots production via pyrolysis of sago waste as potential probe for metal ions sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Li, Y. Solid-phase synthesis of nitrogen and phosphorus co-doped carbon quantum dots for sensing Fe3+ and enhanced photocatalytic degradation of dyes. Sensors Actuators B Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. Carbon nanodots: A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Ed. 2012, 124, 12381–12384. [Google Scholar] [CrossRef]
- Magagula, L.P. Synthesis and Characterization of Agricultural Waste Carbon-Based Structures for Application in Sensing. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2022. Available online: https://wiredspace.wits.ac.za (accessed on 2 January 2023).
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation, and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.-C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Y.; Zhao, X. On-off-on nanosensors of carbon quantum dots derived from coal tar pitch for the detection of Cu2+, Fe3+, and L-ascorbic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119325. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhao, X.; Deng, Y.; Xia, Y. Facile Synthesis of Nitrogen-Doped Carbon Quantum Dots with Chitosan for Fluorescent Detection of Fe3+. Polymers 2019, 11, 1731. [Google Scholar] [CrossRef]
- Ali, M.S.; Bhunia, N.; Ali, M.S.; Karmakar, S.; Mukherjee, P.; Chattopadhyay, D. Fluorescent N-doped carbon quantum dots: A selective detection of Fe3+ and understanding its mechanism. Chem. Phys. Lett. 2023, 825, 140574. [Google Scholar] [CrossRef]
- He, G.; Shu, M.; Yang, Z.; Ma, Y.; Huang, D.; Xu, S.; Wang, Y.; Hu, N.; Zhang, Y.; Xu, L. Microwave formation and photoluminescence mechanisms of multi-state nitrogen doped carbon dots. Appl. Surf. Sci. 2017, 422, 257–265. [Google Scholar] [CrossRef]
- Masemola, C.M. Nitrogen Doped Graphene Quantum Dots Modified Polyaniline for Room Temperature Alcohol Sensing. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2021. Available online: https://wiredspace.wits.ac.za/server/api/core/bitstreams/4be13bf1-8f08-4fc5-9e03-d51ac348dd49/content (accessed on 5 November 2024).
- Bhakare, M.A.; Bondarde, M.P.; Lokhande, K.D.; Dhumal, P.S.; Some, S. Quick transformation of polymeric waste into highly valuable N-self-doped carbon quantum dots for detection of heavy metals from wastewater. Chem. Eng. Sci. 2023, 281, 119150. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors, and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, A.T.; Arockiaraj, S.D.; Rajasekaran, A.; Vasu, A.E. Morinda coreia fruits derived green-emissive nitrogen-doped carbon quantum dots: Selective and sensitive detection of ferric ions from water. Inorg. Chem. Commun. 2024, 164, 112390. [Google Scholar] [CrossRef]
- Elizabeth, A.T.; James, E.; Jesan, L.I.; Arockiaraj, S.D.; Vasu, A.E. Green synthesis of value-added nitrogen doped carbon quantum dots from Crescentia cujete fruit waste for selective sensing of Fe3+ ions in aqueous medium. Inorg. Chem. Commun. 2024, 149, 110427. [Google Scholar] [CrossRef]
- Patra, S.; Singh, M.; Subudhi, S.; Mandal, M.; Nayak, A.K.; Sahu, B.B.; Mahanandia, P. One-step green synthesis of in–situ functionalized carbon quantum dots from Tagetes patula flowers: Applications as a fluorescent probe for detecting Fe3+ ions and as an antifungal agent. J. Photochem. Photobiol. A Chem. 2023, 442, 114779. [Google Scholar] [CrossRef]
- Bi, J.; Wang, H.; Kamal, T.; Zhu, B.-W.; Tan, M. A fluorescence turn-off-on chemosensor based on carbon nanocages for detection of ascorbic acid. RSC Adv. 2017, 7, 30481–30487. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Ma, C.; Yang, T.; Li, L.; Gu, J.; Zhu, C.; Hu, A.; Li, X.; Guan, W.; et al. Carbon quantum dots as a turn-on fluorescent probe for the sensitive detection of Cd2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 317, 124453. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Luo, J.; Guo, Q.; Liu, K.; Liu, K.; Zhao, W.; Lin, Y. Fe3+-functionalized carbon quantum dots: A facile preparation strategy and detection for ascorbic acid in rat brain microdialysates. Talanta 2015, 144, 1301–1307. [Google Scholar] [CrossRef]



| Detection Method | Sensor Material | Synthesis Method | Average Particle Size (nm) | Linear Range (µM) | LOD (µM) | Reference |
|---|---|---|---|---|---|---|
| Fluorescence | N-CQDs from Morinda coreia | Hydrothermal, 180 °C, 24 h | 1.99 | 0–250 | 1.32 | [39] |
| Fluorescence | N-CQDs from Crescentia cujete fruit | Hydrothermal, 200 °C, 10 h | 4.36 | 0–250 | 0.257 | [40] |
| Fluorescence | N-CQDs from Tagetes patula flowers | Hydrothermal, 220 °C, 5 h | 5.15 | 1–4 | 0.32 | [41] |
| Fluorescence | N-CQDs from cellulose nanocrystals | Microwave, 180 °C, 10 min | 5 ± 2 | 0–500 | 0.075 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magagula, L.P.; Masemola, C.M.; Motaung, T.E.; Moloto, N.; Linganiso-Dziike, E.C. Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions. Processes 2025, 13, 257. https://doi.org/10.3390/pr13010257
Magagula LP, Masemola CM, Motaung TE, Moloto N, Linganiso-Dziike EC. Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions. Processes. 2025; 13(1):257. https://doi.org/10.3390/pr13010257
Chicago/Turabian StyleMagagula, Lindokuhle P., Clinton M. Masemola, Tshwafo E. Motaung, Nosipho Moloto, and Ella C. Linganiso-Dziike. 2025. "Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions" Processes 13, no. 1: 257. https://doi.org/10.3390/pr13010257
APA StyleMagagula, L. P., Masemola, C. M., Motaung, T. E., Moloto, N., & Linganiso-Dziike, E. C. (2025). Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions. Processes, 13(1), 257. https://doi.org/10.3390/pr13010257








