Abstract
Oral disintegration films (ODFs) offer a convenient alternative for administering active compounds with quick absorption, no need for water, customizable formulation, and promising pharmaceutical applications. This study aimed to develop chia mucilage films as a new polymer to carry vitamin C. Chia mucilage was extracted using the method of immersing the seeds in water, separated by vacuum filtration and using a sieve to remove the mucilaginous gel, then centrifuged and finally freeze-dried, with the mucilage obtained being used to produce films using the casting technique. The formulations included a control and a 1% vitamin C variant, with glycerol as a plasticizer. The produced films showed high solubility, pH close to the oral and a disintegration time of 53.17 s for the formulation with 1% vitamin C. The presence of vitamin C in the polymer matrix, as well as the interactions between them, were confirmed by DSC and FTIR spectra. On the first day of storage, after 1 min of reaction at 30 °C, the vitamin C concentration obtained was 477.50 mg/g, while at 40 °C was 411.28 mg/g. After 35 days of storage, the films showed a reduction in vitamin C concentration. Chia mucilage proved to be a promising polymer in the production of ODFs carrying vitamin C.
1. Introduction
Oral disintegrating films (ODFs) have attracted considerable attention as an alternative way to ingest pharmaceutical and food ingredients due to their efficiency and rapid dissolution in the oral cavity. In addition to not requiring water for administration, they have a larger contact surface and greater bioavailability due to high blood flow and permeability of the oral mucosa [1]. ODF is normally composed of a water-soluble polymeric matrix capable of adhering to the oral mucosa (gingival, sublingual, and palatine), allowing systemic transmucosal absorption [2]. The possibility of sublingual and buccal release and absorption, in addition to not experiencing first-pass effects, can increase the bioavailability of some drugs, use smaller doses, and reduce adverse effects. In addition, they can be used to achieve local or systemic effects [1,3]. ODFs can be produced by the casting process (solvent evaporation) [4,5]. This process involves mixing the polymer in solution with a substrate, followed by evaporation of the solvent, which provides the molecular orientation of the polymer molecules, resulting in film formation [6]. The dose of a bioactive compound can be determined based on the thickness and size of the film formed after drying. Several possible matrices are used to produce ODFs, such as sodium alginate [5], gelatin, starch [7], chitosan [8], and hydroxypropylmethyl cellulose [9], in addition to chia mucilage, which has been explored as a new polymer for ODFs.
Salvia hispanica (chia seeds) is a renewable resource with great potential for the production of edible and biodegradable films, due to their composition [10]. Chia mucilage is composed of a combination of xylose, glucose and glucuronic acid, forming a branched polysaccharide. This gel, with its strongly cohesive structure, is released on the surface of the seeds when immersed in water [11]. Mucilage extraction is performed with whole seeds immersed in water, and the mixture is stirred to generate three distinct phases: water, seed residues, and mucilage. Subsequently, mucilage is separated from the seeds and water can be eliminated by heating or freeze drying. Currently, chia seed mucilage, a new source of hydrocolloids, has gained more appeal in the food industry due to its biodegradability and biocompatibility. They have different functional properties for industry, such as water retention capacity, emulsifying, thickening, stabilizing, and solubility in hot and/or cold water, and chia seed mucilage is a potential source of hydrocolloids [12,13]. Few studies have been conducted on the use of chia mucilage in the production of oral disintegration films; therefore, we sought to produce a film and evaluate its characteristics. These characteristics are desirable for film applications, such as high solubility in water, ability to form uniform and biodegradable films, and good adhesion to mucosal surfaces.
Vitamin C, also known as ascorbic acid (AA), in its pure form, is water-soluble and represents a structural and functional set of several organic molecules that play crucial roles in maintaining the metabolism, energy, differentiation, and proliferation of cells [14,15]. Vitamin C can minimize the risk of several diseases (cancer, heart disease, and cataracts), and its deficiency is associated with a disease known as scurvy [16]. However, the human body is unable to synthesize and store this vitamin. Therefore, adequate amounts must be ingested through diet or supplements [17]. Thus, looking for a new polymer for ODF films aiming at vitamin C supplementation, the objective of this study was to develop a chia mucilage film with added vitamin C as a possible carrier vehicle for active compounds.
2. Materials and Methods
2.1. Materials
Chia was provided by Produza Foods, glycerol was purchased from Êxodo Científica (Sumaré, Brazil). Vitamin C was purchased from Sigma-Aldrich (São Paulo, Brazil). Analytical grade chemical reagents were used in this study. Chia mucilage was extracted according to the method described by Fernandes and Salas-Mellado [18]. Chia seeds were immersed in distilled water at a seed/water ratio of 1:40 and mechanically shaken using a shaker (Cientec, model CT-712RNT, São Paulo, Brazil) for at least 2 h at 150 rpm and 25 °C. The mucilage was separated from the chia seeds by filtration with a vacuum pump and sieve (18 mesh) to remove the mucilaginous gel strongly bound to the chia seed coat, and subsequently centrifuged for 20 min at 11,600× g (Hanil, model Supra 22 K, Gyeonggi-do, Repulic of Korea). The mucilaginous gel was freeze dried (Liobrás, model L108, São Carlos, SP, Brazil), packaged in plastic containers, and stored at −18 °C for later use.
2.2. Films Production
The films were prepared using the casting technique, according to the method described by Fernandes et al. [10]. 1% (w/v) chia mucilage was hydrated at 25 °C for 1 h, and then glycerol was added. The pH was adjusted to 9 and the temperature was maintained at 80 °C for 30 min under mechanical stirring (1000 rpm). Subsequently, vitamin C (1%) was added to the solution, which was poured into Petri dishes (9 cm in diameter) and dried in an oven with air circulation (BIOPAR, model S36BA, Porto Alegre, RS, Brazil). The films (3 cm × 2 cm) were called chia mucilage films (CMFs) and chia mucilage films containing 1% vitamin C (CMFs + vit C).
2.3. Characterization of Physical, Mechanical and Barrier Properties of the Films
The thickness was measured using a micrometer (Insize, model IP54, Boituva, SP, Brazil) at ten random positions on the film. The mechanical properties of tensile strength (MPa) and elongation at break (%) were determined using a texture analyzer (Stable Micro Systems, model TA.XTplus, Godalming, UK) according to the standard method D-882-02 [19]. The fold endurance of the ODFs was measured by bending the film in place until it broke [20]. Three ODFs samples were tested, and the number of bends was recorded as the fold endurance value. Water vapor permeability (WVP) was evaluated according to the E96-00 method [21]. Water solubility (%) was determined gravimetrically as described by Gontard et al. [22]. Physical, mechanical, and barrier analyses were performed after the films rested for 24 h in a desiccator with a controlled relative humidity of 75%. The color parameters and opacity of the films were evaluated using a colorimeter (Konica Minolta, model CR-400, Tokyo, Japan) and the Illuminant D65 standard. The CIELab scale was applied to determine the L * (luminosity), a * (green/red), and b * (yellow/blue) parameters, which were used to determine the color difference ΔE between the samples. The films were stored in an incubator Biochemical Oxygen Demand (BODs) (Marconi, model MA415, São Paulo, Brazil) at 30 °C and 40 °C, with samples being taken for analysis every 7 days over a 35-day storage period. Correlation was assessed using Pearson’s correlation coefficient (r).
2.4. Surface pH
The surface pH was evaluated according to Prabhu et al. [23] using a phosphate buffer prepared according to Wong et al. [24]. ODFs (3 cm × 2 cm) were placed in contact with the buffered solution (0.5 mL), and the pH was determined after 1 min using a pH measuring electrode (Q400AS). The films were analyzed in triplicate after 24 h of rest in a desiccator with a relative humidity of 75%.
2.5. Disintegration Time
The oral disintegration time of the films was determined, as described by Perumal et al. [25], in a saline phosphate buffer solution (pH = 6.8) that simulates saliva. The films (3 × 2 cm) were placed in 50 mL of phosphate buffer solution at 37 °C and stirred at 100 rpm at a controlled temperature. The films were analyzed in triplicate after 24 h of rest in a desiccator with a relative humidity of 75%.
2.6. Differential Scanning Calorimetry (DSC)
The thermal properties of the films and vitamin C were evaluated by differential scanning calorimetry (DSC) in the temperature range 30–250 °C using a calorimeter (Shimadzu, model TA-60WS, New Castle, DE, USA) at a heating rate of 10 °C/min.
2.7. Fourier Transform-Infrared Spectroscopy (FT-IR)
The functional groups present in the films and vitamin C were determined in the spectra obtained by Fourier transform infrared spectroscopy (FT-IR) in the range 400–4000 cm−1 using an FT-IR spectrometer (Shimadzu, model IRPrestige-21, New Castle, DE, USA). The films were stored for 24 h in a desiccator at a controlled relative humidity of 75%.
2.8. Assessment of the Stability of Vitamin C in Films During the Storage
The chia mucilage films containing 1% vitamin C were placed in closed containers, wrapped in aluminum foil to prevent the passage of light, and stored in BOD incubators at 30 °C and 40 °C for 35 days. Samples were taken every 7 days for quantification of vitamin C. Samples (2 × 2 cm) of the films were weighed, immersed in 50 mL of distilled water, and mechanically shaken (37 °C) in an orbital shaker (Tecnal, model TE420, Piracicaba, SP, Brazil). At predetermined times (1, 5, 15, 30, and 60 min), aliquots (0.5 mL) were removed for vitamin C analysis [7]. The vitamin C present in the films was analyzed using high-performance liquid chromatography (HPLC). Aliquots (20 μL) were injected into a C18 column (Zorbax, model Eclipse XDB, Bellefonte, PA, USA) (4.6 × 150 mm, 5 μM) and its absorbance at 244 nm was monitored. The column was eluted at a flow rate of 1.0 mL/min using ultrapure water (pH 2.6) that was acidified with metaphosphoric acid. The samples were analyzed in duplicate.
2.9. Statistical Analysis
Analysis of variance (ANOVA), followed by Tukey’s test to verify significant differences (p < 0.05) over the storage time (7, 14, 21, 28 and 35 days), and Student’s t-test (p < 0.05) were performed to compare the characteristics of the two samples with each other and in relation to the two storage temperatures. The data were evaluated using a statistical program (version 5.0, StatSoft, Inc., Tulsa, OK, OH, USA).
3. Results
The study aimed to develop a chia mucilage film as a new polymer for the production of ODF films, with the addition of vitamin C as a possible carrier for active compounds. The films were produced by casting and evaluated for their mechanical and barrier properties, color, surface pH, disintegration time, and thermal properties, in addition to investigating the concentration of vitamin C in the films, as well as their stability over a period of storage, wherein the results are presented below.
3.1. Visual Aspects, Mechanical and Barrier Properties of the Films
The control film maintained its integrity and resistance without the occurrence of breaks or tears during removal from the plate, and presented some dark spots, possibly from some chia seeds. For the formulation with vitamin C, the film was homogeneous without bubbles, with good compatibility between mucilage and vitamin C (Figure 1).

Figure 1.
(A) chia mucilage control film (CMF) and (B) chia mucilage film with 1% vitamin C (CMF + Vit C).
The film thickness increased with the addition of vitamin C (Table 1), which was expected because an increase in the vitamin C concentration increased the number of total solids. However, the films were within the rapid dissolution classification according to Jyoti et al. [26].

Table 1.
Thickness, mechanical, barrier, surface pH and disintegration time properties of the films.
The tensile strength and elongation of the films were altered with the addition of vitamin C. The tensile strength of CMF differed significantly (p < 0.05) from the formulation with vitamin C.
The films exhibited values above 300 folds for the folding hardness test.
The films demonstrated complete solubility in water due to the hydrophilic nature of chia mucilage and vitamin C.
High water solubility is a relevant attribute for the application of polymers in the manufacture of controlled-release products of active compounds [27].
The incorporation of vitamin C into the films resulted in a 68% increase in water vapor permeability (WVP). The increase in WVP in films with vitamin C may be related to the crystallinity, molecular orientation, and affinity between the polymer and the active compound [28].
The color parameters for the films with vitamin C are listed in Table 2 at temperatures of 30 °C and 40 °C. High lightness values were observed at both temperatures despite some statistically significant differences. Through ∆E, it was observed that the color changed significantly over time, suggesting that the higher temperature resulted in greater degradation or modification of vitamin C.

Table 2.
Color analysis of chia mucilage films with vitamin C during storage at 30 °C and 40 °C.
By correlating the color parameters and the concentration of vitamin C (Figure 1), significant relationships were observed, varying in intensity depending on the temperature and the parameter evaluated. The luminosity parameter (L *) presented a moderate positive correlation at 30 °C (r = 0.55) and a strong one at 40 °C (r = 0.7), indicating that greater luminosity is associated with greater concentrations of vitamin C, especially in higher temperature conditions. On the other hand, the parameters a * (green/red) and b * (blue/yellow) showed moderate negative correlations at 30 °C (r = −0.55 and r = −0.54 respectively) and strong at 40 °C (r = −0.7 for both), suggesting that changes in hue towards red and yellow accompany the degradation of vitamin C. The parameter ΔE, which reflects the total color variation, showed a strong negative correlation at 30 °C (r = −0.75) and moderate at 40 °C (r = −0.42), reinforcing the finding that more pronounced changes in color are related to a reduction in the concentration of vitamin C. These results highlight the influence of storage conditions on the stability of vitamin C and the appearance of the films.
3.2. Surface pH
The inclusion of vitamin C in the formulation resulted in a reduction in the surface pH value of 5.07 when compared to the control film of 6.45 (Table 1).
3.3. Disintegration Time
The results in Table 1 demonstrate that the control film presented a disintegration time of 39.97 ± 1.41 s. When vitamin C was added to the film, a significant increase in the disintegration time was observed (53.17 ± 1.10 s).
3.4. Differential Scanning Calorimetry (DSC)
The results showed that the addition of vitamin C to the films increased their melting temperature compared to that of the control film (Figure 2). This increase in melting temperature suggests that the presence of ascorbic acid affects the intermolecular forces and molecular structure of the films, leading to a change in the thermal properties, with a higher melting temperature indicating more thermally stable films.
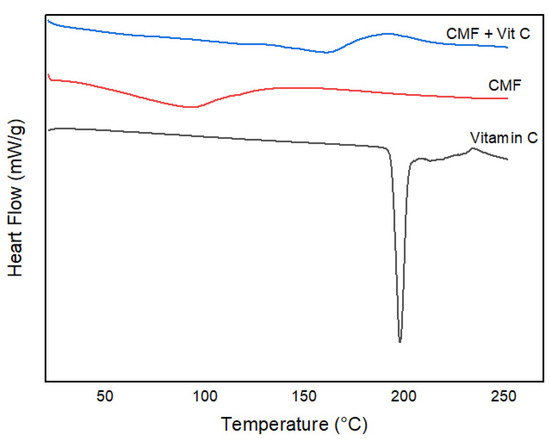
Figure 2.
DSC curves for chia mucilage control film (CMF), chia mucilage film with 1% vitamin C (CMF + Vit C), and pure vitamin C.
3.5. Fourier Transform-Infrared Spectroscopy (FT-IR)
The film with vitamin C presented bands with characteristic peaks of vitamin C present between 1678 and 1116 cm−1 (Figure 3), associated with the C = C stretching and the enol-hydroxyl peak. In addition to the stretching vibrations of C = O, C = C, C = C–OH (enol group), and C–O–C bonds [29,30]. For the control chia mucilage film, the observed bands were characteristic of a polysaccharide, located from 3562 to 1176 cm−1, and were mainly attributed to hydroxyl groups that constitute the carbohydrate structure. In addition to the carboxyl groups of uronic acids, carbonyl groups, and C-O-C glycosidic bonds.
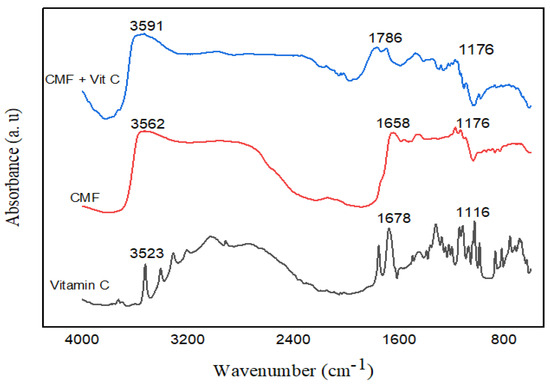
Figure 3.
FT-IR curves of chia mucilage control film (CMF), chia mucilage film with 1% vitamin C (CMF + Vit C), and pure vitamin C.
3.6. Assessment of the Stability of Vitamin C in Films During the Storage
The films were analyzed by observing the stability of vitamin C during storage at 30 and 40 °C (Table 3 or Figure S1 of Supplementary Material). According to the World Health Organization [31], the recommended daily intake of vitamin C is 45 mg/day.

Table 3.
Vitamin C content in chia mucilage films at 30 °C and 40 °C during the storage period.
The results presented in Table 3 demonstrate significant variations in vitamin C concentration during the storage of chia mucilage films. At 30 °C on the first day, the concentration starts at 477.50 mg/g after 1 min of reaction, decreasing to 220.59 mg/g at 60 min. Over the course of 35 days, a noticeable decline in vitamin C stability occurred, with its concentration decreasing to 329.87 mg/g, which was attributed to the degradation of ascorbic acid caused by exposure to heat, light, and oxygen.
At 40 °C, the initial vitamin C concentration was 411.28 mg/g, decreasing to 225.24 mg/g on day 35. The overall loss represents 54.7%, which reflects the sensitivity of the compound to higher temperatures. Despite degradation, the films retained approximately ten times the Recommended Daily Intake (RDI) of vitamin C, highlighting their potential as an effective supplemental source.
4. Discussion
The addition of vitamin C to the films directly influenced several physicochemical properties, including thickness, tensile strength, elongation, and solubility. These results demonstrate the multifunctionality of vitamin C, which acts as a structural and functional additive, in addition to reinforcing the feasibility of its incorporation into biopolymers for applications in biodegradable packaging and orodispersible films.
The increase in film thickness with the addition of vitamin C, observed in this research, is related to the increase in the concentration of total solids in the film-forming solution. This behavior is consistent with the study by Khodaman et al. [32], who reported an increase in the thickness of films composed of chia mucilage, gelatin, and chitosan nanoparticles. Similar values, ranging from 0.096 to 0.140 mm, highlight that the increase in solids content contributes significantly to film thickness. Despite this, the films developed in this study maintained their classification as fast dissolving films, according to the criteria of Jyoti et al. [26], reinforcing its applicability for oral purposes.
Vitamin C significantly reduced the tensile strength of the films while increasing the elongation property. This behavior can be attributed to the function of vitamin C as a plasticizer, decreasing the molecular interactions between the polymer chains and increasing their mobility. Garcia et al. [3] observed similar effects in starch films containing acerola powder, which also resulted in lower tensile strength and greater flexibility. This balance between strength and elasticity is crucial for practical applications, ensuring that the films are not brittle and can withstand handling.
The folding resistance of the developed films, greater than 300 folds, indicates excellent flexibility and structural integrity, essential characteristics to ensure functionality during handling, transportation, and application. This performance is in line with the findings of Sothornvit and Krochta [33], who demonstrated that folding resistance is directly influenced by the interaction between biopolymers and plasticizers, such as glycerin, which increase the mobility of the polymer chains. The high folding resistance also suggests that the films have good mechanical strength without becoming brittle, a critical point for applications such as orodispersible films and biodegradable packaging. Thus, this parameter reinforces the viability of the films as functional and sustainable alternatives to conventional materials.
The high solubility observed in films containing vitamin C is consistent with the results of Khodaman et al. [32], who reported solubility of up to 99.77% in chia mucilage films with chitosan nanoparticles. The combination of high solubility and a disintegration time of less than 60 s, as observed in this study, is essential for applications such as orodispersible films (ODFs). Studies such as that of Sapiee et al. [34] reinforce the importance of adequate morphology and thickness for fast disintegration times, with results ranging from 3.68 to 4.87 s for vitamin C films based on PVA and κ-carrageenan.
The color parameters (L *, a *, b *) indicated significant changes associated with vitamin C concentration and storage conditions. The strong positive correlation between L ∗ and vitamin C concentration at 40 °C (r = 0.7) suggests that brighter films better preserve the compound. In contrast, the negative values for a * and b * suggest a chromatic shift towards red and yellow, accompanying vitamin C degradation, which was reported by Garcia et al. [7] in starch/gelatin films with Camu Camu powder. This pattern confirms the high sensitivity of vitamin C to heat, oxygen, and light, as described by Palezi et al. [5].
The pH of the oral cavity can also impact the stability and release of vitamin C. A difference in pH can affect the dissolution rate and potentially accelerate the degradation of vitamin C, which is less stable in neutral or alkaline environments [35]. Bharti et al. [36] comment that surface pH close to neutral indicates non-irritation of the oral mucosa and greater acceptance by the consumer. Garcia et al. [7] produced gelatin and starch films as vehicles for releasing vitamin C and presented surface pH values between 4.9 and 5.7.
This increase could be attributed to the thickness of the films, as an increase in the number of total solids in the formulation was observed. Even with the increase in the disintegration time, it remained below 60 s, which is important to be considered an ODF [34]. Sapiee et al. [34] developed combined films of κ-carrageenan and, polyvinyl alcohol (PVA) with vitamin C and obtained very short disintegration times for the formulations, ranging from 3.68 to 4.87 s. This behavior correlated well with the morphology and size of the fibers presented in this study.
Thermal analysis (DSC) and FT-IR spectroscopy indicated structural changes induced by the addition of vitamin C. The increase in melting temperature suggests greater thermal stability, consistent with the findings of Palezi et al. [5] who studied the addition of different concentrations of ascorbic acid in a sodium alginate matrix and found endothermic peak values of 72.3 °C for films added with 1% vitamin C. Characteristic bands of polysaccharides observed in the FT-IR spectra, such as those reported by Salazar Vega et al. [37], studied the thermal characterization of biodegradable films of chia mucilage (Salvia hispanica L.) and bands located between 3700 and 950 cm−1 for the hydroxyl group, results similar to those of this study confirm the partial preservation of the chemical structure of the biopolymer even after the incorporation of vitamin C.
The stability of vitamin C in chia mucilage films showed significant variations depending on storage conditions. The results of this study show greater degradation at higher temperatures, with more pronounced losses at 40 °C than at 30 °C. This behavior is related to the intrinsic instability of vitamin C, which is highly sensitive to environmental factors, such as heat, oxygen, and light.
Previous studies corroborate this observation, and work with other polymers also indicates that the choice of matrix influences the stability of vitamin C as Palezi et al. [5], in their study on an orally disintegrating film based on sodium alginate added with vitamin C, observed a concentration of 239.5 mg/g for the formulation containing 1% vitamin C. Garcia et al. [7] produced starch/gelatin film supplemented with Camu Camu powder. The authors reported that the Camu Camu powder used contained 6.75 g of ascorbic acid/100 g, and the developed ODFs showed a reduction of more than 40% in their ascorbic acid content, with values of 40.0 to 22.5 mg/g ODF after 30 days of storage.
To date, no published studies have specifically developed oral disintegration films using chia mucilage with vitamin C. However, chia mucilage has been explored for several applications related to the formation of edible films and biopolymeric materials, due to its film-forming and bioactive properties.
This study presents several strengths, highlighting the innovative approach of incorporating vitamin C into films based on chia mucilage, a biopolymeric matrix with bioactive properties and excellent film-forming capacity. Furthermore, the analysis of mechanical, thermal, optical, and structural properties provides an understanding of the impact of vitamin C, making the results relevant for both industrial applications and academic research. However, some limitations should be acknowledged. The stability of vitamin C was evaluated under controlled temperature conditions, but additional factors, such as exposure to UV light and different humidity levels, could have been explored to simulate real storage conditions. Furthermore, the absence of direct comparison with other polymeric matrices limits the generalizability of the findings. Future studies can expand on these aspects, including controlled-release analyses in biological systems and the evaluation of the biocompatibility of the films. Finally, the manufacture of ODFs requires specific technological processes, such as strict control of thickness and dose uniformity, which can increase production costs compared to more traditional dosage forms. These aspects highlight the need for additional strategies to optimize the formulation of ODFs, such as combination with antioxidants or protective coatings, to maximize the advantages of this pharmaceutical form over tablets, capsules, and liquid solutions.
5. Conclusions
This study developed and characterized chia mucilage-based oral disintegrating films (ODFs) enriched with vitamin C, highlighting their potential as innovative and sustainable delivery systems for bioactive compounds. The films demonstrated desirable properties, such as oral mucosa compatibility and consumer ease of use.
The incorporation of vitamin C significantly influenced the physical and mechanical properties of the films, ensuring durability and adaptability during handling. The films maintained high folding strength (>300 folds), reinforcing their robustness and reliability for practical applications. Furthermore, thermal and spectral analyses confirmed the successful integration of vitamin C into the polymer matrix.
Storage conditions played a critical role in the stability of vitamin C, with significant degradation observed at higher temperatures, particularly at 40 °C. Despite this, the films retained considerable concentrations of vitamin C, highlighting their potential as effective supplementation vehicles. However, the findings emphasize the need to optimize storage protocols to maximize stability and efficacy over time. The use of chia mucilage as a polymer matrix has shown significant promise due to its excellent film-forming ability, biodegradability, and compatibility with active compounds. These attributes position it as a sustainable alternative to conventional polymers in pharmaceutical and nutraceutical applications. However, future studies should focus on improving the chemical stability of vitamin C within the matrix, assessing its in vivo bioavailability, and comparing its performance with other delivery methods. These efforts will further validate the relevance of chia mucilage-based ODFs as a viable and innovative alternative for the delivery of active compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr13010250/s1, Figure S1: (a) Vitamin C content in chia mucilage film at 30 °C; (b) Vitamin C content in chia mucilage film at 40 °C.
Author Contributions
Conceptualization, S.C.P. and V.G.M.; formal analysis, S.C.P., J.M.L. and S.S.F.; data curation, S.C.P., J.M.L., S.S.F. and V.G.M.; writing—original draft preparation, S.C.P., J.M.L., S.S.F. and V.G.M.; writing—review and editing, S.C.P., J.M.L., S.S.F. and V.G.M.; supervision, S.S.F. and V.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Finance code 001) and la ValSe-Food-CYTED (Ref. 119RT0567), and FURG.
Data Availability Statement
The original contributions of this study are included in this article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pezik, E.; Gulsun, T.; Sahin, S.; Vural, İ. Development and Characterization of Pullulan-Based Orally Disintegrating Films Containing Amlodipine Besylate. Eur. J. Pharm. Sci. 2021, 156, 105597. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.G.; De Carvalho, R.A. Orally Disintegrating Films Containing Propolis: Properties and Release Profile. J. Pharm. Sci. 2015, 104, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Garcia, V.A.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally Disintegrating Films Based on Gelatin and Pregelatinized Starch: New Carriers of Active Compounds from Acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]
- Resta, V.G.; Mali, S. Efeito De Sacarose E Glicerol Como Plastificantes Em Filmes Orodispersíveis De Amido E Gelatina. Iniciação Científica Cesumar 2019, 21, 15. [Google Scholar] [CrossRef]
- Palezi, S.C.; Fernandes, S.S.; Barbosa, S.C.; Primel, E.G.; Martins, V.G. Orally Disintegrating Films Based on Sodium Alginate as a Carrier Vehicle for Vitamin C. Int. J. Food Sci. Technol. 2023, 59, 864–871. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of Gelatin/Zein Films Fabricated by Electrospinning vs Solvent Casting; Elsevier: Amsterdam, The Netherlands, 2018; Volume 74, ISBN 8657188982981. [Google Scholar]
- dos Santos Garcia, V.A.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; Lapa-Guimaraes, J.d.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin/Starch Orally Disintegrating Films as a Promising System for Vitamin C Delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Verma, U.; Rajput, R.; Naik, J.B. Development and Characterization of Fast Dissolving Film of Chitosan Embedded Famotidine Using 32 Full Factorial Design Approach. Mater. Today Proc. 2018, 5, 408–414. [Google Scholar] [CrossRef]
- Singh, H.; Singla, Y.P.; Narang, R.S.; Pandita, D.; Singh, S.; Narang, J.K. Frovatriptan Loaded Hydroxy Propyl Methyl Cellulose/Treated Chitosan Based Composite Fast Dissolving Sublingual Films for Management of Migraine. J. Drug Deliv. Sci. Technol. 2018, 47, 230–239. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Romani, V.P.; da Silva Filipini, G.; Martins, V.G. Chia Seeds to Develop New Biodegradable Polymers for Food Packaging: Properties and Biodegradability. Polym. Eng. Sci. 2020, 60, 2214–2223. [Google Scholar] [CrossRef]
- Xiao, Z.; Yan, C.; Jia, C.; Li, Y.; Li, Y.; Li, J.; Yang, X.; Zhan, X.; Ma, C. Structural Characterization of Chia Seed Polysaccharides and Evaluation of Its Immunomodulatory and Antioxidant Activities. Food Chem. X 2023, 20, 101011. [Google Scholar] [CrossRef] [PubMed]
- Balbino, T.R.; Sánchez-Muñoz, S.; Díaz-Ruíz, E.; Rocha, T.M.; Mier-Alba, E.; Custódio Inácio, S.; Jose Castro-Alonso, M.; de Carvalho Santos-Ebinuma, V.; Pereira, J.F.B.; Santos, J.C.; et al. Lignocellulosic Biorefineries as a Platform for the Production of High-Value Yeast Derived Pigments—A Review. Bioresour. Technol. 2023, 386, 129549. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Aguilera, J.M.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and Microstructure of Films Made from Mucilage of Salvia Hispanica and Whey Protein Concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Abd El Azim, H.; Nafee, N.; Ramadan, A.; Khalafallah, N. Liposomal Buccal Mucoadhesive Film for Improved Delivery and Permeation of Water-Soluble Vitamins. Int. J. Pharm. 2015, 488, 78–85. [Google Scholar] [CrossRef]
- Said, H.M. Recent Advances in Carrier-Mediated Intestinal Absorption of Water-Soluble Vitamins. Annu. Rev. Physiol. 2004, 66, 419–446. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Levine, M.; Dutta, A.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and Chromosomal Mapping of the Human Nonfunctional Gene for L-Gulono-γ-Lactone Oxidase, the Enzyme for L-Ascorbic Acid Biosynthesis Missing in Man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.S.; Salas-Mellado, M.d.l.M. Addition of Chia Seed Mucilage for Reduction of Fat Content in Bread and Cakes. Food Chem. 2017, 227, 237–244. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002.
- Carolina Visser, J.; Weggemans, O.A.F.; Boosman, R.J.; Loos, K.U.; Frijlink, H.W.; Woerdenbag, H.J. Increased Drug Load and Polymer Compatibility of Bilayered Orodispersible Films. Eur. J. Pharm. Sci. 2017, 107, 183–190. [Google Scholar] [CrossRef]
- ASTM E96-00; Standard Test Methods for Water Vapor Transmission of Material. ASTM International: West Conshohocken, PA, USA, 2000.
- Gontard, N.; Duchez, C.; Cuq, J.L.; Gilbert, S. Edible Composite Films of Wheat and Lipids: Water Vapor Permeability and Other Physical Properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Prabhu, P.; Malli, R.; Koland, M.; Vijaynarayana, K.; D′Souza, U.; Harish, N.; Shastry, C.; Charyulu, R. Formulation and Evaluation of Fast Dissolving Films of Levocitirizine Di Hydrochloride. Int. J. Pharm. Investig. 2011, 1, 99. [Google Scholar] [CrossRef]
- Wong, C.F.; Yuen, K.H.; Peh, K.K. An In-Vitro Method for Buccal Adhesion Studies: Importance of Instrument Variables. Int. J. Pharm. 1999, 180, 47–57. [Google Scholar] [CrossRef]
- Perumal, V.A.; Lutchman, D.; Mackraj, I.; Govender, T. Formulation of Monolayered Films with Drug and Polymers of Opposing Solubilities. Int. J. Pharm. 2008, 358, 184–191. [Google Scholar] [CrossRef]
- Jyoti, A.; Gurpreet, S.; Seema, S.; Rana, A.C. Fast Dissolving Films: A Novel Approach To Oral Drug Delivery. Int. Res. J. Pharm. 2011, 2, 69–74. [Google Scholar]
- Yu, S.H.; Tsai, M.L.; Lin, B.X.; Lin, C.W.; Mi, F.L. Tea Catechins-Cross-Linked Methylcellulose Active Films for Inhibition of Light Irradiation and Lipid Peroxidation Induced β-Carotene Degradation. Food Hydrocoll. 2015, 44, 491–505. [Google Scholar] [CrossRef]
- Cupone, I.E.; Dellera, E.; Marra, F.; Giori, A.M. Development and Characterization of an Orodispersible Film for Vitamin D3 Supplementation. Molecules 2020, 25, 5851. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Encapsulation of Vitamin C in Tripolyphosphate Cross-Linked Chitosan Microspheres by Spray Drying. J. Microencapsul. 2005, 22, 179–192. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A Green Method for the Synthesis of Copper Nanoparticles Using L-Ascorbic Acid. Rev. Mater. 2014, 19, 197–203. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004; ISBN-10:9241546123/ISBN-13:978-9241546126. [Google Scholar]
- Khodaman, E.; Barzegar, H.; Jokar, A.; Jooyandeh, H. Production and Evaluation of Physicochemical, Mechanical and Antimicrobial Properties of Chia (Salvia hispanica L.) Mucilage-Gelatin Based Edible Films Incorporated with Chitosan Nanoparticles. J. Food Meas. Charact. 2022, 16, 3547–3556. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer Effect on Mechanical Properties of β-Lactoglobulin Films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Sapiee, N.H.; Mat Saufi, M.H.; Abu Bakar, N.F.; Adam, F. Fabrication and Characterization of Electrospun κ-Carrageenan Based Oral Dispersible Film with Vitamin C. Mater. Today Proc. 2023, in press. [CrossRef]
- Wagner, B.A.; Buettner, G.R. Stability of Aqueous Solutions of Ascorbate for Basic Research and for Intravenous Administration. Adv. Redox Res. 2023, 9, 100077. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and Characterization of Fast Dissolving Oral Films Containing Buspirone Hydrochloride Nanoparticles Using Design of Experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Salazar Vega, I.M.; Quintana Owen, P.; Segura Campos, M.R. Physicochemical, Thermal, Mechanical, Optical, and Barrier Characterization of Chia (Salvia hispanica L.) Mucilage-Protein Concentrate Biodegradable Films. J. Food Sci. 2020, 85, 892–902. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).